- 1Department of Neurology, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, China
- 2Department of Neurosurgery, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, China
- 3Department of Neurology, Liverpool Hospital, Sydney, NSW, Australia
- 4Sydney Brain Center, University of New South Wales, Sydney, NSW, Australia
Objective: The study aimed to explore the association between midline shift (MLS) and net water uptake (NWU) within the ischemic penumbra in acute ischemic stroke patients.
Methods: This was a retrospective cohort study that examined patients with anterior circulation stroke. Net water uptake within the acute ischemic core and penumbra was calculated using data from admission multimodal CT scans. The primary outcome was severe cerebral edema measured by the presence of MLS on 24 to 48 h follow-up CT scans. The presence of a significant MLS was defined by a deviation of the septum pellucidum from the midline on follow-up CT scans of at least 3 mm or greater due to the mass effect of ischemic edema. The net water uptake was compared between patients with and without MLS, followed by logistic regression analyses and receiver operating characteristics (ROCs) to assess the predictive power of net water uptake in MLS.
Results: A total of 133 patients were analyzed: 50 patients (37.6%) with MLS and 83 patients (62.4%) without. Compared to patients without MLS, patients with MLS had higher net water uptake within the core [6.8 (3.2–10.4) vs. 4.9 (2.2–8.1), P = 0.048] and higher net water uptake within the ischemic penumbra [2.9 (1.8–4.3) vs. 0.2 (−2.5–2.7), P < 0.001]. Penumbral net water uptake had higher predictive performance than net water uptake of the core in MLS [area under the curve: 0.708 vs. 0.603, p < 0.001]. Moreover, the penumbral net water uptake predicted MLS in the multivariate regression model, adjusting for age, sex, admission National Institutes of Health Stroke Scale (NIHSS), diabetes mellitus, atrial fibrillation, ischemic core volume, and poor collateral vessel status (OR = 1.165; 95% CI = 1.002–1.356; P = 0.047). No significant prediction was found for the net water uptake of the core in the multivariate regression model.
Conclusion: Net water uptake measured acutely within the ischemic penumbra could predict severe cerebral edema at 24–48 h.
Introduction
Stroke is one of the leading causes of death and disability worldwide (1). Cerebral edema after acute ischemic stroke (AIS) often leads to poor clinical outcome and increases mortality (1, 2). Midline shift (MLS) describes the horizontal displacement degree of the septum pellucidum on a CT scan, and it has long been established as a radiologic feature of severe brain edema (3–6). However, MLS often manifests after 24 h of stroke onset, frequently leading to delayed identification (7–9). At the delayed stage, the treatment effect of osmotherapy or surgical decompressive craniectomy would be compromised, and it might be difficult to prevent the rapid progression of edema, which might eventually lead to cerebral herniation or demise in some malignant cases (10–12). Therefore, the detection of early indicators of severe brain edema is essential, as it can allow clinicians to promptly initiate preventive therapeutic strategies.
Net water uptake (NWU) has been reported to help predict brain edema in the hyperacute stage of acute ischemic stroke. It quantifies the degree of hypoattenuation on computed tomography (CT) scans (13, 14). Prior studies have reported that NWU within the ischemic core is a promising early biomarker of malignant cerebral edema (8, 15, 16). The majority of NWU research focuses on the ischemic core, whereas little research has been conducted on the ischemic penumbra. Ischemic penumbra refers to mild-moderate hypoperfusion brain tissues that are salvageable with prompt blood reperfusion (17, 18). It has been the target of reperfusion treatment, either endovascular thrombectomy or intravenous thrombolysis. However, recent studies have shown that reperfusion of the penumbral tissues might lead to reperfusion injury, including edema and bleeding (19). In a recent study published by our group, we showed that NWU within the penumbra predicted symptomatic intracranial hemorrhages (20). This study aimed to explore whether NWU in the penumbral region can predict severe brain edema measured by MLS.
Methods
Study design
This was a retrospective cohort study. Patients were dichotomized into the following two groups: (1) MLS group, including patients with severe brain edema defined by middle line shift (MLS), and (2) non-MLS group, including patients with no MLS. The MLS and non-MLS groups were compared in terms of baseline net water uptake in the region of the ischemic penumbra and core, respectively.
Patients
This study retrospectively screened patients who were diagnosed with their first-ever acute ischemic stroke (within 24 h of symptom onset) in the First Affiliated Hospital of Ningbo University from July 2017 to September 2019. This study selected patients with the following inclusion criteria: (1) acute vessel occlusion of the anterior circulation; (2) multimodal CT imaging completed within 24 h of admission, including non-contrast CT (NCCT) scans, CT angiography (CTA), and CT perfusion (CTP); and (3) follow-up CT scans completed 24 to 48 h after hospitalization. The key exclusion criterion was abnormal density alteration on admission NCCT caused by pre-existing infarction or hemorrhage. This study was approved by the ethics committee of the First Affiliated Hospital of Ningbo University, and all patients signed the informed consent form for the collected data before being included in the study.
Clinical data
Clinical data included age, sex, severity of ischemic stroke on admission measured by the National Institutes of Health Stroke Scale (NIHSS), and medical history.
Image data
The patients received a comprehensive one-stop stroke imaging protocol by multimodal CT scans at admission with NCCT, CTA, and CTP on a 320-slice scanner (Toshiba Aquilion ONE, Canon Medical Imaging, Tokyo, Japan). Collateral status was assessed on the CTA with ASITN/SIR collateral scores ranging from 0 to 4, and poor collateral status was defined as scores ≤2.
The CTP image was spatially segmented into 320 axial sections with 0.5 mm thickness per layer and temporally divided into 19 time points beginning at 4 s after non-ionic iodinated contrast injection into an antecubital vein. The CTP data were processed using commercial software (MI-Star, Apollo Medical Imaging Technology, Melbourne, VIC, Australia). The ischemic core and ischemic penumbra were recognized by CTP and were defined as follows: ischemic core was delay time (DT) >3 s and cerebral blood flow (CBF) <30%, while ischemic penumbra means DT >3 s and CBF >30% according to the corresponding threshold (21).
Net water uptake
Figure 1 shows how net water uptake was measured. First, the regions of interest (ROIs) of the ischemic core and penumbra were manually drawn according to CTP, and then mirrored contralateral ROIs were generated automatically and defined as normal tissue. Both ROIs were superimposed on NCCT and were sampled with CT density between 20 and 80 Hounsfield units (HU) to exclude voxels, including CSF or calcification.
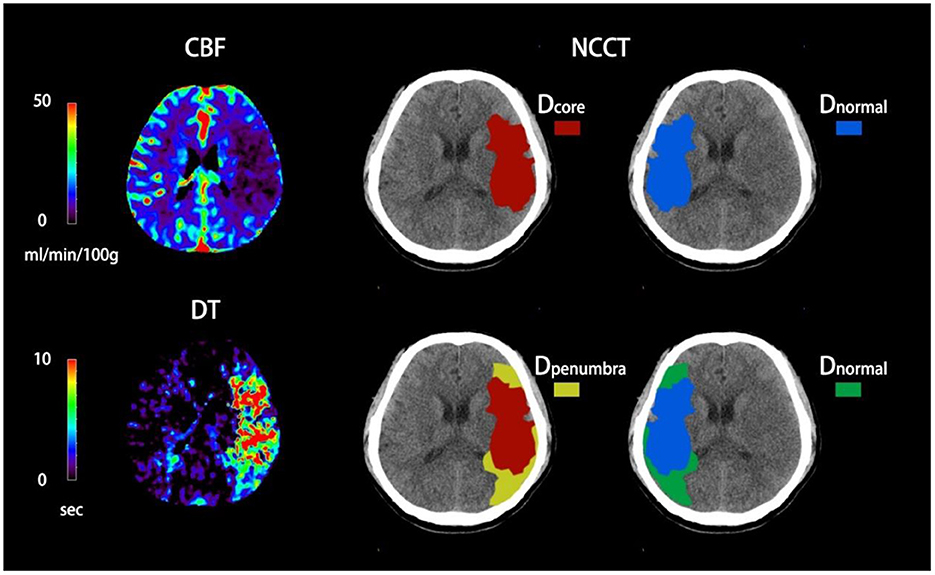
Figure 1. Quantification of NWU-core and NWU-penumbra in admission non-contrast computed tomography (NCCT). The ischemic core and penumbra were identified by cerebral blood flow (CBF) and delay time (DT) maps based on computed tomography perfusion (CTP) with the setting of different thresholds. The mean density of the ischemic core, ischemic penumbra, and normal tissue derived from the mirrored contralateral were then calculated.
NWU-core measured an elevated volume of water uptake per unit volume of ischemic core, while NWU-penumbra represented a higher volume of water uptake per unit volume of ischemic penumbra. The correlative equations were as follows (22, 23):
Outcome
The primary imaging endpoint of this study was the presence of significant MLS. MLS was measured as the maximum horizontal deviation of the line between the anterior and posterior attachment points of the falx cerebri on follow-up CT scans. The presence of significant MLS was defined by a deviation of at least 3 mm or greater due to the mass effect of ischemic edema (24). Two double-blinded clinicians reviewed the CT images to confirm the presence of significant MLS. Any disagreement was resolved by consensus. Patients were dichotomized into two categories: those with MLS and those without MLS.
The secondary outcomes included the following: (1) parenchymal hematoma type 2 (PH2) on follow-up CT scans, defined as hematoma exceeding 30% of the infarcted volume with significant space-occupying effect (25); (2) 3-month poor outcome, defined by a modified Rankin Scale of 5–6; and (3) 3-month mortality. In addition, for patients with thrombectomy, the successful recanalization rate was assessed on the final run of digital subtraction angiography (DSA), defined as the mTICI score scale of 2b-3.
Statistical analysis
The statistical analyses were performed using SPSS (version 26; IBM Corporation). The normality of the distribution was analyzed using the Kolmogorov–Smirnov test. Normally distributed continuous data are presented as mean ± standard deviation and assessed using the independent two-sample t-test. For data that do not follow a normal distribution, we presented the median along with the interquartile range (IQR) and employed the Mann–Whitney U-test for comparisons. Categorical variables are expressed as percentages and calculated using the chi-square test. The confidence interval was set at 95%, and a p-value of < 0.05 was considered statistically significant.
A univariate analysis was performed to assess the prediction of NWU-penumbra and NWU-core in MLS, followed by receiver operating characteristic (ROC) curve analysis. Then, two multivariate regression models, adjusting for patient characteristics, were performed to assess the predictive power of NWU-penumbra and NWU-core in MLS. These showed a statistical significance (p < 0.05 for MLS vs. non-MLS groups) or clinical significance. The NWU-core and NWU-penumbra were tested in two models due to the consideration of their collinearity.
A sensitivity analysis was performed on patients with no PH2. A subgroup analysis was executed on patients stratified by collateral circulation status and with or without mechanical thrombectomy.
Results
Patients
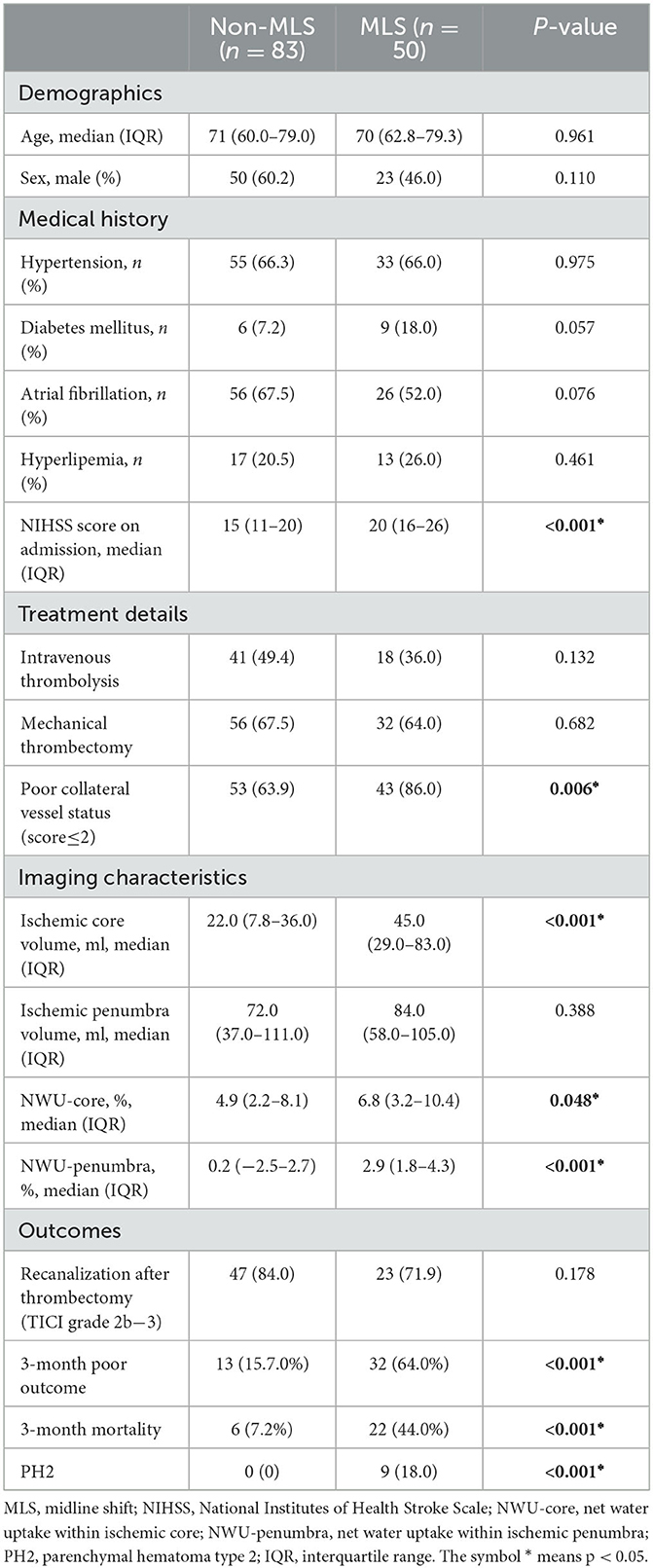
A total of 133 eligible patients with newly diagnosed anterior circulation infarct who arrived at the hospital within 24 h of symptom onset were enrolled for our final analysis. The baseline characteristics of all the participants are shown in Table 1.
Among all the cases, 50 patients (37.6%) were found to have MLS in the follow-up CT scans, whereas 83 did not have MLS (62.4%). Compared with the non-MLS group, patients with MLS had a higher occurrence of poor collateral vessel status [43 (86%) vs. 53 (63.9%), P = 0.006], a larger volume of the ischemic core [45.0 (29.0–83.0) vs. 22.0 (7.8–36.0), p < 0.001], higher NIHSS scores on admission [22 ± 8 vs. 15 ± 7, P < 0.001], a higher NWU within the ischemic core [6.8 (3.2–10.4) vs. 4.9 (2.2–8.1), P = 0.048], and a higher NWU within the penumbra [2.9 (1.8–4.3) vs. 0.2 (−2.5–2.7), P < 0.001].
Patients who had MLS compared to those with no MLS, patients who had MLS had a higher rate of poor clinical outcome (32 (64%) vs. 13 (15.7%), p < 0.001) and a higher rate of 3-month mortality (22 (44%) vs. 6 (7.2%), p < 0.001). For patients with MLS, 9 out of 50 had PH2, whereas no patients had PH2 in the 83 patients without MLS (18.0% vs. 0%, p < 0.001). In addition, 88 (66.2%) patients underwent thrombectomy therapy, of whom 70 (79.5%) presented with successful recanalization. However, the rate of successful recanalization after thrombectomy (mTICI grade 2b−3) between the two groups presented no statistical difference (P > 0.05).
NWU predicts MLS in univariate regression
Univariate analyses showed that the NWU either in the core region or the penumbra predicted the presence of MLS (odds ratio = 1.098 (1.012–1.191) and 1.212 (1.079–1.361), respectively, p = 0.025 and 0.001, respectively). With an increase in NWU in the core by 1%, the odds of having MLS increased by 9.8%, whereas a 1% increase in NWU in the penumbra resulted in the odds of MLS increasing by 21.2%. ROC analysis showed that NWU-penumbra had significantly higher predictive power than NWU-core in MLS (AUC = 0.708 vs. 0.603, p < 0.001, Figure 2).
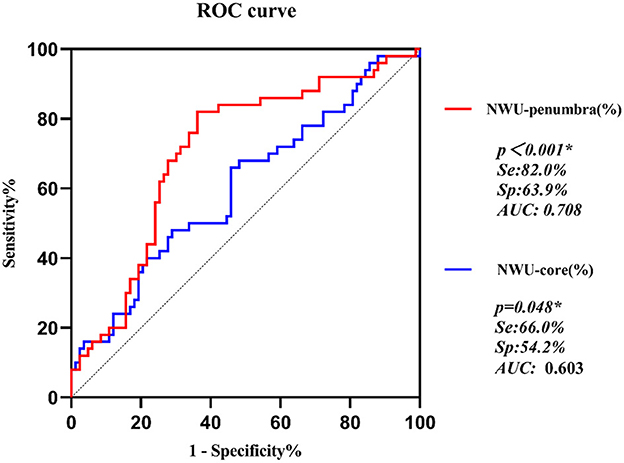
Figure 2. ROC curves of NWU-penumbra and NWU-core on prediction of MLS in acute ischemic stroke patients.
NWU predicts MLS in multivariate regression
The results of multivariate logistic regression models are summarized in Table 2. The multivariate regression models included the following predictors: patient characteristics with clinical significance (age, sex, diabetes mellitus, and atrial fibrillation) or statistical significance (NIHSS on admission, poor collateral vessel status, ischemic core volume, NWU-core, and NWU-penumbra; p < 0.05 for MLS vs. non-MLS group, Table 1). The NWU-core and NWU-penumbra were tested in two models due to the consideration of their collinearity.
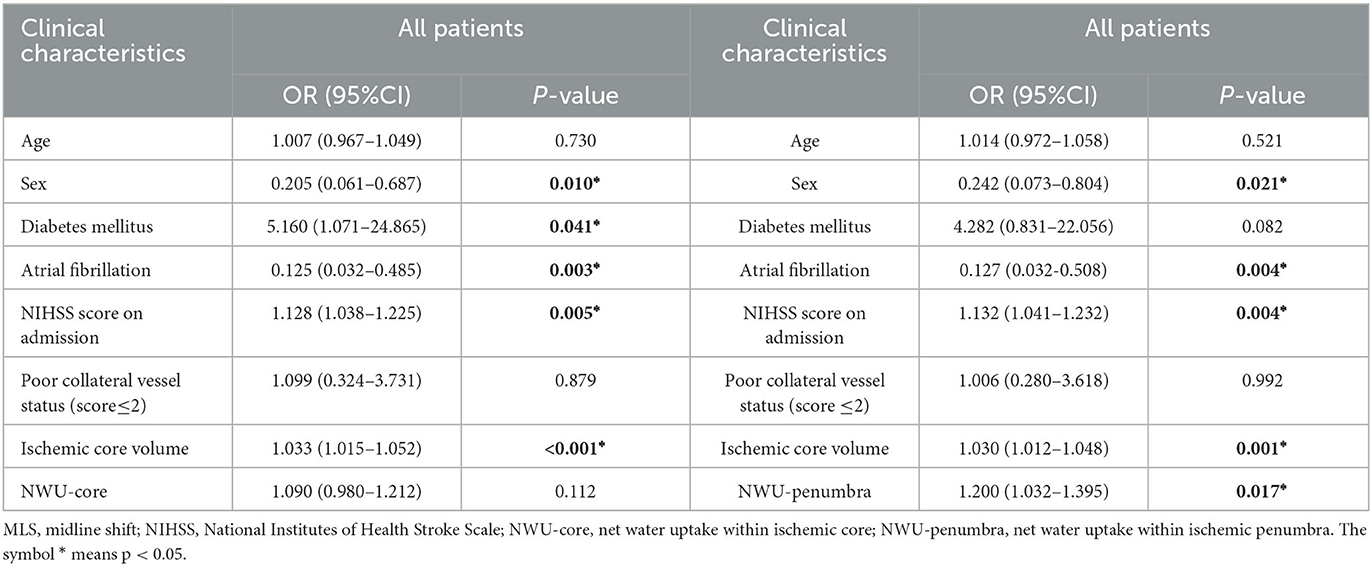
Table 2. Multivariate logistic regression analysis for risk of MLS in anterior circulation infarct patients.
In the multivariate regression model, NWU-penumbra was significantly associated with MLS (adjusted OR = 1.200; 95% CI = 1.032–1.395; P = 0.017). However, NWU-core did not show any significance in predicting MLS in the multivariate regression model (adjusted OR = 1.090; 95% CI = 0.980–1.212; P = 0.112), adjusting for age, sex, diabetes mellitus, atrial fibrillation, NIHSS on admission, poor collateral vessel status, and ischemic core volume. In both models, higher NIHSS scores and a larger volume of the ischemic core also predicted the presence of MLS, while poor collateral status did not show significant predictive power (Table 2).
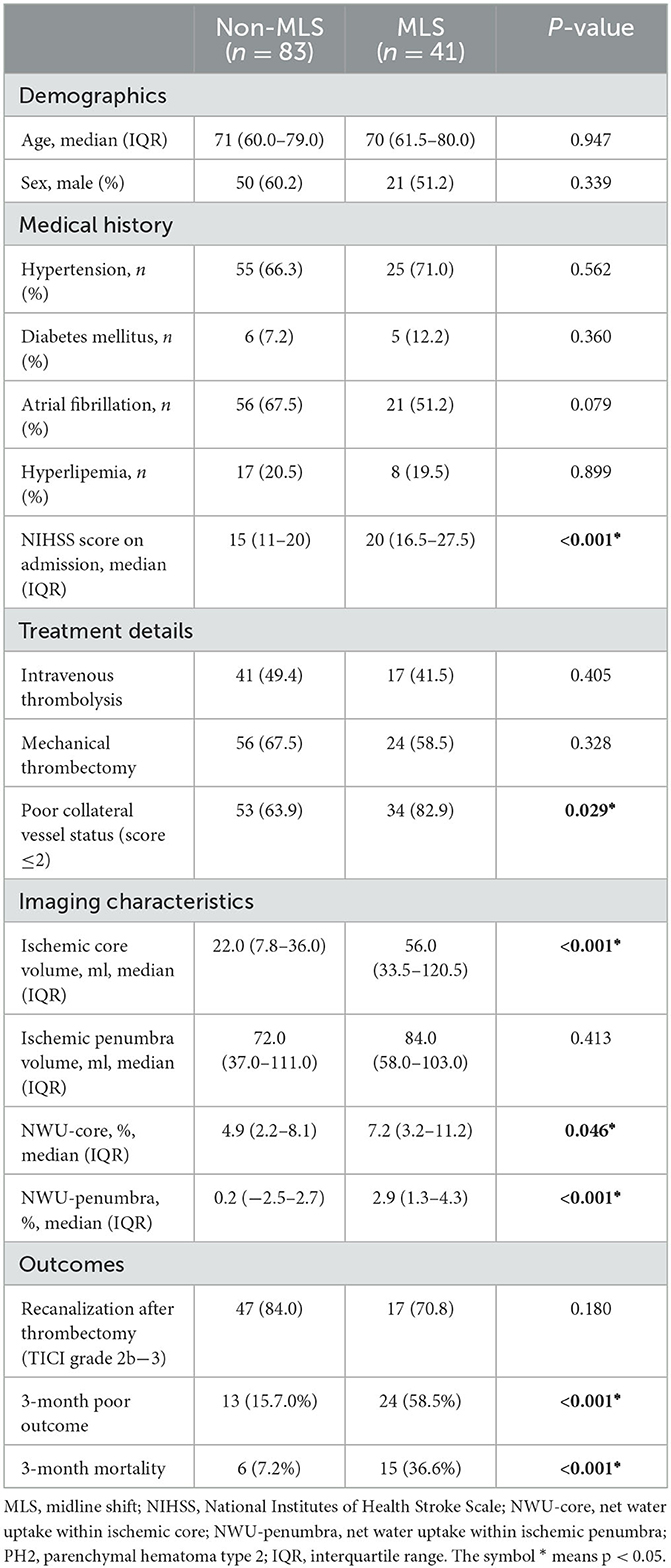
Sensitivity analysis: MLS without large hemorrhages
In this cohort, nine patients had PH2, and all nine patients had MLS. PH2 was significantly associated with MLS (p < 0.001). After excluding patients with PH2, the results of the MLS and non-MLS groups are summarized in Table 3. After excluding patients with PH2, NWU-penumbra still showed a significantly higher value in patients with MLS compared to those without MLS [2.9 (1.3–4.3) vs. 0.2 (−2.5–2.7), P = 0.001]. In the multivariate regression model (Table 4), NWU-penumbra maintained significant predictive power in MLS (OR = 1.165; 95% CI = 1.002–1.356; P = 0.047), adjusting for age, sex, diabetes mellitus, atrial fibrillation, NIHSS on admission, poor collateral vessel status, and ischemic core volume. However, NWU-core had no significant predictive power for MLS in the multivariate regression model (OR = 1.084; 95% CI = 0.967–1.214; P = 0.168).
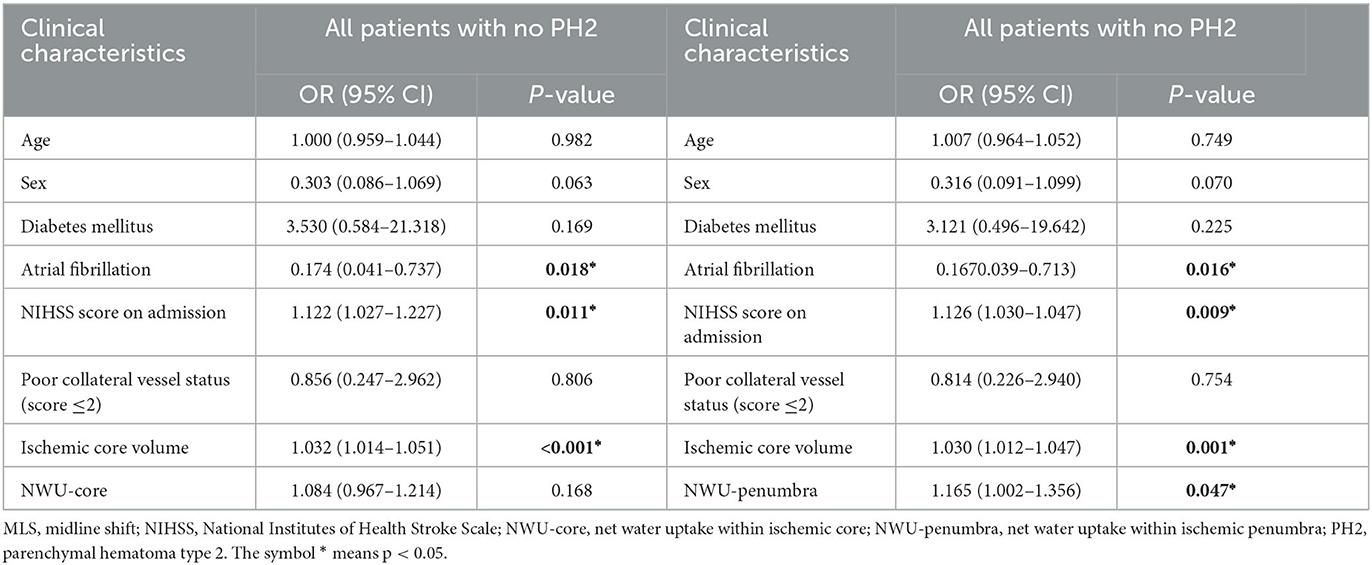
Table 4. Multivariate logistic regression analysis for risk of MLS in anterior circulation infarct patients with no PH2.
Subgroup analysis
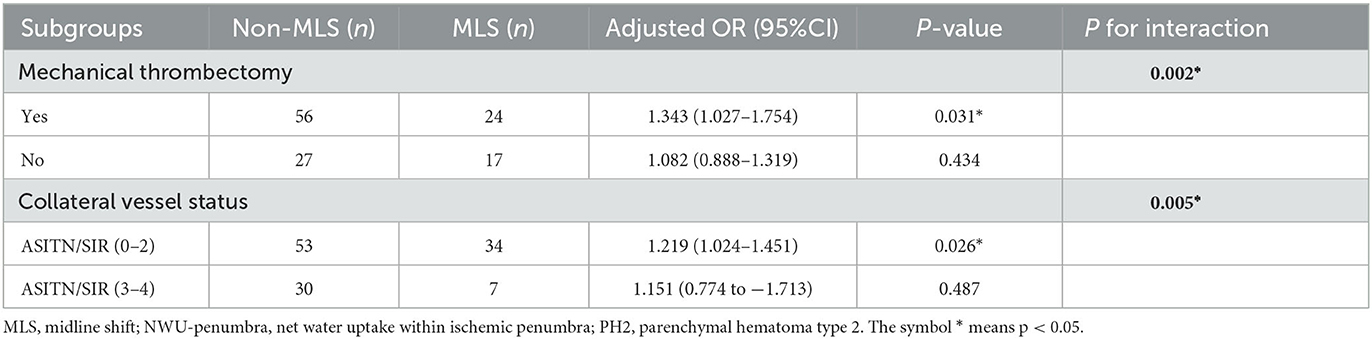
For the 124 patients without PH2, subgroup analyses were further conducted. The results of subgroup analyses are summarized in Table 5. Overall, a total of 80 (64.5%) patients experienced mechanical thrombectomy, and 44 patients (35.5%) did not. For patients with mechanical thrombectomy, NWU in the penumbral region predicted MLS (OR = 1.343, p = 0.031). However, for patients without mechanical thrombectomy, NWU in the penumbral region had no predictive value in MLS (OR = 1.082, p = 0.434).
In addition, 87 patients (65.4%) had poor collateral status, whereas 37 (27.8%) had good collateral status. NWU-penumbra was confirmed as an independent predictor of MLS in those patients with poor collateral status (adjusted OR = 1.219; 95% CI = 1.024–1.451; P = 0.026), whereas it did not predict MLS in patients with good collateral status (adjusted OR = 1.151; 95% CI = 0.774–1.731; P = 0.487, Table 5).
Both mechanical thrombectomy and collateral vessel status had an interaction with NWU-penumbra in predicting MLS (p = 002 and p = 0.005, respectively). Figure 3A demonstrates that the value of NWU-penumbra was only statistically different between the MLS and non-MLS groups when patients had mechanical thrombectomy, while Figure 3B indicates that the value of NWU-penumbra was only statistically different between the MLS and non-MLS groups when patients had poor collateral statuses.
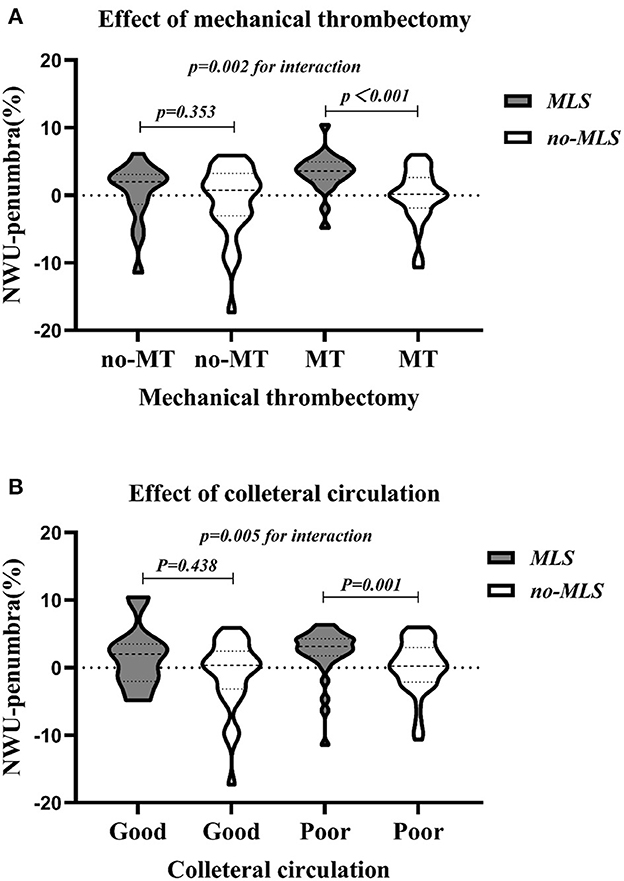
Figure 3. Violin plots showing the dispersion of NWU-penumbra values devised by subgroups. (A) With or without mechanical thrombectomy; (B) good or poor collateral circulation.
Discussion
The main finding of this study is that increased net water uptake within the acute penumbral region was significantly associated with severe brain edema at 24–48 h, measured by MLS on the brain CT. When excluding patients whose MLS was caused by a large hemorrhagic transformation, a significant association still existed between penumbral NWU and MLS.
To the best of our knowledge, this is the first study that reports the relationship between net water uptake within the ischemic penumbra and follow-up severe brain edema. A previous study from our group reported a positive relationship between penumbral net water uptake and hemorrhagic transformation on follow-up images (20). A high reperfusion/recanalization rate has been reported in both studies. Together, the two studies indicate a potential relationship between penumbral NWU and reperfusion injuries. Although the penumbra has been the target of reperfusion treatment, in patients with high NWU of the penumbral region, such treatment might cause reperfusion injuries of edema or hemorrhagic transformation. In this study, for patients with mechanical thrombectomy, NWU-penumbral in the MLS group was significantly higher than in the non-MLS group. In addition, NWU in the penumbral region predicted MLS, but not for patients without mechanical thrombectomy. The findings of this study suggest that the optimal candidate for reperfusion treatment would be patients with penumbral tissues to be salvaged but also with a limited increase of NWU within the penumbral region, which could potentially lead to the maximum benefit and minimum risk from the treatment.
Another novel finding of the study is the interaction of penumbral NWU and collateral vessels in predicting severe brain edema. The association between penumbral NWU and MLS was found to be limited to patients with poor collateral vessel status. One possible explanation is that the penumbral NWU reflects how much edema can expand, and the edema expansion also depends on the collateral status. Such an assumption is supported by the recent studies of collateral status and NWU. Broocks et al. (26) reported that patients with poor collateral status had significantly higher early edema progression rates, while Galego et al. (27) reported that poor collateral circulation was independently associated with significant cerebral edema 24 h after acute ischemic stroke in patients who underwent reperfusion treatment.
This study also found an increased net water uptake in the ischemic core in patients with MLS. However, the core NWU did not predict MLS in the multivariate regression model when adjusting for confounders, including baseline NIHSS and acute core volume. This was different from the findings of the previous studies reporting that the NWU within the ischemic core predicted malignant brain edema (8, 15, 16). One possible explanation is the different definitions of NWU in previous studies. Both image patch-based NWU and ASPECTS-NWU were dependent on NECT rather than CTP and possibly failed to differentiate the penumbra from the ischemic core (8, 15). Another explanation did not account for the effect of parenchymatous hemorrhage on malignant brain edema (16). Parenchymatous hemorrhage with a space-occupying effect may contribute to the overstatement of the predictive ability of NWU-core for malignant brain edema in previous studies. Finally, the CTP-derived parameters have been criticized recently for overestimating the final infarct core (28, 29). Accordingly, the MLS prediction ability of NWU-core, which CTP recognizes, may similarly be biased.
This study has several limitations. First, this is a retrospective and single-center study, which may result in a selection bias of patients with and without MLS. Multivariate regression was performed to adjust for such bias. However, residual confounders might exist. The findings of this study need to be validated in a prospective study and, ideally, with a larger sample size recruited from multiple centers. Second, due to the small sample size, this study did not further grade MLS according to different degrees of horizontal displacement. In future studies, graded MLS can help further explore the relationship between brain edema and net water uptake. Finally, the findings of this study are limited to patients with anterior circulation strokes. The accurate definition of core and penumbra is limited to anterior circulation stroke, whereas limited data are available on how to delineate core and penumbra in posterior circulation stroke. Most NWU studies, including this study, are limited to patients with anterior circulation stroke.
Conclusion
In conclusion, it is important to measure net water uptake in the penumbral region acutely since it can potentially predict severe brain edema, especially in patients with poor collateral status.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Ningbo University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CC: data curation, statistical analysis, and organizing and writing the original draft. JY: project administration. QH, YW, JL, and TX: investigation and resources. JS, XG, and YH: funding acquisition. MP and LL: validation and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Traditional Chinese Medicine Science and Technology Project of Zhejiang Province (2021ZA128), the Ningbo Health Branding Subject Fund (PPXK2018-04), the Ningbo Top Medical and Health Research Program (2022020304), and the Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province (2022E10026).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Global, regional, and national life expectancy, all-cause mortality, and and cause-specific mortality for 249 causes of death 1980–2015: 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1459–544. doi: 10.1161/STROKEAHA.114.006884
2. Rosenberg GA. Ischemic brain edema. Prog Cardiovasc Dis. (1999) 42:209–16. doi: 10.1016/S0033-0620(99)70003-4
3. Zhang X, Huang P, Zhang R. Evaluation and prediction of post-stroke cerebral edema based on neuroimaging. Front Neurol. (2021) 12:763018. doi: 10.3389/fneur.2021.763018
4. Barber PA, Demchuk AM, Zhang J, Kasner SE, Hill MD, Berrouschot J, et al. Computed tomographic parameters predicting fatal outcome in large middle cerebral artery infarction. Cerebrovasc Dis. (2003) 16:230–5. doi: 10.1159/000071121
5. Strbian D, Meretoja A, Putaala J, Kaste M, Tatlisumak T. Cerebral edema in acute ischemic stroke patients treated with intravenous thrombolysis. Int J Stroke. (2013) 8:529–34. doi: 10.1111/j.1747-4949.2012.00781.x
6. Zhang X, Yan S, Zhong W, Yu Y, Lou M. Early nt-probnp (n-terminal probrain natriuretic peptide) elevation predicts malignant edema and death after reperfusion therapy in acute ischemic stroke patients. Stroke. (2021) 52:537–42. doi: 10.1161/STROKEAHA.120.029593
7. Dhar R, Hamzehloo A, Kumar A, Chen Y, He J, Heitsch L, et al. Hemispheric csf volume ratio quantifies progression and severity of cerebral edema after acute hemispheric stroke. J Cereb Blood Flow Metab. (2021) 41:2907–15. doi: 10.1177/0271678X211018210
8. Fu B, Qi S, Tao L, Xu H, Kang Y, Yao Y, et al. Image patch-based net water uptake and radiomics models predict malignant cerebral edema after ischemic stroke. Front Neurol. (2020) 11:609747. doi: 10.3389/fneur.2020.609747
9. Bardutzky J, Schwab S. Antiedema therapy in ischemic stroke. Stroke. (2007) 38:3084–94. doi: 10.1161/STROKEAHA.107.490193
10. Jeon SB, Koh Y, Choi HA, Lee K. Critical care for patients with massive ischemic stroke. Journal of stroke. (2014) 16:146–60. doi: 10.5853/jos.2014.16.3.146
11. Sakai K, Iwahashi K, Terada K, Gohda Y, Sakurai M, Matsumoto Y. Outcome after external decompression for massive cerebral infarction. Neurol Medico-Chirurg. (1998) 38:131–5. doi: 10.2176/nmc.38.131
12. Subramaniam S, Hill MD. Massive cerebral infarction. Neurologist. (2005) 11:150–60. doi: 10.1097/01.nrl.0000159987.70461.d7
13. Broocks G, McDonough R, Meyer L, Bechstein M, Kniep H, Schön G, et al. Reversible ischemic lesion hypodensity in acute stroke ct following endovascular reperfusion. Neurology. (2021) 97:e1075–84. doi: 10.1212/WNL.0000000000012484
14. Han Q, Yang J, Gao X, Li J, Wu Y, Xu Y, et al. Early edema within the ischemic core is time-dependent and associated with functional outcomes of acute ischemic stroke patients. Front Neurol. (2022) 13:861289. doi: 10.3389/fneur.2022.861289
15. Shi J, Wu H, Dong Z, Liang X, Liu Q, Zhu W, et al. Automated quantitative lesion water uptake in acute stroke is a predictor of malignant cerebral edema. Eur Radiol. (2022) 32:2771–80. doi: 10.1007/s00330-021-08443-2
16. Broocks G, Flottmann F, Scheibel A, Aigner A, Faizy TD, Hanning U, et al. Quantitative lesion water uptake in acute stroke computed tomography is a predictor of malignant infarction. Stroke. (2018) 49:1906–12. doi: 10.1161/STROKEAHA.118.020507
17. Yang SH, Liu R. Four decades of ischemic penumbra and its implication for ischemic stroke. Transl Stroke Res. (2021) 12:937–45. doi: 10.1007/s12975-021-00916-2
18. Lin L, Bivard A, Parsons MW. Perfusion patterns of ischemic stroke on computed tomography perfusion. J stroke. (2013) 15:164–73. doi: 10.5853/jos.2013.15.3.164
19. Nie X, Leng X, Miao Z, Fisher M, Liu L. Clinically ineffective reperfusion after endovascular therapy in acute ischemic stroke. Stroke. (2023) 54:873–81. doi: 10.1161/STROKEAHA.122.038466
20. Xu T, Yang J, Han Q, Wu Y, Gao X, Xu Y, et al. Net water uptake, a neuroimaging marker of early brain edema, as a predictor of symptomatic intracranial hemorrhage after acute ischemic stroke. Front Neurol. (2022) 13:903263. doi: 10.3389/fneur.2022.903263
21. Lin L, Bivard A, Krishnamurthy V, Levi CR, Parsons MW. Whole-brain ct perfusion to quantify acute ischemic penumbra and core. Radiology. (2016) 279:876–87. doi: 10.1148/radiol.2015150319
22. Broocks G, Flottmann F, Ernst M, Faizy TD, Minnerup J, Siemonsen S, et al. Computed tomography-based imaging of voxel-wise lesion water uptake in ischemic brain: Relationship between density and direct volumetry. Invest Radiol. (2018) 53:207–13. doi: 10.1097/RLI.0000000000000430
23. Minnerup J, Broocks G, Kalkoffen J, Langner S, Knauth M, Psychogios MN, et al. Computed tomography-based quantification of lesion water uptake identifies patients within 45 h of stroke onset: a multicenter observational study. Ann Neurol. (2016) 80:924–34. doi: 10.1002/ana.24818
24. McKeown ME, Prasad A, Kobsa J, Top I, Snider SB, Kidwell C, et al. Midline shift greater than 3 mm independently predicts outcome after ischemic stroke. Neurocrit Care. (2022) 36:46–51. doi: 10.1007/s12028-021-01341-x
25. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ecass ii). Second european-australasian acute stroke study investigators. Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
26. Broocks G, Kemmling A, Meyer L, Nawabi J, Schön G, Fiehler J, et al. Computed tomography angiography collateral profile is directly linked to early edema progression rate in acute ischemic stroke. Stroke. (2019) 50:3424–30. doi: 10.1161/STROKEAHA.119.027062
27. Galego O, Jesus-Ribeiro J, Baptista M, Sargento-Freitas J, Martins AI, Silva F, et al. Collateral pial circulation relates to the degree of brain edema on ct 24 h after ischemic stroke. Neuroradiol J. (2018) 31:456–63. doi: 10.1177/1971400918769912
28. Haupt W, Meyer L, Wagner M, McDonough R, Elsayed S, Bechstein M, et al. Assessment of irreversible tissue injury in extensive ischemic stroke-potential of quantitative cerebral perfusion. Transl Stroke Res. (2023) 14:562–71. doi: 10.1007/s12975-022-01058-9
Keywords: stroke, anterior circulation infarction, brain edema, midline shift, net water uptake
Citation: Chen C, Yang J, Han Q, Wu Y, Li J, Xu T, Sun J, Gao X, Huang Y, Parsons MW and Lin L (2023) Net water uptake within the ischemic penumbra predicts the presence of the midline shift in patients with acute ischemic stroke. Front. Neurol. 14:1246775. doi: 10.3389/fneur.2023.1246775
Received: 24 June 2023; Accepted: 25 August 2023;
Published: 29 September 2023.
Edited by:
Andre Kemmling, University of Marburg, GermanyReviewed by:
Gabriel Broocks, University of Hamburg, GermanyMatthias Bechstein, University Medical Center Hamburg-Eppendorf, Germany
Copyright © 2023 Chen, Yang, Han, Wu, Li, Xu, Sun, Gao, Huang, Parsons and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Longting Lin, bG9uZ3RpbmcubGluQHVuc3cuZWR1LmF1; Mark W. Parsons, TWFyay5QYXJzb25zQHVuc3cuZWR1LmF1
†These authors have contributed equally to this work
 Cuiping Chen
Cuiping Chen Jianhong Yang1†
Jianhong Yang1† Qing Han
Qing Han Yuefei Wu
Yuefei Wu Tianqi Xu
Tianqi Xu Jie Sun
Jie Sun Xiang Gao
Xiang Gao Yi Huang
Yi Huang Mark W. Parsons
Mark W. Parsons Longting Lin
Longting Lin