- 1Department of Neurology, Affiliated First Hospital of Hunan Traditional Chinese Medical College, Zhuzhou, Hunan, China
- 2The Third Affiliated Hospital of Hunan University of Chinese Medicine, Zhuzhou, Hunan, China
- 3Department of Neurology, Xiangya Hospital, Central South University, Changsha, Hunan, China
Stiff person syndrome (SPS) is a rare central nervous system disorder associated with malignancies. In this review, we retrieved information from PubMed, up until August 2023, using various search terms and their combinations, including SPS, stiff person syndrome spectrum disorders (SPSSDs), paraneoplastic, cancer, and malignant tumor. Data from peer-reviewed journals printed in English were organized to explain the possible relationships between different carcinomas and SPSSD subtypes, as well as related autoantigens. From literature searching, it was revealed that breast cancer was the most prevalent carcinoma linked to SPSSDs, followed by lung cancer and lymphoma. Furthermore, classic SPS was the most common SPSSD subtype, followed by stiff limb syndrome and progressive encephalomyelitis with rigidity and myoclonus. GAD65 was the most common autoantigen in patients with cancer and SPSSDs, followed by amphiphysin and GlyR. Patients with cancer subtypes might have multiple SPSSD subtypes, and conversely, patients with SPSSD subtypes might have multiple carcinoma subtypes. The first aim of this review was to highlight the complex nature of the relationships among cancers, autoantigens, and SPSSDs as new information in this field continues to be generated globally. The adoption of an open-minded approach to updating information on new cancer subtypes, autoantigens, and SPSSDs is recommended to renew our database. The second aim of this review was to discuss SPS animal models, which will help us to understand the mechanisms underlying the pathogenesis of SPS. In future, elucidating the relationship among cancers, autoantigens, and SPSSDs is critical for the early prediction of cancer and discovery of new therapeutic modalities.
1. Introduction
Stiff person syndrome (SPS) is a rare chronic central nervous system (CNS) disorder (1). The clinical manifestations of SPS encompass a wide range of symptoms, including muscle rigidity, sporadic muscle spasms, and chronic muscle pain. It is also characterized by psychiatric symptoms, such as depression and anxiety, and also other neurological symptoms, including horizontal and vertical supranuclear gaze palsy, nystagmus, increased reflexes, and paroxysmal dysautonomic crisis (2, 3).
Recently, SPS spectrum disorders (SPSSDs) have expanded to include a series of diseases with similar signs and symptoms to those of SPS (4).
SPS is associated with malignancies; however, this is not really well-understood. In this review, we retrieved information from PubMed, up until August 2023, using various search terms and their combinations, including SPS, SPSSDs, paraneoplastic, cancer, and malignant. Data from peer-reviewed journals printed in English were organized to explain the possible relationships between different carcinomas and SPSSD subtypes, as well as related autoantigens. An analysis of the literature search revealed that breast cancer was the most prevalent carcinoma linked to SPSSDs, followed by lung cancer and lymphoma. The first aim of this review highlights the complex nature of the relationships among cancers, autoantigens, and SPSSDs as new information in this field continues to be generated globally. The adoption of an open-minded approach to updating information on new cancer subtypes, autoantigens, and SPSSDs is recommended to renew our database. The second aim of this review was to outline SPS animal models, which will help us to understand the mechanisms of pathogenesis of SPS. In future, elucidating the relationship among cancers, autoantigens, and SPSSDs is critical for the early prediction of cancer and the discovery of new therapeutic modalities.
2. Major clinical characteristics of SPSSDs
SPS was first reported by Moersch and Woltman in 1956 (1). In 1999, Brown et al. published the “Diagnostic Criteria for Classic Stiff-Person Syndrome,” which classified SPS into the following two major subtypes: (1) classic SPS, cases without encephalomyelitis; and (2) SPS plus, cases with encephalomyelitis, such as progressive encephalomyelitis with rigidity and myoclonus (PERM), jerking stiff man syndrome, and stiff limb syndrome (SLS) (5). Currently, SPS includes the following three subtypes: (1) glutamic acid decarboxylase 65 (GAD65)-positive SPS associated with other autoimmune conditions; (2) anti-amphiphysin-positive SPS associated with tumors; and (3) seronegative idiopathic SPS (6).
To date, SPSSDs include the following: (1) partial SPS, limited to extremities and often only one limb (stiff limb syndrome, SLS) or the torso; (2) SPS-plus, with classic SPS symptoms that exist in combination with cerebellar and/or brainstem findings; (3) PERM; and (4) some overlapping syndromes, such as classic SPS with epilepsy or limbic encephalitis (LE) (7), classic SPS with myasthenia gravis (8), classic SPS with anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis (NMDARE-SPS) (9), classic SPS with central sleep apnea (10), and classic SPS with pure red blood cell aplasia (11). Most patients with SPSSD are middle-aged females; however, some patients with SPSSD are either pediatric individuals or adult males. For example, among a total of 22 patients, eight older male patients with SPSSD showed early prominent vestibular and ocular motor dysfunction (4, 12).
Several autoantigens are associated with SPSSD. The major SPSSD autoantibodies are antibodies against GAD, amphiphysin, and glycine receptors for PERM (13). GAD65 is the major autoantibody associated with SPSSD and is linked to classic SPS (4, 14). Other autoantigens, such as glycine receptors (linked to PE RM) (15, 16), amphiphysin (linked to cancers) (17), GABAA receptors (18) and its related protein GABAA receptor-associated protein (GABARAP) (19), dipeptidyl-peptidase-like protein-6 (DPPX), and Zic4 (linked to small-cell lung cancer) (20) are also associated with SPSSD. In addition, SPSSD is associated with breast cancer, small-cell lung cancer, and lymphoma (4, 21). Recently, SPSSD has also been reported to be associated with some rare cancers (21–36). In this review, we summarize the current literature on malignant tumor-related SPSSDs.
3. Clinical characteristics of malignant tumor-related SPSSDs
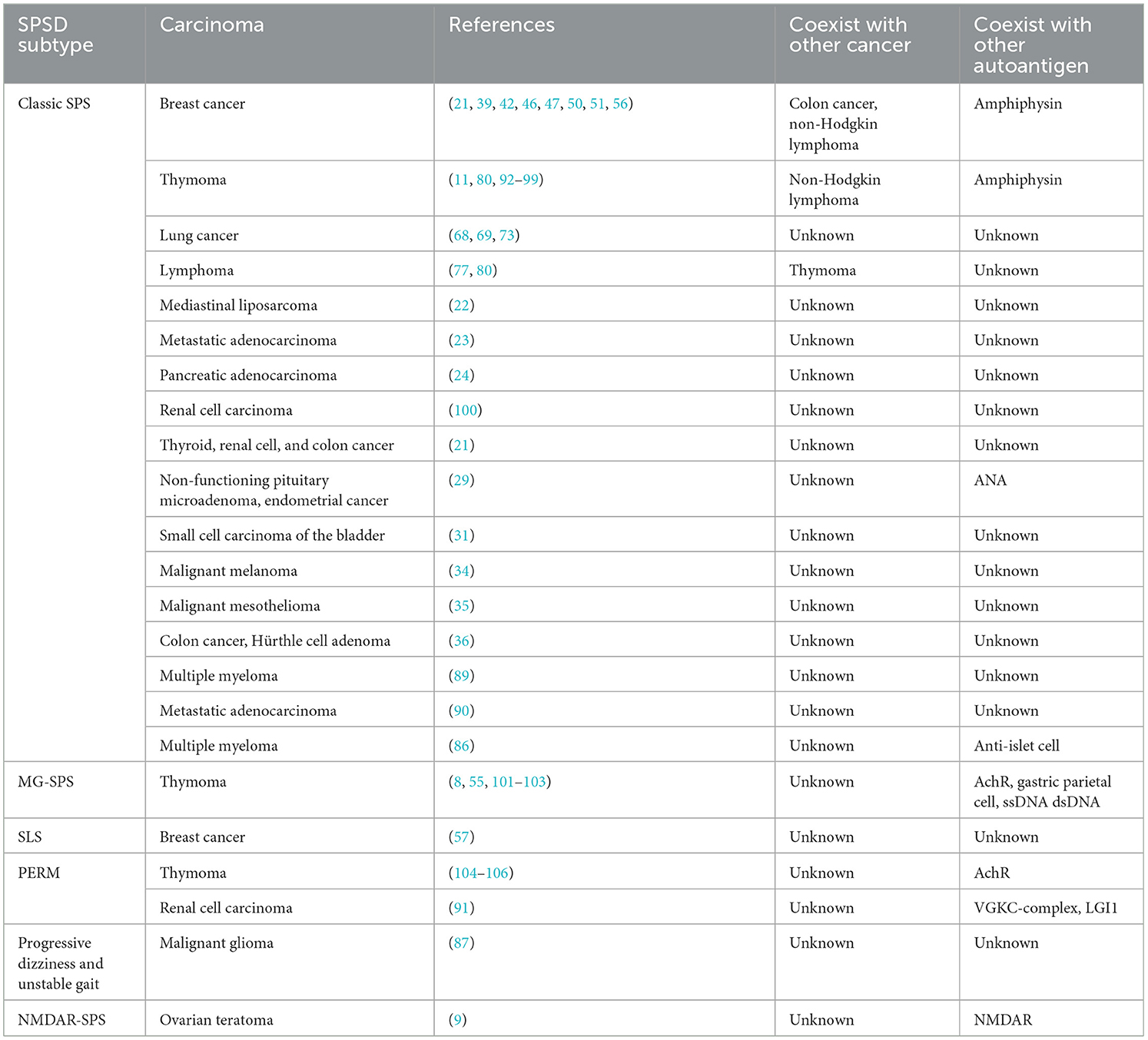
3.1. Breast cancer
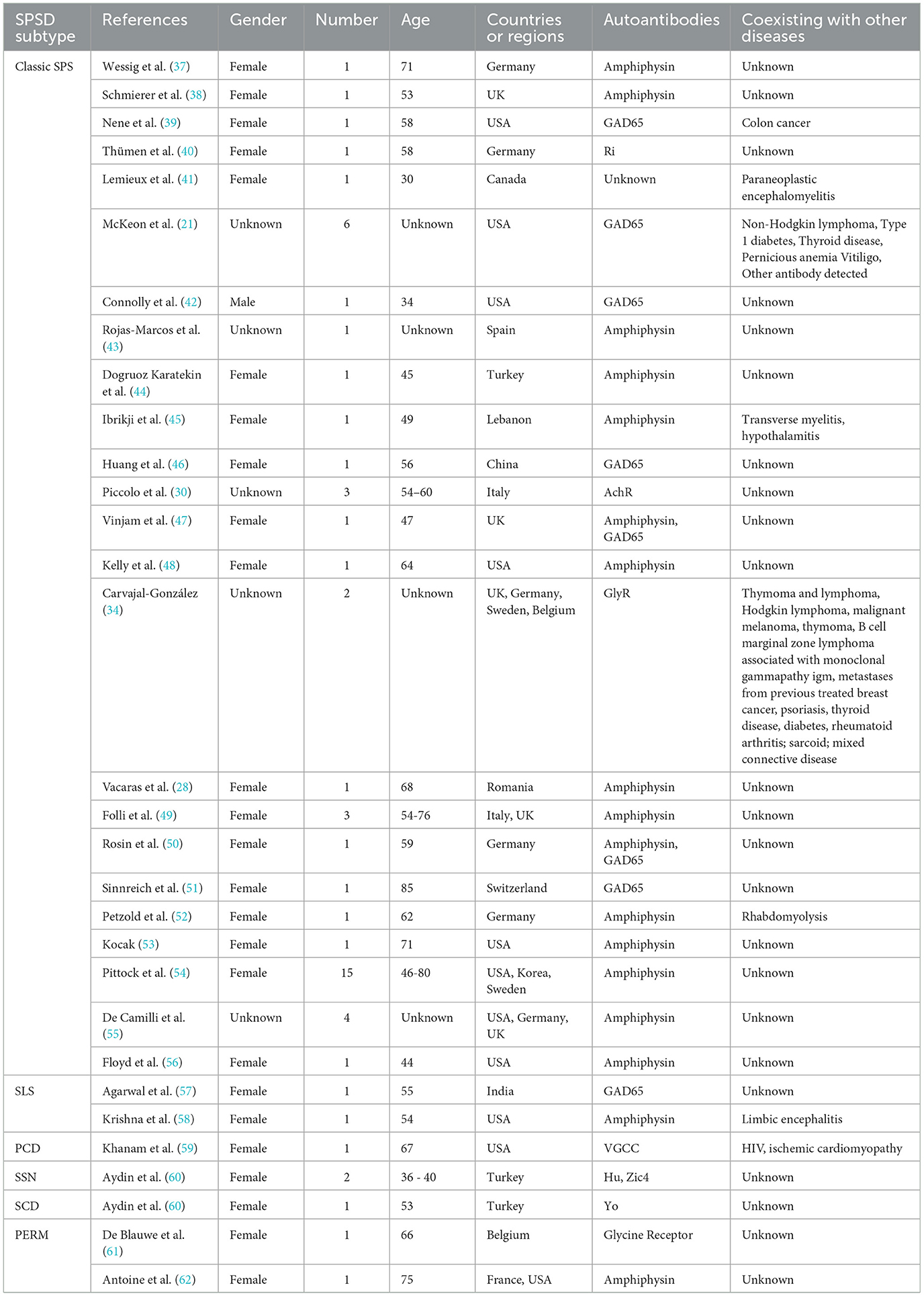
Breast cancer is the most common carcinoma linked to SPSSDs. Table 1 shows that from 29 studies on breast cancer, six SPSSD subtypes, including classic SPS (21, 30, 37–47, 50, 63, 64), SLS (57, 58, 65), paraneoplastic cerebellar degeneration (59), subacute sensory neuronopathy, subacute cerebellar degeneration (60), and PERM (61), among which classic SPS is the major SPSSD subtype, were found to be involved. Patients with breast cancer and PSSD were determined to have other carcinomas, such as colon cancer, non-Hodgkin lymphoma, thymoma and lymphoma, and malignant melanoma (21, 34, 39, 41). Furthermore, patients with breast cancer and SPSSD were found to have other diseases, including autoimmune diseases, such as paraneoplastic encephalomyelitis, type 1 diabetes, thyroid disease, pernicious anemia, vertigo, psoriasis, thyroid disease, rheumatoid arthritis, sarcoidosis, mixed connective disease, limbic encephalitis, myelopathy, HIV, and ischemic cardiomyopathy (21, 34, 41, 45, 58, 59, 65). Amphiphysin (55) is the most common autoantigen in patients with breast cancer and SPSSD, followed by GAD65, Ri, acetylcholine receptor (AChR), and glycine receptor (GlyR). Notably, Connolly et al. reported a 53-year-old male patient with breast cancer and classic SPS who harbored the GAD65 autoantibody (42).
3.2. Lung cancer
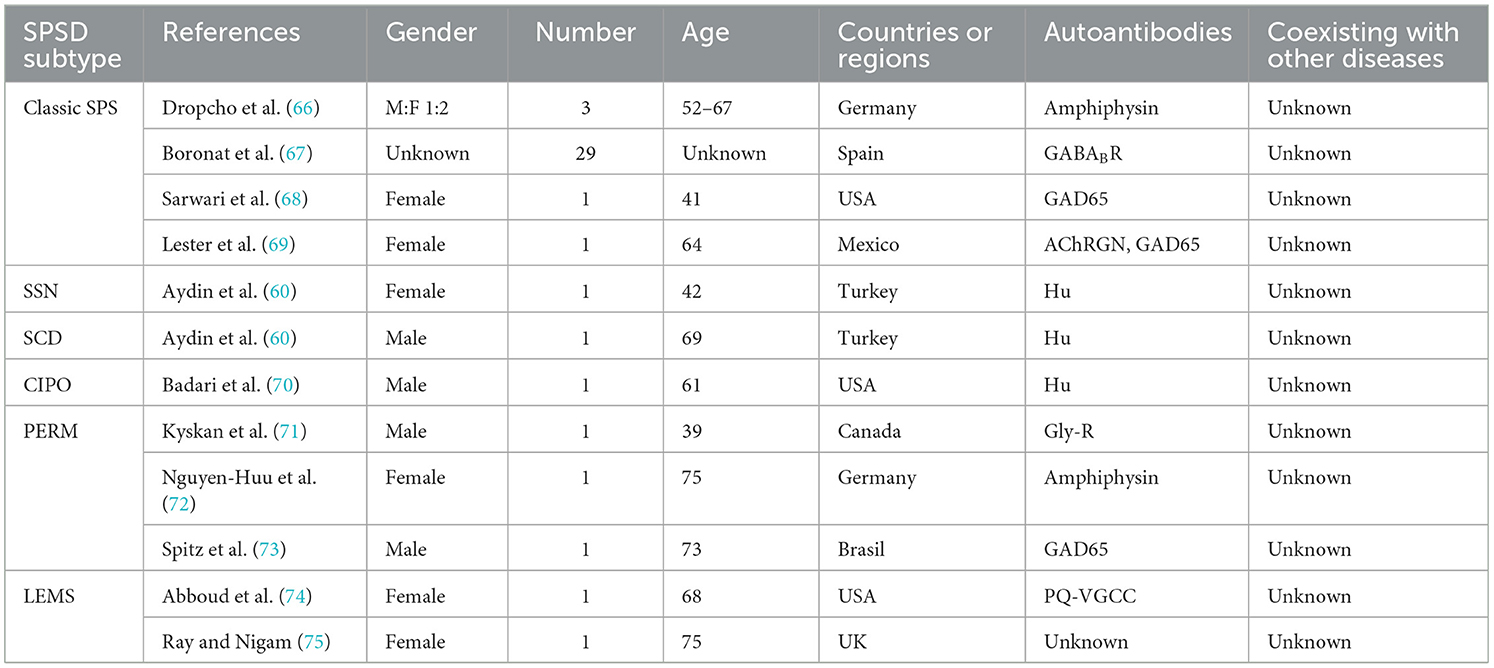
Lung cancer has also been linked to SPSSD. This has been reported in nine studies, involving six SPSSD subtypes, including classic SPS (66–69), subacute sensory neuronopathy, subacute cerebellar degeneration (60), paraneoplastic neurologic syndromes (70), and PERM (71–73) (Table 2). Sinha et al. reported that thymoma coexists with lung cancer and SPSSD (76). Table 2 shows that GAD65 is the most common autoantigen reported in patients with lung cancer and SPSSD, followed by amphiphysin, GABABR, and Hu.
3.3. Lymphoma and similar hematological carcinomas
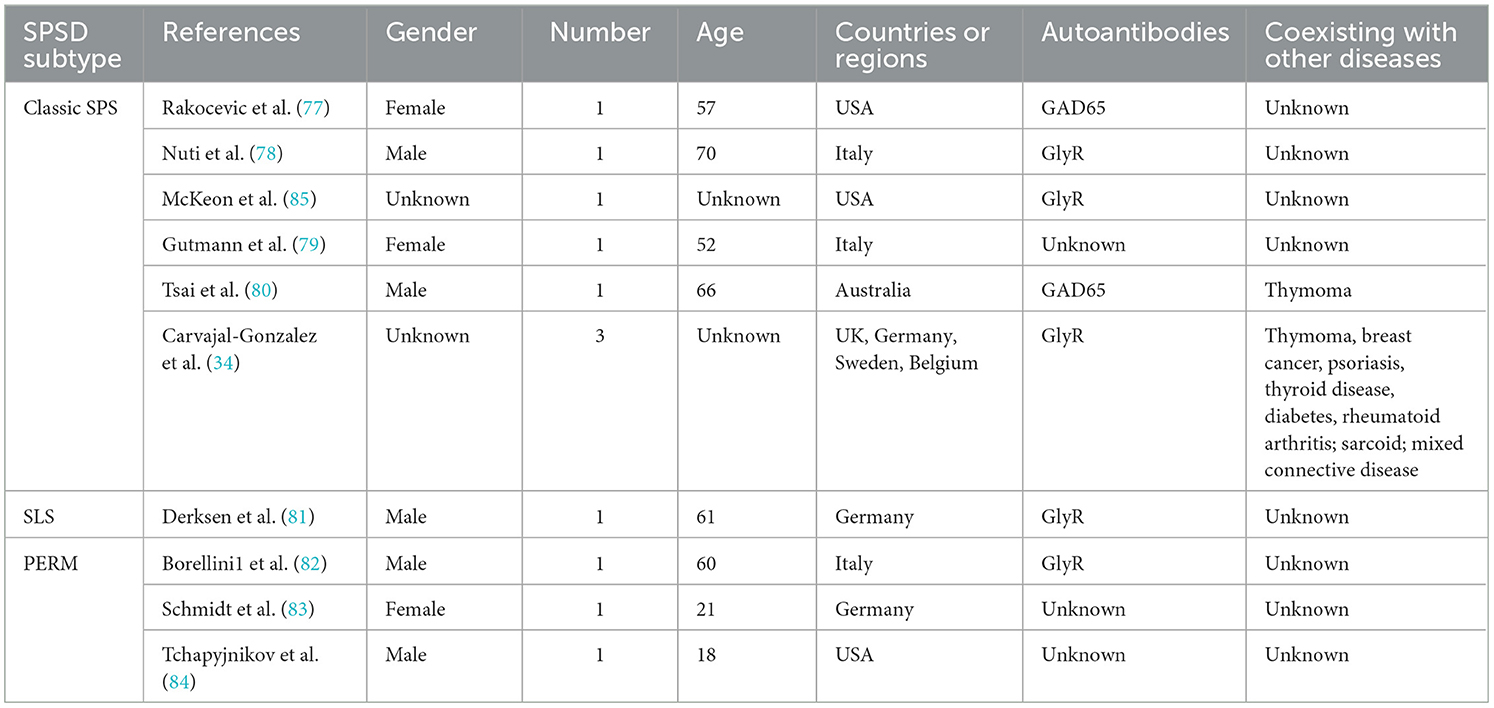
Lymphoma and similar hematological carcinomas have been reported to be associated with SPSSD. In total, 10 studies involving three SPSSD subtypes, such as classic SPS (21, 34, 77–80), SLS (81), and PERM (82–84), have been reported (Table 3). Some authors have reported the coexistence of thymoma and breast cancer with lymphoma and SPSSD (34, 80). Table 3 shows that GlyR is the most commonly reported autoantigen in patients with lymphoma and similar hematological carcinomas and SPSSD, followed by GAD65, PCA-1, PCA-Tr, and striational antibodies.
3.4. Other carcinomas
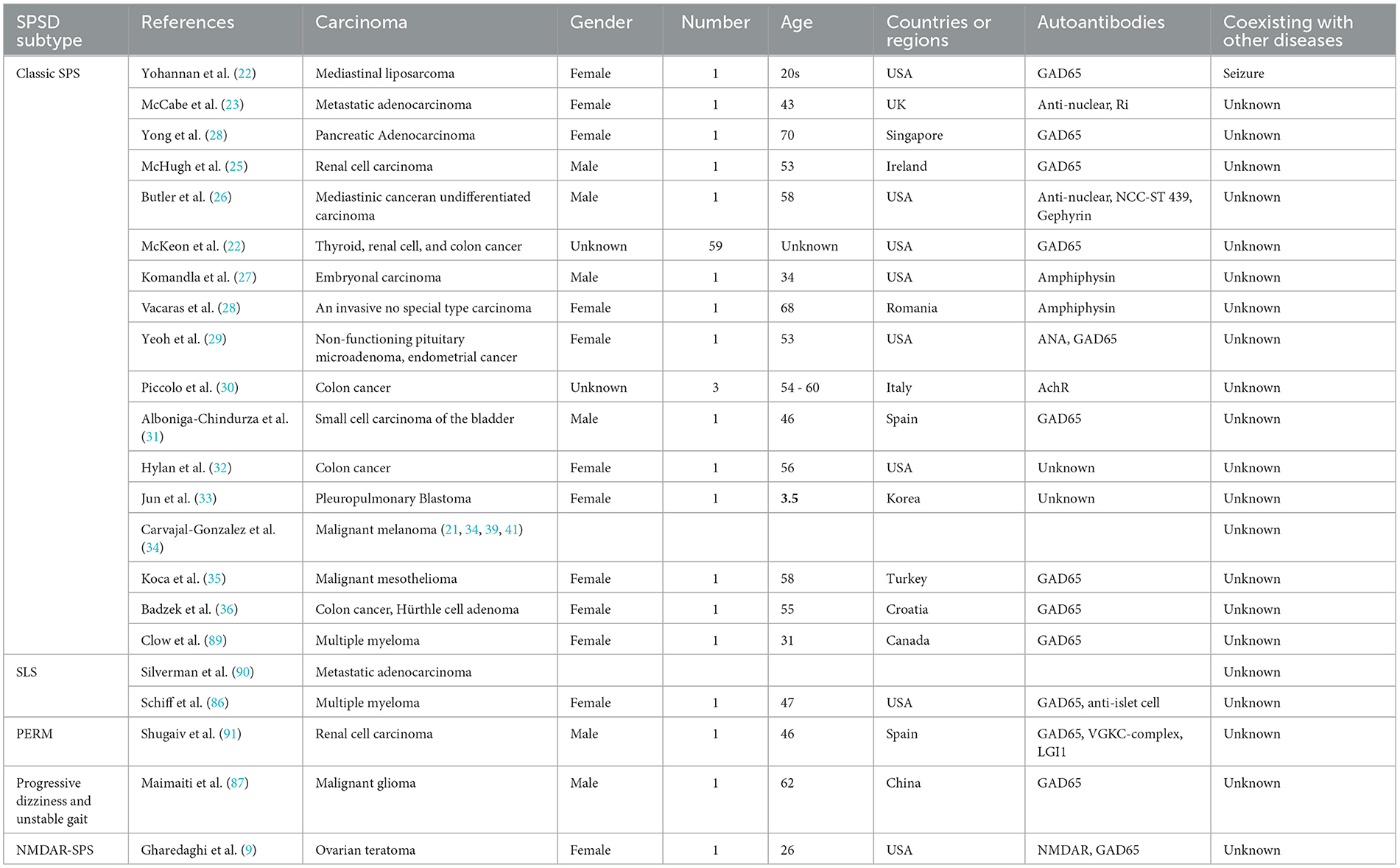
SPSSD is also associated with other carcinomas, such as mediastinal liposarcoma (22), metastatic adenocarcinoma (23), pancreatic adenocarcinoma (24), renal cell carcinoma (25), mediastinal cancer, undifferentiated carcinoma of an undetermined origin (26), multiple myeloma (86), embryonal carcinoma (27), malignant glioma (87), ovarian adenocarcinoma (88), prostate carcinoma (88), testicular seminoma and germ cell neoplasia (88), pancreatic cancer (88), melanoma (88), invasive carcinoma of no special type (28), ovarian teratoma (9), small cell carcinoma of the bladder (31), pleuropulmonary blastoma (33), malignant mesothelioma (35), colon cancer, and Hürthle cell adenoma (36). It is also associated with overlapping cancers, such as breast cancer with colon cancer (30, 39), chronic lymphocytic leukemia (81), thymoma and non-Hodgkin lymphoma (80), non-functioning pituitary microadenoma, and endometrial cancer (29) (Table 4). Table 5 shows the other carcinomas included in 25 studies involving six SPSSD subtypes, namely, classic SPS (21–36), SLS (86, 90), PERM (91), progressive dizziness and unstable gait (87), and NMDAR-SPS (9). Furthermore, thyroid and renal cell cancers reportedly coexist with colon cancer and SPSSD (21). Table 4 shows that GAD65 is the most common autoantigen in patients with other carcinomas and SPSSD, followed by anti-nuclear, Ri, NCC-ST 439, amphiphysin, gephyrin, AchR, anti-islet cell, VGKC-complex, and LGI1 antigens.
4. Possible mechanisms of paraneoplastic SPSSD
As we believe that autoantigens might be good candidates for determining the possible mechanism underlying paraneoplastic SPSSD, we have summarized the detailed information on autoantigens, including GAD and amphiphysin, followed by GlyR, gephyrin, anti-islet cell, and LGI1 (please see Supplementary Table 1).
4.1. GAD
4.1.1. GAD isoform
GAD is predominantly expressed in neurons, which might be linked to SPSSD, and insulin-secreting pancreatic β cells, which might be linked to type I diabetes (107). GAD regulates the decarboxylation of glutamate to gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter within the CNS, and is related to SPSSD (108, 109). There are two GAD isoforms—GAD65 and GAD67. GAD65 is expressed in the presynaptic end of nerve terminals in its inactive form and is converted to its active form at the post-natal stage to rapidly synthesize GABA for synaptic transmission (110). GAD65 is also responsible for packaging GABA after its synthesis (111). Additionally, GAD67 is expressed in the cell body and dendrites and is responsible for synthesizing basal levels of GABA (100).
GAD65 antibody (Ab) titers and epitope specificities are present in different diseases and different subtypes of SPSSD (112, 113). For example, the GAD65 Ab titer is 348 U/mL in type I diabetes, 6.0 × 105 U/mL in cerebellar ataxia (CA), 6.2 × 105 U/mL in LE, and 1.1 × 106 U/mL in SPS (112). GAD65 binding in the presence of rFab b78 is 99% in type I diabetes, 81% in CA, 88% in LE, and 77% in SPS (112). Moreover, positive GAD immunoreactivity is ≥1,800 U/mL in SPS rat brain sections as detected via immunohistochemistry or cell-based assays (7, 114). A high range of GAD65 Ab levels is associated with SPS, whereas a lower one is associated with type I diabetes (13). A possible mechanism underlying this distinction could be that the GAD Ab in type I diabetes primarily reacts with conformational epitopes, whereas GAD antibodies in SPS recognize linear epitopes (115–117). Furthermore, GAD Ab-positive type I diabetes or SPS, CA, and LE are associated with different HLA class II haplotypes (118–121).
4.1.2. Decreased GABAergic activity is the major physiopathological mechanism of SPSSD
GABAergic neurons are responsible for inhibitory signals in the CNS and express high levels of GAD65. They are mainly located in the hippocampus, cerebellum, basal ganglia, brainstem nuclei, and spinal gray matter (122, 123). GABA binds GABAA and GABAB receptors to mediate the hyperpolarization of post-synaptic neurons, comprising an inhibitory signal (124, 125). The GAD Ab inhibits GAD65 to block GABA synthesis, thereby reducing the uptake of newly synthesized GABA in synaptic vesicles and its synaptic release (111, 126–128).
Inhibiting GABA synthesis results in decreased GABAergic transmission, which is linked to neuronal hyperexcitability and is the core pathophysiological mechanism in SPS (129, 130). For example, the possible mechanism underlying SPS might be mediated by the inhibition of GABAergic neurons in the spinal cord, resulting in a state of motor neuron hyperexcitability, ultimately causing the simultaneous contraction of agonist and antagonist muscles (100, 131). GABAergic interneurons are located at different levels of the CNS, other than the spinal cord, leading to other subtypes of SPSSD, such as PERM (128), which has also been supported by animal studies (132, 133). However, this is not the case for LE and temporal lobe epilepsy, owing to insufficient data.
4.1.3. Association with carcinomas or SPSSD Subtypes
The major SPSSD subtype is classic SPS (11, 68, 69, 73, 77, 80, 92–98, 134, 135), followed by SPS with myasthenia gravis (21–25, 29, 31, 34–36, 86, 89, 90, 135, 136), PERM (91), SLS (57), progressive dizziness and unstable gait (87), and NMDAR-SPS (9), as shown in Table 5. Moreover, the major carcinoma associated with SPSSD subtypes is breast cancer (21, 39, 42, 47, 50, 51, 56, 57, 137), followed by thymoma (11, 80, 91–98, 104–106, 134, 135), lymphoma (77, 80), lung cancer (68, 69, 73), and other carcinomas (21–25, 29, 31, 34–36, 86, 89, 90, 135, 136).
4.1.4. Titer of anti-GAD65 Ab in the serum vs. the cerebrospinal fluid of patients with SPSSD
One report showed that the median concentration of anti-GAD65 Ab, measured via ELISA, is 30-fold higher in the serum (74,700 IU/mL) than in the cerebrospinal fluid (CSF) (2,430 IU/mL). However, these data were from 34 patients with classical anti-GAD65-associated syndromes, including SPS, CA, chronic epilepsy, and LE, with overlapping syndromes in some of the cases (138). The serum/CSF ratio of anti-GAD65 Ab was reported to be approximately 20 in patients with SPS (138). Moreover, serum and CSF anti-GAD65 Ab titers decreased, with those of CSF decreasing more rapidly than serum titers after patients with SPS received immunotherapy (138).
4.2. Amphiphysin
4.2.1. Amphiphysin superfamily
Amphiphysins are members of the Bin-Amphiphysin-Rvsp (BAR) family of proteins, which includes the mammalian bridging-integrators (Bin1 and Bin2), amphiphysins, and yeast Rvs161p and Rvs167p (139). Some members of the amphiphysin superfamily have conserved BAR domains, mainly in the N-terminus, and an SH3 domain in the C-terminus (139). Amphiphysin I is expressed in chicken and mammalian brains (140) and is associated with SPS and breast cancer (49, 55). Two members of amphiphysin II are also expressed in the brain. Amphiphysin II, also known as BIN1 (MYC box-dependent interacting protein-1 or bridging integrator-1) or SH3P9, is associated with cancer progression, several myopathies, heart failure, and late-onset Alzheimer's disease (141). Amphiphysin IIa shares a brain-specific domain with amphiphysin I (142, 143), and amphiphysin IIb has a skeletal muscle-specific domain with a tumor suppressor that interacts with the c-Myc oncoprotein (142, 144). In several cancers, such as breast, colon, prostate, and lung cancers, as well as hepatocarcinoma and neuroblastoma, the expression of amphiphysin II is reduced or altered (145–148). In addition, the ablation of amphiphysin II is linked to a poor cancer prognosis and increased metastasis (145, 148–151). Amphiphysin II can also inhibit Myc-dependent transformation and tumorigenesis (145, 148–151).
4.2.2. Association with carcinomas or SPSSD subtypes
The major SPSSD subtype is classic SPS (28, 37, 38, 43–45, 47–50, 52–56, 135), followed by SLS (58, 65) and PERM (62, 72). Moreover, the major carcinoma associated with SPSSD is breast cancer (28, 37, 38, 43–45, 47–50, 52–56, 58, 135), followed by thymoma (66, 95, 99) and lung cancer (66, 72) (Table 6).
4.3. Glycine receptors
4.3.1. Biological studies on GlyR
As an inhibitory neurotransmitter, glycine, as well as its receptor (GlyR), is critical for CNS development (152). Glycine is synthesized via serine hydroxymethyl transferase or a glycine synthase (glycine cleavage, GCS) enzyme, located between carbon dioxide, ammonium ion, N5, N10-methylene tetrahydrofolate, NADH, and a proton, producing glycine, tetrahydrofolate, and NAD+ (153), as confirmed from a rat study (154). Furthermore, the biological function of glycine requires specific transporters such as GlyT1 (glial cells) and GlyT2 (neurons) (155, 156). GlyT1 also regulates glutamatergic neurotransmission through NMDA receptors, affecting brain function and diseases (157).
There are four α subunits and one β unit in GlyR, and these are expressed in the spinal cord and retina, respectively (158–160). Microglia secrete glycine, enhance NMDA receptor-mediated responses (161), and express GlyR to induce membrane depolarization, increasing intracellular calcium and proliferation (162). In addition, glial cells modulate synaptic development by participating in the induction of the action potential conduction in white matter via GlyRs (163). Importantly, glycine has also been linked to rapid cancer cell proliferation due to glycine metabolism (164). For example, α1 and α3 GlyR subunits were found to be expressed in human brain tumor biopsies, and the lack of α1 GlyR protein expression resulted in inhibition of the self-renewal capacity and tumorigenicity of GL261 glioma cells (165). GlyR knockdown can increase P53 tumor suppressor protein expression (166, 167).
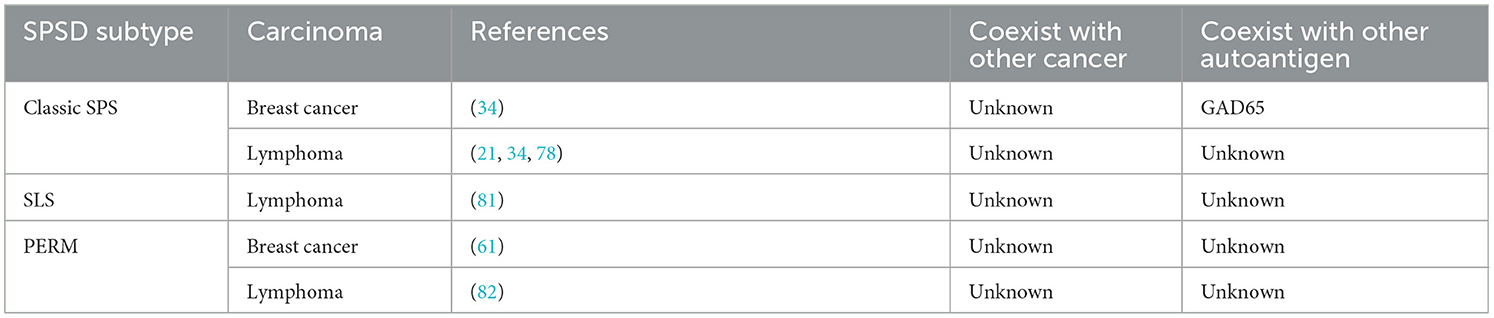
4.4. Association with carcinomas or SPSSD Subtypes
The major associated SPSSD subtype has been reported to be classic SPS (34, 78, 85), followed by SLS (61) and PERM (61, 82) (Table 7). Moreover, the major associated carcinoma is lymphoma (34, 61, 78, 85), followed by breast cancer (34, 61).
5. Clinical characteristics of malignant tumor-related SPSSDs
5.1. Breast Cancer
Breast cancer is the most common carcinoma linked to SPSSDs. Table 1 shows that from 29 studies on breast cancer, six SPSSD subtypes, including classic SPS (21, 30, 37–47, 50, 63, 64), SLS (57, 58, 65), paraneoplastic cerebellar degeneration (59), subacute sensory neuronopathy, subacute cerebellar degeneration (60), and PERM (61), among which classic SPS is the major SPSSD subtype, were found to be involved. Patients with breast cancer and PSSD were determined to have other carcinomas, such as colon cancer, non-Hodgkin lymphoma, thymoma and lymphoma, and malignant melanoma (21, 34, 39, 41). Furthermore, patients with breast cancer and SPSSD were found to have other diseases, including autoimmune diseases, such as paraneoplastic encephalomyelitis, type 1 diabetes, thyroid disease, pernicious anemia, vertigo, psoriasis, thyroid disease, rheumatoid arthritis, sarcoidosis, mixed connective disease, limbic encephalitis, myelopathy, HIV, and ischemic cardiomyopathy (21, 34, 41, 45, 58, 59, 65). Amphiphysin (55) is the most common autoantigen in patients with breast cancer and SPSSD, followed by GAD65, Ri, acetylcholine receptor (AChR), and glycine receptor (GlyR). Notably, Connolly et al. reported a 53-year-old male patient with breast cancer and classic SPS who harbored the GAD65 autoantibody (42).
5.2. Lung cancer
Lung cancer has also been linked to SPSSD. This has been reported in nine studies, involving six SPSSD subtypes, including classic SPS (66–69), subacute sensory neuronopathy, subacute cerebellar degeneration (60), paraneoplastic neurologic syndromes (70), and PERM (71–73) (Table 2). Sinha et al. reported that thymoma coexists with lung cancer and SPSSD (76). Table 2 shows that GAD65 is the most common autoantigen reported in patients with lung cancer and SPSSD, followed by amphiphysin, GABABR, and Hu.
5.3. Lymphoma and similar hematological carcinomas
Lymphoma and similar hematological carcinomas have been reported to be associated with SPSSD. In total, 10 studies involving three SPSSD subtypes, such as classic SPS (21, 34, 77–80), SLS (81), and PERM (82–84), have been reported (Table 3). Some authors have reported the coexistence of thymoma and breast cancer with lymphoma and SPSSD (34, 80). Table 3 shows that GlyR is the most commonly reported autoantigen in patients with lymphoma and similar hematological carcinomas and SPSSD, followed by GAD65, PCA-1, PCA-Tr, and striational antibodies.
5.4. Other carcinomas
SPSSD is also associated with other carcinomas, such as mediastinal liposarcoma (22), metastatic adenocarcinoma (23), pancreatic adenocarcinoma (24), renal cell carcinoma (25), mediastinal cancer, undifferentiated carcinoma of an undetermined origin (26), multiple myeloma (86), embryonal carcinoma (27), malignant glioma (87), ovarian adenocarcinoma (88), prostate carcinoma (88), testicular seminoma and germ cell neoplasia (88), pancreatic cancer (88), melanoma (88), an invasive carcinoma of no special type (28), ovarian teratoma (9), small cell carcinoma of the bladder (31), pleuropulmonary blastoma (33), malignant mesothelioma (35), colon cancer, and Hürthle cell adenoma (36). It is also associated with overlapping cancers, such as breast cancer with colon cancer (30, 39), chronic lymphocytic leukemia (81), thymoma and non-Hodgkin lymphoma (80), non-functioning pituitary microadenoma, and endometrial cancer (29) (Table 4). Table 5 shows the other carcinomas included in 25 studies involving six SPSSD subtypes, namely, classic SPS (21–36), SLS (86, 90), PERM (91), progressive dizziness and unstable gait (87), and NMDAR-SPS (9). Furthermore, thyroid and renal cell cancers reportedly coexist with colon cancer and SPSSD (21). Table 4 shows that GAD65 is the most common autoantigen in patients with other carcinomas and SPSSD, followed by anti-nuclear, Ri, NCC-ST 439, amphiphysin, gephyrin, AchR, anti-islet cell, VGKC-complex, and LGI1 antigens.
6. Treatment and outcomes of paraneoplastic SPSSD
For patients with paraneoplastic SPSSD, the carcinoma is typically detected and identified prior to treatment while concurrently managing and addressing symptoms.
6.1. GABAergic therapy
In patients with SPSSD, antibodies attack the GAD enzyme, which is essential for GABA production. Therefore, drugs targeting GABAergic neurons can be effective in treating SPSSD; by inhibiting the attack on GAD, GABA levels are reduced (168).
6.1.1. Benzodiazepines
Benzodiazepines are the first-line treatment for patients with SPS. These drugs enhance the neurotransmitter effect of GABA at its receptor. Furthermore, benzodiazepines are widely used for their sedative, muscle-relaxant, and anticonvulsant effects (21). Long-term benzodiazepine therapy has been shown to benefit patients with classic or partial SPS and reduce GAD-65-positive Ab-mediated stiffness and spasm symptoms; however, this improvement might also be due to other adjunct medications.
The major drug for SPSSD treatment is diazepam, which results in a good response in most patients at high doses of up to 60 mg daily (169). However, owing to concerns about withdrawal from long-term use and high doses of diazepam therapy, tizanidine has emerged as a good candidate for alternative therapy. As an NMDAR, tizanidine is an α2 inhibitor that inhibits glutamate release and prevents glutamatergic hyperactivity, thereby resolving convulsions in patients with SMS. Nevertheless, the dose of tizanidine should be individualized (21, 169).
6.1.2. Baclofen
Baclofen is an agonist of GABA type B receptors that inhibits reflexive muscle contraction by blocking the release of excitatory neurotransmitters through voltage-gated calcium channels (170). It is also a second-line therapy for patients with SPS. However, to date, the use of oral baclofen therapies is still being debated. In one report, high doses (however, the dose is unknown) of oral baclofen therapy were found to result in serious side effects, such as sedation and respiratory depression (171). However, oral baclofen had good effects on SPS patients without serious side effects. For example, oral baclofen therapy (5 mg, three times per day) plus clonazepam resulted in improvements in a 69-year-old man with SPS and amphiphysin antibodies (172). Symptomatic treatment initiated with oral clonazepam and baclofen (5 mg Bid), followed by intravenous immunoglobulin (IVIG) resulted in improvements in a 60-year-old man with SPS associated with critical illness polyneuropathy (173). Baclofen (30 mg/day) combined with oral diazepam and steroids resulted in improvements in a 55-year-old GAD-Ab-positive female patient with SLS and breast carcinoma (57). For childhood-onset SMS, three SMS patients had good clinical responses with oral baclofen (dose range, 60–80 mg) combined with diazepam, IVIG, plasma exchange or dantrolene, and botulinum toxin (174).
Alternatively, intrathecal therapy is an effective route for baclofen treatment (2). The chronic infusion of intrathecal baclofen can improve SPS patient outcomes, including the pain Numeric Rating Scale, Spasm Frequency Scale, and lower extremity Modified Ashworth Scale (171). Intrathecal baclofen (100 μg) followed by a rehabilitation program resulted in substantial clinical and functional improvements in a 59-year-old female SPS patient, who had no therapeutic response with oral benzodiazepines and botulinum toxin injections (175). In addition, intrathecal baclofen (started from 50 μg/d up to 100 μg/d) improved motor functions in a 48-year-old male GAD-negative SPS patient (176). Baclofen can be used to effectively treat SPS because it is a direct agonist of GABA-B receptors and does not require endogenous GABA to induce presynaptic inhibition (176).
6.1.3. Levetiracetam
Levetiracetam binds to synaptic vesicle glycoprotein 2A (SV2A), resulting in the release of the neurotransmitter stored within the vesicle, rapidly inhibiting firing neurons and potassium and N-type calcium channels (177, 178). In a previous study, three patients with high anti-GAD65 Ab levels did not respond satisfactorily to IVIG and diazepam treatment with or without plasmapheresis (179). These patients were treated with 500 mg oral levetiracetam twice daily, which improved axial rigidity and the disappearance of paroxysmal respiratory arrest within 3 days of therapy initiation, with markedly reduced leg stiffness and ameliorated walking difficulties (179). However, to date, there is no evidence of the effects of long-term levetiracetam therapy. The possible mechanism by which levetiracetam achieves its effects could be by stabilizing and strengthening GABAA and decreasing hyperexcitability in spinal cord neurons (179).
6.1.3. Pregabalin
Structurally, pregabalin is classified as a GABA analog or gabapentinoid (180). In a previous study, a female patient with SMS who did not respond to diazepam treatment, owing to excessive sedation, was successfully treated with a 3-month pregabalin regimen (181). The possible mechanism underlying the effects of pregabalin might be the inhibition of calcium influx and subsequent release of excitatory neurotransmitters, including glutamate and norepinephrine, resulting in compensation for the imbalance between inhibitory and excitatory intracortical circuits (181).
6.1.4. Propofol
The mechanism of action of propofol in the CNS is unclear. Propofol might enhance the function of GABA receptors, evoking the chloride current in central neurons at clinically relevant concentrations, ultimately activating the GABA receptor–chloride ionophore complex (182). Notably, a low dose of propofol improves symptoms in patients with SPS who do not respond to high-dose benzodiazepines, baclofen, corticosteroids, levetiracetam, IVIG, or IV ethanol. Furthermore, propofol is effective for patients with SMS that is refractory to therapy (183). Unfortunately, long-term propofol therapy has unsatisfactory effects in patients with SPS (184).
6.2. Immunotherapy
6.2.1. Rituximab
Rituximab binds to the CD20 antigen on mature B cells, leading to B cell lysis, while sparing precursor B cells. Rituximab improves SPS and other neurological autoimmune disorders, such as Devic's disease, myasthenia gravis, autoimmune neuropathies, and inflammatory myopathies (185). SPS is associated with elevated titers of anti-GAD65 Abs and glycine receptor α-subunits in patients (186). Four reports have demonstrated the benefits of rituximab for patients with SPS (186–189). Although rituximab improved the clinical conditions of patients, the decrease in the anti-GAD titer was inconsistent in different reports. Some reports demonstrated that after rituximab treatment, the anti-GAD titer was rapidly (17 days, from positive to undetectable) or slowly (1 year, from 1,000 to 400 U/mL) reduced (187). However, another case report showed that the anti-GAD Ab titer remained elevated, even during treatment with rituximab (188).
6.2.2. Tacrolimus
Tacrolimus inhibits the calcium calcineurin pathway and exerts its immunosuppressive effect by reducing the proliferation of activated T cells (190). Furthermore, tacrolimus decreases IL-2 levels and impairs T-helper cell functions, finally reducing the activation of B cells to produce antibodies. It also suppresses the function of anti-GAD Abs, thereby blocking GABAergic neurotransmission and interfering with GABA synthesis (191). Tacrolimus directly blocks calcineurin in the GABAergic inhibitory system. Nonetheless, the neuroprotective effect of tacrolimus therapy on SPS demonstrated based on the reduced density of neurons with somal areas and improved pathological conditions, remains debatable (192); evidence that macrolide antibiotics inhibit the function of immunophilins and provide neuroprotective and neuroregenerative effects contradicts this assertion (184). Tacrolimus combined with IVIG or prednisone treatment greatly improved symptoms and reduced Ab titers in two patients who showed no response to other medicines (192). After 4 weeks of treatment with tacrolimus, serum anti-GAD Ab titers in patients with SPS were decreased, with an increase in motor ability, and the patients became completely self-dependent (191).
6.3. IVIG therapy
IVIG is the initial immunomodulator for patients with SPS with severe symptoms or unsatisfactory symptom improvements on other medications (193). IVIG therapy for patients with SPS partially improves symptoms (193) or the patient quality of life (194). It is also safe, with the duration of improvement being 6 weeks to 1 year (2).
6.4. Plasma exchange (plasmapheresis) therapy
Plasma exchange therapy is an option for patients with SPS who have failed to respond to other treatments (194). Plasmapheresis is usually conducted in one cycle with five sessions of plasma exchange. In a previous study, plasma exchange was used to treat two patients with SPS who had failed to respond to other treatments, resulting in improved symptoms and increased anti-GAD levels (195). Albahra et al. reported that among 10 patients with SPS, three had completely resolved symptoms, whereas seven had only partially relieved symptoms (196).
The outcomes of SPS treatment were reported to vary, resulting in a large range of improvements and moderate walking disability (21, 118). Limited reports have shown that patients with CA undergoing treatment have exhibited considerable improvements when assessed using the modified Rankin score. However, walking disability was still observed (197). Unfortunately, there were only modest outcomes for patients with LE following treatment (110, 138, 198–200), with symptoms, such as seizures and cognitive impairment, remaining (199).
6.5. Changes in autoantibody titers after treatment
6.5.1. Anti-GAD65
After immune globulin therapy, 11 patients with SPS showed improvements in their movement disorder and decreased serum anti-GAD65 Ab titers (169). As we previously described, serum and CSF anti-GAD65 Ab titers were found to decrease, with those of CSF decreasing more rapidly than those of serum after patients with SPS received immunotherapy (138).
6.5.2. Ovarian teratoma
A 26-year-old woman with anti-NMDAR encephalitis and SPS with an ovarian teratoma was successfully treated via laparoscopic removal of the ovarian tumor. She received immune-suppressant medications (methylprednisolone followed by a combination with baclofen) preoperatively and postoperatively, and her symptoms were gradually resolved (9).
6.5.3. Breast cancer
A 53-year-old male patient had anti-amphiphysin-positive SPS and breast cancer, as previously mentioned. After undergoing surgery to excise the cancer, he received adjuvant chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil, followed by post-mastectomy radiation and adjuvant endocrine therapy with tamoxifen. After 1 year of surgery, the stiffness in his upper extremity, but not his lower extremities, greatly improved (42). However, for a 30-year-old female patient with anti-amphiphysin, GAD Ab-negative SPS, and breast cancer symptoms were not alleviated following surgery (41).
6.5.4. Lung cancer
A 56-year-old woman with anti-amphiphysin-positive SPS associated with small-cell lung cancer received treatment with benzodiazepines and corticosteroids, followed by cancer therapy with cisplatin/etoposide and radiotherapy. Following treatment, she exhibited signs of improved stiffness and was able to walk independently for short distances (72).
7. Animal models of SPSSD
There are some reports of SPS animal models (13, 113, 114, 201–209). For example, the animal models of anti-GAD65 SPS comprise two major types, specifically an in vitro animal tissue model and an in vivo animal model (13). Some studies have focused on in vitro (113, 114, 201–209) and in vivo SPS animal models (13, 132, 208, 210–213). Unfortunately, the results of these studies were not satisfactory, and further development is needed.
7.1. In vitro animal tissue studies
In vitro SPSSD studies are usually divided into three assays, enzymatic assays, whole-cell patch clamp recordings, and immunofluorescence-using cultures. The major samples in studies using enzymatic assays have been rat pancreatic islet extracts (201), crude rat cerebellar extracts (202), and recombinant human GAD65 (113). These studies demonstrated that high titers of GAD Abs are associated with SPS, whereas few cases (2/12) of high GAD Ab titers were reported in type I diabetes (202). Furthermore, the studies revealed that GAD65 can recognize conformational epitopes in the C-terminus (113).
The major samples for studies using whole-cell patch clamp recordings have been rat cerebellar slices (203, 204), rat hippocampal neurons (205), mouse hippocampal neurons (206), and rat hippocampal slices (207). These studies revealed that presynaptic GABAergic transmission is inhibited by GAD Abs in the CSF of patients with SPS and selectively suppressed (203, 204). In addition, these studies demonstrated that post-synaptic inhibitory potentials are increased by GAD-positive epileptic serum (205) but not by serum from patients with GAD65 Ab-associated LE (206) or with GAD65 Ab-associated epilepsy (206, 207). The major sample for studies using immunofluorescence based on cultures has been rat hippocampal neurons (114, 208, 209). These studies found that GAD Abs from some patients with SPS do not bind to the neuronal surface or that GAD Abs are not internalized by live neurons, suggesting the presence of other Abs specific to unknown antigens, rather than GAD (13).
7.2. In vivo animal model
The two major reported types of in vivo SPSSD animal models are passive transfer animal models, where transfer is induced using the serum or CSF antibodies from patients with SPS, and active immunized animal models induced using the human GAD65 protein (13).
7.2.1. Passive transfer animal model
The main reported methods for passive transfer animal models using rats or mice are single cerebellar or paraspinal injections (132) and intrathecal (210, 211) or intraperitoneal injections (208, 211). Unfortunately, these animal models do not effectively mimic the clinical symptoms of SPS. However, some symptoms, such as paraspinal electrophysiological evidence of continuous motor activity (132), increased anxiety-like behavior (212), worsened rotarod results, and deficits in postural control (211), were partially matched.
7.2.2. Animal model of active immunization
Active immunization using the human GAD65 protein has been effectively performed in type I diabetes studies; however, it has failed for neurologic diseases, including SPS, despite the high titers of GAD Abs (213) developing in these studies. This suggests that the GAD65 protein is also important for the pathogenesis of SPS; however, it is regulated by other autoantigens that contribute to the pathogenesis of SPS.
8. Conclusion
This review demonstrated that the relationship among cancers, autoantigens, and SPSSDs is complicated, and new information in this field is still being revealed globally. Our findings would facilitate the development of an open-minded approach to updating information on novel cancer subtypes, autoantigens, and SPSSDs to renew our database. Future investigations are urgently required to reveal the mechanism by which cancers, autoantigens, and SPSSDs interact, which will facilitate the early prediction of cancer outcomes and the discovery of new therapeutic modalities.
Author contributions
YP received funding support and developed the research hypotheses. YP, HY, Y-hX, QC, HJ, SL, S-yY, and M-qD wrote the main manuscript. The final manuscript is the end product of the joint efforts of all authors. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Scientific Research Project of Hunan Provincial Health Commission, China (No. C202303076574 to YP), Key Plans of Hunan Administration Traditional Chinese Medicine, China (No. A2023039 to YP), University-Hospital Joint-Fund of Hunan University of Chinese Medicine, China (No. 2022XYLH198 to YP), Fund for Creative Research Group of Affiliated First Hospital of Hunan Traditional Chinese Medical College, China (No. 2021B-003 to YP), and Technology Plan Project of Zhuzhou City, Hunan Province, China (No. 2021-009 to YP).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1209302/full#supplementary-material
References
1. Moersch FP, Woltman HW. Progressive fluctuating muscular rigidity and spasm (“stiff-man” syndrome); report of a case and some observations in 13 other cases. Proc Staff Meet Mayo Clin. (1956) 31:421–7.
2. Ortiz JF, Ghani MR, Cox ÁM, Tambo W, Bashir F, Wirth M, et al. Stiff-Person syndrome: a treatment update and new directions. Cureus. (2020) 12:e11995. doi: 10.7759/cureus.11995
3. Baizabal-Carvallo JF, Jankovic J. Stiff-person syndrome: insights into a complex autoimmune disorder. J Neurol Neurosurg Psychiatry. (2015) 86:840–8. doi: 10.1136/jnnp-2014-309201
4. Newsome SD, Johnson T. Stiff person syndrome spectrum disorders; more than meets the eye. J Neuroimmunol. (2022) 369:577915. doi: 10.1016/j.jneuroim.2022.577915
5. Brown P, Marsden CD. The stiff man and stiff man plus syndromes. J Neurol. (1999) 246:648–52. doi: 10.1007/s004150050425
6. Buechner S, Florio I, Capone L. Stiff Person syndrome: a rare neurological disorder, heterogeneous in clinical presentation and not easy to treat. Case Rep Neurol Med. (2015) 2015:278065. doi: 10.1155/2015/278065
7. Saiz A, Blanco Y, Sabater L, González F, Bataller L, Casamitjana R, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. (2008) 131(Pt 10):2553–63. doi: 10.1093/brain/awn183
8. Mehta MP, Sokol LL. The case of a 30-year-old man with subacute gait instability, weakness, and muscle spasms. Annals Clin Translat Neurol. (2020) 7:2535–7. doi: 10.1002/acn3.51181
9. Gharedaghi MH, Khorasani A, Knezevic NN, Ebrahimi F. Anaesthetic management of a patient with a unique combination of anti-N-methyl-D-aspartate receptor encephalitis and stiff-person syndrome. BMJ Case Rep. (2018) 2018:261. doi: 10.1136/bcr-2017-223261
10. Huang J, Zhang G, Liu J. Stiff-person syndrome with central sleep apnea after thymoma excision: report of the first known case. Sleep Med. (2015) 16:1578–9. doi: 10.1016/j.sleep.2015.05.016
11. Kobayashi R, Kaji M, Horiuchi S, Miyahara N, Hino Y, Suemasu K. Recurrent thymoma with stiff-person syndrome and pure red blood cell aplasia. Ann Thorac Surg. (2014) 97:1802–4. doi: 10.1016/j.athoracsur.2013.07.103
12. Wang Y, Tourkevich R, Bosley J, Gold DR, Newsome SD. Ocular motor and vestibular characteristics of antiglutamic acid decarboxylase-associated neurologic disorders. J Neuro-ophthalmol N Am Neuro-Ophthalmol Soc. (2021) 41:e665–71. doi: 10.1097/WNO.0000000000001084
13. Graus F, Saiz A, Dalmau J. GAD antibodies in neurological disorders—Insights and challenges. Nature Reviews Neurol. (2020) 16:353–65. doi: 10.1038/s41582-020-0359-x
14. Martinez-Hernandez E, Ariño H, McKeon A, Iizuka T, Titulaer MJ, Simabukuro MM, et al. Clinical and immunologic investigations in patients with stiff-person spectrum disorder. JAMA Neurol. (2016) 73:714–20. doi: 10.1001/jamaneurol.2016.0133
15. Crisp SJ, Balint B, Vincent A. Redefining progressive encephalomyelitis with rigidity and myoclonus after the discovery of antibodies to glycine receptors. Curr Opin Neurol. (2017) 30:310–6. doi: 10.1097/WCO.0000000000000450
16. Hutchinson M, Waters P, McHugh J, Gorman G, O'Riordan S, Connolly S, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology. (2008) 71:1291–2. doi: 10.1212/01.wnl.0000327606.50322.f0
17. Bataller L, Wade DF, Fuller GN, Rosenfeld MR, Dalmau J. Cerebellar degeneration and autoimmunity to zinc-finger proteins of the cerebellum. Neurology. (2002) 59:1985–7. doi: 10.1212/01.WNL.0000038352.01415.CE
18. Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. (2014) 13:276–86. doi: 10.1016/S1474-4422(13)70299-0
19. Raju R, Rakocevic G, Chen Z, Hoehn G, Semino-Mora C, Shi W, et al. Autoimmunity to GABAA-receptor-associated protein in stiff-person syndrome. Brain. (2006) 129(Pt 12):3270–6. doi: 10.1093/brain/awl245
20. Bernardo F, Rebordão L, Rêgo A, Machado S, Passos J, Costa C, et al. Stiff person spectrum disorders: an illustrative case series of their phenotypic and antibody diversity. J Neuroimmunol. (2020) 341:577192. doi: 10.1016/j.jneuroim.2020.577192
21. McKeon A, Robinson MT, McEvoy KM, Matsumoto JY, Lennon VA, Ahlskog JE, et al. Stiff-man syndrome and variants: clinical course, treatments, and outcomes. Arch Neurol. (2012) 69:230–8. doi: 10.1001/archneurol.2011.991
22. Yohannan B, Sridhar A, Kachira JJ, Jafri SH. Glutamic acid decarboxylase (GAD) antibody-positive paraneoplastic stiff person syndrome associated with mediastinal liposarcoma. BMJ Case Rep. (2022) 15:639. doi: 10.1136/bcr-2022-250639
23. McCabe DJ, Turner NC, Chao D, et al. Paraneoplastic “stiff person syndrome” with metastatic adenocarcinoma and anti-Ri antibodies. Neurology. (2004) 62:1402–4. doi: 10.1212/01.WNL.0000123694.64121.D5
24. Yong SYS, Teo JY, Yong KP, Goh BKP. Paraneoplastic stiff person syndrome secondary to pancreatic adenocarcinoma. J Gastrointestinal Surg. (2018) 22:172–4. doi: 10.1007/s11605-017-3611-y
25. McHugh JC, Murray B, Renganathan R, Connolly S, Lynch T. GAD antibody positive paraneoplastic stiff person syndrome in a patient with renal cell carcinoma. J Mov Disorder Soc. (2007) 22:1343–6. doi: 10.1002/mds.21374
26. Butler MH, Hayashi A, Ohkoshi N, Villmann C, Becker CM, Feng G, et al. Autoimmunity to gephyrin in Stiff-Man syndrome. Neuron. (2000) 26:307–12. doi: 10.1016/S0896-6273(00)81165-4
27. Komandla SR, Vankadari K, Milap MVH, Kandadai RM. 18F-FDG PET/ct findings in a rare case of paraneoplastic vestibulocerebellar syndrome associated with isolated antiamphiphysin antibodies. Clin Nucl Med. (2022) 47:e125–8. doi: 10.1097/RLU.0000000000003868
28. Vacaras V, Cucu EE, Radu R, Muresanu DF. Paraneoplastic Stiff person syndrome in early-stage breast cancer with positive anti-amphiphysin antibodies. Case Rep Neurol. (2020) 12:339–47. doi: 10.1159/000508942
29. Yeoh CB, Lee KJ, Tollinche LE. Anesthetic management of a patient with Stiff-Person Syndrome and endometrial cancer for robotic surgery: a case report. Int J Clin Case Reports Rev. (2020) 3:26. doi: 10.31579/2690-4861/026
30. Piccolo G, Cosi V. Stiff-man syndrome, dysimmune disorder, and cancer. Ann Neurol. (1989) 26:105. doi: 10.1002/ana.410260118
31. de Albóniga-Chindurza A, Riva E, Jiménez-Huete A, Graus F, Franch O. Paraneoplastic stiff person syndrome with small cell carcinoma of the bladder and anti-Ri antibodies. Clin Neurol Neurosurg. (2018) 173:194–5. doi: 10.1016/j.clineuro.2018.08.020
32. Hylan K, Vu AD, Stammen K. Anesthetic considerations of Stiff-person syndrome: a case report. AANA J. (2016) 84:181–7.
33. Jun HO, Yum MS, Kim EH, Lee YJ, Seo JJ, Ko TS, et al. Rare case of childhood stiff person syndrome associated with pleuropulmonary blastoma. Pediatr Neurol. (2015) 53:448–51. doi: 10.1016/j.pediatrneurol.2015.06.015
34. Carvajal-González A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. (2014) 137(Pt 8):2178–92. doi: 10.1093/brain/awu142
35. Koca I, Ucar M, Kalender ME, Alkan S. The horses are the first thought but one must not forget the zebras even if they are rare: Stiff person syndrome associated with malignant mesothelioma. BMJ Case Rep. (2014) 2014:455. doi: 10.1136/bcr-2013-203455
36. Badzek S, Miletic V, Prejac J, Gorsic I, Golem H, Bilic E, et al. Paraneoplastic stiff person syndrome associated with colon cancer misdiagnosed as idiopathic Parkinson's disease worsened after capecitabine therapy. World J Surg Oncol. (2013) 11:224. doi: 10.1186/1477-7819-11-224
37. Wessig C, Klein R, Schneider MF, Toyka KV, Naumann M, Sommer C. Neuropathology and binding studies in anti-amphiphysin-associated stiff-person syndrome. Neurology. (2003) 61:195–8. doi: 10.1212/01.WNL.0000073143.53337.DD
38. Schmierer K, Grosse P, De Camilli P, Solimena M, Floyd S, Zschenderlein R. Paraneoplastic stiff-person syndrome: no tumor progression over 5 years. Neurology. (2002) 58:148. doi: 10.1212/WNL.58.1.148
39. Nene Y, Mehta T, Pawar S, Patil G, Ichaporia NR. A case of anti-glutamic acid decarboxylase-65 antibody positive stiff person syndrome presenting initially as acute peripheral vestibulopathy, leading to delayed diagnosis after multiple hospitalizations. Cureus. (2019) 11:e6083. doi: 10.7759/cureus.6083
40. Thümen A, Moser A. An uncommon paraneoplastic Ri-positive opsoclonus-myoclonus-like syndrome and stiff-person syndrome with elevated glutamate/GABA ratio in the cerebrospinal fluid after breast cancer. J Neurol. (2010) 257:1215–7. doi: 10.1007/s00415-010-5501-z
41. Lemieux J, Provencher L, Brunet D, Hogue JC. Paraneoplastic encephalomyelitis, stiff person syndrome and breast carcinoma. Can J Neurol Sci. (2011) 38:790–2. doi: 10.1017/S0317167100018011
42. Connolly C, Cobain E, Hughes T. Anti-amphiphysin positive stiff-person syndrome due to invasive ductal carcinoma in a male patient. BMJ Case Rep. (2021) 14:7738. doi: 10.1136/bcr-2020-237738
43. Rojas-Marcos I, Rousseau A, Keime-Guibert F, Reñé R, Cartalat-Carel S, Delattre JY, et al. Spectrum of paraneoplastic neurologic disorders in women with breast and gynecologic cancer. Medicine. (2003) 82:216–23. doi: 10.1097/01.md.0000076004.64510.ce
44. Dogruoz Karatekin B, Sahin SN, Icagasioglu A. Rehabilitation in paraneoplastic stiff-person syndrome—Case report. J Musculoskelet Neuronal Interact. (2021) 21:322–5.
45. Ibrikji S, Halabi TE, Sawaya R, Atweh S. Stiff person syndrome, transverse myelitis and hypothalamitis; three paraneoplastic syndromes associated with occult breast cancer. Acta Neurol Belg. (2021) 121:1343–5. doi: 10.1007/s13760-020-01570-5
46. Huang J, Li H, Zhou R, Huang W, Lin W, Chen T, et al. Clinical heterogeneity in patients with glutamate decarboxylase antibody. Neuroimmunomodulation. (2019) 26:234–8. doi: 10.1159/000502695
47. Vinjam MR, Shanmugarajah P, Ford HL. Ophthalmoplegia heralding the onset of anti-amphiphysin related paraneoplastic stiff person syndrome. J Neurol. (2016) 263:1017–8. doi: 10.1007/s00415-016-8078-3
48. Kelly PA, Kuberski C. Stiff person syndrome: a case report. Clin J Oncol Nurs. (2014) 18:465–7. doi: 10.1188/14.CJON.465-467
49. Folli F, Solimena M, Cofiell R, Austoni M, Tallini G, Fassetta G, et al. Autoantibodies to a 128-kd synaptic protein in three women with the stiff-man syndrome and breast cancer. N Engl J Med. (1993) 328:546–51. doi: 10.1056/NEJM199302253280805
50. Rosin L, DeCamilli P, Butler M, Solimena M, Schmitt H-P, Morgenthaler N, et al. Stiff-man syndrome in a woman with breast cancer: an uncommon central nervous system paraneoplastic syndrome. Neurology. (1998) 50:94–8. doi: 10.1212/WNL.50.1.94
51. Sinnreich M, Assal F, Hefft S, Magistris MR, Chizzolini C, Landis T, et al. Anti-GAD antibodies and breast cancer in a patient with stiff-person syndrome: a puzzling association. Eur Neurol. (2001) 46:51–2. doi: 10.1159/000050758
52. Petzold A. Neurodegeneration and multiple sclerosis. In: Galimberti D, Scarpini E, eds Neurodegenerative Diseases: Clinical Aspects, Molecular Genetics and Biomarkers. (London: Springer London) (2014). p. 227–245. doi: 10.1007/978-1-4471-6380-0_14
53. Kocak E, Abdessalam S, Walker MJ, Nuovo GJ, Kissel JT. Stiff-person syndrome: a rare presentation for breast cancer. Breast J. (2004) 10:552–3. doi: 10.1111/j.1075-122X.2004.21506.x
54. Pittock SJ, Lucchinetti CF, Parisi JE, Benarroch EE, Mokri B, Stephan CL, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. (2005) 58:96–107. doi: 10.1002/ana.20529
55. De Camilli P, Thomas A, Cofiell R, Folli F, Lichte B, Piccolo G, et al. The synaptic vesicle-associated protein amphiphysin is the 128-kD autoantigen of Stiff-Man syndrome with breast cancer. J Exp Med. (1993) 178:2219–23. doi: 10.1084/jem.178.6.2219
56. Floyd S, Butler MH, Cremona O, David C, Freyberg Z, Zhang X, et al. Expression of amphiphysin I, an autoantigen of paraneoplastic neurological syndromes, in breast cancer. Mol Med. (1998) 4:29–39. doi: 10.1007/BF03401727
57. Agarwal PA, Ichaporia NR. Glutamic acid decarboxylase antibody-positive paraneoplastic stiff limb syndrome associated with carcinoma of the breast. Neurol India. (2010) 58:449–51. doi: 10.4103/0028-3886.65704
58. Krishna VR, Knievel K, Ladha S, Sivakumar K. Lower extremity predominant stiff-person syndrome and limbic encephalitis with amphiphysin antibodies in breast cancer. J Clin Neuromuscul Dis. (2012) 14:72–4. doi: 10.1097/CND.0b013e31826f0d99
59. Khanam R, Fanous IS, Fadhel EN, Hyder T, Brufsky A. Voltage-gated calcium channel antibody-induced oropharyngeal dysphagia presenting as a paraneoplastic neurological complication in breast cancer. Cureus. (2021) 13:e13677. doi: 10.7759/cureus.13677
60. Aydin Ç, Çelik SY, Içöz S, Ulusoy C, Gündüz T, Demir GA, et al. Prognostic factors in anti-neuronal antibody positive patients. Noro Psikiyatr Ars. (2018) 55:189–94. doi: 10.29399/npa.23033
61. De Blauwe SN, Santens P, Vanopdenbosch LJ. Anti-glycine receptor antibody mediated progressive encephalomyelitis with rigidity and myoclonus associated with breast cancer. Case Rep Neurol Med. (2013) 2013:589154. doi: 10.1155/2013/589154
62. Antoine JC, Absi L, Honnorat J, Boulesteix JM, de Brouker T, Vial C, et al. Antiamphiphysin antibodies are associated with various paraneoplastic neurological syndromes and tumors. Arch Neurol. (1999) 56:172–7. doi: 10.1001/archneur.56.2.172
63. Smith SR, Fu JB. Paraneoplastic stiff person syndrome: Inpatient rehabilitation outcomes of a rare disease from two cancer rehabilitation programmes. J Rehabil Med. (2016) 48:639–42. doi: 10.2340/16501977-2089
64. Sarva H, Deik A, Ullah A, Severt WL. Clinical spectrum of stiff person syndrome: a review of recent reports. Tremor Hyperkin Mov. (2016) 6:340. doi: 10.5334/tohm.316
65. Gogia B, Shanina E, Fang X, He J, Li X. Case report: amphiphysin antibody-associated stiff-limb syndrome and myelopathy: an unusual presentation of breast cancer in an elderly woman. Front Neurol. (2021) 12:735895. doi: 10.3389/fneur.2021.735895
66. Dropcho EJ. Antiamphiphysin antibodies with small-cell lung carcinoma and paraneoplastic encephalomyelitis. Ann Neurol. (1996) 39:659–67. doi: 10.1002/ana.410390516
67. Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. (2011) 76:795–800. doi: 10.1212/WNL.0b013e31820e7b8d
68. Sarwari N, Galili Y, Perez A, Avgeropoulos N, Tseng J. Unusual presentation of lung adenocarcinoma with paraneoplastic stiff person syndrome: role of EGFR tyrosine kinase inhibitors. J Thorac Oncol. (2019) 14:e179–80. doi: 10.1016/j.jtho.2019.03.025
69. Lester J, Cojab J, Klériga E. Stiff Person syndrome and acetylcholine receptor ganglionic neuronal antibodies. Case Rep Neurol. (2020) 12:24–6. doi: 10.1159/000505229
70. Badari A, Farolino D, Nasser E, Mehboob S, Crossland D. A novel approach to paraneoplastic intestinal pseudo-obstruction. Support Care Cancer. (2012) 20:425–8. doi: 10.1007/s00520-011-1305-7
71. Kyskan R, Chapman K, Mattman A, Sin D. Antiglycine receptor antibody and encephalomyelitis with rigidity and myoclonus (PERM) related to small cell lung cancer. BMJ Case Rep. (2013) 2013:27. doi: 10.1136/bcr-2013-010027
72. Nguyen-Huu BK, Urban PP, Schreckenberger M, Dieterich M, Werhahn KJ. Antiamphiphysin-positive stiff-person syndrome associated with small cell lung cancer. J Mov Disord Soc. (2006) 21:1285–7. doi: 10.1002/mds.20910
73. Spitz M, Ferraz HB, Barsottini OG, Gabbai AA. Progressive encephalomyelitis with rigidity: a paraneoplastic presentation of oat cell carcinoma of the lung. Case report. Arq Neuropsiquiatr. (2004) 62(2b):547–9. doi: 10.1590/S0004-282X2004000300033
74. Abboud H, Rossman I, Mealy MA, Hill E, Thompson N, Banerjee A, et al. Neuronal autoantibodies: differentiating clinically relevant and clinically irrelevant results. J Neurol. (2017) 264:2284–92. doi: 10.1007/s00415-017-8627-4
75. Ray D, Nigam A. Paraneoplastic effects on neurophthalmologic function. Otol Neurotol. (2007) 28:860–2. doi: 10.1016/j.athoracsur.2004.02.076
76. Sinha A, Smolik TJ, Roy K, Bollu PC. Neuropsychiatric manifestations of autoimmune encephalitis in a tertiary hospital: a case series and current perspectives. J Clin Psychiatry. (2022) 83:3920. doi: 10.4088/JCP.21nr13920
77. Rakocevic G, Hussain A. Stiff person syndrome improvement with chemotherapy in a patient with cutaneous T cell lymphoma. Muscle Nerve. (2013) 47:938–9. doi: 10.1002/mus.23706
78. Nuti A, Ceravolo R, Salvetti S, Gambaccini G, Bonuccelli U, Capochiani E. Paraneoplastic choreic syndrome during non-Hodgkin's lymphoma. J Mov Disord Soc. (2000) 15:350–60. doi: 10.1002/1531-8257(200003)15:2<350::AID-MDS1029>3.0.CO;2-9
79. Gutmann B, Crivellaro C, Mitterer M, Zingerle H, Egarter-Vigl E, Wiedermann CJ. Paraneoplastic stiff-person syndrome, heterotopic soft tissue ossification and gonarthritis in a HLA B27-positive woman preceding the diagnosis of Hodgkin's lymphoma. Haematologica. (2006) 91(12 Suppl):Ecr59.
80. Tsai T, McGrath R. Lymphoma, thymoma and the wooden man: stiff-person syndrome post-thymoma excision and non-Hodgkin lymphoma remission. Intern Med J. (2012) 42:205–7. doi: 10.1111/j.1445-5994.2011.02688.x
81. Derksen A, Stettner M, Stöcker W, Seitz RJ. Antiglycine receptor-related stiff limb syndrome in a patient with chronic lymphocytic leukaemia. BMJ Case Rep. (2013) 2013:8667. doi: 10.1136/bcr-2013-008667
82. Borellini L, Lanfranconi S, Bonato S, Trezzi I, Franco G, Torretta L, et al. Progressive encephalomyelitis with rigidity and myoclonus associated with Anti-GlyR antibodies and hodgkin's lymphoma: a case report. Front Neurol. (2017) 8:401. doi: 10.3389/fneur.2017.00401
83. Schmidt C, Freilinger T, Lieb M, Rémi J, Klein M, Straube A, et al. Progressive encephalomyelitis with rigidity and myoclonus preceding otherwise asymptomatic Hodgkin's lymphoma. J Neurol Sci. (2010) 291:118–20. doi: 10.1016/j.jns.2009.12.025
84. Tchapyjnikov D, Borst AJ. Immune-related neurological symptoms in an adolescent patient receiving the checkpoint inhibitor nivolumab. J Immunother. (2017) 40:286–8. doi: 10.1097/CJI.0000000000000177
85. McKeon A. Paraneoplastic and other autoimmune disorders of the central nervous system. Neurohospitalist. (2013) 3:53–64. doi: 10.1177/1941874412453339
86. Schiff D, Dalmau J, Myers DJ. Anti-GAD antibody positive stiff-limb syndrome in multiple myeloma. J Neurooncol. (2003) 65:173–5. doi: 10.1023/B:NEON.0000003754.34527.f2
87. Maimaiti B, Mijiti S, Sun H, Xie Y, Jiang T, Meng Q, et al. Are anti-glutamic acid decarboxylase 65-kDa isoform antibodies related to diabetes or brain tumor? Eur J Med Res. (2022) 27:53. doi: 10.1186/s40001-022-00674-3
88. Jones AL, Flanagan EP, Pittock SJ, Mandrekar JN, Eggers SD, Ahlskog JE, et al. Responses to and outcomes of treatment of autoimmune cerebellar ataxia in adults. JAMA Neurol. (2015) 72:1304–12. doi: 10.1001/jamaneurol.2015.2378
89. Clow EC, Couban S, Grant IA. Stiff-person syndrome associated with multiple myeloma following autologous bone marrow transplantation. Muscle Nerve. (2008) 38:1649–52. doi: 10.1002/mus.21153
90. Silverman IE. Paraneoplastic stiff limb syndrome. J Neurol Neurosurg Psychiatry. (1999) 67:126–7. doi: 10.1136/jnnp.67.1.126
91. Shugaiv E, Leite MI, Sehitoglu E, Woodhall M, Çavuş F, Waters P, et al. Progressive encephalomyelitis with rigidity and myoclonus: a syndrome with diverse clinical features and antibody responses. Eur Neurol. (2013) 69:257–62. doi: 10.1159/000342237
92. Aghajanzadeh M, Alavi A, Aghajanzadeh G, Massahania S. Stiff man syndrome with invasive thymic carcinoma. Arch Iran Med. (2013) 16:195–6.
93. Huang C, Kang Y, Zhang B, et al. Anti-N-methyl-d-aspartate receptor encephalitis in a patient with a 7-year history of being diagnosed as schizophrenia: complexities in diagnosis and treatment. Neuropsychiatr Dis Treat. (2015) 11:1437–42. doi: 10.2147/NDT.S82930
94. Zhu X, Zhu J. CD4 T helper cell subsets and related human immunological disorders. Int J Mol Sci. (2020) 21:11. doi: 10.3390/ijms21218011
95. Dupond JL, Essalmi L, Gil H, Meaux-Ruault N, Hafsaoui C. Rituximab treatment of stiff-person syndrome in a patient with thymoma, diabetes mellitus and autoimmune thyroiditis. J Clin Neurosci. (2010) 17:389–91. doi: 10.1016/j.jocn.2009.06.015
96. Qin X, Wang DX, Wu XM. Anesthetic management of a patient with stiff-person syndrome and thymoma: a case report. Chin Med J. (2006) 119:963–5. doi: 10.1097/00029330-200606010-00017
97. Sasaki A, Kato T, Ujiie H. Thymoma-related Stiff-person syndrome with successfully treated by surgery. Ann Thorac Cardiovasc Surg. (2022) 28:448–52. doi: 10.5761/atcs.cr.21-00052
98. Iwata T, Inoue K, Mizuguchi S, Morita R, Tsukioka T, Suehiro S. Thymectomy for paraneoplastic stiff-person syndrome associated with invasive thymoma. J Thorac Cardiovasc Surg. (2006) 132:196–7. doi: 10.1016/j.jtcvs.2006.03.030
99. Essalmi L, Meaux-Ruault N, Hafsaoui C, Gil H, Curlier E, Dupond JL. [Stiff person syndrome associated with thymoma. Eff Thymec Rev Med Interne. (2007) 28:627–30. doi: 10.1016/j.revmed.2007.05.010
100. McKeon A, Tracy JA. GAD65 neurological autoimmunity. Muscle Nerve. (2017) 56:15–27. doi: 10.1002/mus.25565
101. Lee J, Zhou YJ, Ma W, Zhang W, Aljoufi A, Luh T, et al. Lineage specification of human dendritic cells is marked by IRF8 expression in hematopoietic stem cells and multipotent progenitors. Nat Immunol. (2017) 18:877–88. doi: 10.1038/ni.3789
102. Tanaka H, Matsumura A, Okumura M, Kitaguchi M, Yamamoto S, Iuchi K. Stiff man syndrome with thymoma. Ann Thorac Surg. (2005) 80:739–41. doi: 10.1097/MAO.0b013e318064e8fe
103. Hagiwara H, Enomoto-Nakatani S, Sakai K, Ugawa Y, Kusunoki S, Kanazawa I. Stiff-person syndrome associated with invasive thymoma: a case report. J Neurol Sci. (2001) 193:59–62. doi: 10.1016/s0022-510x(01)00602-5
104. Su Y, Cui L, Zhu M, Liang Y, Zhang Y. Progressive encephalomyelitis with rigidity and myoclonus with thymoma: a case report and literature review. Front Neurol. (2020) 11:1017. doi: 10.3389/fneur.2020.01017
105. Ozaki K, Ohkubo T, Yamada T, Yoshioka K, Ichijo M, Majima T, et al. Progressive encephalomyelitis with rigidity and myoclonus resolving after thymectomy with subsequent anasarca: an autopsy case. Internal Med. (2018) 57:3451–8. doi: 10.2169/internalmedicine.1238-18
106. Uehara T, Murai H, Yamasaki R, Kikuchi H, Shigeto H, Ohyagi Y, et al. Thymoma-associated progressive encephalomyelitis with rigidity and myoclonus successfully treated with thymectomy and intravenous immunoglobulin. Eur Neurol. (2011) 66:328–30. doi: 10.1159/000332033
107. Dade M, Berzero G, Izquierdo C, Giry M, Benazra M, Delattre JY, et al. Neurological Syndromes Associated with Anti-GAD Antibodies. Int J Mol Sci. (2020) 21:701. doi: 10.3390/ijms21103701
108. Solimena M, De Camilli P. Autoimmunity to glutamic acid decarboxylase (GAD) in Stiff-Man syndrome and insulin-dependent diabetes mellitus. Trends Neurosci. (1991) 14:452–7. doi: 10.1016/0166-2236(91)90044-U
109. Vincent SR, Hökfelt T, Wu JY, Elde RP, Morgan LM, Kimmel JR. Immunohistochemical studies of the GABA system in the pancreas. Neuroendocrinology. (1983) 36:197–204. doi: 10.1159/000123456
110. Daif A, Lukas RV, Issa NP, Javed A, VanHaerents S, Reder AT, et al. Antiglutamic acid decarboxylase 65 (GAD65) antibody-associated epilepsy. Epilepsy Behav. (2018) 80:331–6. doi: 10.1016/j.yebeh.2018.01.021
111. Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, et al. Demonstration of functional coupling between gamma -aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci U S A. (2003) 100:4293–8. doi: 10.1073/pnas.0730698100
112. Manto M, Honnorat J, Hampe CS, Guerra-Narbona R, López-Ramos JC, Delgado-García JM, et al. Disease-specific monoclonal antibodies targeting glutamate decarboxylase impair GABAergic neurotransmission and affect motor learning and behavioral functions. Front Behav Neurosci. (2015) 9:78. doi: 10.3389/fnbeh.2015.00078
113. Raju R, Foote J, Banga JP, Hall TR, Padoa CJ, Dalakas MC, et al. Analysis of GAD65 autoantibodies in Stiff-Person syndrome patients. J Immunol. (2005) 175:7755–62. doi: 10.4049/jimmunol.175.11.7755
114. Gresa-Arribas N, Ariño H, Martínez-Hernández E, Petit-Pedrol M, Sabater L, Saiz A, et al. Antibodies to inhibitory synaptic proteins in neurological syndromes associated with glutamic acid decarboxylase autoimmunity. PLoS ONE. (2015) 10:e0121364. doi: 10.1371/journal.pone.0121364
115. Kim J, Namchuk M, Bugawan T, Fu Q, Jaffe M, Shi Y, et al. Higher autoantibody levels and recognition of a linear NH2-terminal epitope in the autoantigen GAD65, distinguish stiff-man syndrome from insulin-dependent diabetes mellitus. J Exp Med. (1994) 180:595–606. doi: 10.1084/jem.180.2.595
116. Butler MH, Solimena M, Dirkx R Jr, Hayday A, De Camilli P. Identification of a dominant epitope of glutamic acid decarboxylase (GAD-65) recognized by autoantibodies in stiff-man syndrome. J Exp medicine. (1993) 178:2097–106. doi: 10.1084/jem.178.6.2097
117. Richter W, Shi Y, Baekkeskov S. Autoreactive epitopes defined by diabetes-associated human monoclonal antibodies are localized in the middle and C-terminal domains of the smaller form of glutamate decarboxylase. Proc Natl Acad Sci U S A. (1993) 90:2832–6. doi: 10.1073/pnas.90.7.2832
118. Muñiz-Castrillo S, Ambati A, Dubois V, Vogrig A, Joubert B, Rogemond V, et al. Primary DQ effect in the association between HLA and neurological syndromes with anti-GAD65 antibodies. J Neurol. (2020) 267:1906–11. doi: 10.1007/s00415-020-09782-8
119. Muñiz-Castrillo S, Vogrig A, Honnorat J. Associations between HLA and autoimmune neurological diseases with autoantibodies. Auto Immun Highlights. (2020) 11:2. doi: 10.1186/s13317-019-0124-6
120. Pugliese A, Solimena M, Awdeh ZL, Alper CA, Bugawan T, Erlich HA, et al. Association of HLA-DQB1*0201 with stiff-man syndrome. J Clin Endocrinol Metab. (1993) 77:1550–3. doi: 10.1210/jcem.77.6.8263140
121. Joubert B, García-Serra A, Planagumà J, Martínez-Hernandez E, Kraft A, Palm F, et al. Pregnancy outcomes in anti-NMDA receptor encephalitis: case series. Neurol Neuroimmuno Neuroinflamm. (2020) 7:668. doi: 10.1212/NXI.0000000000000668
122. Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Progress Neurobiol. (1991) 36:35–92. doi: 10.1016/0301-0082(91)90036-Z
123. DeFelipe J, López-Cruz PL, Benavides-Piccione R, Bielza C, Larrañaga P, Anderson S, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. (2013) 14:202–16. doi: 10.1038/nrn3444
124. Frangaj A, Fan QR. Structural biology of GABA(B) receptor. Neuropharmacology. (2018) 136(Pt A):68–79. doi: 10.1016/j.neuropharm.2017.10.011
125. Bowery NG, Bettler B, Froestl W. International union of pharmacology. XXXIII Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev. (2002) 54:247–64. doi: 10.1124/pr.54.2.247
126. Mitoma H, Adhikari K, Aeschlimann D, Chattopadhyay P, Hadjivassiliou M, Hampe CS, et al. Consensus paper: neuroimmune mechanisms of cerebellar ataxias. Cerebellum. (2016) 15:213–32. doi: 10.1007/s12311-015-0664-x
127. Hansen HC, Klingbeil C, Dalmau J, Li W, Weissbrich B, Wandinger KP. Persistent intrathecal antibody synthesis 15 years after recovering from anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol. (2013) 70:117–9. doi: 10.1001/jamaneurol.2013.585
128. Koerner C, Wieland B, Richter W, Meinck HM. Stiff-person syndromes: motor cortex hyperexcitability correlates with anti-GAD autoimmunity. Neurology. (2004) 62:1357–62. doi: 10.1212/01.WNL.0000120543.65812.33
129. Mitoma H, Song SY, Ishida K, Yamakuni T, Kobayashi T, Mizusawa H. Presynaptic impairment of cerebellar inhibitory synapses by an autoantibody to glutamate decarboxylase. J Neurol Sci. (2000) 175:40–4. doi: 10.1016/S0022-510X(00)00272-0
130. Mitoma H, Ishida K, Shizuka-Ikeda M, Mizusawa H. Dual impairment of GABAA- and GABAB-receptor-mediated synaptic responses by autoantibodies to glutamic acid decarboxylase. J Neurol Sci. (2003) 208:51–6. doi: 10.1016/S0022-510X(02)00423-9
131. Levy LM, Dalakas MC, Floeter MK. The stiff-person syndrome: an autoimmune disorder affecting neurotransmission of gamma-aminobutyric acid. Ann Intern Med. (1999) 131:522–30. doi: 10.7326/0003-4819-131-7-199910050-00008
132. Manto MU, Laute MA, Aguera M, Rogemond V, Pandolfo M, Honnorat J. Effects of anti-glutamic acid decarboxylase antibodies associated with neurological diseases. Ann Neurol. (2007) 61:544–51. doi: 10.1002/ana.21123
133. Ishikawa T, Tomatsu S, Tsunoda Y, Lee J, Hoffman DS, Kakei S. Releasing dentate nucleus cells from Purkinje cell inhibition generates output from the cerebrocerebellum. PLoS ONE. (2014) 9:e108774. doi: 10.1371/journal.pone.0108774
134. Ishikawa N, Tajima G, Hyodo S, Takahashi Y, Kobayashi M. Detection of autoantibodies against NMDA-type glutamate receptor in a patient with recurrent optic neuritis and transient cerebral lesions. Neuropediatrics. (2007) 38:257–60. doi: 10.1055/s-2007-1004521
135. Kosseifi SG, Mehta JB, Roy T, Byrd Jr. R, Farrow J. The occurrence of stiff person syndrome in a patient with thymoma: case report and literature review. Tenn Med. (2010) 103:43–7.
136. Thomas S, Critchley P, Lawden M, Farooq S, Thomas A, Proudlock FA, et al. Stiff person syndrome with eye movement abnormality, myasthenia gravis, and thymoma. J Neurol Neurosurg Psychiatry. (2005) 76:141–2. doi: 10.1136/jnnp.2004.036558
137. Huang K, Luo YB, Yang H. Autoimmune channelopathies at neuromuscular junction. Front Neurol. (2019) 10:516. doi: 10.3389/fneur.2019.00516
138. Muñoz-Lopetegi A, de Bruijn MAAM, Boukhrissi S, Bastiaansen AEM, Nagtzaam MMP, Hulsenboom ESP, et al. Neurologic syndromes related to anti-GAD65: clinical and serologic response to treatment. Neurol Neuroimmunol Neuroinflamm. (2020) 7:696. doi: 10.1212/NXI.0000000000000696
139. Zhang B, Zelhof AC. Amphiphysins: raising the BAR for synaptic vesicle recycling and membrane dynamics. Bin-Amphiphysin-Rvsp Traffic. (2002) 3:452–60. doi: 10.1034/j.1600-0854.2002.30702.x
140. Lichte B, Veh RW, Meyer HE, Kilimann MW. Amphiphysin, a novel protein associated with synaptic vesicles. EMBO J. (1992) 11:2521–30. doi: 10.1002/j.1460-2075.1992.tb05317.x
141. Prokic I, Cowling BS, Laporte J. Amphiphysin 2 (BIN1) in physiology and diseases. J Mol Med (Berl). (2014) 92:453–63. doi: 10.1007/s00109-014-1138-1
142. Butler MH, David C, Ochoa GC, Freyberg Z, Daniell L, Grabs D, et al. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J Cell Biol. (1997) 137:1355–67. doi: 10.1083/jcb.137.6.1355
143. Ramjaun AR, Micheva KD, Bouchelet I, McPherson PS. Identification and characterization of a nerve terminal-enriched amphiphysin isoform. J Biol Chem. (1997) 272:16700–6. doi: 10.1074/jbc.272.26.16700
144. Sakamuro D, Elliott KJ, Wechsler-Reya R, Prendergast GC. BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nat Genet. (1996) 14:69–77. doi: 10.1038/ng0996-69
145. Chang MY, Boulden J, Katz JB, Wang L, Meyer TJ, Soler AP, et al. Bin1 ablation increases susceptibility to cancer during aging, particularly lung cancer. Cancer Res. (2007) 67:7605–12. doi: 10.1158/0008-5472.CAN-07-1100
146. Zhong X, Hoelz DJ, Kumar HR, Sandoval JA, Rescorla FJ, Hickey RJ, et al. Bin1 is linked to metastatic potential and chemosensitivity in neuroblastoma. Pediatr Blood Cancer. (2009) 53:332–7. doi: 10.1002/pbc.22068
147. Pan K, Liang X-t, Zhang H-k, Zhao J-j, Wang D-d, Li J-j, et al. Characterization of bridging integrator 1 (BIN1) as a potential tumor suppressor and prognostic marker in hepatocellular carcinoma. Mol Med. (2012) 18:507–18. doi: 10.2119/molmed.2011.00319
148. Ghaneie A, Zemba-Palko V, Itoh H, Itoh K, Sakamuro D, Nakamura S, et al. Bin1 attenuation in breast cancer is correlated to nodal metastasis and reduced survival. Cancer Biol Ther. (2007) 6:192–4. doi: 10.4161/cbt.6.2.3587
149. Ge K, Duhadaway J, Sakamuro D, Wechsler-Reya R, Reynolds C, Prendergast GC. Losses of the tumor suppressor BIN1 in breast carcinoma are frequent and reflect deficits in programmed cell death capacity. Int J Cancer. (2000) 85:376–83. doi: 10.1002/(SICI)1097-0215(20000201)85:3<376::AID-IJC14>3.0.CO;2-1
150. Ge K, Minhas F, Duhadaway J, Mao NC, Wilson D, Buccafusca R, et al. Loss of heterozygosity and tumor suppressor activity of Bin1 in prostate carcinoma. Int J Cancer. (2000) 86:155–61. doi: 10.1002/(sici)1097-0215(20000415)86:2<155::aid-ijc2>3.0.co;2-m
151. Ge K, DuHadaway J, Du W, Herlyn M, Rodeck U, Prendergast GC. Mechanism for elimination of a tumor suppressor: aberrant splicing of a brain-specific exon causes loss of function of Bin1 in melanoma. Proc Natl Acad Sci U S A. (1999) 96:9689–94. doi: 10.1073/pnas.96.17.9689
152. Chalphin AV, Saha MS. The specification of glycinergic neurons and the role of glycinergic transmission in development. Front Mol Neurosci. (2010) 3:11. doi: 10.3389/fnmol.2010.00011
153. Daly EC, Aprison MH. Distribution of serine hydroxymethyltransferase and glycine transaminase in several areas of the central nervous system of the rat. J Neurochem. (1974) 22:877–85. doi: 10.1111/j.1471-4159.1974.tb04312.x
154. Ichinohe A, Kure S, Mikawa S, Ueki T, Kojima K, Fujiwara K, et al. Glycine cleavage system in neurogenic regions. Eur J Neurosci. (2004) 19:2365–70. doi: 10.1111/j.0953-816X.2004.03345.x
155. Eulenburg V, Gomeza J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res Rev. (2010) 63:103–12. doi: 10.1016/j.brainresrev.2010.01.003
156. Zafra F, Giménez C. Glycine transporters and synaptic function. IUBMB Life. (2008) 60:810–7. doi: 10.1002/iub.128
157. Marques BL, Oliveira-Lima OC, Carvalho GA, Chiarelli Rd, Ribeiro RI, Parreira RC, et al. Neurobiology of glycine transporters: From molecules to behavior. Neurosci Biobehav Rev. (2020) 118:97–110. doi: 10.1016/j.neubiorev.2020.07.025
158. Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. (2004) 84:1051–95. doi: 10.1152/physrev.00042.2003
159. Betz H, Langosch D, Rundström N, Bormann J, Kuryatov A, Kuhse J, et al. Structure and biology of inhibitory glycine receptors. Ann N Y Acad Sci. (1993) 707:109–15. doi: 10.1111/j.1749-6632.1993.tb38046.x
160. Sánchez-Chávez G, Velázquez-Flores M, Ruiz Esparza-Garrido R, Salceda R. Glycine receptor subunits expression in the developing rat retina. Neurochem Int. (2017) 108:177–82. doi: 10.1016/j.neuint.2017.03.013
161. Hayashi Y, Nakanishi H. Synaptic plasticity and synaptic reorganization regulated by microglia. Nihon Shinkei Seishin Yakurigaku Zasshi. (2013) 33:211–6.
162. Domingues AM, Taylor M, Fern R. Glia as transmitter sources and sensors in health and disease. Neurochem Int. (2010) 57:359–66. doi: 10.1016/j.neuint.2010.03.024
163. Constantinou S, Fern R. Conduction block and glial injury induced in developing central white matter by glycine, GABA, noradrenalin, or nicotine, studied in isolated neonatal rat optic nerve. Glia. (2009) 57:1168–77. doi: 10.1002/glia.20839
164. Pan S, Fan M, Liu Z, Li X, Wang H. Serine, glycine and one-carbon metabolism in cancer (Review). Int J Oncol. (2021) 58:158–70. doi: 10.3892/ijo.2020.5158
165. Förstera B, Dzaye O, Winkelmann A, Semtner M, Benedetti B, Markovic DS, et al. Intracellular glycine receptor function facilitates glioma formation in vivo. J Cell Sci. (2014) 127(Pt 17):3687–98. doi: 10.1242/jcs.146662
166. Samarut E, Bekri A, Drapeau P. Transcriptomic analysis of purified embryonic neural stem cells from zebrafish embryos reveals signaling pathways involved in glycine-dependent neurogenesis. Front Mol Neurosci. (2016) 9:22. doi: 10.3389/fnmol.2016.00022
167. Samarut E, Chalopin D, Riché R, Allard M, Liao M, Drapeau P. Individual knock out of glycine receptor alpha subunits identifies a specific requirement of glra1 for motor function in zebrafish. PLoS ONE. (2019) 14:e0216159. doi: 10.1371/journal.pone.0216159
168. Zdziarski P. A Case of Stiff Person syndrome: immunomodulatory effect of benzodiazepines. Succ Rituximab Tizanidine Therapy. (2015) 94:e954. doi: 10.1097/MD.0000000000000954
169. Dalakas MC, Fujii M, Li M, Lutfi B, Kyhos J, McElroy B. High-dose intravenous immune globulin for stiff-person syndrome. N Engl J Med. (2001) 345:1870–6. doi: 10.1056/NEJMoa01167
170. Hasnat MJ, Rice JE. Intrathecal baclofen for treating spasticity in children with cerebral palsy. Cochrane Database Sys Rev. (2015) 2015:Cd004552. doi: 10.1002/14651858.CD004552.pub2
171. Abbatemarco JR, Willis MA, Wilson RG, Nagel SJ, Machado AG, Bethoux FA. Case series: intrathecal baclofen therapy in Stiff-Person syndrome. Neuromodulation. (2018) 21:655–9. doi: 10.1111/ner.12765
172. Xie YY, Meng HM, Zhang FX, Maimaiti B, Jiang T, Yang Y. Involuntary movement in stiff-person syndrome with amphiphysin antibodies: a case report. Medicine (Baltimore). (2021) 100:e24312. doi: 10.1097/MD.0000000000024312
173. Cai Q, Wu C, Xu W, Liang Y, Liao S. Stiff-person syndrome coexisting with critical illness polyneuropathy: a case report. Medicine (Baltimore). (2020) 99:e23607. doi: 10.1097/MD.0000000000023607
174. Clardy SL, Lennon VA, Dalmau J, Pittock SJ, Jr HRJ, Renaud DL, et al. Childhood onset of stiff-man syndrome. JAMA Neurol. (2013) 70:1531–6. doi: 10.1001/jamaneurol.2013.4442
175. Geffen S, Chiang N. Successful treatment of Stiff Person syndrome with intrathecal baclofen. J Rehabil Med Clin Commun. (2019) 2:1000016. doi: 10.2340/20030711-1000016
176. Newton JC, Harned ME, Sloan PA, Salles SS. Trialing of intrathecal baclofen therapy for refractory stiff-person syndrome. Reg Anesth Pain Med. (2013) 38:248–50. doi: 10.1097/AAP.0b013e318288b8f9
177. Klitgaard H, Matagne A, Nicolas JM, et al. Brivaracetam: Rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia. (2016) 57:538–48. doi: 10.1111/epi.13340
178. Madeja M, Margineanu DG, Gorji A, Siep E, Boerrigter P, Klitgaard H, et al. Reduction of voltage-operated potassium currents by levetiracetam: a novel antiepileptic mechanism of action? Neuropharmacology. (2003) 45:661–71. doi: 10.1016/S0028-3908(03)00248-X
179. Sechi G, Barrocu M, Piluzza MG, Cocco GA, Deiana GA, Sau GF. Levetiracetam in stiff-person syndrome. J Neurol. (2008) 255:1721–5. doi: 10.1007/s00415-008-0007-7
180. Greenblatt HK, Greenblatt DJ. Gabapentin and pregabalin for the treatment of anxiety disorders. Clin Pharmacol Drug Develop. (2018) 7:228–32. doi: 10.1002/cpdd.446
181. Squintani G, Bovi T, Ferigo L, Musso AM, Ottaviani S, Moretto G, et al. Efficacy of pregabalin in a case of stiff-person syndrome: clinical and neurophysiological evidence. J Neurol Sci. (2012) 314:166–8. doi: 10.1016/j.jns.2011.10.023
182. Short CE, Bufalari A. Propofol anesthesia. Vet Clin North America Small Animal Pract. (1999) 29:747–78. doi: 10.1016/S0195-5616(99)50059-4
183. Hattan E, Angle MR, Chalk C. Unexpected benefit of propofol in stiff-person syndrome. Neurology. (2008) 70:1641–2. doi: 10.1212/01.wnl.0000284606.00074.f1
184. Vernino S, McEvoy K. Propofol for stiff-person syndrome: learning new tricks from an old dog. Neurology. (2008) 70:1584–5. doi: 10.1212/01.wnl.0000310971.62712.40
185. Kosmidis ML, Dalakas MC. Practical considerations on the use of rituximab in autoimmune neurological disorders. Ther Adv Neurol Disord. (2010) 3:93–105. doi: 10.1177/1756285609356135
186. Dalakas MC, Rakocevic G, Dambrosia JM, Alexopoulos H, McElroy B, A. double-blind, placebo-controlled study of rituximab in patients with stiff person syndrome. Ann Neurol. (2017) 82:271–7. doi: 10.1002/ana.25002
187. Qureshi A, Hennessy M. Stiff person syndrome (SPS) complicated by respiratory failure: successful treatment with rituximab. J Neurol. (2012) 259:180–1. doi: 10.1007/s00415-011-6123-9
188. Lobo ME, Araújo ML, Tomaz CA, Allam N. Stiff-person syndrome treated with rituximab. BMJ Case Rep. (2010) 2010:3021. doi: 10.1136/bcr.05.2010.3021
189. Baker MR, Das M, Isaacs J, Fawcett PR, Bates D. Treatment of stiff person syndrome with rituximab. J Neurol Neurosurg Psychiatry. (2005) 76:999–1001. doi: 10.1136/jnnp.2004.051144
190. Dalakas MC. Stiff person syndrome: advances in pathogenesis and therapeutic interventions. Curr Treat Options Neurol. (2009) 11:102–10. doi: 10.1007/s11940-009-0013-9
191. Nakane S, Fujita K, Shibuta Y, Matsui N, Harada M, Urushihara R, et al. Successful treatment of stiff person syndrome with sequential use of tacrolimus. J Neurol Neurosurg Psychiatry. (2013) 84:1177–80. doi: 10.1136/jnnp-2013-305425
192. Ishizawa K, Komori T, Okayama K, Qin X, Kaneko K, Sasaki S, et al. Large motor neuron involvement in Stiff-man syndrome: a qualitative and quantitative study. Acta Neuropathol. (1999) 97:63–70. doi: 10.1007/s004010050956
193. Dalakas MC. The role of IVIg in the treatment of patients with stiff person syndrome and other neurological diseases associated with anti-GAD antibodies. J Neurol. (2005) 252(Suppl 1):I19–25. doi: 10.1007/s00415-005-1105-4
194. Gerschlager W, Brown P. Effect of treatment with intravenous immunoglobulin on quality of life in patients with stiff-person syndrome. Mov Disord J Mov Dis Soc. (2002) 17:590–3. doi: 10.1002/mds.10043
195. De la Casa-Fages B, Anaya F, Gabriel-Ortemberg M, Grandas F. Treatment of stiff-person syndrome with chronic plasmapheresis. Mov Disord J Mov Dis Soc. (2013) 28:39–7. doi: 10.1002/mds.25167
196. Albahra S, Yates SG, Joseph D, De Simone N, Burner JD, Sarode R. Role of plasma exchange in stiff person syndrome. Transf Apheresis Sci J World Apheresis Assoc J Eur Soc Haemapheresis. (2019) 58:310–2. doi: 10.1016/j.transci.2019.03.015
197. Ariño H, Gresa-Arribas N, Blanco Y, Martínez-Hernández E, Sabater L, Petit-Pedrol M, et al. Cerebellar ataxia and glutamic acid decarboxylase antibodies: immunologic profile and long-term effect of immunotherapy. JAMA Neurol. (2014) 71:1009–16. doi: 10.1001/jamaneurol.2014.1011
198. Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. (2010) 67:470–8. doi: 10.1002/ana.21917
199. Joubert B, Belbezier A, Haesebaert J, Rheims S, Ducray F, Picard G, et al. Long-term outcomes in temporal lobe epilepsy with glutamate decarboxylase antibodies. J Neurol. (2020) 267:2083–9. doi: 10.1007/s00415-020-09807-2
200. Malter MP, Frisch C, Zeitler H, Surges R, Urbach H, Helmstaedter C, et al. Treatment of immune-mediated temporal lobe epilepsy with GAD antibodies. Seizure. (2015) 30:57–63. doi: 10.1016/j.seizure.2015.05.017
201. Björk E, Velloso LA, Kämpe O, Karlsson FA. GAD autoantibodies in IDDM, stiff-man syndrome, and autoimmune polyendocrine syndrome type I recognize different epitopes. Diabetes. (1994) 43:161–5. doi: 10.2337/diab.43.1.161
202. Dinkel K, Meinck HM, Jury KM, Karges W, Richter W. Inhibition of gamma-aminobutyric acid synthesis by glutamic acid decarboxylase autoantibodies in stiff-man syndrome. Ann Neurol. (1998) 44:194–201. doi: 10.1002/ana.410440209
203. Ishida K, Mitoma H, Song SY, Uchihara T, Inaba A, Eguchi S, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol. (1999) 46:263–73. doi: 10.1002/1531-8249(199908)46:2<263::AID-ANA19>3.0.CO;2-0
204. Takenoshita H, Shizuka-Ikeda M, Mitoma H, Song S, Harigaya Y, Igeta Y, et al. Presynaptic inhibition of cerebellar GABAergic transmission by glutamate decarboxylase autoantibodies in progressive cerebellar ataxia. J Neurol Neurosurg Psychiatry. (2001) 70:386–9. doi: 10.1136/jnnp.70.3.386
205. Vianello M, Bisson G, Maschio MD, Vassanelli S, Girardi S, Mucignat C, et al. Increased spontaneous activity of a network of hippocampal neurons in culture caused by suppression of inhibitory potentials mediated by anti-gad antibodies. Autoimmunity. (2008) 41:66–73. doi: 10.1080/08916930701619565
206. Stemmler N, Rohleder K, Malter MP, Widman G, Elger CE, Beck H, et al. Serum from a patient with GAD65 antibody-associated limbic encephalitis did not alter GABAergic neurotransmission in cultured hippocampal networks. Front Neurol. (2015) 6:189. doi: 10.3389/fneur.2015.00189
207. Hackert JK, Müller L, Rohde M, Bien CG, Köhling R, Kirschstein T. Anti-GAD65 containing cerebrospinal fluid does not alter GABAergic transmission. Front Cell Neurosci. (2016) 10:130. doi: 10.3389/fncel.2016.00130
208. Chang T, Alexopoulos H, McMenamin M, Carvajal-González A, Alexander SK, Deacon R, et al. Neuronal surface and glutamic acid decarboxylase autoantibodies in non-paraneoplastic stiff person syndrome. JAMA Neurol. (2013) 70:1140–9. doi: 10.1001/jamaneurol.2013.3499
209. Fouka P, Alexopoulos H, Akrivou S, Trohatou O, Politis PK, Dalakas MC. GAD65 epitope mapping and search for novel autoantibodies in GAD-associated neurological disorders. J Neuroimmunol. (2015) 281:73–7. doi: 10.1016/j.jneuroim.2015.03.009
210. Geis C, Grünewald B, Weishaupt A, Wultsch T, Toyka KV, Reif A, et al. Human IgG directed against amphiphysin induces anxiety behavior in a rat model after intrathecal passive transfer. J Neural Trans. (2012) 119(8):981-985. doi: 10.1007/s00702-012-0773-3
211. Hansen N, Grünewald B, Weishaupt A, Colaço MN, Toyka KV, Sommer C, et al. Human Stiff person syndrome IgG-containing high-titer anti-GAD65 autoantibodies induce motor dysfunction in rats. Exp Neurol. (2013) 239:202–9. doi: 10.1016/j.expneurol.2012.10.013
212. Geis C, Weishaupt A, Grünewald B, Wultsch T, Reif A, Gerlach M, et al. Human stiff-person syndrome IgG induces anxious behavior in rats. PLoS ONE. (2011) 6:e16775. doi: 10.1371/journal.pone.0016775
213. Han G, Li Y, Wang J, Wang R, Chen G, Song L, et al. Active tolerance induction and prevention of autoimmune diabetes by immunogene therapy using recombinant adenoassociated virus expressing glutamic acid decarboxylase 65 peptide GAD (500–585). J Immunol. (2005) 174:4516–24. doi: 10.4049/jimmunol.174.8.4516
Keywords: stiff person syndrome (SPS), stiff person syndrome spectrum disorders (SPSSDs), paraneoplastic, cancer, malignant, autoantigen
Citation: Peng Y, Yang H, Xue Y-h, Chen Q, Jin H, Liu S, Yao S-y and Du M-q (2023) An update on malignant tumor-related stiff person syndrome spectrum disorders: clinical mechanism, treatment, and outcomes. Front. Neurol. 14:1209302. doi: 10.3389/fneur.2023.1209302
Received: 21 June 2023; Accepted: 01 September 2023;
Published: 04 October 2023.
Edited by:
Lidia Sabater, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), SpainReviewed by:
Cheran Elangovan, University of Tennessee Health Science Center (UTHSC), United StatesAndreu Vilaseca, Vall d'Hebron University Hospital, Spain
Copyright © 2023 Peng, Yang, Xue, Chen, Jin, Liu, Yao and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Peng, MTc3OTM0MjQ0NkBxcS5jb20=
 Yong Peng
Yong Peng Huan Yang
Huan Yang Ya-hui Xue1,2
Ya-hui Xue1,2 Shu Liu
Shu Liu