
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 12 July 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1222574
This article is part of the Research TopicReviews in: Multiple Sclerosis and NeuroimmunologyView all 9 articles
Objective: Over the last few decades clinicians have become aware that cognitive impairment might be a major cause of disability, loss of employment and poor quality of life in patients suffering from multiple sclerosis [MS].
The impact of disease modifying therapies [DMTs] on cognition is still a matter of debate. Theoretically, DMTs could exert a substantial beneficial effect by means of reducing neuroinflammation and brain atrophy, which are established correlates of cognitive dysfunction. The aim of the study was to review the evidence concerning the effect of DMTs on cognitive functions.
Methods: PubMed, Scopus, and the European Committee for Treatment and Research in Multiple Sclerosis [ECTRIMS] Library were searched for articles concerning the pediatric and adult populations of patients with multiple sclerosis, including clinical trials and RWD, where psychometric results were analyzed as secondary or exploratory endpoints.
Results: We reviewed a total of 44 studies that were found by our search strategy, analyzed the psychological tests that were applied, the length of the follow-up, and possible limitations. We pointed out the difficulties associated with assessing of DMTs’ effects on cognitive functions, and pitfalls in cognitive tools used for evaluating of MS patients.
Conclusion: There is a need to highlight this aspect of MS therapies, and to collect adequate data to make informed therapeutic decisions, to improve our understanding of MS-related cognitive dysfunction and provide new therapeutic targets.
Multiple sclerosis [MS] is an autoimmune demyelinating disorder of the central nervous system [CNS], typically affecting the people between 20 and 40 years of age. It is considered one of the most common non-traumatic diseases of the brain leading to disability in young adults (1). Not only does MS cause physical disability, but also impairs cognition, the latter affecting patients’ quality of life even more profoundly. Cognitive dysfunction has a prevalence rate of approximately 50%, but for patients it is far from benign and remains highly relevant for daily functioning (2). The most vulnerable phase for the progression of cognitive deficits seems to occur during the first 5 years after disease onset (2). Moreover, the percentage of patients with cognitive decline is likely underestimated since detailed neuropsychological tests are not routinely assessed in clinical practice. Also, the fundamental clinimetric scale used to score MS patients’ disability, namely Expanded Disability Status Scale [EDSS], does not sufficiently reflect cognitive impairment. EDSS is influenced mainly by the assessment of physical disability, especially gait impairment. On the other hand, cognition is scored in EDSS as: normal, decrease in “mentation,” or dementia. In MS severe dementia syndromes with disorientation as to time and place are very rare (score 4 and 5), but score 2–without more accurate psychological testing - does not differentiate well within the largest group of patients with milder cognitive impairment (3). Consequently, it is difficult to establish either cognitive relapses or progressive cognitive decline in MS patients. While we do know cognitive relapses occur (4) they may not necessarily cause any increase in EDSS score, and thus might not be considered as evidence for treatment failure.
Cognitive impairment may be present since the earliest stages of the disease, even in radiologically isolated syndrome [RIS], where patients were shown to score below the mean performance for the healthy population on neuropsychological testing (5). In RIS subjects, the most common deficit was observed in information processing speed, similar to patients with clinically definite MS (5). Cognitive domains that are most severely affected in MS include: information processing speed, learning memory, executive functions and attention (2, 5, 6). More frequent and severe deficits are reported in secondary progressive MS [SPMS] and primary progressive MS [PPMS], especially in the working memory and executive functions domains, which can be associated with predominant gray matter pathology in the progressive stages of the disease (6). The impact of disease modifying therapies [DMTs] on cognition is still a matter of debate. Theoretically, DMTs could exert a substantial beneficial effect by means of reducing neuroinflammation and brain atrophy, which are established correlates of cognitive dysfunction. The aim of the study was to review the evidence concerning the effect of DMTs on cognitive functions and to emphasize on difficulties with the analysis of these functions, resulting from the still small number of studies in this field and lack of one, standard neuropsychological battery.
Cognitive dysfunction is one of the major factors determining the quality of life in patients with MS. It was shown that cognitively impaired patients were more likely unemployed, experienced greater difficulty in performing household tasks and were more often socially withdrawn (7).
Unemployment is common in individuals with MS (7)Cognitive dysfunction impacts employment, and unfortunately EDSS does not predict employment status (8), whereas studies have shown that cognitive performance on SDMT is associated with earnings (9).
Importantly, the commonly used term of “benign MS” is defined as a long-term [> 10 years] disease course with EDSS ≤3, but this definition does not include cognitive functioning (10). However, in one study neuropsychological deficits have been documented in 45% of patients with “benign MS” (10). These patients exhibited significantly higher handicap scores and significant restrictions of their everyday activity (11).
The three most frequently used neuropsychological/psychometric batteries in MS are: [i] The Brief Repeatable Battery of Neuropsychological tests [BRB-N], also known as Rao’s battery, [ii] the minimal assessment of cognitive function in MS [MACFIMS], and [iii] the Brief International Cognitive Assessment for Multiple Sclerosis [BICAMS; ISLAS], they are used especially in clinical trials. In clinical practice, SDMT has recently become the most used psychometric and adopted as screening test in MS, mainly due to its ease of administration, predictive validity, sensitivity and specificity. Also, it is well correlated with significant magnetic resonance imaging [MRI] measures, including brain atrophy, total lesion burden and microstructural pathology (12). Transient reduction of SDMT score was also used in defining cognitive relapses (13). Pardini et al. (13) proposed a definition of the isolated cognitive relapse [ICR] as a transient significant cognitive decline [reduction of SDMT≤4 points] associated with the presence of a gadolinium enhancing lesion on brain MRI (13).
Another important issue to consider is the subjective vs. objective cognitive complaints in MS patients. Therefore, appropriate cognitive testing in all MS subjects should be done on a regular basis. Moreover, we suggest cognitive functioning should be formally included in all MS treatment trials as a secondary outcome.
Cognitive functioning is not used as a standard primary outcome measure in assessing treatment efficacy. However, it should be noted that approximately 50% of patients achieving a classically defined “no evidence of disease activity” [NEDA-3, defined as absence of relapses, disability worsening, and MRI activity] after 2-years of follow-up had a noticeable deterioration in at least 2 cognitive domains (14). Moreover, 25% of patients had a meaningful cognitive decline, defined as a decrease of at least 4 points on SDMT, which was associated with a deterioration of the employment status (14).
Brain atrophy accumulates over the disease course and is a known correlate of current and future cognitive decline in MS patients. Patients with more severe structural damage at baseline are more prone to suffer from cognitive decline, which in early RRMS is predicted mainly by white matter integrity damage, while in late RRMS and progressive MS is predicted most accurately by cortical atrophy (15). However, the studies that looked for a relevant radiological predictor of cognitive decline reveal conflicting results. Uher et al. (16) found that the risk of confirmed cognitive decline over the 2-year follow-up was greater in patients with a high baseline T2 lesion volume and more pronounced baseline brain atrophy, measured as low brain parenchymal fraction [BPF] (16). In another study by Papathanasiou et al. (17) neuropsychological measures had a strong correlation with all MRI atrophy measures [third ventricle width, thalamic and corpus callosum atrophy] and weak or moderate one with total lesion volume. In their study the thalamic area was the most sensitive predictor of memory and psychomotor speed deficits (17).
Significant atrophy of the whole brain, the cortical gray matter [GM], hippocampus, deep GM nuclei, and the white matter [WM] was found in patients with MS-related cognitive impairment versus those who were cognitively preserved, despite similar levels of physical disability (18).
More recent studies have demonstrated a strong association between brain atrophy and cognition, focusing on correlations between specific cognitive tests and characteristic pattern of radiological abnormalities, i.e., SDMT with WM microstructural damage, or reduced PASAT performance with the atrophy of several gray matter regions, i.e., bilateral thalamus putamen and caudate nucleus (19). Our team found associations between regional black hole volumes and several cognitive functions (20). We also managed to identify a distinct regional brain atrophy pattern in multiple sclerosis, as compared with one of the MS mimickers, namely neuropsychiatric systemic lupus erythematosus (21) and this MS-specific pattern of global and subcortical gray matter atrophy correlated with cognitive impairment (22).
The obvious predictors of deteriorating cognitive performance include progressing cortical atrophy, older age and higher EDSS. On the contrary, higher cognitive reserve [CR] in individuals with MS may mediate this process, with higher CR predicting better performance on neuropsychological tests, independent of brain atrophy (23). The cognitive reserve is a phenomenon consisting of years of education, occupational status and all leisure activities that expand our cognitive abilities, e.g., social activities or reading books. In order to improve this protecting, intellectual enrichment, it is recommended to be engaged in leisure activities that require, among many, attention, memory, and planning (2).
Early treatment with disease-modifying therapies [DMTs] should be started immediately after the diagnosis, as it has been proven to decrease the risk of disability and delay conversion to secondary progressive MS (24) Consequently, it also appears to be the possible therapeutic intervention to prevent or delay the development of cognitive disability. DMTs’ beneficial effects on cognition may thus depend on the anti-inflammatory properties of the immune therapies. In the long-term, the protective effect against tissue damage may result not only from preventing the accumulation of the new inflammatory-demyelinating lesions, but also from preventing the progression of brain atrophy (25).
Brain atrophy seems to be the indirect way to assess DMTs’ impact on cognitive decline, especially since some cognitive domains, like information processing speed, are closely associated with global brain atrophy (26).
Another aspect of DMT action could be the promotion of neurotrophic factors production, which has been associated with the use of, i.e., beta-interferons or fingolimod in MS patients (27, 28). In RRMS, Brain-Derived Neurotrophic Factor [BDNF] and beta-Nerve Growth Factor [beta-NGF] are strongly linked to cognitive performance and may exert a neuroprotective role (29, 30). Another neurotrophic factor, namely neurotrophin-3 [NT-3] has been linked to brain atrophy (31).
The aim of the study was to review the evidence regarding how DMTs affect cognitive functions.
In the present clinical review, we included 44 articles written in English and reporting on the adult (>90% studies) and pediatric MS population, focusing on cognitive functions as the outcome of DMTs, 39 studies were peer-reviewed and 5 were abstracts.
We included all DMTs currently approved by FDA in US and EMA in United Europe: interferon beta-1a intra-muscular, interferon beta-1a subcutaneous, interferon beta-1b, peginterferon beta-1a, glatiramer acetate, natalizumab, ocrelizumab, alemtuzumab, ofatumumab, dimethyl fumarate, teriflunomide, fingolimod, cladribine, siponimod and ozanimod. We excluded interventions involving combination and nonpharmacological treatments.
Relevant studies were identified with the use of PubMed, and the European Committee for Treatment and Research in Multiple Sclerosis [ECTRIMS] Library, using the words: “cognition,” “cognitive deficit“, “cognitive outcome“‚ “SDMT” ‚” PASAT,” “DMT” (Table 1). We included double-blind Phase III or IV randomized controlled trials (RCTs), in which any of the DMTs was compared as monotherapy with placebo or another active drug for the treatment of RRMS, or SPMS (siponimod), with regards to neurocognitive functions, including cognitive processing speed, working memory and verbal learning. The neurocognitive domains were examined with the use of the following tests: Paced Auditory Serial Addition Test (PASAT), Symbol Digit Modalities Test (SDMT), The Brief Repeatable Battery (BRB) of Neuropsychological Tests, Brief NP Battery, Stroop Test, Wechsler Memory Scale. The minimum duration of treatment was 6 months. We also included data from real-world and observational open-label studies. Preclinical studies, retrospective studies, case reports, reviews, commentaries, and letters were excluded.
Table 1 contains the summary of the most important studies on cognitive functions and DMTs.
Table 2 contains description of the psychological tests mentioned in the review.
Among all DMTs, platform therapies [beta-interferons, IFN, namely IFN beta-1a and IFN beta-1b, and glatiramer acetate, GA] have provided most studies with the longest follow-up data.
The phase III study with IFN beta -1a administered intramuscularly once a week evaluated the effect of the active compound on cognitive functions, by assessing 166 patients [83 on IFN and 83 on placebo] with the Brief NP Battery (32). In a 2-year period of IFN beta- 1a treatment performance measures of information processing and memory significantly improved relative to placebo, but no treatment effect was evident with regards to verbal abilities and attention span domains.
The COGIMUS study [including 201 patients] confirmed that IFN beta-1a [44 ug dose s.c. three times a week] stabilizes or even delays cognitive impairment over a 5-year period in most patients. The proportion of patients with cognitive impairment was 18% at baseline and 22.6% at year 5, which was significantly lower than the expected deterioration without treatment. Interestingly, the protective effect was greater in women than in men (33).
Mori et al. (34) divided a group of 80 treatment-naive MS patients who were to start IFN beta-1a s.c. treatment, into 2 subgroups depending on their radiological activity on baseline MRI (34). Patients who had gadolinium-enhancing lesions [Gd+] performed worse on the PASAT task compared with patients without active lesions. Both groups were similar with regard to disease duration, age, EDSS score and relapse rate. During 24 months of follow-up, the PASAT score improved in baseline Gd + patients and was stable in the baseline Gd- group. This study is particularly valuable because there are only a few studies concerning patients with isolated cognitive relapses [when gadolinium enhancing lesions are otherwise asymptomatic].
Another study concerning patients treated with IFN beta -1a s.c. was the SKORE observational study, with 300 patients enrolled. Patients were randomized into two sub-groups receiving two different doses of IFN [22 μg and 44 μg, respectively] (35). At all follow-up time points, the average cognitive performance improved. The proportion of patients with an increased or stable PASAT score vs. baseline was 57.7% at 6 months, 68.0% at 12 months and 61.4% at 24 months.
The BENEFIT study, including 468 patients with clinically isolated syndrome [CIS], revealed that improvement in PASAT-3 score from baseline to year 2 was greater for IFN beta-1b than for the placebo group. PASAT-3 is the 3 s-interstimulus interval and is one of the 3 tests included in the Multiple Sclerosis Functional Composite (MSFC), interstimulus intervals may be shorter e.g.2 s in other versions of PASAT. The treatment effect was maintained at year 5 and cognitive improvement was significantly more pronounced among patients that were treated early (36). After 11 years, patients from the BENEFIT trial were reassessed. The early-treatment group still had a better PASAT-3 score, and both groups were stable. These findings further confirm the conclusion that beta-interferons effectively protect from cognitive impairment (37), although the potential practice effect on the PASAT task should be acknowledged. Moreover, the BENEFIT population was generally affected with a mild disease course in both groups.
Other studies, based on the real- world data, usually enrolled small patient populations.
Barak et al. (38) confirmed that 1-year of treatment with IFN beta-1b had a positive effect on cognition measured with the BRB-N (38). The study was limited with a small sample size. It compared 18 MS patients treated with IFN beta-1b with 23 untreated subjects. The patients in the control group deteriorated in 3 out of 5 domains that were tested, while those that were treated improved in 2 domains, especially attention, concentration, and visual–spatial learning.
Lacy et al. (39) revealed a 16-years long effect of IFN beta-1b treatment on cognitive functioning, but again on a small sample. Sixteen IFN beta-1b treated patients remained relatively stable in their cognitive performance, which would not have been expected in the natural course of MS (39).
The first randomized, placebo-controlled study with glatiramer acetate, including 251 patients [125 on glatiramer and 126 on placebo], found no effect of GA treatment on the course of cognitive impairment. There were no differences in BRB-N scores at 24 months. Importantly, from the standpoint of the natural disease course, there was no measurable decline in cognitive functioning in either of the groups over the 2-year period. The authors speculated this was the beneficial effect from extra care and supportive social contact for all patients (40). After 2 years the participants were moved to the open-label extension study. Mean scores of memory and semantic retrieval tests did not change significantly, but both groups declined in attention, and the relapse rate during the first 2 years of the trial was a predictor of the cognitive decline (41).
The COPTIMIZE trial assessed patients after switching to GA from another therapy [>95% patients switched from interferons]. After 2 years patients improved PASAT scores by a mean 4.29 ± 9.28 (p,0.0001) (42). It would be more credible should there be a control group with other treatments or no treatment at all to verify if this improvement was not only due to the practice effect. However, this is a common limitation for other trials and studies. The improvement was observed in both, patients who were switched because of adverse events [AEs], and those who were switched for the lack of efficacy. However, the final scores were higher in the AEs group, which could be explained by the decreased fatigue following beta-interferons withdrawal, as the patients no longer experienced the flu-like side-effects, namely fever, fatigue, muscle pain, and headache.
The QualiCOP was an observational study with patients treated with GA, including subjects that were treatment-naive and those who were previously treated with GA (43). Cognitive outcome was measured with the PASAT and Multiple Sclerosis Inventory Cognition scale [MUSIC]. After 24-months follow-up patients improved significantly in both tests.
The efficacy of all injectable therapies seems to be similar. Cinar et al. (44) compared patients on 3 different injectables: IFN beta-1a s.c., IFN beta-1b s.c. and GA. A total number of 161 newly diagnosed patients were monitored with the use of BiCAMS for a period of 1 year (44). At baseline, the mean scores for all 3 cognitive tests were significantly higher in the control group than in the MS group. At month 12, all three scores improved in all MS groups compared with baseline, and there were no significant differences between the groups.
Of note, there are only few studies assessing cognitive functions in the pediatric MS population. In the BETAPAEDIC study, 68 treatment-naive patients who started IFN beta-1b were stable during 2 years of observation in the scores they obtained on Wechsler Intelligence Scale for Children, Raven’s Standard Progressive Matrices, the d2 Test of Attention and the Beery-Buktenica Developmental Test of Visual-Motor Integration (45). However, early administration of highly effective treatment in pediatric MS may better protect from cognitive decline, as patients who were escalated to natalizumab or fingolimod had cognitive performance preserved or ameliorated, while higher impairment was more prominent in those who remained on the first-line platform therapy. The results obviously need to be interpreted with caution, as they included only 19 participants (46).
The Teri-PRO study was a phase 4, real-world study that enrolled patients with RRMS who switched to teriflunomide from another DMT and received teriflunomide for 48 weeks. The SDMT scores were stable over that 48-week study period. Similarly, cognitive impairment, as recorded by patients on the cognitive domain of the MSPS [Multiple Sclerosis Performance Scale], also remained stable (47).
Importantly, there is a clearly recognized association between brain volume loss [BVL] and long-term accumulation of cognitive disability in the pivotal TEMSO study. Teriflunomide slowed BVL significantly compared to placebo [median BVL from baseline to year 2 was 1.29% for placebo and 0.90% for teriflunomide], which could suggest a neuroprotective role of teriflunomide (48). Patients who received 14 mg teriflunomide early on in the 2-year study experienced significant improvement in processing speed domain, compared to those who were randomized to placebo in the core study part, and benefits were extended for up to 5 years (49). This underscores the important role of early treatment initiation in the MS population.
The DEFINE and CONFIRM trials were designed to compare delayed-release dimethyl fumarate [DMF] with placebo or glatiramer acetate, respectively (50). Brain atrophy analysis revealed 30% reduction in the percentage brain volume change [PBVC] from 6 months to 2 years in patients treated with DMF compared to those treated with placebo (51).
In the observational Italian study that recruited over 200 subjects the effects of DMF treatment on cognition were assessed with the use of BRB-N and Stroop tests. Cognitive impairment at baseline was reported in 22.6% of patients. At 2 years, only 44.1% worsened, and 55.9% did not. However, dataset was incomplete as only 69.3% patients with cognitive impairment at baseline completed the study (52).
Most data from clinical trials with fingolimod provide indirect proof of its impact on cognition. This is mostly related to brain atrophy reduction in MS patients. FREEDOMS and TRANSFORMS studies indicate that the onset of action on BVL and cognition commenced early, within 3 to 6 months of treatment initiation. Fingolimod reduced brain atrophy and improved PASAT scores compared to placebo and IFN beta-1a [FREEDOMS and TRANSFORMS studies, respectively] (53, 54).
The post-hoc analysis of data from FREEDOMS revealed that fingolimod significantly improved PASAT scores versus placebo, which was regardless of baseline cognitive status, and the effect was sustained for up to 120 months (55).
In a trial reported by Ozakbas et al. (56) which included 96 patients and 98 healthy controls, a significant improvement in SDMT, CVLT2 and BVMTR scores was observed 6 months since fingolimod initiation (56).
In a smaller study after 1 year of fingolimod treatment 29 RRMS patients were cognitively stable (57). The participants underwent a comprehensive cognitive assessment with the use of the Mindstream Computerized Global Assessment Battery, measuring verbal and non-verbal memory, executive function, visual spatial perception, verbal function, attention, information processing speed and motor skills (57).
There are few studies comparing fingolimod with another drug with regards to its influence on cognition. Utz et al. (58) compared 33 patients treated with fingolimod or natalizumab using 8 neuropsychological tests. After 1 year follow-up,75% of patients were cognitively stable, and there was no difference between the two drugs (58).
A 48-week PREFERMS study compared 861 patients treated with either fingolimod or injectable DMTs [IFN beta-1a/b, glatiramer acetate]. The brain volume loss was less pronounced with fingolimod than with injectables, but no difference in SDMT scores was noted (59).
The GOLDEN study evaluated the effects of fingolimod and IFN beta-1b on cognitive impairment progression. At month 18, both groups showed improvement in all the measured cognitive parameters, as assessed with the use of Rao’s Brief Repeatable Battery and Delis–Kaplan Executive Function System test (60).
PANGAEA 2.0 is an ongoing real-world study assessing patients switching to fingolimod from other DMTs. In the present interim analysis of 2,428 patients, after 2 years all patients on fingolimod were found to have improved their SDMT scores, with the highest improvement observed in patients without any previous DMTs or with one DMT prior to fingolimod use (61). This analysis included patients switched from injectables [beta-interferons or glatiramer acetate] and oral DMTs [dimethyl fumarate or teriflunomide].
Siponimod is a novel S1P1 and S1P5 receptor modulator, which has been approved for secondary progressive MS. In the preclinical models it was shown to promote remyelination, as S1P receptors are also expressed by oligodendrocytes, neurons, microglia and astrocytes (62). In the EXPAND Core Study siponimod significantly reduced the risk of a meaningful worsening in the cognitive processing speed [defined as ≥4point decline in the SDMT score] versus placebo (63).
The effect was sustained for up to 5 years of observation. Interestingly, in the active SPMS subgroup the benefits were more pronounced, which invariably supports the earlier treatment initiation.
The SUNBEAM study compared ozanimod versus intramuscular IFN beta-1a, and the improvement on the SDMT score at month 12 was greater for ozanimod-treated patients, although the effect size was trivial, and the overall composite MSFC score was not significantly different between both groups (64). We did not find any data regarding cognition for ponesimod.
In the AFFIRM pivotal study of 856 patients, the percentage of patients with confirmed progression of cognitive deficit at 2 years was 7% in the natalizumab group vs. 12% in the placebo group, as assessed by the PASAT test [p = 0.013] (65).
STRIVE was an observational open-label study of 222 natalizumab-treated patients, where additional secondary endpoints included changes in cognition. A clinically significant improvement [an increase in SDMT score of ≥4 points] was observed in 41.9% of patients at year 1, and 49.4% of patients at year 2, which was associated with the general improvement of the work capacity (66).
Gudesblatt et al. (67) measured changes in cognition with the use of NeuroTrax computerized battery of tests in 57 patients who were treated with natalizumab for at least 2 years (67). The percentage of patients experiencing a significant improvement in the Global Cognitive Score increased from 21.6% at year 1 to 32.7% at year 2, regardless of the treatment-naïve or previously treated status. The greatest relative reduction of the deficit was observed in the attention and information processing speed domains.
Jacques et al. (68) demonstrated the long-term impact of natalizumab on cognition. Sixty-two patients [divided into two groups: treated longer and shorter than 2 years] were assessed with SDMT and CogState battery before every natalizumab infusion over a 24-month period. No patient in either group showed evidence of sustained cognitive deterioration. Moreover, in both groups, significant improvement in the mean scores of executive functions, verbal memory and working memory was observed (68).
Kunkel et al. (69) investigated a group of 51 natalizumab-treated patients and showed improvements in responsiveness, divided attention and information processing speed, although the percentage of patients suffering from fatigue increased from 55% at baseline to 61% in the second year (69).
The ENER-G study [89 patients] showed that cognitive performance in Automated Neuropsychological Assessment Metrics [ANAM] tests improved or remained stable up to 48 weeks after initiation of natalizumab. Fatigue was also reduced on therapy (70).
There are fewer studies where natalizumab is compared to other DMTs with regards to cognition. Sundgren et al. (71) demonstrated that natalizumab treatment for 1 year did not significantly improve cognitive functioning in RRMS patients compared to control patients on stable first-line DMT [IFN beta -1a i.m.]. In both groups participants with lower baseline scores had a significantly greater improvement (71). Other small sample studies by Rorsman et al. (72) [34 patients], and Portaccio et al. (73) [16 patients] found that natalizumab was more effective than first line therapy in reducing cognitive deterioration.
In an interesting study concerning NTZ withdrawal, patients who stopped NTZ treatment [because of PML risk] were compared with those who continued therapy. Neuropsychological assessment [BRB and the Stroop test] after 1 year revealed that 63.3% patients discontinuing NTZ presented with a cognitive worsening, whereas in the continuers’ group it was only 7.1% (74). Preziosa et al. (75) compared 30 patients on NTZ with 25 on fingolimod therapy, at month 24 both drugs improving the MSFC score (75).
With the schedule of Extended Interval Dosing [EID] of NTZ more and more frequently used worldwide, McManus et al. (76) assessed the impact of EID on cognitive parameters. In a group of 34 patients on EID-NTZ schedule, improved cognitive Z scores after 28 months of treatment were observed, especially in memory, attention and executive function (76).
Natalizumab seems to preserve cognition also in a pediatric-onset MS, where patients are at a high risk of developing cognitive impairment in their adulthood. In a group of 20 treatment-naive patients started on natalizumab, the SDMT score improved in 13 of them after 24 months of treatment and declined in only 2 patients (77).
In CARE-MS II study of alemtuzumab, annual BVL throughout years 3–5 and cumulative BVL over 5 years was smaller in patients who received alemtuzumab compared to IFN beta-1a s.c,. which might indicate neuroprotective effects (78). However, there was no significant difference in the PASAT scores between both groups (79).
In a smaller study, Riepl et al. (80) assessed 21 patients treated with alemtuzumab. After 15 months, overall cognitive functioning of patients remained stable or improved. The proportion of patients that showed deficits in≥3 tests was reduced from 24% at baseline to 14% at follow-up, especially in the processing speed domain (80). The authors explored whether cognitive change from baseline to follow-up was dependent on clinical changes [EDSS, T2 lesion load, relapse rate], but surprisingly none of them was a significant predictor of cognitive function.
In a small group of 17 alemtuzumab-treated patients Hvid et al. confirmed the effectiveness of the drug on cognitive functioning, observing the improvement in the selective reminding test and SDMT after 24-month of therapy (81).
The trend showing a link between the improvement of cognitive functions and higher potency of immunotherapy was also confirmed by the study where 19 patients receiving autologous hematopoietic stem cell transplantation [aHSCT] were compared with 21 patients receiving alemtuzumab. The wide battery of neuropsychological tests was used i.e., SDMT, Verbal Learning and Memory Test, test of attention [TAP] (82). Patients receiving aHSCT showed improved cognitive functioning [mean follow-up 58.8 months], while alemtuzmab-treated subjects deteriorated in all the tested domains [mean follow-up of 27.6 months].
The OPERA I and OPERA II studies showed that ocrelizumab use was associated with a statistically significant improvement in SDMT scores over 96 weeks, compared with IFN beta-1a s.c (83). Also, ocrelizumab-treated patients had a significantly lower risk of developing sustained SDMT decline over 12 and 24 weeks. Such trend was also observed in subgroups of patients with moderate cognitive impairment at baseline.
Patients on ocrelizumab had 57% [OPERA I] and 64% [OPERA II] lower number of new hypointense lesions on T1-weighted MRI. However, the differences in the percentage of brain-volume loss from week 24 to week 96 were non-confirmatory in the OPERA I and non-significant in the OPERA II studies (84).
In the Phase 3 ASCLEPIOS I/II trials ofatumumab significantly improved the SDMT scores from baseline to Month 24, more patients on ofatumumab had ≥4 point sustained improvement on SDMT versus teriflunomide (25% vs. 19.6%, p = 0.005) (85).
The CLARITY study confirmed the efficacy of cladribine in RRMS, but cognitive functioning was assessed only based on the 4 items from the SF-36 The Short Form 36 Health Survey is a 36-item, patient-reported survey of patient quality of life (86).
The CLADQoL is an ongoing prospective study where one of the secondary objectives is cognitive status assessed with SDMT. The final report is planned on December 2024.
As cognitive impairment in multiple sclerosis may be as devastating as physical disability, maintaining patients’ cognition or improving their cognitive deficits should naturally be our key therapeutic goal, besides reducing relapse rate and radiological activity. The stabilization of cognitive functions could be proposed as another outcome in the NEDA score (87), following atrophy normalization [NEDA-4] (88) and neurofilament levels stabilization.
Natural history studies in patients with MS suggest the rate of cognitive decline is approximately 5% per year (89). One of the studies that assessed a large contemporary cohort of patients on DMT with a long-lasting disease, with cognitive assessment after at least 10 years from disease onset, observed a much lower rate of cognitive impairment than previously reported in the pre-disease modifying treatment era (90). We may presume that DMTs have influenced cognition alongside other MS symptoms. However, there are many difficulties with verifying individual DMT’s potential therapeutic effect on cognition and comparing their effectiveness. Cognitive performance can be influenced by a number of factors, including cognitive reserve, fatigue, medications, infections, depression, comorbidities or cognitive rehabilitation. To date, most studies provide only indirect arguments for cognitive efficacy of DMTs, mainly by pointing to the reduction of brain atrophy rate. Cognitive impairment may start in different stages of the disease. Some patients may be protected longer by their cognitive reserve, and short-term [1 or 2 years] follow-up may not be enough to reveal cognitive decline and the therapeutic effects of DMTs. The time of follow-up cognitive assessment in MS patients should obviously be longer. However, in MS trials the 1–2 years follow-up is a pure reflection of the design of the pharmacoclinical studies. This is a clear limitation.
Early clinical trials of disease-modifying therapies did not include neuropsychological tests. Since cognition improved on validity, most of the current clinical trials use cognitive impairment as an outcome measure, although still only as secondary, tertiary, or exploratory outcomes.
Additionally, different neuropsychological tests were used across clinical trials, which makes it difficult to compare them in this aspect. Also, cognitive impairment is often characterized by overall performance based on several tests within a specific battery, with different patients impaired in different cognitive domains (90). As neuropsychological batteries are time-consuming, many studies have been conducted with the use of a single neuropsychological test. While SDMT seems to be a good, reliable [albeit simplified] screening test, PASAT test was mostly used in older studies and since then was shown to be burdened with the practice effect (91). PASAT measures attentional process as information processing speed, sustained attention but also working memory (91).
On the other hand, post-marketing studies usually have small sample sizes, short follow-up periods and are limited by the absence of the control groups.
Fortunately, neuropsychological assessment is now commonly incorporated into clinical trials, at times even as a primary outcome, like in the ENLIGHTEN trial with ozanimod, where the primary outcome is the increases in the SDMT score [estimated completion date 2025] (92). Since full neuropsychological testing is not feasible, a screening with SDMT has been gradually incorporated into routine clinical practice, at baseline in newly diagnosed patients and at regular follow-ups during the treatment period, but this is still far from the routine.
In summary, there is a line of evidence for cognitive benefits of DMTs, the following drugs are effective in preserving cognitive functions: beta-interferons, teriflunomide, dimethyl fumarate, siponimod, ozanimod, natalizumab, alemtuzumab, ofatumumab, ocrelizumab and cladribine.
Conflicting results concern glatiramer acetate and fngolimod, but more studies, especially in the real-world setting, are urgently needed. Highly effective therapies seem to be more effective in preventing cognitive decline. However, a recently published meta-analysis including 41 studies, while revealing a positive effect of DMTs on cognition, failed to show a statistically significant difference between platform and escalation therapies (93). The authors emphasized that some therapies were underrepresented or not taken into consideration at all, e.g., alemtuzumab, ocrelizumab, teriflunomide or cladribine. Even meta-analyzes collect and analyze data previously acquired from RCTs or RWD. In case of DMT’s effect on cognition good quality data is still lacking. Therefore, the value of such meta-analysis is also limited (94).
It is still a matter of debate whether cognitive impairment should be an argument to switching/escalating DMTs. Clinicians need more clues from clinical studies supporting such decisions. Also, if among contemporary treated population of MS only 50% of patients reach annual NEDA-3, is it feasible for the time being to expect NEDA-4, or even higher? One needs to consider that NEDA might not serve as an all-purpose goal of treatment. Also, there may be a group of patients, where minimizing cognitive impairment would be even more essential than reducing relapse rates or MRI activity.
Since” time is brain,” time is also preserved cognition. It is highly important to identify MS patients with cognitive impairment at baseline (Figure 1) or patients with a high risk of developing cognitive impairment, to make sure they promptly start effective therapy, and to propose a cognitive neurorehabilitation plan so that they maintain their quality of life for as long as possible.
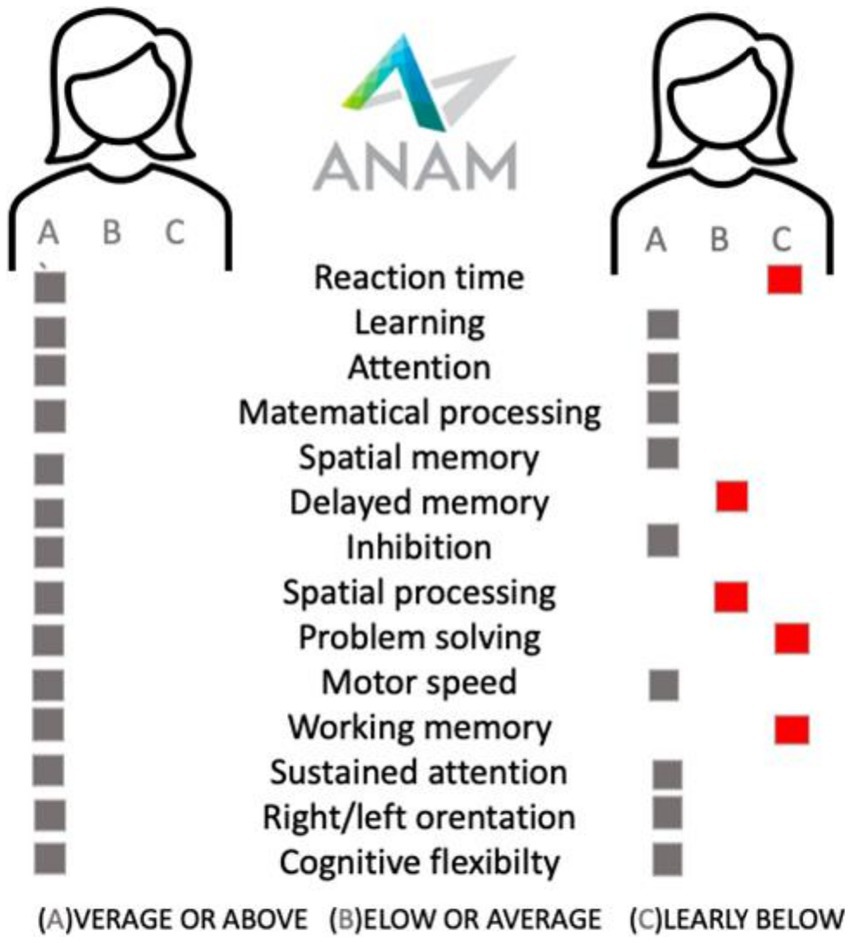
Figure 1. The examplary cognitive battery testing [ANAM, Automated Neuropaychological Assessment Metrics] results in sex, age, education, disease duration, treatment duration, EDSS, and demyelinating lesions volume matched two patients treated with beta-interferon.
KK, WA, AK-Ł, and WK contributed to conception and design of the study. KK organized the database and wrote the first draft of the manuscript. KK, AK-Ł, and WA wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Myhr, K-M . Diagnosis and treatment of multiple sclerosis. Acta Neurol Scand. (2008) 117:12–21. doi: 10.1111/j.1600-0404.2008.01026.x
2. Penner, IK . Evaluation of cognition and fatigue in multiple sclerosis: daily practice and future directions. Acta Neurol Scand. (2016) 134:19–23. doi: 10.1111/ane.12651
3. Lloyd, A, Schofield, H, and Adlard, N. Cognitive decline may not be adequately captured in economic evaluations of multiple sclerosis: are new treatments being undervalued? Curr Med Res Opin. (2020) 36:609–11. doi: 10.1080/03007995.2020.1719393
4. Meli, R, Roccatagliata, L, Capello, E, Bruschi, N, Uccelli, A, Mancardi, G, et al. Ecological impact of isolated cognitive relapses in MS. Mult Scler. (2020) 26:114–7. doi: 10.1177/1352458518813722
5. Achiron, A, Stern, M, and Aloni, R. Cognitive performance in radiologically isolated syndrome. Neurology. (2017) 88:338.
6. Brochet, B, and Ruet, A. Cognitive impairment in multiple sclerosis with regards to disease duration and clinical Penotypes. Front Neurol. (2019) 10:261. doi: 10.3389/fneur.2019.00261
7. Rao, SM, Leo, GJ, Ellington, L, Nauertz, T, Bernardin, L, and Unverzagt, F. Cognitive dysfunction in multiple sclerosis.: II. Impact on employment and social functioning. Neurology. (1991) 41:692–6. doi: 10.1212/WNL.41.5.692
8. Cadden, M, and Arnett, P. Factors associated with employment status in individuals with multiple sclerosis. Int J MS Care. (2015) 17:284–91. doi: 10.7224/1537-2073.2014-057
9. Kavaliunas, A, Danylaite Karrenbauer, V, Gyllensten, H, Manouchehrinia, A, Glaser, A, Olsson, T, et al. Cognitive function is a major determinant of income among multiple sclerosis patients in Sweden acting independently from physical disability. Mult Scler. (2019) 25:104–12. doi: 10.1177/1352458517740212
10. Amato, MP, Zipoli, V, Goretti, B, Portaccio, E, de Caro, MF, Ricchiuti, L, et al. Benign multiple sclerosis. J Neurol. (2006) 253:1054–9. doi: 10.1007/s00415-006-0161-8
11. Macías Islas, M, and Ciampi, E. Assessment and impact of cognitive impairment in multiple sclerosis: an overview. Biomedicine. (2019) 7:22. doi: 10.3390/biomedicines7010022
12. Benedict, RHB, DeLuca, J, Phillips, G, LaRocca, N, Hudson, LD, Rudick, R, et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler J. (2017) 23:721–33. doi: 10.1177/1352458517690821
13. Pardini, M, Uccelli, A, Grafman, J, Yaldizli, Ö, Mancardi, G, and Roccatagliata, L. Isolated cognitive relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry. (2014) 85:1035–7. doi: 10.1136/jnnp-2013-307275
14. Damasceno, A, Damasceno, BP, and Cendes, F. No evidence of disease activity in multiple sclerosis: implications on cognition and brain atrophy. Mult Scler. (2016) 22:64–72. doi: 10.1177/1352458515604383
15. Eijlers, AJC, Van Geest, Q, Dekker, I, Steenwijk, MD, Meijer, KA, Hulst, HE, et al. Predicting cognitive decline in multiple sclerosis: a 5-year follow-up study. Brain. (2018) 141:2605–18. doi: 10.1093/brain/awy202
16. Uher, T, Vaneckova, M, Sormani, MP, Krasensky, J, Sobisek, L, Dusankova, JB, et al. Identification of multiple sclerosis patients at highest risk of cognitive impairment using an integrated brain magnetic resonance imaging assessment approach. Eur J Neurol. (2017) 24:292–301. doi: 10.1111/ene.13200
17. Papathanasiou, A, Messinis, L, Zampakis, P, Panagiotakis, G, Gourzis, P, Georgiou, V, et al. Thalamic atrophy predicts cognitive impairment in relapsing remitting multiple sclerosis. Effect on instrumental activities of daily living and employment status. J Neurol Sci. (2015) 358:236–42. doi: 10.1016/j.jns.2015.09.001
18. Damjanovic, D, Valsasina, P, Rocca, MA, Stromillo, ML, and Gallo, A. Hippocampal and deep gray matter nuclei atrophy is relevant for explaining cognitive impairment in MS: a multicenter study. Am J Neuroradiol. (2017) 38:18–24. doi: 10.3174/ajnr.A4952
19. Riccitelli, GC, Pagani, E, and Rodegher, M. Imaging patterns of gray and white matter abnormalities associated with PASAT and SDMT performance in relapsing-remitting multiple sclerosis. Mult Scler. (2019) 25:204–16. doi: 10.1177/1352458517743091
20. Nowaczyk, N, Kalinowska-Łyszczarz, A, Paprzycki, W, Michalak, S, Kaźmierski, R, and Pawlak, MA. Spatial distribution of white matter degenerative lesions and cognitive dysfunction in relapsing-remitting multiple sclerosis patients. Neurol Neurochir Pol. (2019) 53:18–25. doi: 10.5603/PJNNS.a2018.0001
21. Kalinowska-Lyszczarz, A, Pawlak, MA, Pietrzak, A, Pawlak-Bus, K, Leszczynski, P, Puszczewicz, M, et al. Distinct regional brain atrophy pattern in multiple sclerosis and neuropsychiatric systemic lupus erythematosus patients. Lupus. (2018) 27:1624–35. doi: 10.1177/0961203318781004
22. Kalinowska-Łyszczarz, A, Pawlak, MA, Pietrzak, A, Pawlak-Buś, K, Leszczyński, P, Puszczewicz, M, et al. Subcortical gray matter atrophy is associated with cognitive deficit in multiple sclerosis but not in systemic lupus erythematosus patients. Lupus. (2018) 27:610–20. doi: 10.1177/0961203317735186
23. Amato, MP, Razzolini, L, Goretti, B, Stromillo, ML, Rossi, F, De Giorgio, A, et al. Cognitive reserve and cortical atrophy in multiple sclerosis: a longitudinal study. Neurology. (2013) 80:1728–33. doi: 10.1212/WNL.0b013e3182918c6f
24. Kavaliunas, A, Manouchehrinia, A, Stawiarz, L, Ramanujam, R, Agholme, J, Hedström, AK, et al. Importance of early treatment initiation in the clinical course of multiple sclerosis. Mult Scler. (2017) 23:1233–40. doi: 10.1177/1352458516675039
25. Amato, MP, Portaccio, E, and Goretti, B. Cognitive impairment in early stages of multiple sclerosis. Neurol Sci. (2010) 31:211–S214. doi: 10.1007/s10072-010-0376-4
26. Deloire, MSA, Ruet, A, and Hamel, D. MRI predictors of cognitive outcome in early multiple sclerosis. Neurology. (2011) 76:1161–7. doi: 10.1212/WNL.0b013e318212a8be
27. Biernacki, K, Antel, JP, Blain, M, Narayanan, S, Arnold, DL, and Prat, A. Interferon beta promotes nerve growth factor secretion early in the course of multiple sclerosis. Arch Neurol. (2005) 62:563–8. doi: 10.1001/archneur.62.4.563
28. Golan, M, Mausner-Fainberg, K, Ibrahim, B, Benhamou, M, Wilf-Yarkoni, A, Kolb, H, et al. Fingolimod increases brain-derived neurotrophic factor level secretion from circulating T cells of patients with multiple sclerosis. CNS Drugs. (2019) 33:1229–37. doi: 10.1007/s40263-019-00675-7
29. Kalinowska-Łyszczarz, A, Pawlak, MA, Michalak, S, and Losy, J. Cognitive deficit is related to immune-cell beta-NGF in multiple sclerosis patients. J Neurol Sci. (2012) 321:43–8. doi: 10.1016/j.jns.2012.07.044
30. Yalachkov, Y, Anschütz, V, Jakob, J, Schaller-Paule, MA, Schäfer, JH, Reiländer, A, et al. Brain-derived neurotrophic factor and neurofilament light chain in cerebrospinal fluid are inversely correlated with cognition in multiple sclerosis at the time of diagnosis. Mult Scler Relat Disord. (2022) 63:103822. doi: 10.1016/j.msard.2022.103822
31. Kalinowska-Łyszczarz, A, Pawlak, MA, Michalak, S, Paprzycki, W, and Losy, J. Immune cell NT-3 expression is associated with brain atrophy in multiple sclerosis patients. J Neuroimmunol. (2011) 240-241:109–13. doi: 10.1016/j.jneuroim.2011.10.002
32. Fischer, JS, Priore, RL, and Jacobs, LD. Neuropsychological effects of interferon b-1a in relapsing multiple sclerosis. Ann Neurol. (2000) 48:885–92. doi: 10.1002/1531-8249(200012)48:6<885::AID-ANA9>3.0.CO;2-1
33. Patti, F, Morra, VB, Amato, MP, Trojano, M, Bastianello, S, Tola, MR, et al. Subcutaneous interferon b-1a may protect against cognitive impairment in patients with relapsing–remitting multiple sclerosis: 5-year follow-up of the COGIMUS study. PLoS One. (2013) 8:e74111. doi: 10.1371/journal.pone.0074111
34. Mori, F, Kusayanagi, H, Buttari, F, and Centini, B. Early treatment with high-dose interferon beta-1a reverses cognitive and cortical plasticity deficits in multiple sclerosis. Funct Neurol. (2012) 27:163–8.
35. Benesova, Y, and Tvaroh, A. Cognition and fatigue in patients with relapsing multiple sclerosis treated by subcutaneous interferon β-1a: an observational study SKORE. Ther Adv Neurol Disord. (2017) 10:18–32. doi: 10.1177/1756285616671882
36. Penner, IK, Stemper, B, and Calabrese, P. Effects of interferon beta-1b on cognitive performance in patients with a first event suggestive of multiple sclerosis. Mult Scler. (2012) 18:1466–71. doi: 10.1177/1352458512442438
37. Kappos, L, Edan, G, and Freedman, MS. The 11-year long-term follow-up study from the randomized BENEFIT CIS trial. Neurology. (2016) 87:978–87. doi: 10.1212/WNL.0000000000003078
38. Barak, Y, and Achiron, A. Effect of interferon-beta-1b on cognitive functions in multiple sclerosis. Eur Neurol. (2002) 47:11–4. doi: 10.1159/000047940
39. Lacy, M, Hauser, M, and Pilskin, N. The effects of long-term interferon-beta-1b treatment on cognitive functioning in multiple sclerosis: a 16-year longitudinal study. Mult Scler. (2013) 19:1765–72. doi: 10.1177/1352458513485981
40. Weinstein, A, Schwid, SIL, and Schiffer, RB. Neuropsychological status in multiple sclerosis after treatment with Glatiramer. Eur Neurol. (2002) 47:11–4. doi: 10.1001/archneur.56.3.319
41. Schwid, SR, Goodman, AD, Weinstein, A, McDermott, MP, and Johnson, KP. Cognitive function in relapsing multiple sclerosis: minimal changes in a 10-year clinical trial. J Neurol Sci. (2007) 255:57–63. doi: 10.1016/j.jns.2007.01.070
42. Ziemssen, T, Bajenaru, OA, and Carra, A. A 2-year observational study of patients with relapsing-remitting multiple sclerosis converting to glatiramer acetate from other disease-modifying therapies: the COPTIMIZE trial. J Neurol. (2014) 261:2101–11. doi: 10.1007/s00415-014-7446-0
43. Ziemssen, T, Calabrese, P, Penner, IK, and Apfel, R. QualiCOP: real-world effectiveness, tolerability, and quality of life in patients with relapsing-remitting multiple sclerosis treated with glatiramer acetate, treatment-naïve patients, and previously treated patients. J Neurol. (2016) 263:784–91. doi: 10.1007/s00415-016-8058-7
44. Cinar, BP, Kösehasanoğulları, G, Yigit, P, and Ozakbas, S. Cognitive dysfunction in patients with multiple sclerosis treated with first-line disease-modifying therapy: a multi-center, controlled study using the BICAMS battery. Neurol Sci. (2017) 38:337–42. doi: 10.1007/s10072-016-2775-7
45. Gärtner, J, Brück, W, Weddige, A, Hummel, H, Norenberg, C, and Bugge, J-P. Interferon beta-1b in treatment-naïve paediatric patients with relapsing–remitting multiple sclerosis: two-year results from the BETAPAEDIC study. Mult Scler J Exp Transl Clin. (2017) 3:205521731774762. doi: 10.1177/2055217317747623
46. Johnen, A, Elpers, C, and Riepl, E. Early effective treatment may protect from cognitive decline in paediatric multiple sclerosis. Eur J Paediatr Neurol. (2019) 23:783–91. doi: 10.1016/j.ejpn.2019.08.007
47. Coyle, PK, Khatri, B, Edwards, KR, Meca-Lallana, JE, Cavalier, S, Rufi, P, et al. Patient-reported outcomes in patients with relapsing forms of MS switching to teriflunomide from other disease-modifying therapies: results from the global phase 4 Teri-PRO study in routine clinical practice. Mult Scler Relat Disord. (2018) 26:211–8. doi: 10.1016/j.msard.2018.09.017
48. Radue, EW, Sprenger, T, Gaetano, L, Mueller-Lenke, N, Cavalier, S, Thangavelu, K, et al. Teriflunomide slows BVL in relapsing MS: a reanalysis of the TEMSO MRI data set using SIENA. Neurol Neuroimmunol Neuroinflamm. (2017) 4:e390. doi: 10.1212/NXI.0000000000000390
49. Sprenger, T, Kappos, L, Sormani, MP, Miller, AE, Poole, EM, Cavalier, S, et al. Effects of teriflunomide treatment on cognitive performance and brain volume in patients with relapsing multiple sclerosis: post hoc analysis of the TEMSO core and extension studies. Mult Scler. (2022) 28:1719–28. doi: 10.1177/13524585221089534
50. Giovannoni, G, Gold, R, and Kappos, L. Delayed-release dimethyl fumarate and disability assessed by the multiple sclerosis functional composite: integrated analysis of DEFINE and CONFIRM. Mult Scler J Exp Transl Clin. (2016) 2:205521731663411. doi: 10.1177/2055217316634111
51. Arnold, DL, Gold, R, and Kappos, L. Effects of delayed-release dimethyl fumarate on MRI measures in the phase 3 DEFINE study. J Neurol. (2014) 261:1794–802. doi: 10.1007/s00415-014-7412-x
52. Amato, MP, Goretti, B, Brescia Morra, V, Gallo, P, Zaffaroni, M, Onofrj, M, et al. Effects of 2-year treatment with dimethyl fumarate on cognition and functional impairment in patients with relapsing remitting multiple sclerosis. Neurol Sci. (2020) 41:3185–93. doi: 10.1007/s10072-020-04320
53. Cohen, J, Pelletier, J, Chin, P, Sfikas, N, Karlsson, G, Von Rosenstiel, P, et al. Efficacy of fingolimod in RRMS as measured by multiple sclerosis functional composite: results from the TRANSFORMS, FREEDOMS, and FREEDOMS II phase 3 studies. Mult Scler. (2013) 19:268.
54. Kappos, L, Radue, EW, Chin, P, and Ritter, S. Onset of clinical and MRI efficacy occurs early after fingolimod treatment initiation in relapsing multiple sclerosis. J Neurol. (2016) 263:354–60. doi: 10.1007/s00415-015-7978-y
55. Langdon, DW, Tomic, D, Penner, IK, Calabrese, P, Cutter, G, Häring, DA, et al. Baseline characteristics and effects of fingolimod on cognitive performance in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. (2021) 28:4135–45. doi: 10.1111/ene.15081
56. Ozakbas, S, Cinar, BP, Kosehasanogullari, G, and Yigit, P. Effects of Fingolimod on cognitive status in patients with multiple sclerosis: prospective, controlled trial [P3.071]. Neurology. (2016) 86:16.
57. Barak, Y . Fingolimod [Gilenya] confers cognitive stability in active relapsing-remitting multiple sclerosis patients ECTRIMS online library. Barak Y. 10/11/14; 64026; P322, poster. (n.d.)
58. Utz, KS, Lee, DH, and Lammer, A. Cognitive functions over the course of 1 year in multiple sclerosis patients treated with disease modifying therapies. Ther Adv Neurol Disord. (2016) 9:269–80. doi: 10.1177/1756285616643892
59. Cree, BAC, Arnold, DL, and Cascione, M. Phase IV study of retention on fingolimod versus injectable multiple sclerosis therapies: a randomized clinical trial. Ther Adv Neurol Disord. (2018) 11:1–15. doi: 10.1177/1756286418774338
60. Comi, G, Patti, F, and Rocca, MA. Efficacy of fingolimod and interferon beta 1b on cognitive, MRI, and clinical outcomes in elapsing–remitting multiple sclerosis: an 18 month, open label, rater blinded, randomised, multicentre study [the GOLDEN study]. J Neurol. (2017) 264:2436–49. doi: 10.1007/s00415-017-8642-5
61. Ziemssen, T, and Schulze-Topphoff, U. PANGAEA 2.0: effectiveness of fingolimod in patients with disease activity switching from other DMTs and different treatment frequencies. ECTRIMS. (2019) 2019:EP1544
62. Kipp, M . Does Siponimod exert direct effects in the central nervous system? Cells. (2020) 9:1771. doi: 10.3390/cells9081771
63. Giovannoni, G, Kappos, L, and Fox, RJ. Sustained reduction of disability and cognitive decline with long-term siponimod treatment in patients with active SPMS:EXPAND data up to 5 years. Poster Presentation at the ACTRIMS-ECTRIMS meeting. (2020).
64. DeLuca, J, Schippling, S, Montalban, X, Kappos, L, Cree, BAC, Comi, G, et al. Effect of Ozanimod on symbol digit modalities test performance in relapsing MS. Mult Scler Relat Disord. (2021) 48:102673. doi: 10.1016/j.msard.2020.102673
65. Weinstock-Guttman, B, Galetta, SL, and Giovannoni, G. Additional efficacy endpoints from pivotal natalizumab trials in relapsing-remitting MS. J Neurol. (2012) 259:898–905. doi: 10.1007/s00415-011-6275-7
66. Perumal, J, Fox, RJ, and Balabanov, R. Outcomes of natalizumab treatment within 3 years of relapsing-remitting multiple sclerosis diagnosis: a prespecified 2-year interim analysis of STRIVE. BMC Neurol. (2019) 19:116. doi: 10.1186/s12883-019-1337-z
67. Gudesblatt, M, Wissemann, K, and Zarif, M. Improvement in cognitive function as measured by NeuroTrax in patients with relapsing multiple sclerosis treated with Natalizumab: a 2 year retrospective analysis. CNS Drugs. (2018) 32:1173–81. doi: 10.1007/s40263-018-0553-1
68. Jacques, FH, Harel, BT, Schembri, AJ, Paquette, C, Bilodeau, B, Kalinowski, P, et al. Cognitive evolution in natalizumab-treated multiple sclerosis patients. Mult Scler J Exp Transl Clin. (2016) 2:205521731665711. doi: 10.1177/2055217316657116
69. Kunkel, A, and Fischer, M. Impact of Natalizumab treatment on fatigue, mood, and aspects of cognition in relapsing–remitting multiple sclerosis. Front Neurol. (2015) 6:97. doi: 10.3389/fneur.2015.00097
70. Wilken, J, Kane, RL, and Sullivan, CL. Changes in fatigue and cognition in patients with relapsing forms of multiple sclerosis treated with Natalizumab the ENER-G study. Int J MS Care. (2013) 15:120–8. doi: 10.7224/1537-2073.2012-043
71. Sundgren, M, Piehl, F, Wahlin, A, and Brismar, T. Cognitive function did not improve after initiation of natalizumab treatment in relapsing-remitting multiple sclerosis. A prospective one-year dual control group study. Mult Scler Relat Disord. (2016) 10:36–43. doi: 10.1016/j.msard.2016.08.011
72. Rorsman, I, Peterson, C, and Nilsson, PC. Cognitive functioning following one- year natalizumab treatment: a non- randomized clinical trial. Acta Neurol Scand. (2017) 137:117–24. doi: 10.1111/ane.12833
73. Portaccio, E, Stromillo, ML, and Goretti, B. Natalizumab may reduce cognitive changes and brain atrophyrate in relapsing–remitting multiple sclerosis: a prospective, non-randomized pilot study. Eur J Neurol. (2013) 20:986–90. doi: 10.1111/j.1468-1331.2012.03882.x
74. Iaffaldano, P, Viterbo, RG, and Trojano, M. Natalizumab discontinuation is associated with a rebound of cognitive impairment in multiple sclerosis patients. J Neurol. (2016) 263:1620–5. doi: 10.1007/s00415-016-8177-1
75. Preziosa, P, Rocca, MA, Riccitelli, GC, Moiola, L, Storelli, L, Rodegher, M, et al. Effects of Natalizumab and Fingolimod on clinical, cognitive, and magnetic resonance imaging measures in multiple sclerosis. Neurotherapeutics. (2020) 17:208–17. doi: 10.1007/s13311-019-00781-w
76. McManus, EJ, Clark, KM, Frampton, C, Macniven, JAB, and Schepel, J. Extended interval dosing Natalizumab and impact on neuropsychological deficits in relapsing-remitting multiple sclerosis. Mult Scler J Exp Transl Clin. (2022) 8:205521732110707. doi: 10.1177/20552173211070752
77. Margani, M, Rinaldi, F, and Riccardi, A. No evidence of disease activity including cognition [NEDA 3 plus] in naïve pediatric multiple sclerosis patients treated with natalizumab. J Neurol. (2020) 267:100–5. doi: 10.1007/s00415-019-09554-z
78. Coles, AJ, Cohen, JA, Fox, EJ, Giovannoni, G, Hartung, HP, and Havrdova, E. Alemtuzumab CARE-MS II 5-year follow-up efficacy and safety findings. Neurology. (2017) 89:1117–26. doi: 10.1212/WNL.0000000000004354
79. Giovannoni, G, and CohenJ, CA. Alemtuzumab improves preexisting disability in active relapsing-remitting MS patients. Neurology. (2016) 87:1985–92. doi: 10.1212/WNL.0000000000003319
80. Riepl, E, Pfeuffer, S, and Ruck, T. Alemtuzumab improves cognitive processing speed in active multiple sclerosis-a Lon zgitudinal observational study. Front Neurol. (2018) 8:730. doi: 10.3389/fneur.2017.00730
81. Hvid, LG, Stenager, E, and Dalgas, U. Objectively assessed physiological, physical, and cognitive function along with patient-reported outcomes during the first 2 years of Alemtuzumab treatment in multiple sclerosis: a prospective observational study. J Neurol. (2022) 269:4895–908. doi: 10.1007/s00415-022-11134-7
82. Häußler, V, Ufer, F, Pöttgen, J, Wolschke, C, Friese, MA, Kröger, N, et al. aHSCT is superior to alemtuzumab in maintaining NEDA and improving cognition in multiple sclerosis. Ann Clin Transl Neurol. (2021) 8:1269–78. doi: 10.1002/acn3.51366
83. Cohan, S, Benedict, RHB, and de Seze, J. Time to cognitive worsening in patients with relapsing multiple sclerosis in Ocrelizumab phase III trials. Neurology. (2018) 2018:90.
84. Hauser, SL, Bar-Or, A, Comi, G, Giovannoni, G, and Hartung, H-P. Ocrelizumab versus interferon Beta-1a in relapsing multiple sclerosis. The New England journal of medicine. N Engl J Med. (2017) 376:221–34. doi: 10.1056/NEJMoa1601277
85. Benedict, RH, Penner, IK, Cutter, G, Kappos, L, Coyle, P, Piani-Meier, D, et al. Improvement in cognitive processing speed with Ofatumumab in patients with relapsing multiple sclerosis. Oral Presentation-130EAN (2022).
86. Giovannoni, G, Comi, G, and Cook, S. A placebo-controlled trial of Oral Cladribine for relapsing multiple sclerosis. N Engl J Med. (2010) 362:416–26. doi: 10.1056/NEJMoa0902533
87. Giovannoni, G, Turner, B, Gnanapavan, S, Offiah, C, Schmierer, K, and Marta, M. Is it time to target no evident disease activity [NEDA] in multiple sclerosis? Mult Scler Relat Disord. (2015) 4:329–33. doi: 10.1016/j.msard.2015.04.006
88. Kappos, L, De Stefano, N, Freedman, MS, Cree, BA, Radue, EW, Sprenger, T, et al. Inclusion of brain volume loss in a revised measure of 'no evidence of disease activity' [NEDA-4] in relapsing-remitting multiple sclerosis. Mult Scler. (2016) 22:1297–305. doi: 10.1177/1352458515616701
89. Amato, MP, Ponziani, G, Siracusa, G, and Sorbi, S. Cognitive dysfunction in early-onset multiple sclerosis: a reappraisal after 10 years. Arch Neurol. (2001) 58:1602–6. doi: 10.1001/archneur.58.10.1602
90. Sumowski, JF, Benedict, R, Enzinger, C, Filippi, M, Geurts, JJ, Hamalainen, P, et al. Cognition in multiple sclerosis: state of the field and priorities for the future. Neurology. (2018) 90:278–88. doi: 10.1212/WNL.0000000000004977
91. Tombaugh, TN . A comprehensive review of the paced auditory serial addition test [PASAT]. Arch Clin Neuropsychol. (2006) 21:53–76. doi: 10.1016/j.acn.2005.07.006
92. NCT . (n.d.). Available at: https://clinicaltrials.gov/ct2/show/NCT04140305.
93. Landmeyer, NC, Burkner, PC, Wiendl, H, Ruck, T, Hartung, HP, Holling, H, et al. Disease-modifying treatments and cognition in relapsing-remitting multiple sclerosis. A meta-analysis. Neurology. (2020) 94:e2373–83. doi: 10.1212/WNL.0000000000009522
Keywords: cognitive functions, multiple sclerosis, disease modifying therapies, SDMT, brain atrophy
Citation: Kania K, Ambrosius W, Kozubski W and Kalinowska-Łyszczarz A (2023) The impact of disease modifying therapies on cognitive functions typically impaired in multiple sclerosis patients: a clinician’s review. Front. Neurol. 14:1222574. doi: 10.3389/fneur.2023.1222574
Received: 14 May 2023; Accepted: 28 June 2023;
Published: 12 July 2023.
Edited by:
Douglas Kazutoshi Sato, Pontifical Catholic University of Rio Grande do Sul, BrazilReviewed by:
Brenda Kosaka, Vancouver Coastal Health, CanadaCopyright © 2023 Kania, Ambrosius, Kozubski and Kalinowska-Łyszczarz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karolina Kania, a2Fyb2xpbmEua2FuaWFAdXNrLnBvem5hbi5wbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.