- Department of Neurology, Brigham and Women’s Hospital, Boston, MA, United States
Alzheimer’s disease (AD) is the most common type of dementia and remains an incurable, progressive disease with limited disease-modifying interventions available. In patients with AD, interictal epileptiform discharges (IEDs) have been identified in up to 54% of combined cohorts of mild cognitive impairment (MCI) or mild dementia and are a marker of a more aggressive disease course. Studies assessing the role of IEDs in AD are limited by the lack of standardization in the definition of IEDs or the different neurophysiologic techniques used to capture them. IEDs are an appealing treatment target given the availability of EEG and anti-seizure medications. There remains uncertainty regarding when to treat IEDs, the optimal drug and dose for treatment, and the impact of treatment on disease course. This review covers the state of knowledge of the field of IEDs in AD, and the steps needed to move the field forward.
1. Introduction
Alzheimer’s disease (AD) is the most common type of dementia with devastating effects on cognition in the setting of disrupted synaptic homeostasis, neuronal loss, and impaired neuronal network integrity (1, 2). Clinical studies have suggested a link between AD and epilepsy, given the higher rates of clinical and subclinical seizures in patients with AD (3) and a more aggressive phenotype (early onset and rapid progression) when both disorders are present, or when there is evidence of interictal epileptiform discharges (IEDs) on EEG even in the absence of clinical seizures (4). Seizures can also be one of the first presenting symptoms of AD (4, 5), and an “epileptic variant” of AD is gaining more recognition (6). The accumulation of amyloid-β (a pathogenic hallmark of AD) leads to inhibitory interneuron dysfunction creating a state of network hypersynchrony manifesting as IEDs, clinical and subclinical seizures (7). This raises the appealing prospect of trying to modify the disease course by addressing hypersynchrony with antiseizure medications (ASM).
Electroencephalography (EEG) has proven to be the most accessible and cost-efficient tool to identify epileptiform abnormalities in patients with mild cognitive impairment (MCI) or Alzheimer’s dementia (4, 5). Albeit there may be substantial variability in the interpretation and reporting of the data (8). There is a need for clinicians to understand the EEG findings in patients with AD, its role in the pathogenesis and progression of the disease, and when and whether certain findings should be treated. This review will highlight published data regarding IEDs in AD, and discuss study limitations, and controversies regarding treatment.
2. Illustrative cases
We present 4 cases with different EEG findings in the setting of non-lesional MRIs and highlight the range of abnormalities that a clinician could face, and the challenge with regards to deciding when to initiate treatment.
Case 1 is a 75-year-old male with a history of mild cognitive impairment and no history of spells concerning for seizures. He had a routine EEG revealing “sharp transients in sleep.” An ambulatory EEG showed an isolated left anterior temporal sharp wave in N2 sleep (Figure 1A). Case 2 is a 60-year-old female with a strong family history of AD who presented to the clinic with short-term memory complaints. An ambulatory EEG was obtained revealing occasional periodic left temporal sharp waves in N2 sleep (Figure 1B). Case 3 is an 85-year-old female with a history of mild AD dementia and fluctuating mentation. An ambulatory EEG showed runs of left temporal rhythmic delta activity lasting up to 10 s limited to wakefulness (Figure 1C). Case 4 is a 70-year-old male with a history of mild cognitive impairment and an isolated generalized tonic–clonic seizure; he was maintained on levetiracetam monotherapy. A follow-up ambulatory EEG showed bitemporal independent frequent spike and slow wave discharges in sleep occurring at a frequency of 1/min (Figure 1D).
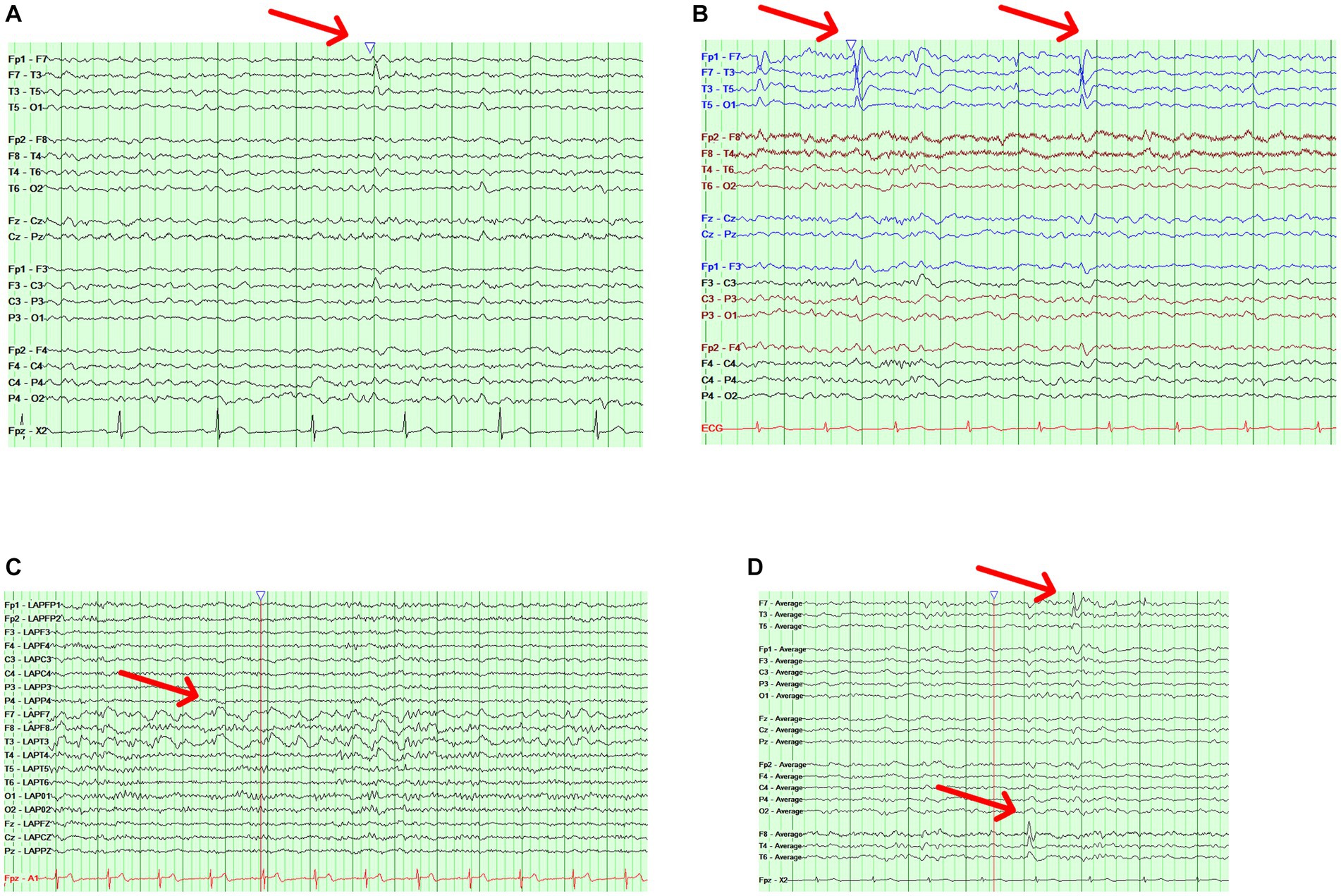
Figure 1. Illustrative cases. (A) Case 1 with an ambulatory EEG showing a bipolar longitudinal montage with a left temporal sharp wave (red arrow) during stage N2 sleep. (B) Case 2 with an ambulatory EEG showing a bipolar longitudinal montage with occasional periodic left temporal sharp waves (red arrow) during stage N2 sleep. (C) Case 3 with an ambulatory EEG showing a Laplacian montage with runs of left temporal rhythmic delta activity (red arrow) lasting up to 10 s. (D) Case 4 with an ambulatory EEG showing an average referential montage with bitemporal independent frequent spike and slow wave discharges (red arrows).
3. EEG findings in AD
Earlier studies in patients with AD suggested that slowing of the occipital peak frequency correlated with the progression of the disease (9). Unfortunately, larger cohort studies failed to confirm this finding (10). Focal slowing on ambulatory EEG is a common finding in older adults in general with a prevalence of up to 63% on ambulatory EEG (11). Focal slowing on a routine EEG is relevant because it is one of the predictors of finding an IED on an ambulatory EEG (11). In the AD literature, the rates of focal slowing range between 44–47% in MCI and mild AD (12), while generalized slowing of the background is seen in 13–30% (3, 12).
More recent studies have highlighted interictal epileptiform discharges (IEDs) as a more relevant electrographic finding in patients with AD or MCI (Table 1); as also previously reviewed by Csernus and colleagues (20). Most of the study findings are limited by the lack of standardization in the definition of IEDs or the variability in the type of study used to capture them (routine vs. long-term vs. ambulatory EEG). An interictal epileptiform abnormality should be defined by at least 4 out of 6 criteria recommended by the international federation for clinical neurophysiology to avoid misinterpretation of normal variants on EEG (19). Common normal variants in older adults that can be easily misinterpreted as pathologic include small sharp spikes, wicket rhythms, and wicket spikes (8). Yet, even when these criteria are applied, there may be substantial interrater variability; the inter-rater reliability regarding IEDs is fair at best even among experts (21). This is problematic because AD patients without a history of seizures tend to have only a limited number of discharges on an ambulatory EEG (12) and can be easily misclassified as having IEDs, as shown in the illustrative cases (Figure 1) where the decision to label an EEG as epileptiform or not rests on an isolated discharge.
Notably, only one study has used expert consensus to evaluate the frequency of IEDs in patients with AD (12), while others screened with spike detection software followed by a visual review (Table 1). Recent studies have suggested a higher accuracy in the identification of IEDs for an ambulatory EEG when compared to one or two routine EEGs (22). This could be relevant in the AD population since most IEDs are present in stage 2 sleep (Table 1). The sensitivity of an EEG is correlated with the length of the recording, which explains why a 20–30 min routine EEG may miss IEDs (23), and why there was a delay in appreciating the true burden of IEDs in AD. Other markers of hyperexcitability such as focal rhythmic slowing (24) have only been studied in one cohort (12). A benign variant, small sharp spikes (SSS), was seen in a subset of patients with AD; some with a high frequency and unilateral predominance (12), suggesting that these features may also indicate underlying irritability given that they represent outliers and also tended to co-occur in EEGs with IEDs. Most of the studies also reported the temporal lobe as the most frequent region for IEDs (Table 1). The temporal-lobe predominance of IEDs could be due to early seeding by amyloid plaques and hyperphosphorylated tau in the limbic system (25).
It must be kept in mind that surface EEG as a neurophysiologic tool has several limitations including its limited ability to detect deep IEDs such as those located in the hippocampus, or IEDs with a tangential dipole (26). This limitation was highlighted in a study in 2016, where 21% out of 42% of the subjects had MEG-only IEDs with no IEDs noted on EEG (3). Similarly, in a case series of 2 subjects with early onset AD with surface EEG and invasive foramen ovale electrodes, 90–100% of the IEDs noted on the invasive electrodes did not have a surface EEG correlate (27).
As illustrated in Table 1 the subjects examined per study with prolonged EEGs have been limited to date with cohorts often including both MCI and mild dementia patients lumped together. We need more studies to explore whether IEDs vary in prevalence depending on disease stage.
4. IEDs and cognition in the epilepsy literature
The association of IEDs on cognition and whether they should be a treatment target has been a matter of debate among epileptologists (28). Transient cognitive impairment secondary to IEDs gained recognition with the advent of computerized testing paradigms. Earlier studies showed that around 50% of subjects with epilepsy exhibited transient impairment coinciding with the occurrence of an IED, and there was a laterality effect with left-sided discharges affecting verbal tasks while right-sided IEDs affecting visual ones (29, 30). The dysfunction was specifically attributed to the after-going slow wave following the discharge (31). IEDs can also affect cognition when occurring in sleep by affecting sleep-dependent memory consolidation. Sleep is essential in transitioning memories from being hippocampal-dependent into more consolidated memories in widespread cortical networks (32). This process is dependent on NREM sleep with slow oscillations and sleep spindles playing a pivotal role (32). In older adults with epilepsy, the frequency of scalp-detected IEDs in NREM sleep was found to negatively correlate with 24 h recall on a visual memory task (33).
Moving on from surface EEG-based studies, a similar theme also emerges with invasive EEG studies. Hippocampal IEDs detected on depths electrodes were associated with impaired maintenance and retrieval but not encoding on a short-term memory task (34), while the frequency of IEDs detected during sleep was associated with impaired one-week long-term recall (35). IEDs even outside of the epileptogenic zone have also been associated with impaired cognition (36).
Invasive EEG studies have also shed light on how IEDs can disrupt cognitive processes; one mechanism is through a transient decrease in global functional connectivity (37), while another is through the impairment of spindle generation (38) and the induction of pathologic hippocampal-cortical coupling (39). IEDs may also alter the firing of hippocampal neurons leading to a state of transient cognitive impairment (40, 41).
Other markers of epileptogenicity that can be detected using scalp EEG, and have been described in epilepsy patients, include high frequency oscillations (HFOs) (42). They are currently divided into physiologic and pathologic HFOs. Physiologic HFOs have been shown to play a central role in information retrieval and sleep dependent memory consolidation (43, 44). On the other hand, one of the features of pathologic HFOs is that they tend to coincide with IEDs and occur during the earliest stages of non-REM sleep (45). HFOs pose methodological challenges in their recording and detection (46), thus limiting their widespread clinical use in patients with AD; especially since it is difficult to disentangle pathologic from physiologic HFOs.
5. IEDs and cognition in the AD literature
Cross-sectional studies of IEDs in AD show a trend for lower mini-mental status exam (MMSE) scores in those with IEDs (14), although this finding was not seen in a study using prolonged ear-EEG recordings (18). Longitudinal studies of AD patients with IEDs have been limited. In a study of 33 patients with AD, those with IEDs had an accelerated decline in their MMSE score and their executive function composite Z-score (a combination of design fluency, information processing speed, and cognitive control from the Stroop test, digit span backward, modified trails and the California verbal learning test) (3). Of note, not all participants had data on the individual tests, and there was no evidence of a decline in the episodic memory, language, or visuospatial function domains (3). The cohort studied predominantly consisted of patients with early-onset AD and 33% with atypical presentations.
In another study, 28 out of 52 AD patients were noted to have IEDs (17). The authors used the cognitive assessments consisting of a Hungarian version of the Addenbrooke Cognitive Examination (ACE); scored from 0–100 and allowing the extraction of MMSE scores, and analysis of the following cognitive subdomains: orientation, attention, memory, verbal fluency, language, and visuospatial ability (17). When compared to AD patients without IEDs, those with IEDs exhibited a faster decline in ACE scores over 3 years (12.15 points per year vs. 8.17 points per year) and on the MMSE (2.71 points per year vs. 2.22 points per year). The study also found a correlation between IED frequency and the rate of decline in the ACE. In comparison, studies evaluating AD patients with comorbid epilepsy treated with anti-seizure medications (ASMs) did not show a change in MMSE scores over at least a 3-year follow-up (47).
6. To treat or not to treat: management of IEDs in AD
Although there is mounting evidence regarding the association between IEDs and impaired cognition and accelerated disease course, there are currently no guidelines to screen for IEDs in AD or to treat IEDs. The goal of the treatment is not seizure prevention because there are no currently anti-epileptogenic medications available. Instead, the aim would be to prevent the possible impact of the IEDs on cognition and memory consolidation. In addition, there is also evidence of neuronal hyperactivity (IEDs being one manifestation of this) causing accelerated neurodegeneration by promoting AD pathology (48). The medication that has garnered the most interest has been levetiracetam. Animal AD mouse models exposed to levetiracetam show IED suppression and improvement in cognition (49). In one of the only randomized trials of the treatment of seizures in AD, levetiracetam (dose range 500-2000 mg) was better tolerated when compared to phenobarbital (dose range 50-100 mg) or lamotrigine (dose range 25-100 mg) and was correlated with improved MMSE scores after 1 year (50). Studies evaluating the IED suppression properties of ASMs in epilepsy also show evidence for lamotrigine and topiramate (51). The downside of treatment is that ASMs in general, as a drug class, are commonly associated with cognitive and fatigue side effects (52). While levetiracetam is associated with prominent neuropsychiatric side effects (53), lamotrigine and other sodium channel blockers are risk factors for falls (54). In addition, benzodiazepines are known to increase the risk of cognitive decline and dementia in the elderly (55).
A recent study trying to tackle the balance between IED suppression and adverse effects of ASMs showed that in children undergoing invasive EEG, reaction time improved with IED suppression (with oxcarbazepine) and worsened with increased IED frequency (56). In this study, levetiracetam did not show a clear benefit (56). In a retrospective analysis of older Japanese patients with IEDs on EEG, treatment with various ASMs improved serial 7 scores and MMSE scores in those with IED suppression (57). The first randomized trial for levetiracetam in AD was published in 2021 (58), and several other trials also exploring levetiracetam are pending. In the trial, 34 patients with AD were treated with levetiracetam at a low dose of 125 mg twice a day vs. placebo and then underwent a washout period and cross-over. Based on overnight EEG and then a 1 h MEG, 13 participants were found to have IEDs. The cognitive battery consisted of the National Institutes of Health Executive Abilities: Measures and Instruments for Neurobehavioral Evaluation and Research (NIH-EXAMINER) which consists of a test measuring executive functions, Stroop color and word test, the Alzheimer’s Disease Assessment Scale—Cognitive Subscale (ADAS-Cog), and a virtual route learning test. There was no improvement on the primary endpoints with the medication, however, a subset analysis of those with IEDs showed that they improved on the virtual route learning test and a subscale of the Stroop test. Notably, there was no evidence of IED suppression with the medications (58).
7. How to deal with IEDs in AD patients in the clinical practice
Ultimately, the clinician caring for patients with AD is faced with decisions regarding when to order an EEG, how to interpret the data, or when to start an ASM. The other challenge is that diagnosis of epilepsy in an elderly population is challenging, requiring a detailed description of suspected events, consideration of atypical events as seizures (i.e., unexplained falls or brief episodes of confusion), and the need for an expert evaluation (59) (Figure 2). Until we have more evidence from randomized trials that levetiracetam will help AD patients, and more so those with IEDs, routine screening of AD patients with EEG is not recommended. However, one should have a low threshold to screen patients with suspected co-morbid seizures, including those with early onset AD because they are at the highest risk. If an EEG is ordered, it should at least have N2 sleep captured, and that is why 24 h EEGs are preferred over routine EEGs. Interictal discharges as exemplified by the illustrative cases lie along a spectrum, with seizures (clinical and subclinical) occurring at the end of that spectrum and representing the extreme manifestation of network hyperexcitability. Features such as a high IED frequency, periodicity, duration, and perhaps morphological features (spikiness, amplitude) should be considered more concerning and should tip the scale toward treatment (cases 2,3,4). In the absence of more data, isolated and equivocal discharges should not be treated (case 1). When a decision is made to treat, the lowest therapeutic dose should be used to ensure tolerability.
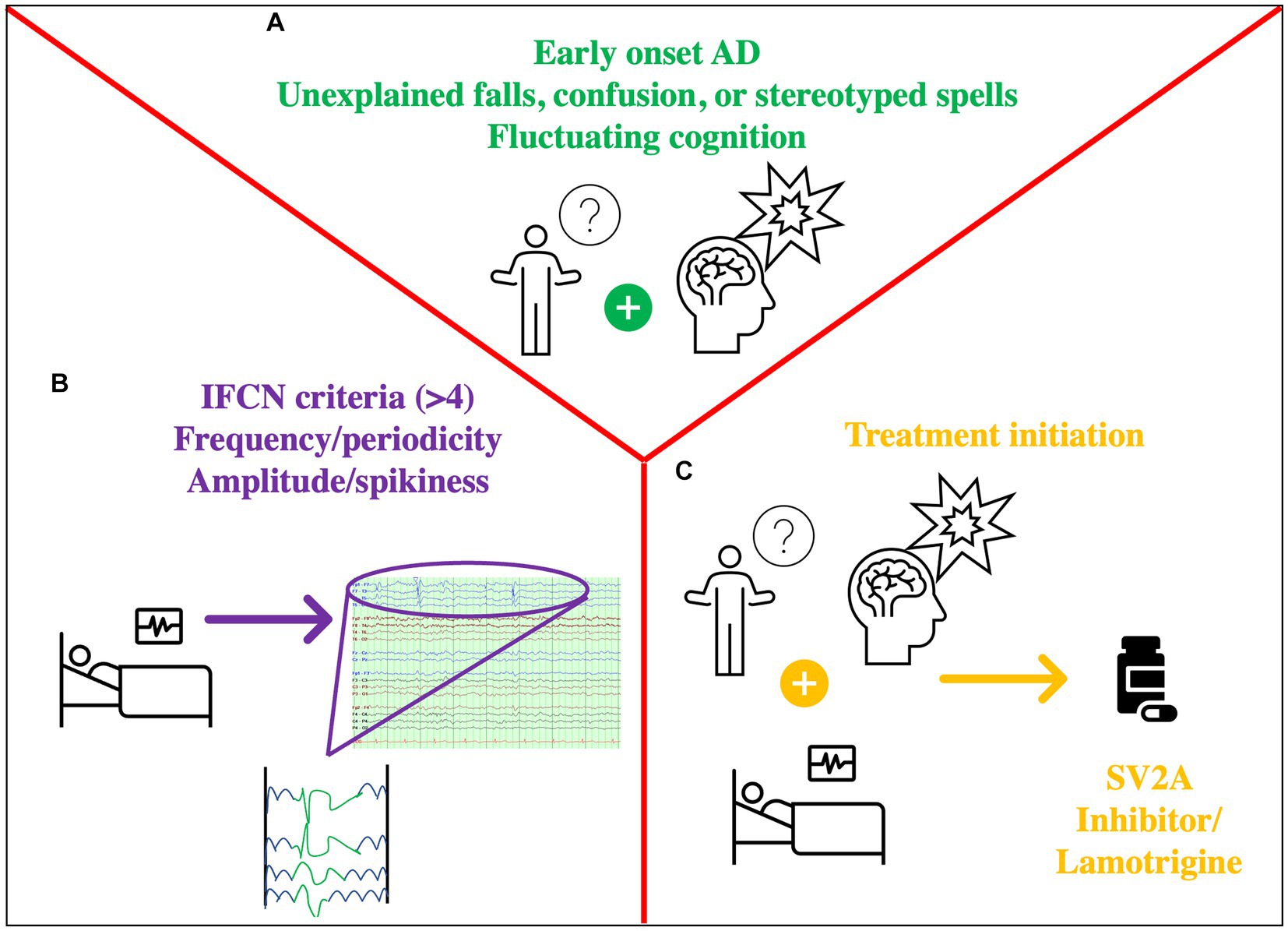
Figure 2. Approach to Hyperexcitability in IED. (A) History prompting the need for EEG: fluctuating cognition, stereotyped symptoms, distinct confusional spells, (possibly) early-onset AD (B) Concerning EEG features: markers of hyperexcitability such as IEDs with >4 out of 6 criteria of the IFCN, unilateral small sharp spikes, temporal rhythmic delta activity. Assess frequency, periodicity, (possibly) amplitude/spikiness. (C) Decision to treat based on A + B: consider an SV2A inhibitor such as levetiracetam/(possibly) brivaracetam or lamotrigine.
8. Conclusion
Network hyperexcitability is a feature of AD, and IEDs are a marker of this phenomenon. They are highly prevalent in AD, are often detected in sleep, and have been linked with deleterious effects on cognition and an accelerated disease course. Limited studies to date show some benefit with treatment, however further evidence is needed to determine whether this should become the standard of care.
Author contributions
HL: Conceptualization, Writing – original draft, Writing – review & editing. RS: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. RS is funded by NINDS grant K23 NS119798.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Querfurth, HW, and Laferla, FM. Alzheimer’s disease. N Engl J Med. (2010) 362:329–44. doi: 10.1056/NEJMra0909142
2. Knopman, DS, Amieva, H, Petersen, RC, Chételat, G, Holtzman, DM, Hyman, BT, et al. Alzheimer disease. Nat Rev Dis Primers. (2021) 7:33. doi: 10.1038/s41572-021-00269-y
3. Vossel, KA, Ranasinghe, KG, Beagle, AJ, Mizuiri, D, Honma, SM, Dowling, AF, et al. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann Neurol. (2016) 80:858–70. doi: 10.1002/ana.24794
4. Vossel, KA, Beagle, AJ, Rabinovici, GD, Shu, H, Lee, SE, Naasan, G, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. (2013) 70:1158–66. doi: 10.1001/jamaneurol.2013.136
5. Sarkis, RA, Dickerson, BC, Cole, AJ, and Chemali, ZN. Clinical and neurophysiologic characteristics of unprovoked seizures in patients diagnosed with dementia. J Neuropsychiatry Clin Neurosci. (2016) 28:56–61. doi: 10.1176/appi.neuropsych.15060143
6. Cretin, B, Sellal, F, Philippi, N, Bousiges, O, Di Bitonto, L, Martin-Hunyadi, C, et al. Epileptic prodromal Alzheimer’s disease, a retrospective study of 13 new cases: expanding the Spectrum of Alzheimer’s disease to an epileptic variant? J Alzheimers Dis. (2016) 52:1125–33. doi: 10.3233/JAD-150096
7. Palop, JJ, and Mucke, L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. (2010) 13:812–8. doi: 10.1038/nn.2583
8. Amin, U, Nascimento, F, Karakis, I, Schomer, D, and Benbadis, S. Normal variants and artifacts: importance in EEG interpretation. Epileptic Disord. (2023). doi: 10.1002/epd2.20040
9. Penttilä, M, Partanen, JV, Soininen, H, and Riekkinen, PJ. Quantitative analysis of occipital EEG in different stages of Alzheimer’s disease. Electroencephalogr Clin Neurophysiol. (1985) 60:1–6. doi: 10.1016/0013-4694(85)90942-3
10. Rae-Grant, A, Blume, W, Lau, C, Hachinski, VC, Fisman, M, and Merskey, H. The electroencephalogram in Alzheimer-type dementia. A sequential study correlating the electroencephalogram with psychometric and quantitative pathologic data. Arch Neurol. (1987) 44:50–4. doi: 10.1001/archneur.1987.00520130042015
11. Tolchin, B, Lee, JW, Pavlova, M, Dworetzky, BA, and Sarkis, RA. Diagnostic yield of ambulatory EEGs in the elderly. Clin Neurophysiol. (2017) 128:1350–3. doi: 10.1016/j.clinph.2017.01.005
12. Lam, AD, Sarkis, RA, Pellerin, KR, Jing, J, Dworetzky, BA, Hoch, DB, et al. Association of epileptiform abnormalities and seizures in Alzheimer disease. Neurology. (2020) 95:e2259–70. doi: 10.1212/WNL.0000000000010612
13. Rao, SC, Dove, G, Cascino, GD, and Petersen, RC. Recurrent seizures in patients with dementia: frequency, seizure types, and treatment outcome. Epilepsy Behav. (2009) 14:118–20. doi: 10.1016/j.yebeh.2008.08.012
14. Liedorp, M, Stam, CJ, Van Der Flier, WM, Pijnenburg, YA, and Scheltens, P. Prevalence and clinical significance of epileptiform EEG discharges in a large memory clinic cohort. Dement Geriatr Cogn Disord. (2010) 29:432–7. doi: 10.1159/000278620
15. Horváth, A, Szűcs, A, Hidasi, Z, Csukly, G, Barcs, G, and Kamondi, A. Prevalence, semiology, and risk factors of epilepsy in Alzheimer’s disease: an ambulatory EEG study. J Alzheimers Dis. (2018) 63:1045–54. doi: 10.3233/JAD-170925
16. Brunetti, V, D’atri, A, Della Marca, G, Vollono, C, Marra, C, Vita, MG, et al. Subclinical epileptiform activity during sleep in Alzheimer’s disease and mild cognitive impairment. Clin Neurophysiol. (2020) 131:1011–8. doi: 10.1016/j.clinph.2020.02.015
17. Horvath, AA, Papp, A, Zsuffa, J, Szucs, A, Luckl, J, Radai, F, et al. Subclinical epileptiform activity accelerates the progression of Alzheimer’s disease: a long-term EEG study. Clin Neurophysiol. (2021) 132:1982–9. doi: 10.1016/j.clinph.2021.03.050
18. Musaeus, CS, Frederiksen, KS, Andersen, BB, Høgh, P, Kidmose, P, Fabricius, M, et al. Detection of subclinical epileptiform discharges in Alzheimer’s disease using long-term outpatient EEG monitoring. Neurobiol Dis. (2023) 183:106149. doi: 10.1016/j.nbd.2023.106149
19. Kural, MA, Duez, L, Sejer Hansen, V, Larsson, PG, Rampp, S, Schulz, R, et al. Criteria for defining interictal epileptiform discharges in EEG: a clinical validation study. Neurology. (2020) 94:e2139–47. doi: 10.1212/WNL.0000000000009439
20. Csernus, EA, Werber, T, Kamondi, A, and Horvath, AA. The significance of subclinical epileptiform activity in Alzheimer’s disease: a review. Front Neurol. (2022) 13:856500. doi: 10.3389/fneur.2022.856500
21. Jing, J, Herlopian, A, Karakis, I, Ng, M, Halford, JJ, Lam, A, et al. Interrater reliability of experts in identifying Interictal epileptiform discharges in electroencephalograms. JAMA Neurol. (2020) 77:49–57. doi: 10.1001/jamaneurol.2019.3531
22. Hernandez-Ronquillo, L, Thorpe, L, Feng, C, Hunter, G, Dash, D, Hussein, T, et al. Diagnostic accuracy of ambulatory EEG vs routine EEG in patients with first single unprovoked seizure. Neurol Clin Pract. (2023) 13:e200160. doi: 10.1212/CPJ.0000000000200160
23. Horváth, A, Szűcs, A, Barcs, G, and Kamondi, A. Sleep EEG detects epileptiform activity in Alzheimer’s disease with high sensitivity. J Alzheimers Dis. (2017) 56:1175–83. doi: 10.3233/JAD-160994
24. Rodriguez Ruiz, A, Vlachy, J, Lee, JW, Gilmore, EJ, Ayer, T, Haider, HA, et al. Association of Periodic and Rhythmic Electroencephalographic Patterns with Seizures in critically ill patients. JAMA Neurol. (2017) 74:181–8. doi: 10.1001/jamaneurol.2016.4990
25. Trejo-Lopez, JA, Yachnis, AT, and Prokop, S. Neuropathology of Alzheimer’s disease. Neurotherapeutics. (2022) 19:173–85. doi: 10.1007/s13311-021-01146-y
26. Beniczky, S, and Schomer, DL. Electroencephalography: basic biophysical and technological aspects important for clinical applications. Epileptic Disord. (2020) 22:697–715. doi: 10.1684/epd.2020.1217
27. Lam, AD, Deck, G, Goldman, A, Eskandar, EN, Noebels, J, and Cole, AJ. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat Med. (2017) 23:678–80. doi: 10.1038/nm.4330
28. Sánchez Fernández, I, Loddenkemper, T, Galanopoulou, AS, and Moshé, SL. Should epileptiform discharges be treated? Epilepsia. (2015) 56:1492–504. doi: 10.1111/epi.13108
29. Aarts, JH, Binnie, CD, Smit, AM, and Wilkins, AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. (1984) 107:293–308. doi: 10.1093/brain/107.1.293
30. Holmes, GL, and Lenck-Santini, PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. (2006) 8:504–15. doi: 10.1016/j.yebeh.2005.11.014
31. Shewmon, DA, and Erwin, RJ. Focal spike-induced cerebral dysfunction is related to the after-coming slow wave. Ann Neurol. (1988) 23:131–7. doi: 10.1002/ana.410230205
32. Klinzing, JG, Niethard, N, and Born, J. Mechanisms of systems memory consolidation during sleep. Nat Neurosci. (2019) 22:1598–610. doi: 10.1038/s41593-019-0467-3
33. Sarkis, RA, Lam, AD, Pavlova, M, Locascio, JJ, Putta, S, Puri, N, et al. Epilepsy and sleep characteristics are associated with diminished 24-h memory retention in older adults with epilepsy. Epilepsia. (2023). doi: 10.1111/epi.17707
34. Kleen, JK, Scott, RC, Holmes, GL, Roberts, DW, Rundle, MM, Testorf, M, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. (2013) 81:18–24. doi: 10.1212/WNL.0b013e318297ee50
35. Lambert, I, Tramoni-Negre, E, Lagarde, S, Roehri, N, Giusiano, B, Trebuchon-Da Fonseca, A, et al. Hippocampal Interictal spikes during sleep impact long-term memory consolidation. Ann Neurol. (2020) 87:976–87. doi: 10.1002/ana.25744
36. Ung, H, Cazares, C, Nanivadekar, A, Kini, L, Wagenaar, J, Becker, D, et al. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain. (2017) 140:2157–68. doi: 10.1093/brain/awx143
37. Bou Assi, E, Zerouali, Y, Robert, M, Lesage, F, Pouliot, P, and Nguyen, DK. Large-scale desynchronization during Interictal epileptic discharges recorded with intracranial EEG. Front Neurol. (2020) 11:529460. doi: 10.3389/fneur.2020.529460
38. Frauscher, B, Bernasconi, N, Caldairou, B, Von Ellenrieder, N, Bernasconi, A, Gotman, J, et al. Interictal hippocampal spiking influences the occurrence of hippocampal sleep spindles. Sleep. (2015) 38:1927–33. doi: 10.5665/sleep.5242
39. Gelinas, JN, Khodagholy, D, Thesen, T, Devinsky, O, and Buzsáki, G. Interictal epileptiform discharges induce hippocampal-cortical coupling in temporal lobe epilepsy. Nat Med. (2016) 22:641–8. doi: 10.1038/nm.4084
40. Reed, CM, Mosher, CP, Chandravadia, N, Chung, JM, Mamelak, AN, and Rutishauser, U. Extent of single-neuron activity modulation by hippocampal Interictal discharges predicts declarative memory disruption in humans. J Neurosci. (2020) 40:682–93. doi: 10.1523/JNEUROSCI.1380-19.2019
41. Landi, S, Petrucco, L, Sicca, F, and Ratto, GM. Transient Cognitive Impairment in Epilepsy. Front Mol Neurosci. (2018) 11:458.
42. Noorlag, L, Van Klink, NEC, Kobayashi, K, Gotman, J, Braun, KPJ, and Zijlmans, M. High-frequency oscillations in scalp EEG: a systematic review of methodological choices and clinical findings. Clin Neurophysiol. (2022) 137:46–58. doi: 10.1016/j.clinph.2021.12.017
43. Norman, Y, Raccah, O, Liu, S, Parvizi, J, and Malach, R. Hippocampal ripples and their coordinated dialogue with the default mode network during recent and remote recollection. Neuron. (2021) 109:2767–2780.e5. doi: 10.1016/j.neuron.2021.06.020
44. Geva-Sagiv, M, Mankin, EA, Eliashiv, D, Epstein, S, Cherry, N, Kalender, G, et al. Augmenting hippocampal-prefrontal neuronal synchrony during sleep enhances memory consolidation in humans. Nat Neurosci. (2023) 26:1100–10. doi: 10.1038/s41593-023-01324-5
45. Von Ellenrieder, N, Dubeau, F, Gotman, J, and Frauscher, B. Physiological and pathological high-frequency oscillations have distinct sleep-homeostatic properties. Neuroimage Clin. (2017) 14:566–73. doi: 10.1016/j.nicl.2017.02.018
46. Liu, AA, Henin, S, Abbaspoor, S, Bragin, A, Buffalo, EA, Farrell, JS, et al. A consensus statement on detection of hippocampal sharp wave ripples and differentiation from other fast oscillations. Nat Commun. (2022) 13:6000. doi: 10.1038/s41467-022-33536-x
47. Hautecloque-Raysz, G, Sellal, F, Bousiges, O, Phillipi, N, Blanc, F, and Cretin, B. Epileptic prodromal Alzheimer’s disease treated with Antiseizure medications: medium-term outcome of seizures and cognition. J Alzheimers Dis. (2023) 94:1057–74. doi: 10.3233/JAD-221197
48. Targa Dias Anastacio, H, Matosin, N, and Ooi, L. Neuronal hyperexcitability in Alzheimer’s disease: what are the drivers behind this aberrant phenotype? Transl Psychiatry. (2022) 12:257. doi: 10.1038/s41398-022-02024-7
49. Sanchez, PE, Zhu, L, Verret, L, Vossel, KA, Orr, AG, Cirrito, JR, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci U S A. (2012) 109:E2895–903. doi: 10.1073/pnas.1121081109
50. Cumbo, E, and Ligori, LD. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy Behav. (2010) 17:461–6. doi: 10.1016/j.yebeh.2010.01.015
51. Guida, M, Iudice, A, Bonanni, E, and Giorgi, FS. Effects of antiepileptic drugs on interictal epileptiform discharges in focal epilepsies: an update on current evidence. Expert Rev Neurother. (2015) 15:947–59. doi: 10.1586/14737175.2015.1065180
52. Sarkis, RA, Goksen, Y, Mu, Y, Rosner, B, and Lee, JW. Cognitive and fatigue side effects of anti-epileptic drugs: an analysis of phase III add-on trials. J Neurol. (2018) 265:2137–42. doi: 10.1007/s00415-018-8971-z
53. Tao, K, Chen, H, Chen, Y, Gu, Y, and Wang, X. Levetiracetam induces severe psychiatric symptoms in people with epilepsy. Seizure. (2022). doi: 10.1016/j.seizure.2022.12.002
54. Marson, AG, Kadir, ZA, and Chadwick, DW. New antiepileptic drugs: a systematic review of their efficacy and tolerability. BMJ. (1996) 313:1169–74. doi: 10.1136/bmj.313.7066.1169
55. He, Q, Chen, X, Wu, T, Li, L, and Fei, X. Risk of dementia in long-term benzodiazepine users: evidence from a Meta-analysis of observational studies. J Clin Neurol. (2019) 15:9–19. doi: 10.3988/jcn.2019.15.1.9
56. Warsi, NM, Wong, SM, Gorodetsky, C, Suresh, H, Arski, ON, Ebden, M, et al. Which is more deleterious to cognitive performance? Interictal epileptiform discharges vs anti-seizure medication. Epilepsia. (2023) 64:e75–81. doi: 10.1111/epi.17556
57. Shiozaki, K, and Kajihara, S. Anti-epileptic drugs improved serial 7s scores on the Mini-mental state examination in elderly with cognitive impairment and epileptiform discharge on electroencephalography. Psychogeriatrics. (2019) 19:38–45. doi: 10.1111/psyg.12362
58. Vossel, K, Ranasinghe, KG, Beagle, AJ, La, A, Ah Pook, K, Castro, M, et al. Effect of Levetiracetam on cognition in patients with Alzheimer disease with and without epileptiform activity: a randomized clinical trial. JAMA Neurol. (2021) 78:1345–54. doi: 10.1001/jamaneurol.2021.3310
Keywords: Alzheimer’s disease, dementia, electroencephalography, interictal discharges, epileptogenesis
Citation: Lemus HN and Sarkis RA (2023) Interictal epileptiform discharges in Alzheimer’s disease: prevalence, relevance, and controversies. Front. Neurol. 14:1261136. doi: 10.3389/fneur.2023.1261136
Edited by:
Keith Vossel, Mary S. Easton Center for Alzheimer’s Disease Research at UCLA, United StatesReviewed by:
Anna Szucs, Queen Victoria Hospital, United KingdomAnna Kucharska-Newton, University of North Carolina at Chapel Hill, United States
Copyright © 2023 Lemus and Sarkis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rani A. Sarkis, cnNhcmtpc0Bid2guaGFydmFyZC5lZHU=
 Hernan Nicolas Lemus
Hernan Nicolas Lemus Rani A. Sarkis
Rani A. Sarkis