- Department of Neurology, Dignity Health, St Joseph’s Hospital and Medical Center, Lewis Headache Clinic, Barrow Neurological Institute, Phoenix, AZ, United States
Background: Refractory migraine is a poorly described complication of migraine in which migraine has chronified and become resistant to standard treatments. The true prevalence is unknown, but medication resistance is common in headache clinic patient populations. Given the lack of response to treatment, this patient population is extremely difficult to treat with limited guidance in the literature.
Objective: To review the diagnostic, pathophysiological, and management challenges in the refractory migraine population.
Discussion: There are no accepted, or even ICHD-3 appendix, diagnostic criteria for refractory migraine though several proposed criteria exist. Current proposed criteria often have low bars for refractoriness while also not meeting the needs of pediatrics, lower socioeconomic status, and developing nations. Pathophysiology is unknown but can be hypothesized as a persistent “on” state as a progression from chronic migraine with increasing central sensitization, but there may be heterogeneity in the underlying pathophysiology. No guidelines exist for treatment of refractory migraine; once all guideline-based treatments are tried, treatment consists of n-of-1 treatment trials paired with non-pharmacologic management.
Conclusion: Refractory migraine is poorly described diagnostically, its pathophysiology can only be guessed at by extension of chronic migraine, and treatment is more the art than science of medicine. Navigating care of this refractory population will require multidisciplinary care models and an emphasis on future research to answer these unknowns.
Introduction
Refractory migraine is poorly understood but likely represents migraine progression. These patients are resistant to guideline-based treatment, though the threshold for refractory is a matter of debate. Refractory is the most common term used though previous publications have used the term intractable and recently the European Headache Federation (EHF) proposed resistant migraine as a stage before refractory migraine (1–8).
Migraine is present in 14–15% of the population with a female preponderance (9). Chronic migraine has at least 15 headache days per month, of which 8 are migraine days, and represents 6.6–8.8% of patients with migraine (10). The proportion of patients refractory to treatment is unknown as no consistent diagnostic criteria have been accepted into the International Classification of Headache Disorders 3rd edition (ICHD-3) (11). Headache disorders are rated the second most disabling condition worldwide based on years lived with a disability (12). Those with refractory migraine are likely among the most disabled of the migraine population.
The purpose of this review article is to provide a comprehensive overview of the current knowledge and theories regarding refractory migraine. It will focus on the diagnosis, hypothesized pathophysiology, and management of refractory migraine. Additionally, the review will highlight the existing gaps in understanding and suggest future directions for research in this field.
Diagnostic challenges
Differentiating refractory migraine from other headache disorders
Refractory migraine is likely a subtype or progression from chronic migraine, though some argue that episodic migraine could be refractory depending on criteria used (13). The main headache disorder to differentiate from refractory migraine is medication overuse headache (MOH) (7).
Medication overuse itself does not exclude a diagnosis of refractory migraine, but MOH should be ruled out as a mimicker. MOH often presents as a chronic daily headache that can be refractory to both acute and preventive migraine treatments. It has long been known that MOH is major risk factor for conversion of episodic migraine into chronic migraine (14). There may be an increased risk of MOH in females, those with lower socioeconomic status, comorbid depression or anxiety, comorbid chronic pain disorders, and in the setting of cannabis use (15–17). MOH does not appear to be drug class specific, but rather occurs in predisposed patients with a primary headache disorder like migraine in the setting of medication overuse; however medication overuse does not automatically denote the disorder of MOH (11). To confirm the diagnosis of MOH, withdrawal of the causative medication(s) leading to significant improvement in headache is required (11). Hence if overused acute medication is withdrawn but no improvement occurs after a period of time, then MOH is unlikely. MOH relapses are more common in those with overuse of opioids, ergotamines, caffeine-containing medications, and combination medications (18). Those with MOH are less likely to respond to treatment hence can mimic refractory migraine, but some patients with MOH will improve with initiation of preventive treatments especially from migraine-specific medications like the calcitonin gene-related peptide (CGRP) monoclonal antibodies (19, 20). Some patients with MOH will spontaneously remit (21). Clinical trials have shown that various MOH management approaches work, but the best approach is to start a preventive treatment with or without planned medication withdrawal (22, 23). Patients unable to successfully withdraw overused acute treatments may need inpatient detoxification (24).
Beyond MOH, it is important to ensure secondary disorders like a cerebrospinal fluid (CSF) leak have been ruled out (25). There is also a possibility that some patients diagnosed with refractory migraine have underlying etiologies yet to be discovered, as evidenced by the case series of Nutcracker syndrome presenting with isolated chronic daily headache (26).
Criteria for diagnosing refractory migraine
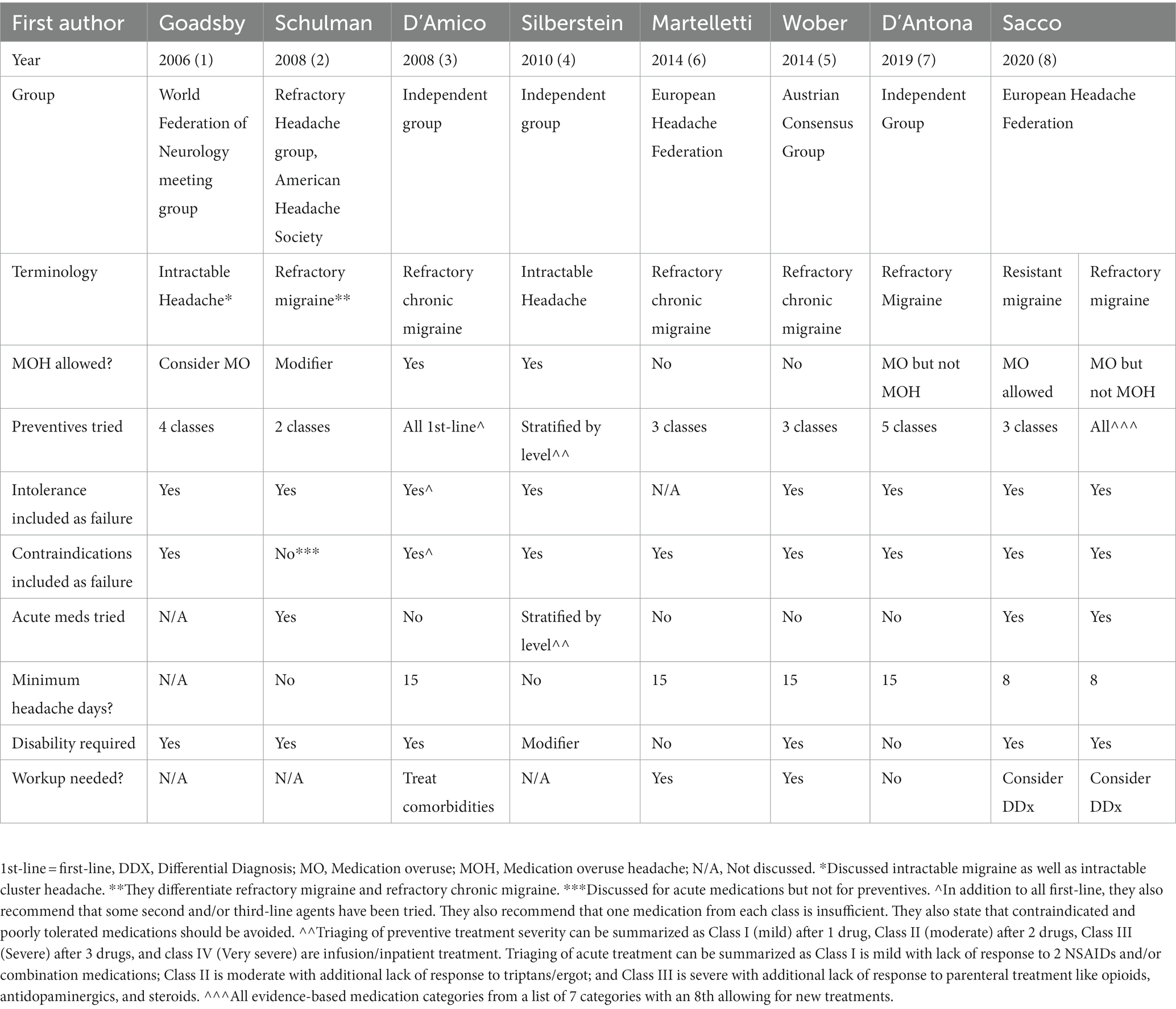
Multiple diagnostic criteria for refractory migraine have been proposed. The most recent diagnostic criteria are those proposed by the European Headache Federation (EHF) (8). They differentiate between resistant versus refractory migraine to provide two levels of severity with resistant migraine being debilitating despite trial of 3 drug classes while refractory migraine remains debilitating despite trying all drug classes. The authors noted that in many European countries, access to new drug classes like CGRP medications is already restricted to difficult to treat patients with one example provided that in Germany CGRP medications are restricted to those patients with episodic migraine who have tried 5 medications or with chronic migraine who have tried 6 medications including onabotulinumtoxinA.
Refractory migraine
A. Established diagnosis of 1.1 Migraine without aura and/or 1.2 Migraine with aura or 1.3 Chronic migraine according to ICHD-III criteria.
B. Debilitating headache for at least 8 days per month for at least 3 months.
C. Failure and/or contraindication to all classes with established evidence for migraine prevention, given at an appropriate dose for an appropriate duration.
They define a debilitating headache impairing daily activity despite at least 2 ineffective triptan trials (8). Their recognized drug classes are antidepressants (amitriptyline and venlafaxine), antiepileptics (topiramate and valproate), beta blockers (atenolol, metoprolol, propranolol, timolol), calcium channel blockers (flunarizine or cinnarizine), CGRP medications (monoclonal antibodies or gepants), angiotensin pathway blockers (candesartan or lisinopril), onabotulinumtoxinA, and allowance for newly developed medications. Note that in the United States we do not have access to calcium channel blockers like flunarizine. Lack of tolerance and contraindications can count toward its failure in the EHF proposed criterion C.
Other refractory migraine criteria have been proposed including Goadsby et al. (1), American Headache Society (2), D’Amico et al. (3), Silberstein et al. (4), Austrian Consensus Group (5), European Headache Federation (6), as well as D’Antona and Matharu (7). See Table 1 for a comparison. Prior to then Valencia et al. (27) described poorly controlled primary headaches. Many groups are actively developing criteria as well. In the meantime, large variations exist in how refractory migraine is defined. Pharmaceutical trials define a “refractory” population as 2 to 4 prior preventives (28–31). However, this range does not mirror the reality of subspecialty clinics where our refractory patients may have tried 20 or higher without response.
Potential pitfalls
When diagnosing refractory migraine, the focus is on lack of response to preventive treatment. However, some criteria like EHF’s argue that lack of response to acute treatments needs consideration (8). I would argue that refractory migraine is a chronic disease state with resistance to preventive treatment. Response to acute treatment focuses on individual attacks and is more relevant to status migrainosus considerations (11).
Another pitfall is treatment access. The threshold for refractory migraine may look different in the Unites States versus in Africa where access to treatment like CGRP monoclonal antibodies is unlikely (32). Also consider pediatric versus adults with migraine; the threshold of refractoriness in an 8-year-old may need to be different than in a 45-year-old (33). Finally, even in the United States, the state of health insurance limits access to medication due to unaffordable copays (34). The threshold for refractoriness may need to be malleable enough to apply to country, age group, and socioeconomic group.
Another issue to consider is whether intolerance or contraindications count toward refractoriness. Refractory is defined as “resistant to treatment or cure.” (35) Sensitivity to multiple medications is common in a headache clinic, but that patient simply has many inadequate trials preventing evaluation of refractoriness. In fact, studies have demonstrated that clinical trials for migraine preventive treatment show nocebo rates of 42.78%, and one study looking at a specific nocebo of delayed headache after placebo infusion was 15.5% (36, 37). Relative versus absolute contraindications can also be an issue. For example, avoiding divalproex in a female of child-bearing age does not mean she would not respond to it. Sacco et al., in discussing resistant versus refractory migraine, specifically mention the hypothetical situation in which a patient has contraindications to all evidence-based classes (8). These situations may be appropriate for the ICHD-3’s use of “probable” diagnoses. True refractory migraine likely relies on ineffective treatment while probable refractory migraine may allow intolerance or contraindications. If using resistant migraine, the preferable use of the proposed diagnostic criteria would be 3 drug classes that are ineffective rather than relying on those not tried due to contraindications (8).
The role of comorbidities
Co-morbid pain conditions are very common in migraine with one study reporting that 51% of patients with migraine have one or more concurrent pain condition(s) (38). That number increases past 70% in patients with chronic migraine (38). Fibromyalgia has been reported in 10–30% of patients with migraine with one study finding increased headache frequency to be predictive (38). One might expect even higher rates in those with refractory migraine, but this association has not been studied. There is a dose response relationship between allodynia and the number of comorbid pain conditions in migraine (39). Migraine is also associated with non-pain conditions including depression, anxiety, insomnia, psoriasis, allergy, diabetes, and asthma (40). More concurrent comorbidities is associated with increasing migraine attack frequency (40). Entities commonly seen in clinic include postural orthostatic tachycardia syndrome (POTS) and hypermobility; migraine is seen in one-third of POTS and in half of patients with hypermobility (41–44). While this dose response relationship has not been studied in refractory migraine, it could be predicted that a similar relationship would be seen.
Pathophysiology of refractory migraine
Current understanding of migraine mechanisms
Migraine is a complex sensory processing disorder with dysfunction of the trigeminovascular system including activation of trigeminal pathways, neurogenic inflammation, and release of neuropeptides like CGRP. Disease progression leads to increasing frequency of attacks and allodynia (45, 46). How this process progresses to refractory is unknown.
In looking at the mechanisms that explain progression from episodic to chronic migraine, the hypothalamus is often mentioned, it shows increased activation and connectivity to the spinal trigeminal nucleus in chronic migraine on functional magnetic resonance imaging (fMRI) (47). The hypothalamus is integral to migraine attack generation; hence one theory of refractory migraine pathophysiology is that enhanced hypothalamic activation perpetuates the active migraine state preventing treatment response (48). Progression is also associated with volume changes in regions of interests (ROIs), but the exact patterns still need to be elucidated (47). Over time there appears to be at least two broad underlying mechanisms for progression including prolonged nociceptive activity and neurogenic inflammation leading to hyperexcitability from sensitization as well as lack of habituation due to dysfunction of inhibitory brainstem pain control (47). The resulting central sensitization causes a brain state with increased spontaneous neural activity and reduced activation thresholds causing hypersensitivity to stimuli, reduced nocioceptive inhibition, and larger nocioceptive receptive fields (49).
Central sensitization represents hyperactivation of nociceptive pathways with dysfunction of thalamocortical modulation (45). Reduced functional connective on fMRI of both the default mode and executive networks has been associated with allodynia, a marker of central sensitization, without volumetric gray matter changes suggesting that functional changes precede any structural changes (50). fMRI has also shown evidence of hyperactivity of the spinal trigeminal nucleus and posterior thalamus with loss of descending pain inhibition (51). Allodynia predicts migraine chronification and is associated with longer disease duration, higher headache frequency, and worse outcomes (46, 52, 53). Treatment like triptans are most effective early in an attack prior to development of central sensitization (54). Allodynia also predicts lack of response to treatments like galcanezumab or onabotulinumtoxinA (55, 56). It follows that clinical and radiologic indicators of central sensitization should be predictive of refractory migraine, but further study is needed to assess that hypothesis.
Risk factors for progression
Episodic migraine progresses to chronic migraine at a rate of 2% per year; the rate of progression to refractory migraine is unknown (57). Predictors of conversion from episodic to chronic migraine include cutaneous allodynia (45, 46), depression (58), MOH (59, 60), pain catastrophizing with a poor internal locus of control (61–63), lower socioeconomic status (64), and having multiple comorbidities (65). It is unknown if these risk factors also apply to refractory migraine however some studies have looked at predictors of response to specific treatments, which can be used as an indirect way to assess refractoriness in general. For instance, poor response to CGRP monoclonal antibodies is predicted by having prior ineffective treatments (66, 67). A different study on the use of erenumab in chronic migraine with concurrent MOH found that non-responders had 7.86 ± 1.85 prior ineffective treatments compared to 5.06 ± 1.62 in responders (p < 0.0001) (68). It should be noted that prior ineffective treatment does not negate the possibility of a response; a study from Germany showed that even with 5 or more prior ineffective treatments there was still at least a 50% response in 41.9% of patients with chronic migraine (69). In fact, even a lack of response to one CGRP monoclonal antibody does not negate response to a different one demonstrating the complexity in predicting treatment response (70). As a further complication to assessing refractoriness, relying on response at 3 month using a 50% responder rate may exclude approximately 16% of patient who would ultimately respond (71).
In looking at super-responders (75–100% responders) to a specific treatment category like CGRP monoclonal antibodies, studies demonstrate that they are more likely to have typical migraine features like unilateral pain, throbbing quality and vomiting; they also tended to have episodic migraine and a good triptan response (59). Studies looking at factors predicting at least a 50% response to CGRP monoclonal antibodies show that treatment responders are younger, have a lower headache frequency, unilateral pain ± unilateral allodynia but no interictal allodynia, unilateral cranial autonomic symptoms, more nausea/vomiting, more photophobia, lack of obesity, better response to triptans, less MOH, less pain catastrophizing, and less depression (67, 72–75). Conversely, a chronic daily headache at baseline is predictive of a poor response (67). MOH may not only be predictive of poor treatment response, but duration of MOH and the number of overused analgesia may also be predictive (68). One study found that cluster C personality disorders and significant life stressors predict poor response to erenumab (76). Based on a neuroimaging study, a lower baseline cerebral blood flow velocity in the middle cerebral arteries may predict a good response to CGRP monoclonal antibodies (77).
Poor treatment response to onabotulinumtoxinA has been associated with longer disease duration (78). There may be less of a decrease in headache days from onabotulinumtoxinA in those with allodynia, MOH and depression (56, 79). However a different study found improved response to onabotulinumtoxinA in patients with allodynia or pericranial muscle tenderness (80). One interesting study found that 74% of responders to onabotulinumtoxinA describe an imploding headache (i.e., external force sensation) and 13% described ocular pain while 92% of non-responders describe an exploding headache (internal pressure sensation) (81). Increased onabotulinumtoxinA response with ocular pain was also seen in a second study (82). At the biochemical level, certain plasma protein levels may predict response to onabotulinumtoxinA including CGRP, vasoactive intestinal peptide (VIP) and pentraxin 3 (PTX3) (79). A neuroimaging study found that iron deposition in the periaqueductal gray, a finding associated with chronic migraine as well as endothelial dysfunction and disrupted blood–brain barrier, was associated with worse onabotulinumtoxinA response (83, 84).
Based on the above findings, one could hypothesize that refractory migraine is more likely in older patients with longer disease duration who have bilateral imploding headache with less throbbing, pericranial muscle tenderness, triptan response and cranial autonomic symptoms, but more nausea/vomiting, allodynia, depression, stressors, and pain catastrophizing as well as higher rates of obesity, cluster C personality disorders and MOH. While not known, there may even be a dose response with more treatment failures corresponding to worse refractoriness. While we typically rely on assessment after 3 months of treatment, these patients may need longer trials and may benefit from trying another medication from a category previously tried. Further studies using biochemical and neuroimaging analysis are needed but some features may be predictive of refractory migraine like plasma protein levels of CGRP, blood flow velocities and the presence of iron deposition.
Pharmacogenomics
Pharmacogenomics to predict treatment response in migraine is limited and not used clinically. Other than rare monogenic migraine like familial hemiplegic migraine, migraine is polygenetic with each gene having a small effect size but overall disease heritability of 35 to 60% (83). First degree relatives have a higher risk of migraine that increases with higher pain severity and attack frequency (83, 85). There are at least 180 loci associated with migraine (83, 86, 87). In a pharmacogenomics migraine study, verapamil-responders were compared to non-responders with 6 gene polymorphisms predictive of response. Polymorphisms of the 5-HT1B receptor gene are associated with sumatriptan response (88). Otherwise, studies on genetics and refractoriness are absent.
Neuroimaging insights
In a 2023 systematic review and meta-analysis, 40 migraine studies (n = 3297 patients) using voxel-based morphometry to compare migraine to healthy controls were assessed (89). Coordinate-based meta-analysis via 2 separate methodologies (anisotropic effect size-signed differential mapping and activation likelihood estimation) was used. Between these two methodologies, they found increased gray matter volume of the bilateral amygdala, bilateral parahippocampus, bilateral temporal poles, bilateral superior temporal gyri, left hippocampus, left middle temporal gyrus, right superior frontal gyrus but decreased volume of the left insula, bilateral cerebellum, right dorsal medulla, bilateral Rolandic operculum, right middle frontal gyrus, and right inferior parietal gyrus. The main finding found across both methodologies was gray matter increase in the left parahippocampus but decrease in the left insula. Broader variation in gray matter volumes were seen when subgroups like migraine with versus without aura or episodic versus chronic migraine were assessed. Further information on imaging findings in migraine is found when reviewing multivariate analysis for comparison to healthy controls or between migraine subgroups. In a 2016 study looking at structural and functional MRI findings using a multi-feature classification approach to compare migraine without aura (n = 21) to healthy control (n = 28), there was accuracy of 83.67% with sensitivity of 92.86% and specificity 71.43% (90). Discriminative structures include the anterior cingulate cortex, prefrontal cortex, orbitofrontal cortex and the insula (90). MRI can also differentiate episodic migraine from chronic migraine with 84.2% accuracy based on regional cortical thickness, cortical surface area, and volume (91). Further studies are needed to see if imaging can distinguish refractory migraine from chronic and episodic though at least one study demonstrated that treatment resistance is associated with more white matter hyperintensities (92).
Management of patients with refractory migraine
Evidence-based preventive treatment
The 2021 American Headache Society (AHS) Consensus Statement for the treatment of migraine is the most up to date guideline for the United States (93). The established preventive treatments from this consensus statement are erenumab, eptinezumab, fremanezumab, galcanezumab, onabotulinumtoxinA, candesartan, divalproex/valproate, propranolol, metoprolol, timolol, and topiramate. Amitriptyline, atenolol, lisinopril, memantine, nadolol, and venlafaxine are considered probably effective. Frovatriptan is an established peri-menstrual preventive treatment, and the guidelines advocate for neuromodulation devices. Nerve blocks are a standard treatments in many headache clinics but are not formally in the guidelines (94). Since the publication of this consensus statement, rimegepant and atogepant have been approved for the treatment of migraine (95). Beyond these options, small studies support the use of many other medications though many have conflicting or low quality evidence.
Approach to pharmacologic management
The first step is to ensure the diagnosis is correct including ruling out MOH and that an adequate trial of evidence-based treatment was done. An adequate trial is 2 to 3 months at an adequate dose (93). The next appropriate step is to consider rational polypharmacy (96, 97). The AHS consensus statement advocates for the combination of a CGRP monoclonal antibody and onabotulinumtoxinA as a possibly effective therapy (93, 96). There is also increasing evidence that gepants can safely be used with CGRP monoclonal antibodies as another consideration for rational polypharmacy in patients with refractory migraine (98–102). The combination of a gepant and onabotulinumtoxinA has also been proposed as another example (103). While there are surprisingly few trials on combinations of the older non-specific treatments, there is some evidence for layering medications like topiramate and amitriptyline (104). The next step is the n-of-1 trial recognizing that by virtue of going outside guideline-based evidence these considerations have limited evidence (105).
Ideas for n-of-1 trials
Preventive non-guideline treatments tried in migraine despite variable or limited evidence include anti-seizure medications like gabapentin, pregabalin, carbamazepine, oxcarbazepine, lamotrigine, levetiracetam and zonisamide (106, 107); calcium channel blockers like verapamil (108); anti-depressants like duloxetine, nortriptyline, doxepin, and phenelzine (109–111); atypical antipsychotic like olanzapine (112); and ergots like methylergonovine (113). Mirtazapine is an evidence-based treatment for tension-type headache, so could be tried for migraine (114). Acetazolamide is occasionally tried, especially in vestibular or hemiplegic migraine (115, 116). Amantadine, an NMDA receptor antagonist like memantine, has been tried in post-traumatic headache and migraine (117, 118). Observational studies suggest benefit from baclofen or tizanidine (119). Cannabinoids are often tried due to high public acceptance and there is good theoretical support for targeting the endocannabinoid system, however the risk of MOH has been raised (17, 120, 121). Despite the risk of MOH, occasionally daily triptans or NSAIDs are tried (122–124). Recently low-dose psilocybin has even been studied in a small cohort of patients with episodic migraine with more data available for the use of psychedelics in cluster headache (125, 126). Refractory migraine is a common indication for inpatient treatment using intravenous dihydroergotamine (DHE), ketamine, lidocaine, or propofol (127–130). In those responding to DHE, methylergonovine may be particularly of consideration (131). In those responding to lidocaine, mexiletine was often tried (132). A recent pilot study found that the ketogenic diet may be another consideration (133).
Opioids are occasionally considered despite low evidence, and may be initially started for non-cephalic pain (38, 134). However in headache medicine, opioids are a taboo due to MOH risk, especially if used greater than 9 days per month (11, 135). Opioid-related MOH represents only 4% of MOH-causing medications (136), but are high risk for central sensitization, MOH, progression from episodic to chronic migraine, increased healthcare utilization, worse disability and higher rates of mood disorders (137–139). Opioids are less effective than prochlorperazine, metoclopramide and dihydroergotamine when used acutely for migraine and may even impact treatment response (140–142). However, a limited group of patients with migraine do report improvement on opioids (143). Interesting the combination of NMDA receptor antagonism and opioids, like methadone or buprenorphine, may potentiate analgesia while reducing tolerance and hyperalgesia (144–146). Prospective cohort study has suggested that methadone, a racemic mixture of R and S-isomers as well as an NMDA receptor antagonist, may be beneficial daily at low doses for refractory chronic migraine (145). Further study is needed, but research into the use of delta opioid receptor agonists in migraine is also being looked into (147). Headache neurologists are hesitant to prescribe opioids due to these risks but there is a debate worth having of whether this group should have a trial of opioids, especially methadone or buprenorphine, with careful monitoring for the development of MOH over a pre-defined period like 3 months.
In patients with refractory migraine, surgical options, like invasive occipital nerve stimulation (ONS), may be considered. ONS has possible support from systematic review and meta-analyzes (17, 148, 149), however the studies are prone to bias due to small size and difficulty blinding with safety concerns including lead migration, infection and pain (150, 151). Long-term studies on ONS are limited in migraine, with the majority done in cluster headache, but persistence of benefit is reported (152, 153). Intuitively, response to occipital nerve block should predict ONS response but thus far it does not (154, 155). Some centers add supraorbital stimulation to ONS for better response though at least one study suggests that response is not sustained (156–158). Deep brain stimulation has been used in headache disorders, but there is no evidence for its use in migraine (159, 160). Finally, occipital nerve decompression is reported as a potentially effective treatment for some patients (161, 162).
Multidisciplinary treatment plans
Beyond pharmacologic management, these patients require non-pharmacologic and multidisciplinary care. Behavioral treatments are highly recommended given cognitive constructs like pain catastrophizing, avoidance, and cephalalgiaphobia (163–165). Biofeedback and cognitive behavioral therapy (CBT) are mainstays of treatment often combined with techniques like mindfulness and relaxation therapy (166, 167). These treatments may be combined with pharmacologic treatments. For instance, CBT and amitriptyline have been shown to be synergistic (168, 169). Treatments like physical therapy, manual therapy, acupuncture, dry needling, and exercise are often used (170–174). In fact, exercise may have a synergistic benefit when used in combination with amitriptyline (175). Beyond strength training or aerobic exercise, yoga also has evidence for use (176). Finally other lifestyle interventions may be tried like trigger elimination, diet alterations, hydration, and sleep optimization (177, 178). Patients with refractory migraine will likely need a combination of these treatments.
Preventing refractory migraine
At this time, we do not know how to prevent refractory migraine. Even for conversion from episodic to chronic migraine, there is conflicting evidence on the importance of starting preventive treatment (179, 180). For instance, studies have shown that the use of topiramate in patients with episodic migraine may prevent progression based on pooled results across 3 studies in which 2.1% (8/384) of patients on topiramate (100 mg) progressed to chronic migraine, while 4.3% (16/372) in the placebo group progressed over 26 weeks (179). Comparatively, the INTREPID study looked at topiramate (100 mg) in patients with high frequency episodic migraine and found no significant difference in the rate of conversion to chronic daily headache at 6 months when compared to the placebo group (180). There is also evidence that optimizing acute treatment of migraine may help prevent progression to chronic migraine (181). NSAIDs may even have a protective effect for those with less than 10–14 headache days per month (57). Beyond treating early with pharmacologic management, treatment of modifiable risk factors for progression like managing comorbidities and avoiding medication overuse may help prevent progression to at least chronic migraine (182, 183). Whether this data on preventing conversion from episodic to chronic migraine is relevant to preventing refractory migraine is unknown.
Knowledge gaps and future research
The top research priority for refractory migraine is the development and acceptance of ICHD diagnostic criteria. Without a standard guide for diagnosis, all studies on epidemiology, pathophysiology, and treatment will not use a homogenous population. Once diagnostic criteria are accepted, research can be undertaken to clarify disease burden, which is likely high and represents a substantial proportion of subspecialty headache clinic patients. Pathophysiology can then be investigated using genetic studies, risk factor analysis, neuroimaging, and biochemical analysis. Once we clarify who we are treating (diagnosis) and what we are treating (pathophysiology) then studies can identify rational targets for therapy allowing for randomized controlled trials and ultimately guideline development. Research may even allow identification of these patients prior to becoming refractory, allowing early intervention to prevent this disease state or avoid years of ineffective treatment trials. This future state is a long way off, and the headache community cannot advocate enough for first pinning down accepted diagnostic criteria.
Conclusion
Refractory migraine, representing the most debilitated and complex migraine population, has been largely overlooked. The urgent need for established diagnostic criteria is paramount to advancing research on pathophysiology and developing effective treatments. Currently, there are multiple proposed criteria without an official diagnosis in the ICHD3. Pathophysiology can only be hypothesized, and treatment approaches vary widely with reliance on low quality evidence driving n-of-1 treatment trials. Management of refractory migraine requires the art of medicine while awaiting scientific advancements.
Author contributions
JR: Conceptualization, Data curation, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
JR discloses grant support from Barrow Neurological foundation, investigator support from Eli Lilly and Abbvie, paid advisory board for Abbvie, speaker for Impel, as well as paid Editorial relationship with MedLink Neurology and Neurodiem. JR also discloses that a family member has partial ownership of Scottsdale Providence Recovery Center.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Goadsby, PJ, Schoenen, J, Ferrari, MD, Silberstein, SD, and Dodick, D. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. (2006) 26:1168–70. doi: 10.1111/j.1468-2982.2006.01173.x
2. Schulman, EA, Lake, AE, Goadsby, PJ, Peterlin, BL, Siegel, SE, Markley, HG, et al. Defining refractory migraine and refractory chronic migraine: proposed criteria from the refractory headache special interest section of the American headache society. Headache. (2008) 48:778–82. doi: 10.1111/j.1526-4610.2008.01132.x
3. D’Amico, D, Leone, M, Grazzi, L, and Bussone, G. When should “chronic migraine” patients be considered “refractory” to pharmacological prophylaxis? Neurol Sci. (2008) 29:S55–8. doi: 10.1007/s10072-008-0888-3
4. Silberstein, SD, Dodick, DW, and Pearlman, S. Defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. (2010) 50:1499–506. doi: 10.1111/j.1526-4610.2010.01764.x
5. Wöber, C, and Wessely, P, Austrian Consensus Group on Refractory Chronic Migraine. Comment on: Martelletti et al. Refractory chronic migraine: a consensus statement on clinical definition from the European headache federation. J Headache Pain. (2014) 15:77. doi: 10.1186/1129-2377-15-77
6. Martelletti, P, Katsarava, Z, Lampl, C, Magis, D, Bendtsen, L, Negro, A, et al. Refractory chronic migraine: a consensus statement on clinical definition from the European headache federation. J Headache Pain. (2014) 15:47. doi: 10.1186/1129-2377-15-47
7. D'Antona, L, and Matharu, M. Identifying and managing refractory migraine: barriers and opportunities? J Headache Pain. (2019) 20:89. doi: 10.1186/s10194-019-1040-x
8. Sacco, S, Braschinsky, M, Ducros, A, Lampl, C, Little, P, van den Brink, AM, et al. European headache federation consensus on the definition of resistant and refractory migraine: developed with the endorsement of the European Migraine and Headache Alliance (EMHA). J Headache Pain. (2020) 21:76. doi: 10.1186/s10194-020-01130-5
9. Steiner, TJ, and Stovner, LJ. Global epidemiology of migraine and its implications for public health and health policy. Nat Rev Neurol. (2023) 19:109–17. doi: 10.1038/s41582-022-00763-1
10. Lipton, RB, Manack Adams, A, Buse, DC, Fanning, KM, and Reed, ML. A comparison of the chronic migraine epidemiology and outcomes (CaMEO) study and American migraine prevalence and prevention (AMPP) study: demographics and headache-related disability. Headache: the journal of head and face. Pain. (2016) 56:1280–9. doi: 10.1111/head.12878
11. Headache Classification Committee of the International Headache Society (IHS) . Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
12. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
13. Lipton, RB, and Bigal, ME. Toward an epidemiology of refractory migraine: current knowledge and issues for future research. Headache. (2008) 48:791–8. doi: 10.1111/j.1526-4610.2008.01131.x
14. Mathew, NT, Stubits, E, and Nigam, MP. Transformation of episodic migraine into daily headache: analysis of factors. Headache: the journal of head and face. Pain. (1982) 22:66–8. doi: 10.1111/j.1526-4610.1982.hed2202066.x
15. Laskar, S, Kalita, J, and Misra, UK. Comparison of chronic daily headache with and without medication overuse headache using ICHD II R and ICHD 3 beta criteria. Clin Neurol Neurosurg. (2019) 183:105382. doi: 10.1016/j.clineuro.2019.105382
16. González-Oria, C, Belvís, R, Cuadrado, ML, Díaz-Insa, S, Guerrero-Peral, AL, Huerta, M, et al. Document of revision and updating of medication overuse headache (MOH). Neurologia. (2020) 36:229–40. doi: 10.1016/j.nrl.2020.04.029
17. Zhang, N, and Woldeamanuel, YW. Medication overuse headache in patients with chronic migraine using cannabis: a case–referent study. Headache: the journal of head and face. Pain. (2021) 61:1234–44. doi: 10.1111/head.14195
18. Grazzi, L, Grignani, E, D'Amico, D, Sansone, E, and Raggi, A. Is medication overuse drug specific or not? Data from a review of published literature and from an original study on Italian MOH patients. Curr Pain Headache Rep. (2018) 22:71. doi: 10.1007/s11916-018-0729-x
19. Krymchantowski, A, Jevoux, C, Krymchantowski, AG, and Silva-Néto, RP. Medication overuse headache, chronic migraine and monoclonal antibodies anti-CGRP: a real-world study. Clin Neuropharmacol. (2023) 2023:559. doi: 10.1097/WNF.0000000000000559
20. Giri, S, Tronvik, E, Linde, M, Pedersen, SA, and Hagen, K. Randomized controlled studies evaluating Topiramate, botulinum toxin type a, and mABs targeting CGRP in patients with chronic migraine and medication overuse headache: a systematic review and meta-analysis. Cephalalgia. (2023) 43:156922. doi: 10.1177/03331024231156922
21. Favoni, V, Mascarella, D, Giannini, G, Bauleo, S, Torelli, P, Pierangeli, G, et al. Prevalence, natural history and dynamic nature of chronic headache and medication overuse headache in Italy: the SPARTACUS study. Cephalalgia. (2023) 43:3331024231157677. doi: 10.1177/03331024231157677
22. Carlsen, LN, Munksgaard, SB, Nielsen, M, Engelstoft, IMS, Westergaard, ML, Bendtsen, L, et al. Comparison of 3 treatment strategies for medication overuse headache: a randomized clinical trial. JAMA Neurol. (2020) 77:1069–78. doi: 10.1001/jamaneurol.2020.1179
23. Schwedt, TJ, Hentz, JG, Sahai-Srivastava, S, Murinova, N, Spare, NM, Treppendahl, C, et al. Patient-centered treatment of chronic migraine with medication overuse: a prospective, randomized, pragmatic clinical trial. Neurology. (2022) 98:e1409–21. doi: 10.1212/WNL.0000000000200117
24. Diener, HC, Holle, D, Solbach, K, and Gaul, C. Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol. (2016) 12:575–83. doi: 10.1038/nrneurol.2016.124
25. Jennifer Robblee, KAS, Alhilali, LM, and Knievel, KL. Spontaneous intracranial hypotension. Pract Neurol. (2020):41–52.
26. Rozen, TD, Devcic, Z, Toskich, B, Caserta, MP, Sandhu, SJS, Huynh, T, et al. Nutcracker phenomenon with a daily persistent headache as the primary symptom: case series and a proposed pathogenesis model based on a novel MRI technique to evaluate for spinal epidural venous congestion. J Neurol Sci. (2022) 434:120170. doi: 10.1016/j.jns.2022.120170
27. Valença, M, Andrade-Valença, L, Bordini, C, Farias da Silva, W, and Speciali, J. Poor control headache. Migraneas Cefaleias. (2003) 6:117–20.
28. Michel Dominique, F, Uwe, R, Peter, JG, Paiva, G, Da Silva, L, Subhayan, M, et al. Two-year efficacy and safety of erenumab in participants with episodic migraine and 2–4 prior preventive treatment failures: results from the LIBERTY study. J Neurol Neurosurg Psychiatry. (2022) 93:254. doi: 10.1136/jnnp-2021-327480
29. Ferrari, MD, Diener, HC, Ning, X, Galic, M, Cohen, JM, Yang, R, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. (2019) 394:1030–40. doi: 10.1016/S0140-6736(19)31946-4
30. Mulleners, WM, Kim, BK, Láinez, MJA, Lanteri-Minet, M, Pozo-Rosich, P, Wang, S, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. (2020) 19:814–25. doi: 10.1016/S1474-4422(20)30279-9
31. Barbanti, P, Goadsby, PJ, Lambru, G, Ettrup, A, Christoffersen, CL, Josiassen, MK, et al. Effects of eptinezumab on self-reported work productivity in adults with migraine and prior preventive treatment failure in the randomized, double-blind, placebo-controlled DELIVER study. J Headache Pain. (2022) 23:153. doi: 10.1186/s10194-022-01521-w
32. Mortel, D, Kawatu, N, Steiner, TJ, and Saylor, D. Barriers to headache care in low- and middle-income countries. eNeurologicalSci. (2022) 29:100427. doi: 10.1016/j.ensci.2022.100427
33. Oskoui, M, Pringsheim, T, Billinghurst, L, Potrebic, S, Gersz, EM, Gloss, D, et al. Practice guideline update summary: pharmacologic treatment for pediatric migraine prevention: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology and the American headache society. Neurology. (2019) 93:500–9. doi: 10.1212/WNL.0000000000008105
34. Reynolds, EL, Gallagher, G, Hill, CE, Banerjee, M, Mante, A, Esper, GJ, et al. Costs and utilization of new-to-market neurologic medications. Neurology. (2023) 100:e884–98. doi: 10.1212/WNL.0000000000201627
35. Refractory . (2023). Available at: https://www.merriam-webster.com/dictionary/refractory. (Accessed April 5, 2023).
36. Mitsikostas, DD, Mantonakis, LI, and Chalarakis, NG. Nocebo is the enemy, not placebo. A meta-analysis of reported side effects after placebo treatment in headaches. Cephalalgia. (2011) 31:550–61. doi: 10.1177/0333102410391485
37. Ghanizada, H, Iljazi, A, Ashina, H, Do, TP, Al-Karagholi, MA-M, Amin, FM, et al. Nocebo response in human models of migraine: a systematic review and meta-analysis of randomized, double-blind, placebo-controlled, two-way crossover trials in migraine without aura and healthy volunteers. Cephalalgia. (2020) 41:99–111. doi: 10.1177/0333102420970489
38. Henningsen, P, Hausteiner-Wiehle, C, and Häuser, W. Migraine in the context of chronic primary pain, chronic overlapping pain disorders, and functional somatic disorders: a narrative review. Headache. (2022) 62:1272–80. doi: 10.1111/head.14419
39. Tietjen, GE, Brandes, JL, Peterlin, BL, Eloff, A, Dafer, RM, Stein, MR, et al. Allodynia in migraine: association with comorbid pain conditions. Headache. (2009) 49:1333–44. doi: 10.1111/j.1526-4610.2009.01521.x
40. Buse, DC, Reed, ML, Fanning, KM, Bostic, R, Dodick, DW, Schwedt, TJ, et al. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (MAST) study. J Headache Pain. (2020) 21:23. doi: 10.1186/s10194-020-1084-y
41. Raj, SR, Bourne, KM, Stiles, LE, Miglis, MG, Cortez, MM, Miller, AJ, et al. Postural orthostatic tachycardia syndrome (POTS): priorities for POTS care and research from a 2019 National Institutes of Health expert consensus meeting - part 2. Auton Neurosci. (2021) 235:102836. doi: 10.1016/j.autneu.2021.102836
42. Kanjwal, K, Saeed, B, Karabin, B, Kanjwal, Y, and Grubb, BP. Comparative clinical profile of postural orthostatic tachycardia patients with and without joint hypermobility syndrome. Indian Pacing Electrophysiol J. (2010) 10:173–8.
43. Ray, JC, Pham, X, Foster, E, Cheema, S, Corcoran, SJ, Matharu, MS, et al. The prevalence of headache disorders in postural tachycardia syndrome: a systematic review and meta-analysis of the literature. Cephalalgia. (2022) 42:1274–87. doi: 10.1177/03331024221095153
44. Zloof, Y, Simchoni, M, Derazne, E, Tsur, AM, Tzur, D, Braun, M, et al. Hypermobility spectrum disorders and active migraine in Israeli adolescents: a nationwide study. Headache. (2023) 63:934–41. doi: 10.1111/head.14526
45. Mínguez-Olaondo, A, Quintas, S, Morollón Sánchez-Mateos, N, López-Bravo, A, Vila-Pueyo, M, Grozeva, V, et al. Cutaneous allodynia in migraine: a narrative review. Front Neurol. (2021) 12:831035. doi: 10.3389/fneur.2021.831035
46. Louter, MA, Bosker, JE, van Oosterhout, WP, van Zwet, EW, Zitman, FG, Ferrari, MD, et al. Cutaneous allodynia as a predictor of migraine chronification. Brain. (2013) 136:3489–96. doi: 10.1093/brain/awt251
47. Rattanawong, W, Rapoport, A, and Srikiatkhachorn, A. Neurobiology of migraine progression. Neurobiol. Pain. (2022) 12:100094. doi: 10.1016/j.ynpai.2022.100094
48. Lerebours, F, Boulanouar, K, Barège, M, Denuelle, M, Bonneville, F, Payoux, P, et al. Functional connectivity of hypothalamus in chronic migraine with medication overuse. Cephalalgia. (2019) 39:892–9. doi: 10.1177/0333102419833087
49. Latremoliere, A, and Woolf, CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. (2009) 10:895–926. doi: 10.1016/j.jpain.2009.06.012
50. Russo, A, Silvestro, M, Trojsi, F, Bisecco, A, De Micco, R, Caiazzo, G, et al. Cognitive networks disarrangement in patients with migraine predicts cutaneous allodynia. Headache. (2020) 60:1228–43. doi: 10.1111/head.13860
51. Maleki, N, Szabo, E, Becerra, L, Moulton, E, Scrivani, SJ, Burstein, R, et al. Ictal and interictal brain activation in episodic migraine: neural basis for extent of allodynia. PLoS One. (2021) 16:e0244320. doi: 10.1371/journal.pone.0244320
52. Seo, J-G, and Park, S-P. Clinical significance of sensory hypersensitivities in migraine patients: does allodynia have a priority on it? Neurol Sci. (2019) 40:393–8. doi: 10.1007/s10072-018-3661-2
53. Mathew, NT, Kailasam, J, and Seifert, T. Clinical recognition of allodynia in migraine. Neurology. (2004) 63:848–52. doi: 10.1212/01.WNL.0000137107.27585.F7
54. Burstein, R, Collins, B, and Jakubowski, M. Defeating migraine pain with Triptans: a race against the development of cutaneous allodynia. Ann Neurol. (2004) 55:19–26. doi: 10.1002/ana.10786
55. Ashina, S, Melo-Carrillo, A, Szabo, E, Borsook, D, and Burstein, R. Pre-treatment non-ictal cephalic allodynia identifies responders to prophylactic treatment of chronic and episodic migraine patients with galcanezumab: a prospective quantitative sensory testing study (NCT04271202). Cephalalgia. (2023) 43:147881. doi: 10.1177/03331024221147881
56. Young, WB, Ivan Lopez, J, Rothrock, JF, Orejudos, A, Manack Adams, A, Lipton, RB, et al. Effects of onabotulinumtoxinA treatment in patients with and without allodynia: results of the COMPEL study. J Headache Pain. (2019) 20:10. doi: 10.1186/s10194-018-0952-1
57. Bigal, ME, Serrano, D, Buse, D, Scher, A, Stewart, WF, and Lipton, RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. (2008) 48:1157–68. doi: 10.1111/j.1526-4610.2008.01217.x
58. Ashina, S, Serrano, D, Lipton, RB, Maizels, M, Manack, AN, Turkel, CC, et al. Depression and risk of transformation of episodic to chronic migraine. J Headache Pain. (2012) 13:615–24. doi: 10.1007/s10194-012-0479-9
59. Raffaelli, B, Fitzek, M, Overeem, LH, Storch, E, Terhart, M, and Reuter, U. Clinical evaluation of super-responders vs. non-responders to CGRP(−receptor) monoclonal antibodies: a real-world experience. J Headache Pain. (2023) 24:16. doi: 10.1186/s10194-023-01552-x
60. Scher, AI, Lipton, RB, Stewart, WF, and Bigal, M. Patterns of medication use by chronic and episodic headache sufferers in the general population: results from the frequent headache epidemiology study. Cephalalgia. (2010) 30:321–8. doi: 10.1111/j.1468-2982.2009.01913.x
61. Seng, EK, Buse, DC, Klepper, JE, Mayson, SJ, Grinberg, AS, Grosberg, BM, et al. Psychological factors associated with chronic migraine and severe migraine-related disability: an observational study in a tertiary headache center. Headache. (2017) 57:593–604. doi: 10.1111/head.13021
62. Kocakaya, H, Say, B, Yörübulut, S, and Ergün, U. Emotion dysregulation in migraine patients: can it be a hallmark the probability of the transformation from episodİc to chronic? Neurol Res. (2023) 45:610–8. doi: 10.1080/01616412.2023.2176089
63. Koechlin, H, Coakley, R, Schechter, N, Werner, C, and Kossowsky, J. The role of emotion regulation in chronic pain: a systematic literature review. J Psychosom Res. (2018) 107:38–45. doi: 10.1016/j.jpsychores.2018.02.002
64. Burch, R, Rizzoli, P, and Loder, E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. (2018) 58:496–505. doi: 10.1111/head.13281
65. Lipton, RB, Fanning, KM, Buse, DC, Martin, VT, Hohaia, LB, Adams, AM, et al. Migraine progression in subgroups of migraine based on comorbidities: results of the CaMEO study. Neurology. (2019) 93:e2224–36. doi: 10.1212/WNL.0000000000008589
66. Ihara, K, Ohtani, S, Watanabe, N, Takahashi, N, Miyazaki, N, Ishizuchi, K, et al. Predicting response to CGRP-monoclonal antibodies in patients with migraine in Japan: a single-Centre retrospective observational study. J Headache Pain. (2023) 24:23. doi: 10.1186/s10194-023-01556-7
67. Hong, JB, Lange, KS, Overeem, LH, Triller, P, Raffaelli, B, and Reuter, U. A scoping review and Meta-analysis of anti-CGRP monoclonal antibodies: predicting response. Pharmaceuticals (Basel). (2023) 16:934. doi: 10.3390/ph16070934
68. Baraldi, C, Castro, FL, Cainazzo, MM, Pani, L, and Guerzoni, S. Predictors of response to erenumab after 12 months of treatment. Brain Behav. (2021) 11:e2260. doi: 10.1002/brb3.2260
69. Scheffler, A, Messel, O, Wurthmann, S, Nsaka, M, Kleinschnitz, C, Glas, M, et al. Erenumab in highly therapy-refractory migraine patients: first German real-world evidence. J Headache Pain. (2020) 21:84. doi: 10.1186/s10194-020-01151-0
70. Iannone, LF, Burgalassi, A, Vigani, G, Tabasso, G, De Cesaris, F, Chiarugi, A, et al. Switching anti-CGRP(R) monoclonal antibodies in multi-assessed non-responder patients and implications for ineffectiveness criteria: a retrospective cohort study. Cephalalgia. (2023) 43:160519. doi: 10.1177/03331024231160519
71. De Icco, R, Vaghi, G, Allena, M, Ghiotto, N, Guaschino, E, Martinelli, D, et al. Does MIDAS reduction at 3 months predict the outcome of erenumab treatment? A real-world, open-label trial. J Headache Pain. (2022) 23:123. doi: 10.1186/s10194-022-01480-2
72. Lee, HC, Cho, S, and Kim, BK. Predictors of response to galcanezumab in patients with chronic migraine: a real-world prospective observational study. Neurol Sci. (2023) 44:2455–63. doi: 10.1007/s10072-023-06683-2
73. Barbanti, P, Egeo, G, Aurilia, C, Altamura, C, d'Onofrio, F, Finocchi, C, et al. Predictors of response to anti-CGRP monoclonal antibodies: a 24-week, multicenter, prospective study on 864 migraine patients. J Headache Pain. (2022) 23:138. doi: 10.1186/s10194-022-01498-6
74. Frattale, I, Caponnetto, V, Casalena, A, Assetta, M, Maddestra, M, Marzoli, F, et al. Association between response to triptans and response to erenumab: real-life data. J Headache Pain. (2021) 22:1. doi: 10.1186/s10194-020-01213-3
75. Silvestro, M, Tessitore, A, Scotto di Clemente, F, Battista, G, Tedeschi, G, and Russo, A. Refractory migraine profile in CGRP-monoclonal antibodies scenario. Acta Neurol Scand. (2021) 144:325–33. doi: 10.1111/ane.13472
76. Bottiroli, S, De Icco, R, Vaghi, G, Pazzi, S, Guaschino, E, Allena, M, et al. Psychological predictors of negative treatment outcome with Erenumab in chronic migraine: data from an open label long-term prospective study. J Headache Pain. (2021) 22:114. doi: 10.1186/s10194-021-01333-4
77. Nowaczewska, M, Straburzyński, M, Waliszewska-Prosół, M, Meder, G, Janiak-Kiszka, J, and Kaźmierczak, W. Cerebral blood flow and other predictors of responsiveness to Erenumab and Fremanezumab in migraine-a real-life study. Front Neurol. (2022) 13:895476. doi: 10.3389/fneur.2022.895476
78. Eross, EJ, Gladstone, JP, Lewis, S, Rogers, R, and Dodick, DW. Duration of migraine is a predictor for response to botulinum toxin type a. Headache. (2005) 45:308–14. doi: 10.1111/j.1526-4610.2005.05067.x
79. Schiano di Cola, F, Caratozzolo, S, Liberini, P, Rao, R, and Padovani, A. Response predictors in chronic migraine: medication overuse and depressive symptoms negatively impact Onabotulinumtoxin-a treatment. Front Neurol. (2019) 10:678. doi: 10.3389/fneur.2019.00678
80. Mathew, NT, Kailasam, J, and Meadors, L. Predictors of response to botulinum toxin type a (BoNTA) in chronic daily headache. Headache. (2008) 48:194–200. doi: 10.1111/j.1526-4610.2007.00914.x
81. Jakubowski, M, McAllister, PJ, Bajwa, ZH, Ward, TN, Smith, P, and Burstein, R. Exploding vs. imploding headache in migraine prophylaxis with botulinum toxin a. Pain. (2006) 125:286–95. doi: 10.1016/j.pain.2006.09.012
82. Lin, KH, Chen, SP, Fuh, JL, Wang, YF, and Wang, SJ. Efficacy, safety, and predictors of response to botulinum toxin type a in refractory chronic migraine: a retrospective study. J Chin Med Assoc. (2014) 77:10–5. doi: 10.1016/j.jcma.2013.09.006
83. Domínguez Vivero, C, Leira, Y, Saavedra Piñeiro, M, Rodríguez-Osorio, X, Ramos-Cabrer, P, Villalba Martín, C, et al. Iron deposits in periaqueductal gray matter are associated with poor response to OnabotulinumtoxinA in chronic migraine. Toxins (Basel). (2020) 12:479. doi: 10.3390/toxins12080479
84. Domínguez, C, López, A, Ramos-Cabrer, P, Vieites-Prado, A, Pérez-Mato, M, Villalba, C, et al. Iron deposition in periaqueductal gray matter as a potential biomarker for chronic migraine. Neurology. (2019) 92:e1076–85. doi: 10.1212/WNL.0000000000007047
85. Polderman, TJ, Benyamin, B, de Leeuw, CA, Sullivan, PF, van Bochoven, A, Visscher, PM, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. (2015) 47:702–9. doi: 10.1038/ng.3285
86. Choquet, H, Yin, J, Jacobson, AS, Horton, BH, Hoffmann, TJ, Jorgenson, E, et al. New and sex-specific migraine susceptibility loci identified from a multiethnic genome-wide meta-analysis. Commun Biol. (2021) 4:864. doi: 10.1038/s42003-021-02356-y
87. Hautakangas, H, Winsvold, BS, Ruotsalainen, SE, Bjornsdottir, G, Harder, AVE, Kogelman, LJA, et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet. (2022) 54:152–60. doi: 10.1038/s41588-021-00990-0
88. MaassenVanDenBrink, A, Vergouwe, MN, Ophoff, RA, Saxena, PR, Ferrari, MD, and Frants, RR. 5-HT1B receptor polymorphism and clinical response to Sumatriptan. Headache: the journal of head and face. Pain. (1998) 38:288–91. doi: 10.1046/j.1526-4610.1998.3804288.x
89. Zhang, X, Zhou, J, Guo, M, Cheng, S, Chen, Y, Jiang, N, et al. A systematic review and meta-analysis of voxel-based morphometric studies of migraine. J Neurol. (2023) 270:152–70. doi: 10.1007/s00415-022-11363-w
90. Zhang, Q, Wu, Q, Zhang, J, He, L, Huang, J, Zhang, J, et al. Discriminative analysis of migraine without Aura: using functional and structural MRI with a multi-feature classification approach. PLoS One. (2016) 11:e0163875. doi: 10.1371/journal.pone.0163875
91. Schwedt, TJ, Chong, CD, Wu, T, Gaw, N, Fu, Y, and Li, J. Accurate classification of chronic migraine via brain magnetic resonance imaging. Headache. (2015) 55:762–77. doi: 10.1111/head.12584
92. Ahmed, SR, Mohamed, AAM, Salem, HH, Helmy, S, Moustafa, RR, and Borham, SMF. Association of white matter hyperintensities with migraine phenotypes and response to treatment. Acta Neurol Belg. (2022). doi: 10.1007/s13760-022-02015-x
93. Ailani, J, Burch, RC, and Robbins, MS, the Board of Directors of the American Headache S. The American headache society consensus statement: update on integrating new migraine treatments into clinical practice. Headache: the journal of head and face. Pain. (2021) 61:1021–39. doi: 10.1111/head.14153
94. Stern, JI, Chiang, CC, Kissoon, NR, and Robertson, CE. Narrative review of peripheral nerve blocks for the management of headache. Headache. (2022) 62:1077–92. doi: 10.1111/head.14385
95. Silvestro, M, Orologio, I, Siciliano, M, Trojsi, F, Tessitore, A, Tedeschi, G, et al. Emerging drugs for the preventive treatment of migraine: a review of CGRP monoclonal antibodies and gepants trials. Expert Opin Emerg Drugs. (2023) 28:79–96. doi: 10.1080/14728214.2023.2207819
97. Peterlin, BL, Calhoun, AH, Siegel, S, and Mathew, NT. Rational combination therapy in refractory migraine. Headache: the journal of head and face. Pain. (2008) 48:805–19. doi: 10.1111/j.1526-4610.2008.01142.x
98. Berman, G, Croop, R, Kudrow, D, Halverson, P, Lovegren, M, Thiry, AC, et al. Safety of Rimegepant, an Oral CGRP receptor antagonist, plus CGRP monoclonal antibodies for migraine. Headache. (2020) 60:1734–42. doi: 10.1111/head.13930
99. Mullin, K, Kudrow, D, Croop, R, Lovegren, M, Conway, CM, Coric, V, et al. Potential for treatment benefit of small molecule CGRP receptor antagonist plus monoclonal antibody in migraine therapy. Neurology. (2020) 94:e2121–5. doi: 10.1212/WNL.0000000000008944
100. Freitag, F, Tolebeyan, A, and Sivakumar, D. CGRP monoclonal antibodies along with CGRP receptor antagonists are safe and effective together and compared to standard of care. Hoboken, NJ: Wiley (2021).
101. Mueller, L . Gepant efficacy when combined with a CGRP monoclonal antibody: A retrospective chart review. Hoboken, NJ: Wiley (2021).
102. Tolebeyan, A, Sivakumar, D, Freitag, F, Shumate, D, and Schloemer, F. CGRP monoclonal antibodies along with CGRP receptor antagonists are safe and effective together and compared to standard of care. AHS annual scientific meeting (2021).
103. Melo-Carrillo, A, Strassman, AM, Schain, AJ, Adams, AM, Brin, MF, and Burstein, R. Combined onabotulinumtoxinA/atogepant treatment blocks activation/sensitization of high-threshold and wide-dynamic range neurons. Cephalalgia. (2021) 41:17–32. doi: 10.1177/0333102420970507
104. Keskinbora, K, and Aydinli, I. A double-blind randomized controlled trial of topiramate and amitriptyline either alone or in combination for the prevention of migraine. Clin Neurol Neurosurg. (2008) 110:979–84. doi: 10.1016/j.clineuro.2008.05.025
105. Lillie, EO, Patay, B, Diamant, J, Issell, B, Topol, EJ, and Schork, NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med. (2011) 8:161–73. doi: 10.2217/pme.11.7
106. Parikh, SK, and Silberstein, SD. Current status of antiepileptic drugs as preventive migraine therapy. Curr Treat Options Neurol. (2019) 21:16. doi: 10.1007/s11940-019-0558-1
107. Lionetto, L, Negro, A, Palmisani, S, Gentile, G, Del Fiore, MR, Mercieri, M, et al. Emerging treatment for chronic migraine and refractory chronic migraine. Expert Opin Emerg Drugs. (2012) 17:393–406. doi: 10.1517/14728214.2012.709846
108. Markley, HG . Verapamil and migraine prophylaxis: mechanisms and efficacy. Am J Med. (1991) 90:48s–53s. doi: 10.1016/0002-9343(91)90486-h
109. Taylor, AP, Adelman, JU, and Freeman, MC. Efficacy of duloxetine as a migraine preventive medication: possible predictors of response in a retrospective chart review. Headache. (2007) 47:1200–3. doi: 10.1111/j.1526-4610.2007.00886.x
110. Punay, NC, and Couch, JR. Antidepressants in the treatment of migraine headache. Curr Pain Headache Rep. (2003) 7:51–4. doi: 10.1007/s11916-003-0010-8
111. Merikangas, KR, and Merikangas, JR. Combination monoamine oxidase inhibitor and beta-blocker treatment of migraine, with anxiety and depression. Biol Psychiatry. (1995) 38:603–10. doi: 10.1016/0006-3223(95)00077-1
112. Silberstein, SD, Peres, MFP, Hopkins, MM, Shechter, AL, Young, WB, and Rozen, TD. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache: the journal of head and face. Pain. (2002) 42:515–8. doi: 10.1046/j.1526-4610.2002.02126.x
113. Graff-Radford, SB, and Bittar, GT. The use of methylergonovine (Methergine) in the initial control of drug induced refractory headache. Headache. (1993) 33:390–3. doi: 10.1111/j.1526-4610.1993.hed3307390.x
114. Ashina, S, Mitsikostas, DD, Lee, MJ, Yamani, N, Wang, SJ, Messina, R, et al. Tension-type headache. Nat Rev Dis Primers. (2021) 7:24. doi: 10.1038/s41572-021-00257-2
115. Lauritsen, CG, and Marmura, MJ. Current treatment options: vestibular migraine. Curr Treat Options Neurol. (2017) 19:38. doi: 10.1007/s11940-017-0476-z
116. Russell, MB, and Ducros, A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. (2011) 10:457–70. doi: 10.1016/S1474-4422(11)70048-5
117. Conor, R, Ivan, C, Britta, B, Michael, C, and Rodolfo, S. Amantadine use in post-concussive headaches: an exploratory retrospective review (P5.024). Neurology. (2018) 90:24.
118. Kawase, Y, Ikeda, K, and Iwasaki, Y. Amantadine for migraine. Headache: the journal of head and face. Pain. (2008) 48:1380. doi: 10.1111/j.1526-4610.2008.01155.x
119. Freitag, FG . Preventative treatment for migraine and tension-type headaches: do drugs having effects on muscle spasm and tone have a role? CNS Drugs. (2003) 17:373–81. doi: 10.2165/00023210-200317060-00001
120. Duarte, RA, Dahmer, S, Sanguinetti, SY, Forde, G, Duarte, DP, and Kobak, LF. Medical Cannabis for headache pain: a primer for clinicians. Curr Pain Headache Rep. (2021) 25:64. doi: 10.1007/s11916-021-00974-z
121. Greco, R, Demartini, C, Zanaboni, AM, Francavilla, M, De Icco, R, Ahmad, L, et al. The endocannabinoid system and related lipids as potential targets for the treatment of migraine-related pain. Headache: the journal of head and face. Pain. (2022) 62:227–40. doi: 10.1111/head.14267
122. Spierings, ELH . Daily Triptan use for intractable migraine. Headache: the journal of head and face. Pain. (2014) 54:155–62. doi: 10.1111/head.12275
123. Pardutz, A, and Schoenen, J. NSAIDs in the acute treatment of migraine: a review of clinical and experimental data. Pharmaceuticals (Basel). (2010) 3:1966–87. doi: 10.3390/ph3061966
124. Rapoport, AM, Bigal, ME, Volcy, M, Sheftell, FD, Feleppa, M, and Tepper, SJ. Naratriptan in the preventive treatment of refractory chronic migraine: a review of 27 cases. Headache. (2003) 43:482–9. doi: 10.1046/j.1526-4610.2003.03094.x
125. Schindler, EAD . The potential of psychedelics for the treatment of episodic migraine. Curr Pain Headache Rep. (2023) 27:489–95. doi: 10.1007/s11916-023-01145-y
126. Sewell, RA, Halpern, JH, and Pope, HGJr . Response of cluster headache to psilocybin and LSD. Neurology. (2006) 66:1920–2. doi: 10.1212/01.wnl.0000219761.05466.43
127. Mojica, JJ, Schwenk, ES, Lauritsen, C, and Nahas, SJ. Beyond the Raskin protocol: ketamine, lidocaine, and other therapies for refractory chronic migraine. Curr Pain Headache Rep. (2021) 25:77. doi: 10.1007/s11916-021-00992-x
128. Piatka, C, and Beckett, RD. Propofol for treatment of acute migraine in the emergency department: a systematic review. Acad Emerg Med. (2019) 27:148–60. doi: 10.1111/acem.13870
129. Shafqat, R, Flores-Montanez, Y, Delbono, V, and Nahas, SJ. Updated evaluation of IV Dihydroergotamine (DHE) for refractory migraine: patient selection and special considerations. J Pain Res. (2020) 13:859–64. doi: 10.2147/JPR.S203650
130. Mendes, PM, Silberstein, SD, Young, WB, Rozen, TD, and Paolone, MF. Intravenous Propofol in the treatment of refractory headache. Headache: the journal of head and face. Pain. (2002) 42:638–41. doi: 10.1046/j.1526-4610.2002.02151.x
131. Haque, N, and Tariq, N. Short term Oral Methylergonovine maleate prophylaxis for status Migrainosus. Case series and review of literature. Front Neurol. (2019) 10:201. doi: 10.3389/fneur.2019.00201
132. Marmura, MJ, Passero, FC, and Young, WB. Mexiletine for refractory chronic daily headache: a report of nine cases. Headache. (2008) 48:1506–10. doi: 10.1111/j.1526-4610.2008.01234.x
133. Lovati, C, d'Alessandro, CM, Ventura, SD, Muzio, F, and Pantoni, L. Ketogenic diet in refractory migraine: possible efficacy and role of ketone bodies-a pilot experience. Neurol Sci. (2022) 43:6479–85. doi: 10.1007/s10072-022-06311-5
134. Saper, JR, Lake, AE, Bain, PA, Stillman, MJ, Rothrock, JF, Mathew, NT, et al. A practice guide for continuous opioid therapy for refractory daily headache: patient selection, physician requirements, and treatment monitoring. Headache: the journal of head and face. Pain. (2010) 50:1175–93. doi: 10.1111/j.1526-4610.2010.01733.x
135. Loder, E, Weizenbaum, E, Frishberg, B, and Silberstein, S. Choosing wisely in headache medicine: the American headache Society's list of five things physicians and patients should question. Headache. (2013) 53:1651–9. doi: 10.1111/head.12233
136. Schwedt, TJ, Hentz, JG, Sahai-Srivastava, S, Spare, NM, Martin, VT, Treppendahl, C, et al. Headache characteristics and burden from chronic migraine with medication overuse headache: cross-sectional observations from the medication overuse treatment strategy trial. Headache: the journal of head and face. Pain. (2021) 61:351–62. doi: 10.1111/head.14056
137. Westergaard, ML, Munksgaard, SB, Bendtsen, L, and Jensen, RH. Medication-overuse headache: a perspective review. Ther Adv Drug Saf. (2016) 7:147–58. doi: 10.1177/2042098616653390
138. Ho, TW, Rodgers, A, and Bigal, ME. Impact of recent prior opioid use on rizatriptan efficacy. A post hoc pooled analysis. Headache. (2009) 49:395–403. doi: 10.1111/j.1526-4610.2009.01346.x
139. Buse, DC, Pearlman, SH, Reed, ML, Serrano, D, Ng-Mak, DS, and Lipton, RB. Opioid use and dependence among persons with migraine: results of the AMPP study. Headache. (2012) 52:18–36. doi: 10.1111/j.1526-4610.2011.02050.x
140. Friedman, BW, Irizarry, E, Solorzano, C, Latev, A, Rosa, K, Zias, E, et al. Randomized study of IV prochlorperazine plus diphenhydramine vs IV hydromorphone for migraine. Neurology. (2017) 89:2075–82. doi: 10.1212/WNL.0000000000004642
141. Friedman, BW, Kapoor, A, Friedman, MS, Hochberg, ML, and Rowe, BH. The relative efficacy of meperidine for the treatment of acute migraine: a meta-analysis of randomized controlled trials. Ann Emerg Med. (2008) 52:705–13. doi: 10.1016/j.annemergmed.2008.05.036
142. Griffith, JD, Mycyk, MB, and Kyriacou, DN. Metoclopramide versus hydromorphone for the emergency department treatment of migraine headaches. Ann Emerg Med. (2004) 44:S60. doi: 10.1016/j.jpain.2007.09.001
143. Piekos, K, and Spierings, EL. Management of daily headache unresponsive to preventive treatment: daily triptans versus daily opioids. Rev Neurol Dis. (2009) 6:E121–30.
144. Wiesenfeld-Hallin, Z . Combined opioid-NMDA antagonist therapies. What advantages do they offer for the control of pain syndromes? Drugs. (1998) 55:1–4. doi: 10.2165/00003495-199855010-00001
145. Benemei, S, Lupi, C, De Cesaris, F, Lombardi, N, Bettiol, A, Chiarugi, A, et al. Low-dose methadone for refractory chronic migraine accompanied by medication-overuse headache: a prospective cohort study. Neurol Sci. (2021) 42:987–94. doi: 10.1007/s10072-020-04602-3
146. Silverman, SM . Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. (2009) 12:679–84.
147. Bertels, Z, and Pradhan, AAA. Emerging treatment targets for migraine and other headaches. Headache. (2019) 59:50–65. doi: 10.1111/head.13585
148. Göbel, CH, Göbel, A, Niederberger, U, Heinze, A, Heinze-Kuhn, K, Meinecke, C, et al. Occipital nerve stimulation in chronic migraine: the relationship between perceived sensory quality, perceived sensory location, and clinical efficacy-a prospective, observational, non-interventional study. Pain Ther. (2020) 9:615–26. doi: 10.1007/s40122-020-00194-0
149. Cadalso, RTJr , Daugherty, J, Holmes, C, Ram, S, and Enciso, R. Efficacy of electrical stimulation of the occipital nerve in intractable primary headache disorders: a systematic review with Meta-analyses. J Oral Facial Pain Headache. (2018) 32:40–52. doi: 10.11607/ofph.1784
150. Schwedt, TJ, Green, AL, and Dodick, DW. Occipital nerve stimulation for migraine: update from recent multicenter trials. Prog Neurol Surg. (2015) 29:117–26. doi: 10.1159/000434662
151. Raoul, S, Nguyen, JM, Kuhn, E, de Chauvigny, E, Lejczak, S, Nguyen, JP, et al. Efficacy of occipital nerve stimulation to treat refractory occipital headaches: a single-institution study of 60 patients. Neuromodulation. (2020) 23:789–95. doi: 10.1111/ner.13223
152. Rodrigo, D, Acin, P, and Bermejo, P. Occipital nerve stimulation for refractory chronic migraine: results of a long-term prospective study. Pain Physician. (2017) 20:E151–e9.
153. Montenegro, MM, and Kissoon, NR. Long term outcomes of occipital nerve stimulation. Front Pain Res (Lausanne). (2023) 4:1054764. doi: 10.3389/fpain.2023.1054764
154. Kinfe, TM, Schuss, P, and Vatter, H. Occipital nerve block prior to occipital nerve stimulation for refractory chronic migraine and chronic cluster headache: myth or prediction? Cephalalgia. (2015) 35:359–62. doi: 10.1177/0333102414541685
155. Schwedt, TJ, Dodick, DW, Trentman, TL, and Zimmerman, RS. Response to occipital nerve block is not useful in predicting efficacy of occipital nerve stimulation. Cephalalgia. (2007) 27:271–4. doi: 10.1111/j.1468-2982.2006.01251.x
156. Reed, KL, Black, SB, Banta, CJ2nd , and Will, KR. Combined occipital and supraorbital neurostimulation for the treatment of chronic migraine headaches: initial experience. Cephalalgia. (2010) 30:260–71. doi: 10.1111/j.1468-2982.2009.01996.x
157. Clark, SW, Wu, C, Boorman, DW, Chalouhi, N, Zanaty, M, Oshinsky, M, et al. Long-term pain reduction does not imply improved functional outcome in patients treated with combined supraorbital and occipital nerve stimulation for chronic migraine. Neuromodulation. (2016) 19:507–14. doi: 10.1111/ner.12400
158. Tepper, SJ, Grosberg, B, Daniel, O, Kuruvilla, DE, Vainstein, G, Deutsch, L, et al. Migraine treatment with external concurrent occipital and trigeminal neurostimulation–a randomized controlled trial. Headache: the journal of head and face. Pain. (2022) 62:989–1001. doi: 10.1111/head.14350
159. Leone, M . Deep brain stimulation in headache. Lancet Neurol. (2006) 5:873–7. doi: 10.1016/S1474-4422(06)70575-0
160. Miller, S, Sinclair, AJ, Davies, B, and Matharu, M. Neurostimulation in the treatment of primary headaches. Pract Neurol. (2016) 16:362–75. doi: 10.1136/practneurol-2015-001298
161. Blake, P, and Burstein, R. Emerging evidence of occipital nerve compression in unremitting head and neck pain. J Headache Pain. (2019) 20:76. doi: 10.1186/s10194-019-1023-y
162. Ducic, I, Felder, JM3rd , and Fantus, SA. A systematic review of peripheral nerve interventional treatments for chronic headaches. Ann Plast Surg. (2014) 72:439–45. doi: 10.1097/SAP.0000000000000063
163. Castelnuovo, G, Giusti, EM, Manzoni, GM, Saviola, D, Gatti, A, Gabrielli, S, et al. Psychological considerations in the assessment and treatment of pain in neurorehabilitation and psychological factors predictive of therapeutic response: evidence and recommendations from the Italian consensus conference on pain in neurorehabilitation. Front Psychol. (2016) 7:468. doi: 10.3389/fpsyg.2016.00468
164. Rogers, DG, Protti, TA, and Smitherman, TA. Fear, avoidance, and disability in headache disorders. Curr Pain Headache Rep. (2020) 24:33. doi: 10.1007/s11916-020-00865-9
165. Giannini, G, Zanigni, S, Grimaldi, D, Melotti, R, Pierangeli, G, Cortelli, P, et al. Cephalalgiaphobia as a feature of high-frequency migraine: a pilot study. J Headache Pain. (2013) 14:49. doi: 10.1186/1129-2377-14-49
166. Pfaller, A . Efficacy of biofeedback in the treatment of migraine and tension type headaches. Pain Physician. (2010) 13:94–6.
167. Penzien, DB, and Irby, MB. Insights on treatment mechanisms of cognitive-behavioral migraine therapy. Headache. (1439) 54:1439–40. doi: 10.1111/head.12433
168. Powers, SW, Kashikar-Zuck, SM, Allen, JR, LeCates, SL, Slater, SK, Zafar, M, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA. (2013) 310:2622–30. doi: 10.1001/jama.2013.282533
169. Kroon Van Diest, AM, Ramsey, RR, Kashikar-Zuck, S, Slater, S, Hommel, K, Kroner, JW, et al. Treatment adherence in child and adolescent chronic migraine patients: results from the cognitive-behavioral therapy and amitriptyline trial. Clin J Pain. (2017) 33:892–8. doi: 10.1097/AJP.0000000000000481
170. Carvalho, GF, Schwarz, A, Szikszay, TM, Adamczyk, WM, Bevilaqua-Grossi, D, and Luedtke, K. Physical therapy and migraine: musculoskeletal and balance dysfunctions and their relevance for clinical practice. Braz J Phys Ther. (2020) 24:306–17. doi: 10.1016/j.bjpt.2019.11.001
171. Muñoz-Gómez, E, Serra-Añó, P, Mollà-Casanova, S, Sempere-Rubio, N, Aguilar-Rodríguez, M, Espí-López, GV, et al. Potential add-on effects of manual therapy techniques in migraine patients: a randomised controlled trial. J Clin Med. (2022) 11:4686. doi: 10.3390/jcm11164686
172. Li, YX, Xiao, XL, Zhong, DL, Luo, LJ, Yang, H, Zhou, J, et al. Effectiveness and safety of acupuncture for migraine: an overview of systematic reviews. Pain Res Manag. (2020) 2020:3825617. doi: 10.1155/2020/3825617
173. Vázquez-Justes, D, Yarzábal-Rodríguez, R, Doménech-García, V, Herrero, P, and Bellosta-López, P. Effectiveness of dry needling for headache: a systematic review. Neurologia (Engl Ed). (2022) 37:806–15. doi: 10.1016/j.nrl.2019.09.010
174. Woldeamanuel, YW, and Oliveira, ABD. What is the efficacy of aerobic exercise versus strength training in the treatment of migraine? A systematic review and network meta-analysis of clinical trials. J Headache Pain. (2022) 23:134. doi: 10.1186/s10194-022-01503-y
175. Lemmens, J, De Pauw, J, Van Soom, T, Michiels, S, Versijpt, J, van Breda, E, et al. The effect of aerobic exercise on the number of migraine days, duration and pain intensity in migraine: a systematic literature review and meta-analysis. J Headache Pain. (2019) 20:16. doi: 10.1186/s10194-019-0961-8
176. Anheyer, D, Klose, P, Lauche, R, Saha, FJ, and Cramer, H. Yoga for treating headaches: a systematic review and Meta-analysis. J Gen Intern Med. (2020) 35:846–54. doi: 10.1007/s11606-019-05413-9
177. Robblee, J, and Starling, AJ. SEEDS for success: lifestyle management in migraine. Cleve Clin J Med. (2019) 86:741–9. doi: 10.3949/ccjm.86a.19009
178. Marmura, MJ . Triggers, protectors, and predictors in episodic migraine. Curr Pain Headache Rep. (2018) 22:81. doi: 10.1007/s11916-018-0734-0
179. Limmroth, V, Biondi, D, Pfeil, J, and Schwalen, S. Topiramate in patients with episodic migraine: reducing the risk for chronic forms of headache. Headache. (2007) 47:13–21. doi: 10.1111/j.1526-4610.2007.00648.x
180. Lipton, RB, Silberstein, S, Dodick, D, Cady, R, Freitag, F, Mathew, N, et al. Topiramate intervention to prevent transformation of episodic migraine: the topiramate INTREPID study. Cephalalgia. (2011) 31:18–30. doi: 10.1177/0333102410372427
181. Lipton, RB, Fanning, KM, Serrano, D, Reed, ML, Cady, R, and Buse, DC. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology. (2015) 84:688–95. doi: 10.1212/WNL.0000000000001256
182. Dodick, DW . Review of comorbidities and risk factors for the development of migraine complications (infarct and chronic migraine). Cephalalgia. (2009) 29:7–14. doi: 10.1177/03331024090290S303
Keywords: refractory migraine, intractable migraine, chronic migraine, chronic daily headache, resistant migraine, diagnosis, management
Citation: Robblee J (2023) Breaking the cycle: unraveling the diagnostic, pathophysiological and treatment challenges of refractory migraine. Front. Neurol. 14:1263535. doi: 10.3389/fneur.2023.1263535
Edited by:
David M. Niddam, National Yang Ming Chiao Tung Unviersity, TaiwanReviewed by:
Raffaele Ornello, University of L’Aquila, ItalyCopyright © 2023 Robblee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer Robblee, TmV1cm9wdWJAYmFycm93bmV1cm8ub3Jn
 Jennifer Robblee
Jennifer Robblee