
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 16 June 2023
Sec. Neuro-Otology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1208902
This article is part of the Research TopicStroke and Balance DisordersView all 11 articles
 Martina Wüthrich1
Martina Wüthrich1 Zheyu Wang2,3
Zheyu Wang2,3 Carlos Mario Martinez4
Carlos Mario Martinez4 Sergio Carmona5
Sergio Carmona5 Georgios Mantokoudis6
Georgios Mantokoudis6 Alexander Andrea Tarnutzer1,7*
Alexander Andrea Tarnutzer1,7*Objectives: For the assessment of patients presenting with acute prolonged vertigo meeting diagnostic criteria for acute vestibular syndrome (AVS), bedside oculomotor examinations are essential to distinguish peripheral from central causes. Here we assessed patterns of spontaneous nystagmus (SN) observed in AVS and its diagnostic accuracy at the bedside.
Methods: MEDLINE and Embase were searched for studies (1980–2022) reporting on the bedside diagnostic accuracy of SN-patterns in AVS patients. Two independent reviewers determined inclusion. We identified 4,186 unique citations, examined 219 full manuscripts, and analyzed 39 studies. Studies were rated on risk of bias (QUADAS-2). Diagnostic data were extracted and SN beating-direction patterns were correlated with lesion locations and lateralization.
Results: Included studies reported on 1,599 patients, with ischemic strokes (n = 747) and acute unilateral vestibulopathy (n = 743) being most frequent. While a horizontal or horizontal-torsional SN was significantly more often found in peripheral AVS (pAVS) than in central AVS (cAVS) patients (672/709 [94.8%] vs. 294/677 [43.4%], p < 0.001), torsional and/or vertical SN-patterns were more prevalent in cAVS than in pAVS (15.1 vs. 2.6%, p < 0.001). For an (isolated) vertical/vertical-torsional SN or an isolated torsional SN specificity (97.7% [95% CI = 95.1–100.0%]) for a central origin etiology was high, whereas sensitivity (19.1% [10.5–27.7%]) was low. Absence of any horizontal SN was more frequently observed in cAVS than in pAVS (55.2 vs. 7.0%, p < 0.001). Ipsilesional and contralesional beating directions of horizontal SN in cAVS were found at similar frequency (28.0 vs. 21.7%, p = 0.052), whereas for pAVS a contralesional SN was significantly more frequent (95.2 vs. 2.5%, p < 0.001). For PICA strokes presenting with horizontal SN, beating direction was ipsilesional more often than contralesional (23.9 vs. 6.4%, p = 0.006), while the opposite was observed for AICA strokes (2.2 vs. 63.0%, p < 0.001).
Conclusions: (Isolated) vertical and/or torsional SN is found in a minority (15.1%) of cAVS patients only. When present, it is highly predictive for a central cause. A combined torsional-downbeating SN-pattern may be observed in pAVS also in cases with isolated lesions of the inferior branch of the vestibular nerve. Furthermore, in cAVS patients the SN beating direction itself does not allow a prediction on the lesion side.
Acute vertigo or dizziness is amongst the most frequent causes for primary care physician visits or emergency department (ED) consults, reflecting about 2.1–4.4% of all ED presentations (1–3). With its differential diagnosis being very broad and cutting across organ systems and specialties and ~4.4 million such visits to US EDs every year, resulting healthcare costs surpassed $10 billion (4). While in the majority of cases, underlying causes are benign and self-limited, at least 15% of all ED patients with these symptoms suffer from a dangerous underlying cause (2). The most common dangerous cause is stroke, accounting for ~3–5% of cases (5).
For patients presenting with acute prolonged vertigo or dizziness, meeting diagnostic criteria for acute vestibular syndrome (AVS), defined as a clinical syndrome of acute-onset, continuous vertigo, dizziness, or unsteadiness lasting days to weeks, and generally including features suggestive of new, ongoing vestibular system dysfunction [e.g., vomiting, nystagmus, severe postural instability; (6)], the combined use of targeted neuro-otologic bedside examination techniques such as HINTS (Head Impulse test, Nystagmus exam, Test of Skew) (7), HINTS+ (which adds a bedside test of hearing) (8), and assessing for gait/truncal instability (9) has been promoted. While HINTS(+) have excellent diagnostic accuracy for distinguishing benign, self-limited peripheral causes from dangerous, potentially life-threatening central causes (mostly strokes) when performed by appropriately trained clinicians, being superior than MRI-DWI in the first 24–48 h (10), its application by frontline health-care providers such as ED physicians or neurology residents lacking dedicated training may be not be feasible. This raises the question about the diagnostic accuracy of other bedside examination techniques that are more accessible to frontline healthcare providers such as assessing gait/truncal instability or describing spontaneous nystagmus (SN) patterns. While recent studies underline the value of grading gait/truncal instability in AVS patients (11, 12), the role of SN in acutely dizzy patients is less clear. Horizontal or horizontal-torsional SN beating away from the affected ear is a hallmark sign in patients with acute unilateral vestibulopathy (13) and may also be observed in patients with vertebrobasilar stroke (14). While it has been proposed that the SN pattern may be useful in the distinction between peripheral and central causes, with torsional and vertical SN pointing to latter one (15), the diagnostic accuracy of distinct SN patterns has not been systematically assessed.
Thus, the primary aim of this systematic review and meta-analysis was to report on the prevalence and diagnostic yield of SN patterns in acutely dizzy patients at the bedside. Furthermore, we also asked the question, to which extent the lesion location has an impact on the SN pattern and the beating direction (ipsilesional vs. contralesional) in lateralized lesions. We hypothesized that the presence of isolated torsional/torsional-vertical or vertical SN predicts a central cause and that the beating direction in cAVS patients critically depend on the location of the lesion.
We searched MEDLINE and Embase for English-language articles, using the following strategy and components: (1) vertigo/dizziness, (2) diagnostic accuracy of SN as assessed at the bedside, and (3) acute vestibular syndrome (including ischemic stroke, acute peripheral vestibulopathy). We also performed a manual search of reference lists from eligible articles and contacted corresponding authors where necessary. We did not seek to identify research abstracts from meeting proceedings or unpublished studies. We limited our search to articles published since 1980, when neuroimaging for stroke first became routine. Our search was updated through September 1st, 2022.
Being a systematic review of the literature and a meta-analysis, no ethical approval was necessary.
Articles were selected by two independent screeners using pre-determined inclusion criteria and a structured process. Our focus was on studies examining the diagnostic accuracy of SN for distinguishing between peripheral and central causes of AVS in patient populations in the ED or on an acute inpatient ward in the acute stage. Not all studies provided specific numbers on delay from symptom onset to clinical examination of SN at the bedside and a subset of patients may have received delayed (i.e., after more than 72 h) examination only. In those studies providing single subject data, only those tested within 72 h were included. When aggregated numbers were provided only, we required testing within 72 h in at least 75% of patients. In those studies that did not provide specific numbers, patients presented with acute symptoms to the emergency department and bedside testing was performed as part of the initial assessment at the ED. Thus, it is reasonable to assume that these were patients with acute (i.e., < 72 h duration) symptoms.
We calculated interrater agreement on full-text inclusion using Cohen's kappa (16). We assessed the risk of bias and applicability concerns for all studies using QUADAS-2 criteria (17). The reference standard for “ruling out” stroke in a peripheral vestibular case was delayed (i.e., more than 48 h after symptom onset) magnetic resonance imaging with diffusion-weighted images (MRI-DWI); strokes could be “ruled in” using confirmatory neuroimaging, including computed tomography (CT) in the appropriate clinical context, but an unconfirmed clinical diagnosis was considered high risk of bias.
We report the diagnostic accuracy of various SN patterns observed at the bedside at primary (i.e., straight-ahead) gaze by visual inspection with fixation either preserved or removed, distinguishing horizontal/horizontal-torsional, vertical [upbeat nystagmus (UBN), downbeat nystagmus (DBN)] and torsional [clockwise (CW), counter-clockwise (CCW)] beating directions. Furthermore, we assess the beating direction of the horizontal component of the SN in relation to the side of the lesion (in case of lateralized lesions)—ipsilesional vs. contralesional—and evaluate the impact of the lesion location (e.g., in the vascular territory of the posterior inferior cerebellar artery [PICA] vs. the anterior inferior cerebellar artery [AICA]).
We calculated sensitivity, specificity, negative likelihood ratio (LR-) and positive likelihood ratio (LR+) for ED or acute-ward diagnoses for any central condition [including stroke, but not limited to this entity as the clinical presentation of other causes of cAVS is often overlapping (e.g., vestibular migraine)]. We present estimated proportions and, where appropriate, 95% confidence intervals (95% CI). Confidence intervals for sensitivities and specificities are calculated based on the Wilson method for binomial counts (18). For a study with zero cells, a continuity correction of 0.5 is added to all cells of that study. Confidence intervals for positive and negative likelihood ratios are calculated based on (19). A summary measure for each “finding” was calculated using a random effect model using the DerSimonian-Laird estimator (20, 21). Sensitivity of a study was calculated (and contributed to the random effect model) even when all other measures were not available (missing false-positives or true-negatives). Tests of heterogeneity were conducted based on Cochran's Q-test. Heterogeneity statistics (Cochran's Q-test) were calculated using R v4.2.1 (Foundation for statistical computing, Vienna, Austria) by a PhD biostatistician. For comparison of proportions, Fisher's exact test was used (Matlab R2022a, The MathWorks, USA) (22). This review is reported in accordance with PRISMA guidelines.
Source data used for meta-analysis will be made available to others upon request to the corresponding author.
Our search identified 4,186 unique citations, of which 3,967 (94.8%) were excluded at the abstract level. Our independent raters had moderate initial agreement on inclusion of full-text manuscripts (kappa value 0.44). After resolving initial disagreements, 39/219 studies were considered eligible (Figure 1—PRISMA flow chart), representing 1.0% of the total. Among the 37 studies included in the final meta-analysis [two studies reporting preliminary data (7) or overlapping data (23) were excluded], the risk of bias and applicability concerns using the QUADAS-2 rating system was judged “high” or “unclear” in one (n = 10), two (n = 8), three (n = 10), or more than three (n = 8) of the seven QUADAS-2 bias/applicability categories (see Supplementary Table 1).

Figure 1. Citation search and selection flow diagram. *MEDLINE was accessed via PubMed; Embase was accessed via embase.com. †Hand search of citation lists from selected studies and investigator files identified 10 additional manuscripts for review. ‡Abstracts coded as “yes” or “maybe” by at least one reviewer were included in full-text review. §After full-text evaluation by two reviewers, any differences were resolved by discussion and adjudication by a third, independent reviewer.
Included studies (n = 37) reported on 1,599 unique patients [747 stroke; 13 intracranial hemorrhages; 26 other central causes (including vestibular migraine, multiple sclerosis, brainstem encephalitis); 743 acute unilateral vestibulopathy; 6 benign paroxysmal positional vertigo (BPPV) and 64 other], full study details are provided in Supplementary Table 2. Eleven studies reported on mixed populations with both peripheral and central causes, whereas the other 26 studies were focusing on either cAVS patients (n = 22) or pAVS patients (n = 4) only. The study design was cross-sectional in all but 3 studies and data collection was retrospective in the majority (n = 23 studies). Bedside testing was performed most frequently by expert neuro-otologists (17 out of 37 studies), but various other specialists were involved as well in other studies (see Table 1 for details). Information on whether SN was assessed with fixation preserved or suppressed (e.g., by use of Frenzel goggles) was provided in 13 manuscripts only, with fixation suppressed in two studies, fixation preserved in four studies, and examinations performed both with and without fixation in seven studies.
While in some studies results from both the initial bedside oculomotor examination and a subsequent dedicated quantitative oculomotor examination are reported, we focused on the findings from the clinical bedside oculomotor exam. In two studies initially included, no bedside testing could be identified, thus these studies were excluded from further analysis (30, 31). In another study included it remained unclear if reported qualitative spontaneous nystagmus patterns were based on bedside testing or on quantitative VOG recordings (32).
Taking into account all 35 studies reporting on bedside SN characteristics in either plane, a SN was observed in almost all pAVS patients and in about half of all cAVS patients [684/701 (97.6%) vs. 293/672 (43.6%)]. Overall, 31 studies did not require presence of a horizontal SN as an inclusion criterion. One of those 31 studies was excluded as 3 out of 4 cells were empty (33). In the remaining 30 studies, absence of any horizontal SN was more frequently observed in cAVS patients than in pAVS patients (350/634 [55.2%] vs. 28/398 [7.0%], p < 0.001). Isolated torsional (12/555, 2.2%) or isolated vertical SN (27/555, 4.9%) was noted in very few participants only, but again was significantly more often found in cAVS patients than in pAVS patients (p < 0.001; see Table 2 for details).
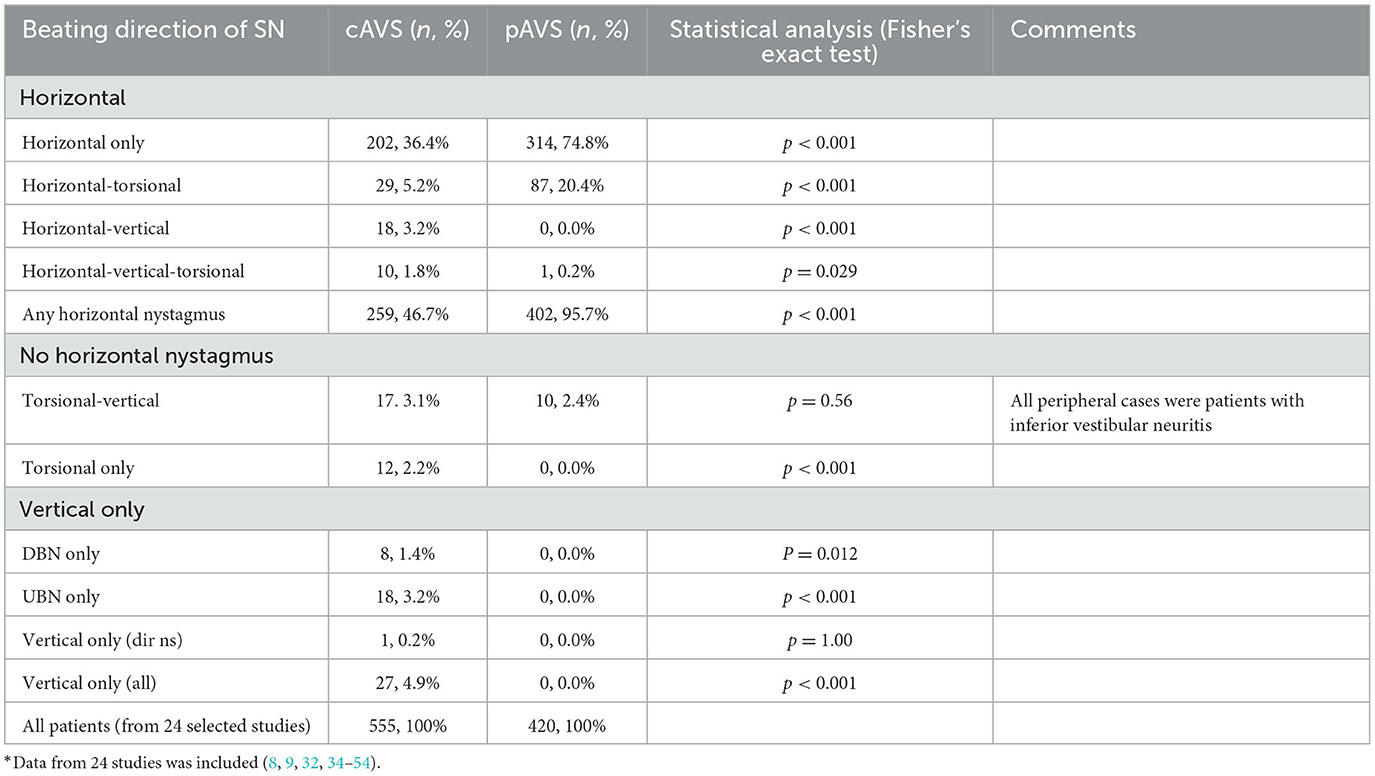
Table 2. Nystagmus patterns in AVS patients with SN (including only those 24 studies that reported on SN in all planes)*.
Eleven studies reported on horizontal SN only, with vertical/torsional SN being either considered an exclusion criterion (33, 55, 56), focusing on the reporting of horizontal SN (24, 25, 57, 58), or not providing any information on whether patients were assessed for the presence/absence of vertical and torsional SN (27–29, 37). These manuscripts were excluded from all analyses concerning the prevalence and distribution of vertical/torsional SN in AVS patients. The remaining 26 studies reported on SN in all planes (horizontal, vertical, and torsional). From those 26 studies, two studies only provided quantitative SN data (30, 31), leaving 24 studies and 975 patients (pAVS = 420; cAVS = 555) for the analysis of bedside SN in all three planes. Focusing on those 24 studies that reported on SN patterns in all planes, a horizontal or horizontal-torsional SN was significantly more often found in pAVS than in cAVS patients (401/420 [95.5%] vs. 231/555 [41.6%], p < 0.001), isolated torsional, isolated torsional-vertical and/or vertical SN patterns were more prevalent in cAVS than in pAVS (84/555 [15.1%] vs. 11/420 [2.6%], p < 0.001; see Table 2 for details).
Diagnostic accuracy of SN patterns for predicting a central origin varied significantly with the SN beating pattern used. For an (isolated) vertical/vertical-torsional SN or an isolated torsional SN, specificity (97.7% [95% CI = 95.1–100.0%]) for a central origin etiology was high, whereas sensitivity (19.1% [10.5–27.7%]) was low (see Table 3 and Figure 2 [forest plots]). When being slightly more inclusive, i.e., using a SN beating pattern of vertical, torsional, vertical-torsional SN with or without horizontal SN, sensitivity was somewhat higher (19.8% [10.7–28.9%]) and specificity was identical (97.7% [95.1–100.0%]). When using more restrictive SN beating patterns, sensitivity further decreased, whereas specificity remained very high. This was true both for selecting a vertical SN pattern (with or without an accompanying horizontal SN; sensitivity = 12.9% [7.6–18.3%]; specificity = 98.2 [96.2–100.0%]) and an isolated torsional SN pattern (sensitivity = 1.0% [0.0–1.8%]; specificity = 99.4 [98.6–100.0%]). Absence of a horizontal SN was highly predictive for a central cause (specificity = 89.8% [84.3–95.4%]), whereas sensitivity was moderate only (sensitivity = 51.6% [3,920–64.1%]; see Table 3 for details).
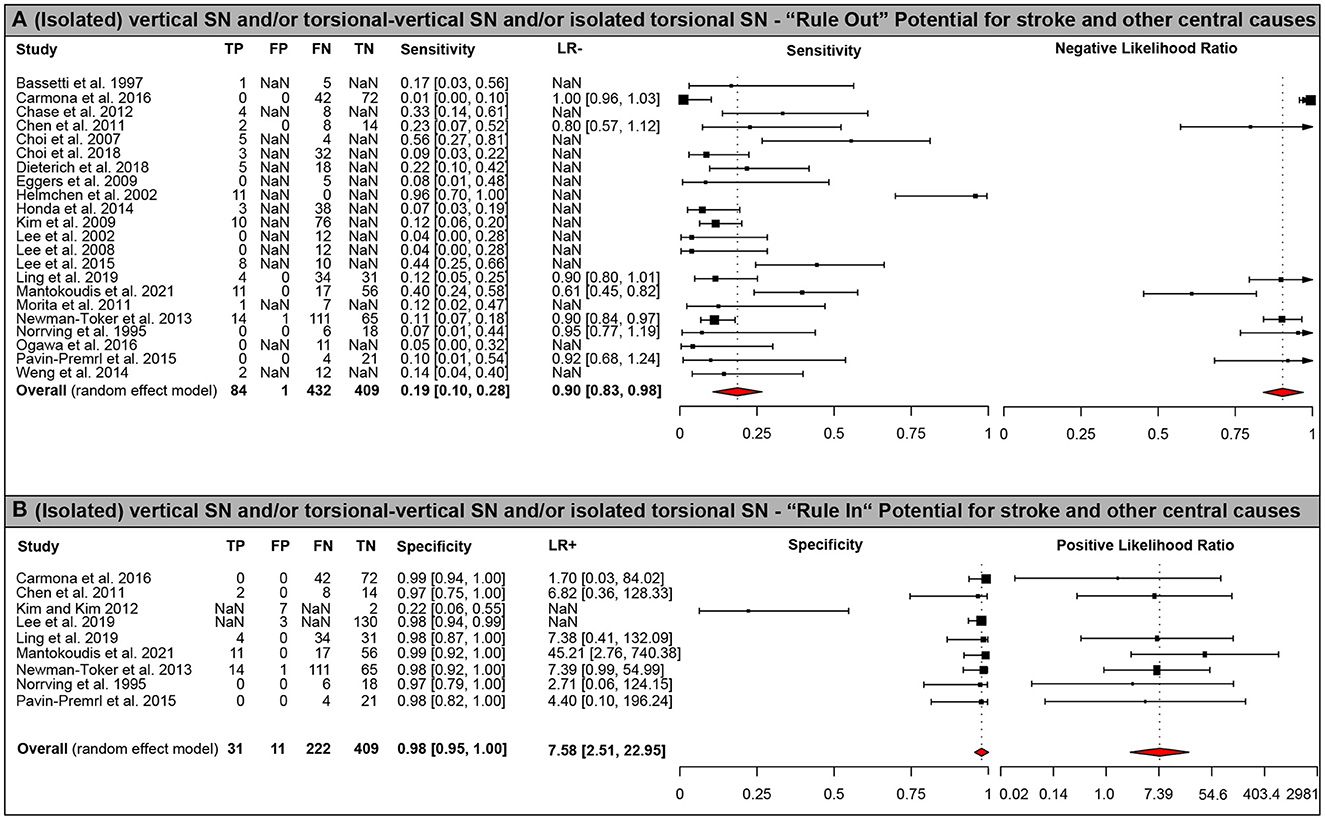
Figure 2. Forest plots of diagnostic test properties. Forest plots of diagnostic test properties for an (isolated) vertical/vertical-torsional or torsional SN. (A) sensitivity and negative likelihood ratio (LR-) including 95% confidence intervals (CI); (B) specificity and positive likelihood ratio (LR+) including 95% CI. Single study results and aggregated values for all test properties (including 95% CI) are provided for all studies included (n = 25). Summary measures were calculated using a random effects model using the DerSimonian-Laird estimator (20, 21). Heterogeneity amongst all studies was significant both for ruling out (i.e., sensitivity, p < 0.001) and for ruling in (i.e., specificity, p < 0.001) central (mostly vertebrobasilar ischemic stroke) causes using Cochran's Q. Note that the axis for positive likelihood ratio uses an exponential scale.
Amongst all patients with SN, the beating direction strongly depended on the underlying cause (peripheral vs. central) and the lesion location for central causes. For central lesions, the distribution of SN patterns varied amongst different affected vascular territories and anatomical lesion locations as shown in Supplementary Table 3. Specifically, the vast majority of PICA strokes (77/89) presented with horizontal only SN, whereas for AICA strokes a horizontal-torsional SN was found at the frequency than a horizontal only SN (20 cases each). Looking at different brainstem strokes, mesencephalic lesions and pontine lesions resulted in various SN patterns. In contrast, the SN pattern critically depended on the type of medullary lesion. Whereas, medial medullary lesions demonstrated mostly with horizontal SN only or UBN only patterns (38/43 patients), lateral medullary strokes presented with a combined horizontal-torsional-vertical SN or a purely horizontal SN at similar rates (36 vs. 40%). Compared to AICA strokes, fractions of patients with any SN were significantly smaller in PICA strokes (65.0 vs. 82.0%, p = 0.031), but not in SCA strokes (62.5 vs. 82.0%, p = 0.34). At the level of the medulla oblongata, medial medullary strokes presented with any SN less often than (dorso-)lateral medullary strokes (44.8 vs. 80.6%, p < 0.001).
SN beating direction in relation to the type and location of the underlying disease could be retrieved in a total of 1,062 patients originating from 35 studies (cAVS = 378, pAVS = 684). Ipsilesional and contralesional beating directions of horizontal (or torsional) SN in cAVS were found at similar frequency (106/378 [28.0%] vs. 82/378 [21.7%], p = 0.052), whereas for pAVS a contralesional SN was significantly more frequent (651/684 [95.2%] vs. 17/684 [2.5%], p < 0.001). Noteworthy, beating direction in relation to the lesion was not reported in 41.5% of all cAVS patients, whereas this fraction was much smaller in pAVS patients (2.3%). Furthermore, no assessment of SN beating direction in relation to the lesion location was possible in those cAVS cases with bilateral lesions (n = 10), non-focal lesions (n = 8), or purely vertical SN patterns (n = 15), as shown in Table 4.
When focusing on cAVS patients with SN and lateralized and focal infratentorial (i.e., cerebellar or brainstem) lesions, the lesion location had a significant impact on the SN beating direction patterns observed. Specifically, for PICA strokes presenting with horizontal (or torsional) SN, beating direction was ipsilesional more often than contralesional (26/109 [23.9%] vs. 7/109 6.4%], p = 0.006), while the opposite was observed for AICA strokes (1/46 [2.2%] vs. 29/46 [63.0%], p < 0.001) as shown in Table 5. Numbers for SCA lesions or combined PICA-AICA and PICA-SCA lesions were too small to reveal statistical significances. For lesions restricted to the medulla oblongata, the lesion location had a critical impact on the SN beating direction. Whereas, in those patients with lesions of the medial parts of the medulla an ipsilesional beating direction of SN was significantly more often observed than a contralesional beating direction (29/43 [67.4%] vs. 2/43 [4.7%], p < 0.001), the opposite was noted in patients with lesions of the (dorso-)lateral medulla oblongata (7/26 [26.9%] vs. 15/26 [57.7%], p = 0.048). For pontine or mesencephalic lesions, ipsilesional and contralesional SN beating directions were observed at similar frequencies.
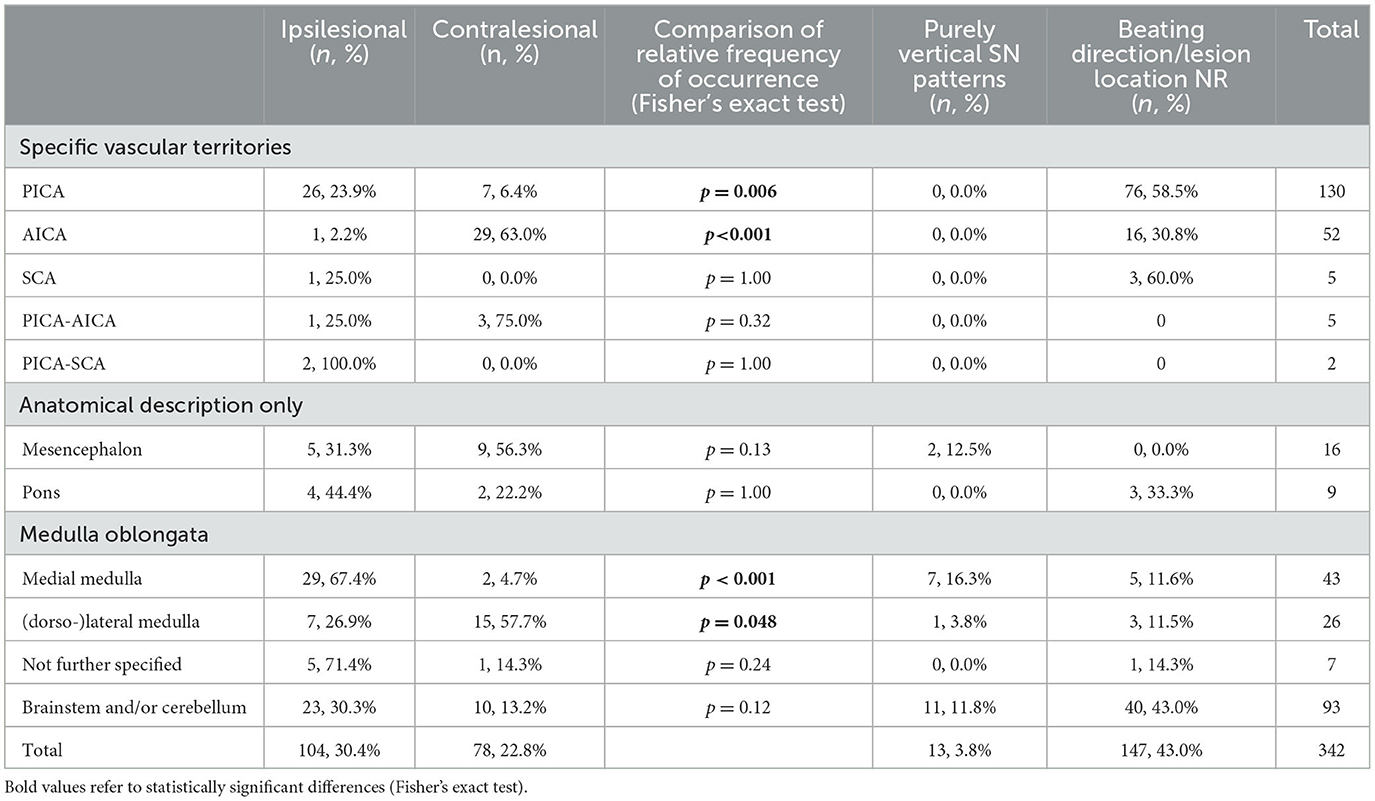
Table 5. Distribution of SN beating direction in cAVS patients with focal lateralized brainstem and/or cerebellar lesions.
Only eight out of 35 studies reported on SN patterns both with fixation and without fixation at the bedside. Whereas, in six studies all patients were evaluation under both conditions (32, 36, 38, 49, 50, 54), in the remaining two studies only in a subset of patients SN was assessed both with fixation preserved and removed (8, 51). The effect of fixation on SN was reported in five studies only (36, 38, 50, 51, 54). Another study reported on SN fixation suppression only after caloric irrigation (32) and additional two studies did not provide any information on the effect on fixation suppression in those patients assessed in both conditions (8, 49).
In four studies, the effect of fixation suppression was reported qualitatively only. In a case series of 18 patients with dorso-lateral medullary stroke, 13 out of 18 patients showed an increase in horizontal–torsional SN with removal of visual fixation (qualitatively, at the bedside) (50). In another case series reporting on patients with (dorso-)lateral medullary infarction, the horizontal and vertical components of the SN were found to be markedly suppressed by visual fixation, whereas the authors noted that the torsional component was less effectively suppressed (38). In patients with mesencephalic stroke and predominantly torsional SN, the authors noted no increase of SN in darkness or with Frenzel goggles (36). In another case series with both pAVS and cAVS patients, fixation suppression of SN was found in eight of the 11 vestibular neuritis patients (73%) where fixation-suppression was tested. In the single stroke patient (PICA stroke) assessed, no fixation suppression of SN was found (51).
In two studies the impact of visual fixation removal was assessed quantitatively. Mantokoudis and co-workers reported a complete nystagmus fixation suppression in 49.5% of patients with AVS, with fractions in patients with vestibular neuritis being lower than in patients with vestibular strokes (40 vs. 62.5%). Whereas, the ocular fixation index (OFI) score calculated had no predictive value for detecting strokes, a nystagmus reduction of < 2°/s was linked to a central cause of AVS, with a sensitivity of 62.2% and a specificity of 84.8% in detecting a stroke (resulting in an overall diagnostic accuracy of 76.9% (95% confidence interval = 59–89%) (54). In another study mean horizontal nystagmus slow-phase velocity (SPV) with fixation in posterior circulation stroke patients (1.6 ± 2.5°/s, range 0–9.6°/s) was significantly slower than in acute vestibular neuritis patients (6.4 ± 5.2°/s, range 0–28.3°/s; p < 0.001) (31). In this study also a greater increase in nystagmus SPV with visual fixation suppression in acute vestibular neuritis compared to posterior circulation stroke was reported, with the mean absolute nystagmus SPV difference between no visual fixation and fixation conditions being significantly higher in acute vestibular neuritis patients compared to posterior circulation stroke patients (5.9 ± 4.7°/s vs. 0.7 ± 2.3°/s, p < 0.001).
Few studies provided a longitudinal assessment of SN characteristics in AVS patients. In one study the authors reported a significant difference in the duration of SN in acute vestibular neuritis patients depending on the extent of canal paresis in caloric irrigation. Specifically, a significantly faster recovery from SN was noted in patients with < 25% canal paresis compared to those patients with more profound canal paresis (i.e., >25%; SN duration: 3.0 ± 1.7 days vs. 4.2 ± 1.9 days, p < 0.001) (28). In another study, time to SN remission was significantly (p < 0.05) shorter in patients with isolated involvement of the inferior branch of the vestibular nerve (inferior vestibular neuritis; SN remission time = 10.3 ± 5.9 days) compared to patients with involvement of either the superior branch of the vestibular nerve (superior vestibular neuritis, 21.5 ± 6.9 days) or both the inferior and the superior branch (total vestibular neuritis, 21.0 ± 13.4 days) (53). In a study reporting on 10 patients with cAVS (and SN in 4/10) initially misdiagnosed as being of peripheral origin, SN duration was reported in three out of four patients only. Specifically, SN disappeared after 1 or 2 days in two patients, whereas it was found to be persistent in another patient (52).
In this systematic review and meta-analysis, we addressed the diagnostic accuracy of spontaneous nystagmus (SN) patterns in patients with acute vestibular syndrome (AVS) of either peripheral (pAVS) or central (cAVS) origin. A SN was observed in almost all pAVS patients and in about half of all cAVS patients (97.6 vs. 43.6%). In those AVS patients demonstrating a SN (with fixation preserved or removed), the SN pattern varied depending on the underlying cause. While a horizontal or horizontal-torsional SN was significantly more often found in pAVS than in cAVS patients (94.8 vs. 43.4%, p < 0.001), isolated torsional, isolated torsional-vertical and/or vertical SN patterns were more prevalent in cAVS than in pAVS (15.1 vs. 2.6%, p < 0.001). When present, it was highly predictive for a central cause (specificity = 97.7% [95% CI = 95.1–100.0%]). The SN beating direction was contralesional in the vast majority of pAVS cases (95.2%), whereas in cAVS cases with focal, lateralized lesions fractions of patients with ipsilesional and contralesional beating direction were similar (28.0 vs. 21.7%, p = 0.052). Thus, in cAVS patients the SN beating direction itself does not allow a prediction on the lesion side. However, for given lesion locations including the medulla oblongata and certain vascular patterns (PICA strokes, AICA strokes), a predominance of either ipsilesional or contralesional horizontal/torsional SN was noted.
In those 24 studies (reporting on a total of 975 patients) providing information on SN beating direction in any plane (horizontal, vertical and torsional), a vertical and/or torsional SN (with or without an accompanying horizontal SN component) was found in a minority (20.4%) of cAVS patients only. When excluding those patients with a horizontal SN component in addition to the presence of a torsional or torsional-vertical nystagmus (i.e., focusing on isolated torsional, isolated torsional-vertical and vertical SN patterns), such a pattern was found in 15.1% of all cAVS patients. While its sensitivity (for ruling out stroke) was low (19.1% [10.5–27.7%]), its specificity (i.e., for ruling in stroke) was very high (97.7% [95.1–100.0%]). Noteworthy, most of the few false-positive cases were linked to inferior vestibular neuritis (IVN), resulting in a combined torsional-downbeating (contralesional) SN pattern, as reported by two studies in our systematic review (49, 53). In IVN, vestibular deficits may be accompanied by ipsilesional, new-onset hearing loss as well. Furthermore, in these patients the horizontal head-impulse test may be normal, resulting in a “central” HINTS pattern (7) and even when adding a fourth sign to the HINTS [new-onset unilateral hearing loss, HINTS “plus” (8)], the pattern may be interpreted as being of central origin. For such cases, obtaining a quantitative video-head-impulse test for all six canals is crucial (23, 59), as it allows canal-specific testing of the vestibulo-ocular reflex (60) and thus should demonstrate isolated impairment of the posterior semicircular canal in case of IVN (49).
In the clinical assessment and in combination with other subtle oculomotor findings, the presence of a horizontal SN indeed may provide valuable. For example, a horizontal/horizontal-torsional SN beating toward the side demonstrating an abnormal head-impulse test points to a central cause (e.g., due to an AICA stroke) rather than to an acute peripheral vestibulopathy (expecting the SN to beat away from the side with the deficient head-impulse test).
Importantly, even in those studies that reported on SN with fixation removed, there was a significant fraction of cAVS patients that did not demonstrate any SN, with fractions in the range of 33–63% (9, 32, 38). Thus, the absence of any SN in a patient with prolonged acute vertigo or dizziness meeting diagnostic criteria for AVS is highly predictive of a central cause with a specificity of 97.9% (95% CI = 96.9–98.9%) and should be considered a central sign in this clinical situation as emphasized by (31).
In patients with underlying central causes of AVS, the SN pattern varied depending on the lesion location, emphasizing distinct contributions to ocular stability by different brainstem and cerebellar structures. Specifically, a torsional component of the SN was observed more frequently in AICA strokes than in PICA strokes and in lateral medullary lesions than in medial medullary lesions. For a detailed discussion of the anatomical-nystagmus correlation with regards to the vascular territory and the lesion sites as observed in the literature, we would like to refer to a recent publication by Nham and colleagues (31).
For peripheral-vestibular lesions, by far most often an acute vestibular neuropathy (vestibular neuritis) was diagnosed (743/813, 91.4%). In these cases, a contralesional nystagmus, i.e., with the fast-phase of the SN beating away from the affected ear, is observed (13). This was reflected in the distribution of pAVS cases, with 95% demonstrating a contralesional SN pattern. In contrast, the lateralizing value of a horizontal/horizontal-torsional SN in central lesions is limited. Overall, patients with focal, lateralized lesions demonstrated ipsilesional and contralesional horizontal/torsional SN beating directions in similar fractions (28.0 vs. 21.7%, p = 0.052). Thus, in cAVS patients the SN beating direction itself does not allow a prediction on the lesion side. However, for given lesion locations such as medial or (dorso-)lateral medullary strokes and certain vascular patterns (PICA strokes, AICA strokes), a predominance of either ipsilesional or contralesional horizontal/torsional SN was noted in our systematic review. In combination with other focal neurologic findings (e.g., pointing to a dorsolateral medullary stroke), the SN beating direction may be valuable in confirming the side of the lesion in these patients. A further differentiation of the beating direction in PICA strokes has been discussed by Nham and colleagues (31) using quantitative SN measurements, with an ipsilesional horizontal SN in those PICA strokes affecting the cerebellum only and a contralesional beating direction in those PICA strokes with brainstem involvement only. Noteworthy, for our meta-analysis we could not retrieve more detailed information on the lesion pattern (brainstem vs. cerebellum) in PICA strokes. With an overall preferentially ipsilesional SN pattern in PICA strokes in our dataset, this would suggest that the majority of PICA stroke cases included here were restricted to the cerebellum.
A decrease (or even cessation) of SN by allowing the acutely dizzy patient to fixate and an enhanced SN by removal of visual fixation is considered an important finding in peripheral-vestibular causes such as acute VN (13). In contrast, a lacking impact of fixation on SN intensity has been associated with central causes in neurology textbooks (61), whereas a systematic review of the literature has emphasized lacking evidence on the accuracy of such a clinical suppression sign (15). More recent publications identified in this systematic review now provide a more detailed picture. Quantitative data on the impact of visual fixation removal was available from two studies, demonstrating that a complete nystagmus fixation suppression may be observed in both patients with acute peripheral vestibulopathy and with vestibular strokes at similar frequency (40 vs. 62.5%) (54). Importantly, the magnitude of nystagmus reduction was helpful in distinguishing peripheral from central causes. Specifically, a nystagmus reduction of < 2°/s was linked to a central cause of AVS (sensitivity = 62.2%, specificity = 84.8%) in one study (54). In another study, a greater increase in nystagmus slow-phase velocity (SPV) with visual fixation suppression in acute vestibular neuritis compared to posterior circulation stroke was reported (5.9 ± 4.7°/s vs. 0.7 ± 2.3°/s, p < 0.001) (31). Noteworthy, in this study the mean horizontal nystagmus SPV with fixation was significantly slower in posterior circulation stroke patients than in acute vestibular neuritis patients (1.6 ± 2.5°/s vs. 6.4 ± 5.2°/s, p < 0.001) (31).
At the bedside, upon removal of visual fixation an increase in horizontal-torsional SN intensity was noted in stroke patients with (dorso-)lateral medullary lesions (50). Noteworthy, the effect of visual fixation seems to depend on the SN plane, as observed in another study with dorso-lateral medullary stroke patients (38). In this study, the horizontal and vertical components of the SN were found to be markedly suppressed by visual fixation, whereas the authors noted that the torsional component was less effectively suppressed (38). A minor impact of visual fixation on torsional SN was also reported in patients with mesencephalic stroke and predominantly torsional SN; specifically the authors noted no increase of SN in darkness or with Frenzel goggles (36).
In summary, the value of SN fixation suppression for distinguishing peripheral from central AVS patients is limited and usually requires a quantitative (VOG-based) assessment of the SN-pattern (54). At the bedside, a qualitative assessment may be not sensitive enough to depict differences in magnitude of response to fixation and fixation removal. When examining the impact of visual fixation removal in this setting, however, clinicians should focus on changes in horizontal and/or vertical SN intensity. Importantly, the presence of fixation suppression does not rule out a central cause of AVS as emphasized by Mantokoudis and co-workers (54).
In this systematic review few included studies reported on serial SN assessments, providing information on the time course of SN. Two of those three studies that did so focused on vestibular neuritis patients (49, 53), whereas in only very few cAVS patients from a single study serial SN assessments were obtained (52). Thus, it remains unclear to which extent delayed presentation to the ED impacts the diagnostic accuracy of SN pattern assessment.
This study has several limitations that need to be further discussed. First, only a minority of studies reported on relatively unselected acutely dizzy patient populations, whereas a majority of studies was at increased risk for a selection bias by including subpopulations such as medial medullary strokes or mesencephalic strokes only, potentially overestimating the presence of certain SN patterns. Over 90% of all peripheral-vestibular cases included were diagnosed with an acute vestibular neuritis, whereas other diagnoses such as Menière's disease which may present with distinct SN patterns (including an irritative ipsilesional SN) were scarce. Not all studies provided specific numbers on delay from symptom onset to clinical examination of spontaneous nystagmus at the bedside and a subset of patients may have received delayed (i.e., after more than 72 h) examination only. While we excluded single patients that received oculomotor bedside testing delayed, this information was not available in all studies. When aggregated numbers were provided, we required that at least 75% of patients received SN evaluation within 72 h after symptom onset. Furthermore, we assumed that patients with obvious stroke symptoms such as hemiparesis or hearing loss generally present to the ED within 24 h after symptom onset and will receive initial clinical examination within hours after presentation to the ED. This pragmatic approach reflects real-life conditions. Note that by potentially including a subset of patients with subacute presentation, spontaneous nystagmus may have disappeared already, thus will result in an underestimation of sensitivity and specificity of SN.
Second, the clinical background of the assessing physicians varied significantly, ranging from subspecialist neuro-otologists to general neurologists or ENT-specialists and emergency physicians. Thus, the level of granularity in describing SN patterns and the amount of expertise likely varied from study to study and physicians not appropriately trained in characterizing a SN may have missed more subtle SN patterns (such as e.g., isolated torsional SN). Third, we did not analyze nystagmus at different gaze positions, which may provide useful also in distinguishing peripheral from central type nystagmus (e.g., by assessing whether the nystagmus follows Alexander's law or not or whether the nystagmus is encoded in a head-fixed or an eye-fixed reference frame). Fourth, the description of lesion locations in relation to the vascular territory affected and/or the definitions of anatomical regions of the brain involved varied amongst studies. This led to more clustered subgroups with both lesion locations based on the vascular supply and the anatomical lesion site, potentially overshading more general patterns, e.g., with regards to the beating direction of SN. Fifth, data on the time course of SN recovery were scarce, thus no conclusions on the time course of SN especially in cAVS patients could be drawn. Sixth, there is a variety in the equipment used for bedside qualitative nystagmus assessments (e.g., Frenzel glasses or videofrenzels) with differences in the sensitivity of detecting nystagmus. For example, the sensitivity in detecting nystagmus with Frenzel glasses is lower than with video frenzels (62).
Unlike other subtle oculomotor bedside findings (such as testing for gaze-evoked nystagmus, vertical divergence, the integrity of the vestibulo-ocular reflex and new-onset unilateral hearing loss), which allow a differentiation between a peripheral and a central cause with high diagnostic accuracy [HINTS+-sensitivity = 99.2% [96.1–100.0%], specificity = 97.0% [90.4–99.5%] (8)], the interpretation of the SN pattern observed in acutely dizzy patients is less straight-forward. Whereas, in those patients that present with an (isolated) vertical and/or torsional SN, a central cause is highly likely (Specificity = 97.7% [95.1–100.0%]) and absence of a horizontal SN (even with fixation removed) strongly suggests a central origin of the patient's AVS (Specificity = 89.8% [84.3–95.4%]), the presence of a horizontal or horizontal-torsional SN does not allow a distinction between a peripheral or a central cause. Furthermore, such an (isolated) vertical and/or torsional SN pattern is found in a minority (15.1%) of central AVS patients only, as reflected by a low sensitivity (19.1% [10.5–27.7%]) and a combined torsional-downbeating SN pattern may be observed in peripheral AVS also in cases with isolated lesions of the inferior branch of the vestibular nerve. Furthermore, the lateralizing value of a horizontal and/or torsional SN in suspected focal lesions is limited at the bedside. While certain lesion locations (anatomical or vascular territory) are associated more often with an ipsilesional or contralesional SN, this cannot be generalized. Additionally, presence of visual fixation suppression does not rule out a central cause of AVS.
In conclusion, a detailed characterization and documentation of the SN pattern observed initially is essential. With a majority of such patients presenting to the emergency department, dedicated training of frontline providers including ED-physicians and neurology/ENT residents in describing and interpreting SN patterns is of great importance.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
MW coded abstract and full-text studies, helped in the analysis, and the interpretation of the data. ZW conducted all analysis and oversaw the interpretation. CM and GM helped abstracting the data and analyzing the results. SC helped develop study protocols, helped abstracting the data, and analyzing the results. AT performed or directly oversaw all aspects of study from conception through completion (principal investigator), designed and conducted the literature search strategy, coded abstract and full-text studies, led analysis, and interpretation of data. All authors critically reviewed and edited the manuscript, seen, and approved the final version.
GM was funded by the Swiss National Science Foundation #320030_173081.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1208902/full#supplementary-material
1. Newman-Toker DE, Cannon LM, Stofferahn ME, Rothman RE, Hsieh YH, Zee DS. Imprecision in patient reports of dizziness symptom quality: a cross-sectional study conducted in an acute care setting. Mayo Clin Proc. (2007) 82:1329–40. doi: 10.4065/82.11.1329
2. Newman-Toker DE, Hsieh YH, Camargo CA Jr, Pelletier AJ, Butchy GT, Edlow JA. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. (2008) 83:765–75. doi: 10.4065/83.7.765
3. Ljunggren M, Persson J, Salzer J. Dizziness and the acute vestibular syndrome at the emergency department: a population-based descriptive study. Eur Neurol. (2018) 79:5–12. doi: 10.1159/000481982
4. Newman-Toker DE. Missed stroke in acute vertigo and dizziness: it is time for action, not debate. Ann Neurol. (2016) 79:27–31. doi: 10.1002/ana.24532
5. Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. (2015) 33, 577–99. doi: 10.1016/j.ncl.2015.04.011
6. WHO. ICD-11 (Mortality and Morbidity Statistics) [Online] (2019). Available online at: https://icd.who.int/dev11/lm/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1462112221 (accessed March 28, 2023).
7. Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. (2009) 40:3504–10. doi: 10.1161/STROKEAHA.109.551234
8. Newman-Toker DE, Kerber KA, Hsieh YH, Pula JH, Omron R, Saber Tehrani AS, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. (2013) 20:986–96. doi: 10.1111/acem.12223
9. Carmona S, Martinez C, Zalazar G, Moro M, Batuecas-Caletrio A, Luis L, et al. The diagnostic accuracy of truncal ataxia and HINTS as cardinal signs for acute vestibular syndrome. Front Neurol. (2016) 7:125. doi: 10.3389/fneur.2016.00125
10. Tarnutzer AA, Gold D, Wang Z, Robinson KA, Kattah JC, Mantokoudis G, et al. Impact of clinician training background and stroke location on bedside diagnostic accuracy in the acute vestibular syndrome -a meta-analysis. Ann Neurol. (2023) doi: 10.1002/ana.26661
11. Carmona S, Martínez C, Zalazar G, Koohi N, Kaski D. Acute truncal ataxia without nystagmus in patients with acute vertigo. Eur J Neurol. (2023) 30:1785–90. doi: 10.1111/ene.15729
12. Kattah JC, Martinez C, Zalazar G, Batuecas Á, Lemos J, Carmona S. Role of incubitus truncal ataxia, and equivalent standing grade 3 ataxia in the diagnosis of central acute vestibular syndrome. J Neurol Sci. (2022) 441:120374. doi: 10.1016/j.jns.2022.120374
13. Strupp M, Bisdorff A, Furman J, Hornibrook J, Jahn K, Maire R, et al. Acute unilateral vestibulopathy/vestibular neuritis: diagnostic criteria. J Vestib Res. (2022) 32:389–406. doi: 10.3233/VES-220201
14. Eggers SDZ, Bisdorff A, Von Brevern M, Zee DS, Kim JS, Perez-Fernandez N, et al. Classification of vestibular signs and examination techniques: nystagmus and nystagmus-like movements. J Vestib Res. (2019) 29:57–87. doi: 10.3233/VES-190658
15. Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ. (2011) 183:E571–592. doi: 10.1503/cmaj.100174
16. Cohen J. A coefficient for agreement for nominal scales. Educ Psychol Meas. (1960) 20:37–46. doi: 10.1177/001316446002000104
17. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
18. Brown LD, Cai TT, Dasgupta A. Interval estimation for a binomial proportion. Stat Sci. (2001) 16:101–17. doi: 10.1214/ss/1009213286
19. Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. (1991) 44:763–70. doi: 10.1016/0895-4356(91)90128-V
20. Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
21. Raudenbush SW. Analyzing effect sizes: random-effects models. In:H. Cooper, L. V. Hedges, and J. C. Valentine, , editors. The Handbook of Research Synthesis and Meta-Analysis. New York, NY: Russell Sage Foundation (2009). p. 295–315.
22. Fisher RA. Statistical Methods for Research Workers, 5th Edn. Edinburgh: Oliver and Boyd (1934).
23. Nham B, Reid N, Bein K, Bradshaw AP, Mcgarvie LA, Argaet EC, et al. Capturing vertigo in the emergency room: three tools to double the rate of diagnosis. J Neurol. (2022) 269:294–306. doi: 10.1007/s00415-021-10627-1
24. Kim CH, Yang YS, Im D, Shin JE. Nystagmus in patients with unilateral acute otitis media complicated by serous labyrinthitis. Acta Otolaryngol. (2016) 136:559–63. doi: 10.3109/00016489.2015.1132845
25. Guler A, Karbek Akarca F, Eraslan C, Tarhan C, Bilgen C, Kirazli T, et al. Clinical and video head impulse test in the diagnosis of posterior circulation stroke presenting as acute vestibular syndrome in the emergency department. J Vestib Res. (2017) 27:233–42. doi: 10.3233/VES-170620
26. Lee H, Sohn SI, Cho YW, Lee SR, Ahn BH, Park BR, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology. (2006) 67:1178–83. doi: 10.1212/01.wnl.0000238500.02302.b4
27. Ogawa K, Suzuki Y, Oishi M, Kamei S, Shigihara S, Nomura Y. Clinical study of medial area infarction in the region of posterior inferior cerebellar artery. J Stroke Cerebrovasc Dis. (2013) 22:508–13. doi: 10.1016/j.jstrokecerebrovasdis.2013.02.006
28. Kim HJ, Kim DY, Hwang JH, Kim KS. Vestibular neuritis with minimal canal paresis: characteristics and clinical implication. Clin Exp Otorhinolaryngol. (2017) 10:148–52. doi: 10.21053/ceo.2016.00948
29. Ogawa K, Suzuki Y, Takahashi K, Akimoto T, Kamei S, Soma M. Clinical study of seven patients with infarction in territories of the anterior inferior cerebellar artery. J Stroke Cerebrovasc Dis. (2017) 26:574–81. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.118
30. Choi JH, Seo JD, Choi YR, Kim MJ, Kim HJ, Kim JS, et al. Inferior cerebellar peduncular lesion causes a distinct vestibular syndrome. Eur J Neurol. (2015) 22:1062–7. doi: 10.1111/ene.12705
31. Nham B, Akdal G, Young AS, Ozcelik P, Tanriverdizade T, Ala RT, et al. Capturing nystagmus in the emergency room: posterior circulation stroke versus acute vestibular neuritis. J Neurol. (2023) 270:632–41. doi: 10.1007/s00415-022-11202-y
32. Ling X, Sang W, Shen B, Li K, Si L, Yang X. Diagnostic value of eye movement and vestibular function tests in patients with posterior circulation infarction. Acta Otolaryngol. (2019) 139:135–45. doi: 10.1080/00016489.2018.1552367
33. Lee JY, Kim CH, Park JS, Kim MB. Peripheral vestibulopathy presenting as acute vertigo and spontaneous nystagmus with negative video head impulse test. Otolaryngol Head Neck Surg. (2019) 160:894–901. doi: 10.1177/0194599818825458
34. Norrving B, Magnusson M, Holtas S. Isolated acute vertigo in the elderly; vestibular or vascular disease? Acta Neurol Scand. (1995) 91:43–8. doi: 10.1111/j.1600-0404.1995.tb06987.x
35. Bassetti C, Bogousslavsky J, Mattle H, Bernasconi A. Medial medullary stroke: report of seven patients and review of the literature. Neurology. (1997) 48:882–90. doi: 10.1212/WNL.48.4.882
36. Helmchen C, Rambold H, Kempermann U, Buttner-Ennever JA, Buttner U. Localizing value of torsional nystagmus in small midbrain lesions. Neurology. (2002) 59:1956–64. doi: 10.1212/01.WNL.0000038387.90128.8D
37. Lee H, Sohn SI, Jung DK, Cho YW, Lim JG, Yi SD, et al. Sudden deafness and anterior inferior cerebellar artery infarction. Stroke. (2002) 33:2807–12. doi: 10.1161/01.STR.0000038692.17290.24
38. Choi KD, Oh SY, Park SH, Kim JH, Koo JW, Kim JS. Head-shaking nystagmus in lateral medullary infarction: patterns and possible mechanisms. Neurology. (2007) 68:1337–44. doi: 10.1212/01.wnl.0000260224.60943.c2
39. Lee H, Yi HA, Lee SR, Lee SY, Park BR. Ocular torsion associated with infarction in the territory of the anterior inferior cerebellar artery: frequency, pattern, and a major determinant. J Neurol Sci. (2008) 269:18–23. doi: 10.1016/j.jns.2007.12.009
40. Eggers C, Fink GR, Moller-Hartmann W, Nowak DA. Correlation of anatomy and function in medulla oblongata infarction. Eur J Neurol. (2009) 16:201–4. doi: 10.1111/j.1468-1331.2008.02381.x
41. Kim JS, Han YS. Medial medullary infarction: clinical, imaging, and outcome study in 86 consecutive patients. Stroke. (2009) 40:3221–5. doi: 10.1161/STROKEAHA.109.559864
42. Chen L, Lee W, Chambers BR, Dewey HM. Diagnostic accuracy of acute vestibular syndrome at the bedside in a stroke unit. J Neurol. (2011) 258:855–61. doi: 10.1007/s00415-010-5853-4
43. Morita S, Suzuki M, Iizuka K. False-negative diffusion-weighted MRI in acute cerebellar stroke. Auris Nasus Larynx. (2011) 38:577–82. doi: 10.1016/j.anl.2011.01.017
44. Chase M, Joyce NR, Carney E, Salciccioli JD, Vinton D, Donnino MW, et al. ED patients with vertigo: can we identify clinical factors associated with acute stroke? Am J Emerg Med. (2012) 30:587–91. doi: 10.1016/j.ajem.2011.02.002
45. Honda S, Inatomi Y, Yonehara T, Hashimoto Y, Hirano T, Ando Y, et al. Discrimination of acute ischemic stroke from nonischemic vertigo in patients presenting with only imbalance. J Stroke Cerebrovasc Dis. (2014) 23:888–95. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.029
46. Weng YC, Young YH. Mapping affected territory of anterior/posterior inferior cerebellar artery infarction using a vestibular test battery. Acta Otolaryngol. (2014) 134:268–74. doi: 10.3109/00016489.2013.851797
47. Choi JH, Oh EH, Park MG, Baik SK, Cho HJ, Choi SY, et al. Early MRI-negative posterior circulation stroke presenting as acute dizziness. J Neurol. (2018) 265:2993–3000. doi: 10.1007/s00415-018-9097-z
48. Dieterich M, Glasauer S, Brandt T. Why acute unilateral vestibular midbrain lesions rarely manifest with rotational vertigo: a clinical and modelling approach to head direction cell function. J Neurol. (2018) 265:1184–98. doi: 10.1007/s00415-018-8828-5
49. Kim JS, Kim HJ. Inferior vestibular neuritis. J Neurol. (2012) 259:1553–60. doi: 10.1007/s00415-011-6375-4
50. Lee SU, Park SH, Park JJ, Kim HJ, Han MK, Bae HJ, et al. Dorsal medullary infarction: distinct syndrome of isolated central vestibulopathy. Stroke. (2015) 46:3081–7. doi: 10.1161/STROKEAHA.115.010972
51. Pavlin-Premrl D, Waterston J, Mcguigan S, Infeld B, Sultana R, O'sullivan R, et al. Importance of spontaneous nystagmus detection in the differential diagnosis of acute vertigo. J Clin Neurosci. (2015) 22:504–7. doi: 10.1016/j.jocn.2014.09.011
52. Ogawa Y, Otsuka K, Hagiwara A, Inagaki T, Shimizu S, Nagai N, et al. Clinical evaluation of acute phase nystagmus associated with cerebellar lesions. J Laryngol Otol. (2016) 130:536–40. doi: 10.1017/S0022215116001079
53. Lee JY, Park JS, Kim MB. Clinical characteristics of acute vestibular neuritis according to involvement site. Otol Neurotol. (2019) 40:797–805. doi: 10.1097/MAO.0000000000002226
54. Mantokoudis G, Wyss T, Zamaro E, Korda A, Wagner F, Sauter TC, et al. Stroke prediction based on the spontaneous nystagmus suppression test in dizzy patients: a diagnostic accuracy study. Neurology. (2021) 97:e42–51. doi: 10.1212/WNL.0000000000012176
55. Braun EM, Tomazic PV, Ropposch T, Nemetz U, Lackner A, Walch C. Misdiagnosis of acute peripheral vestibulopathy in central nervous ischemic infarction. Otol Neurotol. (2011) 32:1518–21. doi: 10.1097/MAO.0b013e318238ff9a
56. Calic Z, Nham B, Taylor RL, Young AS, Bradshaw AP, Mcgarvie LM, et al. Vestibular migraine presenting with acute peripheral vestibulopathy: clinical, oculographic and vestibular test profiles. Cephal Rep. (2020) 3:2515816320958175. doi: 10.1177/2515816320958175
57. Kim GW, Heo JH. Vertigo of cerebrovascular origin proven by CT scan or MRI: pitfalls in clinical differentiation from vertigo of aural origin. Yonsei Med J. (1996) 37:47–51. doi: 10.3349/ymj.1996.37.1.47
58. Lee SJ, Lee SA, Kim BG, Hong HS, Lee JY, Lee JD. Feasibility of magnetic resonance imaging in the differential diagnosis of isolated acute audiovestibular loss. J Vestib Res. (2018) 28:385–91. doi: 10.3233/VES-190649
59. Carmona S, Grinstein G, Weinschelbaum R, Zalazar G. Topodiagnosis of the inner ear: illustrative clinical cases. Ann Otolaryngol Rhinol. (2018) 5:1201.
60. Macdougall HG, Mcgarvie LA, Halmagyi GM, Curthoys IS, Weber KP. The video Head Impulse Test (vHIT) detects vertical semicircular canal dysfunction. PLoS ONE. (2013) 8:e61488. doi: 10.1371/journal.pone.0061488
61. Baloh M, Faan Robert W, Honrubia M, Dmsc V, Kerber M, Kevin A. Baloh and Honrubia's Clinical Neurophysiology of the Vestibular System. New York, NY: Oxford University Press (2011). doi: 10.1093/med/9780195387834.001.0001
Keywords: bedside diagnostic accuracy, nystagmus, stroke, vestibular, vertigo, dizziness
Citation: Wüthrich M, Wang Z, Martinez CM, Carmona S, Mantokoudis G and Tarnutzer AA (2023) Systematic review and meta-analysis of the diagnostic accuracy of spontaneous nystagmus patterns in acute vestibular syndrome. Front. Neurol. 14:1208902. doi: 10.3389/fneur.2023.1208902
Received: 19 April 2023; Accepted: 05 June 2023;
Published: 16 June 2023.
Edited by:
James Robert Lackner, Brandeis University, United StatesReviewed by:
Sun-Uk Lee, Korea University Medical Center, Republic of KoreaCopyright © 2023 Wüthrich, Wang, Martinez, Carmona, Mantokoudis and Tarnutzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Andrea Tarnutzer, YWxleGFuZGVyLnRhcm51dHplckBhY2Nlc3MudXpoLmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.