
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 19 October 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1257896
This article is part of the Research Topic Current issues in diagnosis and therapy of cerebral amyloid angiopathy View all 5 articles
 Yuyi Zhu1,2†
Yuyi Zhu1,2† Lu Liu3†
Lu Liu3† Luyao Zhong1,2
Luyao Zhong1,2 Yajun Cheng1,2
Yajun Cheng1,2 Shihong Zhang1,2
Shihong Zhang1,2 Bo Wu1,2
Bo Wu1,2 Deren Wang1,2*
Deren Wang1,2* Mangmang Xu1,2*
Mangmang Xu1,2*Objective: To determine the association between the burden of cerebral small vessel disease (CSVD) due to hypertensive angiopathy (HA) and cerebral amyloid angiopathy (CAA) on MRI in patients with primary intracerebral hemorrhage (ICH).
Methods: Patients with primary ICH admitted to our center from March 2012 to November 2021 were consecutively enrolled. We used multivariate binary and ordinal regression analyses to assess the association between HA-CSVD burden and CAA-CSVD burden. Lobar cerebral microbleeds (CMBs) were categorized into three level of severity: 0–1, 2–4, and ≥ 5 lobar CMBs. A high CAA-CSVD score was defined as a CAA-CSVD score of ≥3.
Results: Overall, 222 participants (mean age 59.88 ± 13.56) were included into analysis. Age and ICH etiology differed among different lobar CMB severity and between the presence and absence of high CAA-CSVD score (all p < 0.05). Positive associations between HA-related markers and both lobar CMB severity and high CAA-CSVD score (p < 0.05 for the presence of lacune, deep CMBs ≥5, the presence of WMH, and HA-CSVD score) were observed in univariate analysis. These associations remained significant after adjusting for age, sex, ICH etiology, and potential vascular risk factors. The distribution of CAA-CSVD score was significantly different between patients with and without CMBs ≥5 (adjusted OR 2.351, 95% CI 1.242–4.455, p = 0.009) after correcting for age, sex, ICH etiology, and vascular risk factors.
Conclusion: Our study provides evidence of an association between HA-CSVD and CAA-CSVD in patients with primary ICH, which needs to be verified in future studies.
Cerebral small vessel disease (CSVD) is caused by the impairment of small perforating arteries and venules in the brain (1). Hypertensive angiopathy (HA) and cerebral amyloid angiopathy (CAA) are the two most common pathologies of CSVD (1, 2), and are the main etiologies of primary intracerebral hemorrhage (ICH) (3). In general, HA causes deep ICH or cerebral microbleeds (CMBs), while CAA results in lobar ICH/CMBs (4).
Despite the different pathogeneses of HA and CAA, hypertension is frequently observed in CAA patients (5). Previous experimental research has suggested that hypertension could accelerate CAA accumulation (6) and promote ICH occurrence in Tg2576 mice (a CAA mouse model) (7). Furthermore, histopathological studies have shown that HA-related arteriolosclerosis and CAA pathological changes often coexist (8, 9). Therefore, there might be an association between HA and CAA.
Recent researches have suggested that CSVD makers, such as CMBs, cortical superficial siderosis (cSS), enlarged perivascular spaces (EPVS), and white matter hyperintensities (WMH), could help to determine the etiology of primary ICH (3, 10). These features have been selected as MRI features in the Boston criteria version 2.0 (11). In addition, the CAA-CSVD score, which incorporates CAA CSVD markers such as lobar CMBs, cSS, centrum semiovale-EPVS (CSO-EPVS), and WMH into an ordinal scale, has been found to be associated with the presence of CAA-related pathological changes (12). Hence, using CSVD markers to investigate the relationship between HA and CAA might be a practical approach. In this study, we aimed to investigate the association between the burden of CSVD due to HA and the severity of CAA on MRI in patients with primary ICH.
We performed a retrospective analysis using data from our ongoing longitudinal database at West China Hospital of Sichuan University. We included patients who were admitted to our center between March 2012 and November 2021, with a diagnosis of primary ICH and with brain MRI available, including T1-, T2-, and fluid-attenuated inversion recovery sequence (FLAIR), and susceptibility-weighted imaging (SWI) sequences. All subjects were aged ≥18 years at the time of stroke onset. We excluded patients with ICH due to vascular malformation, aneurysm, transformation of an ischemic infarct, tumor, or systematic disease, such as liver cirrhosis and thrombocytopenia, according to the SMASH-U system (13). This study was approved by the Ethics Committee on Biomedical Research at West China Hospital of Sichuan University. Patient consent was waived because of the retrospective chart review.
We collected demographic characteristics (age, sex), medical history of vascular risk factors, including history of hypertension, diabetes mellitus, hyperlipidemia, smoking, and alcohol intake. We also collected laboratory examination variables, which included blood glucose, triglycerides, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol (LDL-C) on admission.
All patients underwent brain CT to confirm the presence of ICH, as well as CTA or MRA to exclude vascular structural lesions. We rated the presence and severity of CSVD markers on MRI according to the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE) criteria (14). Lacune, CMBs, cSS, WMH, and EPVS were rated for all included patients. The severity of WMH was evaluated using the Fazekas scale. We rated HA-CSVD score and CAA-CSVD score in line with previous studies (12, 15, 16). Table 1 shows the markers and their corresponding allocated points in the HA-CSVD score (ranging from 0 to 4 points) and CAA-CSVD score (ranging from 0 to 6).
The location of CMBs was classified into lobar CMBs and deep CMBs. In our present study, lobar CMBs were defined as involving the lobes or cerebellum. We rated lobar CMB severity as 0 for 0–1 lobar CMB, 1 for 2–4 lobar CMBs, and 3 for ≥5 lobar CMBs. Deep CMBs were defined when the CMBs were located in deep brain regions such as basal ganglia, thalamus, internal capsule, external capsule, and brain stem. The presence of WMH was defined if periventricular WMH scored 3 or/and deep WMH scored ≥2 according to the Fazekas scale. HA burden was assessed using multiple variables, including the presence of lacune, the presence of deep CMB, deep CMBs ≥5, the number of deep CMBs, severe periventricular WMH, and moderate-to-severe WMH in deep regions, as well as the HA-CSVD score. CAA burden in our present study was evaluated by assessing lobar CMB severity and CAA-CSVD score. The interrater reliability of ICH characteristics and CSVD markers was good (17).
Regarding to the categorization for lobar CMBs, we adopted the classification of 0–1, 2–4, and ≥ 5 as reported by Andreas Charidimou et al. (12). They found that the combined CAA-CSVD score, which included these lobar CMB categories, was associated with pathological evidence of CAA-associated vasculopathic changes. In defining the cut-off of CAA-score score, we took into account previous research (12) indicating that pathologically-defined CAA-ICH had a median score of 3 on the CAA-CSVD score. Regarding to the cut-off of HA-CSVD score, we used its median value of 2 in our present study.
Intracerebral hemorrhage etiology of ICH in our study was categorized into four types: HA-ICH, CAA-ICH, mixed-ICH, and undetermined type in line with previous studies (3, 9, 18). HA-ICH was defined when the hemorrhage was located in deep regions, with and without deep CMBs, but without lobar CMBs. CAA-ICH was defined when the patients were aged 55 or older, and had ICH involved in the lobes or cerebellum, with or without lobar CMBs, but without deep CMBs per the modified Boston criteria (19). Mixed-ICH was defined if the patient had ICH/CMBs which were located in both deep and lobar regions. If a patient had lobar ICH without deep CBMs, and was under 55 years of age, the type of ICH was classified as undetermined (9).
All analyses in this study were performed using SPSS (version 23). Student t-test, one-way ANOVA, Mann–Whitney test, or Kruskal Wallis test were used for continuous variables, as appropriate. Pearson Chi-Square or Fisher’s exact test was used for categorical variables, as appropriate. As the test of parallel lines did not pass for the association between HA burden and lobar CMB severity and CAA-CSVD score using ordinal logistic regression, we instead assessed the association between HA burden and lobar CMBs ≥5 or high CAA-CSVD score (defined as a CAA-CSVD score of ≥3) (20) using binary regression analysis. Patients with undetermined type of ICH were excluded from the multivariate analysis due to their undetermined etiology. A value of p < 0.05 was considered significantly different.
After screening 301 consecutive ICH patients for potential eligibility, 70 patients were excluded due to lack of CTA or MRA to confirm the absence of structural lesions, and nine patients with prior ischemic stroke or primary intraventricular hemorrhage. Finally, 222 cases of primary ICH were included in the analysis. HA-ICH (42.8%, 95/222) accounted the most among the included patients, followed by mixed-ICH (40.5%, 90/222), and CAA-ICH (13.1%, 29/222). Eight patients (3.6%) were diagnosed with an undetermined etiology. Specifically for CAA-ICH, the age of patients with CAA ranged from 56 to 88, with a median of 70 and interquartile range of 64–77.
Table 2 presents the clinical characteristics and CSVD markers of the general population and subgroups stratified by lobar CMB severity or the presence/absence of high CAA-CSVD score. Among patients with different level of lobar CMB severity, age (p = 0.044) and ICH etiology (p < 0.001) differed among those with lobar CMB 0–1, lobar CMBs 2–4, and lobar CMBs ≥5. There were no significant differences in vascular risk factors and laboratory examination variables. Lobar CMB severity was positively associated with a high prevalence of HA related CSVD markers such as the presence of lacune, the presence of deep CMB, deep CMBs ≥5, periventricular WMH scored 3, deep WMH scored ≥2, and the presence of WMH, as well as higher number of deep CMBs and a higher HA-CSVD score (all p < 0.001). Also, patients with high CAA-CSVD score were older (p < 0.001) and were more likely to have a high burden of HA (p < 0.001 for each of the HA-related CSVD markers). ICH etiology differed between patients with and without high CAA-CSVD score (p < 0.001). Concerning laboratory examination variables, blood glucose (p = 0.030), total cholesterol (p = 0.011), and LDL-C levels (p = 0.049) on admission were lower in patients with high CAA-CSVD score as compared with those without high CAA-CSVD score.
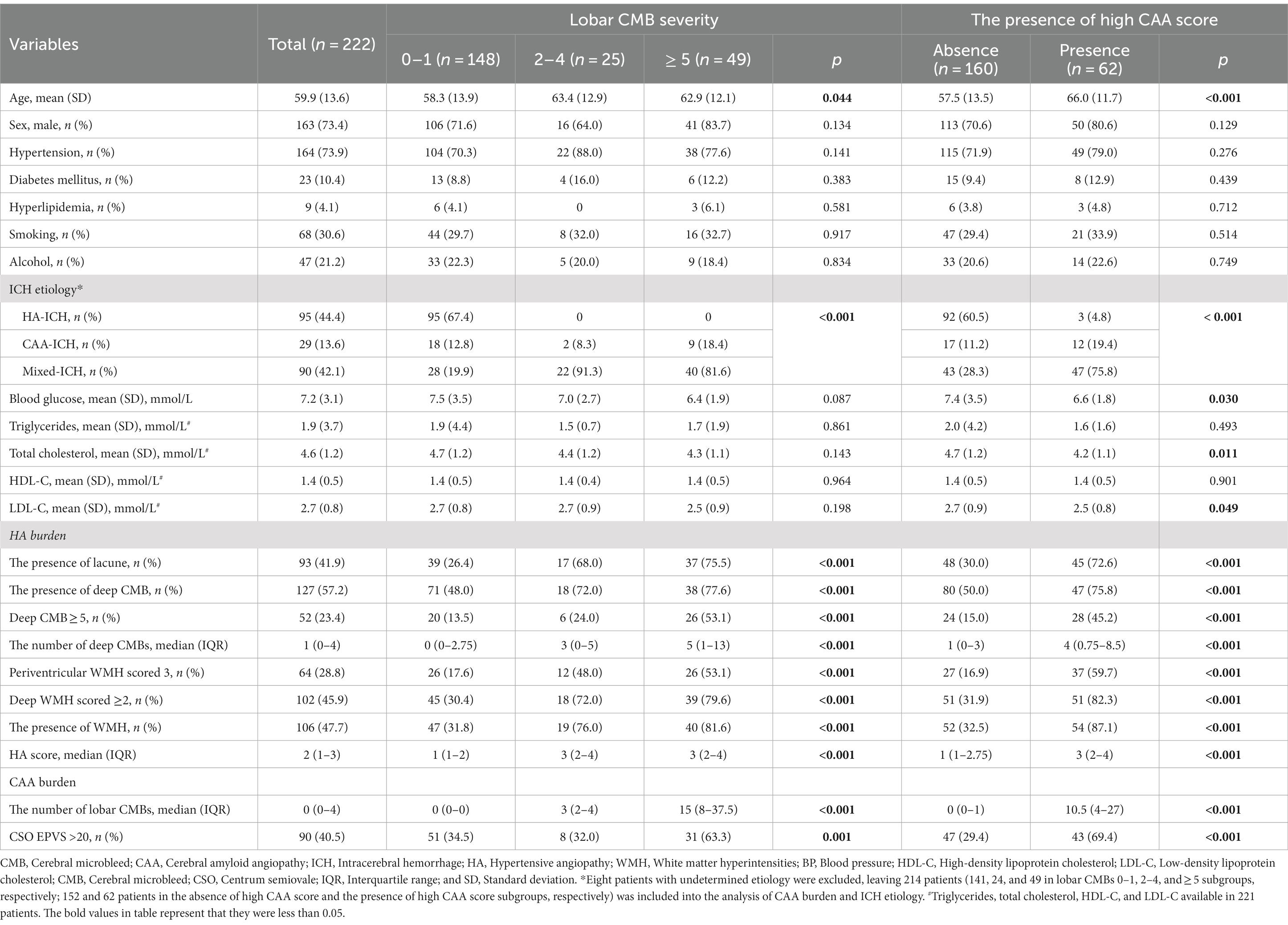
Table 2. The association between HA burden and lobar CMB severity and the presence of high CAA score.
In order to explore the association between HA burden and vascular risk factors, we generated a Supplementary Table 1 to show the distribution of vascular risk factors across different deep CMB numbers and between HA-CSVD score ≥ 2 and HA-CSVD score < 2. The results indicated that as the number of deep CMB increased, the proportion of hypertension also increased (p < 0.001). Also, patients with HA-CSVD score ≥ 2 exhibited a higher proportion of hypertension compared to those with HA-CSVD score < 2 (p = 0.002). In the multivariate analyses, we adjusted for age, male sex, vascular risk factors, including hypertension, diabetes mellitus, hyperlipidemia, smoking, and alcohol, as well as ICH etiology, to examine the association between each of the HA-related CSVD markers and CAA (Table 3). The data revealed that the presence of lacune (adjusted odds ratio [ad OR] 3.909, 95% CI 1.587–9.625, p = 0.003), deep CMBs ≥5 (ad OR 4.600, 95% CI 1.811–11.687, p = 0.001), the number of deep CMBs (ad OR 1.057, 95% CI 1.002–1.116), the presence of WMH (ad OR 5.425, 95% CI 1.911–15.398), and HA-CSVD score (ad OR 2.317, 95% CI 1.483–3.621) were independently associated with the presence of lobar CMBs ≥5. In addition, high CAA-CSVD score was found to be significantly associated with each of the HA-related CSVD markers and the HA-CSVD score, although the association between a higher CAA-CSVD score and the number of deep CMBs was borderline significant.
Due to the significant differences in blood glucose, total cholesterol, and LDL-C levels between groups with and without a high CAA-CSVD score as observed in the univariate analysis, we additionally conducted a multivariate binary logistic regression analysis to assess the association between each of the HA-related CSVD markers and CAA by adjusting for age, male sex, hypertension, blood glucose, total cholesterol, smoking, alcohol, and ICH etiology (see Supplementary Table 2). The results were similar to that in Table 3. Specifically, the presence of lobar CMBs ≥5 or a high CAA-CSVD score was independently related with the presence of HA-related markers and a higher HA-CSVD score, with the exception of the presence of deep CMB.
Figure 1 illustrates the distribution of CAA-CSVD score in patients with and without deep CMBs ≥5 (ad OR 2.351, 95% CI 1.242–4.455, p = 0.009, as determined by ordinal analysis after correcting for age, sex, ICH etiology, and vascular risk factors). The heatmap (Figure 2) displays a correlation between CAA-CSVD score and HA-CSVD score (Spearman correlation coefficients 0.631, p < 0.001). Furthermore, when we stratified the patients based on age and ICH etiology, HA-CSVD score was correlated with CAA-CSVD score or lobar CMB severity in each subgroup (Table 4).
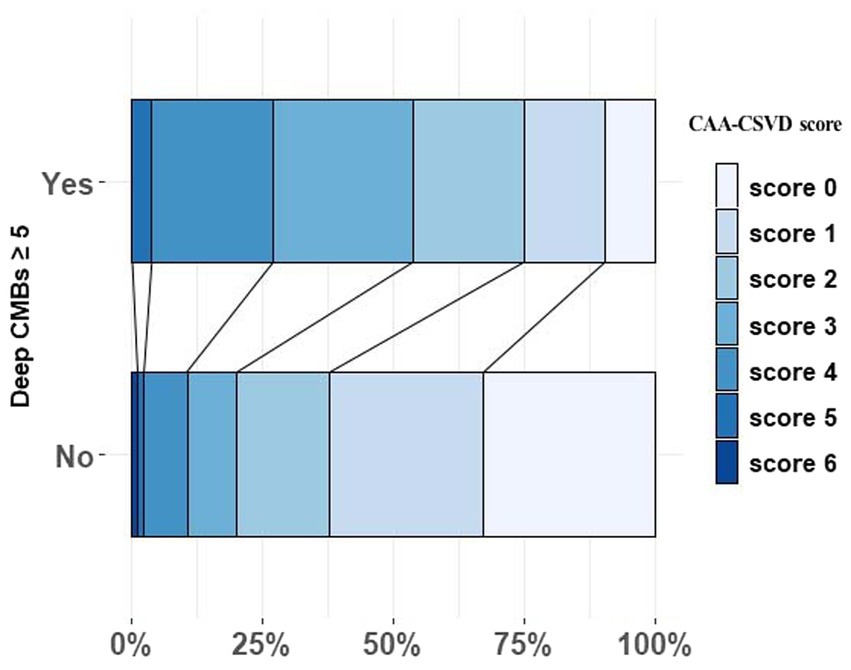
Figure 1. The distribution of CAA-CSVD score in patients with and without deep CMBs ≥ 5. CAA, Cerebral amyloid angiopathy; CSVD, Cerebral small vessel disease; and CMB, Cerebral microbleeds.
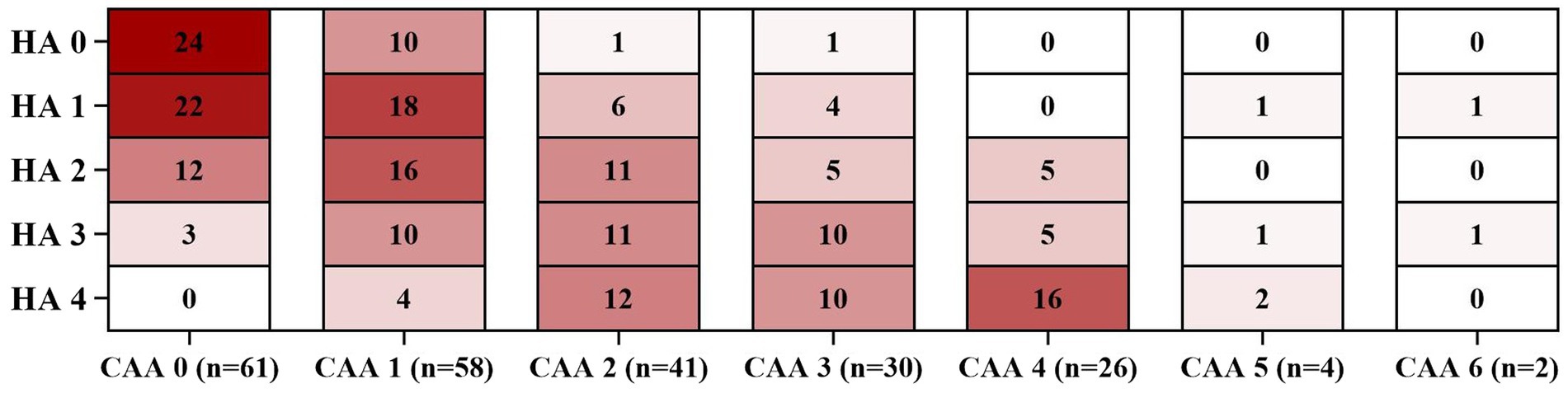
Figure 2. Heatmaps of the frequency distribution of each level of HA-CSVD score stratified by CAA-CSVD score. HA, Hypertensive angiopathy; CAA, Cerebral amyloid angiopathy.
Our study demonstrates that the MRI markers of HA, for instance, the presence of lacune, deep CMBs ≥5, and the presence of WMH, are strongly associated with lobar CMB severity, and further reveals that HA-CSVD score and CAA-CSVD score are closely correlated. These associations are independent of age, sex, ICH etiology, and potential vascular risk factors. Notably, our results supported that the definition of HA based on imaging makers reflected the modifiable vascular risk factors, particularly hypertension, which is the most important risk factor for ICH (21). Specifically for the etiology of CAA, it was diagnosed in 13.1% (29/222) of the included patients, falling within the prevalence spectrum of 8.8% ~ 33% for primary ICH (22–24).
To the best of our knowledge, our study firstly systematically examined the association between HA and CAA based on the severity of their CSVD markers in ICH patients. Although Pasi et al. (15) previously reported that lobar CMBs were correlated with deep CMBs, periventricular WMH, and deep WMH in patients with ICH determined by Spearman correlation analysis. Those associations were not adjusted for confounding factors in this study (15). In our study, we not only confirmed the association between lobar CMBs and HA-related CSVD markers by adjusting for age, sex and potential vascular risk factors, but also observed a correlation between HA-CSVD score and lobar CMB severity, as well as CAA-CSVD score in patients with primary ICH. Interestingly, Ii et al. (20) have reported a positive correlation between HA-CSVD score and CAA-CSVD score in patients undergoing cognitive impairment workup. Meanwhile, the other two studies (25, 26) reported that WMH volume and lacune were positively associated with both lobar and deep CMBs, where lacune was considered as a marker of hypertensive CSVD. Taken together with our findings, the overlap between HA and CAA is common.
Recently, we performed a systematic review and meta-analysis (27) to explore the association between arteriolosclerosis [also named hypertensive small vessel disease (4)] and CAA on pathology by including studies that enrolled patients with primary ICH with pathological examination. And this study (27) found that severe CAA pathological changes were associated with arteriolosclerosis; however, this association was not independent of age and sex. This weak association might be attributed to the small sample size of patient with pathology-proven etiology, or might be attributed to increasing age, a shared risk factor for both HA and CAA (4). Accordingly, we performed a subgroup analysis by stratifying patients into different age groups in our present study. The data demonstrated the association between HA-CSVD score and CAA-CSVD score in each age group. In addition, Kim et al. (25) performed a 3-year longitudinal positron emission topography (PET) study and found that baseline and longitudinal lacune number were associated with Pittsburgh compound retention on longitudinal lobar CMBs in patients with mild cognitive impairment, suggesting that HA had a synergistic effect on the progression of lobar CMBs. Therefore, CAA and HA might have a interact link, which needs to be investigated further in future studies.
Another important finding was that blood glucose, total cholesterol, and LDL-C levels on admission were different between patients with and without high CAA score. After correcting for age and sex, high CAA score was associated with lower total cholesterol (ad OR 0.721, 95% CI 0.527–0.988, p = 0.042, as determined by multivariate binary regression analysis), but not blood glucose and LDL-C. MUCH-Italy study (17) and one previous meta-analysis (21) have reported that low total cholesterol is associated with ICH occurrence, including lobar ICH which is mainly due to CAA. Also, total cholesterol in CAA-ICH is reported to be lower than that in Alzheimer’s disease or controls (age and sex matched healthy subjects) (12). Therefore, lipid levels might be involved in the progression of CAA-CSVD markers in patients with CAA-ICH, which warrants further investigation.
The strength of our study is the systematic assessment of CSVD markers associated with HA and CAA in ICH patients, as well as the use of validated CSVD scores to quantify the overall burden of HA-CSVD and CAA-CSVD. Our findings may help to guide efforts in understanding if hypertensive small vessel disease plays a role in CAA in patients with primary ICH. However, there are several limitations in this study. First, although CSVD markers and the combined CSVD score could help to identify the etiology of CSVD, the lack of neuropathological examination is a major limitation of the present study. Fazekas et al. (9) performed a pathologic examination and postmortem MR imaging in ICH patients, and found that patients with lipofibrohyalinosis but without amyloid angiopathy on pathology could also have cortico-subcortical microbleeds and WMH. Therefore, the component of CAA-CSVD score, such as WMH and lobar CMB could also potentially reflect a severe hypertensive arteriosclerosis. Future studies with pathological evidence of CAA or amyloid-PET imaging are warranted to validate our findings. Second, we only included patients with brain MRI for CSVD study, potentially limiting the generalizability of our results to those with large hematoma volume or severe stroke conditions precluding MRI acquisition. Finally, According to both the classic Boston criteria and modified Boston criteria (19), the presence of multiple hemorrhages in lobes (cerebellar hemorrhage allowed), in together with an age of ≥55 years could be diagnosed with probable CAA. In addition, findings by Pasi et al. (9) suggest that superficial cerebellar ICH might be associated with strict lobar CMB, which is an established marker of CAA. However, cerebellum is also a cite of hypertensive hemorrhage (26). Pasi et al. (25) found that pathologically confirmed CAA was identified in 96% of patients with superficial cerebellar CMBs and in 25% of those with deep/mixed cerebellar CMBs. Therefore, there might be a distinction in etiology between superficial and deep cerebellar CMBs. Future studies with a specific focus on lobar CMBs or with an investigation that assesses lobar CMBs along with superficial cerebellar CMB are needed to confirm our results.
In summary, our study identified a significant association between HA-CSVD burden and CAA-CSVD burden in ICH patients. In addition, HA-CSVD score was correlated with CAA-CSVD score or lobar CMB severity when stratified by age and ICH etiology. These findings suggest that there might be a synergistic effect between HA and CAA in primary ICH, which needs further investigation in future studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Ethics Committee on Biomedical Research at West China Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective chart review.
YZ: Writing – original draft, Writing – review & editing, Formal analysis, and Data curation. LL: Writing – original draft, Writing – review & editing, and Data curation. LZ: Writing – review & editing and Data curation. YC: Writing – review & editing and Data curation. SZ: Writing – review & editing. BW: Writing – review & editing. DW: Writing – review & editing, Funding acquisition, Conceptualization, and Supervision. MX: Writing – review & editing, Funding acquisition, Conceptualization, Supervision, Methodology, and Project administration.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82001250), the Natural Science Foundation of Science and Technology Department of Sichuan Province (2023NSFSC1561), the 1·3·5 Project for Disciplines of Excellence–Clinical Research Incubation Project of West China Hospital (2018HXFH041), and the China Postdoctoral Science Foundation (2020M683322 and 2021T140488).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1257896/full#supplementary-material
1. Wardlaw, JM, Smith, C, and Dichgans, M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. (2019) 18:684–96. doi: 10.1016/S1474-4422(19)30079-1
2. Markus, HS, and de Leeuw, FE. Cerebral small vessel disease: recent advances and future directions. Int J Stroke. (2023) 18:4–14. doi: 10.1177/17474930221144911
3. Charidimou, A, Boulouis, G, Haley, K, Auriel, E, van Etten, ES, Fotiadis, P, et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. (2016) 86:505–11. doi: 10.1212/WNL.0000000000002362
4. Pantoni, L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. (2010) 9:689–701. doi: 10.1016/S1474-4422(10)70104-6
5. Zhang, S, Wang, Z, Zheng, A, Yuan, R, Shu, Y, Zhang, S, et al. Blood pressure and outcomes in patients with different etiologies of intracerebral hemorrhage: a multicenter cohort study. J Am Heart Assoc. (2020) 9:e016766. doi: 10.1161/JAHA.120.016766
6. Stanisavljevic, A, Schrader, JM, Zhu, X, Mattar, JM, Hanks, A, Xu, F, et al. Impact of non-pharmacological chronic hypertension on a transgenic rat model of cerebral amyloid Angiopathy. Front Neurosci. (2022) 16:811371. doi: 10.3389/fnins.2022.811371
7. Passos, GF, Kilday, K, Gillen, DL, Cribbs, DH, and Vasilevko, V. Experimental hypertension increases spontaneous intracerebral hemorrhages in a mouse model of cerebral amyloidosis. J Cereb Blood Flow Metabol. (2016) 36:399–404. doi: 10.1177/0271678X15606720
8. Jolink, WMT, van Veluw, SJ, Zwanenburg, JJM, Rozemuller, AJM, van Hecke, W, Frosch, MP, et al. Histopathology of cerebral microinfarcts and microbleeds in spontaneous intracerebral hemorrhage. Transl Stroke Res. (2022) 14:174–84. doi: 10.1007/s12975-022-01016-5
9. Fazekas, F, Kleinert, R, Roob, G, Kleinert, G, Kapeller, P, Schmidt, R, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. (1999) 20:637–42.
10. Pasi, M, Boulouis, G, Fotiadis, P, Auriel, E, Charidimou, A, Haley, K, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology. (2017) 88:2162–8. doi: 10.1212/WNL.0000000000004007
11. Charidimou, A, Boulouis, G, Frosch, MP, Baron, JC, Pasi, M, Albucher, JF, et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. (2022) 21:714–25. doi: 10.1016/S1474-4422(22)00208-3
12. Charidimou, A, Martinez-Ramirez, S, Reijmer, YD, Oliveira-Filho, J, Lauer, A, Roongpiboonsopit, D, et al. Total magnetic resonance imaging burden of small vessel disease in cerebral amyloid Angiopathy: An imaging-pathologic study of concept validation. JAMA Neurol. (2016) 73:994–1001. doi: 10.1001/jamaneurol.2016.0832
13. Meretoja, A, Strbian, D, Putaala, J, Curtze, S, Haapaniemi, E, Mustanoja, S, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke. (2012) 43:2592–7. doi: 10.1161/STROKEAHA.112.661603
14. Wardlaw, JM, Smith, EE, Biessels, GJ, Cordonnier, C, Fazekas, F, Frayne, R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
15. Pasi, M, Sugita, L, Xiong, L, Charidimou, A, Boulouis, G, Pongpitakmetha, T, et al. Association of cerebral small vessel disease and cognitive decline after intracerebral hemorrhage. Neurology. (2021) 96:e182–92. doi: 10.1212/WNL.0000000000011050
16. Doubal, FN, MacLullich, AM, Ferguson, KJ, Dennis, MS, and Wardlaw, JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. (2010) 41:450–4. doi: 10.1161/STROKEAHA.109.564914
17. Xu, M, Cheng, Y, Song, Q, Yuan, R, Zhang, S, Hao, Z, et al. Total burden of cerebral small vessel disease in recurrent ICH versus first-ever ICH. Aging Dis. (2019) 10:570–7. doi: 10.14336/AD.2018.0804
18. Tsai, HH, Pasi, M, Tsai, LK, Chen, YF, Lee, BC, Tang, SC, et al. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds: a PiB-PET study. Neurology. (2019) 92:e774–81. doi: 10.1212/WNL.0000000000006953
19. Linn, J, Halpin, A, Demaerel, P, Ruhland, J, Giese, AD, Dichgans, M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. (2010) 74:1346–50. doi: 10.1212/WNL.0b013e3181dad605
20. Ii, Y, Ishikawa, H, Matsuyama, H, Shindo, A, Matsuura, K, Yoshimaru, K, et al. Hypertensive Arteriopathy and cerebral amyloid Angiopathy in patients with cognitive decline and mixed cerebral microbleeds. J Alzheimer's Dis. (2020) 78:1765–74. doi: 10.3233/JAD-200992
21. An, SJ, Kim, TJ, and Yoon, BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. (2017) 19:3–10. doi: 10.5853/jos.2016.00864
22. Seiffge, DJ, Polymeris, AA, Law, ZK, Krishnan, K, Zietz, A, Thilemann, S, et al. Cerebral amyloid Angiopathy and the risk of hematoma expansion. Ann Neurol. (2022) 92:921–30. doi: 10.1002/ana.26481
23. Rodrigues, MA, Samarasekera, N, Lerpiniere, C, Humphreys, C, McCarron, MO, White, PM, et al. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol. (2018) 17:232–40. doi: 10.1016/S1474-4422(18)30006-1
24. Roh, D, Sun, CH, Schmidt, JM, Gurol, E, Murthy, S, Park, S, et al. Primary intracerebral hemorrhage: a closer look at hypertension and cerebral amyloid angiopathy. Neurocrit Care. (2018) 29:77–83. doi: 10.1007/s12028-018-0514-z
25. Pasi, M, Pongpitakmetha, T, Charidimou, A, Singh, SD, Tsai, HH, Xiong, L, et al. Cerebellar microbleed distribution patterns and cerebral amyloid Angiopathy. Stroke. (2019) 50:1727–33. doi: 10.1161/STROKEAHA.119.024843
26. Dinsdale, HB. Spontaneous hemorrhage in the posterior FOSSA.A study of primary cerebellar and pontine hemorrhages with observations on their pathogenesis. Arch Neurol. (1964) 10:200–17. doi: 10.1001/archneur.1964.00460140086011
Keywords: cerebral amyloid angiopathy, primary intracerebral hemorrhage, cerebral small vessel disease, hypertensive angiopathy, MRI
Citation: Zhu Y, Liu L, Zhong L, Cheng Y, Zhang S, Wu B, Wang D and Xu M (2023) The association between hypertensive angiopathy and cerebral amyloid angiopathy in primary intracerebral hemorrhage. Front. Neurol. 14:1257896. doi: 10.3389/fneur.2023.1257896
Received: 13 July 2023; Accepted: 15 September 2023;
Published: 19 October 2023.
Edited by:
Hsin-Hsi Cynthia Tsai, National Taiwan University, TaiwanReviewed by:
Haruhiko Hoshino, Saiseikai Central Hospital, JapanCopyright © 2023 Zhu, Liu, Zhong, Cheng, Zhang, Wu, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mangmang Xu, eHUyMW1hbmdtYW5nQHFxLmNvbQ==; Deren Wang, d2FuZ2RlcmVuQHdjaHNjdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.