
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 15 November 2023
Sec. Endovascular and Interventional Neurology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1239199
Middle cerebral artery (MCA) aneurysms are complex and widely distributed throughout the course of the MCA. Various types of aneurysms can occur in the MCA. Ruptured as well as unruptured MCA aneurysms may require treatment to avoid bleeding or rebleeding. Currently, clipping is regarded as the first-line choice for the treatment of MCA aneurysms. However, endovascular treatment (EVT) is emerging as an alternative treatment in selected cases. EVT techniques vary. Therefore, it is necessary to review EVT for MCA aneurysms. In this review, the following issues were discussed: MCA anatomy and anomalies, classifications of MCA aneurysms, the natural history of MCA aneurysms, EVT status and principle, deployments of traditional coiling techniques and flow diverters (FDs), and deployments and prospects of intrasaccular flow disruptors and stent-like devices. According to the review and our experience, traditional coiling EVT is still the preferred therapy for most MCA aneurysms. FD deployment can be used in selective MCA aneurysms. Parent artery occlusion (PAO) can be used to treat distal MCA aneurysms. In addition, new devices can be used to treat MCA aneurysms, such as intrasaccular flow disruptors and stent-like devices. In general, EVT is gaining popularity as an alternative treatment option; however, there is still a lack of evidence regarding EVT, and longer-term data are not currently available for most EVT devices.
The middle cerebral artery (MCA) is the largest and most complex cerebral artery, and it is a common site for aneurysms, accounting for approximately 20% of all intracranial aneurysms (1). MCA aneurysms occur along the course of the MCA, giving them a wide distribution; in addition, various types of aneurysms can occur in the MCA. Ruptured as well as unruptured MCA aneurysms may require treatment to avoid bleeding or rebleeding. Currently, open surgery to clip MCA aneurysms is the first-line therapy. However, endovascular treatment (EVT) is becoming an attractive alternative (2–4). MCA aneurysms tend to have a wide neck and incorporate one or both of the branch vessels, which makes the EVT difficult. EVT techniques for the treatment of MCA aneurysms vary and include traditional coiling EVT, parent artery occlusion (PAO), deployment of a flow diverter (FD), and deployment of intrasaccular flow disruptors and stent-like devices (5, 6). Since the EVT technique for the treatment of MCA aneurysms is complex, a review of existing literature is necessary.
Eligible English language literature was searched in the PubMed database from 1 January 2000 to 15 August 2023. The keywords included “middle cerebral artery and anatomy, or anomaly,” “middle cerebral artery aneurysms and classification, or natural history, or endovascular treatment, or clipping, or parent artery occlusion, or flow diverter, or intrasaccular flow disruptor, or stent-like device.” A flow chart displaying the literature collection is shown in Figure 1.
The MCA is divided into the M1 (sphenoidal), M2 (insular), M3 (opercular), and M4 (cortical) segments (7). The M1 segment belongs to the proximal part, and the M2–M4 segments belong to the distal part (8). A bifurcation-type MCA is common; single- or multiple-trunk-type MCAs are less common (7). Some cortical arteries can arise from the M1 segment, including the early temporal branch (ETB) and the early frontal branch (EFB) (9). MCA gives rise to lenticulostriate arteries (LSAs), most from the M1 segment (7, 9). MCA can have several anomalies, including fenestration, twig-like MCA, duplication, and accessory MCA (9–12).
MCA aneurysms are commonly described in six types: (1–2) M1 bifurcation and trifurcation, (3–5) LSA, ETB, and EFB takeoff, and (6) distal MCA (13). Elsharkawy et al. simplified the classification of MCA aneurysms into proximal, bifurcation, and distal aneurysms (14). MCA bifurcation aneurysms can also include off-bifurcation aneurysms that arise within 5 mm of either side of the MCA bifurcation (15).
MCA aneurysms can be divided into saccular and non-saccular aneurysms. Saccular aneurysms were true and tended to occur at branch takeoff from the proximal MCA and bifurcation (2). Non-saccular aneurysms were from the dissection that presented with fusiform, irregular dilation of the MCA and tended to occur at the distal MCA. MCA aneurysms may be complex, have a large or giant size, wide neck (a dome/neck ratio of <2 or a neck diameter of >4 mm), or incorporate MCA branches (16).
Based on the International Subarachnoid Aneurysm Trial (ISAT), MCA aneurysms can be divided into small (<7 mm), medium (7–12 mm), large (>12–25 mm), or giant (>25 mm) (17). There may be mirror-like MCA aneurysms (18). In addition, MCA aneurysms can be flow-related and located on feeding arteries to brain arteriovenous malformations (BAVMs) (19).
In Korja et al.’s study of the natural history of ruptured but untreated intracranial aneurysms, 510 patients were enrolled; 34% of patients had MCA aneurysms, and the 1-year mortality rate was 65% (20). Therefore, the risk of rebleeding in ruptured MCA aneurysms is high. The bleeding risk in unruptured MCA aneurysms increased with size (12, 21). In the unruptured cerebral aneurysm study (UCAS) in Japan, the annual rate of rupture of MCA aneurysms was 0.2% in sizes of 3–4 mm, 0.3% in sizes of 5–6 mm, 1.6% in sizes of 7–9 mm, 4.1% in sizes of 10–24 mm, and 16.9% in sizes greater than or equal to 25 mm (22). Therefore, for ruptured MCA aneurysms, and for some that are large, aggressive treatment may be necessary to avoid rebleeding and bleeding. For flow-related MCA aneurysms, the natural history is unpredictable, and due to high hemodynamic stress, they may grow (23). The size of flow-related aneurysms in supratentorial BAVMs may influence the rupture risk, especially for aneurysms with diameters ≥5 mm (24).
The superficial location and features of MCA aneurysms are suitable for open surgery (Figure 2) (25). Based on a meta-analysis and clinical trials from 2015 to 2022, for MCA aneurysms, surgical clipping is still recommended first (26–29). Especially, cerebral bypass continues to be a useful tool to tackle complex MCA aneurysms (30). However, with the development of techniques and products, EVT is becoming an attractive therapy for MCA aneurysms due to its minimally invasive characteristics and safety/efficacy (31–34). In carefully selective cases, more and more MCA aneurysms can be treated by EVT (35).

Figure 2. Clipping of MCA aneurysm. (A) CTA showing a right MCA bifurcation aneurysm, with three blebs (arrows 1, 2, and 3). (B) Upper panel: Intraoperative CTA image showing the aneurysm with three blebs (arrows 1, 2, and 3), MCA trunk had been clipped temporarily; Lower panel: Intraoperative image showing three blebs (arrows 1, 2, and 3) of the aneurysm that was clipped. CTA, computed tomography angiography; MCA, middle cerebellar artery; M1, first segment of the MCA.
For proximal MCA aneurysms, the goal of EVT was to occlude the aneurysm and preserve the LSA, ETB, EFB, and integrated branch into the aneurysm. For distal MCA aneurysms, EVT can be performed using reconstructive or deconstructive EVT (2). For flow-related MCA aneurysms, proximal aneurysms should be treated with reconstructive EVT, and distal aneurysms can be treated with PAO (24).
Until now, coiling EVT has remained the favorite therapy for MCA aneurysms, including dual-catheter-assisted, balloon-assisted, and stent-assisted coiling techniques (Figure 3) (32). Balloon-assisted coiling with a single microcatheter or dual microcatheter is a simple technique that offers a safe and effective solution in the management of MCA aneurysms (36, 37). Recently, the low-profile Neuroform Atlas stent (Stryker Neurovascular, Fremont, California, United States) has improved the treatment of MCA aneurysms because it allows safe catheterization of vessels as small as 1 mm (38).
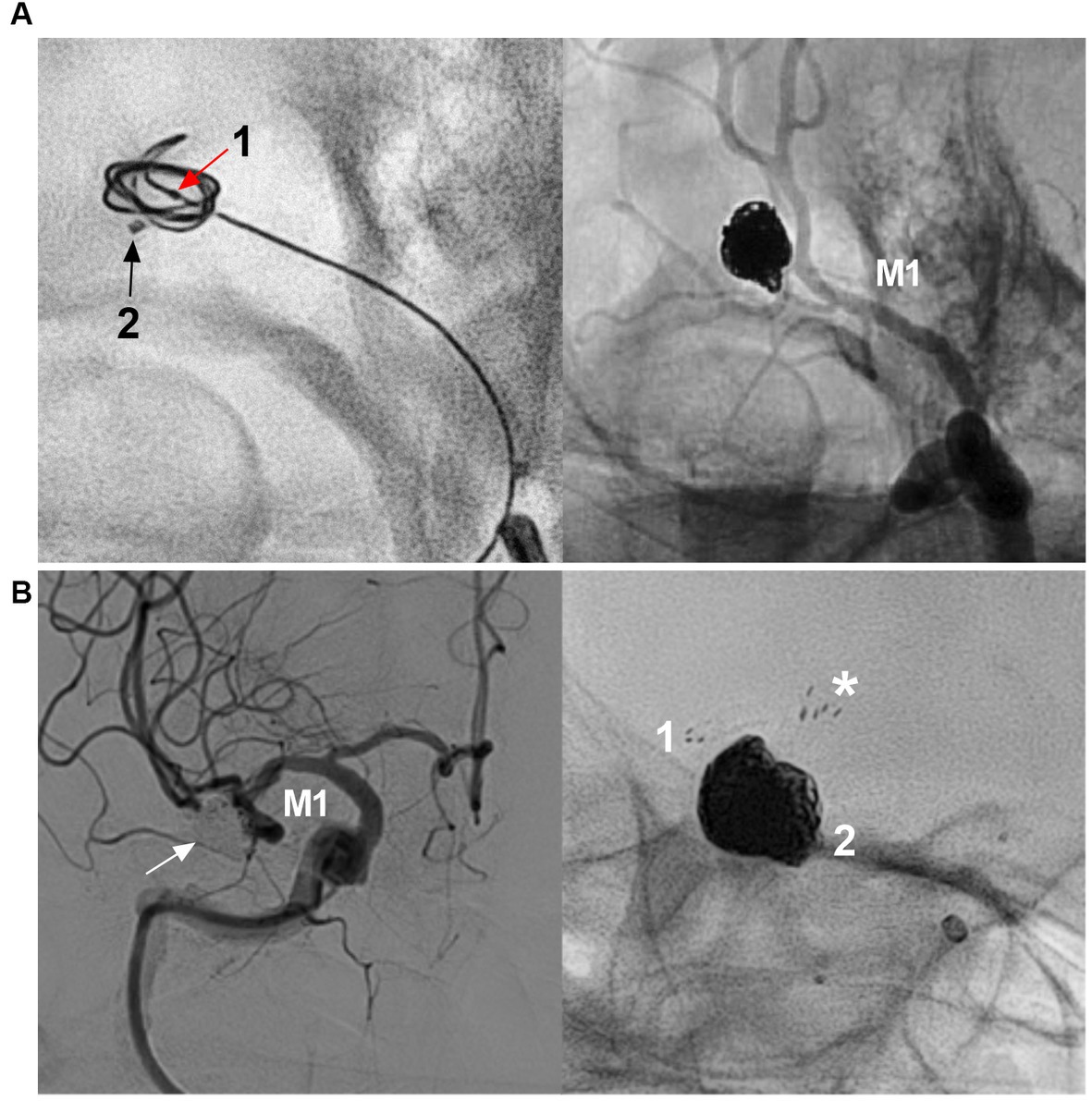
Figure 3. Traditional coiling EVT for MCA aneurysms. (A) Left panel: X-ray image showing two microcatheters into the aneurysm (arrows 1 and 2), microcatheter 1 was used for coiling first; Right panel: unsubtracted DSA showing that the aneurysm was coiled completely by the two microcraters alternately. (B) Left panel: DSA showing that the MCA bifurcation aneurysm (arrow) was completely coiled; Right panel: X-ray image showing that the “Y configuration” stents were used, asterisk indicated the proximal markers of the two stents. Numbers 1 and 2 indicate the distal markers of the two stents. CTA, computed tomography angiography; DSA, digital subtraction angiography; EVT, endovascular treatment, MCA, middle cerebellar artery; M1, M3, first and third segments of the MCA.
Traditional EVT for MCA aneurysms has been associated with adequate (complete occlusion and nearly complete) aneurysm occlusion and good clinical outcomes (modified Rankin Scale (mRS) score of 0–2) (27, 36). In Brinjikji et al.’s meta-analysis of 1,030 patients with 1,076 MCA aneurysms treated by coiling EVT, the morbidity and mortality rates were 5.1 and 6.0% for unruptured and ruptured aneurysms, respectively, and 82.4% of aneurysms had adequate occlusion (39). Traditional EVT for MCA aneurysms is associated with procedure-related complications (Figure 4) (2, 40, 41). EVT for ruptured MCA aneurysms had a higher rate of intraprocedural rupture. In Brinjikji et al.’s meta-analysis, the rates of intraprocedural rupture were 1.7 and 4.8% in unruptured and ruptured aneurysms, respectively (39). In Zhang et al.’s meta-analysis of 1,004 ruptured MCA aneurysms treated by coiling EVT, the overall complication rate was 22.7%, and the rates of procedure-related hemorrhagic and ischemic complications were 5 and 15.4%, respectively (42). However, most ischemic complications are asymptomatic (42). Stent-assisted coiling for EVT significantly increases the risk of procedural complications of MCA aneurysms because MCA catheterization is often difficult (12, 37).

Figure 4. Complication of traditional EVT for MCA aneurysm. (A) Left panel: DSA showing a wide-necked MCA bifurcation aneurysm (arrow); right panel: DSA showing that the aneurysm was coiled with trunk occlusion (arrowhead). (B) Left panel: Three-dimensional DSA showing a wide-necked MCA trunk aneurysm (arrow); Right panel: DSA showing intraoperative aneurysm rupture during coiling. The arrowhead indicates active contrast extravasation. (C) Left panel: Roadmap image showing an MCA bifurcation aneurysm. The lower branch was catheterized to wait for stenting. Right panel: DSA showing thrombosis (arrows) in the stent during coiling of the aneurysm. DSA, digital subtraction angiography; EVT, endovascular treatment, MCA, middle cerebellar artery; M1, first segment of the MCA.
In general, traditional EVT was appropriate for MCA bifurcation and EFB and ETB saccular aneurysms. In future, EVT can still be a useful technique for MCA aneurysms, for which the pros and cons are summarized in Table 1.
For fusiform or dissecting aneurysms of the distal MCA, PAO can still be used (43). However, PAO for MCA aneurysms should be performed cautiously (4). If the aneurysms are located on the inferior trunk of the MCA and are giant or serpentine with thrombi, the distal MCA may experience ischemic preconditioning, and branch occlusion may be safe (Figure 5) (44). PAO for aneurysms of the M3-4 segments can be performed due to adequate leptomeningeal and pial collaterals; however, this also depends on the eloquence of the affected area/branch (44). The pros and cons of PAO for distal MCA aneurysms are summarized in Table 1.
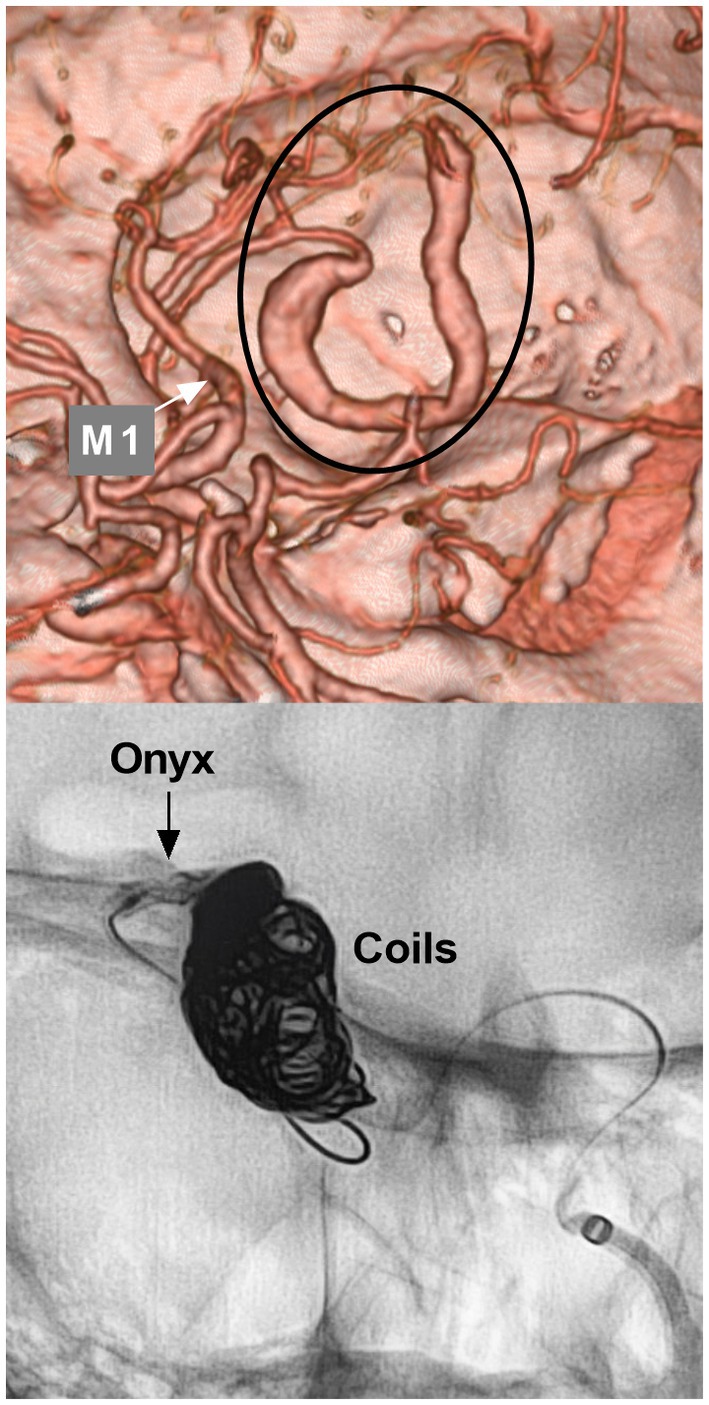
Figure 5. Parent artery occlusion in MCA aneurysm. Upper panel: CTA showing a serpentine aneurysm of the distal MCA; Lower panel: X-ray image showing parent artery occlusion of the aneurysm by coiling and Onyx casting. CTA, computed tomography angiography; MCA, middle cerebellar artery; M1, first segment of the MCA.
FD was effective for MCA aneurysms in selected cases (Figure 6) (4, 45–49). In Cagnazzo et al.’s meta-analysis (2017) of 244 MCA aneurysms that were treated by FD, 76.3% of aneurysms were located at the bifurcation or M2, aneurysms at M1-early cortical branches were 23.7%, the rate of adequate aneurysm occlusion was approximately 80%, the rupture rate per aneurysm-year was 0.4%, and the mortality rate was close to 2% (50). In Salem et al.’s multicenter cohort (2022) of 87 MCA aneurysms, good clinical outcomes were obtained in 96.8% of patients (49). The MCA aneurysm occlusion after FD deployment was progressive. Successful aneurysm healing depended on the occlusion of the branch beside the aneurysm (51).
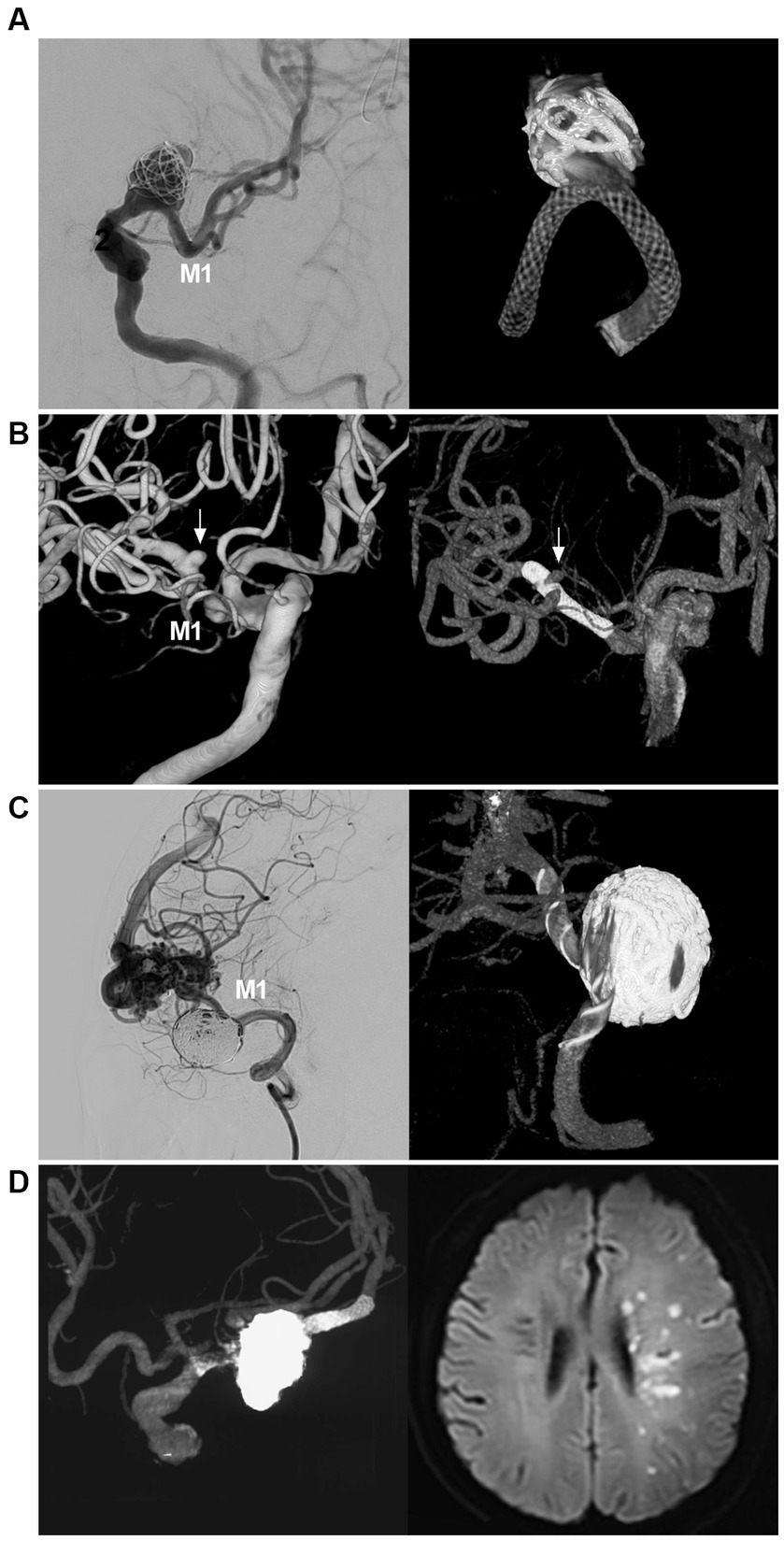
Figure 6. FD deployment in MCA aneurysm. (A) Left panel: DSA showing a wide-necked proximal MCA aneurysm (arrow); right panel: reconstructive CT showing FD deployment in the aneurysm in which the aneurysm was coiled loosely. (B) Left panel: Three-dimensional DSA showing an MCA bifurcation aneurysm (arrow); Right panel: Vaso-3D-DSA showing FD deployment across the bifurcation of the MCA aneurysm (arrow). (C) Left panel: DSA showing that a saccular flow-related aneurysm of the proximal MCA was coiled; right panel: Vaso-3D-DSA showing FD deployment in the aneurysm and that the aneurysm was coiled. (D) Left panel: Vaso-3D-DSA showing that an MCA bifurcation aneurysm was coiled under FD assistance; right panel: postoperative MRI showing acute ischemia of the ipsilateral hemisphere. DSA, digital subtraction angiography; FD, flow diverter, MCA, middle cerebellar artery; M1, first segment of the MCA; MRI, magnetic resonance imaging.
Yavuz et al. proposed three phases of aneurysm occlusion after FD deployment, which was also appropriate for MCA aneurysms: (1) significant decrease in aneurysm filling (1–3 months), (2) infundibulum-like appearance due to the branch with a bulking origin caused by aneurysm shrinkage, the so-called “remodeled artery” (3–12 months), and (3) complete occlusion of both the covered branch and the aneurysm (6–18 months) (52). The Cekirge–Saatci grading scale can also be used to categorize MCA aneurysm occlusion: class 1: complete aneurysm occlusion, class 2: aneurysm neck filling, class 3: incomplete aneurysm filling, class 4: aneurysm filling, and class 5: stable remodeling with flow modification. When a branch comes off the aneurysm, class 1 can be subdivided: 1A as complete aneurysm occlusion with full patency of the branch, 1B as complete aneurysm occlusion with the branch reduced in caliber, and 1C as aneurysm complete occlusion with no antegrade filling of the branch (53).
Smaller bifurcating branches may exhibit less of a sump effect, remodel more rapidly, and lead to more rapid aneurysm occlusion due to low blood flow from the related branches (47, 54). Therefore, for aneurysms of the LSA, ETB, and EFB takeoff and the MCA trunk, the rate of aneurysm occlusion is high (51, 55, 56). For MCA aneurysms with a large covered bifurcating branch subjected to FD deployment, the aneurysm curative course may stop at Yavuz’s phase 1–2 or at class 3–5 of the Cekirge–Saatci grading scale (53, 57, 58). Due to the lack of direct collaterals, FD deployment does not cure all MCA aneurysms. However, reduced blood flow and aneurysm shrinkage can protect the aneurysm from rupture (52).
FD deployment in MCA aneurysms may be associated with a high complication rate (59). In Salem et al.’s multicenter cohort, the overall rates of ischemic and hemorrhagic complications were 8 and 1.1%, respectively, and symptomatic and permanent complications were encountered in 5.7 and 2.3% of patients, respectively (49). In Cagnazzo et al.’s meta-analysis, the rate of complications was 20.7%, and most were ischemic complications, often from branch occlusion and slow flow (50). To reduce or avoid ischemic complications, single FD coverage and slight oversizing were favored in consideration of decreasing mesh density to obtain slower progressive aneurysm occlusion with less risk of abrupt occlusion of the vessel coming out of the sac (53, 55). Therefore, FD may be a viable option for the EVT of MCA aneurysms. In future, with newer production developments, the safety of FD deployment in MCA aneurysms can be improved. The pros and cons of FD deployment for MCA aneurysms are summarized in Table 1.
Intrasaccular flow disruptor devices can disrupt the intra-aneurysmal flow and create thrombosis, which seems to be a promising technique for the treatment of MCA aneurysms (5, 60). Intrasaccular flow disruptor devices include the Woven Endobridge (WEB) device (Aliso Viejo, CA, United States), the Cerus Endovascular Neqstent device, Contour Neurovascular System (CNS) (Cerus Endovascular, Fremont, CA, United States), the Luna/Artisse embolization system (Medtronic, Irvine, CA, United States), and the Medina Embolic Device (MED) (Medtronic, Irvine, CA, United States) (Currently, this device is not widely available) (5, 61–63).
Currently, the WEB device has evolved to a low-profile single layer with enhanced visualization (64, 65). Several landmark studies have confirmed its safety and efficacy, including WEBCAST (2016), WEBCAST-2 (2017), WEB-IT (2017), and the French Observatory (2016) (66–70). The WEB device is feasible in the treatment of MCA aneurysms (Figure 7). In particular, the current WEB-17 system is shifting its usage toward small, ruptured, and atypical aneurysms and even sidewall aneurysms (65, 71, 72).
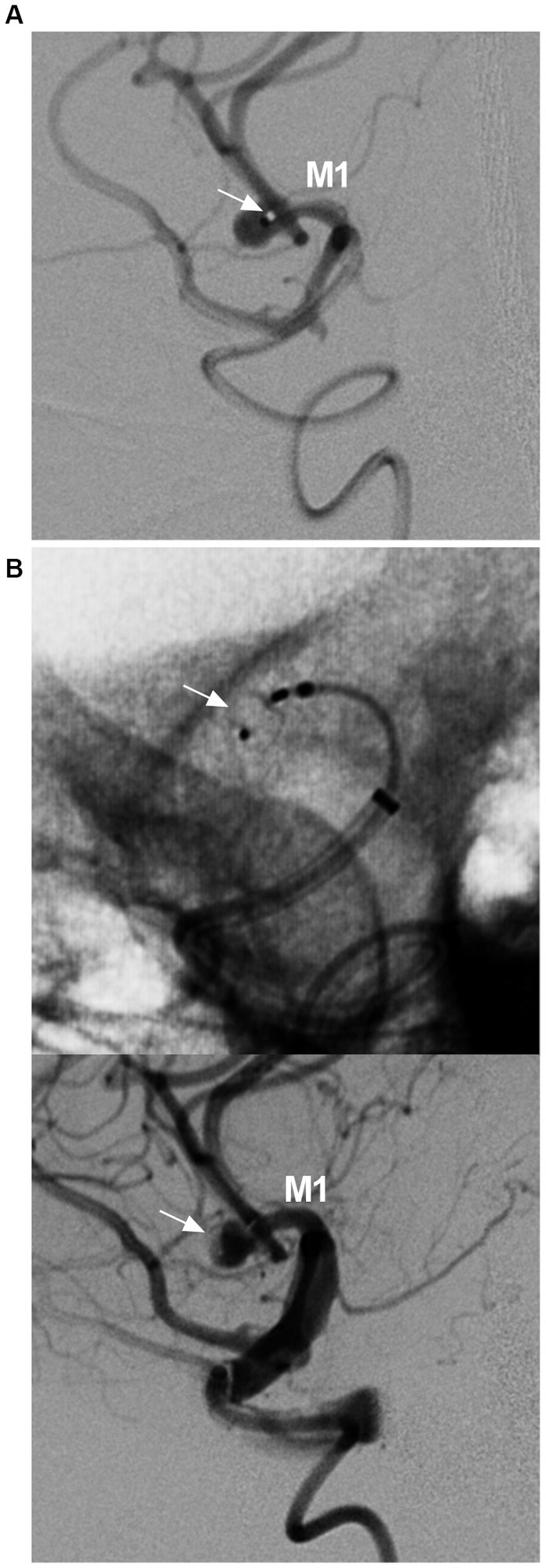
Figure 7. WEB device deployment in MCA aneurysm. (A) DSA showing the position of the VIA microcatheter (arrow) in the aneurysm. (B) Unsubtracted DSA (upper panel) and DSA (lower panel) showing the WEB deployed in the aneurysm (arrows). DSA, digital subtracted angiography; MCA, middle cerebellar artery; M1, first segment of the MCA; WEB, Woven endobridge.
In Adeeb et al.’s study of 206 MCA aneurysms treated by the WEB device, the adequate occlusion rate was 84.7, and 90.7% of patients had good clinical outcomes at the last follow-up (73). However, because the WEB device preserves its shape-memory property, it may not be suitable for every MCA aneurysm. Approximately 10% of WEB device-treated aneurysms may need retreatment due to compression and/or migration of the WEB device during follow-up, especially ruptured aneurysms (73–76).
The use of a WEB device with an oversized width was an independent predictor of aneurysm occlusion, and it was recommended to select a WEB device 1 mm larger than the average width and 1 mm smaller than the average height (77, 78). It is generally believed that treating the recurrence of MCA aneurysms after WEB device deployment can be more challenging. The retreatment consisted of FD deployment, stent-assisted coiling, and second WEB placement, and open surgery was the last resort (79, 80). Therefore, there is a long way to go for WEB to replace traditional EVT for MCA bifurcation aneurysms. The pros and cons of WEB device deployment for MCA aneurysms are summarized in Table 1.
The CNS is a dual-layer nitinol memory mesh that provides flow diversion and flow disruption at the neck of the aneurysm. On deployment, it adapts to the lower half of the aneurysm, covering the neck (81). The CNS seems to be safe and effective in the treatment of MCA aneurysms. The success rate was >90% for the technique and > 80% for adequate aneurysm occlusion, as reported in Ghozy et al.’s meta-analysis (2022) (82), in Liebig et al.’s CERUS study (83), and in Biondi et al.’s study (84). Although good outcomes were shown, the main limitations are the small sample size and lack of long-term and randomized data.
The Neqstent device is a derivative of the CNS. After positioning at the neck of an aneurysm, the coiling microcatheter crosses the mesh into the aneurysm (61, 62). The Neqstent device stays within the aneurysm neck and maintains the coils within the aneurysm (85). According to the design, Neqstent can be applied to MCA aneurysms; however, reports are rare, and more experience is needed.
Luna named Artisse in the newest version (5, 62). Previous studies showed that the Luna/Artisse embolization system was safe and effective (86, 87). However, further study with a large sample is needed.
Stent-like devices include the pCONus device (Phenox GmbH, Bochum, Germany), the Barrel device (Medtronic/Covidien, Irvine, California, United States), the eCLIP (Endovascular Clip System) device (Evasc Medical Systems, Vancouver, Canada), and the PulseRider device (Cerenovus, Irvine, CA, United States) (currently, this device is not widely available) (6).
Compared with other devices, pCONus devices were popularly used, including pCONus 1, pCONus 2, pCONus 2 HPC, and pCANvas devices. The pCONus devices are stent-like self-expanding nitinol implants that blossom like a flower inside the aneurysm to facilitate the “waffle-cone technique” (88–92). Some reports have shown that the use of a pCONus device to assist in coiling of MCA aneurysms successfully treats more than 95% of cases and achieves adequate aneurysm occlusion in more than 80% of cases, such as in Ulfert et al.’s report (93), in the pToWin trial (94), in Morales-Caba et al.’s report (91), and in Yeomans et al.’s report (92).
Although the above outcomes were attractive, there were some concerns about pCONus device deployment, especially regarding thromboembolic complications. In Krupa et al.’s meta-analysis of eight studies (198 patients with 200 aneurysms, including 89 MCA aneurysms), the intraprocedural complication rate was 17.3%, and the most frequent complication was thromboembolic events, occurring in 12.1% of all procedures (6). Therefore, there is a need for consensus regarding the most effective antiplatelet regimen that can be applied to reduce the incidence of thromboembolic events. In addition, more evidence is necessary to support the efficacy of the pCONus device for MCA bifurcation aneurysms.
The barrel device is a closed-cell microstent that can be used to reconstruct the aneurysm neck, and its key feature is a barrel section that herniates over the aneurysmal ostium (95). It can be used to assist in the coiling of MCA bifurcation aneurysms, such as in Gory et al.’s report (2018) (96) and Kabbasch et al.’s report (97). Although good outcomes were shown in the above reports, the sample size was small. A further study is needed.
The eCLIP device is a hybrid device with both neck bridging and flow-diversion properties (98). Recent studies have confirmed its effect in assisting coiling aneurysms, such as in de Vries et al.’s study (99) and in Diestro et al.’s report (100). The eCLIP device can be used for the treatment of MCA bifurcation aneurysms; however, it has been less frequently reported. More evidence is needed.
Currently, for MCA aneurysms, clipping is still regarded as the first-line therapy; EVT can be an alternative, and coiling EVT is still the preferred therapy. FD deployment can be used for the selective treatment of MCA, especially for dissecting aneurysms. PAO can be used to treat distal MCA aneurysms. In addition, some new devices can be used, such as intrasaccular flow disruptors and stent-like devices, of which the WEB device and pCONus device are advanced products. Regarding other new products for the treatment of MCA aneurysms, evidence is lacking, and longer-term data for most EVT devices are not available.
JY contributed to the conception and design of the review. ZZ and WL collected data. JY and ZZ contributed to drafting the text and preparing the figures. All authors contributed to the article and approved the submitted version.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank Kan Xu for contribution to the conception and designation of the review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Inagawa, T . Prevalence of cerebral aneurysms in autopsy studies: a review of the literature. Neurosurg Rev. (2022) 45:2565–82. doi: 10.1007/s10143-022-01783-7
2. Mortimer, AM, Bradley, MD, Mews, P, Molyneux, AJ, and Renowden, SA. Endovascular treatment of 300 consecutive middle cerebral artery aneurysms: clinical and radiologic outcomes. AJNR Am J Neuroradiol. (2014) 35:706–14. doi: 10.3174/ajnr.A3776
3. Hagen, F, Maurer, CJ, and Berlis, A. Endovascular treatment of unruptured mca bifurcation aneurysms regardless of aneurysm morphology: short- and long-term follow-up. AJNR Am J Neuroradiol. (2019) 40:503–9. doi: 10.3174/ajnr.A5977
4. Cimflova, P, Özlük, E, Korkmazer, B, Ahmadov, R, Akpek, E, Kizilkilic, O, et al. Long-term safety and efficacy of distal aneurysm treatment with flow diversion in the M2 segment of the middle cerebral artery and beyond. J Neurointerv Surg. (2021) 13:631–6. doi: 10.1136/neurintsurg-2020-016790
5. Heiferman, DM, Goyal, N, Inoa, V, Nickele, CM, and Arthur, AS. A new era in the treatment of wide necked bifurcation aneurysms: Intrasaccular flow disruption. Interv Neuroradiol. (2022):159101992210943. doi: 10.1177/15910199221094390
6. Krupa, K, Brzegowy, P, Kucybała, I, Łasocha, B, Urbanik, A, and Popiela, TJ. Endovascular embolization of wide-necked bifurcation aneurysms with the use of pCONus device: A systematic review and meta-analysis. Clin Imaging. (2021) 70:81–8. doi: 10.1016/j.clinimag.2020.10.025
7. Rhoton, AL . The supratentorial arteries. Neurosurgery. (2002) 51:S53–S120. doi: 10.1097/00006123-200210001-00003
8. Rodríguez-Hernández, A, Zador, Z, Rodríguez-Mena, R, and Lawton, MT. Distal aneurysms of intracranial arteries: application of numerical nomenclature, predilection for cerebellar arteries, and results of surgical management. World Neurosurg. (2013) 80:103–12. doi: 10.1016/j.wneu.2012.09.010
9. Tanriover, N, Kawashima, M, Rhoton, AL Jr, Ulm, AJ, and Mericle, RA. Microsurgical anatomy of the early branches of the middle cerebral artery: morphometric analysis and classification with angiographic correlation. J Neurosurg. (2003) 98:1277–90. doi: 10.3171/jns.2003.98.6.1277
10. Hou, K, Xu, K, Liu, H, Li, G, and Yu, J. The Clinical Characteristics and Treatment Considerations for Intracranial Aneurysms Associated With Middle Cerebral Artery Anomalies: A Systematic Review. Front Neurol. (2020) 11:564797. doi: 10.3389/fneur.2020.564797
11. Yu, J . Current state and confusion of twig-like middle cerebral artery. Interv Neuroradiol. (2022):159101992211213. doi: 10.1177/15910199221121380
12. Zaidat, OO, Castonguay, AC, Teleb, MS, Asif, K, Gheith, A, Southwood, C, et al. Middle cerebral artery aneurysm endovascular and surgical therapies: comprehensive literature review and local experience. Neurosurg Clin N Am. (2014) 25:455–69. doi: 10.1016/j.nec.2014.04.005
13. Ulm, AJ, Fautheree, GL, Tanriover, N, Russo, A, Albanese, E, Rhoton, AL, et al. Microsurgical and angiographic anatomy of middle cerebral artery aneurysms: prevalence and significance of early branch aneurysms. Neurosurgery. (2008) 62:ONS344. doi: 10.1227/01.neu.0000326018.22434.ed
14. Elsharkawy, A, Lehečka, M, Niemelä, M, Billon-Grand, R, Lehto, H, Kivisaari, R, et al. A new, more accurate classification of middle cerebral artery aneurysms: computed tomography angiographic study of 1,009 consecutive cases with 1,309 middle cerebral artery aneurysms. Neurosurgery. (2013) 73:94–102. doi: 10.1227/01.neu.0000429842.61213.d5
15. Kadkhodayan, Y, Delgado Almandoz, JE, Fease, JL, Scholz, JM, Blem, AM, et al. Endovascular treatment of 346 middle cerebral artery aneurysms: results of a 16-year single-center experience. Neurosurgery. (2015) 76:54–60. doi: 10.1227/NEU.0000000000000562
16. Zhu, W, Liu, P, Tian, Y, Gu, Y, Xu, B, Chen, L, et al. Complex middle cerebral artery aneurysms: a new classification based on the angioarchitecture and surgical strategies. Acta Neurochir. (2013) 155:1481–91. doi: 10.1007/s00701-013-1751-8
17. Molyneux, AJ, Kerr, RS, Yu, LM, Clarke, M, Sneade, M, Yarnold, JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. (2005) 366:809–17. doi: 10.1016/S0140-6736(05)67214-5
18. Zhou, Z, and Yu, J. Endovascular treatment of a supraclinoid internal carotid artery fenestration aneurysm: A case report and literature review. Heliyon. (2023) 9:e17605. doi: 10.1016/j.heliyon.2023.e17605
19. Srinivasa, R, Shwetha, K, Pravin, S, Daisy, S, Eilene, B, Mathuriya, SN, et al. Microsurgical Anatomy of Middle Cerebral Artery in Northwest Indian Population: A Cadaveric Brain Dissection Study. Asian J Neurosurg. (2021) 16:785–91. doi: 10.4103/ajns.ajns_189_21
20. Korja, M, Kivisaari, R, Rezai Jahromi, B, and Lehto, H. Natural History of Ruptured but Untreated Intracranial Aneurysms. Stroke. (2017) 48:1081–4. doi: 10.1161/STROKEAHA.116.015933
21. Ikawa, F, Morita, A, Tominari, S, Nakayama, T, Shiokawa, Y, Date, I, et al. Rupture risk of small unruptured cerebral aneurysms. J Neurosurg. (2019) 132:69–78. doi: 10.3171/2018.9.JNS181736
22. Investigators, UJ, Morita, A, Kirino, T, Hashi, K, Aoki, N, Fukuhara, S, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. (2012) 366:2474–82. doi: 10.1056/NEJMoa1113260
23. Świątnicki, W, Boecher-Schwarz, H, and Standhardt, H. Growth of flow-related aneurysms following occlusion of cerebral arteriovenous malformation. J Neurol Surg A Cent Eur Neurosurg. (2023). doi: 10.1055/a-2037-6079
24. Stein, KP, Wanke, I, Forsting, M, Zhu, Y, Moldovan, AS, Dammann, P, et al. Associated aneurysms in supratentorial arteriovenous malformations: impact of aneurysm size on haemorrhage. Cerebrovasc Dis. (2015) 39:122–9. doi: 10.1159/000369958
25. Metayer, T, Leclerc, A, Borha, A, Derrey, S, Langlois, O, Barbier, C, et al. Microsurgical Clipping of Middle Cerebral Artery Aneurysms: Complications and Risk Factors for Complications. World Neurosurg. (2022) 168:e87–96. doi: 10.1016/j.wneu.2022.09.044
26. Zijlstra, IA, Verbaan, D, Majoie, CB, Vandertop, P, and van den Berg, R. Coiling and clipping of middle cerebral artery aneurysms: a systematic review on clinical and imaging outcome. J Neurointerv Surg. (2016) 8:24–9. doi: 10.1136/neurintsurg-2014-011478
27. Alreshidi, M, Cote, DJ, Dasenbrock, HH, Acosta, M, Can, A, Doucette, J, et al. Coiling Versus Microsurgical Clipping in the Treatment of Unruptured Middle Cerebral Artery Aneurysms: A Meta-Analysis. Neurosurgery. (2018) 83:879–89. doi: 10.1093/neuros/nyx623
28. Toccaceli, G, Diana, F, Cagnazzo, F, Cannizzaro, D, Lanzino, G, Barbagallo, GMV, et al. Microsurgical Clipping Compared with New and Most Advanced Endovascular Techniques in the Treatment of Unruptured Middle Cerebral Artery Aneurysms: A Meta-Analysis in the Modern Era. World Neurosurg. (2020) 137:451–64.e1. doi: 10.1016/j.wneu.2019.12.118
29. Sturiale, CL, Scerrati, A, Ricciardi, L, Rustemi, O, Auricchio, AM, Norri, N, et al. Clipping versus coiling for treatment of middle cerebral artery aneurysms: a retrospective Italian multicenter experience. Neurosurg Rev. (2022) 45:3179–91. doi: 10.1007/s10143-022-01822-3
30. Shenoy, VS, Miller, C, Sen, RD, McAvoy, M, Montoure, A, Kim, LJ, et al. High-Flow Bypass and Clip Trapping of a Giant Fusiform Middle Cerebral Artery (M1) Aneurysm: Technical Case Instruction. Oper Neurosurg (Hagerstown). (2023) 25:e183–7. doi: 10.1227/ons.0000000000000785
31. Duan, G, Zhang, Y, Yin, H, Wu, Y, Zhang, X, Zhao, R, et al. Predictors of recurrence and complications for the endovascular treatment of unruptured middle cerebral artery aneurysm: A high-volume center experience over 12 years. Eur J Radiol. (2023) 163:110833. doi: 10.1016/j.ejrad.2023.110833
32. Hanel, RA, Yoon, N, Sauvageau, E, Aghaebrahim, A, Lin, E, Jadhav, AP, et al. Neuroform Atlas Stent for Treatment of Middle Cerebral Artery Aneurysms: 1-Year Outcomes From Neuroform Atlas Stent Pivotal Trial. Neurosurgery. (2021) 89:102–8. doi: 10.1093/neuros/nyab090
33. Zaidat, OO, Hanel, RA, Sauvageau, EA, Aghaebrahim, A, Lin, E, Jadhav, AP, et al. Pivotal Trial of the Neuroform Atlas Stent for Treatment of Anterior Circulation Aneurysms: One-Year Outcomes. Stroke. (2020) 51:2087–94. doi: 10.1161/STROKEAHA.119.028418
34. Darsaut, TE, Keough, MB, Boisseau, W, Findlay, JM, Bojanowski, MW, Chaalala, C, et al. Middle Cerebral Artery Aneurysm Trial (MCAAT): A Randomized Care Trial Comparing Surgical and Endovascular Management of MCA Aneurysm Patients. World Neurosurg. (2022) 160:e49–54. doi: 10.1016/j.wneu.2021.12.083
35. Diestro, JDB, Li, Y, Kishore, K, Omar, AT, Montanera, W, Sarma, D, et al. A shift from open to endovascular repair in the treatment of ruptured middle cerebral artery aneurysms: a single institution experience. Neuroradiology. (2023) 65:1353–61. doi: 10.1007/s00234-023-03195-w
36. Xenofontos, A, Raffalli-Ebezant, H, Madhavan, A, Khan, H, Mastan, A, Russell, I, et al. Simple endovascular coiling: An effective long-term solution for wide-necked ruptured middle cerebral artery aneurysms? A 10-years retrospective study. Neuroradiol J. (2022) 35:573–9. doi: 10.1177/19714009211067406
37. Gory, B, Rouchaud, A, Saleme, S, Dalmay, F, Riva, R, Caire, F, et al. Endovascular treatment of middle cerebral artery aneurysms for 120 nonselected patients: a prospective cohort study. AJNR Am J Neuroradiol. (2014) 35:715–20. doi: 10.3174/ajnr.A3781
38. Aydin, K, Balci, S, Sencer, S, Barburoglu, M, Umutlu, MR, and Arat, A. Y-Stent-Assisted Coiling With Low-Profile Neuroform Atlas Stents for Endovascular Treatment of Wide-Necked Complex Intracranial Bifurcation Aneurysms. Neurosurgery. (2020) 87:744–53. doi: 10.1093/neuros/nyz516
39. Brinjikji, W, Lanzino, G, Cloft, HJ, Rabinstein, A, and Kallmes, DF. Endovascular treatment of middle cerebral artery aneurysms: a systematic review and single-center series. Neurosurgery. (2011) 68:397–402. doi: 10.1227/NEU.0b013e318201d7f4
40. Bracard, S, Abdel-Kerim, A, Thuillier, L, Klein, O, Anxionnat, R, Finitsis, S, et al. Endovascular coil occlusion of 152 middle cerebral artery aneurysms: initial and midterm angiographic and clinical results. J Neurosurg. (2010) 112:703–8. doi: 10.3171/2009.6.JNS09483
41. Suzuki, S, Tateshima, S, Jahan, R, Duckwiler, GR, Murayama, Y, Gonzalez, NR, et al. Endovascular treatment of middle cerebral artery aneurysms with detachable coils: angiographic and clinical outcomes in 115 consecutive patients. Neurosurgery. (2009) 64:876–89. doi: 10.1227/01.NEU.0000343534.05655.37
42. Zhang, X, Zhou, Y, Zuo, Q, Duan, G, Tang, H, Yang, P, et al. Endovascular Treatment of Ruptured Middle Cerebral Artery Aneurysms: A Single-Arm Meta-Analysis and Systematic Review. World Neurosurg. (2019) 127:559–66. doi: 10.1016/j.wneu.2019.01.066
43. Calvacante, T, Derrey, S, Curey, S, Langlois, O, Fréger, P, Gérardin, E, et al. Distal middle cerebral artery aneurysm: A proposition of microsurgical management. Neurochirurgie. (2013) 59:121–7. doi: 10.1016/j.neuchi.2013.04.007
44. Baltacioğlu, F, Cekirge, S, Saatci, I, Oztürk, H, Arat, A, Pamir, N, et al. Distal middle cerebral artery aneurysms. Endovascular treatment results with literature review. Interv Neuroradiol. (2002) 8:399–407. doi: 10.1177/159101990200800409
45. Cagnazzo, F, di Carlo, DT, Cappucci, M, Lefevre, PH, Costalat, V, and Perrini, P. Acutely Ruptured Intracranial Aneurysms Treated with Flow-Diverter Stents: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. (2018) 39:1669–75. doi: 10.3174/ajnr.A5730
46. Diestro, JDB, Adeeb, N, Dibas, M, Boisseau, W, Harker, P, Brinjikji, W, et al. Flow Diversion for Middle Cerebral Artery Aneurysms: An International Cohort Study. Neurosurgery. (2021) 89:1112–21. doi: 10.1093/neuros/nyab365
47. Soydemir, E, Gündoğmuş, CA, Türeli, D, Andaç Baltacıoğlu, N, Bayri, Y, and Baltacıoğlu, F. Safety and efficacy of flow diverter stents in the treatment of middle cerebral artery aneurysms: a single-center experience and follow-up data. Diagn Intervent Radiol (Ankara, Turkey). (2023) 29:350–8. doi: 10.4274/dir.2022.211050
48. Liang, F, Yang, Y, Luo, L, Liao, B, Zhang, G, Ou, S, et al. Endovascular treatment of complex middle cerebral artery aneurysms using TuBridge flow diverters. Interv Neuroradiol. (2020) 26:539–46. doi: 10.1177/1591019920946216
49. Salem, MM, Khorasanizadeh, M, Lay, SV, Renieri, L, Kuhn, AL, Sweid, A, et al. Endoluminal flow diverting stents for middle cerebral artery bifurcation aneurysms: multicenter cohort. J Neurointerv Surg. (2022) 14:1084–9. doi: 10.1136/neurintsurg-2021-018224
50. Cagnazzo, F, Mantilla, D, Lefevre, PH, Dargazanli, C, Gascou, G, and Costalat, V. Treatment of Middle Cerebral Artery Aneurysms with Flow-Diverter Stents: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. (2017) 38:2289–94. doi: 10.3174/ajnr.A5388
51. Piano, M, Lozupone, E, Milonia, L, Pero, G, Cervo, A, Macera, A, et al. Flow diverter devices in the treatment of complex middle cerebral artery aneurysms when surgical and endovascular treatments are challenging. J Stroke Cerebrovasc Dis. (2022) 31:106760. doi: 10.1016/j.jstrokecerebrovasdis.2022.106760
52. Yavuz, K, Geyik, S, Saatci, I, and Cekirge, HS. Endovascular treatment of middle cerebral artery aneurysms with flow modification with the use of the pipeline embolization device. AJNR Am J Neuroradiol. (2014) 35:529–35. doi: 10.3174/ajnr.A3692
53. Cekirge, HS, and Saatci, I. A New Aneurysm Occlusion Classification after the Impact of Flow Modification. AJNR Am J Neuroradiol. (2016) 37:19–24. doi: 10.3174/ajnr.A4489
54. Lena, J, and Fargen, KM. Flow Diversion and Middle Cerebral Artery Aneurysms: Is Successful Aneurysm Occlusion Dependent on Branch Occlusion? World Neurosurg. (2016) 90:630–1. doi: 10.1016/j.wneu.2016.01.006
55. Iosif, C, Mounayer, C, Yavuz, K, Saleme, S, Geyik, S, Cekirge, HS, et al. Middle Cerebral Artery Bifurcation Aneurysms Treated by Extrasaccular Flow Diverters: Midterm Angiographic Evolution and Clinical Outcome. AJNR Am J Neuroradiol. (2017) 38:310–6. doi: 10.3174/ajnr.A5022
56. Lauzier, DC, Root, BK, Kayan, Y, Almandoz, JED, Osbun, JW, Chatterjee, AR, et al. Pipeline embolization of proximal middle cerebral artery aneurysms: A multicenter cohort study. Interv Neuroradiol. (2022) 28:50–7. doi: 10.1177/15910199211015578
57. Daou, B, and Jabbour, P. Flow Diversion for Treating Middle Cerebral Artery Aneurysms. World Neurosurg. (2016) 90:627–9. doi: 10.1016/j.wneu.2016.01.003
58. Topcuoglu, OM, Akgul, E, Daglioglu, E, Topcuoglu, ED, Peker, A, Akmangit, I, et al. Flow Diversion in Middle Cerebral Artery Aneurysms: Is It Really an All-Purpose Treatment? World Neurosurg. (2016) 87:317–27. doi: 10.1016/j.wneu.2015.11.073
59. Kashkoush, A, El-Abtah, ME, Petitt, JC, Glauser, G, Winkelman, R, Achey, RL, et al. Flow diversion for the treatment of intracranial bifurcation aneurysms: a systematic review and meta-analysis. J Neurointerv Surg. (2023):20582. doi: 10.1136/jnis-2023-020582
60. Naamani, KE, Chen, CJ, Abbas, R, Sweid, A, Sioutas, GS, Badih, K, et al. Woven EndoBridge versus stent-assisted coil embolization of cerebral bifurcation aneurysms. J Neurosurg. (2022) 137:1786–93. doi: 10.3171/2022.3.JNS2217
61. Fatania, K, and Patankar, DT. Comprehensive review of the recent advances in devices for endovascular treatment of complex brain aneurysms. Br J Radiol. (2022) 95:20210538. doi: 10.1259/bjr.20210538
62. Hecker, C, Broussalis, E, Griessenauer, CJ, and Killer-Oberpfalzer, M. A mini-review of intrasaccular flow diverters. J Neurointerv Surg. (2023) 15:70–4. doi: 10.1136/neurintsurg-2021-018426
63. Dmytriw, AA, Salem, MM, Yang, VXD, Krings, T, Pereira, VM, Moore, JM, et al. Endosaccular Flow Disruption: A New Frontier in Endovascular Aneurysm Management. Neurosurgery. (2020) 86:170–81. doi: 10.1093/neuros/nyz017
64. Lee, KB, Suh, CH, Song, Y, Kwon, B, Kim, MH, Yoon, JT, et al. Trends of Expanding Indications of Woven EndoBridge Devices for the Treatment of Intracranial Aneurysms: A Systematic Review and Meta-analysis. Clin Neuroradiol. (2023) 33:227–35. doi: 10.1007/s00062-022-01207-5
65. Pagano, P, Cortese, J, Soize, S, Caroff, J, Manceau, PF, Moret, J, et al. Aneurysm Treatment with Woven EndoBridge-17: Angiographic and Clinical Results at 12 Months from a Retrospective, 2-Center Series. AJNR Am J Neuroradiol. (2023) 44:467–73. doi: 10.3174/ajnr.A7830
66. Fiorella, D, Molyneux, A, Coon, A, Szikora, I, Saatci, I, Baltacioglu, F, et al. Demographic, procedural and 30-day safety results from the WEB Intra-saccular Therapy Study (WEB-IT). J Neurointerv Surg. (2017) 9:1191–6. doi: 10.1136/neurintsurg-2016-012841
67. Pierot, L, Costalat, V, Moret, J, Szikora, I, Klisch, J, Herbreteau, D, et al. Safety and efficacy of aneurysm treatment with WEB: results of the WEBCAST study. J Neurosurg. (2016) 124:1250–6. doi: 10.3171/2015.2.JNS142634
68. Pierot, L, Gubucz, I, Buhk, JH, Holtmannspötter, M, Herbreteau, D, Stockx, L, et al. Safety and Efficacy of Aneurysm Treatment with the WEB: Results of the WEBCAST 2 Study. AJNR Am J Neuroradiol. (2017) 38:1151–5. doi: 10.3174/ajnr.A5178
69. Pierot, L, Moret, J, Turjman, F, Herbreteau, D, Raoult, H, Barreau, X, et al. WEB Treatment of Intracranial Aneurysms: Clinical and Anatomic Results in the French Observatory. AJNR Am J Neuroradiol. (2016) 37:655–9. doi: 10.3174/ajnr.A4578
70. Pierot, L, Moret, J, Turjman, F, Herbreteau, D, Raoult, H, Barreau, X, et al. WEB Treatment of Intracranial Aneurysms: Feasibility, Complications, and 1-Month Safety Results with the WEB DL and WEB SL/SLS in the French Observatory. AJNR Am J Neuroradiol. (2015) 36:922–7. doi: 10.3174/ajnr.A4230
71. Sabuzi, F, Cortese, J, Da Ros, V, Mihalea, C, Chalumeau, V, Moret, J, et al. How a decade of aneurysms embolization with the Woven EndoBridge has changed our understanding and practices? J Neuroradiol. (2023) 50:518–22. doi: 10.1016/j.neurad.2023.02.006
72. Adeeb, N, Dibas, M, Diestro, JDB, Cuellar-Saenz, HH, Sweid, A, Kandregula, S, et al. Multicenter Study for the Treatment of Sidewall versus Bifurcation Intracranial Aneurysms with Use of Woven EndoBridge (WEB). Radiology. (2022) 304:372–82. doi: 10.1148/radiol.212006
73. Adeeb, N, Dibas, M, Diestro, JDB, Phan, K, Cuellar-Saenz, HH, Sweid, A, et al. Comparing treatment outcomes of various intracranial bifurcation aneurysms locations using the Woven EndoBridge (WEB) device. J Neurointerv Surg. (2023) 15:558–65. doi: 10.1136/neurintsurg-2022-018694
74. Al Saiegh, F, Velagapudi, L, Khanna, O, Sweid, A, Mouchtouris, N, Baldassari, MP, et al. Predictors of aneurysm occlusion following treatment with the WEB device: systematic review and case series. Neurosurg Rev. (2022) 45:925–36. doi: 10.1007/s10143-021-01638-7
75. Goertz, L, Liebig, T, Siebert, E, Dorn, F, Pflaeging, M, Forbrig, R, et al. Long-term clinical and angiographic outcome of the Woven EndoBridge (WEB) for endovascular treatment of intracranial aneurysms. Sci Rep. (2022) 12:11467. doi: 10.1038/s41598-022-14945-w
76. Pierot, L, Szikora, I, Barreau, X, Holtmannspoetter, M, Spelle, L, Klisch, J, et al. Aneurysm treatment with the Woven EndoBridge (WEB) device in the combined population of two prospective, multicenter series: 5-year follow-up. J Neurointerv Surg. (2023) 15:552–7. doi: 10.1136/neurintsurg-2021-018414
77. Cortese, J, Caroff, J, Chalumeau, V, Gallas, S, Ikka, L, Moret, J, et al. Determinants of cerebral aneurysm occlusion after embolization with the WEB device: a single-institution series of 215 cases with angiographic follow-up. J Neurointerv Surg. (2023) 15:446–51. doi: 10.1136/neurintsurg-2022-018780
78. Dmytriw, AA, Diestro, JDB, Dibas, M, Phan, K, Sweid, A, Cuellar-Saenz, HH, et al. International Study of Intracranial Aneurysm Treatment Using Woven EndoBridge: Results of the WorldWideWEB Consortium. Stroke. (2022) 53:e47–9. doi: 10.1161/STROKEAHA.121.037609
79. Caroff, J, Janot, K, Soize, S, Marnat, G, Cortese, J, Mihalea, C, et al. Management of aneurysmal recurrence after Woven EndoBridge (WEB) treatment. J Neurointerv Surg. (2022) 15:939–42. doi: 10.1136/jnis-2022-019645
80. Srinivasan, VM, Dmytriw, AA, Regenhardt, RW, Vicenty-Padilla, J, Alotaibi, NM, Levy, E, et al. Retreatment of Residual and Recurrent Aneurysms After Embolization With the Woven EndoBridge Device: Multicenter Case Series. Neurosurgery. (2022) 90:569–80. doi: 10.1227/neu.0000000000001883
81. Akhunbay-Fudge, CY, Deniz, K, Tyagi, AK, and Patankar, T. Endovascular treatment of wide-necked intracranial aneurysms using the novel Contour Neurovascular System: a single-center safety and feasibility study. J Neurointerv Surg. (2020) 12:987–92. doi: 10.1136/neurintsurg-2019-015628
82. Ghozy, S, Lashin, BI, Elfil, M, Bilgin, C, Kobeissi, H, Shehata, M, et al. The safety and effectiveness of the Contour Neurovascular System for the treatment of wide-necked aneurysms: A systematic review and meta-analysis of early experience. Interv Neuroradiol. (2022):159101992211395. doi: 10.1177/15910199221139546
83. Liebig, T, Killer-Oberpfalzer, M, Gal, G, Schramm, P, Berlis, A, Dorn, F, et al. The Safety and Effectiveness of the Contour Neurovascular System (Contour) for the Treatment of Bifurcation Aneurysms: The CERUS Study. Neurosurgery. (2022) 90:270–7. doi: 10.1227/NEU.0000000000001783
84. Biondi, A, Primikiris, P, Vitale, G, and Charbonnier, G. Endosaccular flow disruption with the Contour Neurovascular System: angiographic and clinical results in a single-center study of 60 unruptured intracranial aneurysms. J Neurointerv Surg. (2022) 15:838–43. doi: 10.1136/jnis-2022-019271
85. Diana, F, de Dios, LM, Peschillo, S, Raz, E, Yoshimura, S, Requena Ruiz, M, et al. Intrasaccular Flow Disruptor-Assisted Coiling of Intracranial Aneurysms Using the Novel Contour Neurovascular Systems and NEQSTENT: A Single-Center Safety and Feasibility Study. Brain Sci. (2022) 12:991. doi: 10.3390/brainsci12080991
86. Piotin, M, Biondi, A, Sourour, N, Mounayer, C, Jaworski, M, Mangiafico, S, et al. The LUNA aneurysm embolization system for intracranial aneurysm treatment: short-term, mid-term and long-term clinical and angiographic results. J Neurointerv Surg. (2018) 10:e34. doi: 10.1136/neurintsurg-2018-013767
87. Piotin, M, Fahed, R, Redjem, H, Smajda, S, Desilles, JP, Escalard, S, et al. The ARTISSE intrasaccular device for intracranial aneurysm treatment: short-term, mid-term and long-term clinical and angiographic results. J Neurointerv Surg. (2022) 14:957–61. doi: 10.1136/neurintsurg-2021-017806
88. Sirakov, A, Aguilar-Perez, M, AlMatter, M, and Henkes, H. Complex Wide-necked and lobulated aneurysm of the middle cerebral artery bifurcation: treatment with a pconus2 neck bridging device and p48mw flow modulation device. Clin Neuroradiol. (2020) 30:633–7. doi: 10.1007/s00062-019-00862-5
89. Guenego, A, Mine, B, Bonnet, T, Elens, S, Vazquez Suarez, J, Jodaitis, L, et al. Long-term follow-up of the pCONus device for the treatment of wide-neck bifurcation aneurysms. Interv Neuroradiol. (2022) 28:455–62. doi: 10.1177/15910199211040279
90. Gory, B, Aguilar-Pérez, M, Pomero, E, Turjman, F, Weber, W, Fischer, S, et al. One-year Angiographic Results After pCONus Stent-Assisted Coiling of 40 Wide-Neck Middle Cerebral Artery Aneurysms. Neurosurgery. (2017) 80:925–33. doi: 10.1093/neuros/nyw131
91. Morales-Caba, L, Lylyk, I, Vázquez-Añón, V, Bleise, C, Scrivano, E, Perez, N, et al. The pCONUS2 and pCONUS2 HPC neck bridging devices: results from an international multicenter retrospective study. Clin Neuroradiol. (2023) 33:129–36. doi: 10.1007/s00062-022-01191-w
92. Yeomans, J, Gatt, S, Habeeb Mohamed, E, Crossley, R, Keston, P, Minks, D, et al. pCONUS 2 and pCONUS 2-HPC in the treatment of wide-necked intracranial aneurysms: Multicentre UK experience. Interv Neuroradiol. (2023):159101992211504. doi: 10.1177/15910199221150467
93. Ulfert, C, Pfaff, J, Schönenberger, S, Bösel, J, Herweh, C, Pham, M, et al. The pCONus device in treatment of wide-necked aneurysms: technical and midterm clinical and angiographic results. Clin Neuroradiol. (2018) 28:47–54. doi: 10.1007/s00062-016-0542-z
94. Aguilar Pérez, M, Henkes, H, Kurre, W, Bleise, C, Lylyk, PN, Lundquist, J, et al. Results of the pToWin Study: Using the pCONUS Device for the Treatment of Wide-Neck Intracranial Aneurysms. J Clin Med. (2022) 11:884. doi: 10.3390/jcm11030884
95. Mühl-Benninghaus, R, Simgen, A, Reith, W, and Yilmaz, U. The Barrel stent: new treatment option for stent-assisted coiling of wide-necked bifurcation aneurysms-results of a single-center study. J Neurointerv Surg. (2017) 9:1219–22. doi: 10.1136/neurintsurg-2016-012718
96. Gory, B, Blanc, R, Turjman, F, Berge, J, and Piotin, M. The Barrel vascular reconstruction device for endovascular coiling of wide-necked intracranial aneurysms: a multicenter, prospective, post-marketing study. J Neurointerv Surg. (2018) 10:969–74. doi: 10.1136/neurintsurg-2017-013602
97. Kabbasch, C, Mpotsaris, A, Maus, V, Altenbernd, JC, and Loehr, C. The barrel vascular reconstruction device: a retrospective, observational multicentric study. Clin Neuroradiol. (2019) 29:295–301. doi: 10.1007/s00062-017-0660-2
98. De Vries, J, Boogaarts, HD, Sørensen, L, Holtmannspoetter, M, Benndorf, G, Turowski, B, et al. eCLIPs bifurcation remodeling system for treatment of wide neck bifurcation aneurysms with extremely low dome-to-neck and aspect ratios: a multicenter experience. J Neurointerv Surg. (2021) 13:438–42. doi: 10.1136/neurintsurg-2020-016354
99. de Vries, J, Aquarius, R, Sørensen, L, Boogaarts, HD, Turowski, B, van Zwam, WH, et al. Safety and efficacy of the eCLIPs bifurcation remodelling system for the treatment of wide necked bifurcation aneurysms: 1 year results from the European eCLIPs Safety, Feasibility, and Efficacy Study (EESIS). J Neurointerv Surg. (2023) 15:163–71. doi: 10.1136/neurintsurg-2021-018460
Keywords: MCA aneurysm, endovascular treatment, prognosis, complication, review
Citation: Zhou Z, Lan W and Yu J (2023) Endovascular treatment of middle cerebral artery aneurysms: current status and future prospects. Front. Neurol. 14:1239199. doi: 10.3389/fneur.2023.1239199
Received: 13 June 2023; Accepted: 20 October 2023;
Published: 15 November 2023.
Edited by:
Alberto Maud, Texas Tech University Health Sciences Center El Paso, United StatesReviewed by:
Thomas K. Mattingly, University of Rochester, United StatesCopyright © 2023 Zhou, Lan and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinlu Yu, amx5dUBqbHUuZWR1LmNu; amlubHV5dUBob3RtYWlsLmNvbQ==
†ORCID: Jinlu Yu, https://orcid.org/0000-0003-2329-7946
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.