- 1Department of Neurology, Sichuan Taikang Hospital, Chengdu, Sichuan, China
- 2Department of Respiratory, Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi, China
- 3Department of Rehabilitation, Affiliated Hospital of Yunnan University, Kunming, Yunnan, China
Background: There is mounting evidence suggesting that autoimmune encephalitis (AE) can be observed as a neurological complication in patients with COVID-19. This review aimed to summarize the clinical manifestations, types, and outcomes of COVID-19-associated AE.
Methods: A systematic search was conducted in the PubMed, Embase, and Web of Science databases to identify case reports and case series related to COVID-19-associated AE from 1 January 2020 to 31 March 2023. After a thorough screening and evaluation, irrelevant articles were excluded. Relevant information concerning types, clinical manifestations, and outcomes was extracted and synthesized.
Results: A total of 37 studies, comprising 34 case reports and 3 case series, were included in this review. Among the 42 COVID-19-associated AE patients, 21 (50%) cases were classified as an unknown antibodies (Ab) type of COVID-19-associated AE, 10 (23.80%) cases as anti-N-methyl-D-aspartate (NMDA) encephalitis, 4 (9.5%) cases as limbic encephalitis, and 3 (7.1%) cases as anti-myelin-oligodendrocyte-glycoprotein encephalitis, along with other rare types of AE. Disturbance of consciousness, seizures, and psychiatric symptoms were identified as the main clinical manifestations of COVID-19-associated AE. While the symptoms of AE displayed variation, most patients achieved full recovery although a few experienced residual symptoms of neurological damage.
Conclusion: This systematic review comprehensively describes the characteristics of COVID-19-associated AE. The main type of COVID-19-associated AE identified in this study is an unknown Ab type of COVID-19-associated AE. Despite the potentially life-threatening risks of COVID-19-associated AE, the majority of patients survived, with some patients reporting residual neurological symptoms.
1. Introduction
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease caused by a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (1). It has placed an unprecedented burden on economies and healthcare systems worldwide since the beginning of the COVID-19 pandemic (2). Recent studies have demonstrated that both COVID-19 patients and those with autoimmune diseases exhibit similar immune responses affecting multiple organs and systems, including the respiratory, neurological, cardiovascular, hematologic, and endocrine systems (3). Overproduction of cytokines and overactivation of immune cells by the immune system lead to uncontrolled immune reactions resulting in organ damage and the production of autoantibodies due to the breakdown of immune tolerance (3). Additionally, molecular mimicry triggered by SARS-CoV-2 infection may induce autoimmunity in COVID-19 patients (4).
During the initial stages of the COVID-19 outbreak, several studies indicated that 36.4% of patients experienced neurological symptoms, affecting various parts of the nervous system such as the central nervous system, peripheral nervous system, and skeletal muscles (5). Furthermore, COVID-19 exerts a significant impact on the neuropsychological health of both patients and caregivers. Olfactory and gustatory deficits are considered the most common manifestations of peripheral nerve damage in COVID-19 patients, with incidence rates as high as 41.0 and 38.2%, respectively (6, 7). Peripheral nervous system involvement can also lead to symptoms such as facial nerve palsy and oculomotor abnormalities in COVID-19 patients (7, 8). A study of mental and psychological burdens in 204 countries estimated that the global prevalence of depressive disorders increased by 28% and anxiety disorders by 26% during the COVID-19 pandemic (9).
The neurological impacts of COVID-19 extend beyond dizziness, headaches, facial paralysis, and strokes. A mounting body of evidence has documented cases of COVID-19-associated autoimmune encephalitis (AE) (10). Initially, some symptoms may appear mild, such as fever, headache, dizziness, and lethargy, which could be easily overlooked or mistaken for medication side effects or other unspecified infections (4). However, as the disease progresses, more severe symptoms may manifest, including mental disorders, abnormal behavior, amnesia, aphasia, clumsiness, convulsions, and, in severe cases, loss of consciousness and coma (11). In a meta-analysis conducted by Siow et al., which encompassed 129,008 patients with COVID-19, the prevalence of encephalitis was found to be 0.215% (11).
COVID-19 patients, especially in severe cases, exhibited abnormal inflammatory responses that led to hyperactive innate immune reactions, excessive inflammation, and increased levels of pro-inflammatory mediators, cytokines, chemokines, ferritin, and D-dimer (12). Lower-than-normal levels of helper T cells and suppressor T cells in severe COVID-19 patients suggest possible lymphocyte damage and the induction of adaptive immune hyperfunction by SARS-CoV-2 (13). Lymphocytes, which express angiotensin-converting enzyme 2 (ACE-2), serve as a binding site for SARS-CoV-2, leading to lymphocyte death and COVID-19-associated AE (14). The mechanisms by which SARS-CoV-2 enters the brain and causes encephalopathy are still a matter of controversy, and several major theories have been proposed. First, it is possible that SARS-CoV-2 may be transmitted retrogradely through axons in the olfactory, respiratory, and enteric nervous system networks (14). Second, it is suggested that SARS-CoV-2 can breach the blood-brain barrier by disrupting the endothelial lining, thereby gaining access to brain tissue (15, 16). Once inside the brain, SARS-CoV-2 can interact with both neuronal and non-neuronal cells that express ACE-2 receptors (17, 18).
Despite accumulating reports of COVID-19-associated AE, few systematic reviews have summarized AE as a neurological complication of COVID-19. To gain a better understanding of COVID-19-associated AE, we performed a comprehensive assessment of the clinical manifestations, types, and outcomes of COVID-19-associated AE.
2. Methods
This review was conducted in accordance with the guidelines for preferred reporting elements for systematic reviews and meta-analyses (PRISMA) (19).
2.1. Search strategy
We conducted an extensive and exhaustive search of case reports and series between 1 January 2020 and 31 March 2023 from PubMed, Embase, and Web of Science. The search terms included “Autoimmune Encephalitis,” “Antibody-Mediated Encephalitis,” “Antibody Mediated Encephalitis,” “limbic encephalitis,” “Anti NMDA,” “Anti MOG,” “Anti LGI1,” “Anti GABA,” “coronavirus disease-19,” “SARS-CoV-2,” “COVID 19,” and “COVID-19.” All English publications describing case reports and case series of confirmed COVID-19 infections with AE were included. Two independent reviewers comprehensively searched and filtered the eligible studies. We excluded all review articles, other types of encephalitis, and unconfirmed cases of AE.
2.2. Inclusion criteria
This case report and case series met the following inclusion criteria: (1) patients tested positive for SARS-CoV-2 in the reverse transcription-polymerase chain reaction (RT-PCR) or had a positive title for SARS-CoV-2 serum antibody and (2) raw data on clinical symptoms, diagnosis, treatment, and outcome of COVID-19-associated AE were provided.
2.3. Study selection and data extraction
Two reviewers independently selected studies by reading the full articles and then extracting data from the eligible articles. The following data were extracted from the included studies: the first author's last name, year of publication, number of patients, participant's age, participant gender, SARS-CoV-2 diagnosis method, neurological manifestations, imaging analysis, cerebrospinal fluid (CSF), types, AE diagnosis method, treatment details, and outcomes.
3. Results
3.1. Searching published literature
A total of 443 articles were yielded after a comprehensive search in our literature. After removing 223 duplicate articles, 153 irrelevant title and abstract articles and 66 full-text articles were further evaluated. Finally, 37 studies were included in our systematic review after excluding 6 review articles, 4 modeling studies, 4 commentary, 3 editorials, and 13 other encephalitides (including herpes simplex virus encephalitis, cytomegalovirus encephalitis, and varicella-zoster virus encephalitis) (Figure 1) (20–56).
3.2. Study characteristics
This article includes 37 studies, consisting of 34 case report studies and 3 case series. The basic information of the included studies is shown in Table 1. A total of 42 participants were included in this systematic review. The case reports and case series were conducted in various countries, including the United States (n = 17), Italy (n = 4), Iran (n = 2), the United Kingdom (n = 2), Spain (n = 2), Korea (n = 2), India (n = 2), Singapore (n = 1), France (n = 1), Egypt (n = 1), Mexico (n = 1), Germany (n = 1), Japan (n = 2), Turkey (n = 1), and China (n = 1).
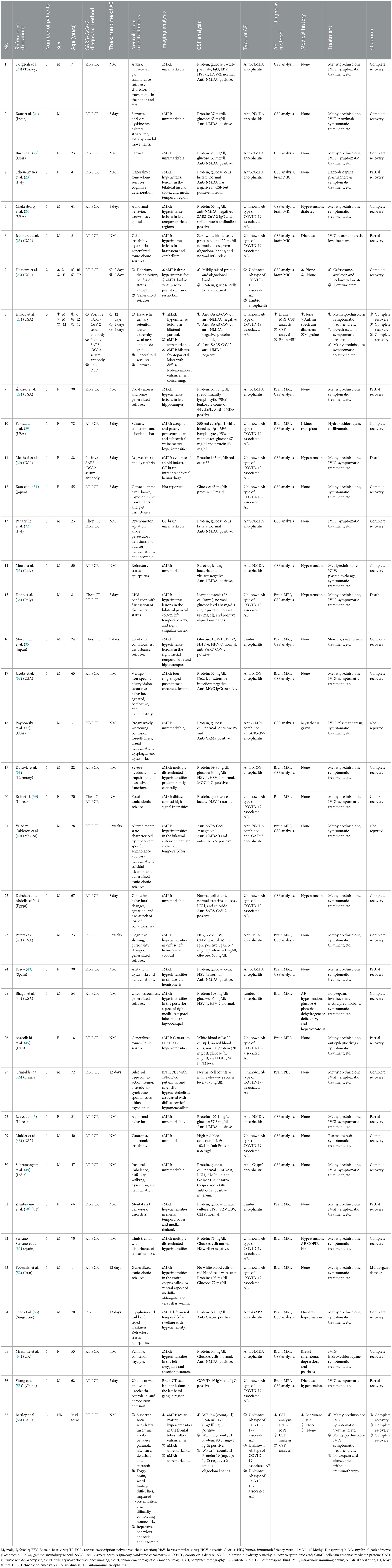
Table 1. Characteristics of included studies for autoimmune encephalitis as a neurological complication in COVID-19 patients.
3.3. Neurological manifestations
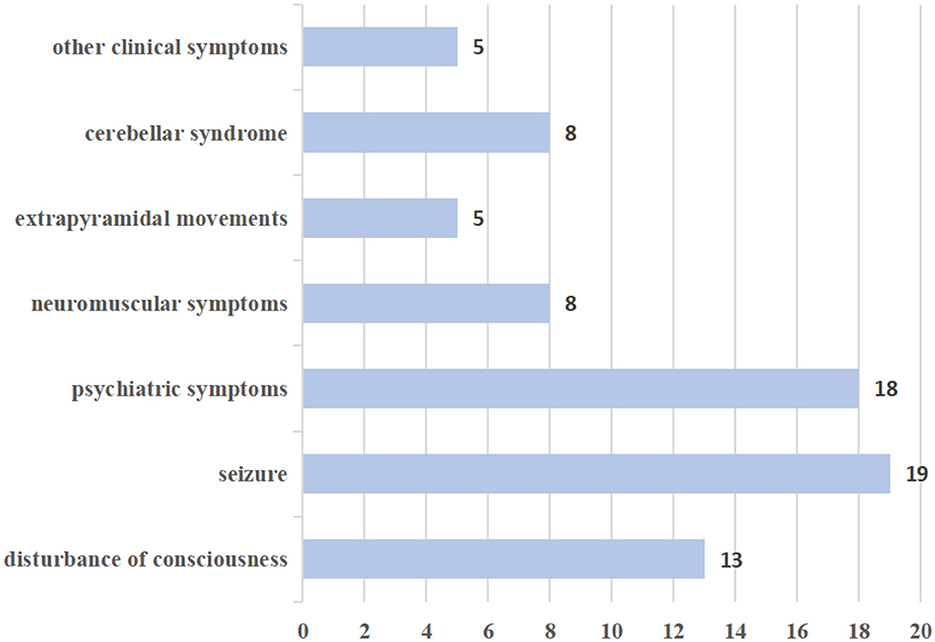
Neurological manifestations in this study were reported in seven categories: (A) disturbance of consciousness; (B) seizures; (C) psychiatric symptoms; (D) neuromuscular symptoms such as myalgia, weakness, and myoclonus; (E) extrapyramidal movements; (F) cerebellar syndrome; and (G) other clinical symptoms. The frequency of various neurological manifestations is presented in Figure 2.
In this review, disturbances of consciousness were observed in 13 patients, primarily characterized by symptoms such as drowsiness, delirium, confusion, and unconsciousness. Additionally, 19 patients exhibited seizures, with 5 experiencing generalized tonic-clonic seizures and 2 experiencing refractory status epilepticus. In all, 18 patients in the study exhibited psychiatric symptoms, which mainly manifested as abnormal behavior, persecutory delusions, and auditory hallucinations. In addition, neuromuscular symptoms were observed, including myalgia, weakness, hypermyotonia, and myoclonus. Notably, many patients displayed extrapyramidal movements, such as choreiform movements in the hands and feet, peri-oral dyskinesias, bilateral upper-limb action tremors, and limb tremors. Eight patients had cerebellar syndrome, characterized by ataxia, dysarthria, and gait disturbance. Other clinical symptoms included ouroclepsia, palilalia, autonomic instability, headache, vertigo, and non-specific blurry vision.
3.4. Types of AE
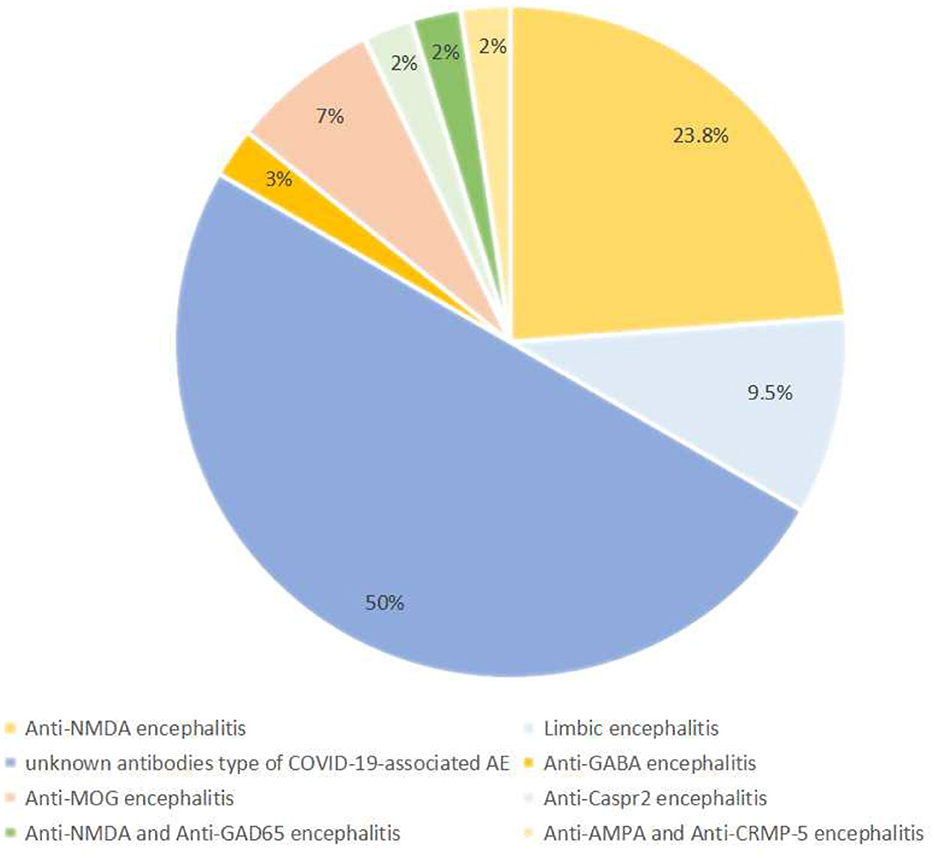
Among the studies included in this analysis, 42 patients were diagnosed with AE, including 21 (50%) cases of an unknown type of COVID-19-associated AE, 10 (23.8%) cases of anti-N-methyl-D aspartate (NMDA) encephalitis, 4 (9.5%) cases of limbic encephalitis, 3 (7.1%) cases of anti-myelin oligodendrocyte glycoprotein (MOG) encephalitis, 1 case of anti-gamma aminobutyric acid (GABA) encephalitis, 1 case of anti-Caspr 2 encephalitis, 1 case of anti-NMDA combined anti-glutamic acid decarboxylase-65 (GAD-65) encephalitis, and 1 case of anti-a-amino-3-hydroxy-5methyl-4-isoxazolepropionic acid (AMPA) combined anti-collapsin response mediator protein-5 (CRMP-5) encephalitis. The frequencies of the various types of AE are depicted in Figure 3.
In this study, the main type of COVID-19-associated AE is an unknown antibody (Ab) type of COVID-19-associated AE, where despite no detection of AE-related Ab in the CSF, a diagnosis of possible AE was made according to Graus criteria: first, they had an acute (< 2 weeks) or subacute (≤ 3 months) onset, accompanied by neurological and psychiatric symptoms or clinical syndrome signs. Second, they showed one or more of the following auxiliary examinations: CSF abnormalities, neuroimaging abnormalities, or electrophysiological abnormalities. Third, other potential causes were reasonably excluded (57). Notably, 21 patients were diagnosed with an unknown Ab type of COVID-19-associated AE; these patients exhibited consciousness disturbance, generalized tonic-clonic seizures, persecution delusion, autonomic instability, and dysarthria (Table 1) (24–27, 29–31, 39, 41, 45, 46, 48, 51, 52, 55, 56). The ages of these patients ranged from 1 to 88 years, with a mean age of 45.3 years. Among this group, 10 cases reported symptom onset ranging from 2 to 12 days (with an average of 8 days) from the positive SARS-CoV-2 RT-PCR.
In all, 10 studies included 10 patients diagnosed with anti-NMDA receptor encephalitis, ranging in age from 1 to 53 years (with an average of 24.2 years) (20–23, 28, 32, 33, 43, 47, 54). The neurological manifestations of these patients mainly included seizures, persecutory delusions, hallucinations, and abnormal behavior.
There were four cases of limbic encephalitis, with the main symptoms being generalized seizures and disturbances of consciousness (26, 35, 44, 50). Three cases reported anti-MOG encephalitis, and prominent clinical manifestations were vertigo, non-specific blurry vision, and assaultive behavior. Notably, the magnetic resonance imaging findings of anti-MOG encephalitis were usually diffuse and multifocal (36, 38, 42).
Additionally, four rare cases of AE were reported, including anti-Caspr 2 encephalitis, anti-NMDA combined anti-GAD65 encephalitis, anti-AMPA combined anti-CRMP-5 encephalitis, and anti-GABA encephalitis (37, 40, 49, 53). These patients mainly manifested hallucinations, postural imbalance, generalized seizures, and dysarthria.
3.5. Cerebrospinal fluid
Among 42 patients, 19 (45.23%) had elevated CSF protein levels, ranging from 49.0 to 838.0 mg/dl. Most of these cases showed mild elevation. The average glucose level in CSF was 81.7 mg/dl (range: 59.0–130.0 mg/dl), indicating raised levels. CSF glucose was found to be elevated in 9 (21.42%) patients, with a maximum value of 78 mg/dl. Additionally, four cases reported elevated levels of IgG in the CSF, and four cases reported the presence of oligoclonal bands.
3.6. MRI results
Among the 42 patients, 36 patients conducted ordinary MRI and 2 conducted enhanced MRI. Of these, 12 (33.33%) patients exhibited no significant abnormalities on ordinary MRI, while 24 (66.66%) patients displayed varying degrees of abnormalities. In the reviewed cases, hyperintensity has been observed in different regions, such as white matter, temporal lobe, parietal lobe, insular cortex, brainstem, cerebellum, and corpus callosum, and in some cases, it has been observed in multiple disseminated regions. Among 21 unknown types of COVID-19-associated AE patients, 12 (57.14%) patients exhibited abnormalities on ordinary MRI, which mainly manifested as hyperintensity in the frontal lobe, corpus callosum, and temporal lobe, and in severe cases, brainstem and multiple diffuse hyperintensities.
3.7. Medical history
The most frequently reported medical history was hypertension, accounting for 19.07% (n = 8), followed by diabetes, accounting for 9.52% (n = 4). Other medical history included autism spectrum disorders, migraine, kidney transplant, myasthenia gravis, glucose-6-phosphate dehydrogenase deficiency, and hepatosteatosis. Among the reviewed cases, in only three patients, COVID-19 vaccination was reported.
3.8. Outcome
Among the 42 patients, 29 (69.04%) achieved complete recovery, 8 (19.04%) showed partial recovery, and 1 experienced multiorgan damage, and in 2 cases the outcome was not reported. Two (4.76%) patients died due to sepsis and intracranial hemorrhage, respectively. Despite the varied symptoms of AE, most patients were able to fully recover or achieve partial recovery, with only a few experiencing residual neurological symptoms.
4. Discussion
AE is one of the neurological complications of COVID-19. This study conducted a review of case series and case reports to evaluate the neurological manifestations, types, and outcomes of COVID-19-associated AE. Previous studies have shown that in COVID-19-associated AE patients, the onset of symptoms can occur several weeks after or even during acute SARS-CoV-2 infection (55). In our review, we observed that the neurological manifestations of patients could be categorized into seven categories, including disturbance of consciousness, seizures, psychiatric symptoms, neuromuscular symptoms, extrapyramidal movements, cerebellar syndrome, and other clinical symptoms. The most common symptoms were seizures (19 cases), disturbance of consciousness (13 cases), and psychiatric symptoms (18 cases). COVID-19-associated AE patients who experienced epileptic seizures predominantly presented with generalized tonic-clonic seizures. COVID-19-associated AE can also manifest as psychiatric symptoms, such as cognitive decline, abnormal behavior, delusions of persecution, and hallucinations. Furthermore, some patients may initially experience neuromuscular symptoms or extrapyramidal movements, including myalgia, weakness, myoclonus, perioral dyskinesias, myoclonus-like movements, and limb tremors. COVID-19-associated AE patients also exhibited other clinical symptoms, including uroclepsia, palilalia, autonomic instability, headache, vertigo, and non-specific blurry vision.
In this review, we identified several types of COVID-19-associated AE, including an unknown type of COVID-19-associated AE, anti-NMDA encephalitis, limbic encephalitis, anti-MOG encephalitis, anti-GABA encephalitis, anti-Caspr-2 encephalitis, anti-NMDA combined anti-GAD-65 encephalitis, and anti-AMPA combined anti-CRMP-5 encephalitis. In our cohort of patients, the most frequently reported COVID-19-associated AE was an unknown Ab type of COVID-19-associated AE (50%), followed by anti-NMDA encephalitis (23.8%), limbic encephalitis (9.5%), and anti-MOG encephalitis (7.1%). Additionally, rare cases of AE were also observed, such as anti-Caspr 2 encephalitis, anti-NMDA combined anti-GAD-65 encephalitis, and anti-AMPA combined anti-CRMP-5 encephalitis.
The mechanism of COVID-19-associated AE is extremely complex and not yet fully understood. One theory suggested that the occurrence of COVID-19-associated AE may be attributed to molecular mimicking between viral proteins and neuronal self-antigens (58). Additionally, it has been observed that severe COVID-19 patients exhibit higher levels of inflammatory cytokines compared to non-severe patients (33, 59). In the case of anti-NMDAR encephalitis, elevated levels of interleukin-6 (IL-6) are commonly found during the inflammatory phase of the disease, which further stimulates the production of autoantibodies (32, 33). Moreover, SARS-CoV-2 infection triggers an inflammatory response in the central nervous system, similar to other pathogens. This immune response involves the activation of neutrophils, monocytes, macrophages, dendritic cells, and natural killer (NK) cells, leading to the release of numerous inflammatory factors (60).
The diagnosis of AE is usually based on a combination of clinical presentation, imaging analysis, CSF analysis, and electroencephalogram (61). In AE patients, MRI scans can detect abnormalities in approximately 60% of cases, while 40% of scans show no significant changes. Among patients with positive MRI findings, most exhibit T2 and Flair high signal shadows on one or both sides of the medial temporal lobe (including the hippocampus and sulcus gyrus), insular lobe, and amygdala (62). These abnormalities may appear progressively but often disappear after immunotherapy. CSF analysis typically lacks specificity, with approximately 30% of results being normal (60, 61). A retrospective multicenter study suggested that 72% of patients did not fulfill AE diagnostic criteria, resulting in a high proportion of mistaken for AE (62). It is worth considering that during the initial phase of the pandemic, the high viral circulation and incidence in the general population may have led to mistakenly associating AEs with COVID-19, resulting in a misinterpretation. The reasons for misdiagnosis may be related to overinterpretation of positive serum antibodies and misinterpretation of functional/psychiatric or non-specific cognitive dysfunction such as encephalopathy (62).
Furthermore, little emphasis has been placed on distinguishing between delirium and seizures caused by metabolic derangements in this review. Metabolic encephalopathy (ME) arises from local or global brain edema, neurotransmitter transmission disorders, and the accumulation of metabolic toxins due to damage to the blood-brain barrier, free radical damage, and apoptosis. It can manifest as coma, hemiplegia, epilepsy, and other symptoms (63). When considering ME, medical history, relevant laboratory tests, and imaging analysis need to be fully integrated. Also, when considering AE, physicians need to differentiate it from ME.
Regarding treatment strategies and outcomes, almost all patients received methylprednisolone, intravenous immunoglobulin, plasmapheresis, and oral prednisone therapy. In our study, we found that despite the potentially life-threatening risks of COVID-19-associated AE, most patients completely or partially recovered, with a few having residual symptoms of neurological damage.
This study has certain limitations. First, this systematic review includes only case reports and case series. In addition, many poor-quality reports have been published during the first stage of the health emergency, and the data are frequently partial or biased in consideration of the high circulation of the virus. Therefore, publication bias is inevitable. Second, we must admit that there are flaws in the inclusion criteria for case and case series reports. Among 42 COVID-19-associated AE patients, 39 (92.85%) cases were RT-PCR positive, and 3 (7.14%) cases were positive for SARS-CoV-2 serum antibody. This discrepancy might introduce bias since not all COVID-19-associated AE patients demonstrated positive RT-PCR either at the onset of AE symptoms or after SARS-CoV-2 infection. Third, most case reports and case series lack detailed descriptions of COVID-19 vaccination. This may lead to detection bias as some diagnoses rely on serum antibodies. Fourth, since the search was limited to articles published in English, some relevant articles in other languages were omitted. Finally, most studies did not have sufficient data, such as the time from onset to recovery and vaccination against COVID-19.
5. Conclusion
This systematic review provides a comprehensive summary of the neurological manifestations, types, and outcomes in patients who developed AE as a complication of COVID-19. The main type of COVID-19-associated AE identified in this study is an unknown Ab type of COVID-19-associated AE. Although COVID-19-associated AE can present potentially life-threatening risks, the majority of patients survived, with some patients reporting residual neurological symptoms.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HX and LZ carried out the conception and design of the research, and HX and DX participated in the acquisition of data. HX carried out the analysis and interpretation of data. HX and HH drafted the manuscript and DX participated in the revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. (2021) 19:141–54. doi: 10.1038/s41579-020-00459-7
2. Muralidar S, Ambi SV, Sekaran S, Krishnan UM. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie. (2020) 179:85–100. doi: 10.1016/j.biochi.2020.09.018
3. Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. (2022) 375:1122–7. doi: 10.1126/science.abm8108
4. El Karoui K, De Vriese AS. COVID-19 in dialysis: clinical impact, immune response, prevention, and treatment. Kidney Int. (2022) 101:883–94. doi: 10.1016/j.kint.2022.01.022
5. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
6. Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. (2020) 95:1621–31. doi: 10.1016/j.mayocp.2020.05.030
7. Perilli L, Fetta M, Capponi M, Guido CA, Grosso S, Iannetti P, et al. Peripheral nervous system involvement in SARS-CoV-2 infection: a review of the current pediatric literature. Front Neurol. (2023) 14:1134507. doi: 10.3389/fneur.2023.1134507
8. Capponi M, Cinicola BL, Brindisi G, Guido CA, Torcé MC, Zicari AM, et al. COVID-19 and abducens nerve palsy in a 9-year-old girl-case report. Ital J Pediatr. (2022) 48:102. doi: 10.1186/s13052-022-01298-3
9. COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. (2021) 398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7
10. Corsi M, Orsini A, Pedrinelli V, Santangelo A, Bertelloni CA, Carli N, et al. PTSD in parents of children with severe diseases: a systematic review to face COVID-19 impact. Ital J Pediatr. (2021) 47:8. doi: 10.1186/s13052-021-00957-1
11. Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A. Encephalitis as a neurological complication of COVID-19: A systematic review and meta-analysis of incidence, outcomes, and predictors. Eur J Neurol. (2021) 28:3491–502. doi: 10.1111/ene.14913
12. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. (2020) 19:767–83. doi: 10.1016/S1474-4422(20)30221-0
13. Brojakowska A, Narula J, Shimony R, Bander J. Clinical implications of SARS-CoV-2 interaction with renin angiotensin system: JACC review topic of the week. J Am Coll Cardiol. (2020) 75:3085–95. doi: 10.1016/j.jacc.2020.04.028
14. Tang KT, Hsu BC, Chen DY. Autoimmune and rheumatic manifestations associated with COVID-19 in adults: an updated systematic review. Front Immunol. (2021) 12:645013. doi: 10.3389/fimmu.2021.645013
15. Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. (2022) 9:815–27. doi: 10.1016/S2215-0366(22)00260-7
16. Angeli F, Reboldi G, Trapasso M, Zappa M, Spanevello A, Verdecchia P. COVID-19, vaccines and deficiency of ACE(2) and other angiotensinases. Closing the loop on the “Spike effect”. Eur J Intern Med. (2022) 103:23–8. doi: 10.1016/j.ejim.2022.06.015
17. McMillan P, Dexhiemer T, Neubig RR, Uhal BD. COVID-19-A theory of autoimmunity against ACE-2 explained. Front Immunol. (2021) 12:582166. doi: 10.3389/fimmu.2021.582166
18. Miners S, Kehoe PG, Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimers Res Ther. (2020) 12:170. doi: 10.1186/s13195-020-00744-w
19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
20. Sarigecili E, Arslan I, Ucar HK, Celik U. Pediatric anti-NMDA receptor encephalitis associated with COVID-19. Childs Nerv Syst. (2021) 37:3919–22. doi: 10.1007/s00381-021-05155-2
21. Kaur P, Vinay MV, Madarkar BS. Infantile Anti-N-Methyl-D-Aspartate receptor encephalitis post-SARS-CoV-2 Infection. Indian Pediatr. (2022) 59:343–4. doi: 10.1007/s13312-022-2506-5
22. Burr T, Barton C, Doll E, Lakhotia A, Sweeney M. N-Methyl-d-Aspartate receptor encephalitis associated with COVID-19 infection in a toddler. Pediatr Neurol. (2021) 114:75–6. doi: 10.1016/j.pediatrneurol.2020.10.002
23. Scheuermeier M, Chaves KQ, Marín-Sanabria D, Acosta-Lazo H, Ulate-Campos A. First pediatric case of autoimmune encephalitis associated with COVID-19 in costa Rica. Cureus. (2022) 14:e30616. doi: 10.7759/cureus.30616
24. Chakraborty AP, Pandit A, Dutta A, Das S, Ganguly G, Dubey S. Transcortical sensory aphasia heralding SARS-CoV-2-induced autoimmune encephalitis with gyral restricted diffusion hyperintensities: a novel case report. Egypt J Neurol Psychiatr Neurosurg. (2022) 58:167. doi: 10.1186/s41983-022-00593-4
25. Jeanneret V, Winkel D, Risman A, Shi H, Gombolay G. Post-infectious rhombencephalitis after coronavirus-19 infection: A case report and literature review. J Neuroimmunol. (2021) 357:577–623. doi: 10.1016/j.jneuroim.2021.577623
26. Hosseini AA, Shetty AK, Sprigg N, Auer DP, Constantinescu CS. Delirium as a presenting feature in COVID-19: Neuroinvasive infection or autoimmune encephalopathy? Brain Behav Immun. (2020) 88:68–70. doi: 10.1016/j.bbi.2020.06.012
27. Hilado M, Banh M, Homans J, Partikian A. Pediatric autoimmune encephalitis following COVID-19 infection. J Child Neurol. (2022) 37:268–72. doi: 10.1177/08830738211069814
28. Álvarez Bravo G, Ramió ITL. Anti-NMDA receptor encephalitis secondary to SARS-CoV-2 infection. Neurologia (Engl Ed). (2020) 35:699–700. doi: 10.1016/j.nrleng.2020.07.011
29. Farhadian S, Glick LR, Vogels CBF, Thomas J, Chiarella J, Casanovas-Massana A, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. (2020) 20:248. doi: 10.1186/s12883-020-01812-2
30. Mekheal E, Mekheal M, Roman S, Mikhael D, Mekheal N, Manickam R. A case report of autoimmune encephalitis: could post-COVID-19 autoimmunity become a lethal health issue? Cureus. (2022) 14:e25910. doi: 10.7759/cureus.25910
31. Kato S, Yoshikura N, Kimura A, Shimohata T. Possible autoimmune encephalitis associated with the severe acute respiratory syndrome coronavirus 2 omicron variant successfully treated with steroids. Intern Med. (2022) 61:3739–41. doi: 10.2169/internalmedicine.0371-22
32. Panariello A, Bassetti R, Radice A, Rossotti R, Puoti M, Corradin M, et al. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: A case report. Brain Behav Immun. (2020) 87:179–81. doi: 10.1016/j.bbi.2020.05.054
33. Monti G, Giovannini G, Marudi A, Bedin R, Melegari A, Simone AM, et al. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. (2020) 81:18–20. doi: 10.1016/j.seizure.2020.07.006
34. Dono F, Carrarini C, Russo M, De Angelis MV, Anzellotti F, Onofrj M, et al. New-onset refractory status epilepticus (NORSE) in post SARS-CoV-2 autoimmune encephalitis: a case report. Neurol Sci. (2021) 42:35–8. doi: 10.1007/s10072-020-04846-z
35. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. (2020) 94:55–8. doi: 10.1016/j.ijid.2020.03.062
36. Jacobs J, Vu P, Liu AK. A patient with myelin oligodendrocyte glycoprotein positive encephalitis with ring enhancing lesions on magnetic resonance imaging (MRI) after COVID-19 exposure. Cureus. (2022) 14:e31844. doi: 10.7759/cureus.31844
37. Raynowska J, Wu V, Kazer M, LaBuzetta JN, Ferrey D, Dunn-Pirio A. COVID-19-associated AMPA-R and CRMP-5 autoimmune encephalitis in a patient with thymoma and myasthenia gravis. Clin Case Rep. (2023) 11:e7064. doi: 10.1002/ccr3.7064
38. Durovic E, Bien C, Bien CG, Isenmann S. MOG antibody-associated encephalitis secondary to Covid-19: case report. BMC Neurol. (2021) 21:414. doi: 10.1186/s12883-021-02449-5
39. Koh S, Kim YS, Kim MH, Choi YH, Choi JY, Kim T-J. Encephalitis with status epilepticus and stroke as complications of non-severe COVID-19 in a young female patient: a case report. BMC Neurol. (2022) 22:253. doi: 10.1186/s12883-022-02782-3
40. Valadez-Calderon J, Ordinola Navarro A, Rodriguez-Chavez E, Vera-Lastro O. Co-expression of anti-NMDAR and anti-GAD65 antibodies. A case of autoimmune encephalitis in a post-COVID-19 patient. Neurologia (Engl Ed). (2022) 37:503–4. doi: 10.1016/j.nrl.2021.09.003
41. Dahshan A, Abdellatef AA. Autoimmune encephalitis as a complication of COVID-19 infection: a case report. Egypt J Intern Med. (2022) 34:32. doi: 10.1186/s43162-022-00119-7
42. Peters J, Alhasan S, Vogels CBF, Grubaugh ND, Farhadian S, Longbrake EE. MOG-associated encephalitis following SARS-CoV-2 infection. Mult Scler Relat Disord. (2021) 50:102857. doi: 10.1016/j.msard.2021.102857
43. Fusco AM, Mármol BB, Bravo GÁ, Sureda NF, Busó MN, Rodríguez AR, et al. A co-registered with MRI imaging of anti-NMDAR encephalitis patient with SARS-CoV-2 infection. Rev Esp Med Nucl Imagen Mol (Engl Ed). (2022) 41:S46–s47. doi: 10.1016/j.remnie.2021.09.004
44. Bhagat R, Kwiecinska B, Smith N, Peters M, Shafer C, Palade A, et al. New-onset seizure with possible limbic encephalitis in a patient with COVID-19 infection: a case report and review. J Investig Med High Impact Case Rep. (2021) 9:2324709620986302. doi: 10.1177/2324709620986302
45. Ayatollahi P, Tarazi A, Wennberg R. Possible autoimmune encephalitis with claustrum sign in case of acute SARS-CoV-2 infection. Can J Neurol Sci. (2021) 48:430–2. doi: 10.1017/cjn.2020.209
46. Grimaldi S, Lagarde S, Harlé J-R, Boucraut J, Guedj E. Autoimmune encephalitis concomitant with SARS-CoV-2 infection: insight from (18)F-FDG PET imaging and neuronal autoantibodies. J Nucl Med. (2020) 61:1726–9. doi: 10.2967/jnumed.120.249292
47. Lee H, Jeon JH, Choi H, Koh SH, Lee KY, Lee YJ, et al. Anti-N-methyl-D-aspartate receptor encephalitis after coronavirus disease 2019: A case report and literature review. Medicine (Baltimore). (2022) 101:e30464. doi: 10.1097/MD.0000000000030464
48. Mulder J, Feresiadou A, Fällmar D, Frithiof R, Virhammar J, Rasmusson A, et al. Autoimmune encephalitis presenting with malignant catatonia in a 40-year-old male patient with COVID-19. Am J Psychiatry. (2021) 178:485–9. doi: 10.1176/appi.ajp.2020.20081236
49. Subramanyam M, Gupta SB, Chamraj S. Caspr2 autoimmune Encephalitis with COVID-19 Infection. J Assoc Physicians India. (2022) 70:11–12.
50. Zambreanu L, Lightbody S, Bhandari M, Hoskote C, Kandil H, Houlihan CF, et al. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J Neurol Neurosurg Psychiatry. (2020) 91:1229–30. doi: 10.1136/jnnp-2020-323839
51. Serrano-Serrano B, López-Hernández N, Dahl-Cruz F, Elvira-Soler E, Díaz-Marín C. Multifocal encephalitis as a neurological manifestation of COVID-19 infection. Rev Neurol. (2020) 71:351–2. doi: 10.33588/rn.7109.2020226
52. Poorshiri B, Raeisi S, Barzegar M. A Toddler with acute encephalitis associated with COVID-19: a case report. Clin Pediatr (Phila). (2022) 61:232–5. doi: 10.1177/00099228211059347
53. Shen JY, Ng GJ, Yeo T. Anti-GABAB receptor encephalitis after COVID-19 infection. QJM. (2022) 115:686–8. doi: 10.1093/qjmed/hcac211
54. McHattie AW, Coebergh J, Khan F, Morgante F. Palilalia as a prominent feature of anti-NMDA receptor encephalitis in a woman with COVID-19. J Neurol. (2021) 268:3995–7. doi: 10.1007/s00415-021-10542-5
55. Wang M, Li T, Qiao F, Wang L, Li C, Gong Y. Coronavirus disease 2019 associated with aggressive neurological and mental abnormalities confirmed based on cerebrospinal fluid antibodies: A case report. Medicine (Baltimore). (2020) 99:e21428. doi: 10.1097/MD.0000000000021428
56. Bartley CM, Johns C, Ngo TT, Dandekar R, Loudermilk RL, Alvarenga BD, et al. Anti-SARS-CoV-2 and autoantibody profiles in the cerebrospinal fluid of 3 teenaged patients with COVID-19 and subacute neuropsychiatric symptoms. JAMA Neurol. (2021) 78:1503–9. doi: 10.1001/jamaneurol.2021.3821
57. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
58. Zamani R, Pouremamali R, Rezaei N. Central neuroinflammation in COVID-19: a systematic review of 182 cases with encephalitis, acute disseminated encephalomyelitis, and necrotizing encephalopathies. Rev Neurosci. (2022) 33:397–412. doi: 10.1515/revneuro-2021-0082
59. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. (2020) 526:135–40. doi: 10.1016/j.bbrc.2020.03.044
60. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80. doi: 10.1016/j.cell.2020.02.052
61. Pennisi M, Lanza G, Falzone L, Fisicaro F, Ferri R, Bella R. SARS-CoV-2 and the nervous system: from clinical features to molecular mechanisms. Int J Mol Sci. (2020) 21:5475. doi: 10.3390/ijms21155475
62. Flanagan EP, Geschwind MD, Lopez-Chiriboga AS, Blackburn KM, Turaga S, Binks S, et al. Autoimmune encephalitis misdiagnosis in adults. JAMA Neurol. (2023) 80:30–9. doi: 10.1001/jamaneurol.2022.4251
Keywords: COVID-19, autoimmune encephalitis, SARS-CoV-2, systematic review, anti-N-methyl-D-aspartate encephalitis
Citation: Xue H, Zeng L, He H, Xu D and Ren K (2023) Autoimmune encephalitis in COVID-19 patients: a systematic review of case reports and case series. Front. Neurol. 14:1207883. doi: 10.3389/fneur.2023.1207883
Received: 18 April 2023; Accepted: 22 August 2023;
Published: 13 September 2023.
Edited by:
Beatrice Paradiso, University of Milan, ItalyReviewed by:
Jerome Graber, University of Washington, United StatesLorenzo Perilli, University of Siena, Italy
Copyright © 2023 Xue, Zeng, He, Xu and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Xue, eHVlaDE4OTVAMTYzLmNvbQ==
 Hua Xue
Hua Xue Li Zeng2
Li Zeng2