
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 27 October 2023
Sec. Endovascular and Interventional Neurology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1226220
This article is part of the Research TopicNeurosonology in Stroke Medicine and Neurocritical careView all 8 articles
Introduction: Redo carotid endarterectomy (CEA) and carotid stenting (CAS) are often performed when there is evidence of post-procedural restenosis. The incidence of restenosis after carotid reconstruction is not negligible, ranging from 5 to 33%. The diagnosis of significant internal carotid artery (ICA) restenosis is usually based on duplex ultrasound (US) criteria, mostly on peak-systolic flow velocity (PSV). However, there have been no generally accepted duplex US criteria for carotid restenosis after CAS or CEA.
Methods: In this systematic review, the PubMed/ Medline and Scopus databases were screened to find trials that reported duplex US criteria for significant restenosis after CEA and/or CAS. Only those reports were analyzed in which the restenoses were also assessed by CT/MR or digital subtraction angiography as comparators for duplex US.
Results: Fourteen studies met the predetermined search criteria and were included in this review. In most studies, PSV thresholds for significant in-stent ICA restenosis after CAS were higher than those for significant stenosis in non-procedurally treated (native) ICA. Many fewer studies investigated the US criteria for ICA restenosis after CEA. Despite the heterogeneous data, there is a consensus to use higher flow velocity thresholds for assessment of stenosis in stented ICA than in native ICA; however, there have been insufficient data about the flow velocity criteria for significant restenosis after CEA. Although the flow velocity thresholds for restenosis after CAS and CEA seem to be different, the large studies used the same duplex criteria to define restenosis after the two procedures. Moreover, different studies used different flow velocity thresholds to define ICA restenosis, leading to variable restenosis rates.
Discussion: We conclude that (1) further examinations are warranted to determine appropriate duplex US criteria for restenosis after CAS and CEA, (2) single duplex US parameter cannot be used to reliably determine the degree of ICA restenosis, (3) inappropriate US criteria used in large studies may have led to false restenosis rates, and (4) studies are required to determine if there is a benefit from redo carotid artery procedure, such as redo-CEA or redo-CAS, starting with prospective risk stratification studies using current best practice non-invasive care alone.
Redo carotid endarterectomy (CEA) and carotid stenting (CAS) are often performed when there is evidence of post-procedural restenosis. The incidence of restenosis after carotid reconstruction is not negligible, ranging from 5 to 33%. The frequency of carotid restenosis after CAS and CEA depends on the definition and assessment criteria of restenosis and the duration of follow-up.
A meta-analysis of the prevalence of stroke in asymptomatic patients with severe restenosis (>70%) after CAS showed no higher risk of ipsilateral stroke than in patients without severe in-stent restenosis. However, after a mean follow-up of 37 months after CEA, the rate of ipsilateral stroke in asymptomatic patients with severe internal carotid artery (ICA) restenosis, although low (5.2%), was higher than in patients without severe restenosis (1.5%) (1). Although the treatment of carotid restenosis is highly controversial, current guidelines endorse redo CEA or CAS in a selected subgroup of symptomatic patients with restenosis between 50 and 99% (2). However, it has to be highlighted that there is no current evidence of benefit from a redo carotid artery procedure in restenosis compared to non-invasive management alone either in asymptomatic or symptomatic patients. Non-invasive care for carotid artery disease includes the identification of arterial disease risk factors and lowering arterial disease risk using healthy lifestyle practices and appropriate medication.
Carotid duplex ultrasound (US) is a popular non-invasive screening test that is used for follow-up after CEA or CAS, while computed tomography angiography (CTA) or contrast-enhanced magnetic resonance angiography (MRA) serves as an independent assessment. In most studies, restenosis rates reported after carotid artery surgery or stenting were based on duplex ultrasound findings (3–5). However, duplex ultrasound criteria for significant restenosis after CEA or CAS may differ from each other and from those used in non-procedurally treated ICA stenosis (native ICA stenosis) (6–10). Nevertheless, a number of large studies investigating restenosis rates used identical duplex US criteria to identify restenosis after CAS and CEA (3–5). Moreover, different trials applied different duplex US criteria for the definition of significant restenosis, potentially leading to false restenosis values and variable and unreliable restenosis rates after carotid stenting and carotid surgery (3–5).
In this report, we aimed to review the literature to examine the ultrasound criteria for carotid restenosis developed after CAS or CEA. Only those reports were selected, in which duplex US criteria were examined for significant restenoses estimated by CTA, MRA, or digital subtraction angiography (DSA).
A systematic review was conducted according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The PubMed/Medline and Scopus databases were independently searched by three investigators (IS, FP and ZRM) to identify prospective or retrospective trials involving ultrasound criteria for restenosis after CEA and/or CAS. The oldest publication date of the searched articles was January 1990, while the date of the last search was 1 May 2023. Only those reports were selected for analysis, in which the ultrasound criteria were examined for at least a 50% ICA restenosis that was also estimated by CTA, MRA, or DSA. Due to the highly variable and insufficient data, quantitative analysis could not be performed.
The keywords for the search were the following:
• carotid endarterectomy AND
restenosis AND
(duplex OR Doppler OR ultrasound) AND
(criteria OR threshold OR cut-off)
• carotid AND
stent AND
restenosis AND
(duplex OR Doppler OR ultrasound) AND
(criteria OR threshold OR cut-off)
Data retrieved from each constituent trial included the type of carotid intervention (CEA, CAS), the method for determining the degree of stenosis (European Carotid Surgery Trial /ECST/ or North American Symptomatic Carotid Endarterectomy Trial /NASCET/), the presence or absence of restenosis greater than a certain degree, and the ultrasound criteria. The ultrasound criteria contained cutoff values for peak systolic flow velocity (PSV), end-diastolic flow velocity (EDV), and internal carotid artery and common carotid artery peak systolic flow velocity ratio (ICA/CCA PSV ratio).
The literature search found 148 potentially relevant records after duplicates were removed. After screening titles and abstracts, 36 articles were selected for full-text evaluation. Fourteen studies met the predetermined search criteria and were included in this systematic review (6–9, 11–20), as shown in the PRISMA flow diagram (Figure 1). A total of 3,186 patients with previous carotid procedures were included in the studies. Tables 1–3 show the number of carotid arteries examined in each study.
The degree of restenosis in all studies was also estimated by DSA (n = 8), CTA (n = 2), and DSA or CTA (n = 4). All studies used the NASCET criteria for evaluating the degree of stenosis. There was heterogeneity in the definition of significant carotid stenosis: ≥50% or ≥ 60% stenosis was used for the definition of a less severe ICA restenosis (Tables 1, 3) and ≥ 70% or ≥ 80% for a more severe ICA restenosis (Tables 2, 3). All studies reported at least one of the PSV, EDV, and ICA/CCA ratio cutoff values for a certain degree of ICA restenosis that was also measured by CTA or DSA after CAS or CEA. MRA was not used in any of the studies included in this review.
Eleven studies reported the flow velocity cutoff values for in-stent restenosis after CAS (6, 7, 9, 11–18). Duplex ultrasound findings were compared with the results of DSA (n = 7), CTA (n = 2), and DSA or CTA (n = 2). A total of 2,852 patients with previous carotid stenting were included in the studies (Tables 1, 2).
For moderate (≥50%) in-stent restenosis (6, 7, 9, 11–16), PSV cutoff values varied between 125 and 240 cm/s, while EDV thresholds ranged from 50 to 88 cm/s. The ICA/CCA ratio indicating ≥50% in-stent restenosis was between 1.5 and 3.43 (Table 1 and Figure 2). Although the flow velocity thresholds for moderate in-stent restenosis showed substantial variability, most studies found that both the PSV thresholds (6, 7, 11–16) and EDV cutoff values (6, 13, 14, 16) for ≥50% in-stent restenoses after CAS were significantly higher compared to the standard PSV cutoff value for ≥50% stenosis in native ICAs. Moreover, the ICA/CCA ratio was also higher in moderate in-stent ICA restenosis (6, 7, 11, 13) than the ICA/CCA PSV ratio threshold of 2 for ≥50% native ICA stenosis (Table 1 and Figure 2).
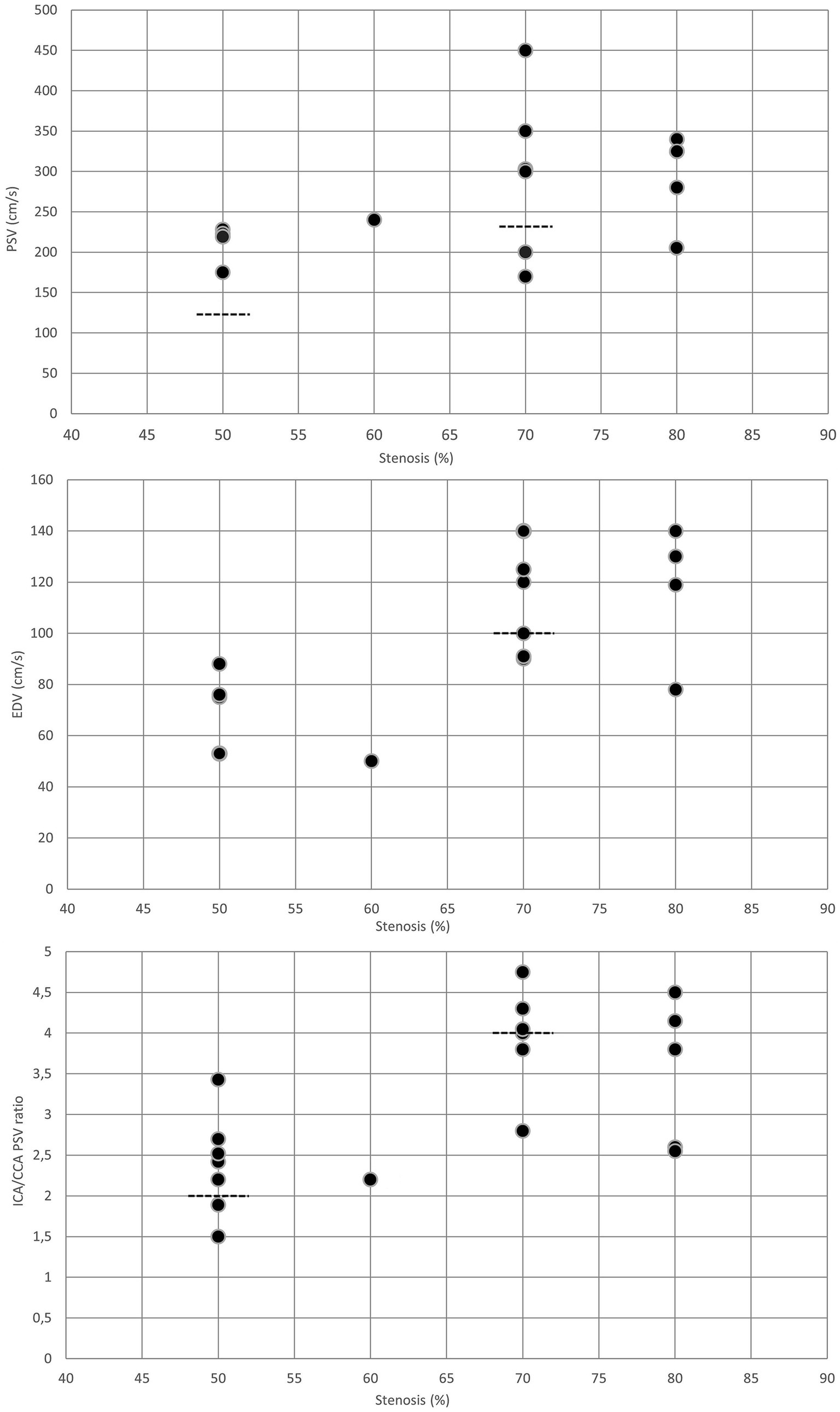
Figure 2. Duplex criteria for different degrees of in-stent restenosis after CAS. Dotted lines represent the standard threshold values for PSV, EDV, and ICA/CCA ratio in the corresponding graphs for ≥50% and ≥ 70% stenoses of the native internal carotid artery. CAS, carotid artery stenting; CCA, common carotid artery; EDV, end-diastolic velocity; ICA, internal carotid artery; PSV, peak systolic velocity.
In severe (≥70%) in-stent restenosis (6, 7, 11–14, 16–18), PSV and EDV thresholds were reported to vary between 170–450 and 78–140 cm/s, respectively. The ICA/CCA ratio for severe restenosis ranged from 2.55 to 4.75 (Table 2 and Figure 2). Similar to the moderate in-stent restenosis, the PSV cutoff values for severe in-stent restenosis were also higher (6, 11, 12, 18) than the PSV cutoff value of 230 cm/s used for classifying ≥70% stenosis in native ICA (10). EDV thresholds were also reported to be higher in severe in-stent ICA restenosis (6, 12, 17) than the standard EDV threshold of 100 cm/s for ≥70% stenosis in native ICA (10) (Table 2 and Figure 2). However, the ICA/CCA PSV ratios in severe in-stent ICA restenoses were similar (7, 11–13, 18), higher (6), or lower (14, 16) in different studies compared to the ICA/CCA PSV ratio threshold of 4 for ≥70% native ICA stenosis (Table 2 and Figure 2).
Many fewer studies (n = 3) evaluated the duplex criteria for post-surgery restenosis after CEA (Table 3) (8, 19, 20) than for in-stent restenosis after CAS (n = 11). Moreover, AbuRahma et al. investigated the same patients in their two reports (8, 20). The degree of post-CEA restenosis was estimated by DSA in one study and by DSA or CTA in two studies. A total of 334 patients with previous carotid endarterectomy were included in the studies (Table 3).
For moderate (≥50%) post-surgical restenosis, the PSV cutoff value was 213 cm/s (8, 20), while the EDV threshold was 60 cm/s (8, 20). The ICA/CCA ratio indicating ≥50% restenosis after CEA was reported to be 2.25 (8, 20) (Table 3 and Figure 3). These cutoff values for ≥50% restenosis after CEA are higher than the corresponding duplex ultrasound threshold values used for classifying ≥50% stenosis in native ICA.
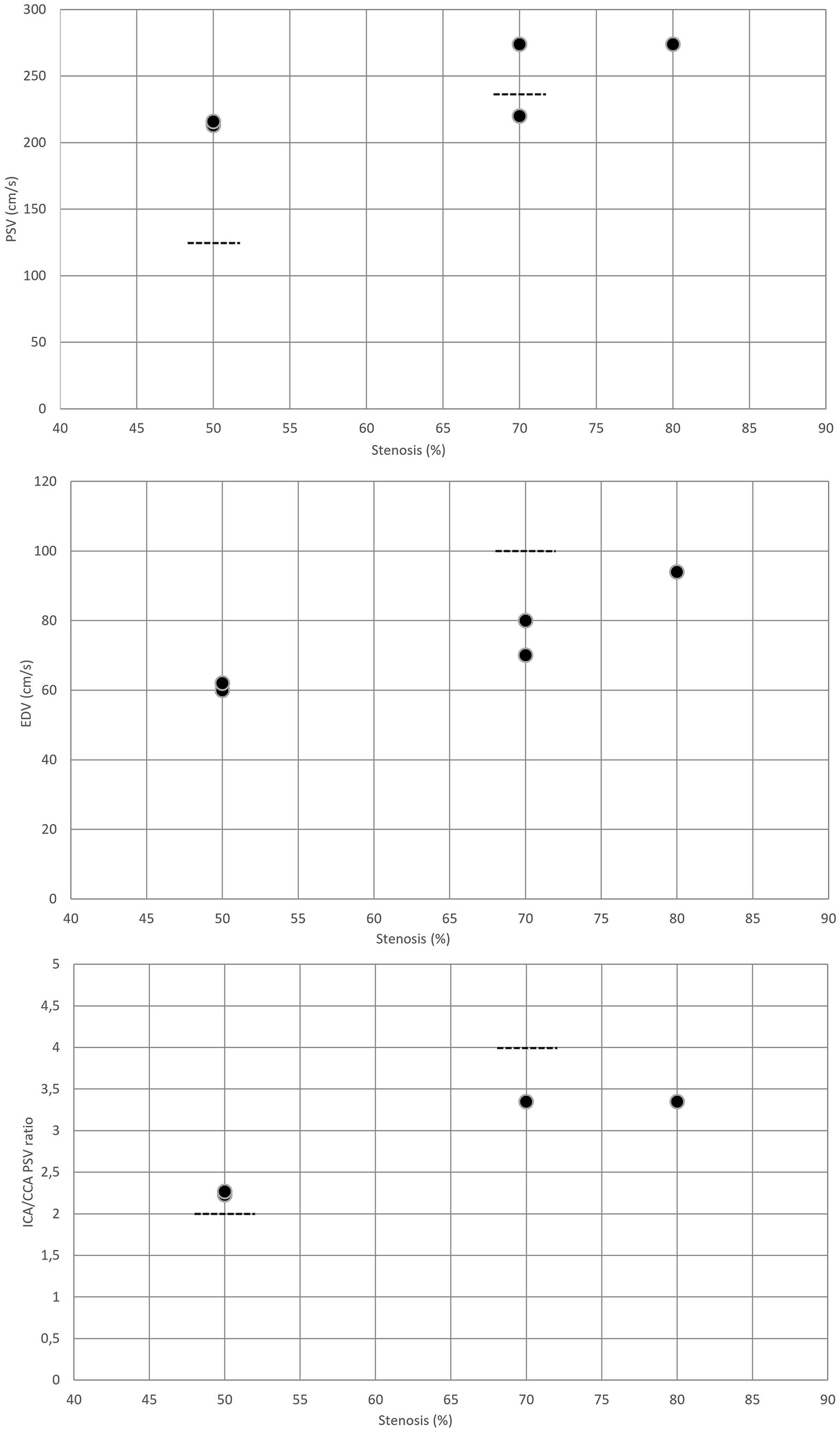
Figure 3. Duplex criteria for different degrees of restenosis after CEA. Dotted lines represent the standard threshold values for PSV, EDV, and ICA/CCA ratio in the corresponding graphs for ≥50% and ≥ 70% stenoses of the native internal carotid artery. CAS, carotid artery stenting; CCA, common carotid artery; EDV, end-diastolic velocity; ICA, internal carotid artery; PSV, peak systolic velocity.
In severe (≥70%) post-CEA restenosis, PSV thresholds were reported to be 274 and 220 cm/s (8, 19). The EDV cutoff values indicating ≥70% restenosis were 70 and 80 cm/s, while the ICA/CCA ratio was 3.35 (Table 3 and Figure 3). These cutoff values for ≥70% post-CEA restenosis are difficult to interpret. Compared to the standard duplex ultrasound criteria for classifying ≥70% stenosis in native ICA, the PSV cutoff values reported for ≥70% post-CEA restenosis were similar or higher; however, the EDV threshold and ICA/CCA ratio were lower.
It should be emphasized, however, that due to the low number of duplex ultrasound studies investigating restenosis after CEA, no conclusions can be drawn about the ultrasound criteria for classifying post-surgical restenosis.
As our aim was to investigate the duplex US criteria for significant carotid restenosis, we analyzed those duplex US studies that used an independent imaging modality including DSA (n = 8), CTA (n = 2), and DSA or CTA (n = 4) to assess the severity of carotid restenosis and to serve as a comparator for US data. In in-stent restenosis studies, the comparator method was DSA in seven, CTA in two, and DSA or CTA also in two studies. In post-CEA restenosis trials, DSA was used in one study and DSA or CTA in two studies.
Many reports confirmed that the flow velocity thresholds for ≥50% and ≥ 70% in-stent restenoses after CAS are higher than for significant ICA stenoses in native arteries. However, due to the few studies investigating the ultrasound criteria for restenosis after carotid surgery, we have insufficient data on cutoff values for significant post-CEA restenosis. The available data after CEA suggested that the flow velocity thresholds for ≥50% restenosis are also higher, while they show no consistent trends for ≥70% restenosis compared to the standard duplex criteria for native ICA. The data also showed that despite the same method (NASCET) used for evaluation, the duplex criteria for restenosis after CAS and CEA are highly variable. The high variability of flow velocity thresholds suggests that no single duplex ultrasound parameter can be used to reliably determine the degree of ICA restenosis after CAS or CEA. Similar to the assessment of the severity of stenosis in native ICA (10), combinations of different flow velocity criteria (PSV, EDV, or ICA/CCA ratio), B-mode and color imaging, and parameters influencing cerebral hemodynamics should be considered for a more accurate evaluation of the degree of restenosis after CEA and CAS (21).
In the era of the first carotid endarterectomy trials, DSA was the only imaging technique to evaluate the degree of carotid stenosis. Two basic calculation methods were used to measure the percentage reduction in the luminal diameter of ICA on DSA images: the European Carotid Surgery Trial (ECST) and the North American Symptomatic Carotid Endarterectomy Trial (NASCET) methods (22, 23). Both techniques use the luminal diameter measured at the site of the most severe stenosis, which is compared to the estimated vessel diameter at the level where the residual luminal diameter is measured in the ECST method and to the plaque-free distal ICA segment in the NASCET method. The NASCET method is more widely used, and the relevant publications we found used this method too, without exception. Therefore, the differences in the method used for the measurements of the degree of restenosis did not interfere with our analysis.
It has to be mentioned, however, that the development and spread of non-invasive imaging techniques is increasingly displacing DSA. As the results of non-invasive methods are correlated with the findings of DSA to a variable degree (24), the use of different non-invasive imaging techniques makes the evaluation of carotid stenosis difficult.
Currently, there is no internationally accepted standard for grading carotid stenosis. Previously, catheter angiography was considered the gold standard for measuring the severity of carotid stenosis, but it has been replaced by non-invasive imaging techniques in the last decades, including MRA, CTA, and duplex ultrasound. It should be noted, however, that all carotid imaging techniques including DSA have limitations, and no method has an absolute advantage over the others (24–26). Contrast-enhanced MRA (24), which is considered the most sensitive non-invasive method, is expensive, less readily available, often overestimates the degree of carotid stenosis (27), and movement artifacts may worsen the image quality. Extensive calcification and dental amalgam may reduce the accuracy of CTA. CTA is also reported to overestimate the severity of ICA stenosis (28), while DSA, due to the limited number of projections, may lead to an underestimation of the degree of asymmetric, eccentric carotid stenosis. Although the carotid duplex is an easily available and safe technique, it can be hampered by the tortuous course of the arteries and the acoustic shadow behind calcified plaques. In addition, flow velocity measurement at the site of the stenosis is affected by contralateral ICA occlusion, collateral circulation, and the length of the stenosis, leading to a considerable variation of flow velocity values at a certain degree of ICA stenosis (10).
As Table 1 demonstrates, the PSV and EDV flow velocity cutoff values, as well as the ICA/CCA PSV ratio thresholds were very heterogeneous for moderate (≥50%) in-stent restenosis after CAS. The lowest PSV cutoff value was 125 cm/s (9), just the same as for native ICA, while the highest one was 240 cm/s (11, 15), regardless of whether DSA or CTA was used for comparison. PSV thresholds for severe ICA in-stent restenosis (≥70%) were also variable, ranging from 170 to 450 cm/s, when using CTA or DSA as a comparator method (Table 2). Similar to PSV cutoff values, the EDV and ICA/CCA ratio thresholds also showed high variability for either moderate or severe in-stent restenosis.
It has to be emphasized that fewer studies investigated the duplex US criteria for ICA restenosis after CEA than after CAS (Table 3). Searching the literature, we have found only three studies that compared the PSV criteria for significant post-CEA restenosis with the findings of other imaging modalities (8, 19, 20), including DSA (19) and CTA or DSA (8, 20). The EDV and ICA/CCA thresholds in post-CEA restenosis were only published by AbuRahma et al. in their two articles; moreover, the results of both reports were based on data from the same patients (8, 20).
Using CTA or DSA for comparison, the PSV cutoff value for ≥50% post-CEA restenosis was 213 cm/s. Surprisingly, the PSV threshold for ≥50% restenosis (213 cm/s) was barely lower than those for ≥70% restenosis after CEA (220 and 274 cm/s, respectively).
It should be highlighted that most of the studies examined patients after CEA with patch closure (8, 19, 20), and very few data are available for restenosis after eversion carotid endarterectomy (29). However, as the standard technique for CEA today is an eversion technique, further studies are needed to determine which ultrasound criteria best predict significant restenosis after eversion CEA.
Although the cutoff values for significant in-stent restenosis were variable, there was a consensus to use higher flow velocity criteria for assessment of the degree of in-stent restenosis after CAS than in native ICA (2). Based on data from Lal et al. (7) and Stanziale et al. (6), the Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS) (2) reported a 220 cm/s PSV threshold for ≥50% and a 300 cm/s cutoff value for ≥70% in-stent restenosis, compared with the 125 cm/ and 230 cm/s PSV thresholds for ≥50% and ≥ 70% stenoses in native ICAs (10), respectively. The higher flow velocity values in in-stent restenosis may lead to an overestimation of the degree of restenosis when using duplex US criteria reported for native arteries (30).
Higher flow velocity in in-stent restenosis after CAS may be due to changes in the biomechanical properties of the artery, including higher stiffness of the arterial wall, rendering the stented carotid artery similar to a rigid tube. This process decreases arterial compliance, leading to a reduced alteration of the volume of the arterial segment during different phases of the pulse wave (31).
As Table 3 shows, AbuRahma et al. (8, 20) found significantly higher PSV cut-off values for ≥50% restenosis after CEA (213 cm/s), compared to the corresponding threshold value for ≥50% stenosis in native ICA (125 cm/s) (10). The PSV threshold for ≥70% carotid restenosis was found to be similar or slightly higher (220 and 274 cm/s, respectively) (8, 19) than the cut-off flow velocity for ≥70% stenosis in native ICA (230 cm/s) (10). Based on these data, the Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS) suggested different duplex US criteria for moderate and severe post-CEA restenoses compared to those in native carotid arteries (2). The ESVS guideline reported a 213 cm/s PSV threshold for ≥50% and a 274 cm/s cut-off value for ≥70% post-CEA restenosis, which values are greater than the corresponding threshold values in native carotid arteries. However, due to insufficient data for the duplex US criteria of ICA restenosis after CEA, there has been no consensus on the flow velocity thresholds indicating ≥50% or ≥ 70% post-CEA restenosis.
The use of higher thresholds in post-CEA restenosis compared to native arteries was confirmed by data from Benzing et al. (29), who investigated the duplex US criteria after longitudinal arteriotomy with patch closure and after eversion CEA. They found that using the standard 125 cm/s PSV threshold, which indicates ≥50% stenosis in native arteries, overestimated the degree of post-CEA restenosis after both surgical techniques. In carotid arteries with a PSV >125 cm/s, the percentage of the stenosis measured by CTA or MRA was only 8 ± 11 and 8% ± 35% after eversion CEA and after longitudinal arteriotomy with patch closure, respectively.
Change of conformation of carotid bifurcation, neo-intimal hyperplasia, and vascular remodeling after carotid surgery are considered to be the most likely explanations for why the standard duplex US criteria used for stenosis of native ICA do not match the criteria for ICA restenosis after carotid surgery (32).
As flow velocities in significant ICA restenosis after both CAS and CEA are higher than in native ICA stenosis, the use of standard duplex US criteria developed for native arteries may overestimate the degree of restenosis after CAS and CEA. Moreover, PSV threshold values after CAS seem to be higher than after CEA for estimating ≥50% and ≥ 70% restenoses. This observation is in line with the results of Lucatelly et al. who monitored ICA flow velocity changes in the 1st year after CAS and CEA and found higher flow velocities in the CAS than in the CEA group (33).
Although the definition of restenosis in large randomized controlled restenosis trials is variable, moderate and severe carotid restenoses are mostly defined by at least 50 and 70% diameter reductions, respectively. The major prospective, randomized, multicenter trials comparing the safety of CEA versus CAS were the CAVATAS (Carotid and Vertebral Artery Transluminal Angioplasty Study), ICSS (International Carotid Stenting Study), SPACE (Stent-Protected Angioplasty Versus Carotid Endarterectomy), EVA-3S (Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis), and CREST (Carotid Revascularization Endarterectomy versus Stenting Trial) trials (3, 4, 30, 34–40). Table 4 shows the diagnostic methods for estimating carotid stenosis in native arteries and restenosis in procedurally treated carotid arteries in these studies (3, 4, 30, 34–40). The duplex ultrasound criteria for severe restenosis after CAS and CEA published in these trials are also shown. While the stenosis in the native carotid arteries was usually estimated by angiography (DSA, MRA, or CTA) or by a combination of duplex ultrasound and CTA or MRA, the definition of restenosis after CEA or CAS was always based on duplex ultrasound alone (Table 4).
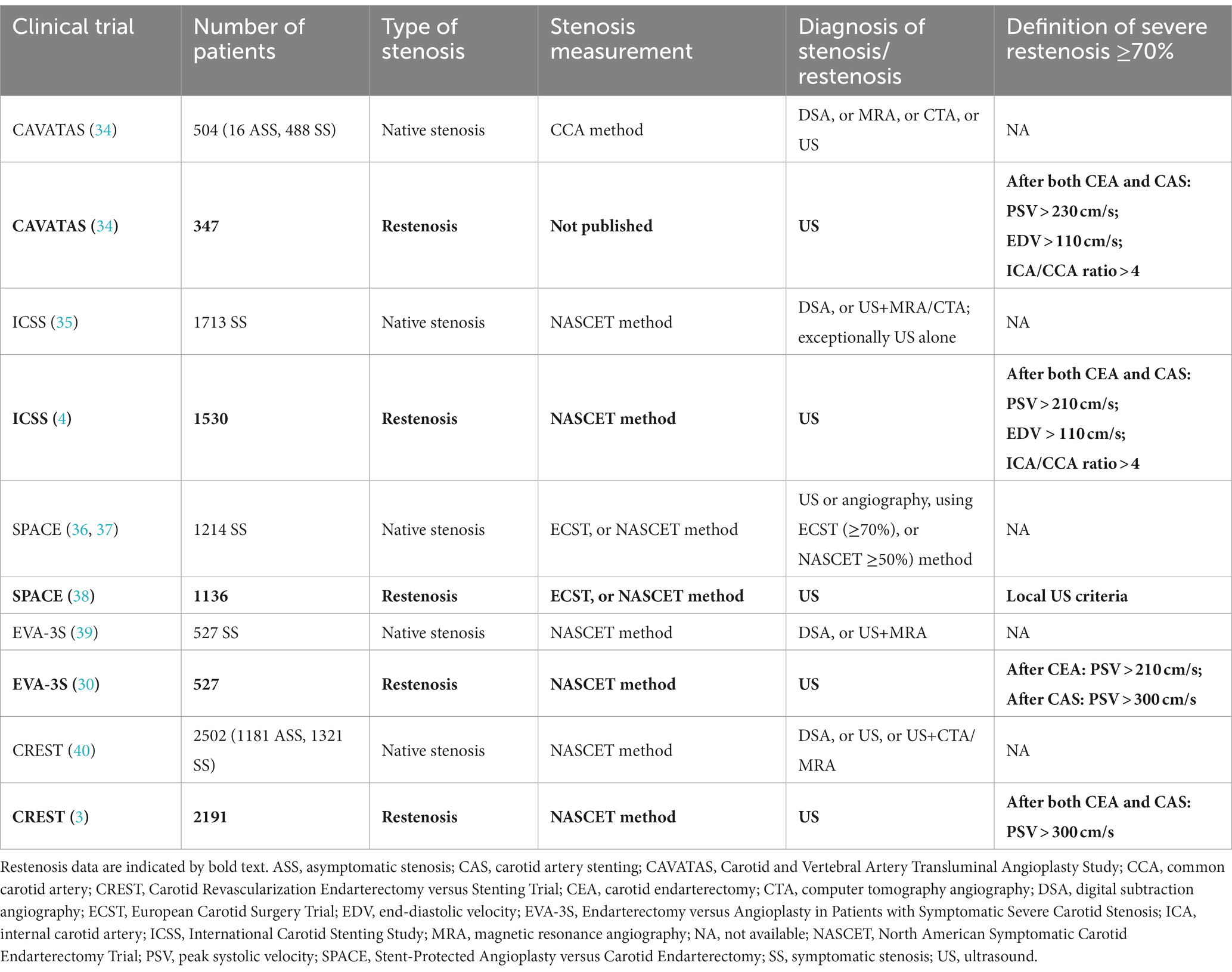
Table 4. Diagnosis of native ICA stenosis and carotid restenosis in major prospective, randomized, multicentre trials comparing the safety of CEA versus CAS.
Due to the lack of consensus on duplex criteria for restenosis, the flow velocity thresholds for severe carotid artery restenosis were highly variable. While the PSV threshold for defining carotid artery restenosis of at least 70% was 210 cm/s in the ICSS study (4) and 300 cm/s in the CREST trial (3) after both CEA and CAS, this PSV threshold indicating ≥70% restenosis in the EVA-3S study was 210 cm/s after CEA and 300 cm/s after CAS (30). Furthermore, strict duplex ultrasound criteria for significant carotid artery restenosis in the CAVATAS and SPACE studies were not reported, but the use of local or standard ultrasound criteria was recommended (34, 38). These data show that the PSV cutoff value indicating ≥70% carotid artery restenosis was very variable in the different studies, making the reliable comparison of restenosis rates between the trials impossible. Moreover, the above studies, with one exception (30), ignored the observation that the flow velocity cutoff values for significant restenosis might be different after CAS and CEA. The use of identical flow velocity criteria after the two procedures may lead to false restenosis rates and incorrect conclusions about the risk of restenosis after CAS and CEA. Prospective clinical studies are needed to validate the flow velocity thresholds for severe restenosis after invasive carotid interventions and to answer whether ultrasound criteria for stenosis in the native ICA differ from those for restenosis after CEA or CAS.
Currently, there is no randomized trial data, which demonstrates a benefit of a carotid procedure compared to best practice non-invasive intervention alone in symptomatic or asymptomatic patients with carotid restenosis after CAS or CEA.
In asymptomatic patients with severe in-stent restenosis receiving non-invasive treatment alone, a meta-analysis showed a very low ipsilateral stroke rate (0.8%) compared to patients without severe in-stent restenosis (2.0%) during a 50-month follow-up after primary CAS (1, 2). Patients with in-stent restenosis were recruited into the meta-analysis studies between 2001 and 2014. Moreover, the studies included in the meta-analysis did not define the nature of non-invasive treatment alone. Therefore, ipsilateral stroke rates would most likely be even lower with current non-invasive care than as represented in the meta-analysis studies. Due to the very low stroke rate, current guidelines do not recommend an invasive carotid procedure in asymptomatic patients with severe carotid restenosis after CAS (2).
Quite the contrary, a meta-analysis revealed a higher ipsilateral stroke rate (5.2%) in non-invasively treated asymptomatic patients with severe post-CEA restenosis than in those without restenosis (1.5%) during a 37-month follow-up after primary CEA (1, 2). It should be noted, however, that the 5.2% ipsilateral stroke rate in patients with severe post-CEA restenosis in a 37-month period is considered very low. Taking into account that the studies included in the meta-analysis did not focus on non-invasive care and were performed between 1998 and 2014, the non-invasive treatment received by patients in these trials can be considered suboptimal by today’s standard. The importance of non-invasive care is further supported by this meta-analysis, showing that 97% of late ipsilateral strokes after CAS and 85% after CEA occurred in patients without evidence of significant restenosis. These data highlight that stroke rate in patients with carotid restenosis could primarily be reduced by improved non-invasive care (1).
In addition to not defining the criteria for non-invasive care, another limitation of the meta-analysis was the use of inappropriate ultrasound criteria to define severe ICA restenosis after CAS and CEA in the included studies (1). Despite the ESVS guideline recommends the use of 300 cm/s PSV as the cutoff value for diagnosing ≥70% in-stent restenosis after CAS and the PSV threshold for severe in-stent restenosis is considered higher than for severe post-CEA restenosis (2), most of the meta-analysis studies used the same PSV threshold for in-stent and post-CEA restenoses, which was much lower than the recommended 300 cm/s (1). The use of a lower PSV cutoff value may have mainly resulted in an overestimation of severe carotid in-stent restenosis, which may have led to a high rate of false positive severe in-stent restenosis, explaining the higher restenosis rate after CAS (10.0%) than after CEA (5.8%). Moreover, the overestimation of carotid in-stent restenosis might have resulted in selecting patients to a severe restenosis group without having severe restenosis, which may have contributed to the very low stroke rate in patients with severe in-stent restenosis (0.8% at 50 months; 0.19%/year) compared to those with post-CEA restenosis (5.2% at 37 months; 1.69%/year).
It should also be emphasized that the higher ipsilateral stroke rate in non-invasively treated patients with severe post-CEA restenosis compared to those without significant restenosis does not justify the benefit of invasive carotid procedures because these observational studies are out of date and did not take into account the risk of periprocedural complications (stroke, death, and myocardial infarction) of redo CEA and CAS. The benefits of invasive carotid interventions can only be proven if randomized controlled trials show their superiority over the current best medical intervention alone; however, these trials are missing. Furthermore, randomized procedural trials are not indicated, and are unethical, if average annual ipsilateral stroke rates are sufficiently close to zero with non-invasive care alone.
Currently, there is no evidence of the benefit of either redo CEA or CAS for severe carotid restenosis, but there is data on the significant risk of periprocedural complications in invasive carotid procedures. Although we did not perform systematic analysis, we evaluated those trials and analyses from the PubMed database which reported the early periprocedural rates after redo CEA or carotid stenting for post-CEA restenosis and included more than 100 patients (41–56). Table 5 demonstrates that both reoperative surgery (3.3–5.0%) and CAS (0.6–5.1%) carry a significant 30-day stroke or death rate (41–56), but the 30-day myocardial infarction rate is also not negligible (Table 5).
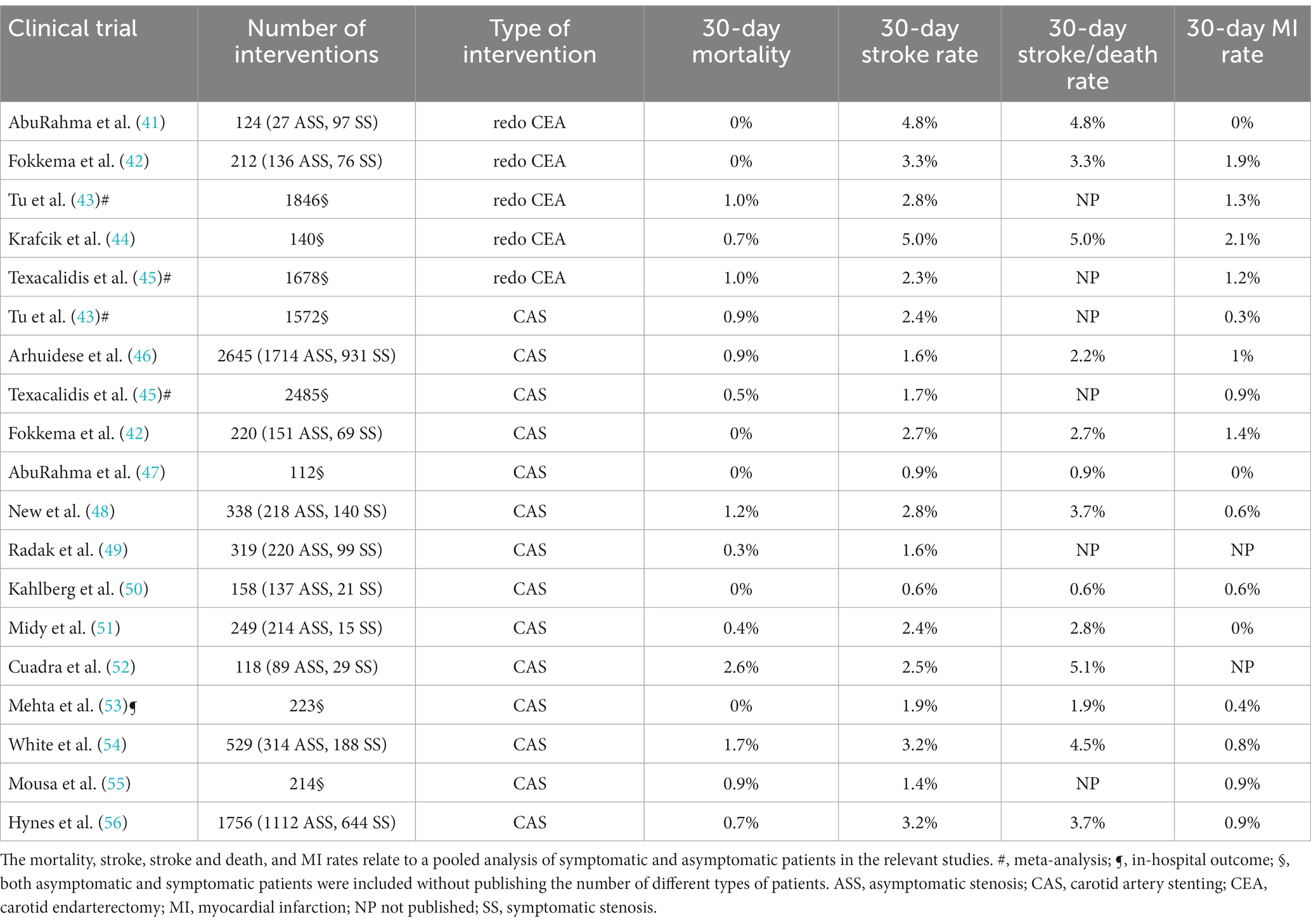
Table 5. 30-day or in-hospital periprocedural complication rates of redo CEA and CAS in patients with post-CEA restenosis in a series describing more than 100 patients.
Table 6 shows the results of studies that separately reported the 30-day stroke or death rate of CAS and redo CEA in symptomatic and asymptomatic patients with post-CEA restenosis (41, 42, 46, 51, 54, 56). Similar to the risk of carotid procedures in patients with significant native ICA stenosis (57), the 30-day stroke or death rate of invasive carotid interventions was also higher in symptomatic (2.6–5.9%) compared with asymptomatic (2.0–3.8%) patients with severe post-CEA restenosis (Table 6). However, while stenting of native ICA stenosis carries a 1.3–1.9 times higher 30-day stroke or death rate than primary CEA in asymptomatic and a 1.8–3.0 times larger rate in symptomatic patients (57), no such difference can be found between redo CEA and CAS in patients with post-surgical restenosis. Available data show that the 30-day periprocedural stroke or death rate of redo CEA (2.9–3.7%) is similar to that of CAS (2.0–3.8%) in asymptomatic and also in symptomatic (redo CEA: 3.9–5.1%; CAS: 2.6–5.9%) patients with post-CEA restenosis (Table 6).
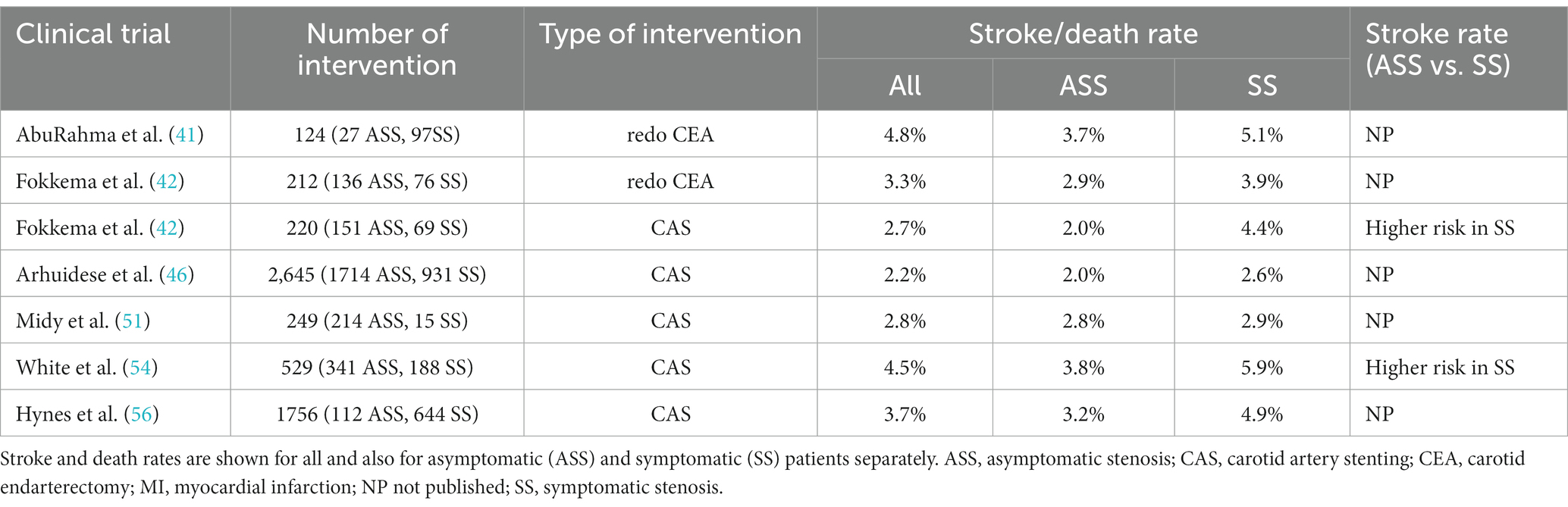
Table 6. 30-day or in-hospital periprocedural stroke or death rates of redo CEA and CAS in symptomatic (SS) and asymptomatic (ASS) patients with post-CEA restenosis.
Using data from Abbott’s study for comparison (57), we found that the 30-day stroke or death rate for redo CEA in post-CEA restenosis was similar to that for primary CEA in native ICA stenosis in both asymptomatic (2.9–3.7% versus 1.4–4.6%, respectively) and symptomatic patients (3.9–5.1% versus 3.2–10.0%, respectively). However, the 30-day periprocedural stroke or death rate for CAS in post-CEA restenosis was lower than that for primary CAS in native ICA stenosis (2.6–5.9% versus 6.0–12.1%, respectively) in symptomatic patients, while these rates were comparable in asymptomatic patients (2.0–3.8% versus 2.5–5.4%, respectively) (Table 6).
As mentioned before, transfemoral carotid artery stenting (CAS) in native carotid stenosis is associated with a higher 30-day periprocedural stroke or death rate compared to CEA (39, 40, 58), which has been attributed to embolization from the aortic arch or from the carotid plaque. In 2004, a new technique called transcarotid artery revascularization (TCAR) with flow reversal in the carotid artery was developed to avoid the manipulation of the guidewire through the aortic arch and to prevent embolization from the carotid plaque (59). Although TCAR has been rapidly adopted in the US, randomized trials were not performed to compare the efficacy and safety of TCAR with CAS, CEA, or best non-invasive care in patients with native carotid stenosis or carotid restenosis (60, 61).
There is evidence that non-invasive best medical intervention is highly effective in carotid stenosis. Moreover, optimal treatment of vascular risk factors decreases not only the stroke risk but also all arterial disease complications, including the risk of myocardial infarction and vascular death. Abbott showed that the benefit from non-invasive medical intervention alone in patients with carotid stenosis has improved over the last 4 decades, leading to a substantial decrease of average annual ipsilateral stroke rates below 1%/year (approximately 0.8%/year) in patients with advanced asymptomatic carotid stenosis (57, 62–64).
Studying symptomatic patients with carotid stenosis awaiting revascularisation in recent randomized controlled trials (EVA-3S, SPACE, ICSS, and CREST) and in medical arms of earlier randomized controlled trials (NASCET, ECST, Veteran Affairs Cooperative Study) revealed that modern non-invasive care alone halved the stroke risk compared to that of earlier studies (65). Available data also showed that urgent best non-invasive treatment alone in symptomatic patients with significant carotid stenosis (66, 67) or with intracranial arterial stenosis was associated with a dramatic decrease in stroke risk (68).
Although the “optimal medical treatment” is described in detail in the Clinical Practice Guidelines of the European Society for Vascular Surgery from 2017, none of the large restenosis studies (4, 30, 34, 38, 40) highlighted the importance or defined the criteria of non-invasive best medical intervention. It means that the significance of optimal medical treatment was probably underestimated, which might have led to suboptimal non-invasive care and a higher rate of arterial disease complications. When designing new trials to compare the efficacy of invasive carotid procedures combined with non-invasive best medical treatment versus non-invasive best medical intervention alone, this issue should be treated as a priority.
Invasive carotid artery procedures in severe carotid restenosis after CEA or CAS are currently not justified. However, despite the lack of evidence for the benefit of CAS or CEA compared to the best non-invasive care alone, current guidelines endorse invasive procedures for severe carotid restenosis that is defined by PSV thresholds of 274 and 300 cm/s after CEA and CAS, respectively (2). The European Society for Vascular Surgery (ESVS) guideline (2), just adopting the same treatment criteria for carotid restenosis as for native stenosis, recommends invasive carotid procedures for severe symptomatic restenosis after CAS or CEA and suggests considering invasive treatment also for severe asymptomatic restenosis after CEA. However, it should be highlighted that there are no consensus criteria for diagnosing significant restenosis after carotid procedures, and the treatment criteria are based on the results of CEA trials performed 3–4 decades ago in patients with severe native ICA stenosis diagnosed by DSA (23, 24, 69). However, both imaging techniques and the best medical treatment options have significantly changed since that time: DSA was replaced by non-invasive imaging methods, and new and highly effective non-invasive stroke prevention strategies were introduced. As the current guideline recommendations are not supported by relevant study results, completely new clinical research is required to determine the diagnostic criteria and the best treatment strategy for carotid restenosis after CAS or CEA.
The current priority is to measure the average annual ipsilateral stroke rate in symptomatic and asymptomatic patients with severe carotid restenosis after CEA or CAS treated with the current best non-invasive medical care alone. A subgroup of patients with a sufficiently high risk of ipsilateral stroke despite the best non-invasive medical treatment (≈3%) should be considered for future randomized trials to answer whether redo CEA or CAS provides an additional stroke risk reduction compared to the best non-invasive medical treatment alone. Therefore, routine invasive carotid procedures should be stopped in patients with ICA restenosis after CAS or CEA in order to begin observational studies with long-term follow-up (at least 3–4 years) and to stratify the risk of ipsilateral stroke in patient subgroups with different restenosis severity. Although we focused on carotid restenosis in this report, the constant and significant improvement of non-invasive treatment alone and the outdated results of randomized carotid stenosis trials performed 3–4 decades ago (23, 24, 69) urge new research approach in patients with native carotid stenosis (57).
As risk stratification of ipsilateral stroke in patients with different restenosis severity is essential, reliable diagnostic criteria for significant carotid restenosis after CAS and CEA must be clarified. Being non-invasive, harmless, and easily available, duplex US could be the first-line tool for monitoring carotid restenosis. However, the lack of consensus on flow velocity thresholds for carotid restenosis requires new diagnostic studies. The comparison of complex US data (B-mode-imaging, PSV, EDV, and IC/CCA ratio) with the degree of restenosis assessed by angiographic methods may answer which duplex US criteria predict best the ≥50% and ≥ 70% carotid restenosis after CAS and CEA and whether these duplex US criteria are different in post-CEA and in-stent restenosis.
The accurate evaluation of carotid restenosis is essential to diagnose significant carotid restenosis and to determine and compare the real restenosis rates in different carotid procedure trials. Due to the lack of consensus on duplex US criteria for carotid restenosis, further examination is warranted to find complex ultrasound criteria suitable for identifying significant ICA restenosis after invasive carotid artery procedures. However, it should be highlighted that a meaningful definition of clinically significant carotid restenosis that determines the management approach to best reduce the risk of ipsilateral stroke depends on the associated risk of stroke with the current best standards of non-invasive care alone and, subsequently, any benefit from a redo carotid procedure in addition to current best non-invasive medical care.
Our manuscript demonstrates the lack of reliable data on annual ipsilateral stroke rate in patients with severe carotid restenosis treated with current best non-invasive medical care alone, which is essential to identify a subgroup of patients with high risk of ipsilateral stroke. This could open the way for new randomized trials to determine whether redo CEA or CAS provides an additional stroke risk reduction compared to the best non-invasive medical treatment alone in this subgroup. Due to the significant periprocedural complication rate of invasive carotid artery procedures in severe carotid restenosis and the low and continuously decreasing ipsilateral stroke risk with best medical intervention alone, choosing to use current best practice non-invasive care alone is recommended until there is clear evidence that adding a carotid artery procedure improves patient outcomes.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
IS, FP, and ZRM systematically screened the literature and extracted the data. IS, CD, and LO processed the articles. IS, LC, and LO wrote the article. All authors have read and agreed to the published version of the article.
The authors thank Ádám Jávorkúti for his valuable assistance with English language editing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kumar, R , Batchelder, A , Saratzis, A , Abu Rahma, AF , Ringleb, P , Lal, BK, et al. Restenosis after carotid interventions and its relationship with recurrent ipsilateral stroke: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. (2017) 53:766–75. doi: 10.1016/j.ejvs.2017.02.016
2. Naylor, AR , Ricco, JB , de Borst, GJ , Debus, S , de Haro, J , Halliday, A, et al. Editor’s choice – Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. (2018) 55:3–81. doi: 10.1016/j.ejvs.2017.06.021
3. Lal, BK , Beach, KW , Roubin, GS , Lutsep, HL , Moore, WS , Malas, MB, et al. And CREST Investigators, restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol. (2012) 11:755–63. doi: 10.1016/S1474-4422(12)70159-X
4. Bonati, LH , Gregson, J , Dobson, J , McCabe, DJH , Nederkoorn, PJ , van der Worp, HB, et al. And international carotid stenting study investigators, restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the international carotid stenting study (ICSS): secondary analysis of a randomised trial. Lancet Neurol. (2018) 17:587–96. doi: 10.1016/S1474-4422(18)30195-9
5. Mas, JL , Arquizan, C , Calvet, D , Viguier, A , Albucher, JF , Piquet, P, et al. Chatellier, and EVA-3S. Investigators, Long-term follow-up study of endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis trial. Stroke. (2014) 45:2750–6. doi: 10.1161/STROKEAHA.114.005671
6. Stanziale, SF , Wholey, MH , Boules, TN , Selzer, F , and Makaroun, MS . Determining in-stent stenosis of carotid arteries by duplex ultrasound criteria. J Endovasc Ther. (2005) 12:346–53. doi: 10.1583/04-1527.1
7. Lal, BK , Hobson, RW 2nd, Tofighi, B , Kapadia, I , Cuadra, S , and Jamil, Z . Duplex ultrasound velocity criteria for the stented carotid artery. J Vasc Surg. (2008) 47:63–73. doi: 10.1016/j.jvs.2007.09.038
8. AbuRahma, AF , Stone, P , Deem, S , Dean, LS , Keiffer, T , and Deem, E . Proposed duplex velocity criteria for carotid restenosis following carotid endarterectomy with patch closure. J Vasc Surg. (2009) 50:286–291.e1-2; discussion 291. doi: 10.1016/j.jvs.2009.01.065
9. Bosch, FT , Hendrikse, J , Davagnanam, I , Bonati, LH , van der Lugt, A , van der Worp, HB, et al. Optimal cut-off criteria for duplex ultrasound compared with computed tomography angiography for the diagnosis of restenosis in stented carotid arteries in the international carotid stenting study. Eur Stroke J. (2017) 2:37–45. doi: 10.1177/2396987316678361
10. von Reutern, GM , Goertler, MW , Bornstein, NM , Del Sette, M , Evans, DH , Hetzel, A, et al. And Neurosonology Research Group of the World Federation of neurology, grading carotid stenosis using ultrasonic methods. Stroke. (2012) 43:916–21. doi: 10.1161/STROKEAHA.111.636084
11. Chi, YW , White, CJ , Woods, TC , and Goldman, CK . Ultrasound velocity criteria for carotid in-stent restenosis. Catheter Cardiovasc Interv. (2007) 69:349–54. doi: 10.1002/ccd.21032
12. Setacci, C , Chisci, E , Setacci, F , Iacoponi, F , and de Donato, G . Grading carotid intrastent restenosis: a 6-year follow-up study. Stroke. (2008) 39:1189–96. doi: 10.1161/STROKEAHA.107.497487
13. AbuRahma, AF , Abu-Halimah, S , Bensenhaver, J , Dean, LS , Keiffer, T , Emmett, M, et al. Optimal carotid duplex velocity criteria for defining the severity of carotid in-stent restenosis. J Vasc Surg. (2008) 48:589–594.e2. doi: 10.1016/j.jvs.2008.04.004
14. Cumbie, T , Rosero, EB , Valentine, RJ , Modrall, JG , Clagett, GP , and Timaran, CH . Utility and accuracy of duplex ultrasonography in evaluating in-stent restenosis after carotid stenting. Am J Surg. (2008) 196:623–8. doi: 10.1016/j.amjsurg.2008.07.008
15. Bitsko, LJ , Ryer, EJ , Penn, EP , Salzler, GG , Major, M , Irvan, J, et al. Defining duplex ultrasound criteria for in-stent restenosis of the carotid artery using computed tomographic angiography. Cureus. (2022) 14:e26700. doi: 10.7759/cureus.26700
16. Liu, Z , Pan, Y , Zhang, Q , and Wang, X . Predictive value of quantitative duplex ultrasound analysis for in-stent carotid artery restenosis. Altern Ther Health Med. (2023) 29:52–7.
17. Peterson, BG , Longo, GM , Kibbe, MR , Matsumura, JS , Blackburn, D , Astleford, P, et al. Duplex ultrasound remains a reliable test even after carotid stenting. Ann Vasc Surg. (2005) 19:793–7. doi: 10.1007/s10016-005-7976-0
18. Zhou, W , Felkai, DD , Evans, M , McCoy, SA , Lin, PH , Kougias, P, et al. Ultrasound criteria for severe in-stent restenosis following carotid artery stenting. J Vasc Surg. (2008) 47:74–80. doi: 10.1016/j.jvs.2007.09.031
19. Telman, G , Kouperberg, E , Sprecher, E , Gruberg, L , Beyar, R , Hoffman, A, et al. Duplex ultrasound verified by angiography in patients with severe primary and restenosis of internal carotid artery. Ann Vasc Surg. (2006) 20:478–81. doi: 10.1007/s10016-006-9049-4
20. Aburahma, AF . Duplex criteria for determining >/=50% and >/=80% internal carotid artery stenosis following carotid endarterectomy with patch angioplasty. Vascular. (2011) 19:15–20. doi: 10.1258/vasc.2010.oa0245
21. Benzing, T , Wilhoit, C , Wright, S , McCann, PA , Lessner, S , and Brothers, TE . Standard duplex criteria overestimate the degree of stenosis after eversion carotid endarterectomy. J Vasc Surg. (2015) 61:1457–63. doi: 10.1016/j.jvs.2015.01.039
22. Gornik, HL , Rundek, T , Gardener, H , Benenati, JF , Dahiya, N , Hamburg, NM, et al. Optimization of duplex velocity criteria for diagnosis of internal carotid artery (ICA) stenosis: a report of the intersocietal accreditation commission (IAC) vascular testing division carotid diagnostic criteria committee. Vasc Med. (2021) 26:515–25. doi: 10.1177/1358863X211011253
23. European Carotid Surgery Trialists’ Collaborative Group . MRC European carotid surgery trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. European carotid surgery trialists’ collaborative group. Lancet. (1991) 337:1235–43. doi: 10.1016/0140-6736(91)92916-P
24. North American Symptomatic Carotid Endarterectomy Trial CollaboratorsBarnett, HJM , Taylor, DW , Haynes, RB , Sackett, DL , Peerless, SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. (1991) 325:445–53.
25. Wardlaw, JM , Chappell, FM , Best, JJ , Wartolowska, K , and Berry, E, NHS Research and Development Health Technology Assessment Carotid Stenosis Imaging Group . Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta-analysis. Lancet. (2006) 367:1503–12. doi: 10.1016/S0140-6736(06)68650-9
26. Adla, T , and Adlova, R . Multimodality imaging of carotid stenosis. Int J Angiol. (2015) 24:179–84. doi: 10.1055/s-0035-1556056
27. Del Brutto, VJ , Gornik, HL , and Rundek, T . Why are we still debating criteria for carotid artery stenosis? Ann Transl Med. (2020) 8:1270. doi: 10.21037/atm-20-1188a
28. Townsend, TC , Saloner, D , Pan, XM , and Rapp, JH . Contrast material-enhanced MRA overestimates severity of carotid stenosis, compared with 3D time-of-flight MRA. J Vasc Surg. (2003) 38:36–40. doi: 10.1016/S0741-5214(03)00332-X
29. Horev, A , Honig, A , Cohen, JE , Goldbart, A , Dizitzer, Y , Star, M, et al. Overestimation of carotid stenosis on CTA - real world experience. J Clin Neurosci. (2021) 85:36–40. doi: 10.1016/j.jocn.2020.12.018
30. Arquizan, C , Trinquart, L , Touboul, PJ , Long, A , Feasson, S , Terriat, B, et al. Investigators, restenosis is more frequent after carotid stenting than after endarterectomy: the EVA-3S study. Stroke. (2011) 42:1015–20. doi: 10.1161/STROKEAHA.110.589309
31. Lal, BK , Hobson, RW 2nd, Goldstein, J , Chakhtoura, EY , and Duran, WN . Carotid artery stenting: is there a need to revise ultrasound velocity criteria? J Vasc Surg. (2004) 39:58–66. doi: 10.1016/j.jvs.2003.10.043
32. Stilo, F , Montelione, N , Calandrelli, R , Distefano, M , Spinelli, F , Di Lazzaro, V, et al. The management of carotid restenosis: a comprehensive review. Ann Transl Med. (2020) 8:1272. doi: 10.21037/atm-20-963
33. Lucatelli, P , Fanelli, F , Cirelli, C , Sacconi, B , Anzidei, M , Montisci, R, et al. Carotid endarterectomy versus stenting: does the flow really change? An echo-color-doppler analysis. Int J Cardiovasc Imaging. (2015) 31:773–81. doi: 10.1007/s10554-015-0623-0
34. CAVATAS Investigators . Endovascular versus surgical treatment in patients with carotid stenosis in the carotid and vertebral artery transluminal angioplasty study (CAVATAS): a randomised trial. Lancet. (2001) 357:1729–37. doi: 10.1016/S0140-6736(00)04893-5
35. Featherstone, RL , Brown, MM , and Coward, LJ, ICSS Investigators . International carotid stenting study: protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovasc Dis. (2004) 18:69–74. doi: 10.1159/000078753
36. SPACE Collaborative GroupRingleb, PA , Allenberg, J , Bruckmann, H , Eckstein, HH , Fraedrich, G, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. (2006) 368:1239–47. doi: 10.1016/S0140-6736(06)69122-8,
37. Ringleb, PA , Kunze, A , Allenberg, JR , Hennerici, MG , Jansen, O , Maurer, PC, et al. And steering committee of the SPACE study, the stent-supported percutaneous angioplasty of the carotid artery vs. endarterectomy trial. Cerebrovasc Dis. (2004) 18:66–8. doi: 10.1159/000078752
38. Eckstein, HH , Ringleb, P , Allenberg, JR , Berger, J , Fraedrich, G , Hacke, W, et al. Results of the stent-protected angioplasty versus carotid endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. (2008) 7:893–902. doi: 10.1016/S1474-4422(08)70196-0
39. Mas, JL , Chatellier, G , Beyssen, B , Branchereau, A , Moulin, T , Becquemin, JP, et al. And EVA-3S investigators, endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. (2006) 355:1660–71. doi: 10.1056/NEJMoa061752
40. Brott, TG , Hobson, RW 2nd, Howard, G , Roubin, GS , Clark, WM , Brooks, W, et al. And CREST investigators, stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. (2010) 363:11–23. doi: 10.1056/NEJMoa0912321
41. AbuRahma, AF , Jennings, TG , Wulu, JT , Tarakji, L , and Robinson, PA . Redo carotid endarterectomy versus primary carotid endarterectomy. Stroke. (2001) 32:2787–92. doi: 10.1161/hs1201.099649
42. Fokkema, M , de Borst, GJ , Nolan, BW , Lo, RC , Cambria, RA , Powell, RJ, et al. Carotid stenting versus endarterectomy in patients undergoing reintervention after prior carotid endarterectomy. J Vasc Surg. (2014) 59:e1–2. doi: 10.1016/j.jvs.2013.06.070
43. Tu, J , Wang, S , Huo, Z , Wu, R , Yao, C , and Wang, S . Repeated carotid endarterectomy versus carotid artery stenting for patients with carotid restenosis after carotid endarterectomy: systematic review and meta-analysis. Surgery. (2015) 157:1166–73. doi: 10.1016/j.surg.2015.02.005
44. Krafcik, BM , Cheng, TW , Farber, A , Kalish, JA , Rybin, D , Doros, G, et al. Perioperative outcomes after reoperative carotid endarterectomy are worse than expected. J Vasc Surg. (2018) 67:793–8. doi: 10.1016/j.jvs.2017.08.053
45. Texakalidis, P , Giannopoulos, S , Jonnalagadda, AK , Kokkinidis, DG , Machinis, T , Reavey-Cantwell, J, et al. Carotid artery endarterectomy versus carotid artery stenting for restenosis after carotid artery endarterectomy: a systematic review and meta-analysis. World Neurosurg. (2018) 115:421–429.e1. doi: 10.1016/j.wneu.2018.02.196
46. Arhuidese, IJ , Rizwan, M , Nejim, B , and Malas, M . Outcomes of primary and secondary carotid artery stenting. Stroke. (2017) 48:3086–92. doi: 10.1161/STROKEAHA.117.016963
47. AbuRahma, AF , Abu-Halimah, S , Bensenhaver, J , Nanjundappa, A , Stone, PA , Dean, LS, et al. Primary carotid artery stenting versus carotid artery stenting for postcarotid endarterectomy stenosis. J Vasc Surg. (2009) 50:1031–9. doi: 10.1016/j.jvs.2009.06.051
48. New, G , Roubin, GS , Iyer, SS , Vitek, JJ , Wholey, MH , Diethrich, EB, et al. Safety, efficacy, and durability of carotid artery stenting for restenosis following carotid endarterectomy: a multicenter study. J Endovasc Ther. (2000) 7:345–52. doi: 10.1177/152660280000700501
49. Radak, D , Tanaskovic, S , Sagic, D , Antonic, Z , Babic, S , Popov, P, et al. Carotid angioplasty and stenting is safe and effective for treatment of recurrent stenosis after eversion endarterectomy. J Vasc Surg. (2014) 60:645–51. doi: 10.1016/j.jvs.2014.03.288
50. Kahlberg, A , Ardita, V , Spertino, A , Mascia, D , Bertoglio, L , Baccellieri, D, et al. Propensity-matched comparison for carotid artery stenting in primary stenosis versus after carotid endarterectomy restenosis. Ann Vasc Surg. (2021) 70:332–40. doi: 10.1016/j.avsg.2020.06.063
51. Midy, D , Berard, X , Becquemin, JP , Patra, P , Alric, P , Derrider, P, et al. Multicentric retrospective study of endovascular treatment for restenosis after open carotid surgery. Eur J Vasc Endovasc Surg. (2011) 42:742–50. doi: 10.1016/j.ejvs.2011.08.008
52. Cuadra, S , Hobson, RW , Lal, BK , Goldstein, J , Chakhtoura, E , and Jamil, Z . Outcome of carotid artery stenting for primary versus restenotic lesions. Ann Vasc Surg. (2009) 23:330–4. doi: 10.1016/j.avsg.2008.05.013
53. Mehta, RH , Zahn, R , Hochadel, M , Ischinger, T , Jung, J , Hauptmann, KE, et al. Comparison of in-hospital outcomes of patients with versus without previous carotid endarterectomy undergoing carotid stenting (from the German ALKK CAS Registry). Am J Cardiol. (2007) 99:1288–93. doi: 10.1016/j.amjcard.2006.12.047
54. White, RA , Sicard, GA , Zwolak, RM , Sidawy, AN , Schermerhorn, ML , Shackelton, RJ, et al. Society of vascular surgery vascular registry comparison of carotid artery stenting outcomes for atherosclerotic vs nonatherosclerotic carotid artery disease. J Vasc Surg. (2010) 51:1116–23. doi: 10.1016/j.jvs.2009.11.082
55. Mousa, AY , AbuRahma, AF , Bozzay, J , Broce, M , Kali, M , Yacoub, M, et al. Long-term comparative outcomes of carotid artery stenting following previous carotid endarterectomy vs De novo lesions. J Endovasc Ther. (2015) 22:449–56. doi: 10.1177/1526602815581597
56. Hynes, BG , Kennedy, KF , Ruggiero, NJ 2nd, Kiernan, TJ , Margey, RJ , Rosenfield, K, et al. Carotid artery stenting for recurrent carotid artery restenosis after previous ipsilateral carotid artery endarterectomy or stenting: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. (2014) 7:180–6. doi: 10.1016/j.jcin.2013.11.004
57. Abbott, AL . Extra-cranial carotid artery stenosis: an objective analysis of the available evidence. Front Neurol. (2022) 13:739999. doi: 10.3389/fneur.2022.739999
58. Mantese, VA , Timaran, CH , Chiu, D , Begg, RJ , and Brott, TG, CREST Investigators . The carotid revascularization endarterectomy versus stenting trial (CREST): stenting versus carotid endarterectomy for carotid disease. Stroke. (2010) 41:S31–4. doi: 10.1161/STROKEAHA.110.595330
59. Criado, E , Doblas, M , Fontcuberta, J , Orgaz, A , Flores, A , Wall, LP, et al. Transcervical carotid stenting with internal carotid artery flow reversal: feasibility and preliminary results. J Vasc Surg. (2004) 40:476–83. doi: 10.1016/j.jvs.2004.06.026
60. de Borst, GJ . Transcarotid artery stenting: hype or hope? Stroke. (2022) 53:108–10. doi: 10.1161/STROKEAHA.121.036464
61. de Borst, GJ . Transcarotid artery revascularization. Br J Surg. (2023) 110:127–8. doi: 10.1093/bjs/znac421
62. Abbott, A . Asymptomatic carotid stenosis and stroke risk. Lancet Neurol. (2021) 20:698–9. doi: 10.1016/S1474-4422(21)00199-X
63. Abbott, AL , Brunser, AM , Giannoukas, A , Harbaugh, RE , Kleinig, T , Lattanzi, S, et al. Misconceptions regarding the adequacy of best medical intervention alone for asymptomatic carotid stenosis. J Vasc Surg. (2020) 71:257–69. doi: 10.1016/j.jvs.2019.04.490
64. Abbott, AL , Silvestrini, M , Topakian, R , Golledge, J , Brunser, AM , de Borst, GJ, et al. Optimizing the definitions of stroke, transient ischemic attack, and infarction for research and application in clinical practice. Front Neurol. (2017) 8:537. doi: 10.3389/fneur.2017.00537
65. Fisch, U , von Felten, S , Wiencierz, A , Jansen, O , Howard, G , Hendrikse, J, et al. Editor’s choice – risk of stroke before revascularisation in patients with symptomatic carotid stenosis: a pooled analysis of randomised controlled trials. Eur J Vasc Endovasc Surg. (2021) 61:881–7. doi: 10.1016/j.ejvs.2021.02.024
66. Shahidi, S , Owen-Falkenberg, A , Hjerpsted, U , Rai, A , and Ellemann, K . Urgent best medical therapy may obviate the need for urgent surgery in patients with symptomatic carotid stenosis. Stroke. (2013) 44:2220–5. doi: 10.1161/STROKEAHA.111.000798
67. Shahidi, S , Owen-Falkenberg, A , Gottschalksen, B , and Ellemann, K . Risk of early recurrent stroke in symptomatic carotid stenosis after best medical therapy and before endarterectomy. Int J Stroke. (2016) 11:41–51. doi: 10.1177/1747493015609777
68. Chimowitz, MI , Lynn, MJ , Derdeyn, CP , Turan, TN , Fiorella, D , Lane, BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. (2011) 365:993–1003. doi: 10.1056/NEJMoa1105335
Keywords: carotid artery stenting, carotid endarterectomy, carotid restenosis, ultrasound, systematic review
Citation: Szegedi I, Potvorszki F, Mészáros ZR, Daniel C, Csiba L and Oláh L (2023) Role of carotid duplex in the assessment of carotid artery restenosis after endarterectomy or stenting. Front. Neurol. 14:1226220. doi: 10.3389/fneur.2023.1226220
Received: 30 May 2023; Accepted: 05 October 2023;
Published: 27 October 2023.
Edited by:
Michele Romoli, Maurizio Bufalini Hospital, ItalyReviewed by:
Anne Louise Abbott, Monash University, AustraliaCopyright © 2023 Szegedi, Potvorszki, Mészáros, Daniel, Csiba and Oláh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: László Oláh, b2xhaEBtZWQudW5pZGViLmh1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.