
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 22 September 2023
Sec. Neuro-Otology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1256826
 Sang Hee Ha1,2
Sang Hee Ha1,2 Dong Kyu Lee3
Dong Kyu Lee3 Gayoung Park1
Gayoung Park1 Bum Joon Kim1
Bum Joon Kim1 Jun Young Chang1
Jun Young Chang1 Dong-Wha Kang1
Dong-Wha Kang1 Sun U. Kwon1
Sun U. Kwon1 Jong S. Kim4
Jong S. Kim4 Hong Ju Park3*†
Hong Ju Park3*† Eun-Jae Lee1*†
Eun-Jae Lee1*†Background: Video head impulse tests (vHITs), assessing the vestibulo-ocular reflex (VOR), may be helpful in the differential diagnosis of acute dizziness. We aimed to investigate vHITs in patients with acute posterior circulation stroke (PCS) to examine whether these findings could exhibit significant abnormalities based on lesion locations, and to evaluate diagnostic value of vHIT in differentiating dizziness between PCS and vestibular neuritis (VN).
Methods: We prospectively recruited consecutive 80 patients with acute PCS and analyzed vHIT findings according to the presence of dorsal brainstem stroke (DBS). We also compared vHIT findings between PCS patients with dizziness and a previously studied VN group (n = 29). Receiver operating characteristic (ROC) analysis was performed to assess the performance of VOR gain and its asymmetry in distinguishing dizziness between PCS and VN.
Results: Patients with PCS underwent vHIT within a median of 2 days from stroke onset. Mean horizontal VOR gain was 0.97, and there was no significant difference between PCS patients with DBS (n = 15) and without (n = 65). None exhibited pathologic overt corrective saccades. When comparing the PCS group with dizziness (n = 40) to the VN group (n = 29), patients with VN demonstrated significantly lower mean VOR gains in the ipsilesional horizontal canals (1.00 vs. 0.57, p < 0.001). VOR gain and their asymmetry effectively differentiated dizziness in the PCS from VN groups, with an area under the ROC curve of 0.86 (95% CI 0.74–0.98) and 0.91 (95% CI 0.83–0.99, p < 0.001), respectively.
Conclusion: Significantly abnormal vHIT results were rare in patients with acute PCS, even in the presence of DBS. Moreover, vHIT effectively differentiated dizziness between PCS and VN, highlighting its potential for aiding differential diagnosis of acute dizziness.
Fast and accurate diagnosis of stroke among patients presenting with acute dizziness is important. Stroke as a central cause of acute vestibular syndrome can be distinguished from peripheral causes using the bedside HINTS (head impulse test, nystagmus, test of skew) examination (1, 2). Especially, the head impulse test (HIT), which evaluates the vestibulo-ocular reflex (VOR), has been suggested as the best stroke predictor because an intact VOR during HIT may outperform even magnetic resonance imaging (MRI) in differentiating central from peripheral causes of acute vestibular syndrome (3, 4). However, bedside HIT may be technically demanding to non-experts, with interpretation varying across clinicians in the emergency department (5). Accordingly, the use of video head impulse test (vHIT), which can easily and objectively measure VOR, has been suggested as an “ECG for the eyes” and has showed promising results (1).
Decreases in VOR gain and subsequent catch-up saccades develop after damages to the peripheral vestibular system with disruption of vestibular input signals (6, 7). The VOR is mediated by various parts of the dorsal brainstem, which includes central structures, such as the medial longitudinal fasciculus (MLF) and the nuclei of the cranial nerves III, IV, and VI (8). Central lesions affecting these structures can also induce VOR impairment (9). Recent studies that evaluated HITs with magnetic search coils or video-based techniques have reported frequent VOR abnormalities in patients with central vestibular disorders (8, 10). These findings raise questions on whether vHIT can be used as a screening tool to differentiate between central and peripheral causes of acute dizziness in clinical settings.
In this prospective study, we aimed to investigate the vHITs in consecutive patients with acute posterior circulation stroke (PCS). First, we determined whether these findings could show significant abnormalities according to the presence of dorsal brainstem stroke (DBS). Additionally, we evaluated whether VOR gains in patients with DBS differ along the longitudinal axis of brainstem (i.e., midbrain vs. pons vs. medulla), because the vestibular pathway spreads out and becomes sparse as it ascends from the caudal to the rostral part. Finally, we evaluated the diagnostic value of vHITs in the differential diagnosis of dizziness between PCS and acute vestibular neuritis (VN).
Of the consecutive patients who were admitted to the stroke unit of Asan Medical Center (Seoul, South Korea) between July 2021 and April 2022, we prospectively recruited those affected by acute PCS (within 24 h of stroke onset) with mild neurologic deficits [National Institutes of Health Stroke Scale (NIHSS) score ≤ 7] (11). Because we aimed to comprehensively analyze vHIT results in acute stroke patients with lesions in the posterior circulation territory, we included patients regardless of the presence of acute dizziness. We excluded individuals with concomitant acute lesions in the anterior circulation, as well as those exhibiting altered mental status (characterized by disorientation, confusion, or diagnosed loss of consciousness by neurologists), and those with active systemic diseases (such as infection or active cancer) (12). These exclusions were implemented due to the potential for compromised cooperation and safety concerns during the testing procedure. The included patients underwent vHIT after the stabilization of neurologic symptoms, which were defined at the discretion of attending physicians.
We also reviewed patients with VN diagnosed in our institution between March 2014 and December 2014 as the control group (13). These patients were enrolled in a randomized controlled 98 trial and prospectively underwent vHIT (13). Patients with VN were included as following criteria: (1) presenting with acute persistent vertigo with unidirectional nystagmus without hearing loss of middle ear pathology on clinical examination, (2) had undergone both vHIT and caloric test finding (abnormal finding: canal paresis ≥ 20%), (3) and no central vestibular diseases from neurological examination or magnetic resonance imaging (13). The study protocol was approved by the local ethics committee (IRB approval number: 2021-0901), and all patients provided written informed consent.
Demographic information and risk factors, along with the chief symptom of acute dizziness were obtained at admission. The symptoms of acute dizziness were defined as follows: (1) vertigo, which is an illusion of movement, either of the person or the visual surrounding, (2) dysequilibrium without vertigo, (3) presyncope (near-faint), and (4) psychophysiological dizziness, which is often associated with anxiety and panic (14). Examinations were conducted by an experienced neurologist. The cause of stroke was categorized according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification (15). Neurological deficit caused by stroke was evaluated based on the NIHSS score at admission.
All patients underwent MRI using a 3.0-T Philips scanner (Philips Healthcare, Eindhoven, Netherlands) that included diffusion-weighted imaging and MR angiography. The locations of acute lesions were evaluated using diffusion-weighted imaging and classified as follows: cerebellar [superior cerebellar, anterior inferior cerebellar, and posterior inferior cerebellar (PICA) artery territory], medulla oblongata, pons, midbrain, thalamus, and medial tempo-occipital areas. If a patient had ischemic lesions in more than one location, each location was classified as a separate location variable; thus, a single patient could have multiple values for the location of stroke lesions. DBS was defined as the presence of lesions in the dorsal medulla oblongata or tegmentum of the midbrain and pons, which are related to the central vestibular pathway (16). If a patient had DBS, they were categorized into the DBS group irrespective of the presence of lesions outside of the dorsal brainstem area. Lesion locations were consensually determined by two stroke experts (SH Ha and E.-J Lee) who were blinded to clinical information.
Recording of the head and eye movements were conducted using vHIT (SLVNG, SLMED, Seoul, South Korea) for PCS patients and ICS Impulse 3-D vHIT system (GN Otometrics, Taastrup, Denmark) for VN patients. Despite the variation in the machines and examiners, the procedures adhered to the protocol established by our institution. The subjects were seated in a height-adjustable chair which allowed the examiner to adjust the height of each subject’s head to an optimal level for the examination. After calibration, an experienced well-trained technician delivered repetitive passive and rapid head rotations (head rotation: 10°–15°, peak velocity:150°/s–250°/s, duration 150–200 ms) in horizontal and vertical canals with minimum of 10 times in each plane. The device records head and eye velocity traces. VOR gain values were derived by calculating the ratio of the area under the eye velocity curve with respect to the area under the head velocity curve. To reduce artifacts, peak velocities were maintained above 150°/s, and trace oscillations during head movement, pseudo-saccades (blinks), head-motion artifacts, and other tracking errors were excluded from the analysis. Also, neurological saccadic eye movements may interfere with the interpretation of vHIT data. Therefore, if a patient was not cooperative enough to reliably undergo the test, the result was recorded as not applicable (17).
Abnormal VOR gain was defined as a lateral gain <0.8 for lateral canals and a posterior/anterior gain < 0.7 for anterior and posterior canals (5, 18). Gain asymmetry (GA) was evaluated in the plane of the lateral semicircular canal and calculated using a general formula analogous to the formula for caloric canal paresis:
where Gc is the vHIT gain for head impulses exciting the contralateral canal and Gi is the vHIT gain for head impulses exciting the ipsilateral canal (18). Corrective saccades were classified as covert or overt if they occurred before or after the end of the head movement, respectively (19). When comparing between VN and PCS patients, VOR gain was calculated based on the side of the lesion, specifically ipsilesional and contralesional. The definition of ipsilesional VOR gain in stroke patients was derived by considering the direction of the diminished VOR gain value.
Clinical characteristics and vHIT findings were compared in patients with PCS according to the presence of DBS. Also, VOR gains were compared in patients with DBS according to the caudal-to-rostral distribution (i.e., medulla, pons, and midbrain). Furthermore, we analyzed vHIT findings between PCS with acute dizziness and VN groups. Fisher’s exact test was used to analyze categorical variables, and Student’s t-test or the Mann–Whitney U-test was used to analyze continuous variables, as appropriate.
To evaluate the diagnostic performance of vHIT, we conducted receiver operating characteristic (ROC) analysis for VOR gain and VOR gain asymmetry to ascertain sensitivity and specificity, as well as to identify the optimal threshold values. The IBM SPSS Statistics software, version 21.0 (IBM Corp., Armonk, NY, United States) was used for all analyses. A value of p < 0.05 was considered statistically significant.
During the study period, a total of 232 patients with acute PCS were admitted to our center, and 80 (34.5%) patients satisfied the inclusion criteria (Figure 1). The mean age was 65 ± 13 years, 57 (71.3%) patients were men, and the median initial NIHSS score was 2 [interquartile range (IQR), 0–3; Table 1]. Among them, 40 (50.0%) patients had the symptom of acute dizziness. The most common lesion location was posterior inferior cerebellar arterial territory (35.8%), while multiple lesions were observed in 24 (30.0%) patients. All patients were confirmed as stroke with MRI conducted within 24 h following the symptom onset.
DBS was observed in 15 (18.8%) patients. The most common lesion location was the midbrain (n = 5; 33.3%), followed by the medulla oblongata (n = 4; 26.7%). Demographic and risk factors were not significantly different between patients with and without DBS except for hyperlipidemia (12 [80.0%] vs. 29 [44.6%]; p = 0.013) and the symptoms of acute dizziness (12 [80.0%] vs. 28 [43.1%]; p = 0.01), which were more common in patients with DBS.
Skew deviation was detected in 6 patients (7.5%), while extraocular movement limitation was observed in 3 patients (3.8%). Both findings were notably more prevalent in patients with dorsal brainstem (DBS) involvement (Table 2). None of the individuals displayed abnormal bedside head impulse test results with naked eyes. The mean horizontal VOR gain was 0.99 ± 0.12, with no significant distinction between patients with and without DBS (0.99 ± 0.11 vs. 1.00 ± 0.11, p = 0.930). Comparable mean VOR gains were evident across all tested canals.
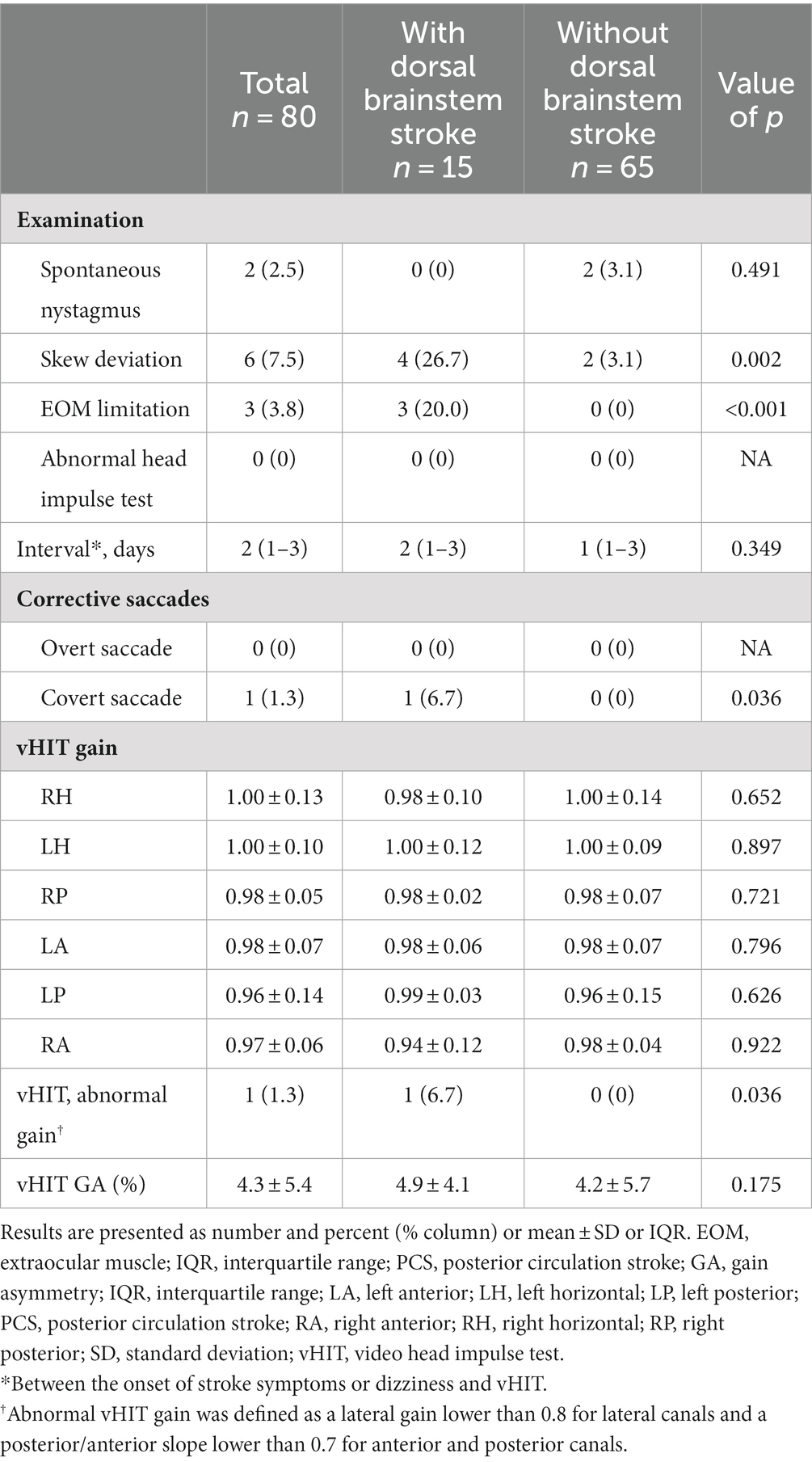
Table 2. Examination and vHIT findings in patients with PCS according to the presence of dorsal brainstem stroke.
Abnormal VOR gain (0.70) in the right horizontal canal was identified in only one patient who had lesions in the vicinity of the MLF. Remarkably, this patient also presented with dysarthria, right-sided weakness, ataxia, and dizziness (Supplementary Table S1). Among the remaining 14 patients, VOR gains were not significantly abnormal even in patients who exhibited clinical symptoms of diplopia or neurological signs related to eye movements (e.g., internuclear ophthalmoplegia) or those with lesions near the central vestibular pathway (e.g., medial vestibular nucleus, vestibular nucleus, nucleus prepositus hypoglossi, and MLF). Regarding pathologic corrective saccades, overt saccades were absent in all cases. However, one patient who exhibited an abnormal VOR gain of 0.70 in the right horizontal canal demonstrated covert saccades.
Finally, we evaluated whether VOR gains differed among patients with DBS according to the rostrocaudal axis of lesion distribution. Five patients had midbrain lesions, whereas six and four patients had pontine and medullary lesions, respectively. Three patients had multiple lesion locations [two patients with pontine and cerebellar (PICA) lesions; one patient with medullary and cerebellar (PICA) lesions]. We found that VOR gains were similar for the midbrain, pons, and medulla groups (Supplementary Table S2), showing that VOR gains were not significantly different throughout the rostrocaudal axis of lesion distribution.
We extended our investigation to assess the diagnostic efficacy of vHIT in differentiating dizziness between PCS and VN. This analysis included only PCS patients with dizziness symptoms (n = 40). We then compared the vHIT results of these individuals with those of VN patients (n = 29) who had been previously examined at our institution. Of the VN patients, 27 (93.1%) underwent MRI confirming the absence of acute stroke lesions, while the remaining two showed significant unilateral vestibular hypofunction confirmed by caloric testing, without other neurological deficits suggestive of stroke. Patients with PCS exhibited vascular risk factors more often than those with VN (Table 3).
Clinically, the VN group displayed a higher incidence of spontaneous nystagmus and abnormal head impulse test results, while the PCS group exhibited skew deviation and restricted extraocular muscle movement (Table 4). In both groups, vHITs were conducted within a median of 2 days after symptom onset. Mean ipsilesional VOR gains were significantly lower across the assessed canals in the VN group compared to the PCS group. Furthermore, the percentage of patients displaying vHIT results with abnormal gain and VOR gain asymmetry was significantly higher in the VN group.
Based on the ROC curves, VOR gain exhibited a sensitivity was 85.0% and specificity was 86.2% at cutoff values of 0.93, with an area under the curve (AUC) of 0.86 (95% CI 0.74–0.98, p < 0.001). Furthermore, VOR asymmetry displayed a sensitivity of 79.3% and specificity of 80.0% at a cutoff value of 6.1%, resulting in an AUC of 0.91 (95% CI 0.83–0.99, p < 0.001) (Figure 2).
In the present study, we prospectively investigated vHITs in patients with acute PCS within 24 h of symptom onset. VOR gains were not significantly affected in these patients, and they did not differ between the patients with and without DBS. Pathologic overt corrective saccades were absent in these patients, with only one individual exhibiting covert corrective saccades. Moreover, VOR gains as well as their asymmetry were effectively differentiated dizziness between PCS and VN. These findings underscore the potential value of vHIT as a diagnostic tool for acute dizziness.
Remarkably, when prospectively administered to acute PCS patients with mild symptoms, vHIT yielded infrequent significant abnormalities. Significant asymmetry and unidirectional corrective saccades are prominent in patients with VN (20, 21); thus, abnormal HIT findings have been suggested as an important sign to rule out central causes. However, the examination process per se may be technically demanding to non-experts, which decreases the clinical utility of this examination. Consequently, despite the high screening efficacy of the HINTs exam in excluding stroke, many emergency room physicians tend to place considerable reliance on costly imaging procedures like DWI (22). vHIT and its relevant application can serve as a valuable diagnostic approach, augmenting the objective measurement of VOR and supporting the HINTs exam (20). However, it has been untested prospectively whether vHIT findings are actually useful in patients with normal in terms of overt corrective saccades in patients with central causes of acute dizziness. In this regard, our results are notable and provide evidence for the concept of using vHIT as an ECG for the eyes in the emergency department. The clinical usefulness of vHIT will be further verified in the ongoing AVERT clinical trial (NCT02483429).
One patient with DBS presented pathologic covert corrective saccades and significantly decreased VOR gains. Lesions involving central VOR pathways may have affected the results in this patient. This finding is in line with several previous studies that showed unilaterally or bilaterally reduced VOR gains in patients with lesions involving the vestibular nucleus, nucleus prepositus hypoglossi, MLF, flocculus (8, 23), dorsolateral medulla, or cerebellar vermis (24). However, the degree of VOR decrease in patients with PCS was only modest in our case (0.70 for the right horizontal canal), as well as in the cited studies (0.88 ± 0.28) (24). Also, the degree of catch-up saccades in patients with stroke was much smaller than that of patients with VN (9, 25, 26). It should also be noted that the patient exhibited not only dizziness but also other stroke symptoms, such as dysarthria and right-sided weakness. Therefore, the diagnosis should be confirmed using other neurological examinations.
In terms of diagnostic performance, vHIT findings demonstrated remarkable success in distinguishing dizziness between VN and PCS. VOR gains and their asymmetry exhibited effectiveness in discerning the etiology of acute dizziness. The performance is comparable to or even surpasses results reported in previous studies (27, 28). These findings imply that VOR gains during vHIT and related parameters may offer valuable diagnostic insights into acute dizziness differentiation. These discoveries warrant validation in subsequent research and clinical application.
The rarity of pathologic vHIT findings even in patients with DBS with MLF lesions warrants further discussion. First, because the central VOR pathway ascends and spreads out bilaterally in the MLF from the medial vestibular nucleus (29), a focal lesion in this pathway may not materially affect the overall VOR process. Given the similar degrees of VOR gains for stroke lesions along the rostrocaudal axis of the brainstem, spreading of the VOR pathway may occur immediately after the vestibular nucleus and may change not substantially thereafter. As discussed above, patients with a considerable MLF lesion may present abnormal findings; however, the degree of vHIT abnormalities in these patients appeared to be modest. Second, vHIT may not be sensitive enough to detect subtle VOR changes in patients with stroke. Compared to search coils, vHIT is susceptible to recording artifacts and is less sensitive (30, 31). In the present study, if we had examined patients using magnetic search coils, abnormal VOR findings may have been detected. However, for a screening tool, being appropriately sensitive may be more helpful to distinguish between central and peripheral causes of acute dizziness, rather than being too sensitive. Moreover, patient discomfort associated with magnetic search coils presents another drawback in their role as a screening tool.
The strength of this study is its prospective design to systemically evaluate patients with PCS regardless of acute dizziness and the inclusion of supra/infratentorial ischemic lesions, such as those in the thalamus and midbrain. Most previous studies have focused on patients with acute dizziness and infratentorial lesions (32); however, dizziness is a vague symptom in patients with acute stroke and may even be present in those with supratentorial lesions (33, 34). Accordingly, several patients with supratentorial lesions [temporo-occipital area (n = 5, 16.1%), thalamus (n = 2, 6.5%), and midbrain (n = 2, 6.5%)] in the present study also had acute dizziness. For using vHIT as a screening tool in patients with acute vestibular syndrome, vHIT data must be collected from both stroke patients with infratentorial lesions and those with supratentorial lesions. Therefore, our results increase the applicability of vHIT for ruling out stroke in clinical practice.
Several limitations should be noted. First, we only included stroke patients with mild neurologic deficits (NIHSS score ≤ 7) for safety reasons, which may have led to the underestimation of the degree of VOR impairment in patients with stroke. However, patients who may mainly benefit from vHIT in the emergency department are those with mild neurologic deficits (e.g., only dizziness). Second, the interval between symptom onset to vHIT was a median of 2 days, and this delay may have affected the results reflecting acute situations in the emergency department (24). This was unavoidable as we tried to include all patients with PCS regardless of acute dizziness, as numerous patients with acute stroke require stabilization to safely undergo vHIT. Thus, we cannot negate the possibility of early abnormal vHIT findings that had normalized prior to vHIT. Moreover, half of the patients with acute strokes visit the emergency department after 24 h of symptom onset (35), and these patients are less likely to have severe strokes as they were brought to the emergency department. Considering that vHIT may be particularly useful in patients with mild stroke, our results still merit attention and should be considered clinically meaningful. Third, not all consecutive patients treated at our center were included for various reasons (Figure 1), which could have led to selection bias. Thus, our results should be interpreted with caution. Fourth, this study was performed at a single center, which limits the generalizability of its results. Specifically, the sample size for assessing lesion distribution in terms of VOR gains may not have been sufficient for conducting a statistically meaningful analysis. Finally, it should also be noted that the VN control group was not recruited in a prospective manner. Consequently, two out of 29 (6.9%) patients with VN did not undergo MRI to rule out stroke diagnosis, and the VN and PCS groups utilized different vHIT devices, despite adhering to the same protocol within the same institution.
Nevertheless, we found that vHITs in patients with acute PCS rarely show abnormal findings even in those with DBS. Importantly, we did not find pathologic overt corrective saccades in any studied patient with acute PCS; only one patient with DBS showed abnormal vHIT findings, such as covert corrective saccades and decreased VOR gains with a modest degree of abnormalities. Moreover, additional vHIT variables, such as VOR gains and their asymmetry, effectively distinguished dizziness between PCS and VN patients (Figure 2). These findings support the concept of using vHIT as a screening tool to differentiate between central and peripheral causes of acute dizziness in clinical settings.
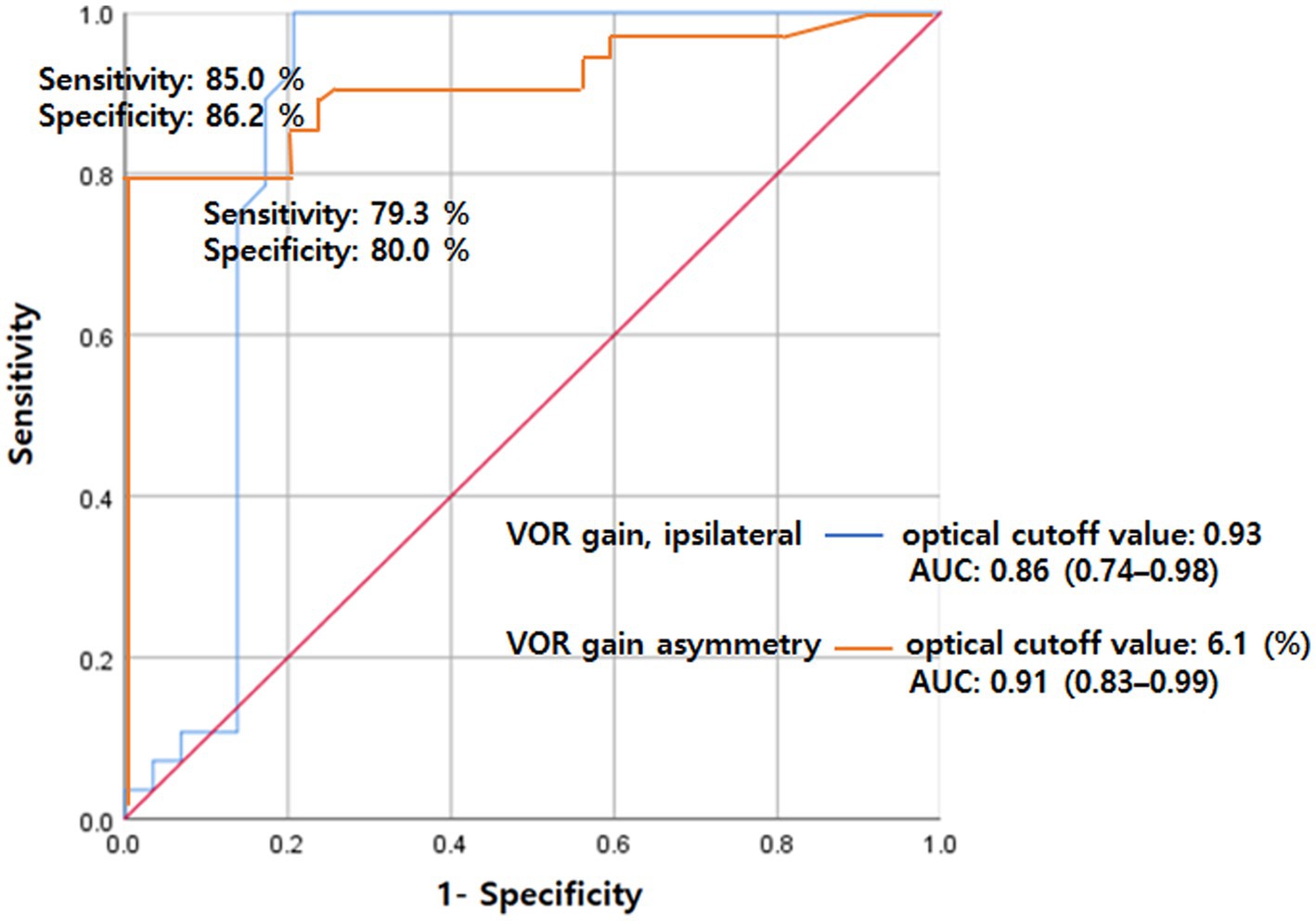
Figure 2. ROC analysis for VOR gain (ipsilateral) and gain asymmetry for separating VN from PCS. The sensitivity was 85.0% and specificity was 86.2% at cutoff values of 0.93 for VOR gain with an AUC of 0.86 (95% CI 0.74–0.98). Also, the sensitivity was 79.3% and specificity was 80.0% at a cutoff value of 6.1 for VOR asymmetry with an AUC of 0.91 (95% CI 0.83–0.99). AUC, area under the curve; PCS, posterior circulation stroke; ROC, receiver operating characteristics; VN, vestibular neuritis; VOR, vestibulo-ocular reflex.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
SH: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft. DL: Methodology, Writing – review & editing. GP: Data curation, Writing – review & editing. BK: Investigation, Methodology, Writing – review & editing. JC: Investigation, Methodology, Writing – review & editing. D-WK: Investigation, Methodology, Writing – review & editing. SK: Investigation, Methodology, Writing – review & editing. JK: Investigation, Methodology, Writing – review & editing. HP: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. E-JL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Ministry of Health and Welfare, Republic of Korea (grant number: HR18C0016).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1256826/full#supplementary-material
1. Newman-Toker, DE, Saber Tehrani, AS, Mantokoudis, G, Pula, JH, Guede, CI, Kerber, KA, et al. Quantitative video-oculography to help diagnose stroke in acute vertigo and dizziness: toward an ECG for the eyes. Stroke. (2013) 44:1158–61. doi: 10.1161/STROKEAHA.111.000033
3. Kattah, JC, Talkad, AV, Wang, DZ, Hsieh, YH, and Newman-Toker, DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. (2009) 40:3504–10. doi: 10.1161/STROKEAHA.109.551234
4. Tarnutzer, AA, Berkowitz, AL, Robinson, KA, Hsieh, Y-H, and Newman-Toker, DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ Open. (2011) 183:E571–92. doi: 10.1503/cmaj.100174
5. Machner, B, Erber, K, Choi, JH, Sprenger, A, Helmchen, C, and Trillenberg, P. A simple gain-based evaluation of the video head impulse test reliably detects Normal Vestibulo-ocular reflex indicative of stroke in patients with acute vestibular syndrome. Front Neurol. (2021) 12:741859. doi: 10.3389/fneur.2021.741859
6. Black, RA, Halmagyi, GM, Thurtell, MJ, Todd, MJ, and Curthoys, IS. The active head-impulse test in unilateral peripheral Vestibulopathy. Arch Neurol. (2005) 62:290–3. doi: 10.1001/archneur.62.2.290
7. van Esch, BF, Nobel-Hoff, GE, van Benthem, PP, van der Zaag-Loonen, HJ, and Bruintjes, TD. Determining vestibular hypofunction: start with the video-head impulse test. Eur Arch Otorhinolaryngol. (2016) 273:3733–9. doi: 10.1007/s00405-016-4055-9
8. Choi, JY, Kim, HJ, and Kim, JS. Recent advances in head impulse test findings in central vestibular disorders. Neurology. (2018) 90:602–12. doi: 10.1212/WNL.0000000000005206
9. Nam, GS, Shin, HJ, Kang, JJ, Lee, NR, and Oh, SY. Clinical implication of corrective saccades in the video head impulse test for the diagnosis of posterior inferior cerebellar artery infarction. Front Neurol. (2021) 12:605040. doi: 10.3389/fneur.2021.605040
10. Chen, L, Todd, M, Halmagyi, GM, and Aw, S. Head impulse gain and saccade analysis in pontine-cerebellar stroke and vestibular neuritis. Neurology. (2014) 83:1513–22. doi: 10.1212/WNL.0000000000000906
11. Liu, YL, Wu, ZQ, Qu, JF, Qiu, DH, Luo, GP, Yin, HP, et al. High neutrophil-to-lymphocyte ratio is a predictor of poor short-term outcome in patients with mild acute ischemic stroke receiving intravenous thrombolysis. Brain Behav. (2020) 10:e01857. doi: 10.1002/brb3.1857
12. Lee, EJ, Nah, HW, Kwon, JY, Kang, DW, Kwon, SU, and Kim, JS. Ischemic stroke in patients with cancer: is it different from usual strokes? Int J Stroke. (2014) 9:406–12. doi: 10.1111/ijs.12124
13. Yoo, MH, Yang, CJ, Kim, SA, Park, MJ, Ahn, JH, Chung, JW, et al. Efficacy of steroid therapy based on symptomatic and functional improvement in patients with vestibular neuritis: a prospective randomized controlled trial. Eur Arch Otorhinolaryngol. (2017) 274:2443–51. doi: 10.1007/s00405-017-4556-1
14. Karatas, M. Central vertigo and dizziness: epidemiology, differential diagnosis, and common causes. Neurologist. (2008) 14:355–64. doi: 10.1097/NRL.0b013e31817533a3
15. Kim, BJ, and Kim, JS. Ischemic stroke subtype classification: an asian viewpoint. J Stroke. (2014) 16:8–17. doi: 10.5853/jos.2014.16.1.8
16. Lee, SU, Park, SH, Park, JJ, Kim, HJ, Han, MK, Bae, HJ, et al. Dorsal medullary infarction: distinct syndrome of isolated central Vestibulopathy. Stroke. (2015) 46:3081–7. doi: 10.1161/STROKEAHA.115.010972
17. Yoo, MH, Kim, SH, Lee, JY, Yang, CJ, Lee, HS, and Park, HJ. Results of video head impulse and caloric tests in 36 patients with vestibular migraine and 23 patients with vestibular neuritis: a preliminary report. Clin Otolaryngol. (2015) 41:813–7. doi: 10.1111/coa.12556
18. Calic, Z, Nham, B, Bradshaw, AP, Young, AS, Bhaskar, S, D’Souza, M, et al. Separating posterior-circulation stroke from vestibular neuritis with quantitative vestibular testing. Clin Neurophysiol. (2020) 131:2047–55. doi: 10.1016/j.clinph.2020.04.173
19. Yang, CJ, Cha, EH, Park, JW, Kang, BC, Yoo, MH, Kang, WS, et al. Diagnostic value of gains and corrective saccades in video head impulse test in vestibular neuritis. Otolaryngol Head Neck Surg. (2018) 159:347–53. doi: 10.1177/0194599818768218
20. Kattah, JC. Use of HINTS in the acute vestibular syndrome. An overview. Stroke Vasc Neurol. (2018) 3:190–6. doi: 10.1136/svn-2018-000160
21. Newman-Toker, DE, Kattah, JC, Alvernia, JE, and Wang, DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology. (2008) 70:2378–85. doi: 10.1212/01.wnl.0000314685.01433.0d
22. Quimby, AE, Kwok, ESH, Lelli, D, Johns, P, and Tse, D. Usage of the HINTS exam and neuroimaging in the assessment of peripheral vertigo in the emergency department. J Otolaryngol Head Neck Surg. (2018) 47:54. doi: 10.1186/s40463-018-0305-8
23. Kim, SH, Kim, HJ, and Kim, JS. Isolated vestibular syndromes due to brainstem and cerebellar lesions. J Neurol. (2017) 264:63–9. doi: 10.1007/s00415-017-8455-6
24. Thomas, JO, Sharobeam, A, Venkat, A, Blair, C, Ozalp, N, Calic, Z, et al. Video head impulse testing to differentiate vestibular neuritis from posterior circulation stroke in the emergency department: a prospective observational study. BMJ Neurol Open. (2022) 4:e000284. doi: 10.1136/bmjno-2022-000284
25. Park, HK, Kim, JS, Strupp, M, and Zee, DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol. (2013) 260:1576–82. doi: 10.1007/s00415-013-6837-y
26. Machner, B, Erber, K, Choi, JH, Trillenberg, P, Sprenger, A, and Helmchen, C. Usability of the head impulse test in routine clinical practice in the emergency department to differentiate vestibular neuritis from stroke. Eur J Neurol. (2021) 28:1737–44. doi: 10.1111/ene.14707
27. Kim, SH, Lee, SU, Cho, BH, Cho, KH, Yu, SW, Kim, BJ, et al. Analyese of head-Impluse test in patients with posterior circulation stroke and vestibular neuritis. Neurology. (2023) 100:e2374–85. doi: 10.1212/WNL.0000000000207299
28. Guler, A, Karbek Akarca, F, Eraslan, C, Tarhan, C, Bilgen, C, Kirazli, T, et al. Clinical and video head impulse test in the diagnosis of posterior circulation stroke presenting as acute vestibular syndrome in the emergency department. J Vestib Res. (2017) 27:233–42. doi: 10.3233/VES-170620
29. Ki-Bum Sung, T-KL. Central mechanisms of the Vestibulo-ocular reflex (VOR). Korean Bal Soc. (2002) 1:55–66.
30. Mantokoudis, G, Saber Tehrani, AS, Wozniak, A, Eibenberger, K, Kattah, JC, Guede, CI, et al. Impact of artifacts on VOR gain measures by video-oculography in the acute vestibular syndrome. J Vestib Res. (2016) 26:375–85. doi: 10.3233/VES-160587
31. Macdougall, HG, McGarvie, LA, Halmagyi, GM, Curthoys, IS, and Weber, KP. The video head impulse test (vHIT) detects vertical semicircular canal dysfunction. PLoS One. (2013) 8:e61488. doi: 10.1371/journal.pone.0061488
32. Oh, SY, Kim, JS, Lee, JM, Shin, BS, Hwang, SB, Kwak, KC, et al. Ocular vestibular evoked myogenic potentials induced by air-conducted sound in patients with acute brainstem lesions. Clin Neurophysiol. (2013) 124:770–8. doi: 10.1016/j.clinph.2012.09.026
33. Wijesinghe, R, Protti, DA, and Camp, AJ. Vestibular interactions in the thalamus. Front Neural Circuits. (2015) 9:79. doi: 10.3389/fncir.2015.00079
34. Lee, E-J, Kim, H-J, and Kim, J-S. Ocular, vestibular, and Otologic syndromes In: JS Kim, editor. Posterior circulation stroke: Advances in understanding and management. Singapore: Springer Singapore (2021). 101–19.
Keywords: corrective saccades, dorsal brainstem stroke, posterior circulation stroke, vestibular neuritis, vestibulo-ocular reflex, video head impulse test
Citation: Ha SH, Lee DK, Park GY, Kim BJ, Chang JY, Kang D-W, Kwon SU, Kim JS, Park HJ and Lee E-J (2023) Prospective analysis of video head impulse tests in patients with acute posterior circulation stroke. Front. Neurol. 14:1256826. doi: 10.3389/fneur.2023.1256826
Received: 12 July 2023; Accepted: 05 September 2023;
Published: 22 September 2023.
Edited by:
Sun-Young Oh, Jeonbuk National University, Republic of KoreaReviewed by:
Niraj Kumar Singh, All India Institute of Speech and Hearing (AIISH), IndiaCopyright © 2023 Ha, Lee, Park, Kim, Chang, Kang, Kwon, Kim, Park and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Ju Park, ZHpuZXNzQGFtYy5zZW91bC5rcg==; Eun-Jae Lee, ZXVuamFlLmxlZUBhbWMuc2VvdWwua3I=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.