
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 17 August 2023
Sec. Movement Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1242484
 Emmanuelle Schmitt1*
Emmanuelle Schmitt1* Bettina Debu1
Bettina Debu1 Anna Castrioto1
Anna Castrioto1 Andrea Kistner1
Andrea Kistner1 Valerie Fraix1
Valerie Fraix1 Martine Bouvard2
Martine Bouvard2 Elena Moro1
Elena Moro1Background: Neuropsychiatric fluctuations (NpsyF) are frequent and disabling in people with Parkinson’s disease (PD). In OFF-medication, NpsyF entail minus neuropsychiatric symptoms (NPS) like anxiety, apathy, sadness, and fatigue. In ON-medication, NpsyF consist in plus NPS, such as high mood, hypomania, and hyperactivity. Accurate identification of these NpsyF is essential to optimize the overall PD management. Due to lack of punctual scales, the neuropsychiatric fluctuation scale (NFS) has been recently designed to assess NpsyF in real time. The NFS comprises 20 items with two subscores for plus and minus NPS, and a total score.
Objective: To evaluate the psychometric properties of the NFS in PD.
Methods: PD patients with motor fluctuations and healthy controls (HC) were assessed. In PD patients, the NFS was administrated in both the ON-and OFF-medication conditions, together with the movement disorders society-unified Parkinson disease rating scale parts I–IV. Depression (Beck depression scale II), apathy (Starkstein apathy scale) and non-motor fluctuations items of the Ardouin scale of behaviour in PD (ASBPD OFF and ON items) were also assessed. NFS internal structure was evaluated with principal component analysis consistency (PCA) in both medication conditions in PD patients and before emotional induction in HC. NFS internal consistency was assessed using Cronbach’s alpha coefficient. NFS convergent and divergent validity was measured through correlations with BDI-II, Starktein, and ASBPD OFF and ON non motor items. Specificity was assessed comparing NFS global score between the HC and PD populations. Sensitivity was evaluated with t-student test comparing the ON-and the OFF-medication conditions for NFS global score and for minus and plus subscores.
Results: In total, 101 consecutive PD patients and 181 HC were included. In PD patients and HC, PCA highlighted one component that explained 32–35 and 42% of the variance, respectively. Internal consistency was good for both the NFS-plus (alpha =0.88) and NFS-minus items (alpha =0.8). The NFS showed a good specifity for PD (p < 0.0001) and a good sensitivity to the medication condition (p < 0.0001).
Conclusion: The satisfactory properties of the NFS support its use to assess acute neuropsychiatric fluctuations in PD patients, adding to available tools.
Non-motor fluctuations are frequent in advanced Parkinson’s disease (PD) and have a major negative impact on patients’ autonomy and quality of life (1, 2). Indeed, disability linked to non-motor fluctuations can be greater than that caused by motor fluctuations (3).
Neuropsychiatric fluctuations (NpsyF) are possibly the most disabling non-motor fluctuations (4–7). In the OFF-medication condition, NpsyF are characterized by minus neuropsychiatric symptoms (NPS), also called hypo-dopaminergic symptoms, like anxiety, fatigue, lack of motivation, sadness, and slowness of thinking. Conversely, in the ON-medication condition, plus NPS, also called hyper-dopaminergic symptoms, can be described by a general feeling of well-being, high mood (even hypomania), and hyperactivity (2). The presence and severity of NpsyF can differ from one patient to another, likely depending on the extent of central dopaminergic depletion and sensitivity of D3-D5 receptors of the mesocorticolimbic pathway (8). NpsyF are also considered to be a risk factor for developing dopaminergic dysregulation syndrome or hypo-dopaminergic syndrome after deep brain stimulation (DBS) of the subtalamic nucleus (STN) (6, 9). Prompt and careful identification and management of these NpsyF play an essential role in the overall management of PD.
Although several retrospective questionnaires and scales are currently available to measure non motor fluctuations in people with PD (6, 10–15), none is specific to punctually assess the presence and severity of NpsyF. To fill this fundamental gap, our group has developed the neuropsychiatric fluctuation scale (NFS) (16). In 18 PD patients with motor fluctuations, we found a positive correlation between NFS-minus subscore and motor symptoms in the OFF medication condition, namely bradykinesia (17). The NFS-minus and NFS-plus subscores also inversely correlated with each other, depending on the medication condition. These findings suggest that the NFS can capture opposite conditions and identify both the ON and OFF non motor symptomes. Although tested in a small sample, the NFS was easily applied, and results matched the expected findings.
To date, the validation of the NFS is ongoing in France (ClinicalTrials.gov Identifier: NCT04455074), and it will be soon completed.
The aim of this study was to further analyze the psychometrics qualities of the NFS scale. We focused on the internal structure of the scale to identify the number of dimensions constituting the NFS, and the way the items are distributed across these dimensions. We studied the internal consistency, i.e., the degree of homogeneity among the items of the NFS, assessing whether they were consistent with one another and measured the same aspect. We also looked at the convergent and divergent valididity, i.e., the degree to which a measure is related to or divergent from another measure of which the underlying construct is conceptually related or unrelated. The specificity, i.e., the ability of the NFS to differentiate the PD population from the HC, and the sensitivity, i.e., the ability of the NFS to detect NpsyF, were also analyzed.
We collected retrospective data from PD patients hospitalised for evaluations before STN DBS surgery at the Movement Disorders center in the Centre Hospitalier Universitaire Grenoble Alpes (CHUGA) of Grenoble, France, from September 2016 to June 2021. Inclusion criteria were: diagnosis of idiopathic PD (18), presence of motor fluctuations, and no dementia.
Healthy controls (HC) were recruited from the general population through advertisements in companies, social networks, and caregiver associations. Inclusion criteria were: age between 40 and 75 years, and the absence of neurological or psychiatric disorders. All HC assessments were conducted online.
The Department of Clinical Research and Innovation (DRCI) of the CHUGA gave its approval for this research. PD patients have been fully informed of the objectives of the study and the nature of the informations collected, including their right to object at any time to the use of collected data. For HC, the ethics committee for research and teaching of the University of Savoie Mont Blanc approuved the study, and participants completed a consent form before starting the study.
All PD patients had an acute levodopa challenge after an overnight medication withdrawal (19). The NFS (see below) was administrated in both the OFF-and ON-medication conditions along with motor examination, using the movement disorders society-unified Parkinson disease rating scale (MDS-UPDRS) part III. Non motor signs (MDS-UPDRS part I), activities of daily living (MDS-UPDRS part 2), and motor complications (MDS-UPDRS part IV) were also collected (20). In the ON condition, patients also filled the Beck depression inventory (BDI-II) (21), the Starkstein apathy scale (22), and the non-motor fluctuations items of the Ardouin Scale for Behavioral Assessment in Parkinson’s Disease (ASBPD OFF and ON items) (23, 24). The cognitive global state and the executive functions were evaluated using the Mattis dementia rating scale (MDRS) (25) and the frontal score (26).
The HC group filled the NFS before and after a simple emotional induction. Since spontaneous and brief emotional fluctuations do not exist in the general population, they were artificially induced through an emotional induction task. The emotional induction procedure consisted of viewing 3 min video clips from the StimFilm database (27). Participants were randomly assigned to one of three experimental conditions (positive, negative, or neutral emotion induction). In addition, mood condition was examined with the Beck depression inventory (BDI-II).
Demographic data (age and gender for patients and HC), PD duration, and levodopa equivalent daily dosage (LEDD) were also recorded.
Figure 1 shows the study design.
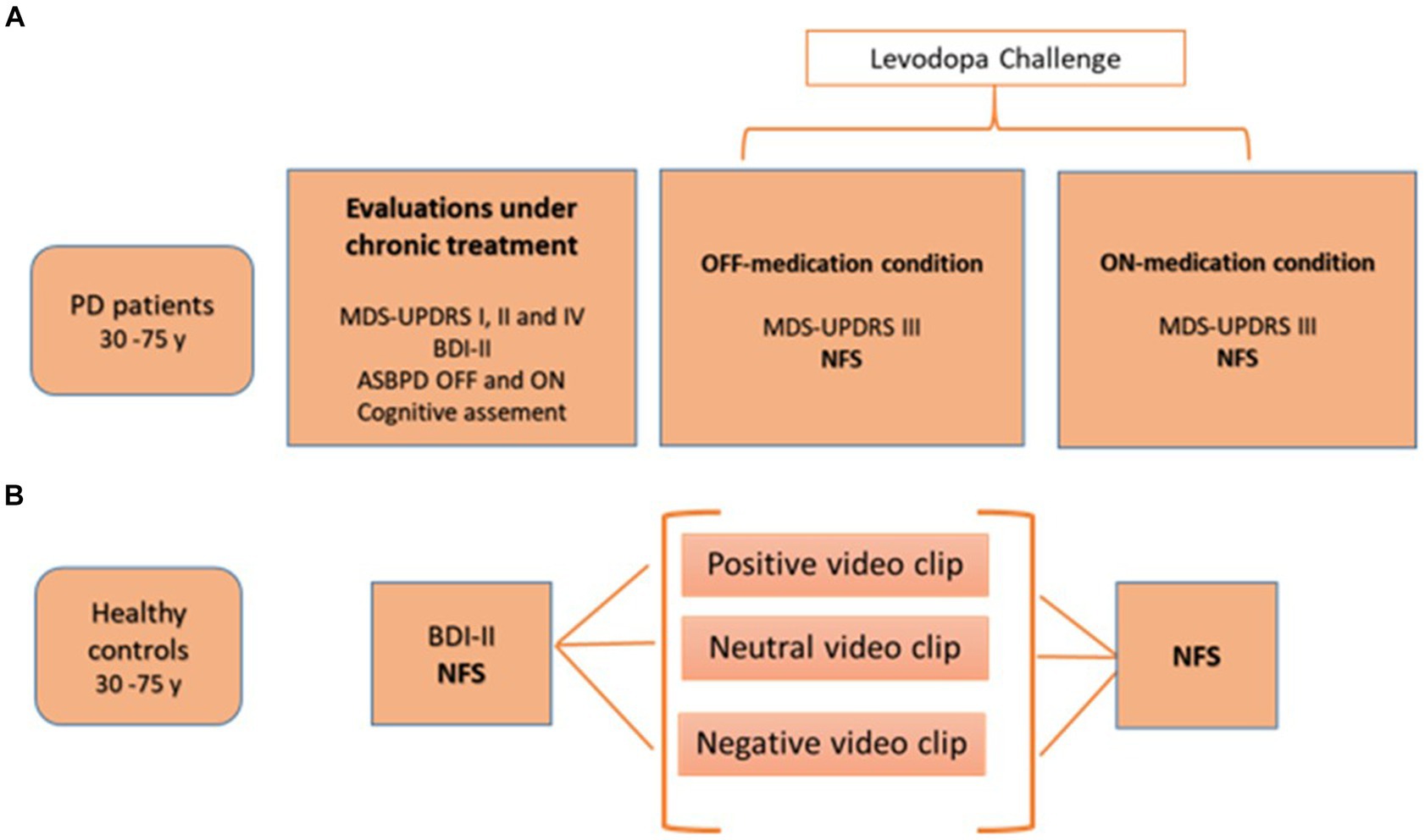
Figure 1. Study design for PD patients (A) and healthy controls (B). ASBPD, Ardouin scale for behavioral assessment in Parkinson’s disease; BDI-II, Beck depression inventory; MDS-UPDRS, movement disorders society-unified Parkinson disease rating scale; NFS, neuropsychiatric fluctuation scale; PD: Parkinson’s disease.
Briefly, the NFS is composed by 20 items, divided in two parts: the NFS-plus and the NFS-minus (16). The NFS-plus part includes NFS hyper-dopaminergic symptoms, i.e., items describing patients’ feelings in the ON-medication condition, such as euphoria and wellbeing. In the scale, the NFS-plus items are number 2, 3, 6, 7, 10, 11, 12,13, 17, and 18. The NFS-minus part corresponds to hypo-dopaminergic symptoms expressed during the OFF-medication condition, i.e., apathy, anxiety, attention issues, depression, and fatigue. In the scale, the NFS-minus items correspond to number 1, 4, 5, 8, 9, 14, 15, 16, 19, and 20. The NFS can be completed in the OFF-and the ON-medication condition. The scale provides two subscores with a maximal total score of 30 points each. The global score ranges from −30 to +30. A negative global score means that the OFF neuropsychological symptoms are predominant, whereas a positive global score reflects a majority of ON-neuropsychological symptoms. See Figure 2 for further details.
The main clinical and neuropsychological characteristics of PD patients and HC were described using means, standard deviations, and max-min data. The two groups were compared by age and depression criteria using t-tests. The normality of distribution for each variable was verified using Shapiro–Wilk test.
Analysis of the internal structure of the questionnaire was performed using a principal component analysis (PCA) with an Oblimin rotation. For PD patients, PCA was applied on global scores in each medication condition; for HC, PCA was applied on the global score before emotional induction.
The internal consistency of the scale was assessed by calculating the Cronbach’s alpha for global scores for HC and the patients (in both the ON-and OFF-medication conditions). Based on the usual recommendations, a 0.70–0.90 outcome was considered a good internal consistency (28).
Convergent and divergent validity of the NFS was measured using Spearman correlations for BDI-II, Starkstein apathy scale, and ASBPD non motor items. In the OFF-medication condition, we hypothesized that the minus subscore was positively correlated with the depression and apathy scales, and the ASBPD OFF item. Conversely, in the ON-medication condition, we expected that the plus subscore would be negatively correlated with these same psychologic scales.
The specificity of the NFS was evaluated by NFS global scores comparisons between: (a) HC after positive induction (HC+) and PD patients in the ON-medication condition; (b) HC after negative induction (HC−) and PD patient in the OFF-medication condition. The Mann Whitney test was used. We hypothesized that NFS global scores between PD patients and HC would be significantly different for each comparison.
Sensitivity of the NFS was assessed by comparing the ON-and the OFF-medication conditions for NFS global scores, and for minus and plus subscores using the Student test. We expected a significant difference between the NFS global scores in each medication condition, and also between the plus and minus subscores in each condition.
Data were analyzed using JASP software, version 0.16.4 (intel).
A total of 101 consecutive PD patients were included from September 2016 to June 2021. The main clinical characteristics of PD patients are detailed in Table 1.
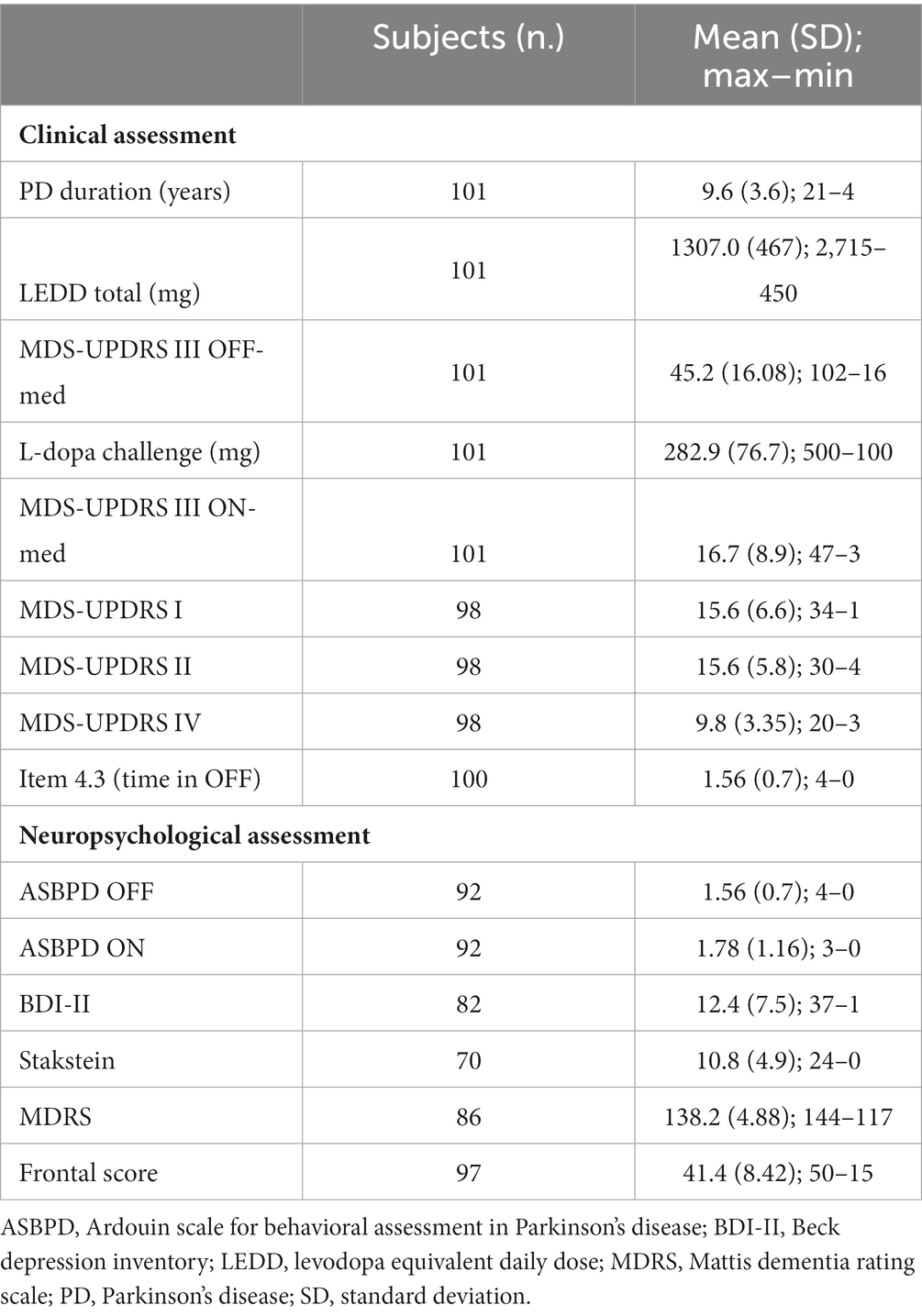
Table 1. Main clinical and neuropsychological characteristics of people with Parkinson’s disease (PD).
One hundred and eighty-one HC were included from January 2020 to November 2022.
Average age and mood condition were significantly different (p < 0.001) between the two groups. Table 2 shows PD and HC clinical caracteristics comparaison.
The NFS global scores did not follow the normal distribution, neither in the HC group (W = 0.94; p < 0.0001) nor in the PD group (OFF-medication condition: W = 0.94; p = 0.002/ON-medication condition: W = 0.95; p = 0.008).
PCA performed in PD patients in the OFF-and the ON-medication conditions, and in HC before emotional induction highlighted one dimension that explained 32, 39 and 42% of the variance, respectively.
In the OFF-medication condition, items 1, 4, 5, 8, 9, 14, 15, 16, 19, and 20 (corresponding to NFS-minus items) were positively related to this unique dimension. On the contrary, items 2, 3, 6, 7, 10, 11, 12, 17, and 18 (corresponding to NFS-plus items) were negatively related to this dimension. Item 13 showed a poor saturation in this dimension (<30).
Conversely, in the ON-medication condition, items 2, 3, 6, 7, 10, 11, 12, 13, 17, and 18 were positively related to the dimension, whereas items 1, 4, 9, 14, 15, 16, 19, and 20 were negatively related. Items 5 and 8 showed a poor saturation in this dimension (<30).
In HC, before emotional induction, items 2, 3, 6, 7, 10, 11, 12, 13, 17 and 18 were positively related to the unique dimension, whereas items 1, 4, 5, 8, 9, 14, 15, 16, and 20 were negatively related. Item 19 showed a poor saturation in this dimension (<30). The items’ distribution in HC was the same as that observed in PD patients in the ON-medication condition.
Table 3 shows the saturation of the NFS items in PD patient in the OFF-and ON-medication conditions, and in HC.
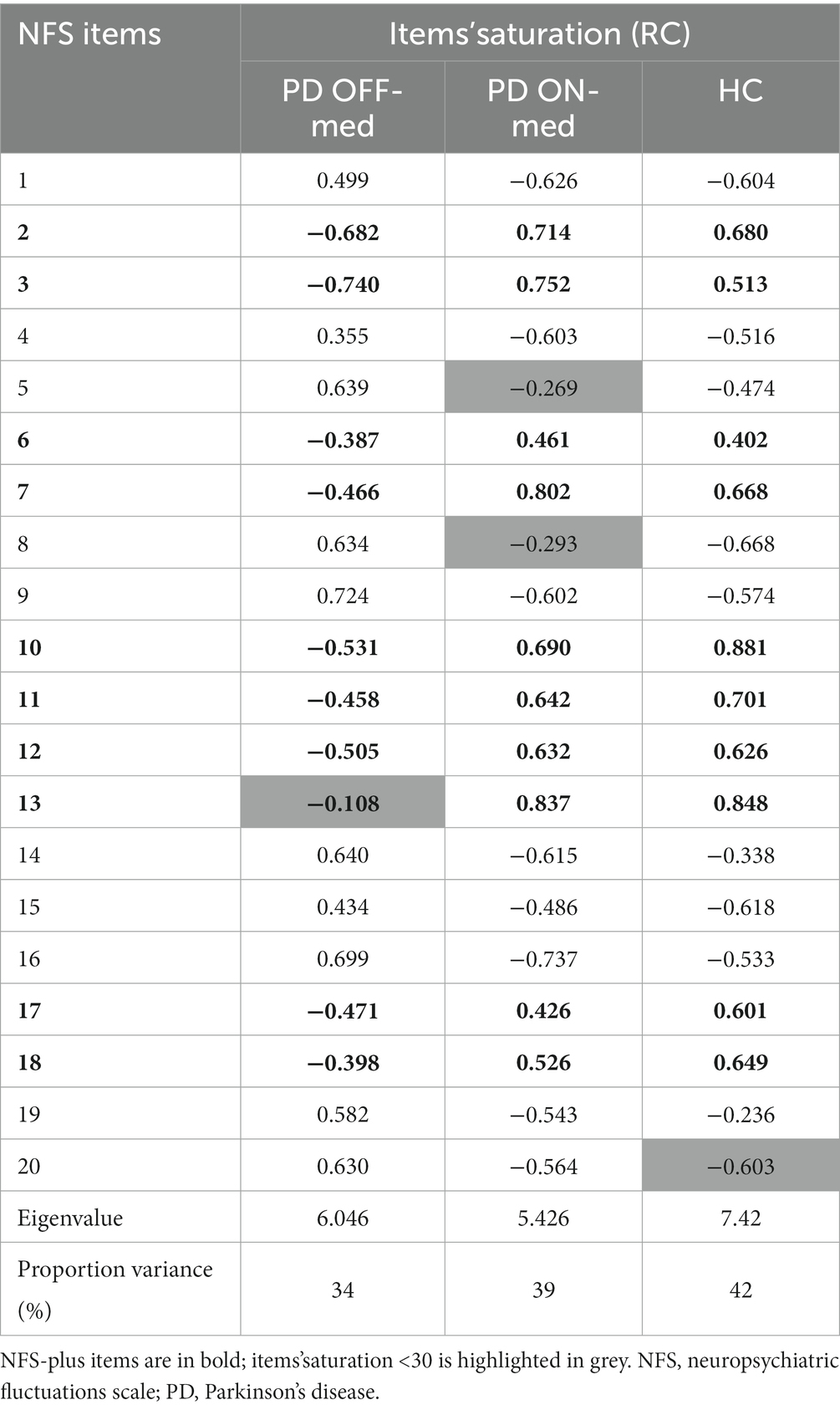
Table 3. Saturation of the NFS items for PD patient in the OFF-medication condition (PD OFF-med), and the ON-medication condition (PD ON-med), and for HC.
In the OFF-and ON-medication conditions, the Cronbach’s alpha coefficient for EFN NFS global scores was 0.88 and 0.89, respectively. For HC, the Cronbach’s alpha coefficient was 0.93.
In the OFF-medication condition, plus subscores did not show significant correlations with emotional scales (apathy, depression, and OFF non-motor fluctuations) whereas minus subscores showed a positive correlation with OFF ASBPD (p = 0.004) and with BDI (p = 0.04), but no correlation with apathy. In the ON-medication condition, only the Starkstein apathy scale correlated negatively with plus subscores (p = 0.0005).
In HC, minus subscores strongly correlated with the BDI-II (p < 0.001) whereas plus subscores were negatively correlated with the BDI-II (p < 0.001).
The NFS global scores comparison between HC+ (N = 54) and PD patients in the ON-medication condition, and between HC− (N = 83) and PD patients in the OFF-medication condition showed significant differences between the two populations in both condition (p < 0.0001). See Figure 3 for more details.
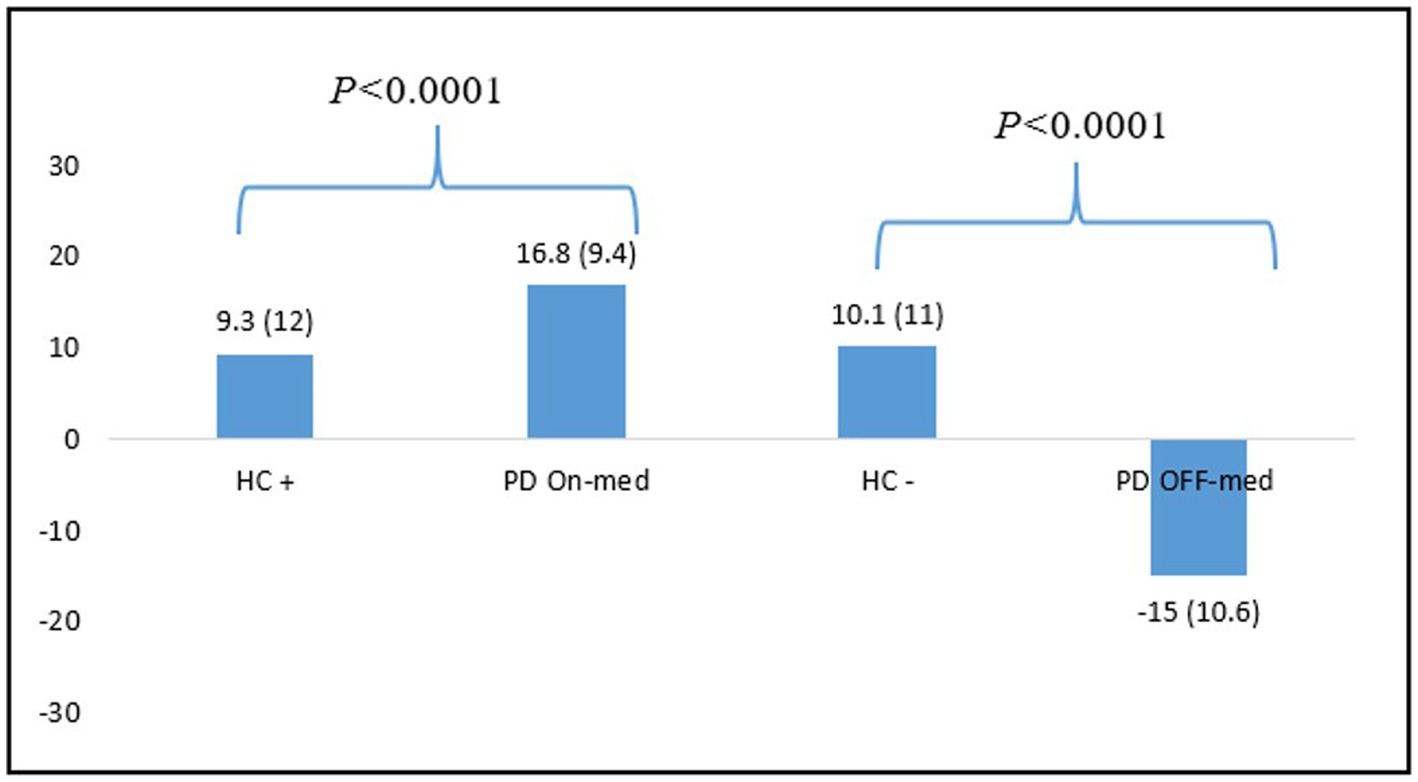
Figure 3. Differences between NFS global scores means for HC and PD patients in the ON-and OFF-medication conditions. HC, healthy controls; PD: Parkinson’s disease.
NFS-minus, NFS-plus, and NFS global scores all significantly differed between the OFF-and the ON-medication conditions (all p < 0.001). For details, see Table 4.
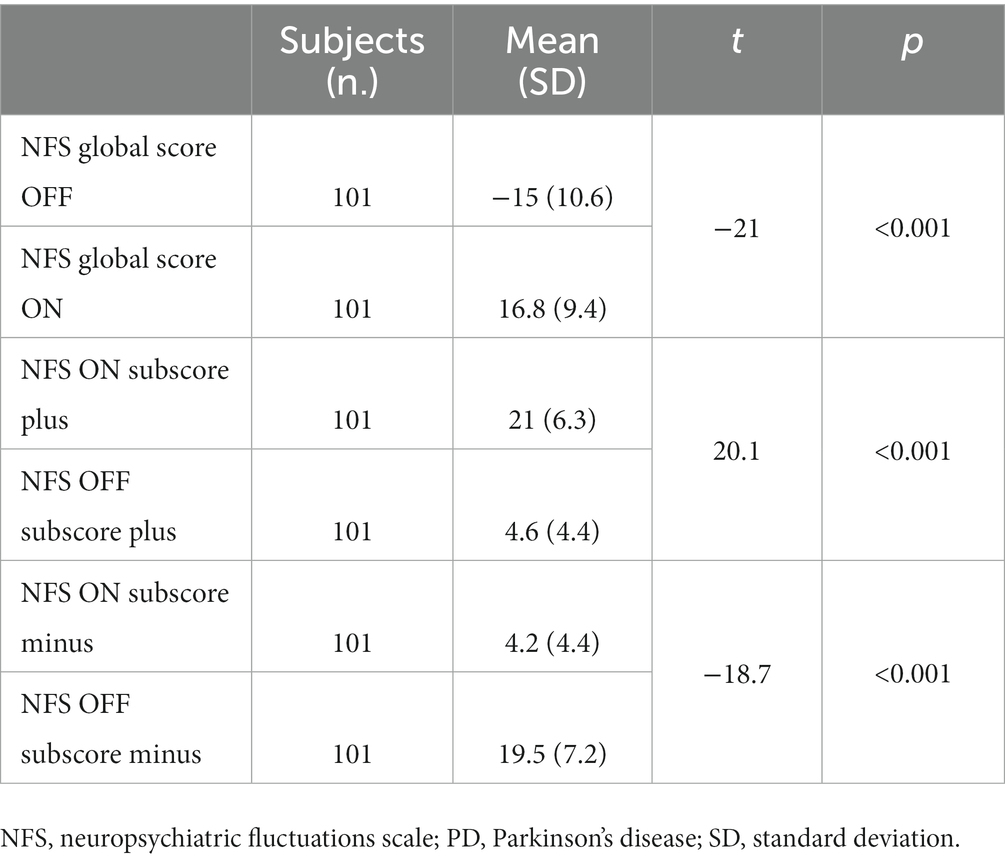
Table 4. Student’ test values between the NFS subscores and global scores in the ON-and OFF-medication conditions in PD patients.
The NFS has been designed to acutely detect and quantify subjective changes in neuropsychological conditions in PD patients with motor fluctuations, especially in acute settings (16). In our study, we have demonstrated that the NFS is sufficiently sensitive to measure neuropsychiatric variations related to dopaminergic status, specifically in PD patients.
This pre-validation of the psychometric qualities of the NFS shows that the internal structure of our tool, measured with the PCA, revolves around a single dimension, with some items positively and others negatively related to the dimension. For PD patients, the distribution of items that change according to the ON and OFF-medication conditions underlines that this unique dimension is probably the dopaminergic state. Therefore, the items’distribution in the OFF-medication condition reflects the hypo-dopaminergic state, whereas the items’ distribution in the ON-medication condition reflects the hyper-dopaminergic state. Several studies showing the effects of L-dopa treatment on mood, motivation, and also cognitive changes in non-demented patients have supported a dopaminergic involvement in NpsyF (29–32). NpsyF are likely linked to changes in the dopaminergic mesolimbic pathway. When the mesolimbic dopamine concentration is low, OFF-NpsyF (fatigue, depression, low motivation, and slowness of thinking) occur (33, 34). In contrast, the increase in dopamine concentration after dopaminergic drugs intake is associated with ON-NpsyF (feeling of well-being, increased motivation, better attentional functions), and also hyper-dopaminergic behaviors (35).
The PCA performed on HC shows the same items’ distribution as observed in PD patients in the ON-medication condition. The items of the NFS are also distributed around a single dimension. This shows consistency in the construction of the scale.
In PD population, the NFS global scores demonstrated a good internal consistency in both the OFF-and ON-medication conditions (alpha >0.80). The NFS internal consistency was also very good in HC (alpha >0.90). These results indicate that the items of the scale are sufficiently interconnected and measure the same construct.
For convergent and divergent validity, the correlations between NFS-plus and-minus subscores, scales measuring depression (BDI-II), apathy (Starktein), and a tool measuring NFpsy (OFF and ON ASBPD) show that, in the OFF-medication condition, the minus subscore is linked to the mood assessment and another scale measuring OFF dysphoria (ASBPD OFF), whereas the plus subscore is negatively correlated with depression. In the ON-medication condition, we found a negative correlation between the plus subscore and the scale measuring apathy. This suggests that the minus subscore highlights OFF dysphoria when PD patients are dopamine deprived, whereas the plus subscore reflects increased motivation and activity seeking when PD patients are under dopaminergic stimulation. This is consistent with previous studies describing NFpsy characteristics (7, 8, 36). We also found this pattern of correlation in HC in whom minus subscore correlated with the depression scale, and a plus subscore negatively correlated on the same measure. This confirms that the NFS’s subscores can reflect two opposite mood states even in HC.
We also demonstrated that the NFS global score allows to properly differentiate HC from the PD patients in each condition. PD patients showed significantly higher scores than the control population. In the OFF-medication condition, patients showed very negative scores, reflecting a hypo-dopaminergic mood, whereas HC still had positive overall scores, showing a stable mood despite the induction. Conversely, in the ON-medication condition, patients had significantly more positive scores than HC after positive induction. These findings show that the NFS captures hyper-dopaminergic euphoria or hypomania specific to fluctuating PD patients, whereas HC stand in a standard positive mood even after positive induction. These results are consistent with data from the literature showing that the prevalence of mood disorders (depression, dysphoria, anxiety) and hypomania is greater in PD than in the general population (37, 38).
Our study has several limitations. First, the HC population and PD participants differed in terms of age and mood condition, which may limit the comparison between the two groups. Moreover, some concerns about the effectiveness of the emotional induction procedure chosen for the HC may arise. Although the presentation of emotional videos guarantees a more robust induction over time than presentation of emotional pictures, we cannot ensure that a single viewing was sufficient to induce a relevant emotional change (39). Furthermore, for the negative-valence videos, a medium valence was chosen in agreement with the ethical committee, to guarantee psychic safety to the participants, which may have contributed to diminishing the effect of the induction. Additionally, although our scale aims to assess acute mood changes, it lacks the evaluation of test-retest stability to have a complete study of the psychometric qualities of the scale.
Nevertheless, our pre-validation study shows that the NFS is an acceptable tool for detecting and evaluating NpsyF in PD. The NFS allows to define different profiles of fluctuating patients: patients with predominant OFF neuropsychiatric symptoms (who experience major dysphoria when treatment is ineffective, and return to a state of emotional homeostasis, without hypomania, when treatment is effective), patients with predominant ON neuropsychiatric symptoms (euphoric mood, compulsive and hyperactive behaviours in the ON phase, and a stable, non-depressive mood in the OFF phase), and also patients with both ON and OFF severe neuropsychiatric symptoms (moving from dysthymic to hypomanic periods, with few intervals of mood stability).This distinction can allow to optimize the medical treatment and overall management of different phenotypes of PD.
The NFS can also be used in chronic situations to detect neuropsychiatric profiles such as hypo-dopaminergic or hyper-dopaminergic syndromes. Overall, we can use the NFS in the same way and in addition to MDS-UPDRS part III, either to capture a chronic condition or to highlight fluctuations by repeating the measurements under different conditions.
Neuropsychiatric fluctuations are frequent in PD. These changes in mood and cognitive status experienced by PD patients severely influence their quality of life and experience of the disease. The NFS seems to be a reliable and handy tool for measuring NpsyF in acute setting in the PD population, thus helping physician to better personalize the patients’ management.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Department of Clinical Research and Innovation (DRCI) of the CHUGA and ethics committee for research and teaching of the University of Savoie Mont Blanc. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
ES, VF, AK, and AC: data collection. ES, BD, MB, and EM: conception, design of the work, data analysis, interpretation, and drafting the article. ES, MB, and EM: critical revision of the article and final approval of the version to be published. All authors contributed to the article and approved the submitted version.
The authors thank to all PD patients for giving their consent the use their medical data. The authors also thank the HC for their participation to our study. The authors thank Paul Krack and Pablo Martinez-Martin for their contribution to the development of the preliminary version of the Neuropsychiatric Fluctuation Scale (not used in this study).
EM has received honoraria from Medtronic for consulting services. She has also received research grant support from the Grenoble Alpes University, Abbott, Ipsen, and France Parkinson.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Armstrong, MJ, Rastgardani, T, Gagliardi, AR, and Marras, C. Impact of off periods on persons with Parkinson disease and care partners: a qualitative study. Neurol Clin Pract. (2021) 11:e232–8. doi: 10.1212/CPJ.0000000000000921
2. Franke, C, and Storch, A. Chapter thirty-three—nonmotor fluctuations in Parkinson’s disease. Int Rev Neurobiol. (2017) 134:947–71. doi: 10.1016/bs.irn.2017.05.021
3. Riley, DE, and Lang, AE. The spectrum of levodopa-related fluctuations in Parkinson’s disease. Neurology. (1993) 43:1459–64. doi: 10.1212/WNL.43.8.1459
4. Witjas, T, Kaphan, E, Azulay, JP, Blin, O, Ceccaldi, M, Pouget, J, et al. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology. (2002) 59:408–13. doi: 10.1212/WNL.59.3.408
5. Seki, M, Takahashi, K, Uematsu, D, Mihara, B, Morita, Y, Isozumi, K, et al. Clinical features and varieties of non-motor fluctuations in Parkinson’s disease: a Japanese multicenter study. Parkinsonism Relat Disord. (2013) 19:104–8. doi: 10.1016/j.parkreldis.2012.08.004
6. Stacy, M, Bowron, A, Guttman, M, Hauser, R, Hughes, K, Larsen, JP, et al. Identification of motor and nonmotor wearing-off in Parkinson’s disease: comparison of a patient questionnaire versus a clinician assessment. Mov Disord. (2005) 20:726–33. doi: 10.1002/mds.20383
7. Storch, A, Schneider, CB, Wolz, M, Stürwald, Y, Nebe, A, Odin, P, et al. Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology. (2013) 80:800–9. doi: 10.1212/WNL.0b013e318285c0ed
8. Martínez-Fernández, R, Schmitt, E, Martinez-Martin, P, and Krack, P. The hidden sister of motor fluctuations in Parkinson’s disease: a review on nonmotor fluctuations. Mov Disord. (2016) 31:1080–94. doi: 10.1002/mds.26731
9. Thobois, S, Ardouin, C, Lhommée, E, Klinger, H, Lagrange, C, Xie, J, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain J Neurol. (2010) 133:1111–27. doi: 10.1093/brain/awq032
10. Faggianelli, F, Loundou, A, Baumstarck, K, Nathalie, S, Auquier, P, Eusebio, A, et al. Validation of a non-motor fluctuations questionnaire in Parkinson’s disease. Rev Neurol. (2021) 178:347–54. doi: 10.1016/j.neurol.2021.06.013
11. Kleiner-Fisman, G, Martine, R, Lang, AE, and Stern, MB. Development of a non-motor fluctuation assessment instrument for Parkinson disease. Parkinsons Dis. (2011) 2011:292719:1–13. doi: 10.4061/2011/292719
12. Martinez-Martin, P, and Hernandez, B, Q10 Study Group. The Q10 questionnaire for detection of wearing-off phenomena in Parkinson’s disease. Parkinsonism Relat Disord. (2012) 18:382–5. doi: 10.1016/j.parkreldis.2011.12.011
13. Martinez-Martin, P, Tolosa, E, Hernandez, B, and Badia, X, ValidQUICK Study Group. Validation of the “QUICK” questionnaire—a tool for diagnosis of “wearing-off” in patients with Parkinson’s disease. Mov Disord. (2008) 23:830–6. doi: 10.1002/mds.21944
14. Stacy, MA, Murphy, JM, Greeley, DR, Stewart, RM, Murck, H, Meng, X, et al. The sensitivity and specificity of the 9-item wearing-off questionnaire. Parkinsonism Relat Disord. (2008) 14:205–12. doi: 10.1016/j.parkreldis.2007.07.013
15. Storch, A, Schneider, CB, Klingelhöfer, L, Odin, P, Fuchs, G, Jost, WH, et al. Quantitative assessment of non-motor fluctuations in Parkinson’s disease using the non-motor symptoms scale (NMSS). J Neural Transm. (2015) 122:1673–84. doi: 10.1007/s00702-015-1437-x
16. Schmitt, E, Krack, P, Castrioto, A, Klinger, H, Bichon, A, Lhommée, E, et al. The neuropsychiatric fluctuations scale for Parkinson’s disease: a pilot study. Mov Disord Clin Pract. (2018, 2018) 5:265. doi: 10.1002/mdc3.12607
17. Del Prete, E, Schmitt, E, Meoni, S, Fraix, V, Castrioto, A, Pelissier, P, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. (2022) 43:3641–7. doi: 10.1007/s10072-021-05833-8
18. Postuma, RB, Berg, D, Stern, M, Werner Poewe, C, Olanow, W, Oertel, W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
19. Albanese, A, Bonuccelli, U, Brefel, C, Chaudhuri, KR, Colosimo, C, Eichhorn, T, et al. Consensus statement on the role of acute dopaminergic challenge in Parkinson’s disease. Mov Disord. (2001) 16:197–201. doi: 10.1002/mds.1069
20. Goetz, CG, Tilley, BC, Shaftman, SR, Stebbins, GT, Fahn, S, Martinez-Martin, P, et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. doi: 10.1002/mds.22340
21. Beck, AT, Steer, RA, Ball, R, and Ranieri, W. Comparison of Beck depression inventories-IA and-II in psychiatric outpatients. J Pers Assess. (1996) 67:588–97. doi: 10.1207/s15327752jpa6703_13
22. Starkstein, SE, Mayberg, HS, Preziosi, TJ, Andrezejewski, P, Leiguarda, R, and Robinson, RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. (1992) 4:134–9. doi: 10.1176/jnp.4.2.134
23. Ardouin, C, Chéreau, I, Llorca, P-M, Lhommée, E, Durif, F, Pollak, P, et al. Assessment of hyper-and hypodopaminergic behaviors in Parkinson’s disease. Rev Neurol. (2009) 165:845–56. doi: 10.1016/j.neurol.2009.06.003
24. Rieu, I, Martinez-Martin, P, Pereira, B, De Chazeron, I, Verhagen Metman, L, Jahanshahi, M, et al. International validation of a behavioral scale in Parkinson’s disease without dementia. Mov Disord. (2015) 30:705–13. doi: 10.1002/mds.26223
25. Marson, DC, Dymek, MP, Duke, LW, and Harrell, LE. Subscale validity of the Mattis dementia rating scale. Arch Clin Neuropsychol. (1997) 12:269–75. doi: 10.1093/arclin/12.3.269
26. Dubois, B, Pillon, B, Legault, F, Agid, Y, and Lhermitte, F. Slowing of cognitive processing in progressive Supranuclear palsy: a comparison with Parkinson’s disease. Arch Neurol. (1988) 45:1194–9. doi: 10.1001/archneur.1988.00520350032011
27. Schaefer, A, Nils, F, Sanchez, X, and Philippot, P. Assessing the effectiveness of a large database of emotion-eliciting films: a new tool for emotion researchers. Cognit Emot. (2010) 24:1153–72. doi: 10.1080/02699930903274322
29. Cools, R, Stefanova, E, Barker, RA, Robbins, TW, and Owen, AM. Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain J Neurol. (2002) 125:584–94. doi: 10.1093/brain/awf052
30. Kulisevsky, J, Avila, A, Barbanoj, M, Antonijoan, R, Berthier, ML, and Gironell, A. Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson’s disease patients at different levodopa plasma levels. Brain. (1996) 119:2121–32. doi: 10.1093/brain/119.6.2121
31. Maricle, RA, Nutt, JG, and Carter, JH. Mood and anxiety fluctuation in Parkinson’s disease associated with levodopa infusion: preliminary findings. Mov Disord. (1995) 10:329–32. doi: 10.1002/mds.870100316
32. Maricle, RA, Nutt, JG, Valentine, RJ, and Carter, JH. Dose-response relationship of levodopa with mood and anxiety in fluctuating Parkinson’s disease: a double-blind, placebo-controlled study. Neurology. (1995) 45:1757–60. doi: 10.1212/WNL.45.9.1757
33. Castrioto, A, Thobois, S, Carnicella, S, Maillet, A, and Krack, P. Emotional manifestations of PD: neurobiological basis. Mov Disord. (2016) 31:1103–13. doi: 10.1002/mds.26587
34. Thobois, S, Lhommée, E, Klinger, H, Ardouin, C, Schmitt, E, Bichon, A, et al. Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with Piribedil. Brain. (2013) 136:1568–77. doi: 10.1093/brain/awt067
35. Béreau, M, Fleury, V, Bouthour, W, Castrioto, A, Lhommée, E, and Krack, P. Hyperdopaminergic behavioral spectrum in Parkinson’s disease: a review. Rev Neurol. (2018) 174:653–63. doi: 10.1016/j.neurol.2018.07.005
36. Lhommée, E, Klinger, H, Thobois, S, Schmitt, E, Ardouin, C, Bichon, A, et al. Subthalamic stimulation in Parkinson’s disease: restoring the balance of motivated Behaviours. Brain J Neurol. (2012) 135:1463–77. doi: 10.1093/brain/aws078
37. Nuti, A, Ceravolo, R, Piccinni, A, Dell’Agnello, G, Bellini, G, Gambaccini, G, et al. Psychiatric comorbidity in a population of Parkinson’s disease patients. Eur J Neurol. (2004) 11:315–20. doi: 10.1111/j.1468-1331.2004.00781.x
38. Vilas, D, Pont-Sunyer, C, and Tolosa, E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. (2012) 18:S80–4. doi: 10.1016/S1353-8020(11)70026-8
Keywords: psychometric characteristics, scale, validation, Parkinson‘s disease, neuropsychiatric fluctuations
Citation: Schmitt E, Debu B, Castrioto A, Kistner A, Fraix V, Bouvard M and Moro E (2023) Fluctuations in Parkinson’s disease and personalized medicine: bridging the gap with the neuropsychiatric fluctuation scale. Front. Neurol. 14:1242484. doi: 10.3389/fneur.2023.1242484
Received: 21 June 2023; Accepted: 02 August 2023;
Published: 17 August 2023.
Edited by:
Emilia Mabel Gatto, Sanatorio de la Trinidad Mitre, ArgentinaReviewed by:
Jesus Perez Perez, Universitat Autònoma de Barcelona, SpainCopyright © 2023 Schmitt, Debu, Castrioto, Kistner, Fraix, Bouvard and Moro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emmanuelle Schmitt, RVNjaG1pdHRAY2h1LWdyZW5vYmxlLmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.