
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 08 August 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1224748
This article is part of the Research TopicMultiple Sclerosis and Related Disorders: Challenges and Approaches to Mechanisms, Biomarkers, and Therapeutic TargetsView all 11 articles
 Lijie Zhang1,2†
Lijie Zhang1,2† Xintong Yu1,2†
Xintong Yu1,2† Yexiang Zheng1,2
Yexiang Zheng1,2 Aiyu Lin1,2
Aiyu Lin1,2 Zaiqiang Zhang3
Zaiqiang Zhang3 Shaowu Li4
Shaowu Li4 Ning Wang1,2*
Ning Wang1,2* Ying Fu1,2,3*
Ying Fu1,2,3*Multiple sclerosis (MS) is a condition that affects the veins and small blood vessels. Previous research suggests that individuals with MS have an increased risk of vascular events and higher mortality rates. However, the relationship between MS and cerebral small vessel disease (CSVD) remains uncertain. This study aims to investigate the association between MS and lacunes. A prospective observational study was conducted, including a total of 112 participants, of which 46 had MS and 66 had CSVD. All participants underwent an MRI scan and a battery of neurological functional assessments. The presence of definite lacunes and black holes was determined through the analysis of T2-weighted, T1-weighted, and FLAIR images. The occurrence of lacunes in MS patients was found to be 19.6%. Notably, the duration of MS was identified as the sole risk factor for the development of lacune lesions in MS patients [odds ratio (OR) = 1.3, 95% confidence interval (CI) = 1.1–1.6, p = 0.008]. Comparatively, MS patients with lacunes exhibited a higher frequency of attacks and larger volumes of T2 lesions compared to MS patients without lacunes. Further analysis using receiver operating characteristic (ROC) curves showed that lacune lesions had limited ability to discriminate between MS and CSVD when disease duration exceeded 6 years. The presence of small arterial lesions in the brain of individuals with MS, along with the duration of the disease, contributes to the development of lacunes in MS patients.
Black holes in multiple sclerosis (MS) are characterized by T1 hypointense and T2 hyperintense lesions on MRI (1). These lesions can be categorized as either acute black holes, which may either progress to permanent black holes or transform into transient black holes (2). The exact etiology and pathogenesis of black holes remain uncertain. Some studies have proposed that CD8-mediated immune damage may contribute to the formation of black holes (3–5).
While MS primarily affects veins and venules, cerebral small vessel disease (CSVD) primarily affects small arteries. Aging, vascular risk factors (VRFs), and chronic inflammation are known to cause damage to the microvascular system, including the small arteries, resulting in hypoperfusion and tissue hypoxia that can influence the extent and distribution of MS-related pathology (6).
Lacunes, which are residual lesions of lacunar infarction or small hemorrhagic foci, serve as prominent imaging features of CSVD, reflecting intracranial arteriole lesions (7). Therefore, in this study, we focused on monitoring lacunar lesions to explore potential associations between CSVD and MS. We aimed to investigate the incidence of lacunes in an MS cohort and evaluate the related risk factors based on demographic and clinical characteristics, shedding light on the potential clinical significance of lacunes in MS patients.
In this ongoing study, we prospectively collected clinical, demographic, and imaging data from patients diagnosed with MS and CSVD. The patients were hospitalized at Tian Tan Hospital of Capital Medical University in Beijing, China, between 2015 and 2017. MS diagnosis was made according to the 2010 McDonald MS standard (8) while CSVD diagnosis followed the 2013 European “Neuroimaging standards for research into small vessel disease” (9). Patients who fell into both categories of MS and CSVD were explicitly excluded from this study. All participants completed a structured questionnaire (10) and underwent physical and neurological assessments during their hospital stay (11).
Criteria for patient selection in our investigation were as follows:
1. Undergoing an MRI examination at least 2 days after the completion of physical and/or neurological examinations during hospitalization.
2. Having a fully documented medical record including information on hypertension, hyperlipidemia, diabetes, atrial fibrillation, current smoking, alcohol use, age at onset, age at baseline, disease duration at baseline, number of attacks, annual recurrence rate (ARR), number of relapses, and the use of any form of disease-modifying therapy (DMT).
3. Undergoing a comprehensive neurological examination, including the Expanded Disability Status Scale (EDSS) and modified Rankin scale (mRS).
Patients were excluded based on the following criteria: (a) MS patients who experienced relapse and receive steroid treatment within 30 days before study entry, (b) Pre-existing medical conditions associated with brain pathologic processes such as cerebrovascular disease or a history of alcohol abuse, (c) Confirmation of ischemic or hemorrhagic infarcts in the brain as observed in MRI, and (d) Pregnancy.
All patients underwent brain MRI using a 3.0-T scanner (Magnetom Trio Tim; Siemens) equipped with a 32-channel head coil. The imaging protocol included diffusion-weighted imaging (DWI), T2-weighted imaging, fluid-attenuated inversion recovery (FLAIR), and T1-weighted imaging (12).
During the analysis, the MR imaging experts were blinded to the physical and neurological conditions of the subjects. Two experienced neuroimagers, also blinded to MR images obtained through other analytical sequences, assessed lacune lesions on T2-, T1-weighted, and FLAIR images. Lacunes were defined as a round or ovoid subcortical fluid-filled cavity (CSF-like signal) between 3 mm and approximately 15 mm in diameter, consistent with a previous acute small subcortical infarct or haemorrhage in the territory of a perforating arteriole. The diameter of the involved perforating arteriole varies from 40 to 850 μm, and the diameter of the associated infarct ranges from 2 to 3 mm to 15 mm or larger (9). Importantly, we distinguished these lesions from “black holes” (13, 14), which were round or elliptical in shape with neat edges and showed a liquid or near-liquid low signal on the T1 image. In addition, there is rarely a low signal on the FLAIR image due to the presence of myelin regeneration in the lesion. T2-, T1-weighted, and FLAIR images (Figure 1) were used to identify definite lacune lesions and black holes. To determine the volumes of individual white matter hyperintensity (WMH) lesions, manual segmentation was performed using MRIcro software based on the T2 images.1
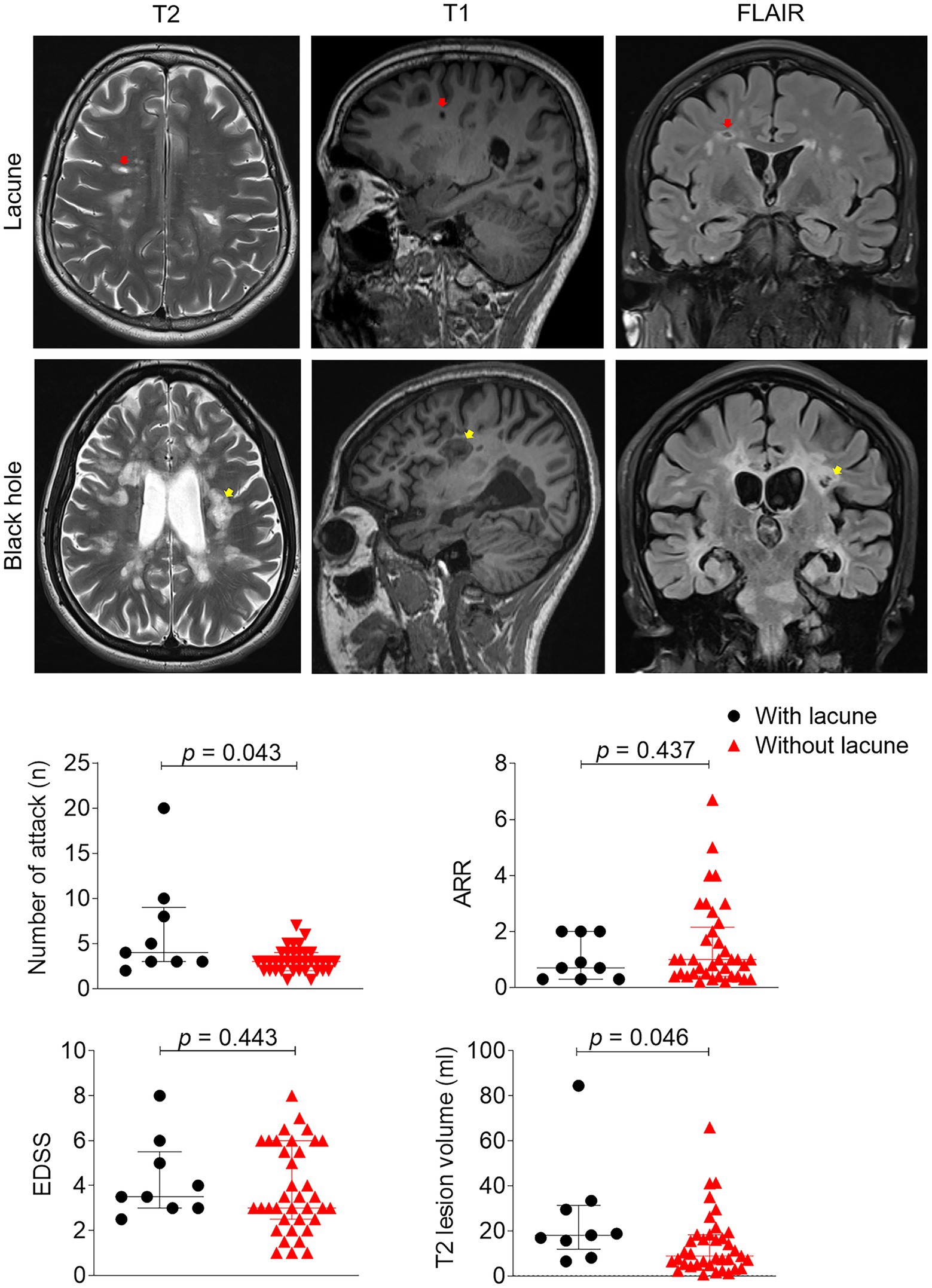
Figure 1. Clinical and imaging differences between patients with or without lacune. Example of lacune (red arrow) and black hole lesion (yellow arrow) in this study: Lacunas defined as round or ovoid, subcortical, fluid-filled cavities 3–15 mm in diameter in the territory of 1 perforating arteriole, specifically, lesions were round or elliptical in shape, and edges were neat, showing a liquid or near-liquid low signal on the T1 image. EDSS, expanded disability status scale, ARR, annual relapse rate; inferrential statistical analysis was performed with Mann–Whitney tests.
To ensure accuracy in identifying lacune lesions, two raters independently assessed their presence. Both raters were blinded to each other’s evaluations and their own previous assessments. Interclass correlation coefficients were calculated, and the values exceeded 0.8. Where there was discrepancy between the two raters, a third rater’s assessment was used.
All statistical analyses were conducted using SPSS 17.0 (SPSS Inc., Chicago, IL). Differences between MS patients with and without lacune lesions were assessed using chi-square and Mann–Whitney tests. We used chi-square tests for gender, hypertension, hyperlipidemia, diabetes, atrial fibrillation, current smoking, and alcohol between MS patients with and without lacune lesions, as well as to compare the frequency of lacune in different groups of patients. Binary logistic regression analysis was performed to adjust for potential confounding factors. The diagnostic value of lacune lesions was determined using receiver operating characteristic (ROC) curves, with stratified evaluation at a time point of 6 years.
We enrolled a total of 46 patients with MS and 66 patients with CSVD through a meticulous selection process. Among the MS patients, the median age was 32 years (range: 25–56 years), and the median disease duration was 5 years (range: 2–7 years). Fifteen (33%) of the MS patients were male (Table 1). Nine out of the 46 MS patients met the imaging threshold for lacune lesions (19.6%) (Supplementary Figure S1). We performed Mann–Whitney tests to examine the clinical and demographic characteristics of patients with and without lacune lesions. No significant differences were observed in age or vascular risk factors between the two groups (Table 1). However, there was a significant difference in disease duration [median (IQR), 4 (1.8–6.0) vs. 10 (4.3–13.5), p = 0.016]. To adjust for age, we conducted binary logistic regression analysis, which revealed a significant association between disease duration (OR = 1.3, 95% CI = 1.1–1.6, p = 0.008) and the presence of lacune lesions. MS patients with lacune lesions exhibited a higher frequency of attacks (6 vs. 3, p = 0.043) and larger T2 lesion volumes (19 vs. 23 mL, p = 0.046) compared to those without lacune lesions (Figure 1). No differences were observed in ARR (0.7 vs. 1.0, p = 0.437), EDSS (3.5 vs. 3.0, p = 0.443) and mRS (1.0 vs. 1.0, p = 0.482).
Comparison between the MS and CSVD groups revealed a higher incidence of traditional VRFs among CSVD patients (Table 1). There was a significant difference in the incidence of lacunes between the CSVD and MS groups (65.1% vs. 19.6%, p < 0.001), with an area under the ROC curve (AUC) of 0.73. However, when stratified by disease duration at a time point of 6 years, the AUC values decreased for patients with a disease course of >6 years (AUC = 0.67) (Figure 2), suggesting a diminishing ability to discriminate lacunar lesions with prolonged disease duration. Notably, the frequency of lacune lesions in MS patients with a disease duration over 6 years was comparable to that of CSVD patients (46.7 vs. 65.1%, p = 0.184).
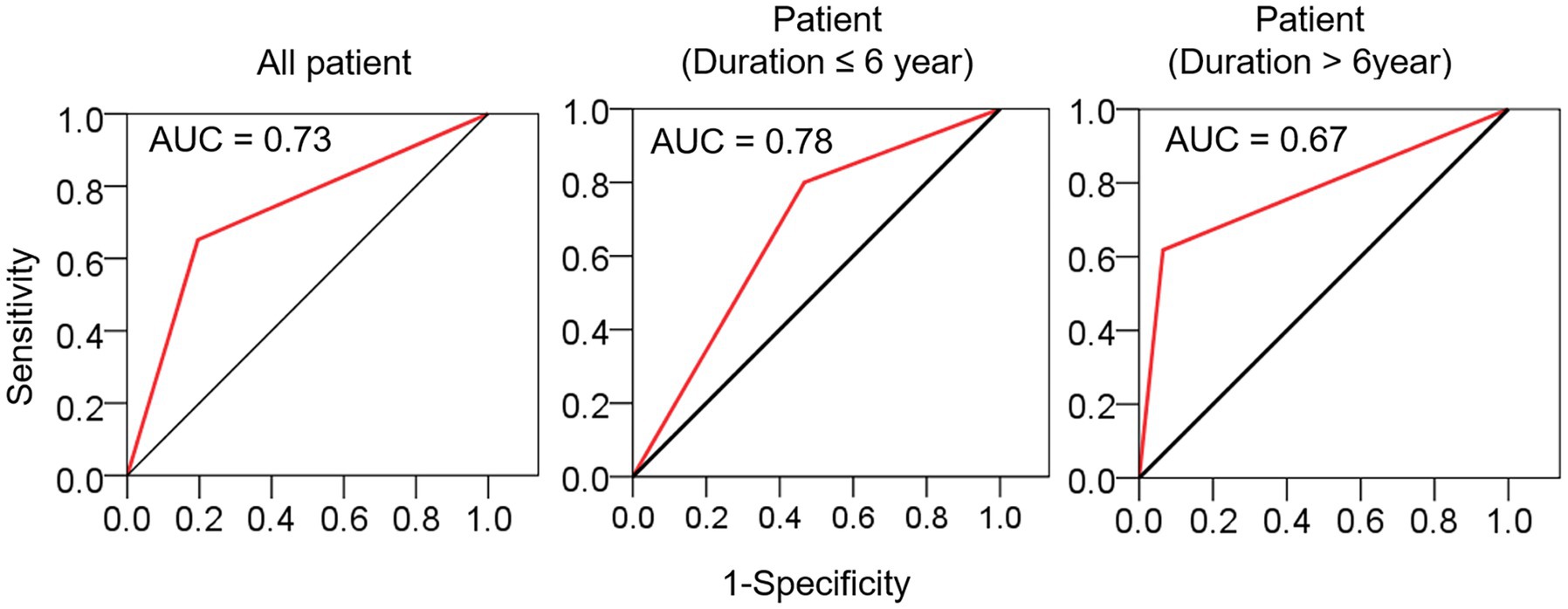
Figure 2. The sensitivity and specificity of lacune in distinguishing MS from CSVD. ROC curves were used to evaluate the diagnostic value of lacune, and the stratified evaluation was performed at a time point of 6 years. AUC, area under curve.
Our study aimed to investigate the frequency and clinical significance of lacune lesions in individuals diagnosed with MS. By comparing the clinical features of MS patients with and without such lesions and analyzing the ROC curve after age stratification, we found that disease duration was the only significant risk factor for the occurrence of lacune lesions in MS patient. Furthermore, these findings suggest that the duration of MS increases the risk of CSVD, highlighting the time sensitivity and the importance of initiating preventive treatments at an appropriate time.
To the best of our knowledge, there are no existing reports exploring the potential relationship between lacune lesions and MS. Classical descriptions of lacunae can include lacune lesions (9), which can be challenging to distinguish from “black holes” in images (13, 14). To overcome this challenge, we further defined the distinguishing characteristics of lacune lesions in the imaging data of our study cohort. Based on our assessment criteria, we determined that the incidence of lacune lesions in the study population was 19.6%. It is important to note that the actual incidence is expected to be higher than the reported value we detected.
Traditional VRFs such as hypertension, diabetes, hyperlipidemia, and smoking were not significant factors in MS patients (with incidence rates of 2.2%, 2.2%, 23.9%, and 2.2% respectively). This could be attributed to the younger age [median (IQR): 31.5 (24.8–36.3)] of the enrolled population in our study compared to the aforementioned study. Several publications have shown an increased rate of ischemic stroke (IS) in MS patients (15, 16). Similarly, CSVD patients also exhibit a higher incidence of IS. Therefore, we were interested in exploring the differences in the development and causal influences of lacunes in MS patients compared to CSVD patients. The level of VRFs was higher in the CSVD cohort compared to the MS cohort, and the overall incidence of lacunes was also higher. When drawing ROC curves using lacune lesions, age-stratified analysis revealed that when the disease duration exceeded 6 years, lacune lesions had limited discriminatory capability between MS and CSVD. This further emphasizes the association between lacune lesions and MS disease duration, suggesting that intervention therapy for CSVD should be initiated at an appropriate time in the MS population.
There are several limitations to our study that should be acknowledged. First, the sample size was relatively small and should be expanded to further support our conclusions. Additionally, the mechanism underlying the increased risk of cerebral small vessel disease in MS patients remains unclear. It is hypothesized that MS disease creates a chronic inflammatory environment that damages small arterioles in the brain (6), but empirical confirmation is needed.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Tian Tan Hospital of Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
YF formulated the study concept. YF and AL acquired funding for the study. YF and NW designed the study. LZ, YZ, ZZ, XY, and SL collected data. SL, YF, LZ, and YZ analyzed the data. YF and YZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the grants U21A20360 (YF) and 82271375 (AL) from the National Natural Science Foundation of China; the grant 2020Y9129 (YF) from the Joint Funds for the innovation of science and Technology of Fujian Province.
The authors thank our patients for participating in this study, the clinical neuroimmunology team for recruiting the patients.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1224748/full#supplementary-material
1. Barkhof, F, McGowan, JC, van Waesberghe, JH, and Grossman, RI. Hypointense multiple sclerosis lesions on T1-weighted spin echo magnetic resonance images: their contribution in understanding multiple sclerosis evolution. J Neurol Neurosurg Psychiatry. (1998) 64:S77–9.
2. Sahraian, MA, Radue, EW, Haller, S, and Kappos, L. Black holes in multiple sclerosis: definition, evolution, and clinical correlations. Acta Neurol Scand. (2010) 122:1–8. doi: 10.1111/j.1600-0404.2009.01221.x
3. Fazekas, F, Strasser-Fuchs, S, Schmidt, H, Enzinger, C, Ropele, S, Lechner, A, et al. Apolipoprotein E genotype related differences in brain lesions of multiple sclerosis. J Neurol Neurosurg Psychiatry. (2000) 69:25–8. doi: 10.1136/jnnp.69.1.25
4. Enzinger, C, Ropele, S, Smith, S, Strasser-Fuchs, S, Poltrum, B, Schmidt, H, et al. Accelerated evolution of brain atrophy and "black holes" in MS patients with APOE-epsilon 4. Ann Neurol. (2004) 55:563–9. doi: 10.1002/ana.20027
5. Pirko, I, Nolan, TK, Holland, SK, and Johnson, AJ. Multiple sclerosis: pathogenesis and MR imaging features of T1 hypointensities in a (corrected) murine model. Radiology. (2008) 246:790–5. doi: 10.1148/radiol.2463070338
6. Geraldes, R, Esiri, MM, DeLuca, GC, and Palace, J. Age-related small vessel disease: a potential contributor to neurodegeneration in multiple sclerosis. Brain Pathol. (2017) 27:707–22. doi: 10.1111/bpa.12460
7. Wardlaw, JM, Smith, C, and Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. (2013) 12:483–97. doi: 10.1016/S1474-4422(13)70060-7
8. Polman, CH, Reingold, SC, Banwell, B, Clanet, M, Cohen, JA, Filippi, M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. (2011) 69:292–302. doi: 10.1002/ana.22366
9. Wardlaw, JM, Smith, EE, Biessels, GJ, Cordonnier, C, Fazekas, F, Frayne, R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
10. Dolic, K, Weinstock-Guttman, B, Marr, K, Valnarov, V, Carl, E, Hagemeier, J, et al. Risk factors for chronic cerebrospinal venous insufficiency (CCSVI) in a large cohort of volunteers. PLoS One. (2011) 6:e28062. doi: 10.1371/journal.pone.0028062
11. Zivadinov, R, Marr, K, Cutter, G, Ramanathan, M, Benedict, RH, Kennedy, C, et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology. (2011) 77:138–44. doi: 10.1212/WNL.0b013e318212a901
12. Pichler, A, Khalil, M, Langkammer, C, Pinter, D, Ropele, S, Fuchs, S, et al. The impact of vascular risk factors on brain volume and lesion load in patients with early multiple sclerosis. Mult Scler. (2019) 25:48–54. doi: 10.1177/1352458517736149
13. van Walderveen, MA, Kamphorst, W, Scheltens, P, van Waesberghe, JH, Ravid, R, Valk, J, et al. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology. (1998) 50:1282–8. doi: 10.1212/WNL.50.5.1282
14. van den Elskamp, IJ, Lembcke, J, Dattola, V, Beckmann, K, Pohl, C, Hong, W, et al. Persistent T1 hypointensity as an MRI marker for treatment efficacy in multiple sclerosis. Mult Scler. (2008) 14:764–9. doi: 10.1177/1352458507087842
15. Allen, NB, Lichtman, JH, Cohen, HW, Fang, J, Brass, LM, and Alderman, MH. Vascular disease among hospitalized multiple sclerosis patients. Neuroepidemiology. (2008) 30:234–8. doi: 10.1159/000128103
Keywords: multiple sclerosis (MS), cerebral small vessel disease (CSVD), lacune, disease duration, arteriolar damage
Citation: Zhang L, Yu X, Zheng Y, Lin A, Zhang Z, Li S, Wang N and Fu Y (2023) Lacunes are associated with late-stage multiple sclerosis comorbidities. Front. Neurol. 14:1224748. doi: 10.3389/fneur.2023.1224748
Received: 18 May 2023; Accepted: 25 July 2023;
Published: 08 August 2023.
Edited by:
Jinzhou Feng, First Affiliated Hospital of Chongqing Medical University, ChinaReviewed by:
Honghao Wang, Guangzhou First People's Hospital, ChinaCopyright © 2023 Zhang, Yu, Zheng, Lin, Zhang, Li, Wang and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Wang, bmluZ3dhbmdAZmptdS5lZHUuY24=; Ying Fu, ZnV5aW5nQGZqbXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.