- 1Department of Physical Therapy, Virginia Commonwealth University, Richmond, VA, United States
- 2Department of Physical Medicine and Rehabilitation, Virginia Commonwealth University, Richmond, VA, United States
- 3Department of Biostatistics, Virginia Commonwealth University, Richmond, VA, United States
- 4Department of Health and Kinesiology, University of Utah, Salt Lake City, UT, United States
- 5Department of Physical Medicine and Rehabilitation Services, James A. Haley Veterans’ Hospital, Tampa, FL, United States
- 6Department of Physical Medicine and Rehabilitation, Michael E. DeBakey VA Medical Center, Houston, TX, United States
- 7Baylor College of Medicine, Houston, TX, United States
- 8Department of Neurology, University of Utah, Salt Lake City, UT, United States
- 9Minneapolis VA Health Care System, Minneapolis, MN, United States
- 10Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis, MN, United States
- 11Hunter Holmes McGuire Veterans Affairs Medical Center, Richmond, VA, United States
Introduction: Among patients with traumatic brain injury (TBI), balance problems often persist alongside hearing and vision impairments that lead to poorer outcomes of functional independence. As such, the ability to regain premorbid independent gait may be dictated by the level of sensory acuity or processing decrements that are shown following TBI assessment. This study explores the relationships between standardized sensory acuity and processing outcomes to postural balance and gait speed.
Methods: Secondary analysis was performed on the Long-Term Impact of Military- Relevant Brain Injury Consortium Chronic Effects of Neurotrauma Consortium LIMBIC (CENC) data set. Separate regression analyses were carried out for each of the balance assessments (via Computerized Dynamic Posturography, CDP) and walking speed.
Discussion: TBI frequency was significantly related to the majority of single CDP outcomes (i.e., Conditions 2–6), while various sensory processing outcomes had task-specific influences. Hearing impairments and auditory processing decrements presented with lower CDP scores (CDP Conditions 3,5,6, and 1–3 respectively), whereas greater visual processing scores were associated with better CDP scores for Conditions 2,5, and 6. In sum, patients with TBI had similar scores on static balance tests compared to non-TBI, but when the balance task got more difficult patients with TBI scored worse on the balance tests. Additionally, stronger associations with sensory processing than sensory acuity measures may indicate that patients with TBI have increased fall risk.
1. Introduction
Patients with traumatic brain injury (TBI) often have chronically persisting symptoms of dizziness, nausea, and postural instability. This includes patients with mild TBI, in whom balance and gait problems can persist for more than 3 months (1–8), especially when caused by blast exposure (e.g., military injury, industrial accidents) (9). Besides balance impairments, blast-related TBI has been associated with a loss of hearing (19%), vision (34%) or both (32%) in a TBI population admitted to a Veterans Affairs (VA) Polytrauma Rehabilitation Center (PRC). When both hearing and vision are impaired, poorer functional independence at discharge has been reported (10). Critically, this is independent of TBI severity. The ability to regain premorbid balance and independent gait may be dictated by the ability to process, interpret, and combine, sensory information. Specifically, gait speed and the ability to maintain balance may be dictated by the perceived sensory information and subsequent sensorimotor integration and motor transformation necessary for successful task execution (11).
Postural control (whether for balance or gait purposes) depends on the integration of information from visual, vestibular, and somatosensory systems (12). In healthy individuals, the weighting of each sensory input adjusts to a decrease or loss in quality from any one input to preserve balance and maintain postural stability (13), and optimize movement efficiency (12). For example, while vision is an important sensory system used to maintain optimal postural stability (14), when visual information is occluded (c.f., closing your eyes) the CNS can adapt the weighting of the visual system, and upregulate the sensitivity of the vestibular and somatosensory inputs to maintain balance (13). However, during more complex (and dynamic) tasks, integration informed by all sensory inputs may be more critical to task success. When walking in cluttered terrain, where multiple obstacles complicate foot-placement (15), visual information can be leveraged in a feed-forward manner to register (1) where the foot needs to be placed safely and (2) ongoing visual monitoring of the foot to safely place the foot.
While few studies have investigated how impaired sensory systems affect the mobility of patients post-TBI, we can gather insights from the known influences to balance [including increased fall risk (16)] and deterioration in gait speed and performance that occur with sensory decline as a function of aging (17, 18). It is well known though that eyesight, hearing, vestibular function (17), and proprioception (18) all decline with age. While evidence indicates that the decline in sensory systems may play a role in the increase of fall risk (16) and deterioration of gait speed, these relationships have not been extensively studied. In the general population, the elderly rely more on their visual system to maintain postural stability, and gait is slower and more variable when the visual system is perturbed (19). Further, visual acuity, contrast sensitivity, and stereo acuity were also associated with greater risk of walking limitations during a 5-year follow-up (20). Finally, impaired hearing is reported to be related to a slower maximal gait speed, self-reported walking difficulties (19), and postural stability (21, 22). This relationship between hearing and gait speed and balance may be explained by the information hearing provides of our surroundings and/or because the vestibular organs share structure and function: they are anatomically closely localized, share fluid-filled bony compartments and blood circulation, are both served by the eighth cranial nerve, and have similar mechanosensory receptor hair cells, which detect sound, head movements, and orientation in space. However, all these findings are in the aging population in general, and it is largely unknown how sensory decline and balance, and mobility impairments are related to central nervous system deficits due to TBI.
Therefore, the aim of this study was to determine relationships amongst balance, gait, and sensory measures in a large cohort study including patients with one or more mild TBIs. It is hypothesized that the quality of gait and balance decline as the number and severity of sensory impairments increase.
2. Methods
2.1. Design
The study utilized an observational design with cross-sectional analyses using hierarchical regression to examine the predictive value of sensory measures of hearing and vision including auditory and visual processing measures on gait and balance.
Methods are described in more detail in van der Veen et al. (23).
2.2. Outcome measures
2.2.1. Sensory-specific balance assessment (via CDP scores)
The computerized dynamic posturography (CDP) protocol on the NeuroCom Smart Balance Master (previously Natus, Inc) was used to assess postural balance. An embedded dual-plate force platform was used to generate equilibrium scores; ranging from 0 (touching a support surface, shifting feet, or falling) to 100 (little or no sway) for six sensory conditions: (1) all sensory inputs available; (2) no visual feedback; (3) distorted visual feedback because visual surround is “center of pressure referenced” (movements are proportional to the anterior–posterior displacement of the COP); (4) distorted somatosensory feedback because supporting platform is “center of pressure referenced”; (5) same as condition 4, but now with eyes closed; and (6) distorted visual and somatosensory feedback because both visual surround and supporting platform are “center of pressure referenced” (Figure 1). Each subject performed three trials for each condition, with an overall Composite CDP score calculated as a weighted average of the 6 scores (i.e., conditions 1 and 2 are weighted 1/3 as much as conditions 3 through 6).
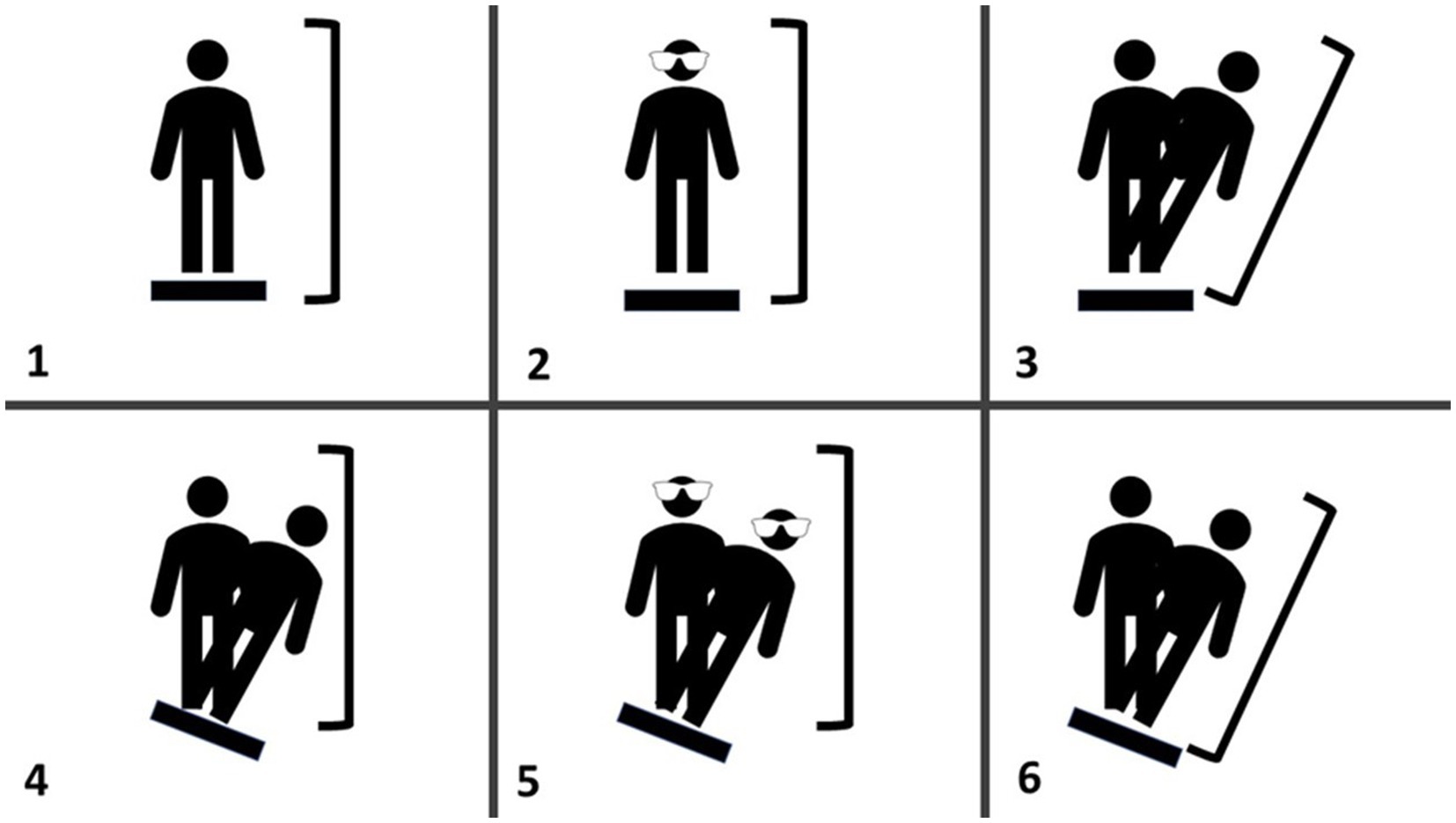
Figure 1. Schematic representation of the CDP the various panels represent the different balance assessments; (1) eyes open with fixed surface and surroundings, (2) eyes closed with fixed surface, (3) eyes open with fixed surface and sway-referenced visual surround, (4) eyes open with sway-referenced surface and fixed visual surround, (5) eyes closed with a sway-referenced surface, and (6) eyes open with sway-referenced surface and visual surround.
2.2.2. Walking speed
Gait was measured as part of the NIH Toolbox by the 4-meter walk score representing gait speed (24). This test is adapted from the 4-meter walk test in the short physical performance battery, an assessment tool for evaluating lower extremity functioning in older persons. Participants were asked to walk 4 meters at their usual pace twice, both attempts were timed in seconds, with the better trial used for scoring (calculation to walking speed in m/s).
2.3. Sensory tests
2.3.1. Corrected visual acuity
Visual acuity is a measure determining clarity of vision with the subject standing 20 feet from the Snellen Eye Chart and the distance at which the participant can read the line of letters (25). If the participant normally wears glasses or contact lenses, the test was performed while wearing glasses or contacts. A left and right visual acuity score was measured and a threshold score for the right eye was met with a visual acuity score of 20/40.
2.3.2. Visual spatial memory
The brief visuospatial memory test-revised (BVMT-R) is a measure of immediate and delayed visual memory (26). It requires the participant to reproduce line figures from memory. The BVMT-R provides twelve scores; three recall performance scores, one for each trial; a delayed recall score; three memory summary scores; three summary learning scores; hits (number of correct ‘yes’ responses) during the delayed recognition tasks; and a false alarm score (number of incorrect ‘yes’ responses) during the delayed recognition task.
2.3.3. Auditory processing
The Scan-3 test is comprised of a screening battery of tests to detect auditory processing disorders in adolescents and adults (27). The test evaluates temporal processing with three subtests: gap detection; auditory figure ground; and competing words.
2.3.4. Hearing handicap
The hearing handicap inventory for adults (HHIA) is a well-studied and widely used self-report measure of the respondent’s perceived hearing difficulty (28). The 11-item screening version used in this study is composed of two subscales (emotional and situational).
2.4. Data analysis
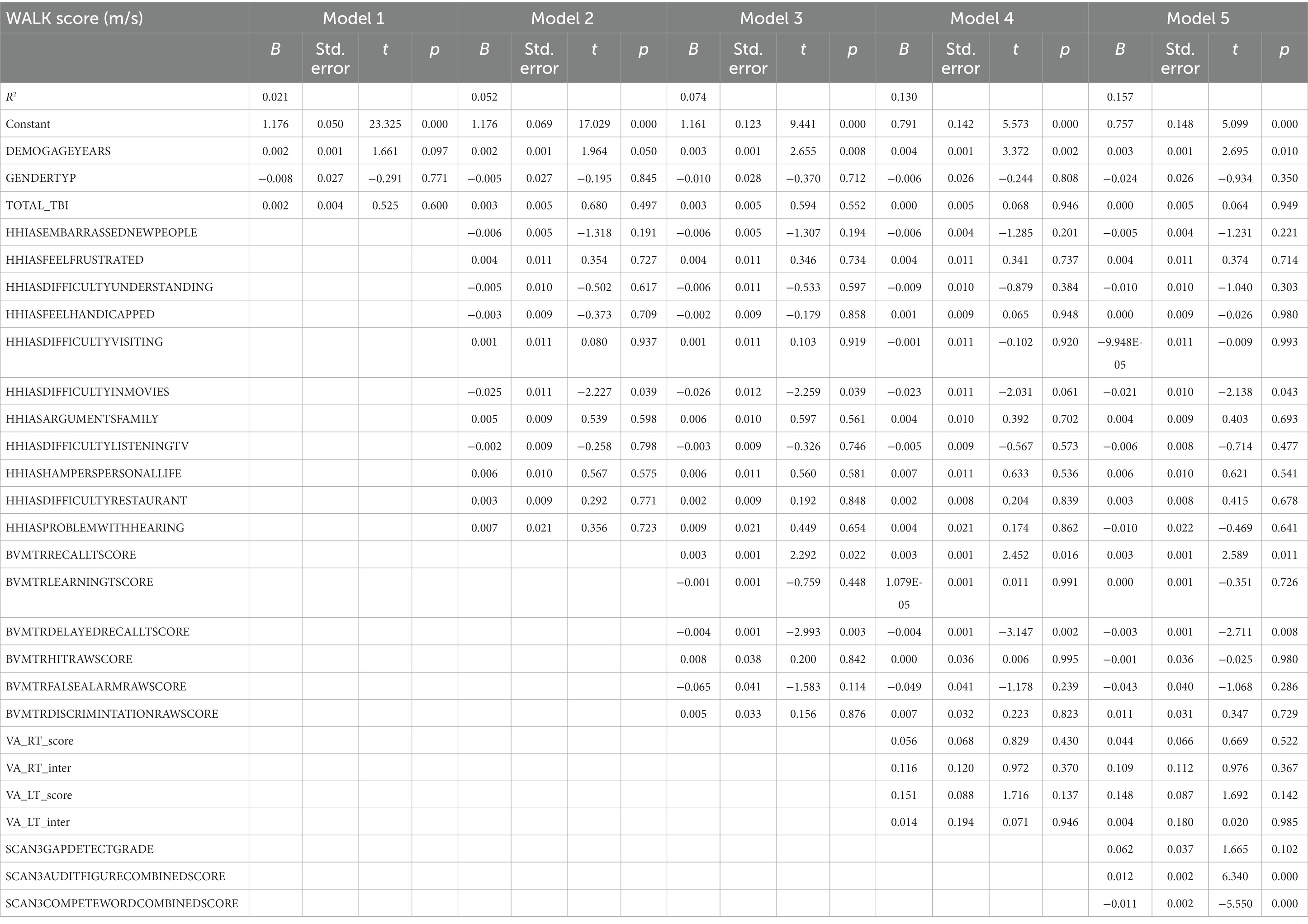
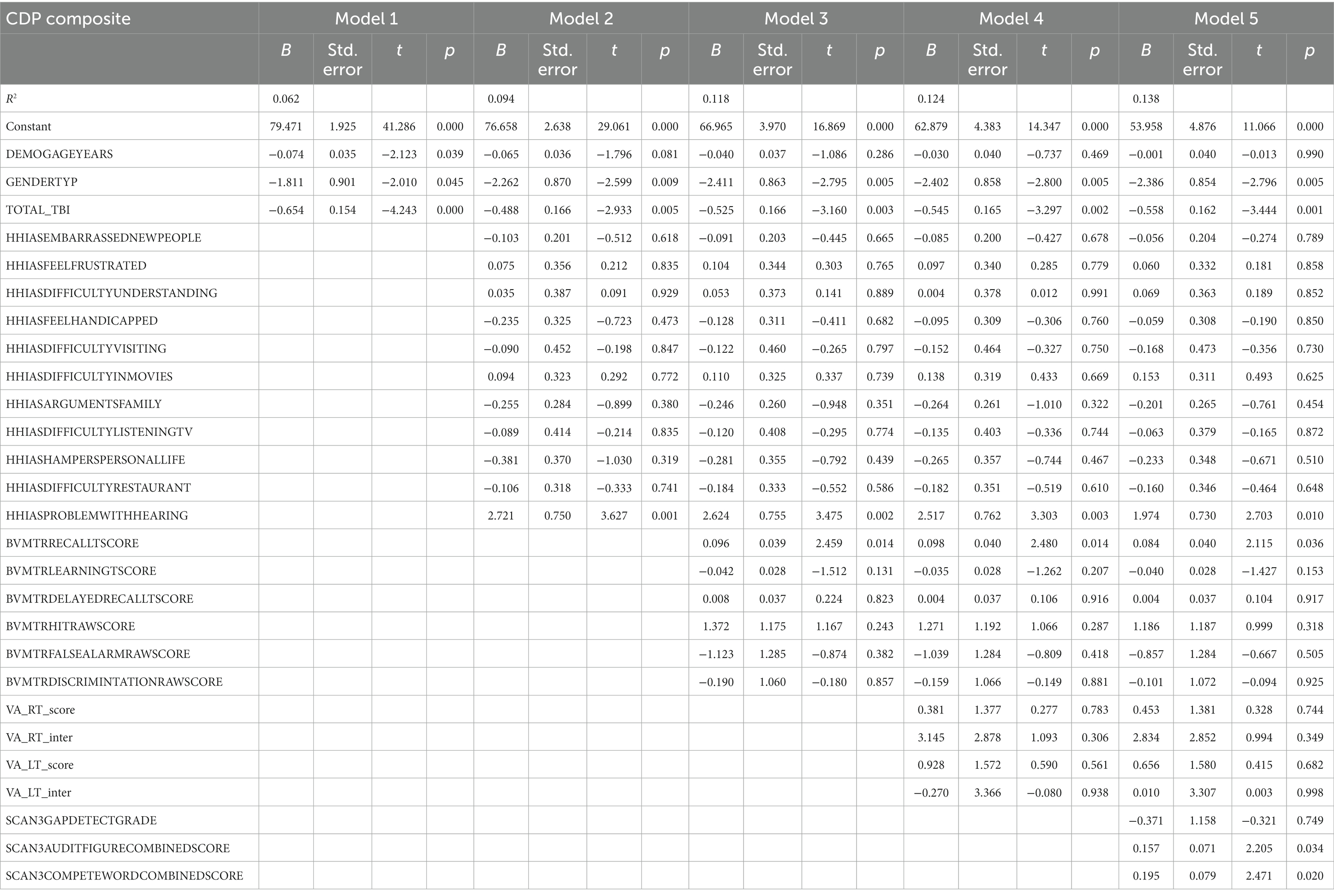
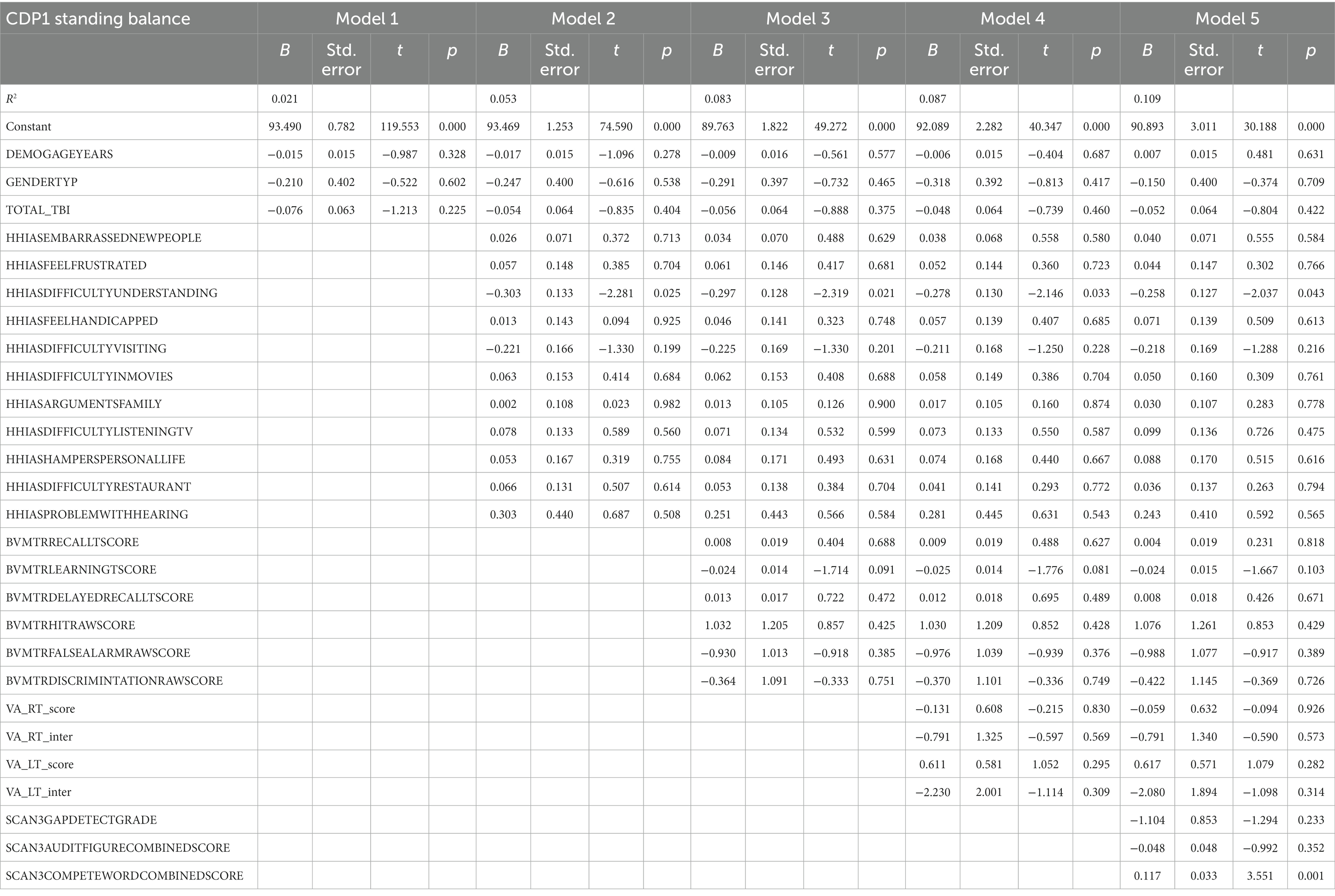
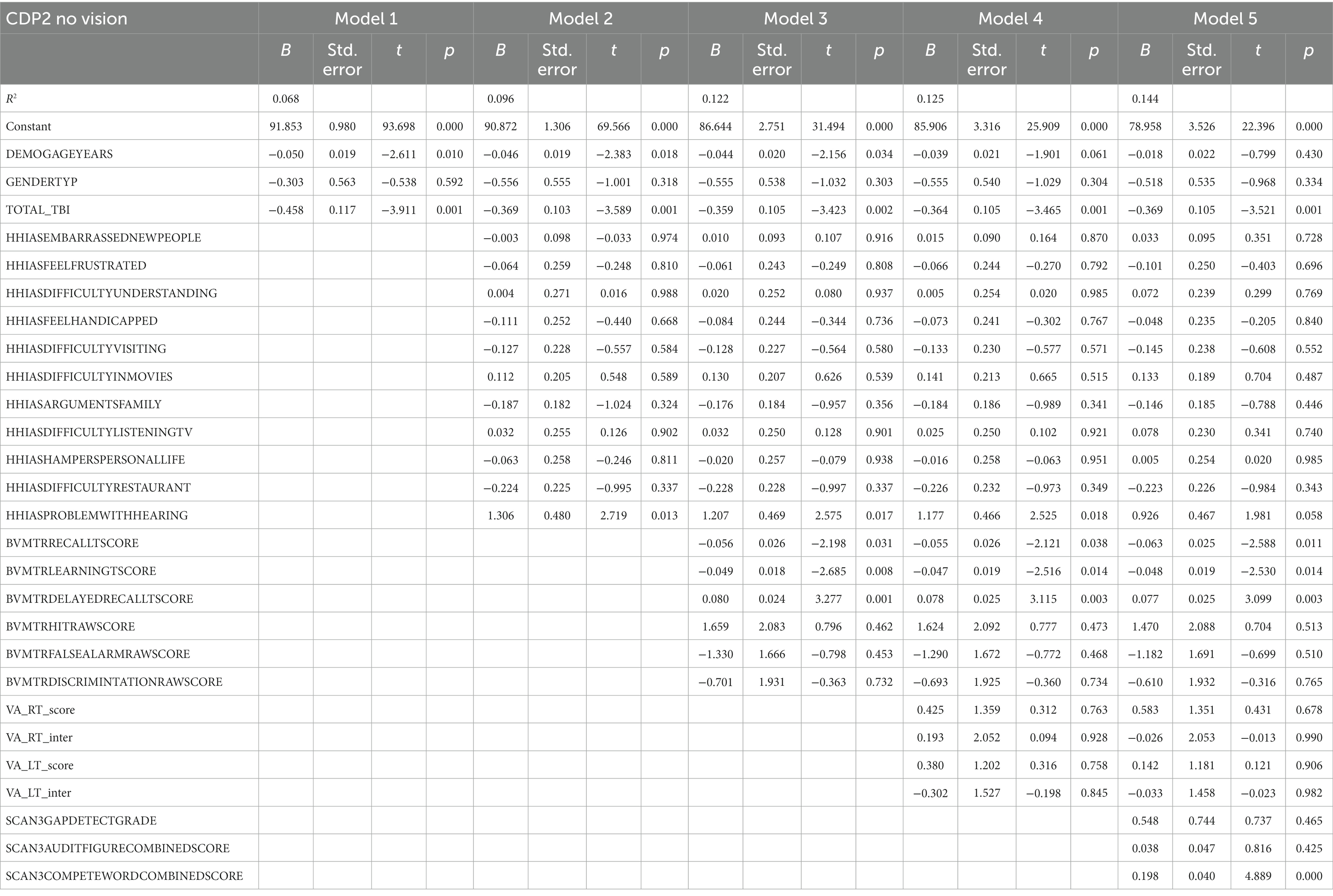
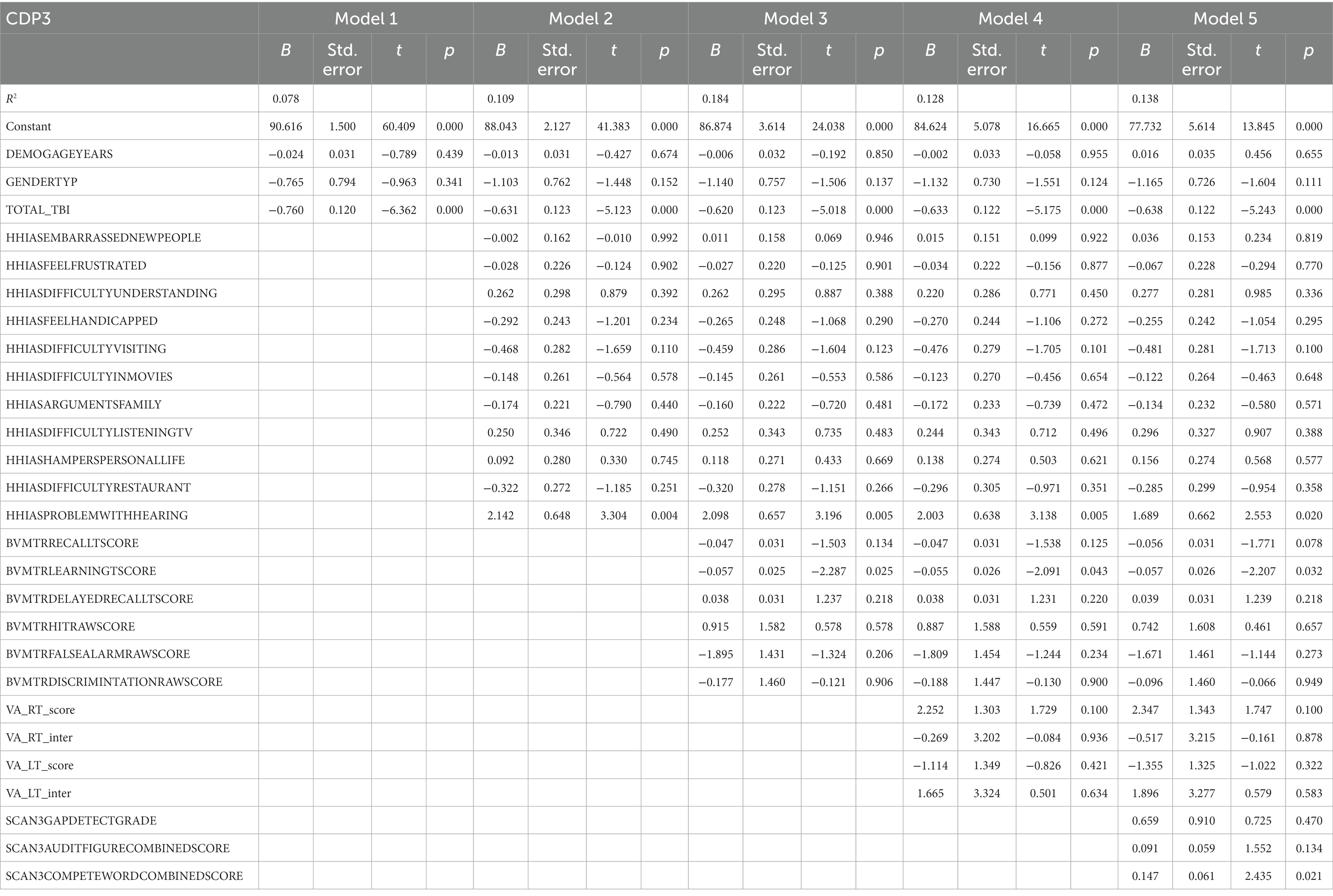
Participant characteristics were summarized using means and standard deviations or frequencies (see Table 1). Missing data was accounted for using multiple imputation using SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp), see percentages in Figure 2. Five imputed datasets were created using a fully condition specification. The estimates were then combined, and standard errors were adjusted to account for the uncertainty due to missingness. Hierarchical regressions were performed using SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp) with TBI classification and covariates of interest grouped in the following 5 steps: (1) the number of TBIs suffered, age, and sex, (2) the separate HHIA items, (3) separate BVMT items, (4) visual acuity, and (5) items of the SCAN3 (see Table 2 for a complete overview of the items entered in the regression). Separate hierarchical regression analyses were carried out for each of the balance assessment outcome measures (i.e., best 4 m walk score, CDP composite, CDP condition 1–6). Sensory measures were removed from the regression equations when collinearity was found (VIF > 10). Statistical significance was determined using a Benjamini-Hochberg correction, where the critical p values were based on the 27 tests per regression and a fall discovery rate of 20%.
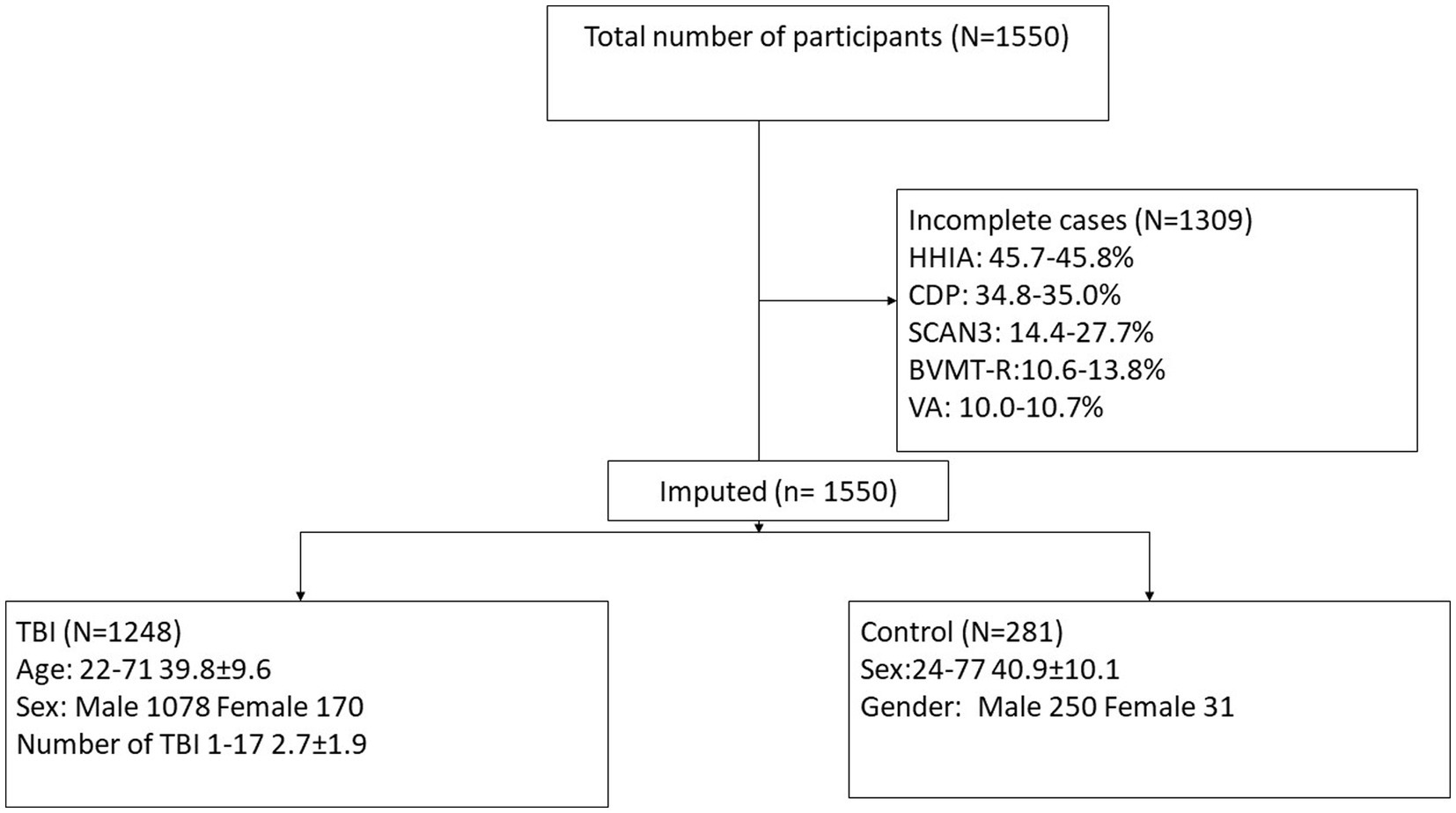
Figure 2. Consort diagram demonstrating participant selection for the current study. HHIA, Hearing Handicap Inventory for Adults; CDP, Computerized Dynamic Posturography; SCAN-3, Tests for Auditory Processing Disorders; BVMT-R, Brief Visuospatial Memory Test-Revised; VA, Visual acuity; TBI, traumatic brain injury.
3. Results
3.1. Participants
The study includes data from 1550 participants, but only 241 (15.55%) cases were complete (see Figure 2). All participants were included in analyses due to the use of multiple imputation. Of these 1550, 1248 suffered at least one TBI and 281 were participants with no history of TBI (non-TBI). For demographics, see Table 1.
3.2. Walking speed
Table 2 presents the complete hierarchical regression results for the 4 m walking speed. Step 2 revealed a negative association between the difficulty with the item “understanding movies” (p = 0.012), “problems with hearing” (p = 0.003), and walk score. Step 3 indicated an increase in valid items recalled after a delay was associated with slower walking speeds; age (p = 0.006) became related to faster walking speeds. Although Step 4 (visual acuity added) increased variance accounted for to 12.0%, none of the visual acuity measures were significant; visual spatial recall memory (BVMT-R delayed recall score, p = 0.013) remained positively related to 4 m walk time. Step 5 added audio processing and increased variance accounted for to 14.0%. The ability to distinguish audio target from noise showed a relation with faster walking speeds (p = 0.001), indicating the ability to distinguish words from noise was related to longer 4 m walk times. See Table 2 for the complete results.
3.3. CDP composite
Table 3 presents the complete hierarchical regression results for the CDP composite score. Step 1 accounted for 6.2% of the variance of the composite CDP score. All demographic measures were found to be related to balance measured with the CDP combined score. Age, sex, and number of TBI are negatively correlated with the CDP composite score, indicating older people (p = 0.039), females (p = 0.045), and people with more TBIs suffered (p < 0.001) have more balance difficulties. Step 2, revealed an association between self-reported absence of difficulty with hearing (p = 0.001) and a better CDP composite score. Step 3 showed visual spatial recall memory (BVMT-R delayed recall score, p = 0.014) was positively related to the CDP composite score. A positive relationship was shown between auditory processing [the ability to distinguish audio target from noise (p = 0.034) and the ability to repeat both words (p = 0.020)] and CDP composite score in step 5. See Table 3 for the individual measures.
3.4. CDP condition 1 eyes open with fixed surface and visual surround
Table 4 represents the complete hierarchical regression results for the CDP condition 1 score. Step 1 accounted for 2.1% of the variance of the CDP condition 1 score. In step 2, an association between increased difficulty understanding new people (p = 0.025) and worse CDP condition 1 score was found. In step 5 a relationship was shown between auditory processing (the ability to repeat both words, p = 0.001) and CDP condition 1. See Table 4 for the individual measures.
3.5. CDP condition 2 eyes closed with a fixed surface
Table 5 represents the complete hierarchical regression results for the CDP condition 2 score. Both Age (p = 0.010) and number of TBI (p = 0.001) were shown to be negatively related to CDP condition 2 score in step 1. Step 2, the absence of difficulty hearing (p = 0.013) was associated with a better CDP condition 2 score. Step 3 revealed a positive association was shown for the delayed recall score (p = 0.001). Step 4 showed visual learning score (p = 0.008) has a negative association with the CDP condition 2. Step 5 showed a positive relationship was shown between auditory processing [the ability to repeat both words (p < 0.001)] and CDP condition 2 scores. See Table 5 for the individual measures.
3.6. CDP condition 3 eyes open with fixed surface and sway-referenced visual surround
Table 6 represents the complete hierarchical regression results for the CDP condition 3 score. In step 1 number of TBI (p = 0.001) was shown to be negatively related to CDP condition 3 scores. Step 2 showed the absence of difficulty hearing (p = 0.004) was associated with a better CDP condition 3 score. A positive relationship was shown between auditory processing [the ability to repeat both words (p = 0.021) and CDP condition 3 score in step 5]. See Table 6 for the individual measures.
3.7. CDP condition 4 eyes open with sway-referenced surface and fixed visual surround
Table 7 represents the complete hierarchical regression results for the CDP condition 4 score. In step number of TBI (p = 0.006) showed a negative relation to CDP condition 4 score. In step 4, visual processing measures showed a positive association with recall score (p = 0.033). See Table 7 for the individual measures.
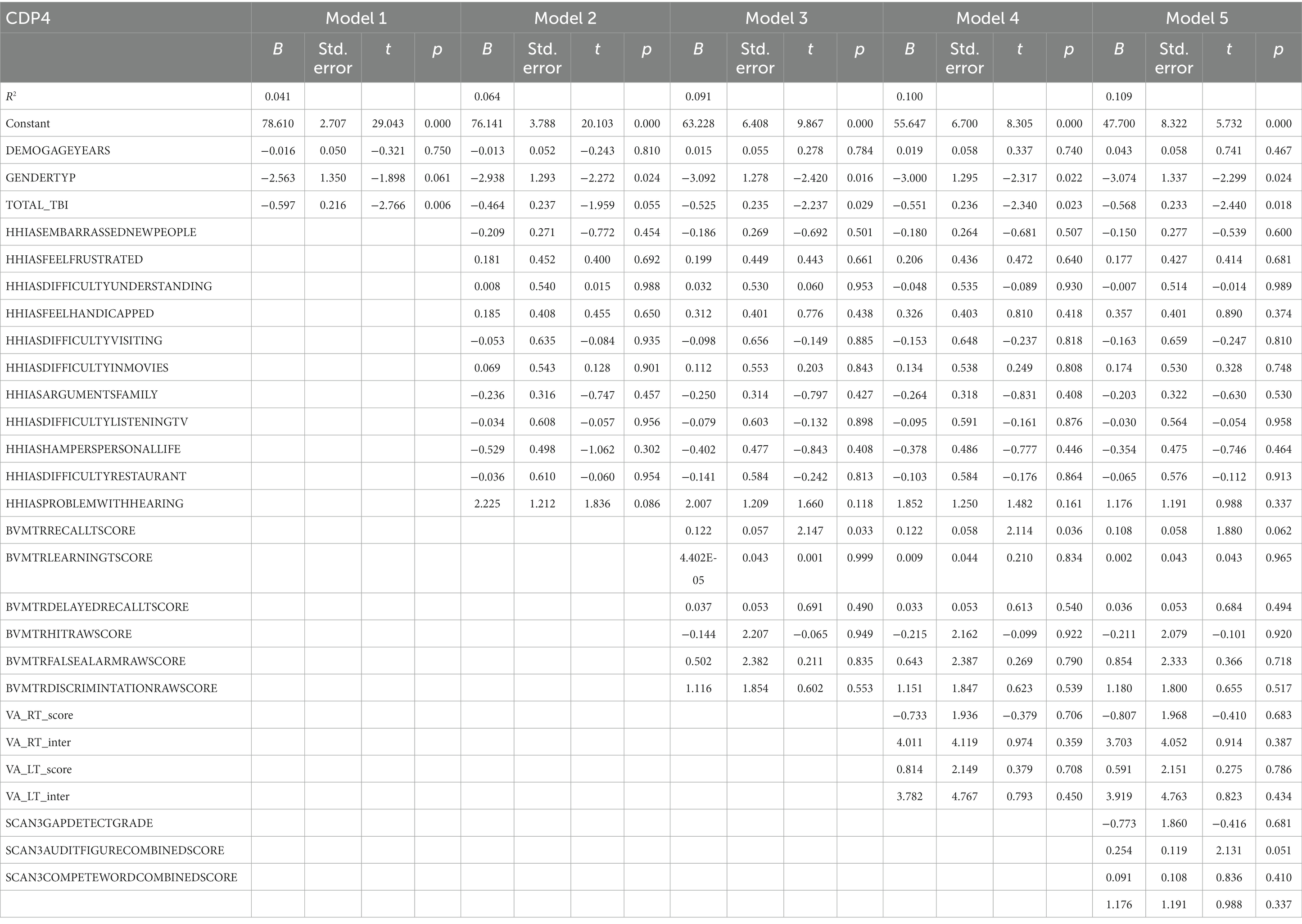
Table 7. Results from the hierarchical regression for CDP condition 4, sway references base of support.
3.8. CDP condition 5 eyes closed with a sway-referenced surface
Table 8 represents the complete hierarchical regression results for the CDP condition 5 score. In step 1 both age (p = 0.020) and number of TBI (p < 0.001) were shown to be negatively related to CDP condition 5 score. Step 2, showed the absence of difficulty hearing (p < 0.001) was associated with a better CDP condition 5 score. In step 4 visual processing measures showed a negative association between recall score (p = 0.004). See Table 8 for the individual measures.
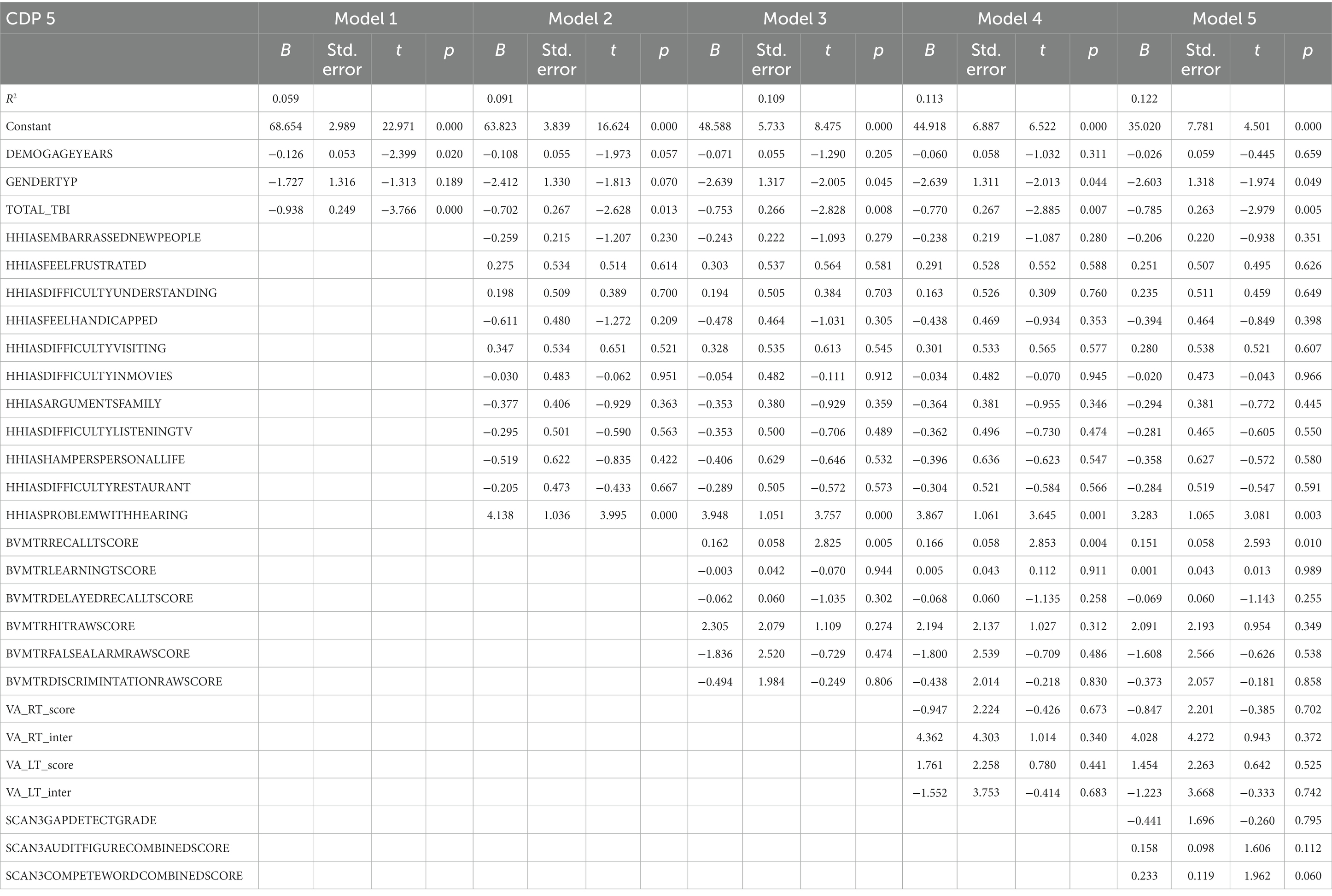
Table 8. Results from the hierarchical regression for CD5, sway references base of support and occluded vision.
3.9. CDP condition 6 eyes open with sway-referenced surface and visual surrounds
Table 9 represents the complete hierarchical regression results for the CDP 6 score. In step 1 both age (p = 0.021) and number of TBI (p = 0.016) were shown to be negatively related to CDP condition 6 score. In step 2 the absence of difficulty hearing (p < 0.001) was associated with better CDP condition 6 score. Step 3 revealed recall score (p = 0.004) showed a positive relation. In step 4 visual processing measure showed a negative association with learning score (p = 0.011). See Table 9 for the individual measures.
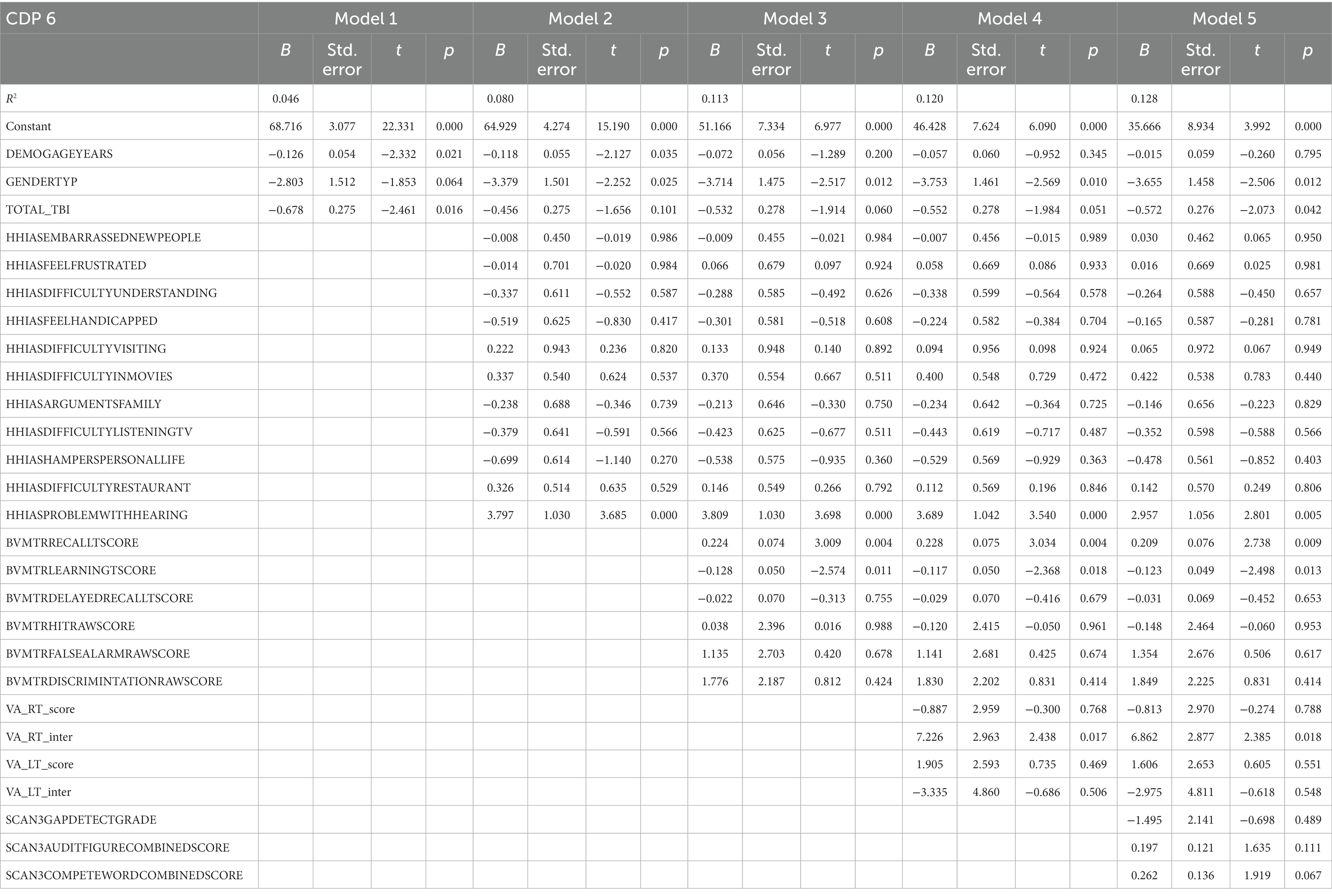
Table 9. Results from the hierarchical regression for CDP condition 6, sway references base of support and vision.
4. Discussion
The goal of this study was to determine the relationships between sensory function and postural balance among current and former combat-exposed service members, with and without a history of mTBI(s). Balance is dependent on the ability to combine and process sensory information, identifying the fidelity of these signals and using this information to adjust the weighting of the sensory information (12). This study reinforces that postural balance is a complex control problem that utilizes multiple sensory systems and requires the ability to successfully process multiple inputs at the executive processing level.
In general, individuals with TBI can reliably maintain postural stability (as evidenced by high CDP scores for Condition 1 in Table 1) and ambulate at similar speeds successfully when sensory input from vision, proprioception, or vestibular systems are unperturbed. However, individuals with TBI have more difficulty when adjustments in the weighting of these sensory inputs are required due to various experimental perturbations; swaying surrounding or base of support, or the occlusion of vision.
The most consistent feature across regression analyses was that sensory disruptions (vision, vestibular, or somatosensory) and subsequent lower balance assessment outcomes (via CDP 2–6 scores) were associated with the number of TBIs reported (29). Additionally, females appear to have more difficulty keeping their balance when proprioception is unreliable (e.g., on a swaying surface) than males. Counterintuitively, age shows to be associated with faster walking speed, however as expected, older participants had lower scores on balance assessments (CDP 2, 5 & 6 and the Composite score).
Surprisingly, variance accounted for by the combination of all demographic and sensory acuity/processing items only attributed 10.9 and 14.0% of the total variability in the balance and gait outcomes. While this may seem limited, many factors affect gait and balance not accounted for in these models. In general, more associations were found between the visual and auditory processing measures compared to specific hearing and vision impairments, more in-depth discussion follows below.
Deficits in hearing as assessed by the self-reported hearing difficulties on the hearing handicap questionnaire (HHIA-S) showed associations with measures of balance (CDP, except CDP1) and gait. Participants who indicated to have problems with hearing showed to have slower walking speeds and lower balance scores (CDP composite and CDP2-6). Additionally, ‘difficulty understanding movies’ showed an association with slower walking speeds. These findings are consistent with previous studies by Viljanen et al. (21) showing that women with poorer hearing have poorer postural control and higher fall risk. Authors have postulated this to be related to the anatomical location of the vestibular system to the auditory system, along with their shared vestibulocochlear nerve, vascular supply, similar mechanosensory receptor hair cells, which detect sound, head movements, and orientation in space, and therefore with balance (21).
Auditory processing (SCAN3) was shown to be associated with gait speed and balance (composite score and CDP 1–3). The ability to better distinguish words from noise (SCAN audio figure score) was associated with faster gait speeds and better CDP composite score, so in general better gait and balance. Additionally, the ability to better repeat word pairs (compete word score) presented to be associated with faster gait speeds and better balance scores while proprioception was not perturbed (i.e., standing surface was stable in CDP1-3). So, when proprioception does not have to be re-weighted, but vision may or may not be perturbed, participants with a better ability to recall word pairs are shown to be better at maintaining balance with all sensory intact (CDP 1), or occluded or perturbed vision (CDP 2 and 3). In previous literature auditory processes have been shown to slow down gait; elderly stop walking when talking (30), and affect foot placement; stroke survivors lag auditory cues for foot falls (31). These findings suggest that the ability to inhibit noise, remember word pairs, and process auditory stimuli benefits gait speed and balance.
Visual acuity (VA) only showed an association with balance when vision and proprioception were sway-referenced, participants with impaired vision on the right had worse balance scores on the CDP6. No associations were found for visual acuity and walking speed, nor the other balance measures. In previous literature relationships of visual acuity among other visual measures and self-reported ability to walk a quarter of a mile or walking up 10 steps (20) were shown in the aging population (70–79). The lack of associations with vision, gait, and balance in this study could be caused by multiple factors. One of these factors may be that other visual functions are more important for balance and gait, like peripheral vision or spatial relations. Secondly, the demographics (age range 22–71) of this cohort did only show impaired vision (20/40 met) of 4.2% in the right eye and 2.7% in the left, where Swenor et al. found 7.4% to have vision impairment when looking with both eyes. Additionally, literature has reported various outcomes on the associations between vision, balance, and gait measures. Many studies have indicated the ability to detect movements (32, 33) or having visual blur (34, 35) affects balance. Visual acuity may not be directly related to gait speed or balance, it has been identified as a risk factor for falls (36–40), however, when adjusting for age these associations were not found (41–48).
Visual processing (BVMT) showed a more complex association with gait speed and balance. The ability to immediately recall a figure was associated with faster gait speed, while a delayed recall was associated with slower gait speed. Doi et al. showed that better visual memory was associated with faster gait speed, especially in participants with mild cognitive impairment (49). This is in agreement with literature showing people slow down (15, 50) and attentional costs increase (51–53) when walking to visual targets, and sway area (an often used balance measure) increases when eyes are closed (54). However, better delayed recall (after a 25-min delay) is associated with slower gait speeds. This association of slower gait speed with delayed memory as increased cortical attention/demand is required to recall, therefore visual processing requires greater attentional resources (55). Better general balance scores (CDP composite) and balance when vision was compromised or occluded and proprioception was compromised (CDP 5 and 6) are associated with better direct visual recall and better balance when vision was occluded (CDP 2) showed associations with delayed recall. Indicating that participants who rely on visuo-spatial memory when visual information is crude may prevent them from indicating what sensory information is reliable and upregulate those systems, affecting their ability to maintain balance. This confirms that visual processing is more important when proprioception is compromised.
4.1. Limitations
A large proportion of the non-TBI participants (53.85%) had relatively low SOT-composite scores (less than 75). In a manufacturer’s stated normative data set only 20% of ‘normal’ individuals had composite scores below 75. The higher proportion in our sample may be due to comorbidities, including chronic pain, PTSD, and sleep apnea in Veterans and Service Members (56), which previous preliminary analyses have linked to lower SOT-composite scores in Veterans and Service Members (9). Given that our sample had all served in the military and was predominantly male, results may not generalize to civilian or female populations and therefore, a similar analysis may be performed with a general public control group in the future. Therefore, relationships between sensory and processing deficits and gait and balance may be underestimated. In the future, similar analysis may be done on a population with greater balance and gait deficits and or when this cohort ages more.
Additionally, a large proportion of the data had to be imputed due to missing values. However, imputing missing values is known to reduce bias and improve efficiency over complete case analysis over excluding missing data (57, 58).
5. Conclusion
In general, individuals with TBI maintained postural stability and ambulation as well as their healthy counterparts, likely showing an ability to adapt to their sensory impairments (shown in acuity and processing outcomes). However, balance deficits may be unmasked when re-weighting inputs is required due to sensory disruption (e.g., during light adaptation to a dimly lit room), and may have greater consequences with more frequent exposure to TBIs. Our findings reinforce that sensory processing (rather than acuity) is more associated with negative balance and gait outcomes and potential increases in fall risk.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the CENC/LIMBIC data board has to approve data availability. Requests to access these datasets should be directed to Sudeep Karki Sudeep S2Fya2lAdmN1aGVhbHRoLm9yZw==.
Ethics statement
The studies involving humans were approved by Richmond VAMC Research and Development Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SV: conceptualize, analyze, and write a first draft. RP: conceptualize analysis, statistical advice, and proofread. LF: EEG data analysis and proofread. AA, KS, SS, EW, AS, JT, and WW: conceptualize analysis, methods, and proofreading. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Assistant Secretary of Defense for Health Affairs and endorsed by the Department of Defense, through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium (LIMBIC) Award/W81XWH-18-PH/TBIRP-LIMBIC under Awards Nos. W81XWH1920067 and W81XWH-13-2-0095, and by the U.S. Department of Veterans Affairs Awards No. I01 CX002097, I01 CX002096, I01 HX003155, I01 RX003444, I01 RX003443, I01 RX003442, I01 CX001135, I01 CX001246, I01 RX001774, I01 RX 001135, I01 RX 002076, I01 RX 001880, I01 RX 002172, I01 RX 002173, I01 RX 002171, I01 RX 002174, and I01 RX 002170. The U.S. Army Medical Research Acquisition Activity, 839 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Coonley-Hoganson, R, Sachs, N, Desai, BT, and Whitman, S. Sequelae associated with head injuries in patients who were not hospitalized: a follow-up survey. Neurosurgery. (1984) 14:315–7.
2. Bazarian, JJ, Wong, T, Harris, M, Leahey, N, and Dombovy, SMM. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj. (1999) 13:173–89.
3. Middelboe, T, Andersen, HS, Birket-Smith, M, and Friis, ML. Minor head injury: impact on general health after 1 year. A prospective follow-up study. Acta Neurol Scand. (2009) 85:5–9. doi: 10.1111/j.1600-0404.1992.tb03987.x
4. Hoge, CW, McGurk, D, Thomas, JL, Cox, AL, Engel, CC, and Castro, CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. New Engl J Med. (2008) 358:453–63. doi: 10.1056/NEJMoa072972
5. Pogoda, TK, Hendricks, AM, Iverson, KM, Stolzmann, KL, Krengel, MH, Baker, E, et al. Multisensory impairment reported by veterans with and without mild traumatic brain injury history. J Rehabil Res Dev. (2012) 49:971–784. doi: 10.1682/jrrd.2011.06.0099
6. Terrio, H, Brenner, LA, Ivins, BJ, Cho, JM, Helmick, K, Schwab, K, et al. Traumatic brain injury screening. J Head Trauma Rehabil. (2009) 24:14–23. doi: 10.1097/HTR.0b013e31819581d8
7. Fino, PC, Raffegeau, TE, Parrington, L, Peterka, RJ, and King, LA. Head stabilization during standing in people with persisting symptoms after mild traumatic brain injury. J Biomech. (2020) 112:110045. doi: 10.1016/j.jbiomech.2020.110045
8. Martini, DN, Parrington, L, Stuart, S, Fino, PC, and King, LA. Gait performance in people with symptomatic, chronic mild traumatic brain injury. J Neurotrauma. (2021) 38:218–24. doi: 10.1089/neu.2020.6986
9. Walker, WC, Nowak, KJ, Kenney, K, Franke, LM, Eapen, BC, Skop, K, et al. Is balance performance reduced after mild traumatic brain injury?: interim analysis from chronic effects of neurotrauma consortium (CENC) multi-centre study. Brain Inj. (2018) 32:1156–68. doi: 10.1080/02699052.2018.1483529
10. Lew, HL, Garvert, DW, Pogoda, TK, Hsu, PT, Devine, JM, White, DK, et al. Auditory and visual impairments in patients with blast-related traumatic brain injury: effect of dual sensory impairment on functional independence measure. J Rehabil Res Dev. (2009) 46:819–26. doi: 10.1682/jrrd.2008.09.0129
11. Fino, PC, Peterka, RJ, Hullar, TE, Murchison, C, Horak, FB, Chesnutt, JC, et al. Assessment and rehabilitation of central sensory impairments for balance in mTBI using auditory biofeedback: a randomized clinical trial. BMC Neurol. (2017) 17:41. doi: 10.1186/s12883-017-0812-7
12. Peterka, RJ. Sensorimotor integration in human postural control. J Neurophysiol. (2002) 88:1097–118. doi: 10.1152/jn.2002.88.3.1097
13. Hillier, SL, and McDonnell, M. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction In: SL Hillier, editor. Cochrane database of systematic reviews. Chichester: John Wiley & Sons, Ltd (2007)
14. Grace Gaerlan, M, Alpert, PT, Cross, C, Louis, M, and Kowalski, S. Postural balance in young adults: the role of visual, vestibular and somatosensory systems. J Am Acad Nurse Pract. (2012) 24:375–81. doi: 10.1111/j.1745-7599.2012.00699.x
15. van der Veen, SM, Hammerbeck, U, and Hollands, KL. Foot-placement accuracy during planned and reactive target stepping during walking in stroke survivors and healthy adults. Gait Posture. (2020) 81:261–7. doi: 10.1016/j.gaitpost.2020.08.114
16. Wolfson, LI, Whipple, R, Amerman, P, Kaplan, J, and Kleinberg, A. Gait and balance in the elderly. Two functional capacities that link sensory and motor ability to falls. Clin Geriatr Med. (1985) 1:649–59.
17. Bermudez Rey, MC, Clark, TK, Wang, W, Leeder, T, Bian, Y, and Merfeld, DM. Vestibular perceptual thresholds increase above the age of 40. Front Neurol. (2016) 7:162. doi: 10.3389/fneur.2016.00162
18. Patel, M, Magnusson, M, Kristinsdottir, E, and Fransson, PA. The contribution of mechanoreceptive sensation on stability and adaptation in the young and elderly. Eur J Appl Physiol. (2009) 105:167–73. doi: 10.1007/s00421-008-0886-4
19. Viljanen, A, Kaprio, J, Pyykko, I, Sorri, M, Koskenvuo, M, and Rantanen, T. Hearing acuity as a predictor of walking difficulties in older women. J Am Geriatr Soc. (2009) 57:2282–6. doi: 10.1111/j.1532-5415.2009.02553.x
20. Swenor, BK, Simonsick, EM, Ferrucci, L, Newman, AB, Rubin, S, Wilson, V, et al. Visual impairment and incident mobility limitations: the health, aging and body composition study. J Am Geriatr Soc. (2015) 63:46–54. doi: 10.1111/jgs.13183
21. Viljanen, A, Kaprio, J, Pyykkö, I, Sorri, M, Pajala, S, Kauppinen, M, et al. Hearing as a predictor of falls and postural balance in older female twins. J Gerontol A Biol Sci Med Sci. (2009) 64:312–7. doi: 10.1093/gerona/gln015
22. Carpenter, MG, and Campos, JL. The effects of hearing loss on balance: a critical review. Ear Hear. (2020) 41:107S–19S. doi: 10.1097/AUD.0000000000000929
23. van der Veen, SM, Perera, RA, Manning-Franke, L, Agyemang, AA, Skop, K, Sponheim, SR, et al. Executive function and relation to static balance metrics in chronic mild TBI: a LIMBIC-CENC secondary analysis. Front Neurol. (2022) 13:906661. doi: 10.3389/fneur.2022.906661
24. Mehmet, H, Robinson, SR, and Yang, AWH. Assessment of gait speed in older adults. J Geriatr Phys Ther. (2020) 43:42–52. doi: 10.1519/JPT.0000000000000224
25. Hetherington, R. The Snellen chart as a test of visual acuity. Psychol Forsch. (1954) 24:349–57. doi: 10.1007/BF00422033
26. Benedict, RHB, Schretlen, D, Groninger, L, Dobraski, M, and Shpritz, B. Revision of the brief visuospatial memory test: studies of normal performance, reliability, and validity. Psychol Assess. (1996) 8:145–53. doi: 10.1037/1040-3590.8.2.145
27. Lovett, BJ, and Johnson, TL. Test review: R. W. Keith SCAN-3 for adolescents and adults: tests for auditory processing disorders. San Antonio, TX: Pearson, 2009. J Psychoeduc Assess. (2010) 28:603–7. doi: 10.1177/0734282909353341
28. Newman, CW, Weinstein, BE, Jacobson, GP, and Hug, GA. Test-retest reliability of the hearing handicap inventory for adults. Ear Hear. (1991) 12:355–7. doi: 10.1097/00003446-199110000-00009
29. Haran, FJ, Slaboda, JC, King, LA, Wright, WG, Houlihan, D, and Norris, JN. Sensitivity of the balance error scoring system and the sensory organization test in the combat environment. J Neurotrauma. (2016) 33:705–11. doi: 10.1089/neu.2015.4060
30. Weerdesteyn, V, van Swigchem, R, van Duijnhoven, HJ, and Geurts, AC. Why stroke patients stop walking when talking. J Am Geriatr Soc. (2007) 55:1691. doi: 10.1111/j.1532-5415.2007.01365.x
31. Roerdink, M, Lamoth, CJ, van Kordelaar, J, Elich, P, Konijnenbelt, M, Kwakkel, G, et al. Rhythm perturbations in acoustically paced treadmill walking after stroke. Neurorehabil Neural Repair. (2009) 23:668–78. doi: 10.1177/1545968309332879
32. Freeman, EE, Broman, AT, Turano, KA, and West, SK, SEE Project. Motion-detection threshold and measures of balance in older adults: the SEE project. Invest Ophthalmol Vis Sci. (2008) 49:5257–63. doi: 10.1167/iovs.07-1106
33. Helbostad, JL, Vereijken, B, Hesseberg, K, and Sletvold, O. Altered vision destabilizes gait in older persons. Gait Posture. (2009) 30:233–8. doi: 10.1016/j.gaitpost.2009.05.004
34. Anand, V, Buckley, JG, Scally, A, and Elliott, DB. Postural stability changes in the elderly with cataract simulation and refractive blur. Invest Ophthalmol Vis Sci. (2003) 44:4670–5. doi: 10.1167/iovs.03-0455
35. Anand, V, Buckley, JG, Scally, A, and Elliott, DB. Postural stability in the elderly during sensory perturbations and dual tasking: the influence of refractive blur. Invest Ophthalmol Vis Sci. (2003) 44:2885–91. doi: 10.1167/iovs.02-1031
36. Tinetti, ME, Williams, TF, and Mayewski, R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. (1986) 80:429–34. doi: 10.1016/0002-9343(86)90717-5
37. Ivers, RQ, Cumming, RG, Mitchell, P, and Attebo, K. Visual impairment and falls in older adults: the Blue Mountains eye study. J Am Geriatr Soc. (1998) 46:58–64. doi: 10.1111/j.1532-5415.1998.tb01014.x
38. Klein, BE, Moss, SE, Klein, R, Lee, KE, and Cruickshanks, KJ. Associations of visual function with physical outcomes and limitations 5 years later in an older population: the beaver dam eye study. Ophthalmology. (2003) 110:644–50. doi: 10.1016/S0161-6420(02)01935-8
39. Lord, SR, and Menz, HB. Visual contributions to postural stability in older adults. Gerontology. (2000) 46:306–10. doi: 10.1159/000022182
40. Nevitt, MC, Cummings, SR, Kidd, S, and Black, D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. (1989) 261:2663–8.
41. Lord, SR, Clark, RD, and Webster, IW. Postural stability and associated physiological factors in a population of aged persons. J Gerontol. (1991) 46:M69–76. doi: 10.1093/geronj/46.3.m69
42. Brocklehurst, JC, Robertson, D, and James-Groom, P. Clinical correlates of sway in old age--sensory modalities. Age Ageing. (1982) 11:1–10. doi: 10.1093/ageing/11.1.1
43. Campbell, AJ, Borrie, MJ, and Spears, GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. (1989) 44:M112–7. doi: 10.1093/geronj/44.4.m112
44. Tinetti, ME, Speechley, M, and Ginter, SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. (1988) 319:1701–7.
45. Coleman, AL, Cummings, SR, Yu, F, Kodjebacheva, G, Ensrud, KE, Gutierrez, P, et al. Binocular visual-field loss increases the risk of future falls in older white women. J Am Geriatr Soc. (2007) 55:357–64. doi: 10.1111/j.1532-5415.2007.01094.x
46. Freeman, EE, Munoz, B, Rubin, G, and West, SK. Visual field loss increases the risk of falls in older adults: the Salisbury eye evaluation. Invest Ophthalmol Vis Sci. (2007) 48:4445–50. doi: 10.1167/iovs.07-0326
47. Robbins, AS, Rubenstein, LZ, Josephson, KR, Schulman, BL, Osterweil, D, and Fine, G. Predictors of falls among elderly people. Results of two population-based studies. Arch Intern Med. (1989) 149:1628–33.
48. Ivers, RQ, Norton, R, Cumming, RG, Butler, M, and Campbell, AJ. Visual impairment and risk of hip fracture. Am J Epidemiol. (2000) 152:633–9. doi: 10.1093/aje/152.7.633
49. Doi, T, Shimada, H, Makizako, H, Tsutsumimoto, K, Uemura, K, Anan, Y, et al. Cognitive function and gait speed under normal and dual-task walking among older adults with mild cognitive impairment. BMC Neurol. (2014) 14:67. doi: 10.1186/1471-2377-14-67
50. Peper, CL, de Dreu, MJ, and Roerdink, M. Attuning one's steps to visual targets reduces comfortable walking speed in both young and older adults. Gait Posture. (2015) 41:830–4. doi: 10.1016/j.gaitpost.2015.02.016
51. Mazaheri, M, Hoogkamer, W, Potocanac, Z, Verschueren, S, Roerdink, M, Beek, PJ, et al. Effects of aging and dual tasking on step adjustments to perturbations in visually cued walking. Exp Brain Res. (2015) 233:3467–74. doi: 10.1007/s00221-015-4407-5
52. Mazaheri, M, Roerdink, M, Bood, RJ, Duysens, J, Beek, PJ, and Peper, CL. Attentional costs of visually guided walking: effects of age, executive function and stepping-task demands. Gait Posture. (2014) 40:182–6. doi: 10.1016/j.gaitpost.2014.03.183
53. Peper, CL, Oorthuizen, JK, and Roerdink, M. Attentional demands of cued walking in healthy young and elderly adults. Gait Posture. (2012) 36:378–82. doi: 10.1016/j.gaitpost.2012.03.032
54. Maheu, M, Behtani, L, Nooristani, M, Houde, MS, Delcenserie, A, Leroux, T, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. (2019) 40:1418–24. doi: 10.1097/AUD.0000000000000720
55. Plotnik, M, Bartsch, RP, Zeev, A, Giladi, N, and Hausdorff, JM. Effects of walking speed on asymmetry and bilateral coordination of gait. Gait Posture. (2013) 38:864–9. doi: 10.1016/j.gaitpost.2013.04.011
56. Pugh, MJ, Finley, EP, Copeland, LA, Wang, CP, Noel, PH, Amuan, ME, et al. Complex comorbidity clusters in OEF/OIF veterans: the polytrauma clinical triad and beyond. Med Care. (2014) 52:172–81. doi: 10.1097/MLR.0000000000000059
57. Lee, KJ, and Carlin, JB. Recovery of information from multiple imputation: a simulation study. Emerg Themes Epidemiol. (2012) 9:3. doi: 10.1186/1742-7622-9-3
Keywords: TBI, balance, sensory functions, auditory, vision
Citation: van der Veen SM, Perera R, Fino PC, Franke LM, Agyemang AA, Skop K, Wilde EA, Sponheim SR, Stamenkovic A, Thomas JS and Walker WC (2023) Sensory functions and their relation to balance metrics: a secondary analysis of the LIMBIC-CENC multicenter cohort. Front. Neurol. 14:1241545. doi: 10.3389/fneur.2023.1241545
Edited by:
Nada Andelic, University of Oslo, NorwayReviewed by:
Mari Storli Rasmussen, Oslo University Hospital, NorwayAlberto Cordova, University of Texas at San Antonio, United States
Copyright © 2023 van der Veen, Perera, Fino, Franke, Agyemang, Skop, Wilde, Sponheim, Stamenkovic, Thomas and Walker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susanne M. van der Veen, c212YW5kZXJ2ZWVuQHZjdS5lZHU=
 Susanne M. van der Veen
Susanne M. van der Veen Robert Perera
Robert Perera Peter C. Fino
Peter C. Fino Laura Manning Franke
Laura Manning Franke Amma A. Agyemang2
Amma A. Agyemang2 Karen Skop
Karen Skop Elisabeth A. Wilde
Elisabeth A. Wilde Scot R. Sponheim
Scot R. Sponheim Alexander Stamenkovic
Alexander Stamenkovic James S. Thomas
James S. Thomas William C. Walker
William C. Walker