- 1Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, United States
- 2Department of Radiology, Montefiore Medical Center and Albert Einstein College of Medicine, Bronx, NY, United States
- 3Department of Neurology, Montefiore Health System and Albert Einstein College of Medicine, Bronx, NY, United States
Introduction: Neurocognitive symptoms and dysfunction of various severities have become increasingly recognized as potential consequences of SARS-CoV-2 infection. Although there are numerous observational and subjective survey-reporting studies of neurological symptoms, by contrast, those studies describing imaging abnormalities are fewer in number.
Methods: This study conducted a metanalysis of 32 studies to determine the incidence of the common neurological abnormalities using magnetic resonance imaging (MRI) in patients with COVID-19.
Results: We also present the common clinical findings associated with MRI abnormalities. We report the incidence of any MRI abnormality to be 55% in COVID-19 patients with perfusion abnormalities (53%) and SWI abnormalities (44%) being the most commonly reported injuries. Cognitive impairment, ICU admission and/or mechanical ventilation status, older age, and hospitalization or longer length of hospital stay were the most common clinical findings associated with brain injury in COVID-19 patients.
Discussion: Overall, the presentation of brain injury in this study was diverse with no substantial pattern of injury emerging, yet most injuries appear to be of vascular origin. Moreover, analysis of the association between MRI abnormalities and clinical findings suggests that there are likely many mechanisms, both direct and indirect, by which brain injury occurs in COVID-19 patients.
Introduction
Coronavirus Disease 2019 (COVID-19) (1, 2) characteristically involves multiple organ systems, including the central and peripheral nervous system. SARS-CoV-2 infection has been associated with a range of neurological phenomena, which are still incompletely understood. Severe acute neurological events include ischemic stroke, intracranial hemorrhage, encephalopathy, seizure disorders, extrapyramidal syndromes, neuromuscular pathologies, various immune-mediated neuroinflammatory disorders, and dysautonomias (3). In this context, neurocognitive symptoms and dysfunction of various severities have become increasingly recognized as potential consequences of SARS-CoV-2 infection. While brain dysfunction might be attributed to the effects of critical care illness among hospitalized patients, emerging data indicate that brain effects are also prevalent among less severely ill, non-hospitalized and even mildly symptomatic patients (4). Although there are numerous observational and subjective survey-reporting studies of neurological symptoms, by contrast, those studies describing imaging abnormalities are fewer in number.
This study conducted a metanalysis to determine the incidence of the common neurological abnormalities using magnetic resonance imaging (MRI) in patients with COVID-19. This study expands on a previous metanalysis of COVID-19 neuroimaging performed early in the pandemic (5) providing a more contemporary and elaborate analysis. We also present the common clinical findings associated with MRI abnormalities.
Methods
Eligibility criteria and evidence search
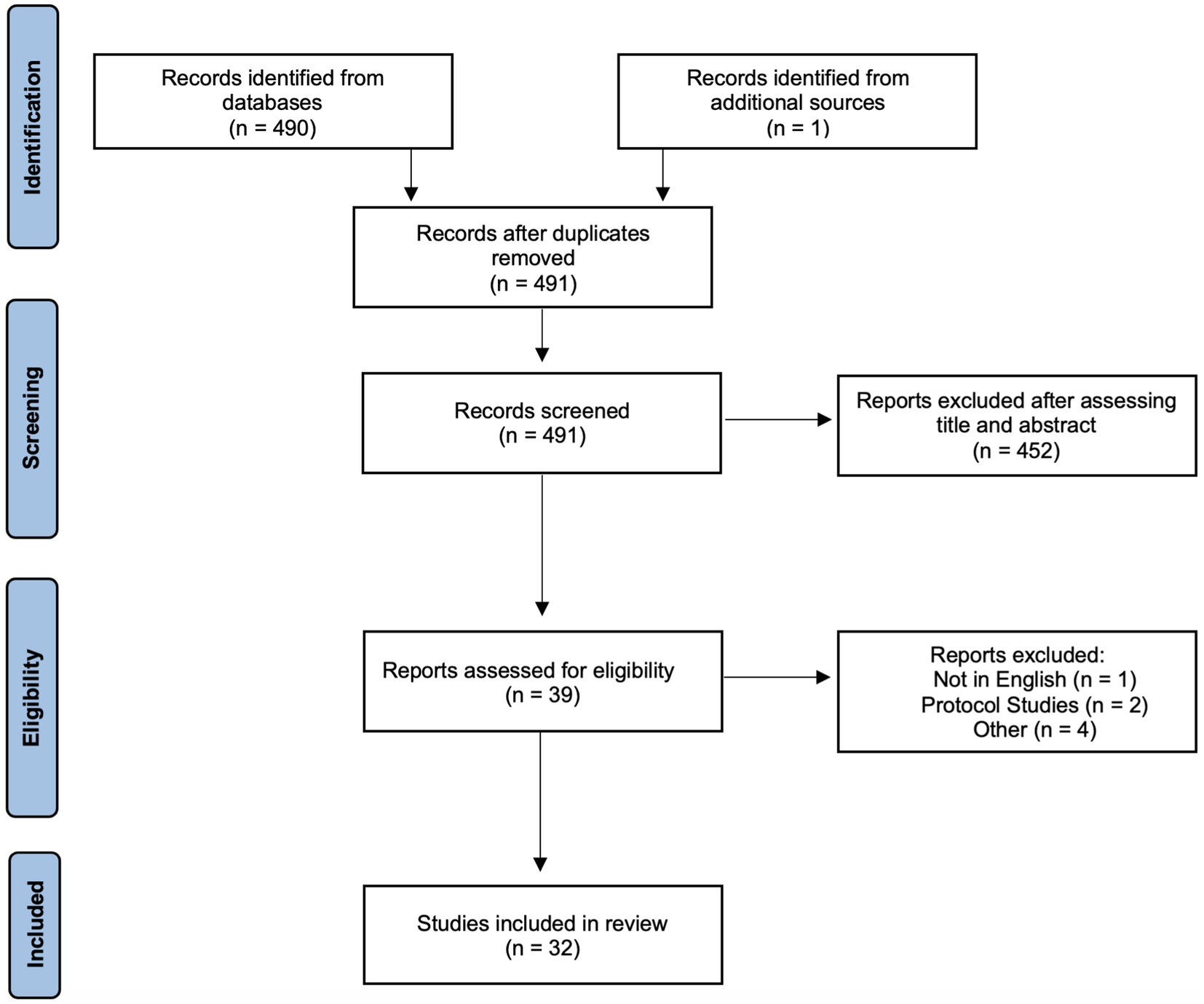
Using Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA), we conducted a systematic review of studies which reported neurological MRI findings in COVID-19 patients (Figure 1). A PubMed, Embase and Google Scholar database search from January 1, 2020, to June 17, 2022, was performed. Additional papers found outside of these searches were added at the authors’ discretion. The search parameters can be found in the Supplementary Information. Cross-sectional, case–control, and cohort studies were included in the analyses. Studies that were excluded included: (1) case reports, case series, review papers, and conference abstracts; (2) papers not written in English; (3) protocol papers, letters to the editor, preprint papers, and healthcare provider surveys without data; and (4) papers that did not use MRI as a data metric.
The title and abstract of papers after the initial search were assessed by two independent reviewers, MB and BM, and only studies approved by both reviewers were included. Disputes regarding the inclusion of a paper were decided by a third reviewer, TD.
Data collection and analysis
Study characteristics, including author, study type, origin, sample size and other qualitative findings were manually collected. The incidence of any brain MRI abnormality as well as the incidence of common specific and subspecific brain MRI abnormalities after SARS-CoV-2 infection were collected manually.
Results
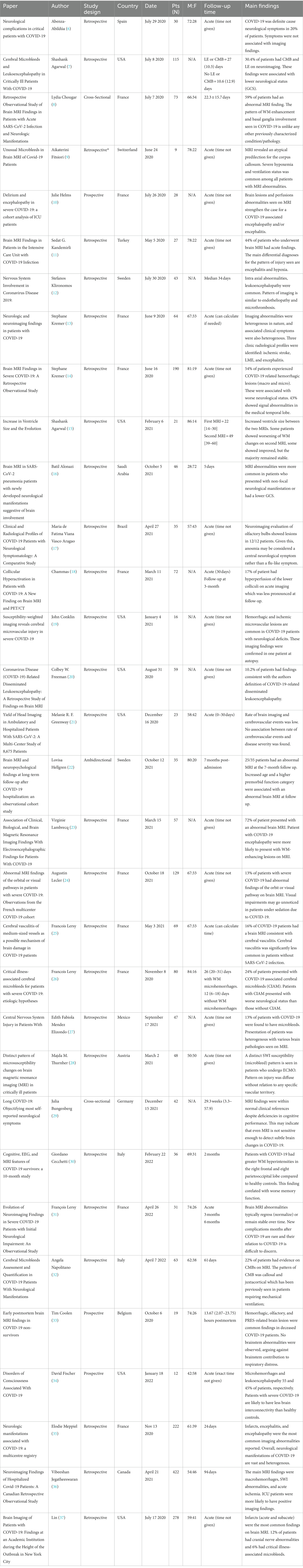
The initial search resulted in 491 articles with no duplicates. After assessing the title and abstract, 452 papers were removed. An additional seven papers which did not meet the inclusion criteria were removed after assessing the entire paper. Thirty-two papers were included in the final study. Table 1 summarizes the study characteristics and main findings.
Incidence of brain MRI abnormalities
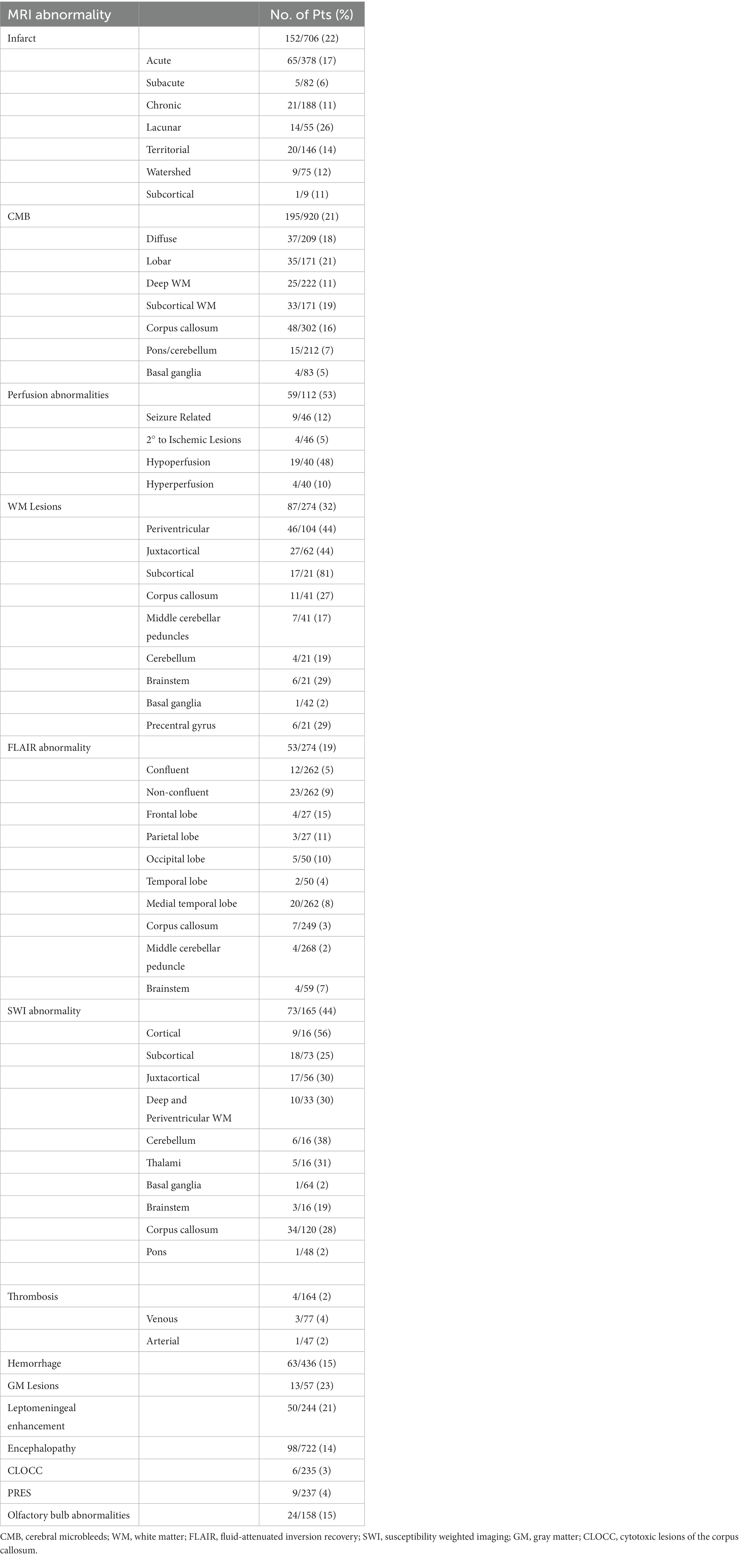
Figure 2 illustrates the incidence of brain MRI abnormalities after SARS-CoV-2 infection. The incidence of any brain MRI abnormality after SARS-CoV-2 infection was 55% (461/837 patients). The most common brain abnormalities in order of incidence were perfusion abnormalities (53%), susceptibility weighted imaging (SWI) abnormality (44%), white matter lesions (32%), gray matter lesions (23%), infarct/ischemia (22%), cerebral microbleeds (CMB; 21%), leptomeningeal enhancement (LME; 21%), fluid attenuated inversion recovery (FLAIR) abnormality (19%), olfactory bulb abnormalities (15%), hemorrhage (15%), encephalopathy (14%), posterior reversible encephalopathy syndrome (PRES; 4%), cytotoxic lesions of the corpus callosum (CLOCC; 3%) and thrombosis (2%). Subspecific information regarding brain MRI abnormalities are presented in Table 2.
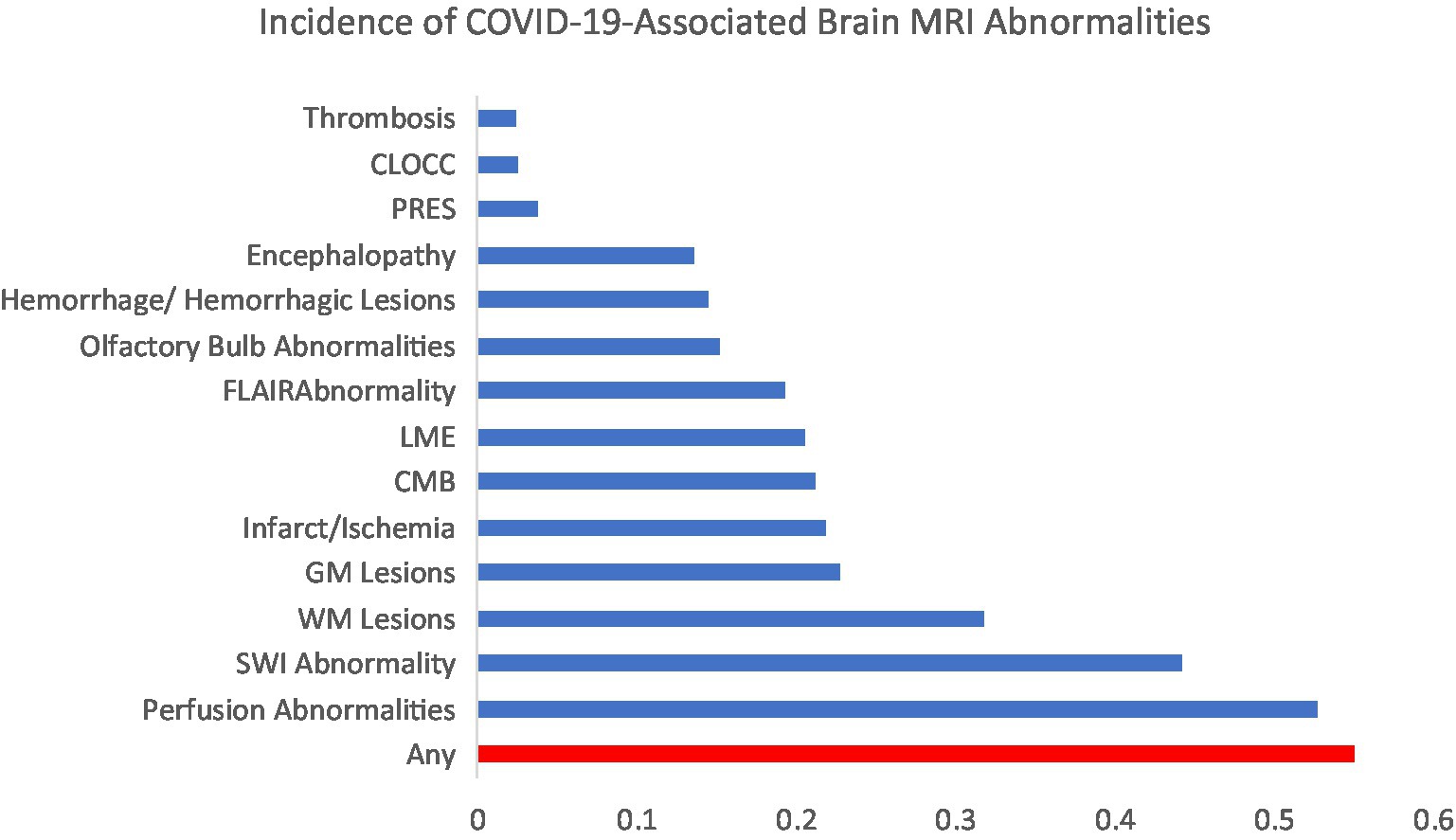
Figure 2. Incidence of the most common COVID-19-associated brain MRI abnormalities. CLOCC, cytotoxic lesions of the corpus callosum; PRES, posterior reversible encephalopathy syndrome; FLAIR, fluid-attenuated inversion recovery; LME, leptomeningeal enhancement; CMB, cerebral microbleeds; GM, gray matter; WM, white matter; SWI, susceptibility weighted imaging.
The incidence of acute infarcts (17%) was more common than chronic (11%) and subacute infarcts (6%). Lacunar infarcts were the most common (26%) followed by territorial arterial infarcts (14%) and watershed infarcts (12%). Cortical stroke was not reported in any studies whereas the incidence of subcortical stroke was found to be 11% in one study.
The incidence of lobar CMBs (21%) was slightly higher than diffuse CMBs (18%). The incidence of CMBs in the subcortical and deep WM was 19 and 11%, respectively. The most commonly affected subcortical structures were the corpus callosum (16%), pons/cerebellum (7%), and basal ganglia (5%).
Hypoperfusion abnormalities (48%) were more common than hyperperfusion abnormalities (10%). The incidence of seizure-related perfusion abnormalities was 12%. Perfusion abnormalities secondary to ischemic lesions was 5%.
The incidence of subcortical WM changes was 81%. The incidence of periventricular and juxtacortical WM changes was 44% each. The most common sites for WM changes were the brainstem (29%), precentral gyrus (29%), corpus callosum (27%), cerebellum (19%), middle cerebellar peduncles (17%), and basal ganglia (2%).
Non-confluent FLAIR abnormalities (9%) were more common than confluent ones (5%). The most common locations were the frontal lobe (15%), parietal lobe (11%), occipital lobe (10%), medial temporal lobe (8%), brainstem (7%), temporal lobe (4%), corpus callosum (3%), and middle cerebellar peduncles (2%).
The incidence of cortical, juxtacortical, and subcortical on DWI was 56, 30, and 25%, respectively. A combined incidence of deep and periventricular WM SWI abnormality (30%) was reported in one study. The most common sites for SWI abnormalities were cerebellum (38%), thalami (31%), corpus callosum (28%), brainstem (19%), basal ganglia (2%), and pons (2%).
The incidence of venous thrombosis (4%) was marginally higher than arterial thrombosis (2%).
Figure 3 shows the inter-study heterogeneity for the commonly reported brain MRI abnormalities.
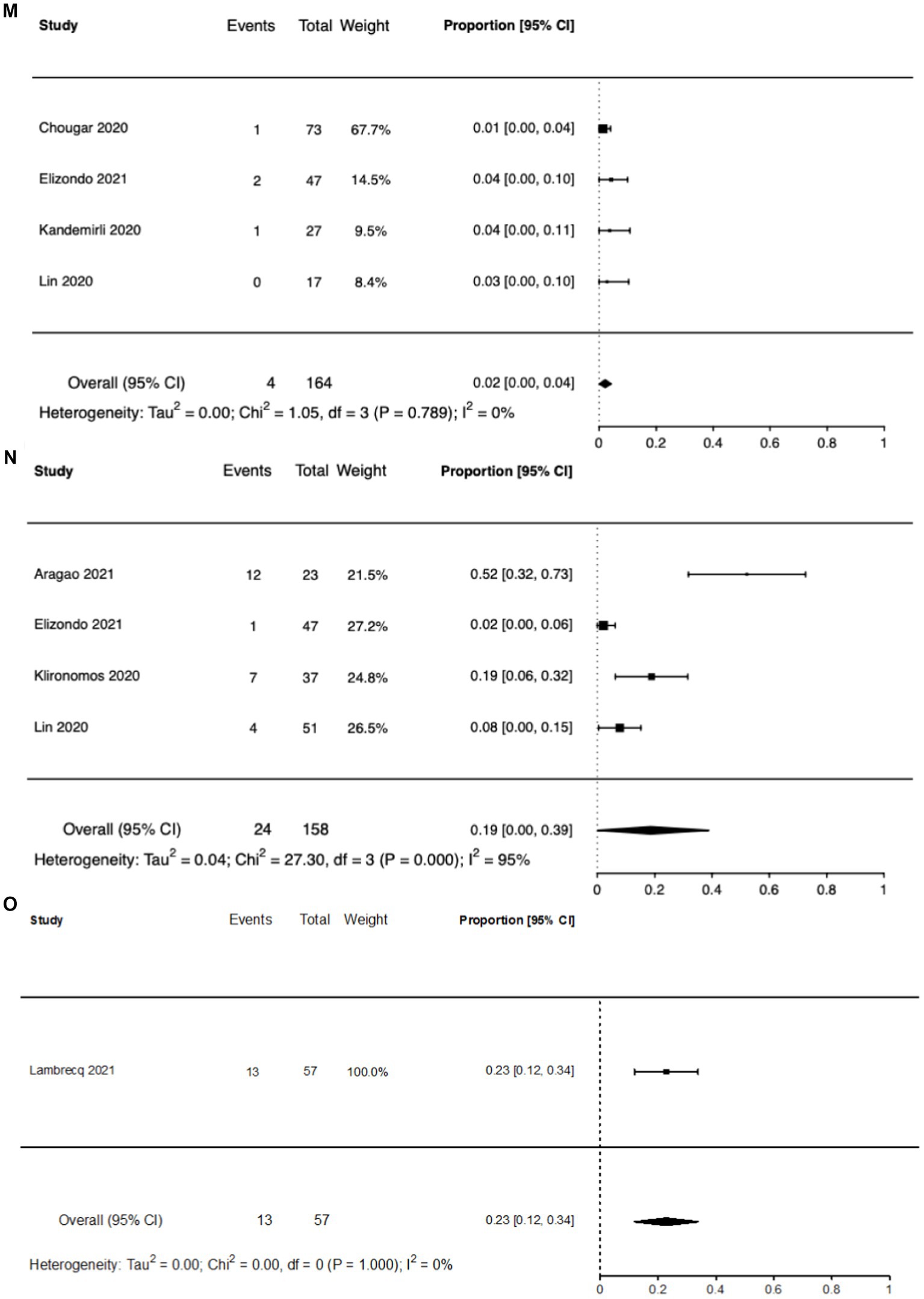
Figure 3. Forest plots of reported brain MRI abnormalities. (A) Any brain MRI abnormality. (B) Cerebral microbleeds. (C) Encephalopathy. (D) Hemorrhage. (E) Infarct. (F) White matter lesions. (G) FLAIR abnormality. (H) SWI Abnormality. (I) Posterior reversible encephalopathy syndrome. (J) Leptomeningeal enhancement. (K) Cytotoxic lesion of the corpus callosum. (L) Perfusion abnormalities. (M) Thrombosis. (N) Olfactory bulb abnormalities. (O) Gray matter lesions.
Clinical measures associated with MRI abnormalities
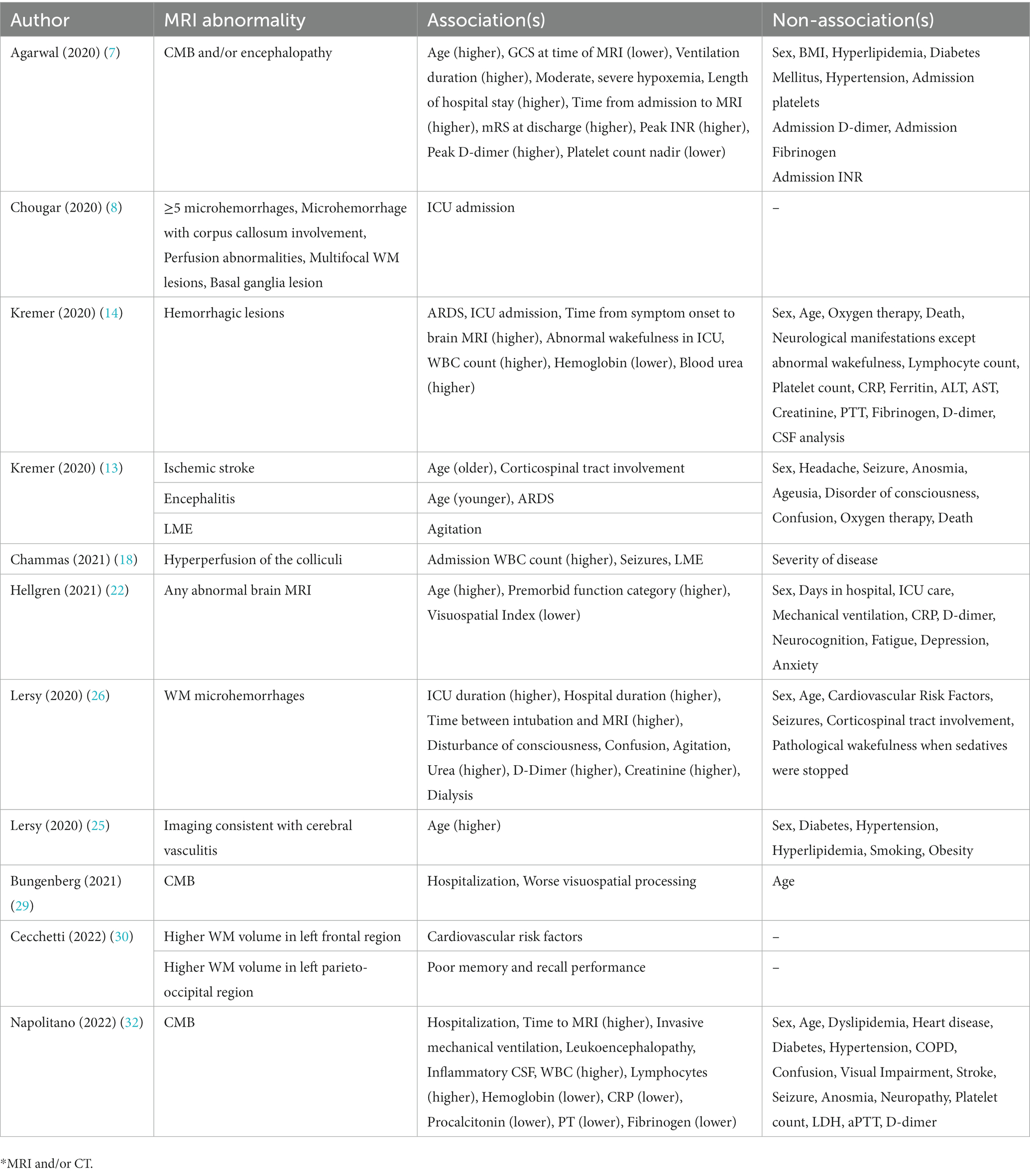
Twelve studies in this review reported a statistical (p < 0.05) association between at least one clinical datapoint and an MRI abnormality. The most commonly reported associations were cognitive impairment (6), followed by ICU and/or mechanical ventilation status (5), older age (4 studies), hospitalization or longer length of hospital stay (4), and ARDS (2). The most commonly reported laboratory marker was elevated WBC count (3), higher D-Dimer (2), higher creatinine (2) and decreased hemoglobin (2). Table 3 qualitatively outlines the MRI abnormality and the associated clinical parameter for each of the 12 studies.
Discussion
In this metanalysis, we report the pooled incidence of the commonly reported brain MRI abnormalities in patients with COVID-19. The pooled incidence of any brain MRI abnormality was found to be 55% [Proportion = 0.65; 95% CI = 54–76%; I2 = 94%]. The five most commonly studied abnormalities were WM lesions [Proportion = 0.39; 95% CI = 11–66%; I2 = 99%], cerebral microbleeds [Proportion = 0.29; 95% CI = 16–38%; I2 = 95%], hemorrhage [Proportion = 0.16; 95% CI = 9–22%; I2 = 74%], infarct [Proportion = 0.18; 95% CI = 11–21%; I2 = 65%], and encephalopathy [Proportion = 0.12; 95% CI = 3–18%; I2 = 94%]. Perfusion abnormalities (53%) and SWI abnormalities (47%) were the two brain abnormalities with the highest incidence. The most reported clinical characteristic and laboratory value with a statistically significant association with at least one brain MRI abnormality was cognitive impairment and elevated WBC count, respectively. Together, these results show that brain MRI abnormalities after SARS-CoV-2 infection are common and that clinical associations may provide insight into identifying at-risk patients as well as possible combinatorial and intersectional mechanisms of brain injury in COVID-19.
There is considerable heterogeneity in the results reported in this meta-analysis, a finding which is similar to a smaller meta-analysis performed earlier in the pandemic (5). There are a few reasons for the observed heterogeneity. First, there is substantial interstudy variation in patient populations, study designs, and end-outcomes. Second, the neurological presentation of COVID-19 is itself very heterogenous. Unlike other pathogens, such as Lyme Disease and Herpes Simplex Virus (38), which may reveal distinct patterns of injury on brain MRI, there is no unanimous pattern of brain injury with SARS-CoV-2, likely due to multifactorial and synergistic mechanisms of direct and indirect injury responses. Indirect effects include respiratory distress, sepsis, hypoxia, cardiovascular distress, host-mediated proinflammatory responses, hypercoagulation, amongst many others. Whether the heterogeneity seen in our study is the result of interstudy variation or the result of the inherent diversity of SARS-CoV-2-mediated brain injury remains to be seen.
The incidence of any brain MRI abnormality was found to be 55%. This number is likely greatly inflated given that the majority of studies included in this meta-analysis looked at patients that had severe COVID-19. Indeed, a previous study found the rate of brain MRI abnormalities to be less than 1% (51/5430) when looking at all COVID-19 patients regardless of severity (39), although this is likely an underestimation given that not all patients in the aforementioned study were referred for MRI analysis. Regardless, the findings of our meta-analysis are likely more useful to the clinician managing a critically ill COVID-19 patient in the ICU than the clinician managing a milder form of the disease.
Clinicians should be aware that the presentation of brain injury in COVID-19 can be diverse, although the majority of brain abnormalities in COVID-19 appear to be cerebrovascular events. The two injuries with the highest prevalence are perfusion abnormalities and SWI abnormalities, the latter of which usually indicates a cerebral microbleed and/or calcification (40). Infarcts, hemorrhages, cerebral microbleeds, and thrombosis are also of cerebrovascular origin. On the other hand, olfactory bulb lesions are likely exclusively associated with nerve damage (17). The source of the rest of the abnormalities can vary.
The question of how much COVID-19 contributes to abnormal brain MRI findings is unclear, especially since the patients indicated for brain MRI are often the sickest patients with several comorbidities that may present as confounders. However, longitudinal studies with multiple time points can provide some insight into this question. Lersy et al. show that 79% of patients had partial or complete regression of abnormal brain MRI findings at 189 days follow-up (31). Furthermore, Chammas et al. showed a marked decreased in collicular hyperintensity at 3-month follow-up (18). It is more likely that this type of dynamic neuro-evolution would be due an acute insult rather than pre-existing chronic conditions. Likewise, Agarwal et al. demonstrated an increase in ventricle size at a 22-day follow-up MRI that is likely due to an acute infectious process rather than chronic processes like alcoholism or neurodegenerative diseases which progress over a longer period of time (15). COVID-19 does contribute to acute brain injury, however, the extent to which the findings reported in brain MRI papers is due to COVID-19 vs. comorbidities is difficult to assess. Future studies should utilize pre- and post-COVID MRI scans as well as matched controls to better determine the extent to which COVID-19 causes brain injury. The UK Biobank study of 785 participants is a good example of such a study (41).
Analysis of the association between MRI abnormalities and clinical findings provides an insight into the mechanism of brain injury in COVID-19. Given that ACE2 receptors and associated SARS-CoV-2 virions are expressed on brain endothelial cells, direct injury mediated by SARS-CoV-2 is theoretically possible (42). However, a direct mechanism of injury is highly unlikely given that only 1.6% (3/184) of patients across 9 studies in our meta-analysis were found to have to have SARS-CoV-2 RNA in their CSF via RT-PCR. Indeed, indirect mechanisms of brain injury seem more plausible, one of which is mechanical ventilation – a known contributor to various neurological injuries including intracranial hemorrhages, ischemic stroke, and hypoxic ischemic encephalopathy (43). This mechanism is supported by multiple papers in our analysis which show an association between mechanical ventilation and the presence of CMBs, WM microhemorrhages, and encephalopathy on MRI (7, 26, 32). It should be noted, however, that patients who do not undergo mechanical ventilation can still present with acute MRI abnormalities suggesting that while mechanical ventilation may contribute to the development neurological abnormalities, it is not the only mechanism at play. The cytokine storm hypothesis is another hypothesis supported by multiple papers which show an association between abnormal MRI findings and elevated inflammatory markers (14, 18, 32). Moreover, a non-specific inflammatory response is more consistent with the heterogenous presentation that is seen on brain MRI. Thrombosis is another potential mechanism for brain injury supported by the association of abnormal MRI findings with elevated D-Dimer, though this association is relatively non-specific (7, 26). Lastly, cerebral vasculitis is another possible mechanism (25). Overall, the mechanism of brain injury in COVID-19 is likely due to multiple, indirect mechanisms, including microvascular infarction and post-infarction hemorrhage.
In addition to possible mechanism of injury, associations between clinical findings and abnormal MRI may provide a predictive model for identifying patients who are likely to present with abnormal MRI findings. Napolitano et al. for example, have determined a CSF inflammatory profile in patients with cerebral microbleeds, though the invasiveness of a lumbar puncture is a large drawback to CSF profiling (32). Future studies should evaluate the possibility of creating such predictive models but using easier to obtain data points.
It is unlikely that specific brain injuries in COVID-19 contribute to acute neurocognitive dysfunction. Many papers in our analysis show an association between lower cognitive functioning and various acute brain MRI abnormalities but no specific imaging pattern has yet emerged. Indeed, these associations are likely the result of confounding bias due to critical care illness. Other modalities, such as EEG and neurocognitive testing, can be used to corroborate MRI findings. A similar conclusion is drawn in terms of long-term cognitive dysfunction, AKA ‘brain fog’ in COVID-19 patients (44, 45). A 7-month follow up study showed no difference in cognitive functioning between patients with and without MRI abnormalities suggesting that ‘brain fog’ cannot routinely be determine by MRI (22). Additional long-term MRI studies are needed to determine (1) whether ‘brain fog’ is due to neurological injury and (2) whether that injury can be identified on MRI analysis.
There are several limitations with this meta-analysis study. First, we chose to analyze only MRI imaging findings to assess the neurological complications of COVID-19 because MRI can detect a broad range of anatomical abnormalities with high sensitivity. CT and other brain imaging modalities should also be explored. Most of the studies included in this analysis did not have propensity-matched control groups and/or pre-COVID-19 brain MRI scans for comparison. Therefore, some findings may be attributable to pre-existing conditions rather than caused or exacerbated by COVID-19. There is publication bias as the patients with more severe COVID-19 disease are more likely to be reported in the literature. Finally, unintentional reporting bias could be present given that virtually all papers in this meta-analysis were retrospective studies.
Conclusion
Improved understanding of the imaging findings associated with neurological signs and symptoms amongst COVID-19 patients and survivors will help to identify common neurological injuries, inform the care of at-risk patients, and understand the mechanism of neurological injury and the progression of brain effects of COVID-19. In this meta-analysis of the neurological MRI findings in COVID-19 patients, we report the incidence of any MRI abnormality to be 55%. The dynamic nature of these abnormalities suggests that the observed brain injury is, at least in part, the result of a SARS-CoV-2 related (para)infectious process rather than chronic comorbidities. Although the presentation of COVID-19 brain injury on MRI is diverse, most injuries appear to be of vascular origin. Moreover, analysis of the association between MRI abnormalities and clinical findings suggests that there are likely many mechanisms by which brain injury occurs in COVID-19. The use of these clinical associations to form predictive models for identifying patients likely to present with MRI abnormalities should be explored by future studies. These studies should also investigate the neurological and neurocognitive manifestations associated with brain MRI abnormalities. Brain MRI studies with longer follow-up intervals are needed to provide detailed assessment of the neurological sequelae of COVID-19. Brain MRI studies analyzing patients with mild COVID-19 are also necessary.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
MB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. BM: Conceptualization, Data curation, Methodology, Validation, Writing – review & editing. WH: Data curation, Visualization, Writing – original draft. MM: Writing – review & editing. TD: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1258352/full#supplementary-material
References
1. Zhu, N , Zhang, D , Wang, W , Li, X , Yang, B , Song, J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Huang, C , Wang, Y , Li, X , Ren, L , Zhao, J , Hu, Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
3. Anand, H , Ende, V , Singh, G , Qureshi, I , Duong, TQ , and Mehler, MF . Nervous system-systemic crosstalk in SARS-CoV-2/COVID-19: a unique Dyshomeostasis syndrome. Front Neurosci. (2021) 15:727060. doi: 10.3389/fnins.2021.727060
4. Liguori, C , Pierantozzi, M , Spanetta, M , Sarmati, L , Cesta, N , Iannetta, M, et al. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun. (2020) 88:11–6. doi: 10.1016/j.bbi.2020.05.037
5. Choi, Y , and Lee, MK . Neuroimaging findings of brain MRI and CT in patients with COVID-19: a systematic review and meta-analysis. Eur J Radiol. (2020) 133:109393. doi: 10.1016/j.ejrad.2020.109393
6. Abenza-Abildúaa, MJ , Ramírez-Prietob, MT , Moreno-Zabaletab, R , Arenas-Vallsb, N , Salvador-Mayab, MA , Algarra-Lucasa, C, et al. Neurological complications in critical patients with COVID-19. Neurologia. (2020) 35:621–7. doi: 10.1016/j.nrl.2020.07.014
7. Agarwal, S , Jain, R , Dogra, S , Krieger, P , Lewis, A , Nguyen, V, et al. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke. (2020) 51:2649–55. doi: 10.1161/STROKEAHA.120.030940
8. Chougar, L , Shor, N , Weiss, N , Galanaud, D , Leclercq, D , Mathon, B, et al. Retrospective observational study of brain MRI findings in patients with acute SARS-CoV-2 infection and neurologic manifestations. Radiology. (2020) 297:E313–23. doi: 10.1148/radiol.2020202422
9. Fitsiori, A , Pugin, D , Thieffry, C , Lalive, P , and Vargas, MI . COVID-19 is associated with an unusual pattern of brain microbleeds in critically ill patients. J Neuroimaging. (2020) 30:593–7. doi: 10.1111/jon.12755
10. Helms, J , Kremer, S , Merdji, H , Schenck, M , Severac, F , Clere-Jehl, R, et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. (2020) 24:491. doi: 10.1186/s13054-020-03200-1
11. Kandemirli, SG , Dogan, L , Sarikaya, ZT , Kara, S , Akinci, C , Kaya, D, et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. (2020) 297:E232–5. doi: 10.1148/radiol.2020201697
12. Klironomos, S , Tzortzakakis, A , Kits, A , Ohberg, C , Kollia, E , Ahoromazdae, A, et al. Nervous system involvement in coronavirus disease 2019: results from a retrospective consecutive neuroimaging cohort. Radiology. (2020) 297:E324–34. doi: 10.1148/radiol.2020202791
13. Kremer, S , Lersy, F , Anheim, M , Merdji, H , Schenck, M , Oesterle, H, et al. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology. (2020) 95:e1868–82. doi: 10.1212/WNL.0000000000010112
14. Kremer, S , Lersy, F , Sèze, J , Ferré, J-C , Maamar, A , Carsin-Nicol, B, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. (2020) 297:E242–51. doi: 10.1148/radiol.2020202222
15. Agarwal, S , Melmed, K , Dogra, S , Jain, R , Conway, J , Galetta, S, et al. Increase in ventricle size and the evolution of white matter changes on serial imaging in critically ill patients with COVID-19. Neurocrit Care. (2021) 35:491–500. doi: 10.1007/s12028-021-01207-2
16. Alonazi, B , Farghaly, AM , Mostafa, MA , Al-Watban, JA , Zindani, SA , Altaimi, F, et al. Brain MRI in SARS-CoV-2 pneumonia patients with newly developed neurological manifestations suggestive of brain involvement. Sci Rep. (2021) 11:20476. doi: 10.1038/s41598-021-00064-5
17. Aragao, M , Leal, MC , Andrade, PHP , Cartaxo Filho, OQ , Aragao, LV , Fonseca, TM, et al. Clinical and radiological profiles of COVID-19 patients with neurological symptomatology: a comparative study. Viruses. (2021) 13. doi: 10.3390/v13050845
18. Chammas, A , Bund, C , Lersy, F , Brisset, JC , Ardellier, FD , Kremer, S, et al. Collicular Hyperactivation in patients with COVID-19: a new finding on brain MRI and PET/CT. AJNR Am J Neuroradiol. (2021) 42:1410–4. doi: 10.3174/ajnr.A7158
19. Conklin, J , Frosch, MP , Mukerji, SS , Rapalino, O , Maher, MD , Schaefer, PW, et al. Susceptibility-weighted imaging reveals cerebral microvascular injury in severe COVID-19. J Neurol Sci. (2021) 421:117308. doi: 10.1016/j.jns.2021.117308
20. Freeman, CW , Masur, J , Hassankhani, A , Wolf, RL , Levine, JM , and Mohan, S . Coronavirus disease (COVID-19)-related disseminated leukoencephalopathy: a retrospective study of findings on brain MRI. AJR Am J Roentgenol. (2021) 216:1046–7. doi: 10.2214/AJR.20.24364
21. Greenway, MRF , Erben, Y , Huang, JF , Siegel, JL , Lamb, CJ , Badi, MK, et al. Yield of head imaging in ambulatory and hospitalized patients with SARS-CoV-2: a multi-center study of 8675 patients. Neurohospitalist. (2021) 11:221–8. doi: 10.1177/1941874420980622
22. Hellgren, L , Birberg Thornberg, U , Samuelsson, K , Levi, R , Divanoglou, A , and Blystad, I . Brain MRI and neuropsychological findings at long-term follow-up after COVID-19 hospitalisation: an observational cohort study. BMJ Open. (2021) 11:e055164. doi: 10.1136/bmjopen-2021-055164
23. Lambrecq, V , Hanin, A , Munoz-Musat, E , Chougar, L , Gassama, S , Delorme, C, et al. Association of Clinical, biological, and brain magnetic resonance imaging findings with electroencephalographic findings for patients with COVID-19. JAMA Netw Open. (2021) 4:e211489. doi: 10.1001/jamanetworkopen.2021.1489
24. Lecler, A , Cotton, F , Lersy, F , Kremer, S , and Heran, F . Group SFsCs. Abnormal MRI findings of the orbital or visual pathways in patients with severe COVID-19: observations from the French multicenter COVID-19 cohort. J Neuroradiol. (2021) 48:331–6. doi: 10.1016/j.neurad.2021.07.004
25. Lersy, F , Anheim, M , Willaume, T , Chammas, A , Brisset, JC , Cotton, F, et al. Cerebral vasculitis of medium-sized vessels as a possible mechanism of brain damage in COVID-19 patients. J Neuroradiol. (2021) 48:141–6. doi: 10.1016/j.neurad.2020.11.004
26. Lersy, F , Willaume, T , Brisset, JC , Collange, O , Helms, J , Schneider, F, et al. Critical illness-associated cerebral microbleeds for patients with severe COVID-19: etiologic hypotheses. J Neurol. (2021) 268:2676–84. doi: 10.1007/s00415-020-10313-8
27. Mendez Elizondo, EF , Valdez Ramirez, JA , Barraza Aguirre, G , Dautt Medina, PM , and Berlanga, EJ . Central nervous system injury in patients with severe acute respiratory syndrome coronavirus 2: MRI findings. Cureus. (2021) 13:e18052. doi: 10.7759/cureus.18052
28. Thurnher, MM , Boban, J , Roggla, M , and Staudinger, T . Distinct pattern of microsusceptibility changes on brain magnetic resonance imaging (MRI) in critically ill patients on mechanical ventilation/oxygenation. Neuroradiology. (2021) 63:1651–8. doi: 10.1007/s00234-021-02663-5
29. Bungenberg, J , Humkamp, K , Hohenfeld, C , Rust, MI , Ermis, U , Dreher, M, et al. Long COVID-19: objectifying most self-reported neurological symptoms. Ann Clin Transl Neurol. (2022) 9:141–54. doi: 10.1002/acn3.51496
30. Cecchetti, G , Agosta, F , Canu, E , Basaia, S , Barbieri, A , Cardamone, R, et al. Cognitive, EEG, and MRI features of COVID-19 survivors: a 10-month study. J Neurol. (2022) 269:3400–12. doi: 10.1007/s00415-022-11047-5
31. Lersy, F , Bund, C , Anheim, M , Mondino, M , Noblet, V , Lazzara, S, et al. Evolution of neuroimaging findings in severe COVID-19 patients with initial neurological impairment: An observational study. Viruses. (2022) 14. doi: 10.3390/v14050949
32. Napolitano, A , Arrigoni, A , Caroli, A , Cava, M , Remuzzi, A , Longhi, LG, et al. Cerebral microbleeds assessment and quantification in COVID-19 patients with neurological manifestations. Front Neurol. (2022) 13:884449. doi: 10.3389/fneur.2022.884449
33. Coolen, T , Lolli, V , Sadeghi, N , Rovai, A , Trotta, N , Taccone, FS, et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. (2020) 95:e2016–27. doi: 10.1212/WNL.0000000000010116
34. Fischer, D , Snider, SB , Barra, ME , Sanders, WR , Rapalino, O , Schaefer, P, et al. Disorders of consciousness associated with COVID-19: a prospective multimodal study of recovery and brain connectivity. Neurology. (2022) 98:e315–25. doi: 10.1212/WNL.0000000000013067
35. Meppiel, E , Peiffer-Smadja, N , Maury, A , Bekri, I , Delorme, C , Desestret, V, et al. Neurologic manifestations associated with COVID-19: a multicentre registry. Clin Microbiol Infect. (2021) 27:458–66. doi: 10.1016/j.cmi.2020.11.005
36. Jegatheeswaran, V , Chan, MWK , Chakrabarti, S , Fawcett, A , and Chen, YA . Neuroimaging findings of hospitalized Covid-19 patients: a Canadian retrospective observational study. Can Assoc Radiol J. (2022) 73:179–86. doi: 10.1177/08465371211002815
37. Lin, E , Lantos, JE , Strauss, SB , Phillips, CD , Campion, TR Jr, Navi, BB, et al. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in new York City. AJNR Am J Neuroradiol. (2020) 41:2001–8. doi: 10.3174/ajnr.A6793
38. Kastrup, OWI , and Maschke, M . Neuroimaging of infections. NeuroRx. (2005) 2:324–32. doi: 10.1602/neurorx.2.2.324
39. Usta, NC , and Bulut, E . Evaluation of intracranial vascular and non-vascular pathologies in patients hospitalized due to COVID-19 infection. Acta Neurol Taiwanica. (2022) 31:91–100.
40. Halefoglu, AM , and Yousem, DM . Susceptibility weighted imaging: clinical applications and future directions. World J Radiol. (2018) 10:30–45. doi: 10.4329/wjr.v10.i4.30
41. Douaud, G , Lee, S , Alfaro-Almagro, F , Arthofer, C , Wang, C , McCarthy, P, et al. SARS-CoV-2 is associated with changes in brain structure in UK biobank. Nature. (2022) 604:697–707. doi: 10.1038/s41586-022-04569-5
42. Hamming, I , Timens, W , Bulthuis, ML , Lely, AT , Navis, G , and van Goor, H . Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. (2004) 203:631–7. doi: 10.1002/path.1570
43. Illum, B , Odish, M , Minokadeh, A , Yi, C , Owens, RL , Pollema, T, et al. Evaluation, treatment, and impact of neurologic injury in adult patients on extracorporeal membrane oxygenation: a review. Curr Treat Options Neurol. (2021) 23:15. doi: 10.1007/s11940-021-00671-7
44. Iosifescu, AL , Hoogenboom, WS , Buczek, AJ , Fleysher, R , and Duong, TQ . New-onset and persistent neurological and psychiatric sequelae of COVID-19 compared to influenza: a retrospective cohort study in a large new York City healthcare network. Int J Methods Psychiatr Res. (2022) 31:e1914. doi: 10.1002/mpr.1914
Keywords: COVID-19, magnetic resonance imaging, brain, neurocognitive, cerebral microbleeds (CMB), infarct
Citation: Boparai MS, Musheyev B, Hou W, Mehler MF and Duong TQ (2023) Brain MRI findings in severe COVID-19 patients: a meta-analysis. Front. Neurol. 14:1258352. doi: 10.3389/fneur.2023.1258352
Edited by:
Thorsten Rudroff, The University of Iowa, United StatesReviewed by:
Lorenzo Muccioli, University of Bologna, ItalyJaime Daniel Mondragón, University Medical Center Groningen, Netherlands
Copyright © 2023 Boparai, Musheyev, Hou, Mehler and Duong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tim Q. Duong, dGltLmR1b25nQGVpbnN0ZWlubWVkLmVkdQ==; Montek S. Boparai, TW9udGVrLkJvcGFyYWlAc3Rvbnlicm9va21lZGljaW5lLmVkdQ==
 Montek S. Boparai
Montek S. Boparai Benjamin Musheyev1
Benjamin Musheyev1