- Eye Hospital China Academy of Chinese Medical Sciences, Beijing, China
Objective: This study aimed to evaluate the retina and microvascular alterations with optical coherence tomography (OCT) or optical coherence tomography angiography (OCTA) in patients with migraine with aura (MA) and migraine without aura (MO).
Methods: PubMed, Embase, and Cochrane Library databases were searched to find relevant literature on patients with MA or MO using OCT/OCTA devices. The eligible data were analyzed by Stata Software (version 15.0).
Results: There were 16 studies identified, involving 379 eyes with MA, 583 eyes with MO, and 658 eyes of healthy controls. The thickness of the peripapillary retinal nerve fiber layer (pRNFL) of patients with MA decreased significantly in most regions. The foveal avascular zone (FAZ) area and perimeter in MA patients significantly enlarged, while the perfusion density (PD) in the macular deep capillary plexus (mDCP) significantly decreased in the whole image and its subregions except for the fovea, with the PD in radial peripapillary capillary (RPC) decreasing inside the disk. Patients with MO demonstrated a significantly decreased thickness of pRNFL in most regions, and the FAZ parameters were significantly enlarged. No statistical significance was observed in the retina and microvascular features of patients with MA and MO.
Conclusion: The eyes affected by MA and MO demonstrated significantly reduced thickness of pRNFL and enlarged FAZ. Patients with MA showed retinal microvascular impairments, including a decreased PD in mDCP. The OCT and OCTA could detect membrane morphology and circulation status in migraine and might provide the basis for the diagnosis and follow-up of patients with migraine.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, CRD42023397653.
1. Introduction
Migraine is a common neurovascular disorder with a prevalence of 15–18% in the population (1). It is clinically characterized by recurrent, mostly unilateral, moderate-to-severe throbbing pain (2). Episodic migraine is clinically classified as migraine with aura (MA) and migraine without aura (MO). Visual aura is reported as the most common aura symptom, often manifesting as flashes of light, dark spots, and decreased visual acuity (3). Previous data were inadequate for the diagnosis and treatment of migraine. Therefore, it is important to determine reliable biomarkers that could be employed for the clinical diagnosis, condition assessment, and prognostic follow-up of patients with migraine.
Although the pathogenesis of migraine is not fully understood, an increasing amount of evidence supports the involvement of the neurovascular system in the development of the disease (4). In 1979, Moskowitz et al. proposed the “trigeminovascular theory,” which suggested that the trigeminal vascular pain pathway is a common pathway that leads to the development of migraine (5). The trigeminovascular system (TGVS) consists of the trigeminal nucleus, the trigeminal ganglion, the trigeminal nerve, and its innervated meningeal and ocular vascular networks. The activation of the TGVS increases the release of neurotransmitters and vasoactive intestinal peptides, which activates injurious receptors that transmit pain signals to the center via the trigeminal nociceptive afferent fibers, resulting in pain (6). It has also been found that during visual aura or migraine attacks, perfusion deficit can be caused by reduced blood flow due to transient vasospasm, which occurs not only in the cranial but also in the ocular vasculature. Note that recurrent migraine attacks can cause permanent damage to the brain and retina (7, 8). Migraine has been reported to be closely associated with various neurovascular eye diseases such as retinal artery occlusion, ischemic optic neuropathy, and glaucoma (9–12). Therefore, it needs to be clarified whether migraine causes ischemia and structural changes in the retina and optic disk, which makes patients with migraine more susceptible to these ocular diseases.
OCT and OCTA can now be used to qualitatively and quantitatively detect the status of the retinal nerve fiber layer (RNFL), retina, and optic nerve papillae circulation. Therefore, they are currently used as clinical biomarkers for various neurological diseases (13, 14). While previous studies on migraine hemodynamics focused on the brain using fMRI (15, 16), several recent clinical studies have reported alterations in the retina and retinal perfusion in patients with migraine, which may provide a way to understand the ocular physiopathology observed with migraine (17, 18).
Although many studies have evaluated and reported retina and microvascular alterations in patients with migraine, different and even contradictory results have been obtained. The present meta-analysis combines the OCT and OCTA results about the retinal structure and perfusion in patients with episodic migraine from different studies to compare the differences between patients with episodic migraine and healthy controls and the differences between the different subtypes of MA and MO.
2. Materials and methods
The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (19). The PRISMA checklist is detailed in Supplementary File S1.
2.1. Search strategy
PubMed, Embase, and Cochrane Library databases were searched for research published from February 2018 to February 2023. The following subject terms and keywords were used to identify relevant studies: “migraine” AND “optical coherence tomography” OR “optical coherence tomography angiography” OR “OCT” OR “OCTA.” Detailed search strategies are provided in Supplementary File S2.
2.2. Inclusion and exclusion criteria
The following inclusion criteria were employed: (1) Patients diagnosed with migraine according to the criteria of the International Classification of Headache Disorders, third version (ICHD-3) (2). The detailed diagnostic criteria for migraine are provided in Supplementary File S3. (3) The OCT or OCTA was used to observe the retina and microvascular alterations.
The following exclusion criteria were applied: (1) absence of any comparative studies; (2) insufficient data for meta-analysis; (3) unmatched study object; and (4) conference abstract.
2.3. Data collection and risk of bias assessments
Literature screening was carried out independently by two researchers and cross-checked. In case of any disagreement, it was discussed by the two researchers or settled through third-party arbitration. The data include details on basic trial information, population, location, sex, age, mean migraine duration, attack numbers per month, MIDAS score, OCT/OCTA device, scan sizes, and outcome variables.
This study used the Newcastle–Ottawa Scale (NOS). Two authors evaluated the risk of bias in the included studies from three submissions: selection, comparability, and outcome. All studies were assigned a scale of 0–9.
2.4. Data analysis
Statistical analysis was performed using Stata Software version 15.0. We estimated the weighted mean differences (WMDs) and 95% confidence intervals (CIs). The heterogeneity was estimated with I2 statistics. If the heterogeneity was substantial (I2 > 50%), a random effect model was employed. Sensitivity or subgroup analysis was used to identify and eliminate the source of the heterogeneity. Descriptive analysis was used if there was still a substantial remaining heterogeneity. Egger’s linear regression test was used to statistically evaluate the potential publication bias.
3. Results
3.1. Search results
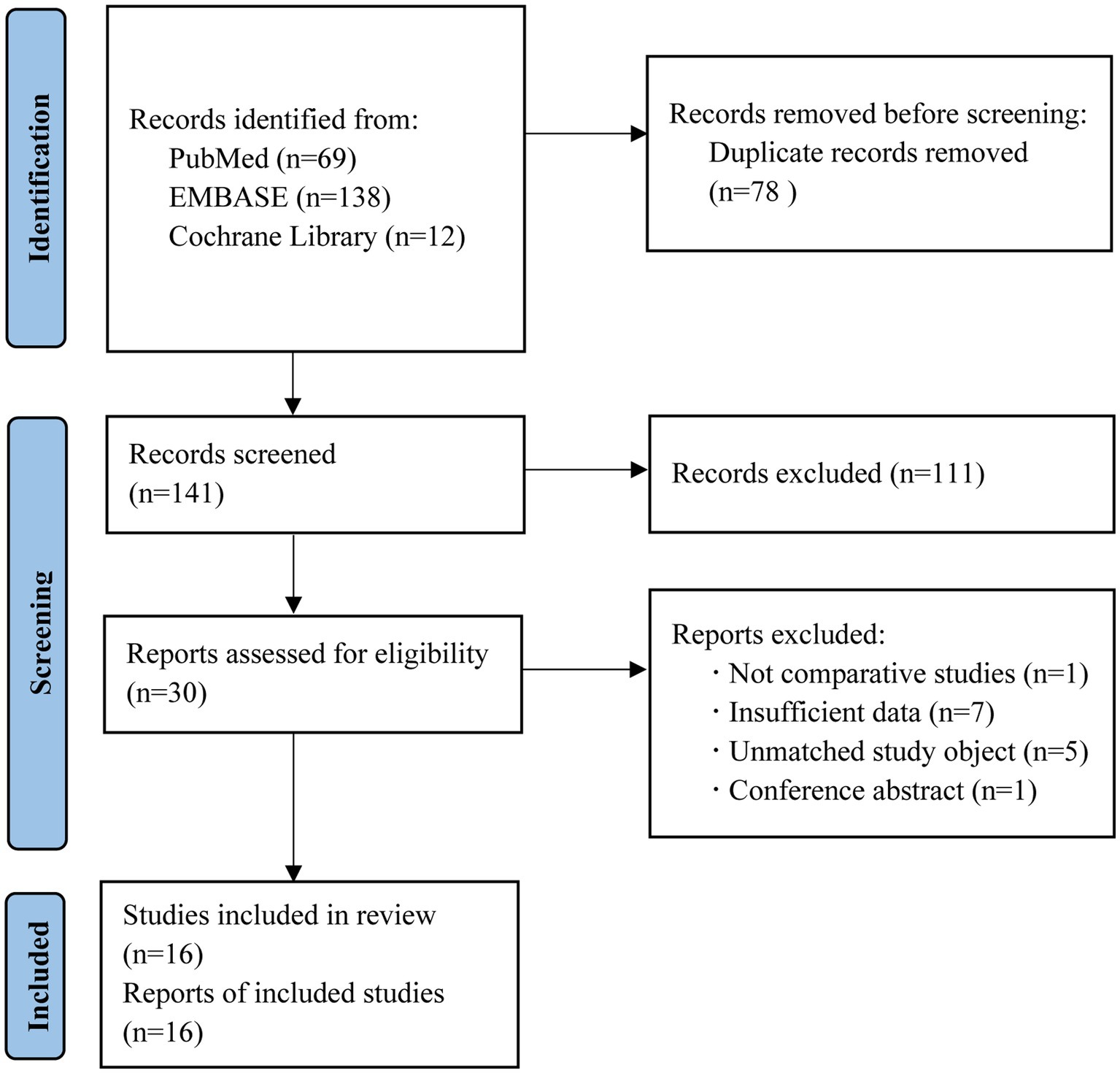
We retrieved 219 records from PubMed, Embase, and Cochrane library databases. Among these, 78 duplicates were removed. Among the remaining 141 records, 111 were deleted after reading their titles and abstracts and 14 were removed after closely reading their full text. Finally, 16 studies were selected. The flowchart of the search is shown in Figure 1.
3.2. Characteristics and risk of bias assessment of included studies
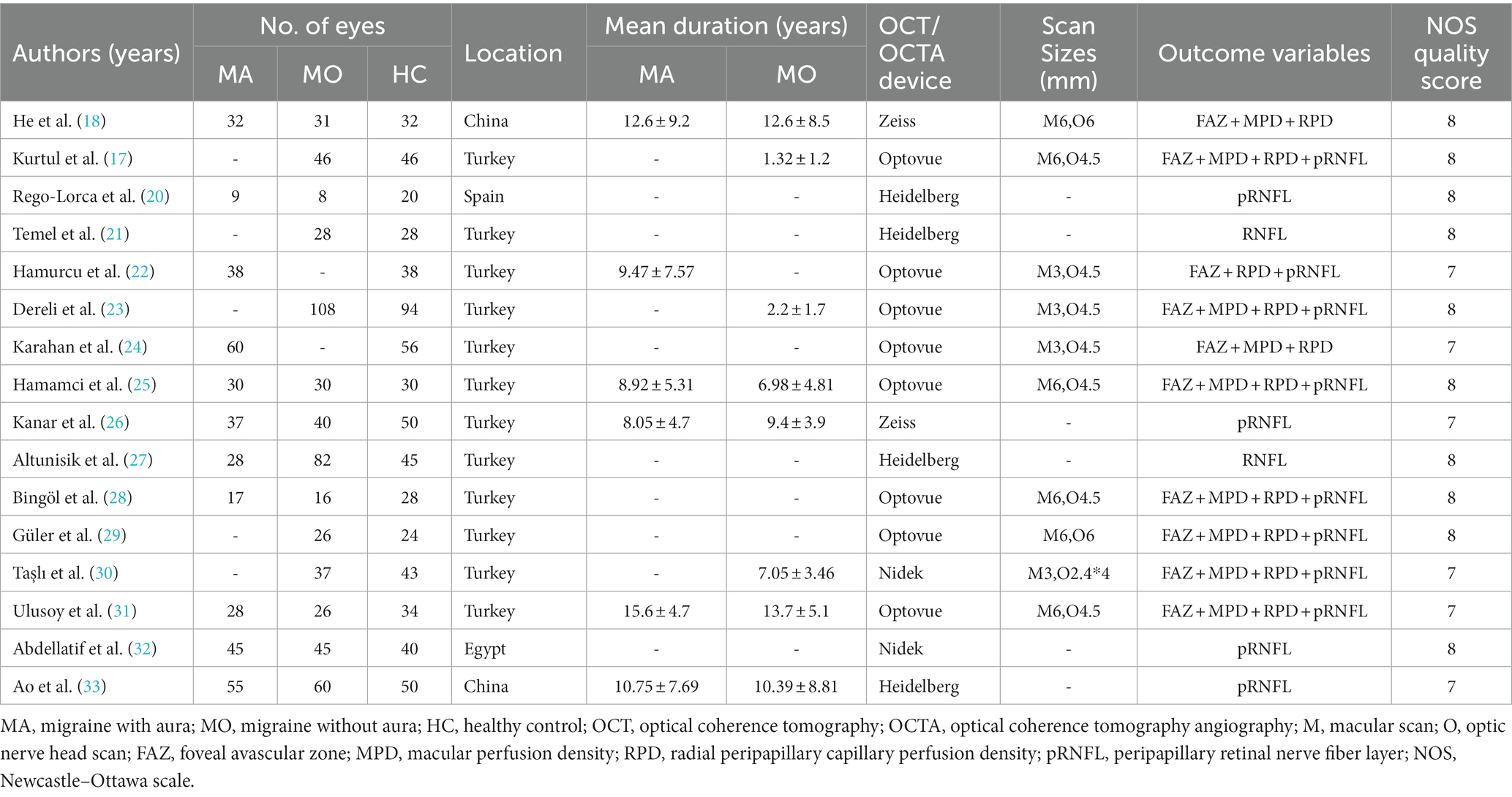
The final 16 studies had 379 eyes with MA, 583 eyes with MO, and 658 eyes with healthy controls. No significant age- or gender-based difference was found between the groups. All of them reported the type of OCT/OCTA devices used (Zeiss, Optovue, Heidelberg, and Nidek). The detailed characteristics of the studies and the location, disease duration, and outcome variables are listed in Table 1.
The results of the quality evaluation are shown in Table 1. The results showed that 10 studies scored 8 and 6 studies scored 7, indicating the high quality of the studies. These details are also listed in Supplementary File S4.
3.3. Meta-analysis
3.3.1. Meta-analysis for MA versus healthy controls
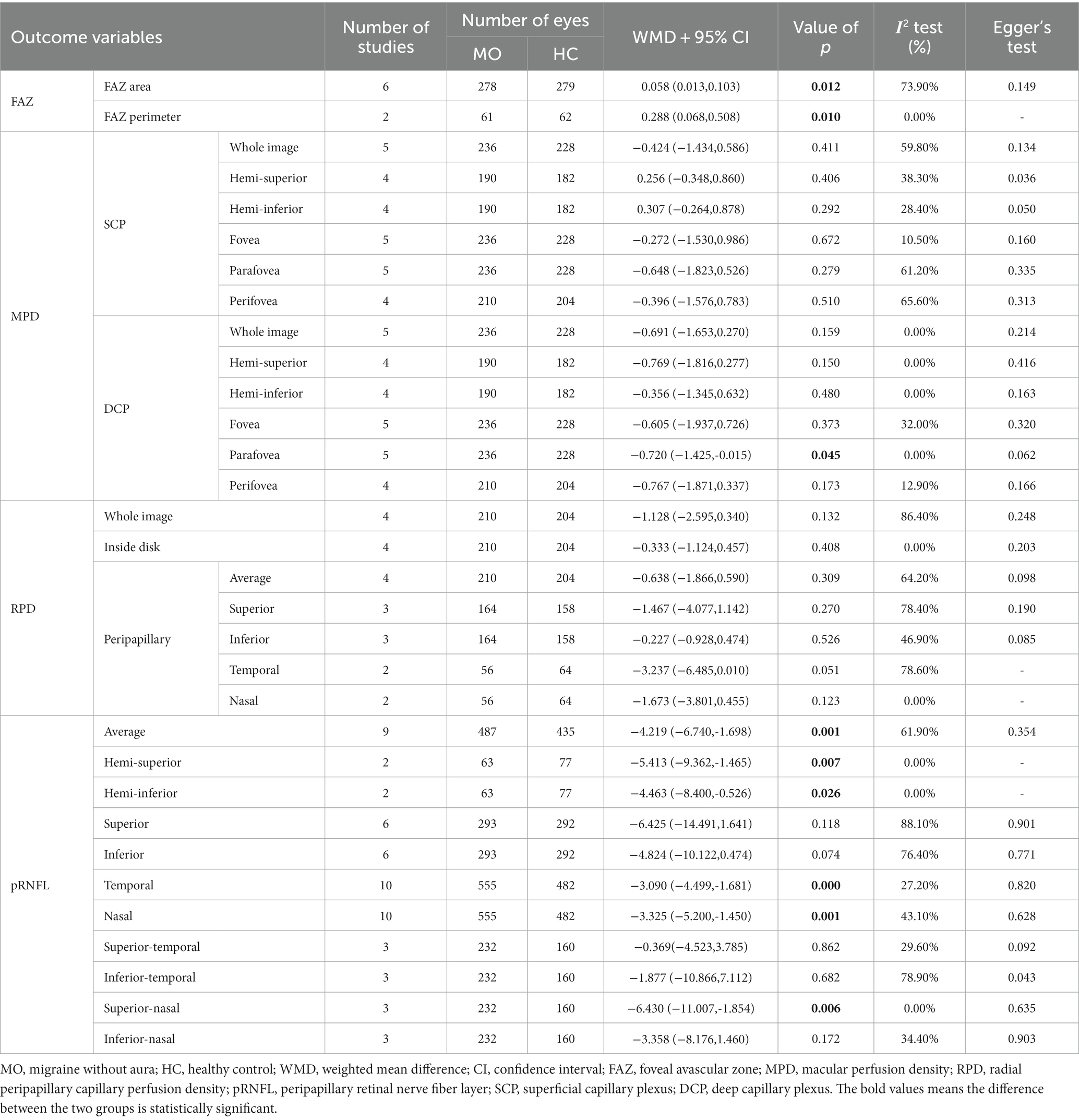
Table 2 shows the main finding of the meta-analysis on the FAZ, the perfusion density (PD) of the macula superficial capillary plexus (mSCP) and macula deep capillary plexus (mSDP), the PD of the radial peripapillary capillary (RPC), and the thickness of the peripapillary RNFL (pRNFL). When compared to that of the healthy controls, a significant increase was observed in the FAZ area and perimeter in patients with MA, while the PD of the mDCP significantly decreased in the whole image and its subregions, except for the fovea. There was little difference between the two groups for the PD in the RPC, except for the PD inside the disk. Additionally, our results demonstrated a considerably reduced thickness of the pRNFL on average as well as that of several peripapillary regions (hemi-superior, hemi-inferior superior, nasal, temporal, inferior-temporal, and inferior-nasal quadrants). The detailed forest plots (MA vs. HC) are shown in Supplementary File S5.
3.3.2. Meta-analysis for MO versus healthy controls
We also performed a meta-analysis on the FAZ and pRNFL thickness and determined the PD of mSCP, mDCP, and RPC of MO eyes and healthy control eyes (Table 3). For FAZ parameters, the area and perimeter were significantly enlarged in patients with MO than those of the eyes of healthy controls. For the OCTA metrics, no significant difference was found in the PD of the macular and RPC regions, except for the PD of mDCP in the parafovea. Moreover, our results showed significantly reduced pRNFL thickness on average, as well as that of several peripapillary regions (hemi-superior, hemi-inferior superior, nasal, temporal, and superior-nasal quadrants). The detailed forest plots (MO vs. HC) are shown in Supplementary File S6.
3.3.3. Meta-analysis for MA versus MO
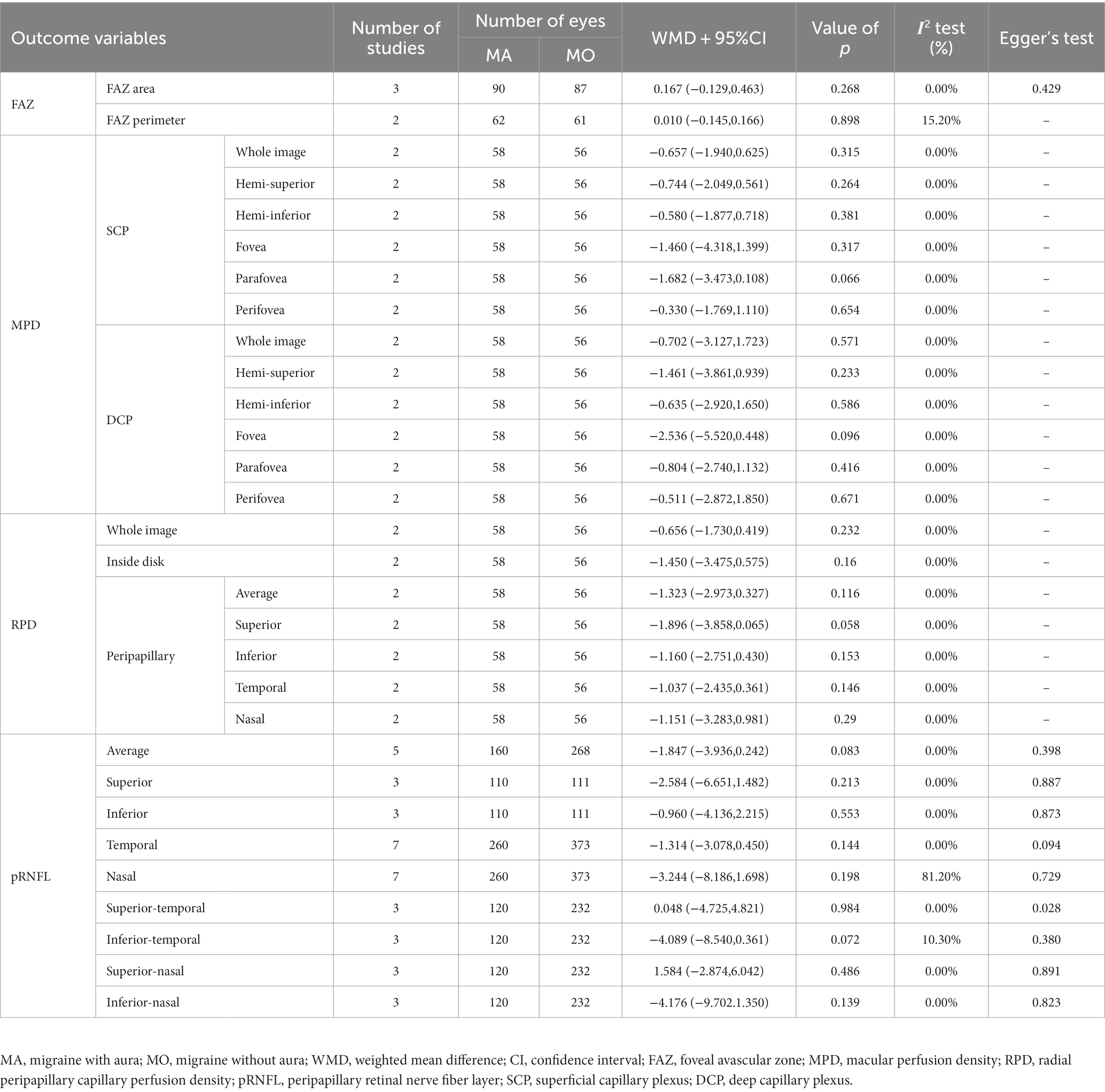
The outcomes of the quantitative synthesis on the eyes with MA and MO included the FAZ, pRNFL thickness, and PD of the mSCP, mDCP, and RPC (Table 4). For all measures, there was no discernible difference between the two groups (p > 0.05).
3.4. Sensitivity analysis and subgroup analysis
We performed sensitivity analysis by removing one study in each of the direct comparisons. When we omitted Karahan et al. (24), the five comparisons (fovea PD in mSCP, parafovea PD in mSCP, fovea PD in mDCP, peripapillary average PD in RPC, and peripapillary-nasal PD in the RPC) became statistically significant (p < 0.05). For the peripapillary-superior and nasal PD in the RPC, the removal of Hamurcu et al. (22) contributed the most to the heterogeneity. However, their absence had no impact on the outcomes. The detailed sensitivity analysis is shown in Supplementary File S7.
After a thorough review of the characteristics, we next performed a subgroup analysis according to the age of patients and OCT/OCTA devices. The results showed that the age and the OCT/OCTA device were the sources of heterogeneity in several comparisons. The detailed subgroup analysis is provided in Supplementary File S8.
3.5. Publication bias
In the majority of the comparisons, Egger’s tests revealed that there was no publication bias (p ≥ 0.05), except for the analysis of the PD of the mSCP in the hemi-superior and fovea regions (MA vs. HC), the PD of the RPC in the peripapillary-inferior quadrant (MA vs. HC), and the pRNFL thickness in the inferior-temporal quadrant (MO vs. HC). Egger’s test results are shown in Tables 2–4.
4. Discussion
The present meta-analysis utilized OCT and OCTA parameters, such as pRNFL thickness, FAZ area, MPD, and PD of RPC, to analyze the retina and microvascular alterations between MA and healthy controls, MO and healthy controls, and MA and MO eyes. The main results are as follows: (1) Compared to healthy controls, the thickness of the pRNFL of patients with MA significantly decreased in most areas except in the inferior, superior-temporal, and superior-nasal quadrants, while the area and circumference of the FAZ significantly increased. The PD of mDCP in the patients with MA significantly decreased in the whole image and its subregions except for the fovea. The PD of the RPC also significantly decreased inside the disk. (2) The average thickness of pRNFL of the patients with MO, as well as that of several peripapillary regions, decreased, while the FAZ parameters significantly increased. The PD of the mSCP, mDCP, and RPC was not significantly different, except for the mDCP in the parafovea. (3) No statistical significance was observed in the retina and microvascular features of the patients with MA and MO. These results suggest that there is a certain degree of optic nerve and retinal microvascular damage in patients with migraine, especially those with MA.
The thinning of RNFL thickness in patients with migraine was first reported by Martinez et al. in 2008 (34) The thinned RNFL was mostly located in specific quadrants around the optic nerve papillae. The present meta-analysis found a significant decrease in the average pRNFL thickness in patients with migraine, consistent with most of the previous studies. Some possible causes for this decrease include abnormal vascular changes such as abnormal vascular regulation and focal cerebral ischemia. To explain the above, Kara et al., in as early as 2003, applied color Doppler ultrasound and observed that the blood flow in the central retinal and posterior ciliary arteries was lower in patients with migraine than that in normal healthy subjects (35). Migraine aura or attack can change the retinal and nerve blood supply, which can lead to ischemic and hypoxic injury, which may in turn damage the retinal nerve. pRNFL is composed of nerve fibers consisting of retinal ganglion cell axons, which are mostly unmyelinated and require more energy supply and therefore are more vulnerable to retinal ischemic injury (36). The present meta-analysis found significant quadrant-specific pRNFL thinning in patients with migraine, concentrated in the superior hemisphere, inferior hemisphere, and temporal and nasal quadrants. This selective pRNFL involvement may be related to differences in the retinal ganglion cell axon sensitivity to the local ischemia and focal perimetric changes. The abnormal blood supply to the fundus due to migraine occurs mostly in these specific regions (37).
The FAZ is a region jointly delineated by both superficial and deep capillaries. This region is round or oval in healthy individuals, with an area of approximately 0.231–0.280 mm2. Any changes in its size and shape can indirectly lead to macular microvascular changes (38, 39). Previous studies have shown that the FAZ enlarges in various systemic diseases (40). However, changes in the FAZ have not been clearly established in patients with migraine. The meta-results showed a significant increase in the area and circumference of the FAZ in patients with migraine, including those with MA and MO, which may be closely related to retinal ischemia caused by vasospastic alterations in these patients. Recurrent attacks of migraine in these patients cause transient spasms of intracranial and intraocular vessels, resulting in acute and chronic microvascular and perfusion changes in retinal vessels, with the consequent enlargement of the FAZ area and circumference. However, clinical studies have also yielded inconsistent results. Kurtul et al. (17) found no significant changes in the FAZ of patients with migraine. Karahan et al. (24) found changes only in the deep FAZ area in patients with MA. These results suggest that there are individual differences in the FAZ size, which are closely related to various factors such as the macular central recess structure, eye axis length, age, race, and gender (41).
To avoid conceptual confusion, we uniformly applied PD to express the ratio between the blood flow area to the total scan area. A major advantage of OCTA over conventional fundus fluoroscopy is that the former allows quantitative measurement of the macular and optic papillary perfusion density. The present meta-analysis showed that compared with healthy controls, the PD of the mDCP in patients with MA significantly decreased in the whole image and its subregions except for the fovea, which may be closely related to the retinal ischemia caused by MA. The development of MA symptoms is currently considered to be a clinical manifestation of cortical spreading depression (CSD), a depolarizing wave that propagates along the cerebral cortex and has an inhibitory effect on the cortical function, which increases the cortical cerebral blood flow and disturbances in neurovascular coupling. Studies have shown that CSD during MA episodes can lead to a change from an initial transient increase in the blood flow to hypoperfusion changes in the cerebral cortex. However, its electrophysiological correlation with ocular vascular nerves has not been confirmed (1, 42). Based on its retinal localization propagation over the visual cortex, the characteristics of visual defects and imaging studies indirectly suggest a correlation with the ocular vasculature (43). Therefore, the above discussion could indicate the presence of intracranial and ocular retinal microvascular injuries and ischemic changes in patients with MA. In the PD of the RPC, our current meta-analysis found almost no significant difference between patients with migraine and healthy controls. A further sensitivity analysis showed significant differences between MA and HC in the average PD of the RPC and in some specific quadrants (superior, inferior, and nasal) after excluding Hamurcu et al. (22) and Karahan et al. (24). Hence, it can be suggested that patients with MA show some degree of reduced PD of the RPC, while the overall difference is not statistically significant. Hence, the optic nerve papilla could be more resistant to ischemia, hypoxia, and inflammation than the central macular sulcus (17). In addition, the small sample size included in the study could explain why PD in the RPC appears unaffected.
The current meta-analysis further compared the retinal and microvascular characteristics of the two subgroups of MA and MO and found that patients with MA had an enlarged FAZ area and circumference and a reduced macular and optic disk PD. However, the differences were not statistically significant, which is somewhat different from the previous meta-analysis results (44). The posterior regions of the cerebral hemispheres were usually more significantly malperfused during migraine attacks in patients with MA compared to patients with MO (45). The lack of statistical difference between the two in the current meta-analysis could be owing to various factors such as the sample size of the included studies, migraine variables, and ethnic differences. Hence, large prospective cohort studies are needed for validating the results of this meta-analysis.
This is a comprehensive assessment of the retina and microvascular alterations in patients with MA and MO using systematic review and meta-analysis, providing an evidence-based basis for the characteristic fundus manifestations of migraine. Compared with previous literature published by Ke W et al. (46), this meta-analysis further compares the thickness of the pRNFL and the optic disk PD to evaluate the retina and optic microvascular alterations. However, the study has some limitations: (1) Sensitivity analysis: exclusion of literature such as Karahan et al. (24) and Hamurcu et al. (22) can lead to alterations in some macular and optic disk PD results; therefore, caution should be taken with the above meta-analysis results. (2) Careful sensitivity analysis and subgroup analysis showed that some comparisons had large heterogeneity possibly because of the sample size, ethnic differences, and migraine variable correlations. (3) Owing to the lack of information in the literature, factors that may affect OCTA measurements were not analyzed. (4) The majority of the studies included in the current meta-analysis were from Turkey, and there is a certain bias, so the results should be analyzed with caution. (5) The sample size of some comparisons was relatively small, which could cause a bias. (6) Few articles reported systemic diseases that may affect retinal microcirculation. Therefore, we could not analyze these potential confounding factors.
5. Conclusion
The eyes of patients with MA and MO demonstrated significant pRNFL thickness impairments in some regions. Patients with MA showed retinal microvascular impairments, including FAZ enlargement and decreased PD in the mDCP. An enlarged FAZ in patients with MO was also shown. The OCT and OCTA could detect membrane morphology and circulation status in migraine and might provide the basis for the diagnosis and follow-up of patients with migraine.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZL: conceptualization, methodology, formal analysis, and writing the original draft. XH and YD: methodology and supervision. WZ, JYW, and YL: visualization and review editing. JWW: language editing and supervision. CJ: conceptualization, funding, and project administration. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China (general program No. 81874494), the Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (No. CI2021A02604), the Natural Science Foundation of Beijing Municipality (No. 7232325), Beijing Traditional Chinese Medicine Technology Development Fund (No. BJZYYB-2023-57).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1241778/full#supplementary-material
References
1. Goadsby, PJ, Holland, PR, Martins-Oliveira, M, Hoffmann, J, Schankin, C, and Akerman, S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. (2017) 97:553–622. doi: 10.1152/physrev.00034.2015
2. Agosti, R. Migraine burden of disease: from the Patient's experience to a socio-economic view. Headache. (2018) 58:17–32. doi: 10.1111/head.13301
3. International Headache Society. Headache classification Committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd Edition. Cephalalgia. (2018):1468–2982. doi: 10.1177/0333102417738202
4. Burstein, R, Noseda, R, and Borsook, D. Migraine: Multiple processes, complex pathophysiology. J Neurosci. (2015) 35:6619–29. doi: 10.1523/JNEUROSCI.0373-15.2015
5. Moskowitz, MA, Reinhard, JF Jr, Romero, J, Melamed, E, and Pettibone, DJ. Neurotransmitters and the fifth cranial nerve: is there a relation to the headache phase of migraine? Lancet. (1979) 2:883–5. doi: 10.1016/s0140-6736(79)92692-8
6. Khan, J, Asoom, LIA, Sunni, AA, Rafique, N, Latif, R, Saif, SA, et al. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed Pharmacother. (2021) 139:111557–6007. doi: 10.1016/j.biopha.2021.111557
7. Tsokolas, G, Tsaousis, KT, Diakonis, VF, Matsou, A, and Tyradellis, S. Optical coherence tomography angiography in neurodegenerative diseases: a review. Eye Brain. (2020) 12:73–87. doi: 10.2147/EB.S193026
8. Fu, T, Liu, L, Huang, X, Zhang, D, Gao, Y, Yin, X, et al. Cerebral blood flow alterations in migraine patients with and without Aura: An arterial spin labeling study. J Headache Pain. (2022) 23:131. doi: 10.1186/s10194-022-01501-0
9. McKendrick, A, and Nguyen, B. The eye in migraine: A review of retinal imaging findings in migraine. Clin Exp Optom. (2022) 105:186–93. doi: 10.1080/08164622.2021.1971045
10. Al-Moujahed, A, Tran, EM, Azad, A, Vail, D, Ludwig, CA, Pasricha, MV, et al. Risk of retinal artery occlusion in patients with migraine. Am J Ophthalmol. (2021) 225:157–65. doi: 10.1016/j.ajo.2020.11.004
11. Chhabra, N, Chiang, C, Di Nome, MA, Houghton, O, Carlin, RE, O'Carroll, CB, et al. Migrainous infarction of the eye: two cases of monocular ischemic complications associated with retinal migraine. Cephalalgia. (2022) 42:553–6. doi: 10.1177/03331024211056286
12. Drance, S, Anderson, DR, and Schulzer, M. Risk factors for progression of visual field abnormalities in Normal-tension Glaucoma. Am J Ophthalmol. (2001) 131:699–708. doi: 10.1016/s0002-9394(01)00964-3
13. Chan, VTT, Sun, Z, Tang, S, Chen, LJ, Wong, A, Tham, CC, et al. Spectral-domain OCT measurements in Alzheimer's disease: a systematic review and meta-analysis. Ophthalmology. (2019) 126:497–510. doi: 10.1016/j.ophtha.2018.08.009
14. Liu, J, Song, S, Gu, X, Li, H, and Yu, X. Microvascular impairments detected by optical coherence tomography angiography in multiple sclerosis patients: a systematic review and meta-analysis. Front Neurosci. (2023) 16:1899. doi: 10.3389/fnins.2022.1121899
15. Messina, R, Gollion, C, Christensen, RH, and Amin, FM. Functional MRI in migraine. Curr Opin Neurol. (2022) 35:328–35. doi: 10.1097/WCO.0000000000001060
16. Bashir, A, Lipton, R, Ashina, S, Ashina, S, Ashina, M, and Ashina, M. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology. (2013) 81:1260–8. doi: 10.1212/WNL.0b013e3182a6cb32
17. Kurtul, BE, Sipal, C, and Akbas, Y. Assessment of the optic disc and retinal microvasculature by optical coherence tomography angiography in patients with pediatric migraine. J Neuroophthalmol. (2022) 43:191–6. doi: 10.1097/WNO.0000000000001697
18. He, N, Shao, H, He, J, Zhang, X, Ye, D, and Lv, Z. Evaluation of retinal vessel and perfusion density in migraine patients by optical coherence tomography angiography. Photodiagn Photodyn Ther. (2022) 40:103060. doi: 10.1016/j.pdpdt.2022.103060
19. Hutton, B, Salanti, G, Caldwell, DM, Chaimani, A, Schmid, CH, Cameron, C, et al. The Prisma extension statement for reporting of systematic reviews incorporating network Meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
20. Rego-Lorca, D, Burgos-Blasco, B, Ginés-Gallego, C, Carrasco-López-Brea, M, de Santos-Moreno, MT, and Santos-Bueso, E. Retinal nerve Fiber layer analysis in children with migraine with and without Aura using optical coherence tomography: a case-control study. J Pediatr Ophthalmol Strabismus. (2022) 60:196–202. doi: 10.3928/01913913-20220516-01
21. Temel, E, Aşikgarip, N, Koçak, Y, Şahin, C, Özcan, G, Kocamiş, Ö, et al. Choroidal vascularity index and retinal nerve fiber layer reflectivity in newly diagnosed migraine patients. Photodiagn Photodyn Ther. (2021) 36:102531. doi: 10.1016/j.pdpdt.2021.102531
22. Hamurcu, MS, Gultekin, BP, Koca, S, and Ece, SD. Evaluation of migraine patients with optical coherence tomography angiography. Int Ophthalmol. (2021) 41:3929–33. doi: 10.1007/s10792-021-01962-3
23. Dereli, CG, Can, ME, and Ekici, A. Evaluation of retinal microvasculature and foveal avascular zone by the optical coherence tomography angiography in pediatric migraine patients. Acta Neurol Belg. (2021) 121:1449–55. doi: 10.1007/s13760-020-01325-2
24. Karahan, M, Erdem, S, Ava, S, Kaya, AA, Demirtas, AA, and Keklikci, U. Evaluation of retinal and optic nerve vasculature by optic coherence tomography angiography in migraine with Aura. J Fr Ophtalmol. (2021) 44:1396–402. doi: 10.1016/j.jfo.2021.02.018
25. Hamamci, M, Songur, MS, Aslan, BS, and Bayhan, HA. Is ocular vascularity affected in young migraine patients? A Pilot Study. J Clin Neurosci. (2021) 91:144–51. doi: 10.1016/j.jocn.2021.06.045
26. Kanar, HS, Toz, HT, and Penbe, A. Comparison of retinal nerve Fiber layer, macular ganglion cell complex and choroidal thickness in patients with migraine with and without Aura by using optical coherence tomography. Photodiagn Photodyn Ther. (2021) 34:102323. doi: 10.1016/j.pdpdt.2021.102323
27. Altunisik, E, and Oren, B. Retinal neurovascular structural changes in optical coherence tomography and the relationship between these changes and white matter Hyperintensities in patients with migraine. Eur Neurol. (2021) 84:460–71. doi: 10.1159/000518380
28. Bingöl, KP, Özcan, G, Özer, F, Togay, IC, and Atilla, H. Evaluation of retinal vessel density and Choriocapillaris flow in migraine patients with and without Aura. Graefes Arch Clin Exp Ophthalmol. (2020) 258:2517–21. doi: 10.1007/s00417-020-04805-6
29. Güler, Ö, Güler, M, Tuğan, YCB, and Hakkoymaz, H. Are retinal and Peripapillary blood flows affected during migraine attack? Neuroophthalmology. (2020) 44:299–306. doi: 10.1080/01658107.2020.1752260
30. Taşlı, NG, and Ersoy, A. Altered macular vasculature in migraine patients without Aura: is it associated with ocular vasculature and white matter Hyperintensities? J Ophthalmol. (2020) 2020:1–8. doi: 10.1155/2020/3412490
31. Ulusoy, MO, Horasanlı, B, and Kal, A. Retinal vascular density evaluation of migraine patients with and without Aura and Association with white matter Hyperintensities. Acta Neurol Belg. (2019) 119:411–7. doi: 10.1007/s13760-019-01094-7
32. Abdellatif, MK, and Fouad, MM. Effect of duration and severity of migraine on retinal nerve Fiber layer, ganglion cell layer, and choroidal thickness. Eur J Ophthalmol. (2018) 28:714–21. doi: 10.1177/1120672117750054
33. Ao, R, Wang, R, Yang, M, Wei, S, Shi, X, and Yu, S. Altered retinal nerve fiber layer thickness and choroid thickness in patients with migraine. Eur Neurol. (2018) 80:130–7. doi: 10.1159/000494671
34. Martinez, A, Proupim, N, and Sanchez, M. Retinal nerve fibre layer thickness measurements using optical coherence tomography in migraine patients. Br J Ophthalmol. (2008) 92:1069–75. doi: 10.1136/bjo.2008.137471
35. Kara, SA, Erdemoğlu, AK, Karadeniz, MY, and Altinok, D. Color Doppler sonography of orbital and vertebral arteries in Migraineurs without Aura. J Clin Ultrasound. (2003) 31:308–14. doi: 10.1002/jcu.10181
36. Wang, L, Dong, J, Cull, G, Fortune, B, and Cioffi, GA. Varicosities of Intraretinal ganglion cell axons in human and nonhuman Primates. Invest Ophthalmol Vis Sci. (2003) 44:2–9. doi: 10.1167/iovs.02-0333
37. Gunes, A, Demirci, S, Tok, L, Tok, O, Demirci, S, and Kutluhan, S. Is retinal nerve Fiber layer thickness change related to headache lateralization in migraine? Korean J Ophthalmol. (2016) 30:134–9. doi: 10.3341/kjo.2016.30.2.134
38. Li, H, Yu, X, Zheng, B, Ding, S, Mu, Z, and Guo, L. Early neurovascular changes in the retina in preclinical diabetic retinopathy and its relation with blood glucose. BMC Ophthalmol. (2021) 21:220. doi: 10.1186/s12886-021-01975-7
39. Tick, S, Rossant, F, Ghorbel, I, Gaudric, A, Sahel, JA, Chaumet-Riffaud, P, et al. Foveal shape and structure in a Normal population. Invest Ophthalmol Vis Sci. (2011) 52:5105–10. doi: 10.1167/iovs.10-7005
40. Zhang, B, Chou, Y, Zhao, X, Yang, J, and Chen, Y. Early detection of microvascular impairments with optical coherence tomography angiography in diabetic patients without clinical retinopathy: a meta-analysis. Am J Ophthalmol. (2021) 222:226–37. doi: 10.1016/j.ajo.2020.09.032
41. Abay, RN, Akdeniz, G, Katipoğlu, Z, and Kerimoğlu, H. Normative data assessment of age-related changes in macular and optic nerve head vessel density using optical coherence tomography angiography. Photodiagn Photodyn Ther. (2022) 37:102624. doi: 10.1016/j.pdpdt.2021.102624
42. Piilgaard, H, and Lauritzen, M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J Cereb Blood Flow Metab. (2009) 29:1517–27. doi: 10.1038/jcbfm.2009.73
43. Lauritzen, M, Dreier, JP, Fabricius, M, Hartings, JA, Graf, R, and Strong, AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. (2011) 31:17–35. doi: 10.1038/jcbfm.2010.191
44. Lin, X, Yi, Z, Zhang, X, Liu, Q, Zhang, H, Cai, R, et al. Retinal nerve Fiber layer changes in migraine: a systematic review and Meta-analysis. Neurol Sci. (2021) 42:871–81. doi: 10.1007/s10072-020-04992-4
45. Romozzi, M, Cuffaro, G, Rollo, E, Mattei, R, Marcelli, S, Rizzo, S, et al. Microvascular involvement in migraine: an optical coherence tomography angiography study. J Neurol. (2023) 270:4024–30. doi: 10.1007/s00415-023-11697-z
Keywords: migraine, microvascular alterations, retinal nerve fiber layer, meta-analysis, OCTA, OCT
Citation: Liu Z, Jie C, Wang J, Hou X, Zhang W, Wang J, Deng Y and Li Y (2023) Retina and microvascular alterations in migraine: a systemic review and meta-analysis. Front. Neurol. 14:1241778. doi: 10.3389/fneur.2023.1241778
Edited by:
Alessandra Rufa, University of Siena, ItalyReviewed by:
Federico Ricardi, University of Turin, ItalyGema Esquiva, University of Alicante, Spain
Copyright © 2023 Liu, Jie, Wang, Hou, Zhang, Wang, Deng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanhong Jie, amllY2h1YW5ob25nQDE2My5jb20=
 Ziqiang Liu
Ziqiang Liu Chuanhong Jie*
Chuanhong Jie*