
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 11 October 2023
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1253915
 Jian Wang1*†
Jian Wang1*† Anbang Zhang2†
Anbang Zhang2† Boya Wang3†
Boya Wang3† Jingmeng Yuan1
Jingmeng Yuan1 Junchi Zhu1
Junchi Zhu1 Mengjiao Li1
Mengjiao Li1 Henli Liu1
Henli Liu1 Lijuan Cheng1
Lijuan Cheng1 Ping Kong1
Ping Kong1Ossified intracranial meningiomas (OIM) and ossified spinal meningiomas (OSM) are rare neoplasms of mesenchymal origin that predominantly manifest in the spinal cord and infrequently in the cranial region, accounting for ~0. 7–5.5% of all meningiomas. It is extremely rare to have multiple intracranial and spinal lesions accompanied by ossification. Herein, we report this rare case for the first time. A 34-year-old woman presented with paresthesia and limb weakness in the right lower limb and gradually worsened. Approximately half a year later, she could only walk with crutches. Magnetic resonance imaging of the brain and spinal cord showed multiple meningiomas, and histopathological examination confirmed multiple OIM and OSM (WHO grade 1). Multiple OIM and OSM are extremely rare with diverse imaging features, and it is easily confused with other tumors. Histopathological examination is the final diagnostic method.
Multiple intracranial and spinal meningiomatosis refer to the simultaneous or sequential occurrence of meningiomas in two or more locations, approximately accounting for 1–10% of all meningiomas (1). Metaplastic meningiomas are a subtype of meningiomas characterized by focal mesenchymal differentiation with osseous, cartilaginous, lipomatous, myxoid, or xanthomatous elements (2). Ossified intracranial meningiomas (OIM) and ossified spinal meningiomas (OSM) are rare subtypes of metaplastic meningiomas characterized by diverse clinical symptoms and slow growth. Its imaging manifestations are easily confused with other tumors. There are only dozens of OIM and OSM case reports worldwide, whereas multiple intracranial and spinal cord lesions accompanied by ossification are extremely rare (3), and no relevant literature has been found. Herein, we have reported the first case of concurrent occurrence of multiple OSM and OIM, along with a comprehensive review of the literature. The case reports were conducted strictly in accordance with the CARE guidelines (4).
We present a case of a 34-year-old woman who complained of paresthesia in the right lower extremity with limb weakness. She described that, initially, she felt paresthesia in her right lower limb, manifested as abnormal sensation and decreased tactile perception on the right plantar. After 1 month, there was no sensation on the right plantar landing, accompanied by numbness and weakness of the right lower limb and ataxia. After half a year, she had to grasp the bed rail with both hands or get support from family members to get up, and she felt weak and unable to support his waist and back while bending over to tie shoelaces. In addition, she had to make repeated attempts to put her right foot into the shoe and had obvious dragging when walking. The patient had been admitted to external hospitals and was diagnosed with lumbar spine disease.
The patient was admitted to our department 73 days later due to the progressive deterioration of symptoms that significantly impacted their activities of daily living. Nervous system physical examination showed that the right lower limb proximal muscle strength was grade IV and the distal muscle strength was grade III, the heel knee tibial test of the right lower limb was unstable, and the deep and shallow sensation of the right lower limb was decreased. In addition, Romberg and Babinski signs were positive, the left biceps and triceps brachii demonstrated active reflex, the bilateral knee and ankle showed hyperactivity, and the bilateral patellar and ankle clonus were positive. Moreover, brain magnetic resonance imaging (MRI) showed multiple round lesions in the left frontoparietal lobe and cerebral longitudinal fissure, accompanied by bone destruction, and the lesion was uniformly enhanced during enhancement (Figure 1). The MRI of the spinal cord showed multiple round and irregular lesions in the cervical and thoracic spinal cord with bone destruction, uniformly enhanced during enhancement (Figure 2). Considering the intricate nature and inherent perils associated with surgical intervention, the patient and his family have expressed their desire for a transfer to an alternative medical facility. According to the patient's recollection, he underwent a partial resection of a meningioma at another hospital, encompassing both intracranial and cervicothoracic spinal regions. The postoperative imaging revealed partial excision of the intracranial meningiomas, resulting in significant alleviation of brain tissue compression and remarkable improvement in cervicothoracic spinal cord compression (Figures 1, 2 illustrate the given information). Histopathological examination of the lesion in the left parietal and cervicothoracic spinal cord suggested OIM and OSM (WHO grade 1). Immunohistochemical examination of the lesion showed the EMA (+), PR (+), CD34 (–), GFAP (–), SSTR2 (+), S100 (+), and Ki67 (~3%) (Figure 3). According to the patient's account, paresthesia and weakness in their right lower limb were entirely alleviated through an intensive 3-month rehabilitation program subsequent to their release.
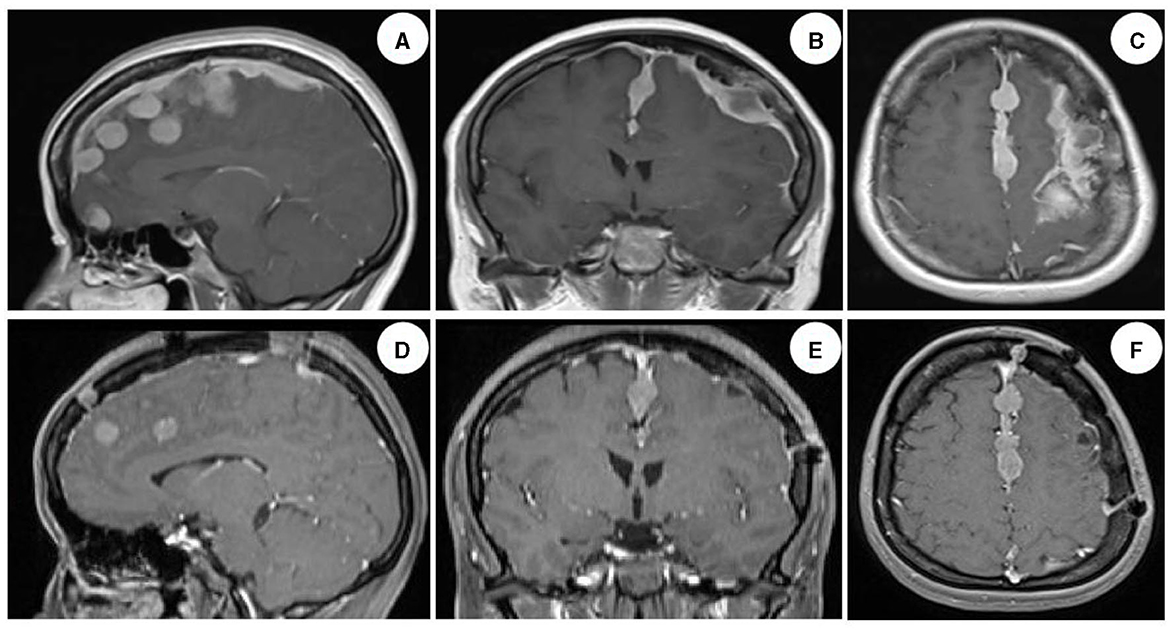
Figure 1. Brain MRI images of the patient. Enhanced images in sagittal, coronal, and transverse views of the brain demonstrated multiple round-like and irregular uniformly enhanced lesions in the left frontoparietal lobe and cerebral longitudinal fissure, with compression and deformation of the brain tissue, deviation of the brain midline, and dural adhesion, accompanied by obvious bone destruction, and no obvious edema around the lesions [(A–C) illustrate the given information]. The majority of meningiomas exhibiting evident space-occupying effects were successfully excised, leading to a significant alleviation of brain tissue compression. The restoration of brain midline symmetry was essentially achieved; however, a complete resection of the tumor along the intracranial midline was not accomplished. This observation was confirmed by postoperative imaging data obtained after 6 months [(D–F) illustrate the given information].
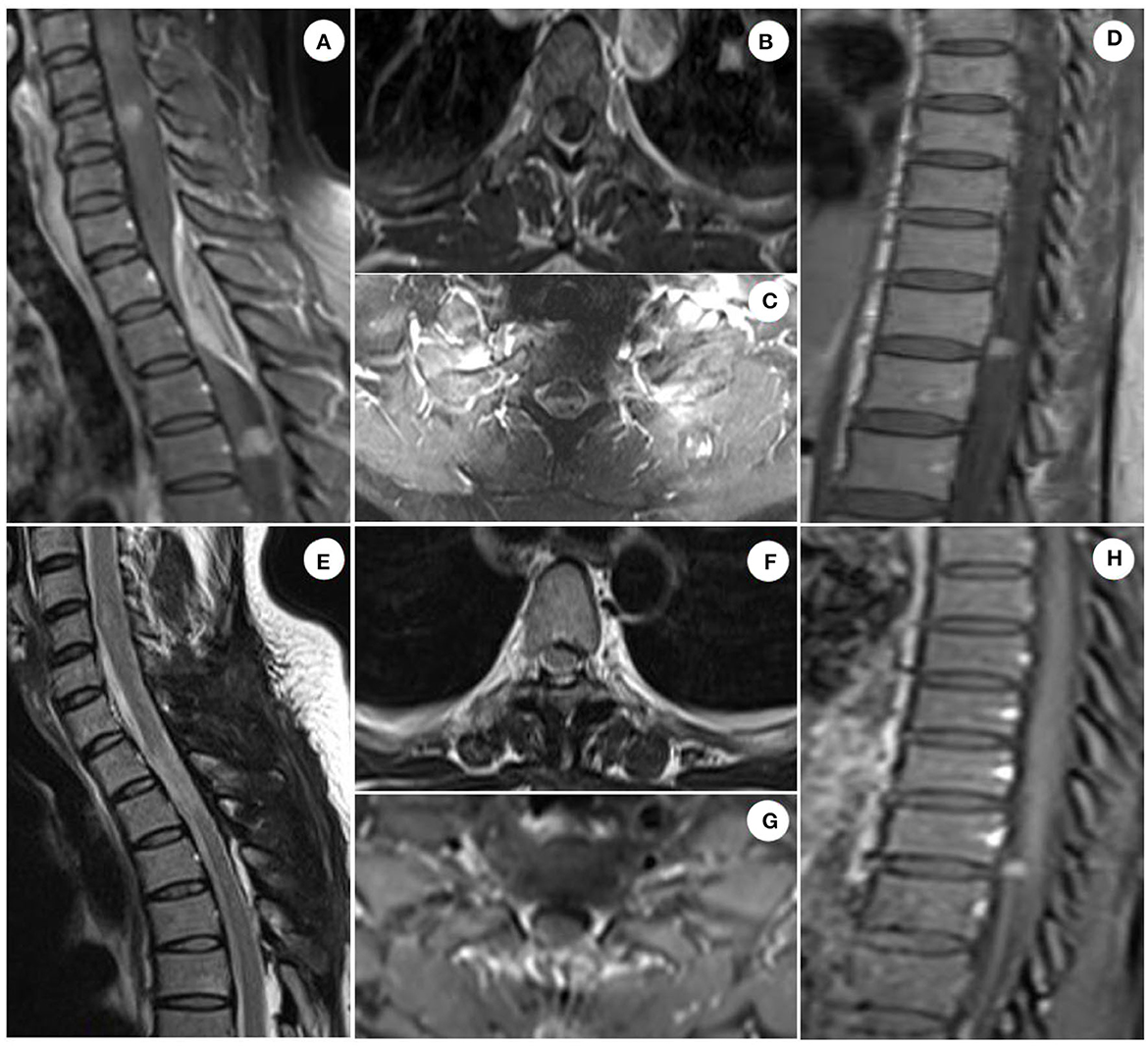
Figure 2. Spinal cord MRI images of patient. Sagittal enhanced T1 scan of the cervical and thoracic spinal cord showed multiple circular and irregular homogeneous enhanced lesions in the spinal cord, and spindle-shaped homogeneous enhanced lesions in the epidural space, with obvious compression and deformation of the spinal cord, accompanied by bone destruction. Axial enhanced T1 scan of the spinal cord demonstrated homogeneous enhancement of the lesion, lower signal intensity in the center of the tumor than that in the periphery, compression, and deformation of the spinal cord, and spinal dural adhesion [(A–D) illustrate the given information]. The cervicothoracic spinal cord meningiomas with evident space-occupying effect were successfully excised, leading to significant alleviation of compression on the spinal cord tissue. However, a complete resection of the tumors in the upper cervical and lower thoracic spinal cord was not achievable. This observation was confirmed by postoperative imaging data obtained 6 months later [(E–H) illustrate the given information].
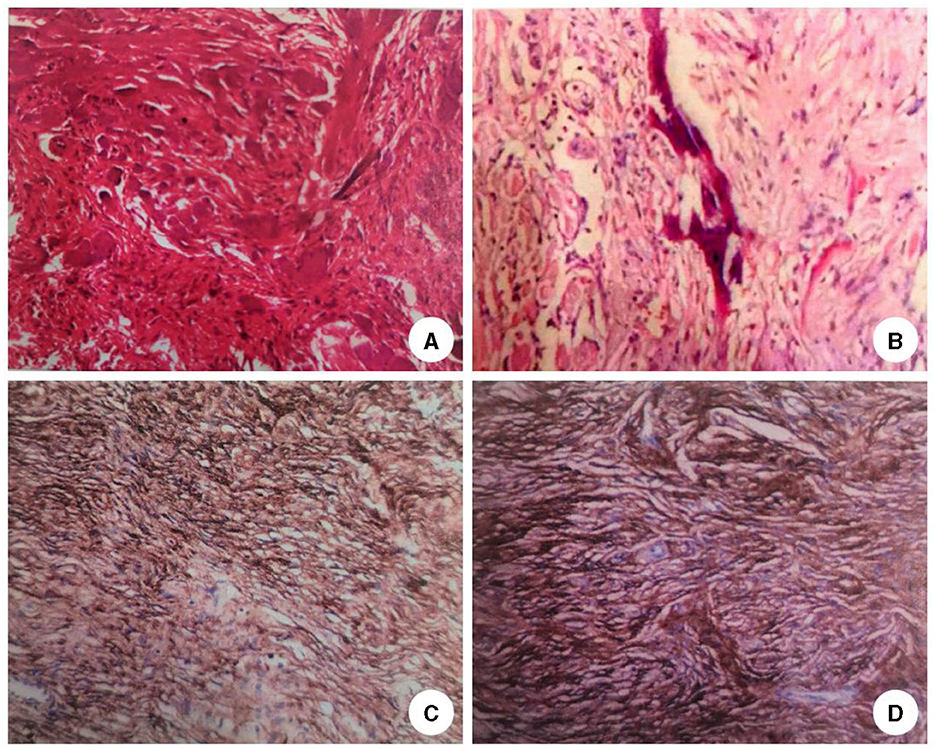
Figure 3. Histopathological results of the lesions. HE staining of the lesion tissue in the left parietal lobe (A) and cervicothoracic spinal cord (B) showed psammoma bodies around the tumor cells and mature bone tissue formation around the tumor cells (H&E stain, original magnification × 40). Immunohistochemical results of the lesions in the left parietal lobe (C) and cervicothoracic spinal cord (D) showed that the neoplastic meningothelial cells are immunoreactive for epithelial membrane antigen (EMA) (×10), progesterone receptor (PR) (×10), somatostatin receptor 2 (SSTR2)(×10), and Ki67 ~5%.
Meningioma is a type of primary central nervous system tumor originating from arachnoid cap cells, accounting for ~25–45% of intracranial tumors, with an incidence rate of 4.7–7.5/100,000 and male/female ratio of 1:2–3.5. In total, 80% of them are benign, sporadic, and solitary (1, 5). Multiple meningiomas, first described by Anfimov and Blumenau in 1889 (6), were defined as the presence of two or more unconnected tumors in the intracranial and extracranial areas without other causes by Cushing and Eisenhardt in 1938, accounting for ~1–10% of all meningiomas. Most of the multiple meningiomas are located in the cranial cavity, whereas few are in the spinal cord (1, 5).
Most meningiomas are sporadic, and familial cases of meningiomas are rare (7). The most common genetic alteration observed in sporadic meningiomas is the deletion of chromosome 22 either in its entirety or distally (7–9). Tumor susceptibility to sporadic meningiomas often arises from heterozygous mutations occurring in the SMARCE1 gene located on chromosome 17q21 (10, 11). Furthermore, heterozygous mutations in SUFU gene (12, 13) on chromosome 10q24 and PDGFB gene (14, 15) on chromosome 22q have also been reported to be associated with the development of meningiomas.
Metaplastic meningioma is a kind of benign tumor originating from arachnoid epithelial cells, which can differentiate into mesenchymal tissues including the bone, cartilage, smooth muscle, and adipose tissues alone or in combination. Most of them grow slowly with a pathological grade of WHO 1, and their clinical symptoms mainly depend on the location of the tumor (16, 17). There is a clear difference between meningiomas ossification and calcification. Calcification is more of an imaging description than a histopathological diagnosis, whereas ossification is a subtype of metaplastic meningiomas characterized by a histopathologic expression of mesenchymal components (18, 19).
OIM and OSM are classified as an uncommon subtype of metaplastic meningiomas, accounting for ~0.7–5.5% of all spinal meningiomas (20). Currently, the mechanisms of OIM and OSM are far from clear. A hypothesis indicated that ossification is caused by the repeated accumulation of hydroxyapatite crystals in the psammoma bodies (21), which has been negated by some reports (22). Most researchers prefer to believe that ossification is secondary to the metaplasia of arachnoid and interstitial cells, which induces a synergistic effect of osteoblasts, fibroblasts, and angiogenic components in bone tissue formation (23–26). Therefore, the theory of mesenchymal differentiation of metaplastic meningioma cells has been proposed (3, 27). The association of the extra-axial mass with dural and osseous reactions, as well as a massive calcified component, may suggest an ossified meningioma. The differential diagnosis may include benign bone processes, such as osteoid osteomas, aneurysmal bone cysts, and fibrous dysplasia, and malignant processes, such as osteogenic sarcomas, chondrosarcoma, and metastatic disease (28, 29).
The expression of epithelial membrane antigen (EMA) and soluble protein-100(S-100) can vary among different meningiomas, which is a well-known phenomenon. However, due to their limited sensitivity and specificity, the combined use of EMA and S-100 is often employed to enhance diagnostic accuracy (30). Progesterone receptor (PR) (31, 32) serves as a highly specific marker for meningiomas. It exhibits high expression in benign meningiomas and low expression in malignant ones. Somatostatin receptor 2 (SSTR2) (33, 34) is currently regarded as the most specific and sensitive biomarker for meningiomas. SSTR2 can be detected in all grades of meningiomas, with high expression observed in benign cases and low expression observed in malignant cases. Studies have shown (34) that the monoclonal antibody for SSTR2a is a highly sensitive and specific marker for meningiomas. SSTR2a is expressed in cases that do not express EMA or PR and that are often considered in the differential diagnosis of meningiomas, including schwannomas, cellular schwannomas, malignant peripheral nerve sheath tumors, and hemangiopericytomas/solitary fibrous tumors. Thus, SSTR2a immunohistochemistry can be useful in establishing the diagnosis of meningiomas, including high-grade meningiomas with poor differentiation. Additionally, Ki-67 (35) is frequently utilized for the evaluation of meningiomas proliferation trend. The expression level of Ki67 demonstrates a positive correlation with the pathological grade, growth rate, peritumoral edema, and recurrence rate of meningiomas. As the size of the meningiomas increases, so does its expression rate; conversely, cases with slower tumor growth and lighter peritumoral edema exhibit lower expression rates. Moreover (36), studies have revealed that patients with recurrent meningiomas exhibit significantly elevated levels of Ki-67 expression compared to those without recurrence, reaching a critical threshold at ~10%.
Numerous evidence demonstrated that most OIM or OSM grow very slowly and are asymptomatic, whereas OIM or OSM occurring in the spinal canal may show clinical symptoms in the early stage due to the narrow space of the spinal canal (2, 37). Compared with ordinary meningiomas, metaplastic meningiomas adhere more heavily to the dura mater or arachnoid membrane, resulting in more difficulty of operation (38, 39).
Tumor resection remains the primary treatment modality for meningiomas (40). However, the surgical management of OIM and OSM is relatively intricate due to extensive tumor adhesion to surrounding brain structures and issues with dural attachment. Additionally, postoperative cerebrospinal fluid leakage and tumor recurrence pose significant challenges to surgical operations, greatly impacting patients' quality of life (40, 41). Therefore, the current recommendation advocates for an individualized approach focusing on achieving maximum and safe resection (40, 42). Studies have indicated that stereotactic radiosurgery (SRS) appears to be a reliable and effective treatment option for recurrent meningiomas and deep-seated lesions where traditional neurosurgical methods are inadequate or ineffective (43, 44). SRS has been clinically applied in various primary and secondary tumors as well as single or multiple meningiomas. With sub-millimeter accuracy, SRS can optimize dose exposure on the target volume compared to conventional radiotherapy techniques while minimizing damage to surrounding critical structures. These characteristics make SRS not only a potential adjunctive therapy but also a valuable alternative in certain cases due to its clinical efficacy and extremely low rate of side effects (45).
The meticulous management of meningiomas is currently under deliberation. The International Consortium on Meningiomas (ICOM) (40) provides several fundamental recommendations. First, although the last decade has witnessed advancements in our understanding of the biology and genomic landscape of meningiomas, further developments are necessary and critical for improving care for patients. Identification of molecular alterations driving the aggressive meningioma phenotype will be critical to advance care for patients and should be done in parallel with the development of reliable preclinical models that allow for rapid translation of discovery to clinical trials. Collaboration with the World Health Organization is needed to advocate for the integration of key molecular alterations that refine standard-of-care classifications to allow for more individualized diagnosis and prognostication such that management and decision-making can be tailored to the patient. In addition to this, standardized core outcomes and definitions that evaluate intervention complication rates, tumor recurrence, seizures, cognitive function, and health-related quality of life are needed to unify language and facilitate the assessment of key metrics in meningiomas. Although most meningiomas requiring treatment will be managed primarily with surgery, particularly challenging cases will likely benefit from review by a multidisciplinary team that can offer the spectrum of various treatment options in meningiomas, including ongoing investigational clinical trials. Lastly, since a subset of patients with meningiomas can have continued impairments that extend beyond the treatment of their tumors, centers of excellence that are able to address the complex needs of these patients in a longitudinal fashion will be key to addressing the unmet needs of this growing population of patients.
However, inspiring, large-scale genomic profiling of meningiomas has uncovered possible driver mutations for a subset of tumors. Several clinical trials are currently underway to evaluate the efficacy of SMO, AKT1, FAK, and mTOR inhibitors in patients with residual, recurrent, or progressive meningiomas (46–48). Furthermore, traditional chemotherapies such as trabectedin are also now being investigated in Phase II trials for use in recurrent higher-grade meningiomas. The increased attention and momentum driving advances in clinical trials in meningiomas are promising and should continue to be a focus of future efforts (40).
In this case, the responsible lesion for clinical symptoms was mainly located in the mass effect of the cervicothoracic spinal cord tumor. Due to its slow growth, the patient presented slowly progressive limb weakness and numbness and was repeatedly diagnosed as “lumbar disease” in other hospitals. A previous study showed that OIM or OSM show are more common in women, and most of the pathological grades are WHO grade 1 (49). Our current study reported a female case pathologically confirmed as OIM and OSM (WHO grade 1), which was consistent with the conclusions of previous studies. The immunohistochemical analysis demonstrated positive expression for EMA, S100, PR, and SSTR2 markers in this patient's sample. Additionally, a low proliferation rate with only 3% Ki67 positivity confirmed the diagnosis as an ossifying meningioma classified as WHO grade 1—indicating its benign nature. Moreover, PR expression predominantly correlated with progesterone levels while maintaining a Ki67 positivity rate below 10%. Notably absent were any signs of significant edema surrounding both intracranial and spinal meningiomas. Considering these imaging and immunohistochemical findings collectively suggests that this patient carries a relatively minimal risk for future recurrence.
The patient was a female with subacute onset, slow progression, and no clinical symptoms at the early stage. With the progression of the disease, the tumor gradually compressed the spinal cord nerves, resulting in paresthesia, limb weakness, and numbness. Histopathological biopsy finally confirmed multiple OIM and OSM (WHO grade 1). According to the latest literature, no more than 50 cases of OIM and OSM have been reported worldwide, and most of them are solitary in the spinal cord (Table 1). It is the first report of multiple OIM and OSM in the spinal cord and cranial cavity. Lastly, it is clear that patients with meningiomas can be affected by both the disease and their treatments, and some have long-lasting effects, resulting in chronic quality-of-life impairments that compound the challenges mentioned above. Consequently, regular brain and spinal cord MRI evaluations have been scheduled annually to closely monitor any potential resurgence.
OIM or OSM is a subtype of metaplastic meningiomas that is extremely rare in clinics, and it is more common in women. Most patients with meningiomas grow slowly, and tumors growing in the spinal canal usually have early clinical symptoms. Its clinical symptoms are mainly associated with the location of the tumor, and the imaging manifestations are complex and diverse. The final diagnosis depends on histopathological examination. Due to a few reports of OIM or OSM and most of them are individual cases, there is no large sample of clinical randomized controlled study data. Therefore, the specific mechanism of the occurrence and evolution of OIM and OSM is far from clear.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Affiliated Aerospace Hospital of Zunyi Medical University. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JW, AZ, and BW treated the patient, reviewed the literature, designed the study, and drafted the manuscript. JY, JZ, ML, HL, LC, and PK contributed to the design and implementation of the research. All authors have read, revised, and approved the final manuscript.
This study was supported by the Science and Technology Fund Project of Guizhou Provincial Health Commission (gzwkj2023-109), the Science and Technology Plan Project of Zunyi City (ZSKHZC-HZ(2020)172), and the Provincial Project: In-hospital Fund of the First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine (GYZYYFY-BS-2021(01)).
The authors are sincerely grateful for the support of the Department of Neurology, the Affiliated Aerospace Hospital of Zunyi Medical University, the First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, and People's Hospital of Fenggang County. In addition, the authors thank the patient and his family for agreeing to publish the case.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
OIM, ossified intracranial meningiomas; OSM, ossified spinal meningiomas; WHO, World Health Organization; CARE, The CAse REport; EMA, epithelial membrane antigen; S-100, soluble protein-100; PR, progesterone receptor; SSTR2, somatostatin receptor 2; SRS, stereotactic radio surgery; ICOM, International Consortium on Meningiomas.
1. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. (2020) 22:iv1–1iv96. doi: 10.1093/neuonc/noaa200
2. Taha MM, Alawamry A, Abdel-Aziz HR. Ossified spinal meningioma: a case report and a review of the literature. Surg J. (2019) 5:e137–41. doi: 10.1055/s-0039-1697634
3. Dong C, Liu Y, Zhu Y, Wei H, Ma Y. Multiple ossified spinal meningiomas in the thoracic spine: a case report and literature review. Front Surg. (2022) 9:965815. doi: 10.3389/fsurg.2022.965815
4. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med. (2013) 2:38–43. doi: 10.7453/gahmj.2013.008
5. Behling F, Hempel JM, Schittenhelm J. Brain invasion in meningioma-A prognostic potential worth exploring. Cancers. (2021) 13:259. doi: 10.3390/cancers13133259
6. Terrier LM, François P. Multiple meningiomas. Neurochirurgie. (2016) 62:128–35. doi: 10.1016/j.neuchi.2015.12.006
7. Zang KD. Meningioma: a cytogenetic model of a complex benign human tumor, including data on 394 karyotyped cases. Cytogenet Cell Genet. (2001) 93:207–20. doi: 10.1159/000056986
8. Zang KD. Cytological and cytogenetical studies on human meningioma. Cancer Genet Cytogenet. (1982) 6:249–74. doi: 10.1016/0165-4608(82)90063-2
9. Zang KD, Singer H. Chromosomal consitution of meningiomas. Nature. (1967) 216:84–5. doi: 10.1038/216084a0
10. Fontaine B, Rouleau GA, Seizinger B, Jewell AF, Hanson MP, Martuza RL, et al. Equal parental origin of chromosome 22 losses in human sporadic meningioma: no evidence for genomic imprinting. Am J Hum Genet. (1990) 47:823–7.
11. Lekanne Deprez RH, Riegman PH, van Drunen E, Warringa UL, Groen NA, Stefanko SZ, et al. Cytogenetic, molecular genetic and pathological analyses in 126 meningiomas. J Neuropathol Exp Neurol. (1995) 54:224–35. doi: 10.1097/00005072-199503000-00009
12. Aavikko M, Li SP, Saarinen S, Alhopuro P, Kaasinen E, Morgunova E, et al. Loss of SUFU function in familial multiple meningioma. Am J Hum Genet. (2012) 91:520–6. doi: 10.1016/j.ajhg.2012.07.015
13. Stone DM, Murone M, Luoh S, Ye W, Armanini MP, Gurney A, et al. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci. (1999) 112:4437–48. doi: 10.1242/jcs.112.23.4437
14. Bolger GB, Stamberg J, Kirsch IR, Hollis GF, Schwarz DF, Thomas GH. Chromosome translocation t(14;22) and oncogene (c-sis) variant in a pedigree with familial meningioma. N Engl J Med. (1985) 312:564–7. doi: 10.1056/NEJM198502283120907
15. Smidt M, Kirsch I, Ratner L. Deletion of Alu sequences in the fifth c-sis intron in individuals with meningiomas. J Clin Invest. (1990) 86:1151–7. doi: 10.1172/JCI114820
16. Alafaci C, Salpietro FM, Grasso G, Montemagno G, Lucerna S, Matalone D, et al. Osteoblastic meningioma of the lateral ventricle. Case illustration J Neurosurg. (1999) 91:1058. doi: 10.3171/jns.1999.91.6.1058
17. Paolini F, Scalia G, Graziano F, Umana GE, Maugeri R, Iacopino DG, et al. Ossified metaplastic meningiomas: a systematic review on a rare subset of “brain stones”. Brain Spine. (2022) 2:101189. doi: 10.1016/j.bas.2022.101189
18. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
19. Bale TA, Rosenblum MK. The 2021 WHO classification of tumors of the central nervous system: an update on pediatric low-grade gliomas and glioneuronal tumors. Brain Pathol. (2022) 32:e13060. doi: 10.1111/bpa.13060
20. Raza SM, Anderson WS, Eberhart CG, Wolinsky JP, Gokaslan ZL. The application of surgical cordectomy in the management of an intramedullary-extramedullary atypical meningioma: case report and literature review. J Spinal Disord Tech. (2005) 18:449–54. doi: 10.1097/01.bsd.0000155032.69394.23
21. Demir MK, Yapicier Ö, Toktaş ZO, Akakin A, Yilmaz B, Konya D. Ossified-calcified intradural and extradural thoracic spinal meningioma with neural foraminal extension. Spine J. (2016) 16:e35–7. doi: 10.1016/j.spinee.2015.08.053
22. Nakayama N, Isu T, Asaoka K, Harata T, Hayashi S, Aoki T, et al. Two cases of ossified spinal meningioma. No Shinkei Geka. (1996) 24:351–5.
23. Niijima K, Huang YP, Malis LI, Sachdev VP. Ossified spinal meningioma en plaque. Spine. (1993) 18:2340–3. doi: 10.1097/00007632-199311000-00036
24. Tahir M, Usmani N, Ahmad FU, Salmani S, Sharma MS. Spinal meningioma containing bone: a case report and review of literature. BMJ Case Rep. (2009) 2009:1186. doi: 10.1136/bcr.11.2008.1186
25. Ju CI, Hida K, Yamauchi T, Houkin K. Totally ossified metaplastic spinal meningioma. J Korean Neurosurg Soc. (2013) 54:257–60. doi: 10.3340/jkns.2013.54.3.257
26. Murakami T, Tanishima S, Takeda C, Kato S, Nagashima H. Ossified metaplastic spinal meningioma without psammomatous calcification: a case report. Yonago Acta Med. (2019) 62:232–5. doi: 10.33160/yam.2019.06.008
27. Almatrafi F, Alomair M, Alojan A, Alkhaldi M, Alsafwani N, Aseeri A, et al. Intradural extramedullary spinal cord meningioma with a rare extradural foraminal extension: a case report. Front Surg. (2023) 10:1077355. doi: 10.3389/fsurg.2023.1077355
28. Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC, et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. (2021) 23:1821–34. doi: 10.1093/neuonc/noab150
29. Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. (2022) 128:47–58. doi: 10.1002/cncr.33918
30. Liang QL, Shi HZ, Qin XJ, Liang XD, Jiang J, Yang HB. Diagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysis. Thorax. (2008) 63:35–41. doi: 10.1136/thx.2007.077958
31. Kuroi Y, Matsumoto K, Shibuya M, Kasuya H. Progesterone receptor is responsible for benign biology of skull base meningioma. World Neurosurg. (2018) 118:e918–24. doi: 10.1016/j.wneu.2018.07.100
32. Poniman J, Cangara MH, Kaelan C, Miskad UA, Arsyadi G, Ghaznawe M, et al. Progesterone receptor expression and score differences in determining grade and subtype of meningioma. J Neurosci Rural Pract. (2020) 11:552–7. doi: 10.1055/s-0040-1714043
33. Wu W, Zhou Y, Wang Y, Liu L, Lou J, Deng Y, et al. Clinical significance of somatostatin receptor (SSTR) 2 in meningioma. Front Oncol. (2020) 10:1633. doi: 10.3389/fonc.2020.01633
34. Menke JR, Raleigh DR, Gown AM, Thomas S, Perry A, Tihan T. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. (2015) 130:441–3. doi: 10.1007/s00401-015-1459-3
35. Tabor JK, O'Brien J, Vasandani S, Vetsa S, Lei H, Jalal MI, et al. Clinical and genomic differences in supratentorial versus infratentorial NF2 mutant meningiomas. J Neurosurg. (2023) 1–9. doi: 10.3171/2023.4.JNS222929
36. Sapkota MR, Yang Z, Zhu D, Zhang Y, Yuan T, Gao J, et al. Evaluation of epidemiologic factors, radiographic features, and pathologic findings for predicting peritumoral brain edema in meningiomas. J Magn Reson Imaging. (2020) 52:174–82. doi: 10.1002/jmri.27046
37. Naderi S, Yilmaz M, Canda T, Acar U. Ossified thoracic spinal meningioma in childhood: a case report and review of the literature. Clin Neurol Neurosurg. (2001) 103:247–9. doi: 10.1016/s0303-8467(01)00157-3
38. Uchida K, Nakajima H, Yayama T, Sato R, Kobayashi S, Mwaka ES, et al. Immunohistochemical findings of multiple ossified en plaque meningiomas in the thoracic spine. J Clin Neurosci. (2009) 16:1660–2. doi: 10.1016/j.jocn.2009.03.013
39. Shah DS, Reddy RV, Dogruel Y, Asfour MZ, Pour-Rashidi A, Haider AS, et al. Calcified spinal meningiomas: a systematic review of clinical characteristics, treatment strategies, and outcomes. J Neurooncol. (2023) 162:295–305. doi: 10.1007/s11060-023-04291-w
40. Nassiri F, Tabatabai G, Aldape K, Zadeh G. Challenges and opportunities in meningiomas: recommendations from the International Consortium on Meningiomas. Neuro Oncol. (2019) 21:i2–2i3. doi: 10.1093/neuonc/noy181
41. Gousias K, Trakolis L, Simon M. Meningiomas with CNS invasion. Front Neurosci. (2023) 17:1189606. doi: 10.3389/fnins.2023.1189606
42. Maiuri F, Del Basso de Caro M. Update on the diagnosis and management of meningiomas. Cancers. (2023) 15:3575. doi: 10.3390/cancers15143575
43. Ganau M, Foroni RI, Gerosa M, Zivelonghi E, Longhi M, Nicolato A. Radiosurgical options in neuro-oncology: a review on current tenets and future opportunities. Part I: therapeutic strategies. Tumori. (2014) 100:459–65. doi: 10.1700/1636.17912
44. Ganau M, Foroni RI, Gerosa M, Ricciardi GK, Longhi M, Nicolato A. Radiosurgical options in neuro-oncology: a review on current tenets and future opportunities. Part II: adjuvant radiobiological tools. Tumori. (2015) 101:57–63. doi: 10.5301/tj.5000215
45. Pikis S, Mantziaris G, Bunevicius A, Islim AI, Peker S, Samanci Y, et al. Stereotactic radiosurgery compared with active surveillance for asymptomatic, parafalcine, and parasagittal meningiomas: a matched cohort analysis from the IMPASSE study. Neurosurgery. (2022) 90:750–7. doi: 10.1227/neu.0000000000001924
46. Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. (2013) 339:1077–80. doi: 10.1126/science.1233009
47. Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. (2013) 45:285–9. doi: 10.1038/ng.2526
48. Clark VE, Harmanci AS, Bai H, Youngblood MW, Lee TI, Baranoski JF, et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet. (2016) 48:1253–9. doi: 10.1038/ng.3651
49. Zevgaridis D, Thomé C. Purely epidural spinal meningioma mimicking metastatic tumor: case report and review of the literature. Spine. (2002) 27:E403-5. doi: 10.1097/00007632-200209150-00021
50. Rogers L. A spinal meningioma containing bone. Br J Surg. (1928) 15:675–7. doi: 10.1002/bjs.1800156015
51. Freidberg SR. Removal of an ossified ventral thoracic meningioma. Case report. J Neurosurg. (1972) 37:728–30. doi: 10.3171/jns.1972.37.6.0728
52. Kandel E, Sungurov E, Morgunov V. Cerebral and two spinal meningiomas removed from the same patient: case report. Neurosurgery. (1989) 25:447–50. doi: 10.1227/00006123-198909000-00021
53. Kitagawa M, Nakamura T, Aida T, Iwasaki Y, Abe H, Nagashima K. Clinicopathologic analysis of ossification in spinal meningioma. Noshuyo Byori. (1994) 11:115–9.
54. Huang TY, Kochi M, Kuratsu J, Ushio Y. Intraspinal osteogenic meningioma: report of a case. J Formos Med Assoc. (1999) 98:218–21.
55. Saito T, Arizono T, Maeda T, Terada K, Iwamoto Y. A novel technique for surgical resection of spinal meningioma. Spine. (2001) 26:1805–8. doi: 10.1097/00007632-200108150-00017
56. Liu C-L, Lai P-L, Jung S-M, Liao C-C. Thoracic ossified meningioma and osteoporotic burst fracture: treatment with combined vertebroplasty and laminectomy without instrumentation: case report. J Neurosurg Spine. (2006) 4:256–9. doi: 10.3171/spi.2006.4.3.256
57. Hirabayashi H, Takahashi J, Kato H, Ebara S, Takahashi H. Surgical resection without dural reconstruction of a lumbar meningioma in an elderly woman. Eur Spine J. (2009) 18(Suppl 2):232–5. doi: 10.1007/s00586-009-0895-y
58. Licci S, Limiti MR, Callovini GM, Bolognini A, Gammone V, Di Stefano D. Ossified spinal tumour in a 58-year-old woman with increasing paraparesis. Neuropathology. (2010) 30:194–6. doi: 10.1111/j.1440-1789.2009.01076.x
59. Chotai SP, Mrak RE, Mutgi SA, Medhkour A. Ossification in an extra-intradural spinal meningioma-pathologic and surgical vistas. Spine J. (2013) 13:e21–6. doi: 10.1016/j.spinee.2013.06.102
60. Taneoka A, Hayashi T, Matsuo T, Abe K, Kinoshita N, Yasui H, et al. Ossified thoracic spinal meningioma with hematopoiesis: a case report and review of the literature. Case Reports Clin Med. (2013) 02:24–8. doi: 10.4236/crcm.2013.21007
61. Yamane K, Tanaka M, Sugimoto Y, Ichimura K, Ozaki T. Spinal metaplastic meningioma with osseous differentiation in the ventral thoracic spinal canal. Acta Med Okayama. (2014) 68:313–6. doi: 10.18926/AMO/52901
62. Chu YW, Cheuk DK, Chung BH, Bowers NL, Ha SY, Chiang AK, et al. A patient with mosaic neurofibromatosis type 2 presenting with early onset meningioma. BMJ Case Rep. (2014) 2014:bcr2014203919. doi: 10.1136/bcr-2014-203919
63. Cochran EJ, Schlauderaff A, Rand SD, Eckardt GW, Kurpad S. Spinal osteoblastic meningioma with hematopoiesis: radiologic-pathologic correlation and review of the literature. Ann Diagn Pathol. (2016) 24:30–4. doi: 10.1016/j.anndiagpath.2016.07.002
64. Xia T, Tian J-W. Entirely ossified subdural meningioma in thoracic vertebral canal. Spine J. (2016) 16:e11. doi: 10.1016/j.spinee.2015.09.005
65. Alafaci C, Grasso G, Granata F, Salpietro FM, Tomasello F. Ossified spinal meningiomas: clinical and surgical features. Clin Neurol Neurosurg. (2016) 142:93–7. doi: 10.1016/j.clineuro.2016.01.026
66. Prakash A, Mishra S, Tyagi R, Attri PC, Bhatnagar A, Kansal S. Thoracic psammomatous spinal meningioma with osseous metaplasia: a very rare case report. Asian J Neurosurg. (2017) 12:270–2. doi: 10.4103/1793-5482.150222
67. Sakamoto K, Tsutsumi S, Nonaka S, Suzuki T, Ishii H, Ito M, et al. Ossified extradural en-plaque meningioma of the cervical spine. J Clin Neurosci. (2018) 50:124–6. doi: 10.1016/j.jocn.2018.01.058
68. Kim DH, Hong Y-K, Jeun S-S, Park J-S, Kim SW, Cho JH, et al. Endoscopic transseptal approach with bilateral nasoseptal flap in challenging skull-base tumors. World Neurosurg. (2018) 115:e178–84. doi: 10.1016/j.wneu.2018.03.224
69. Wang C, Chen Y, Zhang L, Ma X, Chen B, Li S. Thoracic psammomatous meningioma with osseous metaplasia: a controversial diagnosis of a case report and literature review. World J Surg Oncol. (2019) 17:150. doi: 10.1186/s12957-019-1694-5
70. Xu F, Tian Z, Qu Z, Yao L, Zou C, Han W, et al. Completely ossified thoracic intradural meningioma in an elderly patient: a case report and literature review. Medicine. (2020) 99:e20814. doi: 10.1097/MD.0000000000020814
71. Buchanan D, Martirosyan NL, Yang W, Buchanan RI. Thoracic meningioma with ossification: case report. Surg Neurol Int. (2021) 12:505. doi: 10.25259/SNI_643_2021
72. Wong YP, Tan GC, Mukari SAM, Palaniandy K. Heterotopic ossification in psammomatous spinal meningioma: a diagnostic controversy. Int J Clin Exp Pathol. (2021) 14:627–32.
Keywords: ossified spinal meningiomas, ossified intracranial meningiomas, multiple, meningiomatosis, case report
Citation: Wang J, Zhang A, Wang B, Yuan J, Zhu J, Li M, Liu H, Cheng L and Kong P (2023) Multiple ossified intracranial and spinal meningiomas: a rare case report and literature review. Front. Neurol. 14:1253915. doi: 10.3389/fneur.2023.1253915
Received: 06 July 2023; Accepted: 04 September 2023;
Published: 11 October 2023.
Edited by:
Herbert H. Engelhard, University of Illinois Chicago, United StatesReviewed by:
Mario Ganau, Oxford University Hospitals NHS Trust, United KingdomCopyright © 2023 Wang, Zhang, Wang, Yuan, Zhu, Li, Liu, Cheng and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wang, amFja193X3N6eUAxNjMuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.