
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 12 October 2023
Sec. Neuroepidemiology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1263291
This article is part of the Research Topic Epidemiology, Evidence-Based Care, and Outcomes in Spinal Cord Injury View all 15 articles
 Shahin Basiratzadeh1*†
Shahin Basiratzadeh1*† Ramtin Hakimjavadi2
Ramtin Hakimjavadi2 Natalie Baddour3†
Natalie Baddour3† Wojtek Michalowski1
Wojtek Michalowski1 Herna Viktor4
Herna Viktor4 Eugene Wai5,6
Eugene Wai5,6 Alexandra Stratton5,6
Alexandra Stratton5,6 Stephen Kingwell5,6
Stephen Kingwell5,6 Jean-Marc Mac-Thiong7,8
Jean-Marc Mac-Thiong7,8 Eve C. Tsai9,10
Eve C. Tsai9,10 Zhi Wang11
Zhi Wang11 Philippe Phan5,6
Philippe Phan5,6Background: Conducting clinical trials for traumatic spinal cord injury (tSCI) presents challenges due to patient heterogeneity. Identifying clinically similar subgroups using patient demographics and baseline injury characteristics could lead to better patient-centered care and integrated care delivery.
Purpose: We sought to (1) apply an unsupervised machine learning approach of cluster analysis to identify subgroups of tSCI patients using patient demographics and injury characteristics at baseline, (2) to find clinical similarity within subgroups using etiological variables and outcome variables, and (3) to create multi-dimensional labels for categorizing patients.
Study design: Retrospective analysis using prospectively collected data from a large national multicenter SCI registry.
Methods: A method of spectral clustering was used to identify patient subgroups based on the following baseline variables collected since admission until rehabilitation: location of the injury, severity of the injury, Functional Independence Measure (FIM) motor, and demographic data (age, and body mass index). The FIM motor score, the FIM motor score change, and the total length of stay were assessed on the subgroups as outcome variables at discharge to establish the clinical similarity of the patients within derived subgroups. Furthermore, we discussed the relevance of the identified subgroups based on the etiological variables (energy and mechanism of injury) and compared them with the literature. Our study also employed a qualitative approach to systematically describe the identified subgroups, crafting multi-dimensional labels to highlight distinguishing factors and patient-focused insights.
Results: Data on 334 tSCI patients from the Rick Hansen Spinal Cord Injury Registry was analyzed. Five significantly different subgroups were identified (p-value ≤0.05) based on baseline variables. Outcome variables at discharge superimposed on these subgroups had statistically different values between them (p-value ≤0.05) and supported the notion of clinical similarity of patients within each subgroup.
Conclusion: Utilizing cluster analysis, we identified five clinically similar subgroups of tSCI patients at baseline, yielding statistically significant inter-group differences in clinical outcomes. These subgroups offer a novel, data-driven categorization of tSCI patients which aligns with their demographics and injury characteristics. As it also correlates with traditional tSCI classifications, this categorization could lead to improved personalized patient-centered care.
Traumatic spinal cord injury (tSCI) has significant physical, social, and vocational consequences for patients and their families (1). The loss of independence, increased lifelong mortality rates, and high costs for care place a great burden on the individuals and the healthcare system (1–3), making the appropriate treatment of this devastating disorder crucially important. The management of tSCI requires significant health care resource utilization (4), owing to a possible need for short-term intensive acute care and appropriate management of long-term secondary complications (3). Better specialization for managing tSCI is needed to address the unique needs of patients and to better allocate healthcare resources (5). The implementation of targeted care and effective treatment options could produce substantial benefits for both the patient and the healthcare system.
Early research suggests that specialized care, as opposed to general care, can help produce positive outcomes, including decreased length of stay (LOS) and decreased incidence of secondary complications (6–8). However, the optimal model of healthcare delivery for patients with tSCI has not yet been defined (5); despite current advances, the considerable heterogeneity within the tSCI patient population remains a prominent challenge (9). The variety of pathologies, levels of neurological impairment, and different potentials for recovery within tSCI patients (10, 11) makes it difficult to determine the efficacy of management strategies when novel therapies and standards of care are applied to a group with mixed needs and outcome trajectories. The identification of tSCI patient subgroups with clinically similar characteristics should facilitate better communication between patients and providers, guide optimal management, and inform the development of targeted therapies and models of care.
The categorization and management of tSCI have traditionally been guided by established classifications. Most tSCI studies rely on the International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI) to classify patients into groups, which is considered the gold standard for neurological assessment (12, 13). Based on the ISNCSCI, the American Spinal Injury Association Impairment Scale (AIS) is a measure of the neurological severity of injury, and is the most important predictor of recovery in tSCI patients (14). However, classification based on AIS grade alone does not adequately address the heterogeneity observed in the tSCI population; there is considerable variation in spontaneous recovery within each AIS grade (range: A–D), leading to differences in recovery trajectories between patients with presumedly similar initial clinical impairment (11). In other words, knowledge about individual prognostic variables for tSCI provides limited information about complex interactions between other variables and how they may influence prognosis. While the AIS grade itself might be the most important indicator for the prediction of recovery, other clinical factors such as age, injury characteristics, and functional measures have also been reported as significant prognostic variables (15).
As a first step towards understanding the heterogeneity inherent in tSCI, Dvorak and colleagues (9) proposed a classification scheme based on the joint use of baseline neurological level of injury (NLI) and severity of neurological impairment (i.e., AIS grade) – two of the predominant predictors of neurological outcome (13, 15). This approach was deemed the “Canadian Classification” and serves to guide tSCI researchers on how to better classify patients for clinical trials, and how to avoid unrecognized heterogeneity (or imbalances) between treatment groups. Dvorak’s work made several important contributions, including a demonstration that classifying based on the joint distribution of the two baseline characteristics (level and severity of injury), beyond simple univariable classification, can reveal meaningful differences in the recovery potential of patients (9).
The digital age has produced a wealth of healthcare data, providing new opportunities to apply data analytics for improved decision-making by facilitating predictive modeling, treatment pattern identification, and detection of subtle correlations that may be overlooked in traditional methods (16, 17).
Through the lens of data analytics, we aim to build upon previous research by using unsupervised machine learning and specifically spectral clustering (SC), to examine the simultaneous interactions within multiple variables and identify previously unrecognized associations in a data-driven manner. In this approach, the analysis is based on the data itself rather than being influenced by preconceived notions or assumptions about the data (17, 18). Our study therefore represents a data-driven approach to understanding and categorizing tSCI that could potentially guide management of these complex injuries. Notably, such a methodology has previously been applied to research on adult spinal deformity (19).
We hypothesize that a data-driven approach can identify subgroups with clinical similarity within a heterogeneous population of tSCI patients and provide a clinically relevant categorization. To this end, the objectives of this study are to (1) apply an unsupervised machine learning approach, specifically cluster analysis, to identify subgroups of tSCI patients using patient demographics and injury characteristics at baseline, (2) to find clinical similarity within subgroups using etiological variables and outcome variables at discharge, and (3) to categorize patients with clinically similar characteristics by creating multi-dimensional labels.
This was a retrospective study using prospectively collected data from a large national, multicenter SCI registry. It included variables from different time points (e.g., admission, inpatient rehabilitation, and discharge), and was conducted in two phases.
During the first phase, SC was performed on a subset of variables at baseline to identify subgroups of tSCI patients. Clinical similarities were then identified between each subgroup by superimposing the outcome variables at discharge and etiological variables. The rationale behind exclusively forming subgroups based on baseline variables, and then superimposing outcome variables, is to assess the distinction among patient categories, with respect to selected outcomes. This choice protocol results in a subgrouping independent of outcome variables. As such, the identified subgroups can later be studied against a range of outcomes.
During the second phase, the results were interpreted from a point of view of statistical significance between each group. Thereafter, exemplars were used to describe (or “label”) patient’s subgroups qualitatively and systematically to reveal any patient-centred insights that can be drawn from the identified clinically similar subgroups.
The analyzed data set consisted of patients enrolled in the Rick Hansen Spinal Cord Injury Registry (RHSCIR): a Canada-wide, prospectively collected multicenter database (20). RHSCIR collects data on individuals who have sustained an acute tSCI and received care at one of the participating 18 acute or 12 rehabilitation sites. All sites obtained Institutional Research Ethics Board (REB) approval to enroll patients and enter their data into the registry. A wide variety of data was collected from the pre-hospital, surgical, acute, and in-patient rehabilitation phases of the enrolled patients’ care, including but not limited to: socio-demographic factors, medical history, injury details, diagnoses and interventions, neurologic impairment, complications, and patient-reported outcomes. Upon discharge, a survey was conducted at 1, 2, 5, and 10-year intervals (from the date of injury) to obtain patient-reported outcome measures. The registry was created to support research and facilitate the implementation and optimization of best clinical practices (20).
The data for this study was collected from RHSCIR, which enrolled 8,273 patients with acute tSCI between 2004 and 2017. Data were extracted from RHSCIR for all eligible patients with an acute tSCI between the year 2004 to 2017. Patients were included in this study according to the following inclusion criteria:
• The potential participant was at least 18 years old at the time of injury.
• They had complete data for the variables of interest collected at the acute (0–15 days) time stamp.
• They had complete data for the variables of interest collected at discharge.
• They provided explicit consent for specific data collection, including patient-reported outcomes, across all study time points.
Of the original data, 334 patients met the inclusion criteria for the study. The specific numbers of patients adhering to these criteria at each stage of the study are shown in the flowchart in Figure 1.
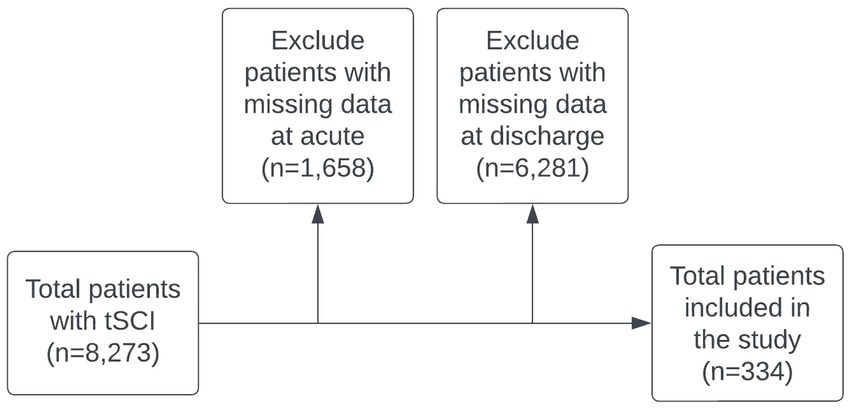
Figure 1. Study population flowchart. Noted that 4,164 patient cases labeled as ‘missing data’ resulted from the absence of explicit consent across all study time points.
While the tSCI patient population is the focus of the present study, we note that all spine trauma patients with injuries from the C1 to L5 vertebrae were considered. The spinal cord terminates at the conus medullaris, most commonly at the L1 vertebral body or L1-2 disk interspace level in adults (21). Injuries to the lumbar vertebral bodies may involve the lumbosacral nerve roots and cause cauda equina syndrome (CES), which is not strictly a type of SCI. We chose to include patients with injuries to all levels of the spine and observe the patterns that emerge from the data. Thus, our population of interest includes all patients with impairment of the spinal cord or cauda equina function resulting from trauma.
Variables related to patient demographic, injury, outcome characteristics, and etiology of tSCI were selected based on supporting clinical literature (1, 3, 15, 22–28), expert opinion, and availability in RHSCIR database. The list of variables selected from the dataset are presented in Table 1. Note that the neurological assessments included in the dataset were conducted within a time frame of less than 15 days following the injury.
Both patients and the healthcare system stand to benefit from more specialized tSCI care (5, 29). While the optimal method of healthcare delivery for this patient population has yet to be fully elucidated, there is emerging evidence that specialized care for tSCI patients is associated with reduced LOS and decreased overall mortality (5). The clustering used in this study, driven by baseline patient- and injury-related characteristics, should itself be able to produce clinically similar subgroups with distinct healthcare needs. In this case, patient needs were evaluated using the total Functional Independence Measure (FIM) motor score at discharge (a prognostic factor for long-term outcomes and economic burden) (30), FIM motor score change (measured as the difference between discharge and admission FIM motor scores), and the total LOS (a surrogate measure for healthcare resource utilization).
In this study, “etiological variables” refers to factors contributing to the cause or origin of the traumatic spinal cord injuries. Our analysis incorporated these variables with the aim of capturing crucial aspects of the injury’s cause and initial impact. These aspects can influence the severity of the injury and the patient’s subsequent recovery.
Two etiological variables were included in our study: “Mechanism of Injury,” and “Energy.” “Mechanism of Injury” describes the initial mechanical force that caused the SCI, such as a fall, a motor vehicle accident, and other types of incidents. “Energy” refers to the intensity of the initial mechanical force that led to a patient’s injury (15, 22).
Cluster analysis (CA) is an unsupervised learning method used for revealing hidden structures in data. Cluster analysis group objects with similar traits into subgroups while minimizing intragroup heterogeneity (16, 18, 31). To find the subgroups of tSCI patients based on baseline variables in the present study (Table 1), multiple clustering algorithms were assessed, and SC was chosen to explore patterns in our data. SC varies from the most commonly used partitioning-based clustering algorithm, K-means clustering (32, 33) as it is not dependent on distance from a centroid and it also does not require clusters to be spherical. Rather, the distance metric used for SC ensures that cluster members are near to one another. As a result, patients in the same subgroups tend to have similar values based on selected characteristics. Unlike K-means, which identifies subgroups with linear borders, SC is capable of forming subgroups with nonlinear boundaries (34).
To identify the optimal number of subgroups, the performance of SC using different numbers of subgroups was evaluated using the Silhouette Coefficient, the Davies-Bouldin index, and the elbow method (35–37).
Statistically significant differences and exemplars were used to interpret key findings of the analysis.
After subgroups were created using SC, continuous variables were analyzed using a Kruskal-Wallis test (not meeting the normality assumption) (38) to identify whether there were statistically significant intergroup differences across all subgroups (considered as p ≤ 0.05). Although the p-value is not traditionally a focal point in cluster analysis, its application here provided deeper insights by enabling us to identify distinguishing factors among subgroups. Variables found to statistically differ were then explored in a pairwise manner between subgroups using Mann–Whitney U tests (39), further enhancing our understanding of the patient subgroups. Continuous variables were presented with means and standard deviations, whereas categorical variables were presented with frequency of occurrence and percentages.
The concept of exemplars is used in this study to provide a tangible illustration of the characteristics of each identified cluster. Exemplar terms are used to represent each subgroup, and are also defined as the medoid of each cluster. Medoids are known as “actual objects” in the data (i.e., a typical patient); the object within a cluster that has the minimum sum of distances to all other objects in the cluster. Therefore, typical patient cases from each subgroup have been provided as a method of interpretation of SC analysis. Using this method, significant findings from statistically significant tests can be demonstrated for each subgroup.
Once patient subgroups were identified using SC analysis, they were then qualitatively described (i.e., “labelled”) to elucidate patient-centred insights that could be learned from the data-driven groupings of patients. Three types of multi-dimensional labels were created to demonstrate the clinical similarity of the following characteristics: (1) patient at presentation, (2) spine injury, and (3) patient at discharge. These multi-dimensional labels served to highlight the distinguishing factors that emerged in each patient subgroup with similar characteristics and to explore whether the “label” (i.e., the pattern of values of distinguishing variables in each subgroup) provided clinically intuitive and plausible characterization in the context of tSCI. Labelling was approached systematically; for each subgroup, only those variables that were determined to have values statistically different from other subgroups were considered as “distinguishing” and thus were used as labels. In addition, we discussed the relevance of the identified subgroups based on the etiological variables and compared them with the literature.
The 334 patients that met the inclusion criteria for the study and were divided into 5 subgroups using SC. These subgroups were verified as clinically relevant through consultation with field experts and literature review. Table 2 shows the variables at baseline that were found to have statistically significant differences (p < 0.05) between the subgroups. Tables 3, 4 present the outcome variables and additional variables related to the mechanism of injury superimposed on each subgroup.
Multi-dimensional labels were created to describe patient demographic and injury characteristics with distinguishing variables at baseline and discharge. Where applicable, specifiers were used to describe variables on a spectrum of severity (i.e., “mild,” “moderate,” “severe” or “extreme”) relative to other subgroups (Table 5). Additionally, a phenotype anatomical figure was constructed to visually highlight the anatomical order of the subgroups, providing a perspective on patient demographics and injury traits (Figure 2). Lastly, exemplars (typical patient case representative of each subgroup) were described.
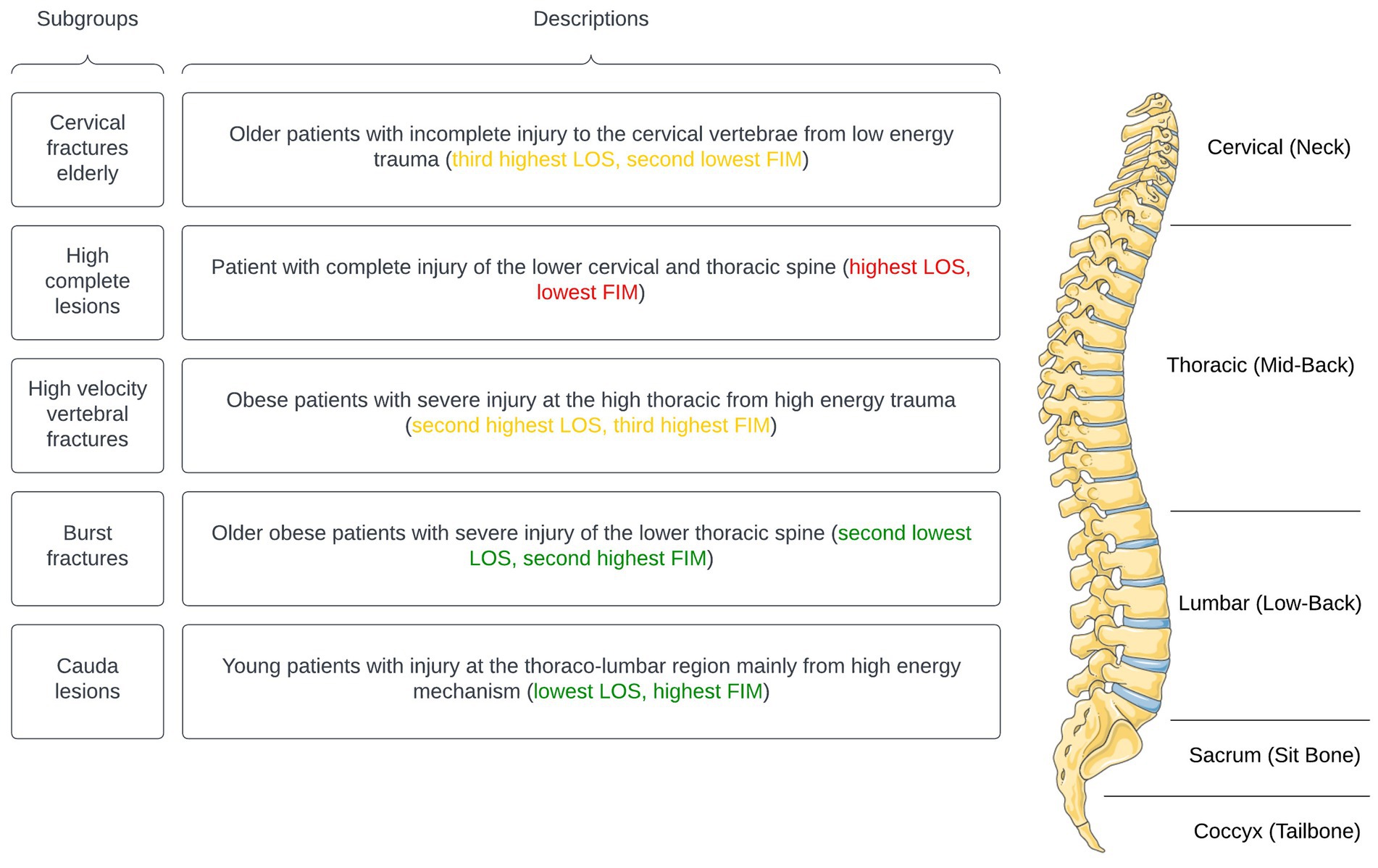
Figure 2. Subgroups with clinically significant phenotypes (parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License).
60-year-old non-obese individual presenting with a motor incomplete injury (AIS D) to the C3-C4 vertebrae, an NLI at C4, and a FIM motor score of 35 at admission. Patient 1’s total length of stay was 102 days before being discharged with a FIM motor score of 87.
37-year-old non-obese individual presenting with a motor and sensory complete injury to the C6-C7 vertebrae, an NLI of C5, and a FIM motor score of 24 at admission. Patient 2’s total length of stay was 212 days before being discharged with a FIM motor score of 75.
36-year-old non-obese individual presenting with a motor and sensory complete injury to the T4-T5 vertebrae, an NLI at T2, and a FIM motor score of 30 at admission. Patient 3’s total length of stay was 112 days before being discharged with a FIM motor score of 73.
47-year-old obese individual presenting with a motor and sensory complete injury to the T11-T12 vertebrae, and NLI at L2, and a FIM motor score of 49 at admission. Patient 4’s total length of stay was 101 days before being discharged with a FIM motor score of 84.
26-year-old non-obese individual presenting with a motor incomplete (AIS D) to the L1 vertebra, an NLI at L2, and a FIM motor score of 50 at admission. Patient 5’s total length of stay was 105 days before being discharged with a FIM motor score of 88.
In the present study, an unsupervised machine learning approach of cluster analysis utilizing SC was deployed to categorize tSCI patients into clinically similar subgroups based on patient demographics and injury characteristics at baseline. Clustering based on baseline data enabled an exploration for latent relationships between patient demographic and injury characteristics without depending on selected outcomes that might not be comprehensive measures of the condition’ behaviour.
The five patient subgroups with clinical similarities were defined with a data-driven approach that did not rely on a priori assumptions or outcome variables, and subgroups were distinguished based on age, BMI, baseline injury severity (AIS grade), PLI, and baseline FIM motor score. The three outcome variables used in the study (FIM motor score at discharge, FIM motor score change, and total LOS) were superimposed on these subgroups to evaluate the distinction of patient subgroups and to explore their clinical relevance. FIM motor score at discharge and total LOS resulted in statistically significant differences across all patient subgroups (Table 3).
The choice of input variables in cluster analysis is a crucial factor that can influence the quality of the analysis and, by extension, the robustness of the conclusions drawn from the study. As part of our research, we carefully selected baseline variables based on their relevance, clinical significance, and prior evidence of impact on patient outcomes (Table 1). This choice ensured that our cluster analysis was grounded in a strong theoretical and empirical basis. Several studies have demonstrated the importance of these variables in patient outcomes. For instance, age plays a significant role in recovery and functional outcomes following tSCI (1, 3). Similarly, the AIS classification at baseline has shown to be a strong predictor of neurological recovery and rehabilitation outcomes (25). The baseline FIM motor score has been identified as a valuable predictor for functional outcomes and discharge planning in tSCI patients (27). While both AIS scores and the total FIM motor score provide insights into a patient’s neurological and functional statuses, their combined use in our research presents a holistic, patient-centered approach. The AIS details specific neurological information, while the total FIM motor score measures patient-reported motor functionality. Together, they furnish a comprehensive understanding of the patient’s condition and experience. Lastly, the primary location of injury (PLI) has been linked to variations in functional outcomes and recovery potential (26).
Though obesity is not a prognostic variable commonly considered in the tSCI patient population (15), it was included in the present study based on several sources of supporting evidence. A study of note by Stenson and colleagues examined the relationship between obesity and inpatient rehabilitation outcomes for patients with tSCI; it found that obese patients had longer hospital stays and less improvement in motor function when compared to non-obese patients (40). Furthermore, this same study concludes that obese patients were less likely to be discharged home and more likely to be discharged to another healthcare facility (40). Additionally, other studies found that obesity not only affects a patient’s recovery process, but also affects the required healthcare resources and rehabilitation needs of these patients (41, 42). By incorporating obesity as a baseline variable in our study, we were able to distinguish between patient groups when evaluating their hospital or rehabilitation facility LOS. Our result is consistent with previous studies reporting a relationship between obesity and prolonged LOS in patients with tSCI (40, 41).
Our sample included tSCI patients with trauma to any vertebral level from C1 to L5 and resultant impairment to the spinal cord or cauda equina (43). In the interest of finding naturally-emerging patterns in the patient sample, we did not prespecify groups based on the anatomical location of their injury. Based on our results, the PLI was among the primary factors that distinguished patient subgroups. Furthermore, when observed from an inter-group perspective, this classification characterized patients into a purely cervical spine trauma (subgroup 1), a mixed lower cervical or/and thoracic spine trauma (subgroup 2), a purely thoracic spine trauma (subgroup 3), a lower thoracic spine trauma (subgroup 4), and a lumbar spine trauma (subgroup 5).
The choice inclusion of SC in our method identified subgroups of patients with a pattern of PLI that was clinically informative; these subgroups separated traumatic injury to the spinal cord (subgroups 1, 2, 3, and 4) from trauma below the spinal cord (subgroup 5), which is more likely to produce cauda equina injury. Our findings extend on previous work examining the factors associated with traumatic cauda equina injury (tCEI), an understudied condition (44). SC identified a patient subgroup with comparable characteristics to those reported by Attabib and colleagues (44), including a similar mean age and a bimodal distribution in initial injury severity (i.e., peaks at AIS A and D). Notably, patients in subgroup 5 demonstrated the most favourable outcome across all patient subgroups, supporting the notion that these patients have a considerable chance of functional recovery after injury (44). However, it is important to note that Attabib and colleagues used the NLI as determined by the ISNCSCI to classify patients as having tCEI, whereas we opted to use PLI as the only anatomical data in this study.
Potential subjectivity in evaluating NLI may impact the accuracy of tSCI patient classification methods such as the groundwork laid by Dvorak and colleagues (45, 46). Our data-driven approach alleviates this reliance on NLI assessment and improves the Canadian Classification method in two primary ways.
First, reliability of assessment of NLI might impact the consistency of data collected for classifying tSCI patients. The Canadian classification’s singular reliance upon the neurological examination to collect baseline characteristics – NLI and injury severity based on AIS grade – have some practical drawbacks. For instance, conducting baseline examinations in an acute setting is challenging, and results can be confounded by patient-specific factors such as intoxication, sedation, or concurrent brain injuries (46–48). This represents real-world clinical challenges that contribute to the complexity of tSCI patients. To address these issues, our analysis incorporated cluster analysis using context-relevant data to explore inherent patterns within our study population and identify clinically relevant patient subgroups. Furthermore, the PLI was found to be a significant factor; a surrogate for the skeletal level of injury referring to the vertebral column level on the radiograph where trauma has taken place. This demonstrates PLI’s potential as an alternative anatomical classification of SCI that is more accurate and reliable than the ISNCSCI neurological assessment, which is susceptible to poor reliability (49).
Second, the Canadian Classification system uses a priori to classify patients (9). This may affect the generalizability in different clinical settings, where data inaccuracies triggered by patient-specific factors can introduce bias. This may in turn influence the applicability of the data, particularly when translating novel therapeutics from animal models to clinical practice (1). In contrast, our study sought to explore the clinical insights obtainable when context-relevant data alone drives the analysis without depending on outcome variables. We discovered patterns linking variables to outcomes that naturally incorporated data applicability. By applying SC to a set of baseline variables, we were able to uncover key interactions among demographic, anthropometric, and clinical variables such as age, body mass index, and injury mechanism/etiology.
Total LOS had high standard deviations in all subgroups comparing to other variables. This high variation within each cluster supports the finding of Craven and colleagues (24), who developed a prediction model for patients’ rehabilitation LOS in Canada. Their findings report that the prediction of rehabilitation LOS is beyond impairment characteristics (e.g., administration, resource allocation etc.). As our subgroups were created based on demographics and injury characteristics, the high variability in LOS is therefore understandable.
Although the rate of change among the subgroups from inpatient rehabilitation to discharge in FIM motor score (“FIM Motor Difference”) was not significantly different, each one showed improved scores. Subgroups 1, 3, 4, and 5 had similar mean FIM motor score changes, while subgroup 2, which had the longest LOS and the smallest FIM motor score at discharge, had the lowest mean FIM Motor Difference. This trend generates several hypotheses: first, considering the variation in different locations of injury in subgroup 2 and the lowest FIM Motor Difference, it is possible that patients in this subgroup may have suffered from multiple traumas as the majority of cases included in this group report high energy injuries. Second, while the FIM Motor Difference was not statistically different among subgroups, the similar mean value across subgroups 1, 3, 4, and 5, demonstrates their similar potential of motor gain for independence. In comparison, the complete injury in subgroup 2, which had a lower mean FIM Motor Difference, seemed to lead to limited independence gain, and therefore results in a longer hospital stay. Further investigation is required to validate these data-driven insights of FIM motor score change.
When additional injury-related variables (i.e., mechanism and energy of injury) were superimposed on the patient subgroups, they exhibited patterns of traumatic injury that were distinct and clinically intuitive (Table 4). For example, subgroup 1 – which counts comparatively more elderly patients – had the highest proportion of injuries attributed to low-energy mechanisms (76.11%), and of injuries specifically caused by falls (56.64%). In contrast, subgroups 2 to 5 had a higher proportion of high-energy mechanisms attributed to transportation, and patients in these subgroups were comparatively younger. This corresponds with the literature on the etiology of tSCI, which reports high-energy impacts (e.g., traffic accidents and sport-related injuries) as more common in younger individuals, and low-energy impacts (e.g., falls) as a more frequent occurence in older adults, who commonly have degenerative changes leading to central cord syndrome or osteoporotic fractures of the cervical spine (50, 51).
To further understand the characteristics and clinical significance of the identified subgroups, we analyzed the data from Tables 4, 5 and compared them to relevant clinical literature. Subgroup 2 predominantly consists of patients with complete spinal cord injuries (tetra/paraplegia) resulting from motor vehicle accidents (52–54). Subgroup 5 includes patients with cauda equina injury or sacral dysraphism following a spinal cord injury (44, 53, 55). Subgroup 4 comprises injuries in the thoraco-lumbar region, often burst fractures (53, 56, 57). Subgroup 3 counts more chance fractures or seat belt fractures resulting in a spinal cord injury (40, 53, 55). Finally, subgroup 1 represents low energy falls in the elderly that result in a spinal cord injury (22, 55, 58, 59).
In our study, a distinctive pattern emerged within subgroup 1, revealing an overlap of injuries across both the upper and lower cervical regions. This observation is particularly prevalent among the elderly participants whose predominant mechanism of injury was falls. This pattern accentuates the vulnerabilities of the elderly demographic to spinal injuries, consistent with established clinical findings (60). While the clinical literature broadly categorizes cervical injuries into distinct upper and lower zones, our data-driven examination highlights the nuances of injury patterns, suggesting that such traditional delineations may manifest differently within specific patient demographics.
Our findings also provide insight into the prognosis of these different patient profiles (Table 5). Older age has a negative impact on neurological and functional recovery (61). However, our analysis shows that older patients with tSCI caused by the prototypical geriatric fall (subgroup 1) have a relatively moderate prognosis when compared to younger patients in other subgroups. This suggests that age should be considered in the context of other factors, such as the energy and location of injury, and ensuing neurological deficit (15) when predicting motor impairment and LOS.
In order to assist clinicians providing care for tSCI patients and allow personalized approaches to treatment, classifications to evaluate patients need to take the heterogeneity of SCIs into consideration (9). Our analysis identified five subgroups of patients that could be described in a simple yet intuitive manner, producing patient and injury-related labels at presentation and discharge (Table 5). Furthermore, our analysis allowed an exemplar case to be drawn from each patient subgroup. We were able to illustrate that baseline clinical factors commonly available in the acute setting (age, BMI, injury-related information) can contribute to a better understanding of individual patient needs, potentially enabling more tailored care. A national survey of Canadian SCI centers revealed that insufficient SCI-specific knowledge, poor recognition of the condition in the acute setting, and communication between clinicians were all major challenges to providing specialized SCI care (29). The identification of clinically similar subgroups of tSCI patients and presenting their clinical characteristics is a step towards addressing these challenges by equipping clinicians with a way to better recognize and communicate this condition. By applying advanced machine learning algorithms to sift through greater combinations of clinical variables without a priori assumptions, it becomes possible to reveal previously unrecognized patterns within the analyzed data or consolidate known associations within the variables. These results could provide clinically relevant insights for clinicians managing patients with tSCI, especially as large multicenter SCI registries accumulate more data.
In the realm of healthcare, the vast potential of AI is undeniable. Similarly, the need for rigorous oversight in AI-driven research is evident (62, 63). Recognizing both the promise and the challenges, in our study, we prioritized clinical relevancy by basing our data selection on clinically accepted practices. We applied unsupervised learning to the data to unveil inherent patterns without making prior assumptions, which resulted in classifications tied to the specific attributes of the cohort. The clinical relevancy of the identified subgroups was examined through a rigorous process involving an extensive review of clinical literature and consultations with medical experts. Thus, our methodology offers a refined perspective on data-driven patient categorization, underscoring the significance of clinical relevancy in the application of AI in healthcare. This insight could promote a more tailored, patient-centric approach to care and treatment strategies.
Our research utilized data drawn from a Canada-wide, prospectively collected registry with a limited number of patients, which may restrict generalizability. Although the variables used in the cluster analysis may differ if replicated elsewhere, the derived patient labels presented are clinically intuitive and might be generalizable across care systems.
While cluster analysis can be a useful tool in new research, it has some limitations that should be considered. Different clustering methods may identify different subgroups, which may be sensitive to dropped cases. In addition, there are limited ways to validate these obtained subgroups. In the case of this study, we used cluster analysis to demonstrate the potential of a data-driven approach to autonomously separate patient populations that are clinically distinct. However, it should be noted that the clustering method does not currently offer a straightforward way to assign a patient into a specific group as it does not use cut-off values, but rather groups patients based on their averaged similarities across the input variables.
In our pursuit to demonstrate the utility of a data-driven methodology as a supplementary approach for patient categorization, we must acknowledge the study’s findings are bound by the dataset’s scope and completeness of the respective data in our study cohort. This accentuates the need for comprehensive data to facilitate nuanced analyses in subsequent research.
Future research on tSCI can focus on improving and expanding the use of cluster analysis in databases, as well as building on its results to develop prediction models for patterns of patient recovery. Future studies could also involve focusing on specific domains of interest, such as patient outcomes, motor and sensory functioning, and could consider additional patient characteristics such as interventions and socio-demographics in the acute clinical evaluation. In addition, incorporating advanced neuroimaging and molecular biomarkers, which are more sensitive to disease processes (64), may provide insight into how these data could contribute to predicting and customizing the individual trajectories of recovery and unique needs of each patient.
We deployed spectral clustering method and identified five subgroups of traumatic spinal cord injury patients with clinical intra-group similarities and statistically significant inter-group differences for baseline demographic and injury characteristics collected at admission and outcome variables at discharge. This data-driven approach resulted in clinically relevant and plausible insights without depending on a priori decisions, a step toward better understanding of the heterogeneity inherent in tSCI. We demonstrated that cluster analysis can be used to further define the patterns or groups of other patient characteristics that exist in the tSCI population, thus contributing to categorizing the tSCI population into subgroups with distinct needs. This data-driven patient categorization holds the potential to support the delivery of more specialized, patient-centered care.
The data analyzed in this study was obtained from the Rick Hansen Spinal Cord Injury Registry (RHSCIR), managed by the Praxis Spinal Cord Institute (https://praxisinstitute.org/research-care/key-initiatives/national-sci-registry/), the following licenses/restrictions apply: access to these datasets is subject to adherence to RHSCIR Data Use and Disclosure Policy. Requests to access these datasets should be directed to the Praxis Spinal Cord Institute, ZGF0YXNlcnZpY2VzQHByYXhpc2luc3RpdHV0ZS5vcmc=.
The studies involving humans were approved by ethics approval was obtained from each contributing site’s Research Ethics Boards (see the acknowledgement section of the manuscript for site details). The studies were conducted in accordance with the local legislation and institutional requirements. Participants either provided written informed consent for participation in this study or had a minimal chart review dataset collected under a consent waiver.
SB: Investigation, Software, Visualization, Conceptualization, Methodology, Validation, Writing – original draft, Writing – review & editing, Formal analysis. RH: Validation, Methodology, Resources, Writing – review & editing. NB: Resources, Supervision, Writing – review & editing, Funding acquisition. WM: Resources, Validation, Supervision, Writing – review & editing, Conceptualization, Funding acquisition, Methodology. HV: Validation, Resources, Supervision, Writing – review & editing. EW: Validation, Resources, Writing – review & editing. AS: Validation, Resources, Writing – review & editing. SK: Validation, Resources, Writing – review & editing. J-MM-T: Validation, Resources, Writing – review & editing. ET: Validation, Resources, Writing – review & editing. ZW: Validation, Writing – review & editing. PP: Methodology, Project administration, Validation, Conceptualization, Investigation, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Rick Hansen Spinal Cord Injury Registry and this work are supported by funding from the Praxis Spinal Cord Institute through the Government of Canada and the Province of British Columbia. For more information about RHSCIR, please visit www.praxisinstitute.org. The authors would like to acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (NSERC), (funding reference numbers: NSERC GR000540 and NSERC 482728-2016-CREATE) through the Government of Canada. For more information about NSERC, please visit www.nserc-crsng.gc.ca.
We would like to thank RHSCIR participants and network, including all the participating local RHSCIR sites: GF Strong Rehabilitation Centre, Vancouver General Hospital, Foothills Hospital, Glenrose Rehabilitation Hospital, Royal Alexandra Hospital, University of Alberta Hospital, Royal University Hospital, Saskatoon City Hospital, Winnipeg Health Sciences Centre, Toronto Western Hospital, Toronto Rehabilitation Institute, St. Michael’s Hospital, Sunnybrook Health Sciences Centre, Hamilton General Hospital, Hamilton Health Sciences Regional Rehabilitation Centre, Victoria Hospital (London), University Hospital (London), Parkwood Hospital (London), The Ottawa Hospital Rehabilitation Centre, The Ottawa Hospital Civic Campus, Hôpital de l’Enfant Jésus, Institut de Réadaptation en Deficience Physique de Quebec, Institut de Réadaptation Gingras-Lindsay-de-Montréal, Hôpital du Sacre Cœur de Montréal, Nova Scotia Rehabilitation Centre, QEII Health Sciences Centre, Saint John Regional Hospital, Stan Cassidy Centre for Rehabilitation, St. John’s Health Sciences Centre, and L.A. Miller Rehabilitation Centre.
Data for this research/project was collected using the Global Research Platform http://www.rhigrp.net/. Neurology data for this research/project was cleaned using the Praxis ISNCSCI Algorithm http://www.isncscialgorithm.com/.
We would like to acknowledge the Alberta Trauma Services Team and the Alberta Trauma Registry in providing data for the Rick Hansen Spinal Cord Injury Registry.
As per the funding section, PP, SB, and RH were supported through a grant from the Praxis Spinal Cord Institute, with funding provided by the Government of Canada. As per the funding section, SB was supported through funding provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) to used to support methodological and analytical research.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ahuja, CS , Wilson, JR , Nori, S , Kotter, MRN , Druschel, C , Curt, A, et al. Traumatic spinal cord injury. Nat Rev Dis Primer. (2017) 3:1–21. doi: 10.1038/nrdp.2017.18
2. Krause, JS , Sternberg, M , Lottes, S , and Maides, J . Mortality after spinal cord injury: an 11-year prospective study. Arch Phys Med Rehabil. (1997) 78:815–21. doi: 10.1016/S0003-9993(97)90193-3
3. Krueger, H , Noonan, VK , Trenaman, LM , Joshi, P , and Rivers, CS . The economic burden of traumatic spinal cord injury in Canada. Chronic Dis Inj Can. (2013) 33:113–22. doi: 10.24095/hpcdp.33.3.01
4. Sekhon, LH , and Fehlings, MG . Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. (2001) 26:S2–S12. doi: 10.1097/00007632-200112151-00002
5. Parent, S , Barchi, S , LeBreton, M , Casha, S , and Fehlings, MG . The impact of specialized centers of care for spinal cord injury on length of stay, complications, and mortality: a systematic review of the literature. J Neurotrauma. (2011) 28:1363–70. doi: 10.1089/neu.2009.1151
6. Senthinathan, A , Tadrous, M , Hussain, S , Craven, BC , Jaglal, SB , Moineddin, R, et al. Spinal injuries admitted to a specialist Centre over a 5-year period: a study to evaluate delayed admission. Spinal Cord. (2023):1–8. doi: 10.1038/s41393-023-00930-1
7. Oakes, DD , Wilmot, CB , Hall, KM , and Sherck, JP . Benefits of early admission to a comprehensive trauma center for patients with spinal cord injury. Arch Phys Med Rehabil. (1990) 71:637–43.
8. Audit of a British Centre for spinal injury – PubMed [internet]. Available at: https://pubmed.ncbi.nlm.nih.gov/9076864/ (Accessed April 9, 2022)
9. Dvorak, MF , Noonan, VK , Fallah, N , Fisher, CG , Rivers, CS , Ahn, H, et al. Minimizing errors in acute traumatic spinal cord injury trials by acknowledging the heterogeneity of spinal cord anatomy and injury severity: an observational Canadian cohort analysis. J Neurotrauma. (2014) 31:1540–7. doi: 10.1089/neu.2013.3278
10. Fawcett, JW , Curt, A , Steeves, JD , Coleman, WP , Tuszynski, MH , Lammertse, D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. (2007) 45:190–205. doi: 10.1038/sj.sc.3102007
11. Marino, RJ , Ditunno, JF , Donovan, WH , and Maynard, F . Neurologic recovery after traumatic spinal cord injury: data from the model spinal cord injury systems. Arch Phys Med Rehabil. (1999) 80:1391–6. doi: 10.1016/S0003-9993(99)90249-6
12. ASIA and ISCoS International Standards Committee . The 2019 revision of the international standards for neurological classification of spinal cord injury (ISNCSCI)-What’s new? Spinal Cord. (2019) 57:815–7. doi: 10.1038/s41393-019-0350-9
13. Kirshblum, S , Snider, B , Rupp, R , and Read, MS . International standards committee of ASIA and ISCoS. Updates of the international standards for neurologic classification of spinal cord injury: 2015 and 2019. Phys Med Rehabil Clin N Am. (2020) 31:319–30. doi: 10.1016/j.pmr.2020.03.005
14. Coleman, WP , and Geisler, FH . Injury severity as primary predictor of outcome in acute spinal cord injury: retrospective results from a large multicenter clinical trial. Spine J. (2004) 4:373–8. doi: 10.1016/j.spinee.2003.12.006
15. Wilson, JR , Cadotte, DW , and Fehlings, MG . Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: a systematic review. J Neurosurg Spine. (2012) 17:11–26. doi: 10.3171/2012.4.AOSPINE1245
16. Ahmad, P , Qamar, S , and Rizvi, SQA . Techniques of data mining in healthcare: a review. Int J Comput Appl. (2015) 120:38–50. doi: 10.5120/21307-4126
17. Abidi, SSR , and Abidi, SR . Intelligent health data analytics: a convergence of artificial intelligence and big data. Healthc Manage Forum. (2019) 32:178–82. doi: 10.1177/0840470419846134
18. Liao, M , Li, Y , Kianifard, F , Obi, E , and Arcona, S . Cluster analysis and its application to healthcare claims data: a study of end-stage renal disease patients who initiated hemodialysis. BMC Nephrol. (2016) 17:1–14. doi: 10.1186/s12882-016-0238-2
19. Phan, P , Mezghani, N , Wai, EK , de Guise, J , and Labelle, H . Artificial neural networks assessing adolescent idiopathic scoliosis: comparison with Lenke classification. Spine J. (2013) 13:1527–33. doi: 10.1016/j.spinee.2013.07.449
20. Noonan, VK , Kwon, BK , Soril, L , Fehlings, MG , Hurlbert, RJ , Townson, A, et al. The Rick Hansen spinal cord injury registry (RHSCIR): a national patient-registry. Spinal Cord. (2012) 50:22–7. doi: 10.1038/sc.2011.109
21. Malas, MA , Salbacak, A , Büyükmumcu, M , Seker, M , Köylüoğlu, B , and Karabulut, AK . An investigation of the conus medullaris termination level during the period of fetal development to adulthood. Kaibogaku Zasshi. (2001) 76:453–9.
22. Wilson, JR , Cronin, S , Fehlings, MG , Kwon, BK , Badhiwala, JH , Ginsberg, HJ, et al. Epidemiology and impact of spinal cord injury in the elderly: results of a fifteen-year population-based cohort study. J Neurotrauma. (2020) 37:1740–51. doi: 10.1089/neu.2020.6985
23. Kao, YH , Chen, Y , Deutsch, A , Wen, H , and Tseng, TS . Rehabilitation length of stay, body mass index, and functional improvement among adults with traumatic spinal cord injury. Arch Phys Med Rehabil. (2022) 103:657–64. doi: 10.1016/j.apmr.2021.09.017
24. Catharine Craven, B , Kurban, D , Farahani, F , Rivers, CS , Ho, C , Linassi, AG, et al. Predicting rehabilitation length of stay in Canada: It’s not just about impairment. J Spinal Cord Med. (2017) 40:676–86. doi: 10.1080/10790268.2017.1368962
25. Waters, RL , Adkins, RH , Yakura, JS , and Sie, I . Motor and sensory recovery following incomplete tetraplegia. Arch Phys Med Rehabil. (1994) 75:306–11. doi: 10.1016/0003-9993(94)90034-5
26. van Middendorp, JJ , Hosman, AJF , Donders, ART , Pouw, MH , Ditunno, JF , Curt, A, et al. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet Lond. (2011) 377:1004–10. doi: 10.1016/S0140-6736(10)62276-3
27. Scivoletto, G , Tamburella, F , Laurenza, L , Torre, M , and Molinari, M . Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front Hum Neurosci. (2014) 8:141. doi: 10.3389/fnhum.2014.00141
28. Hakimjavadi, R , Basiratzadeh, S , Wai, EK , Baddour, N , Kingwell, S , Michalowski, W, et al. Multivariable prediction models for traumatic spinal cord injury: a systematic review. In: Topics in spinal cord injury rehabilitation. (2023).
29. Noonan, VK , Chan, E , Santos, A , Soril, L , Lewis, R , Singh, A, et al. Traumatic spinal cord injury Care in Canada: a survey of Canadian centers. J Neurotrauma. (2017) 34:2848–55. doi: 10.1089/neu.2016.4928
30. Cohen, JT , Marino, RJ , Sacco, P , and Terrin, N . Association between the functional Independence measure following spinal cord injury and long-term outcomes. Spinal Cord. (2012) 50:728–33. doi: 10.1038/sc.2012.50
31. Moore, WC , Meyers, DA , Wenzel, SE , Teague, WG , Li, H , Li, X, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. (2010) 181:315–23. doi: 10.1164/rccm.200906-0896OC
32. Murugesan, N , Cho, I , and Tortora, C . Benchmarking in cluster analysis: a study on spectral clustering, DBSCAN, and K-means. Springer; (2021). p. 175–185.
33. Kannan, R , Vempala, S , and Vetta, A . On clusterings: good, bad and spectral. JACM. (2004) 51:497–515. doi: 10.1145/990308.990313
34. Ng, A , Jordan, M , and Weiss, Y . On spectral clustering: analysis and an algorithm. Adv Neural Inf Proc Syst. (2001) 14
35. Gustriansyah, R , Suhandi, N , and Antony, F . Clustering optimization in RFM analysis based on k-means. Indones J Electr Eng Comput Sci. (2020) 18:470–77. doi: 10.11591/ijeecs.v18.i1.pp470-477
36. Davies, DL , and Bouldin, DW . A cluster separation measure. IEEE Trans Pattern Anal Mach Intell. (1979) PAMI-1:224–7. doi: 10.1109/TPAMI.1979.4766909
37. Aranganayagi, S , and Thangavel, K . Clustering categorical data using silhouette coefficient as a relocating measure. IEEE. (2007):13–7.
38. McKight, PE , and Najab, J . Kruskal-wallis test. Corsini Encycl Psychol. (2010):1. doi: 10.1002/9780470479216.corpsy0491
39. McKnight, PE , and Najab, J . Mann-Whitney U test. Corsini Encycl Psychol. (2010):1. doi: 10.1002/9780470479216.corpsy0524
40. Stenson, KW , Deutsch, A , Heinemann, AW , and Chen, D . Obesity and inpatient rehabilitation outcomes for patients with a traumatic spinal cord injury. Arch Phys Med Rehabil. (2011) 92:384–90. doi: 10.1016/j.apmr.2010.07.235
41. Jain, NB , Ayers, GD , Peterson, EN , Harris, MB , Morse, L , O’Connor, KC, et al. Traumatic spinal cord injury in the United States, 1993-2012. JAMA. (2015) 313:2236–43. doi: 10.1001/jama.2015.6250
42. Gorgey, AS , Dolbow, DR , Dolbow, JD , Khalil, RK , and Gater, DR . The effects of electrical stimulation on body composition and metabolic profile after spinal cord injury–part II. J Spinal Cord Med. (2015) 38:23–37. doi: 10.1179/2045772314Y.0000000244
43. Zhang, S , Wadhwa, R , Haydel, J , Toms, J , Johnson, K , and Guthikonda, B . Spine and spinal cord trauma: diagnosis and management. Neurol Clin. (2013) 31:183–206. doi: 10.1016/j.ncl.2012.09.012
44. Attabib, N , O’Connell, C , Kurban, D , Rivers, CS , Noonan, VK , Ethans, K, et al. 103. Factors associated with motor, sensory, bladder and bowel function recovery after traumatic cauda equina injury (TCEI). Spine J. (2019) 19:S49–50. doi: 10.1016/j.spinee.2019.05.116
45. Marino, RJ , Jones, L , Kirshblum, S , Tal, J , and Dasgupta, A . Reliability and repeatability of the motor and sensory examination of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med. (2008) 31:166–70. doi: 10.1080/10790268.2008.11760707
46. Lee, RS , Noonan, VK , Batke, J , Ghag, A , Paquette, SJ , Boyd, MC, et al. Feasibility of patient recruitment into clinical trials of experimental treatments for acute spinal cord injury. J Clin Neurosci. (2012) 19:1338–43. doi: 10.1016/j.jocn.2012.02.015
47. Burns, AS , Lee, BS , Ditunno, JF , and Tessler, A . Patient selection for clinical trials: the reliability of the early spinal cord injury examination. J Neurotrauma. (2003) 20:477–82. doi: 10.1089/089771503765355540
48. Snyder, R , Verla, T , and Ropper, AE . Practical application of recent advances in diagnostic, prognostic, and therapeutic modalities for spinal cord injury. World Neurosurg. (2020) 136:330–6. doi: 10.1016/j.wneu.2020.01.011
49. Hales, M , Biros, E , and Reznik, J . Reliability and validity of the sensory component of the international standards for neurological classification of spinal cord injury (ISNCSCI): a systematic review. Top Spinal Cord Inj Rehabil. (2015) 21:241–9. doi: 10.1310/sci2103-241
50. Gelb, D , Zarro, C , O’Brien, J , and Ludwig, SC . Spine fractures in the elderly. Elsevier; (2005). p. 165–173.
51. Aguirre, MFI , Tsirikos, AI , and Clarke, A . Spinal injuries in the elderly population. Orthop Trauma. (2020) 34:272–7. doi: 10.1016/j.mporth.2020.06.004
52. Smits, AJ , den Ouden, LP , Deunk, J , and Bloemers, FW, LNAZ Research Group . Incidence of traumatic spinal fractures in the Netherlands: analysis of a Nationwide database. Spine. (2020) 45:1639–48. doi: 10.1097/BRS.0000000000003658
53. Khorasanizadeh, M , Yousefifard, M , Eskian, M , Lu, Y , Chalangari, M , Harrop, JS, et al. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine. (2019) 30:683–99. doi: 10.3171/2018.10.SPINE18802
54. Wang, H , Liu, X , Zhao, Y , Ou, L , Zhou, Y , Li, C, et al. Incidence and pattern of traumatic spinal fractures and associated spinal cord injury resulting from motor vehicle collisions in China over 11 years: an observational study. Medicine (Baltimore). (2016) 95:e5220. doi: 10.1097/MD.0000000000005220
55. Tederko, P , Middleton, J , Mycielski, J , Joseph, C , Pagliacci, MC , Rapidi, CA, et al. Relationship between level of economic development, age, and etiology of spinal cord injury: a cross-sectional survey from 22 countries. Arch Phys Med Rehabil. (2021) 102:1947–58. doi: 10.1016/j.apmr.2021.04.024
57. Skeers, P , Battistuzzo, CR , Clark, JM , Bernard, S , Freeman, BJC , and Batchelor, PE . Acute thoracolumbar spinal cord injury: relationship of cord compression to neurological outcome. J Bone Joint Surg Am. (2018) 100:305–15. doi: 10.2106/JBJS.16.00995
58. Mirzaeva, L , Gilhus, NE , Lobzin, S , and Rekand, T . Incidence of adult traumatic spinal cord injury in Saint Petersburg, Russia. Spinal Cord. (2019) 57:692–9. doi: 10.1038/s41393-019-0266-4
59. Chamberlain, JD , Deriaz, O , Hund-Georgiadis, M , Meier, S , Scheel-Sailer, A , Schubert, M, et al. Epidemiology and contemporary risk profile of traumatic spinal cord injury in Switzerland. Inj Epidemiol. (2015) 2:1–11. doi: 10.1186/s40621-015-0061-4
60. Rubenstein, LZ . Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. (2006) 35:ii37–41. doi: 10.1093/ageing/afl084
61. Kirshblum, S , Snider, B , Eren, F , and Guest, J . Characterizing natural recovery after traumatic spinal cord injury. J Neurotrauma. (2021) 38:1267–84. doi: 10.1089/neu.2020.7473
62. Anghelescu, A , Firan, FC , Onose, G , Munteanu, C , Trandafir, AI , Ciobanu, I, et al. PRISMA systematic literature review, including with Meta-analysis vs. Chatbot/GPT (AI) regarding current scientific data on the Main effects of the calf blood Deproteinized Hemoderivative medicine (Actovegin) in ischemic stroke. Biomedicine. (2023) 11:1623. doi: 10.3390/biomedicines11061623
63. Char, DS , Shah, NH , and Magnus, D . Implementing machine learning in health care — addressing ethical challenges. N Engl J Med. (2018) 378:981–3. doi: 10.1056/NEJMp1714229
Keywords: traumatic spinal cord injury, patient-centric approach, patient categorization, data-driven method, cluster analysis
Citation: Basiratzadeh S, Hakimjavadi R, Baddour N, Michalowski W, Viktor H, Wai E, Stratton A, Kingwell S, Mac-Thiong J-M, Tsai EC, Wang Z and Phan P (2023) A data-driven approach to categorize patients with traumatic spinal cord injury: cluster analysis of a multicentre database. Front. Neurol. 14:1263291. doi: 10.3389/fneur.2023.1263291
Received: 19 July 2023; Accepted: 05 September 2023;
Published: 12 October 2023.
Edited by:
Michelle Hook, Texas A&M University, United StatesReviewed by:
Ann M. Parr, University of Minnesota Twin Cities, United StatesCopyright © 2023 Basiratzadeh, Hakimjavadi, Baddour, Michalowski, Viktor, Wai, Stratton, Kingwell, Mac-Thiong, Tsai, Wang and Phan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shahin Basiratzadeh, QmFzaXJhdHphZGVoQHRlbGZlci51b3R0YXdhLmNh
†ORCID: Shahin Basiratzadeh https://orcid.org/0000-0002-7387-0088
Natalie Baddour https://orcid.org/0000-0002-7025-7501
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.