
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 13 July 2023
Sec. Neurotrauma
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1193406
This article is part of the Research TopicNew Insights and Perspectives on Traumatic Brain Injury: Integration, Translation and Multidisciplinary ApproachesView all 13 articles
Post-traumatic Olfactory Dysfunction (PTOD) consists of a complete or partial loss of olfactory function that may occur after a traumatic brain injury (TBI). PTOD may be linked to some neuropsychiatric features, such as social, cognitive and executive dysfunction, as well as behavioral symptoms, especially when TBI involves the orbito-frontal cortex. The diagnosis of PTOD is based on medical history and clinical data and it is supported by psychometric tests (i.e., subjective tools) as well as electrophysiological and neuroimaging measures (i.e., objective methods). The assessment methods allow monitoring the changes in olfactory function over time and help to establish the right therapeutic and rehabilitative approach. In this context, the use of the olfactory training (OT), which is a non-pharmacological and non-invasive treatment option, could promote olfactory function through top-down (central) and bottom-up (peripheral) processes. To better manage patients with TBI, PTOD should be detected early and properly treated using the various therapeutic rehabilitative possibilities, both conventional and advanced, also taking into consideration the emerging neuromodulation approach.
Traumatic brain injury (TBI) is a leading cause of significant public health problems, since it often causes high disability and mortality rates. Indeed, TBI is often associated with physical and sensory disturbances, including not only the well-known auditory and visual disorders, but also olfactory problems, which are instead poorly investigated (1). Post-traumatic Olfactory Dysfunction (PTOD) is commonly described as the complete or partial loss of olfactory function due to the block of nasal nerve passages, olfactory nerve injury or concussions or hemorrhages in the olfactory centers of the brain (2). Prognosis of olfactory dysfunction depends on the etiologies because conductive smell loss shows a good prognosis after intervention compared to sensory-neural type. Bratt et al. (3) found that 8% of patients with moderate or severe TBI had anosmia, and 14% had olfactory dysfunctions lasting years after the trauma. The likelihood of PTOD has been linked to both the severity of injury and length of post-traumatic amnesia (4). PTOD can occur in 0–16% of patients with mild TBI, 15–19% of those with moderate TBI, and 25–30% of those with severe TBI (5), especially in terms of the perception of smell changes, It has been suggested that the site of injury could be strictly related to olfactory dysfunction. In fact, results from morphological magnetic resonance image (MRI) studies in PTOD patients pointed out that brain lesions were localized in the orbito-frontal, olfactory frontal cortex, and temporal lobes (6). Generally, PTOD is linked to some neuropsychiatric disorders including anxiety, depression, and impulsivity, especially when TBI occurs in the orbito-frontal (ventral prefrontal) areas (7, 8). In this way, PTOD leads to lower quality of life (QoL) compared to TBI patients without olfactory dysfunction, also because of the fear of exposure to hazardous substances (9, 10).
Indeed neuroanatomical and kinetic factors make the peripheral and central olfactory structures more susceptible to damage when TBI occurs, as reflected by the high prevalence (11) of PTOD and the related psychological and cognitive – behavioral symptoms. Traumatic facial and brain injury due to blast and incidents are common causes of smell alterations, including total loss of function (anosmia), decreased sensitivity (hyposmia), alterations in odor quality (dysosmia/parosmia) and hallucination (phantosmia) (12). In some cases, brain damage limited to the primary olfactory areas leads to anosmia, while damage to the orbitofrontal cortex provokes olfactory discrimination and recognition deficits due to the multisensory integration role of this brain area (13). In a more specific way, anosmia can be considered as manifestation of frontal lobe damage, and it is correlated with alterations in verbal fluency abilities and executive functions (14, 15). Other authors (16) found that anosmia is strongly associated with depression symptoms in TBI patients, likely for the anatomical relationship between the two functions. The orbital frontal cortex plays a key role in both mood regulation and recognition and differentiation of odors (17). Moreover, hyposmia could be accompanied by socially disinhibited behavioral alteration, which is likely linked to orbital frontal cortex damage, as for the cognitive deficits (18). Moreover, Neumann et al. (19) reported that 56% of moderate to severe TBI presented dysosmia/parosmia in addition to difficulty in interpreting facial expressions and emotions.
These authors identified that cognitive-emotional networks, which are important for recognition and empathy, were also involved in central olfactory functions suggesting that this may be related to more complex social functions. It has been hypothesized that dysosmia/parosmia and depressive and anxiety symptoms are linked to persisting alterations of frontotemporal structures, such as the hippocampus and the orbitofrontal cortex (20, 21), as demonstrated by neuroimaging studies (22, 23). Phantosmia has been described as an olfactory disturbance in which individuals perceive an odor in the absence of a stimulus that may disappear, improve or worsen over time (24–26).
Various medical treatments have been tried to improve PTOD, including topical and systemic steroids, but well-controlled studies still lack (27).
Other drugs such as Gingko biloba and vitamin B have not proven to be effective to treat olfactory dysfunction (28).
Although there are limited therapeutic options for patients with PTOP, about 16.8 to 27% of patients may experience some degree of spontaneous recovery, which is mainly due to the high degree of neuroplasticity of the olfactory system (29, 30).
Natural smell recovery mostly occurs within 1 year after the traumatic event. However, the chances of improvement are reduced after 2 years from PTOD. Olfactory training might be a promising modality for the treatment of PTOD. In this context, different studies (31, 32) have indicated the effectiveness of olfactory training (OT), i.e., daily exposure to certain odors, thanks to the possibility of boosting neural plasticity of the olfactory system.
The diagnosis of smell disorders is suspected by medical history and supported by clinical data as well as by the results of psychophysiological, electrophysiological and neuroimaging measures. Among the validated psychophysical tests, the Sniffin’ Sticks Test (SST) is the most commonly and widely used tool. A more objective and quantitative measurement of sensory smell loss following TBI can be recorded though the Olfactory Event-Related Potentials (OERPs), which allows one to observe electrophysiologically the function of the olfactory system and its changes (33, 34).
In a forensic traumatic event, the clinical picture and severity of the person need to be determined with the use of objective criteria, although there are still limitations in objectively evaluating olfactory dysfunctions and state the relationship between the event and its cause. Performing both subjective and electrophysiological tests together to detect olfactory dysfunctions that occur after a forensic incident enable provide more reliable results in diagnosis and treatment (35). The OERPs method may provide objective data in the evaluation of post traumatic anosmia from the medicolegal perspective, to identify specific factors and the degree of smell loss (36).
The aim of this review is to investigate the main features of PTOD, focusing on the assessment through subjective and objective tools, emphasizing at the same time, the role of the main rehabilitative approaches to treat smell impairments following TBI.
The studies included in this review were identified by searching on PubMed, Scopus, Web of Science and Cochrane library, using the following keywords: “post-traumatic olfactory dysfunction” OR “olfactory dysfunction in traumatic brain injury” AND “post-traumatic olfactory dysfunctions and cognitive manifestations” AND “post-traumatic olfactory dysfunction diagnosis” OR “post-traumatic olfactory dysfunction assessment” AND “olfactory rehabilitative training in traumatic brain injury” OR “post-traumatic olfactory dysfunction rehabilitation.”
We defined the search terms using the PICO (population, intervention, comparison, outcome) model. We considered patients affected by PTOD as population; intervention included both assessment tools and rehabilitation approaches (conventional or not) for PTOD; comparison consisted in other kind of tool/medication used to assess/treat PTOD; the outcome measures considered were smell recovery, quality of life and any kind of improvement in olfactory function, including neuroplasticity.
The inclusion criteria were (i) patients affected by moderate to severe TBI with OD; (ii) randomized clinical trials (RCT), pilot studies and systematic reviews, case control and retrospective studies published between January 2012 and September 2022; (iii) English language; and (iv) papers published in a peer-reviewed journal. Exclusion criteria were (i) case reports and narrative reviews; (ii) studies describing other kinds of post-traumatic dysfunctions; (iii) studies involving children and adolescents affected by PTOD; (iv) other etiology of OD (i.e., vascular accidents, ischemic and/or hemorrhagic, neurodegenerative).
Besides the papers themselves, we have analyzed the references of the selected articles, (but including only English papers), in order to obtain a complete search. The studies fulfilling our selected criteria and published between 2012 and 2022 were evaluated for possible inclusion (n = 198). Then, we have considered only English papers and removed duplicates (n = 100) (see Figure 1). Two reviewers (RDL and MB), have evaluated articles according to title, abstracts and text, and finally we considered 35 articles that addressed the main PTOD assessment tools and rehabilitative approaches.
In details, two reviewers (R.D.L. and M.B.) extracted data under the following categories: (i) measure characteristics (i.e., purpose, target population, time of test execution), (ii) psychometric properties of each assessment tool according to the information reported by the available studies and (iii) type of rehabilitative intervention used by the selected studies.
Recently, researchers made significant progress in the development of widely available, reliable, and reproducible methods to evaluate olfactory function. The administration of these tools is essential to establish the degree of chemosensory loss and confirm the patient’s complaint of olfactory alteration (37). Indeed, it permits monitoring the OD changes over time in post-TBI patients and helps to establish the right therapeutic and rehabilitative choice, considering also its impact on the patient’s treatment and counseling (38). Two main types of olfactory testing are commonly used: subjective tools, which include psychometric scales/tests (Table 1), and objective methods such as electrophysiological testing (Table 2) (62).
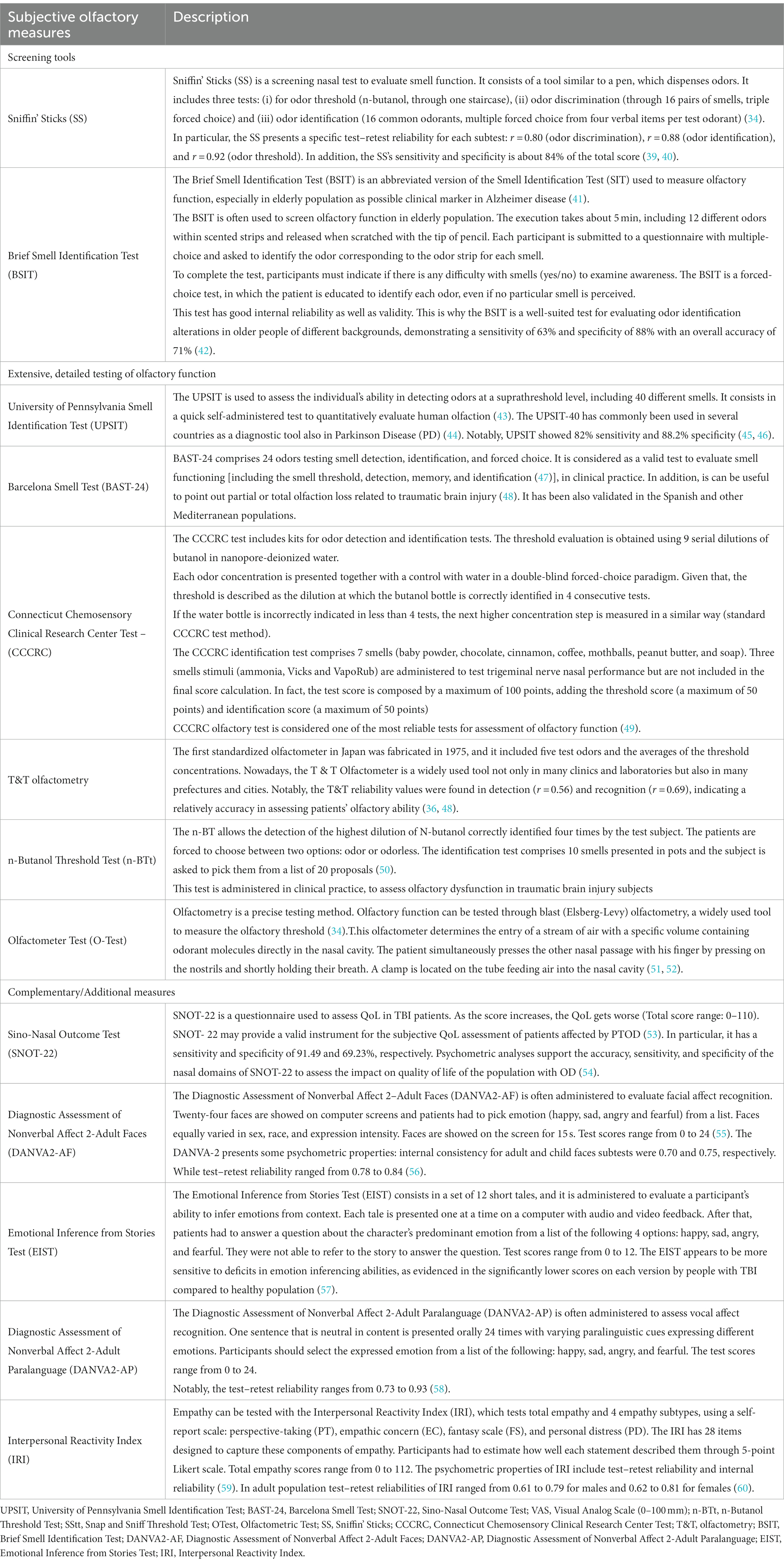
Table 1. The main subjective measures and complementary/additional tools to assess olfactory dysfunctions following TBI.
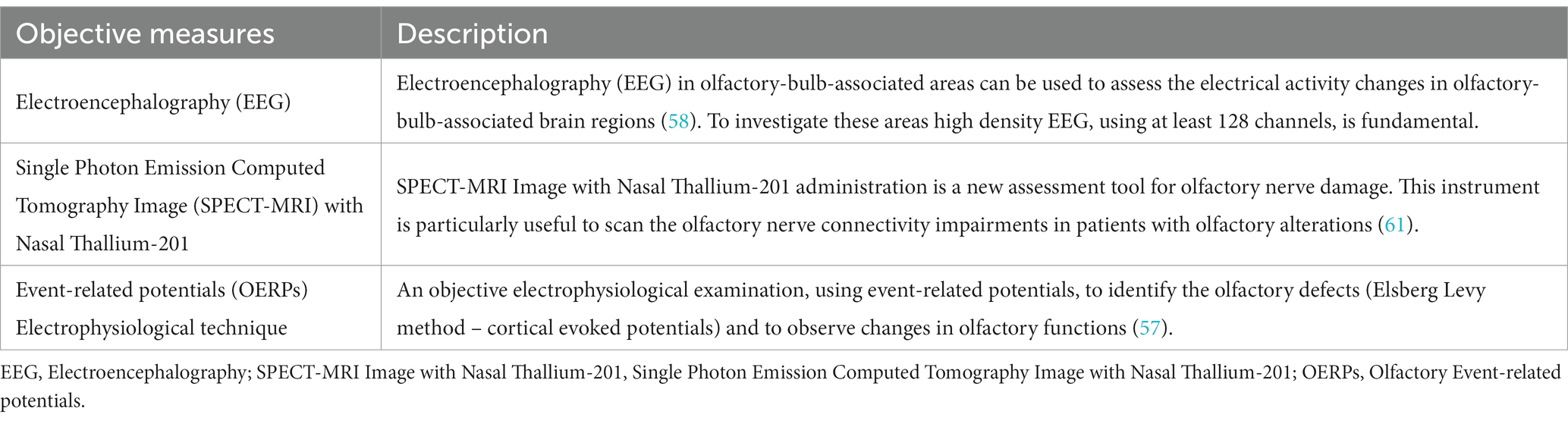
Table 2. Description of the main objective measures to evaluate olfaction in patients affected by TBI.
Among the subjective examinations of olfaction, some screening tools are useful to differentiate easily and rapidly patients with normosmia, hyposmia or anosmia. In clinical practice, the most used screening tool for TBI-related olfactory dysfunctions is the Sniffin’ Sticks test, developed by Hummel in 1997, which contains some marker pens to be smelled, and it takes approximately 4 min to be administered. Concerning the recognition part, this validated test can be easily administered also in patients with language alterations (i.e., in the presence of aphasia) thanks to a wordlist of odors or non-verbal information like photographs/drawings representing smells. However, it requires good cooperation by the patient, who must pay attention during the test (39, 63).
When clinicians need to further investigate odor identification, discrimination and thresholds, a more extensive and detailed testing can be used (64) Indeed, the University of Pennsylvania Smell Identification Test (UPSIT), developed in the early 1980’s, focuses on the comparative ability of individuals to identify odors at the suprathreshold level (40–42).
It was administered also in Parkinson disease’s (PD) population and in patients with COVID-19 to reveal changes in the olfactory function (43–46).
Despite its high reliability (r = 0.94), the UPSIT has shown poor sensitivity for malingering detection in people familiar with the test mechanism.
The Connecticut Test, in which the odor stimulus is contained in suitable glass flasks (47) is quite similar to the Brief-Smell Identification Test (see Table 1), which is administered in older people and can be used for the evaluation and diagnosis of patients with olfactory impairments, considering the advantage of its low cost. An important issue about CCCRC is that low scores can be indicative of TBI, while abnormal detection thresholds may reflect altered olfactory cell function (49). Langdon et al. (48) evaluated severe smell loss in TBI patients using the Barcelona Smell Test (BAST-24), validated for the Catalan and Spanish population. It consists of 24 odors scoring smell detection, identification, and forced choice, and according to Cartesin et al., the tool is a good and reliable method to test the olfactory function in clinical practice (47).
Another specific test that can be administered to determine olfactory function in a rapid and non-invasive manner is the n-Butanol Threshold Test (n-BTt) (48, 65). Denzer et al. (50) used sniffing sticks with n-Butanol to investigate smell function, when generally this test is administered through gas chromatographic methods. The authors revealed that a pen set with n-butanol is an appropriate tool for testing olfactory sensitivity.
During the administration of self-assessment tools, there are three factors to consider: (i) odor threshold, (ii) odor discrimination, and (iii) odor identification (51, 52).In a recent study, Limphaibool et al. (2020) described a subjective olfactory examination, named the blast (Elsberg-Levy) olfactometry, which is a popular method of olfactory threshold measurement (34), in addition to the administration of main Fragrances Used in Olfactometer Test. The specific odors are mint (100% natural menthe piperita oil) and anise (100% natural Illicium Verum Seed Oil) at the temperature of 21 ± 1 degrees Celsius. Notably, anise oil is administered to stimulate the olfactory nerve endings whereas mint oil promotes the activation of both the olfactory and trigeminal nerve endings in the nasal mucosal tissue (66). Despite its usefulness in detecting olfactory thresholds, the blast (Elsberg-Levy) olfactometry may provide false results in smell performance due to the presence of odorant-free air, which could stimulate trigeminal nerve sensors (67).
Interestingly, Sattin (68) investigated olfactory function in patients affected by disorders of consciousness (DOC) due to TBI, using an olfactory discrimination protocol (ODP). This ODP was composed of four odors, selected and dosed according to both the literature on clinical sniff tests and to functional magnetic resonance, assessing the olfactory neural process and pathways (69–71). Given that the olfactory receptors are implicated in processing memory (which involves amygdala, hippocampus, etc.), olfaction could be a simple and direct way to stimulate memory and emotions in DOC.
Langdon et al. (48) administered the SNOT-22 as an additional/complementary outcome measure to investigate QoL in PTOD patients. In fact, the tool seems particularly useful in detecting changes in QoL according to smell symptoms (53, 54). In this vein, Neumann et al. (19) investigated the PTOD in moderate and severe TBI by assessing also emotional sequelae, through the administration of a complete battery, which included: (i) Olfaction (Brief Smell Identification Test-BSIT) (41); (ii) facial affect recognition [Diagnostic Assessment of Nonverbal Affect 2-Adult Faces (DANVA2-AF)] (55); (iii) vocal affect recognition [Diagnostic Assessment of Nonverbal Affect 2-Adult Paralanguage (DANVA2-AP)] (56); (iv) emotional inference [Emotional Inference from Stories Test (EIST)] (57) and (v) empathy [Interpersonal Reactivity Index (IRI)] (58). The authors showed that the detection of olfactory dysfunction may be related to affect and empathy deficits (59, 60). This is why an early assessment of these emotional impairments could be useful to set the most appropriate treatment for PTOD patients.
An objective examination (Table 2) may be indeed useful to more accurately identify olfactory defects.
Olfactory Event-related potentials (OERPs) are a reliable electrophysiological instrument to detect changes in olfactory function in an objective view. The recording device for cortical evoked potentials and odor stimulator, according to the above-mentioned Elsberg Levy method, can be also considered an objective olfactory evaluation (61). Compared to other methods such as MRI or fMRI, OERP measurements also have a higher temporal resolution, and can be conducted at lower cost with a lower degree of invasiveness (72).
Some authors have used OERPs to evaluate olfactory function in different patient populations such as multiple sclerosis (73) Alzheimer’s disease and other dementias (74–76), Parkinson’s disease (77), older people (78, 79), and as a marker for depression (80). However, other authors believe that these techniques are complex, time-consuming and not routinely performed in clinical practice (81). Notably, Shiga et al. used SPECT-MRI with Nasal Thallium-201 administration to identify lesions of the olfactory nerve connectivity in patients with impaired olfaction. In fact, these authors noticed that the degree of axon degeneration in human olfactory mucosa correlates with olfactory function (82).
Olfactory training (OT) can be considered a non-pharmacological and non-invasive treatment option for patients affected by TBI with consequences in olfactory functioning (83), but also in individuals with signs of depression (84), neurodegenerative diseases such as Parkinson’s disease and older adults (85). Generally, OT consists in the administration of specific fragrances (i.e., floral, fruity, and more intense aromas such as eucalyptus) inhaled, which can stimulate the olfactory nerve and promote neuroplasticity (Figure 2).
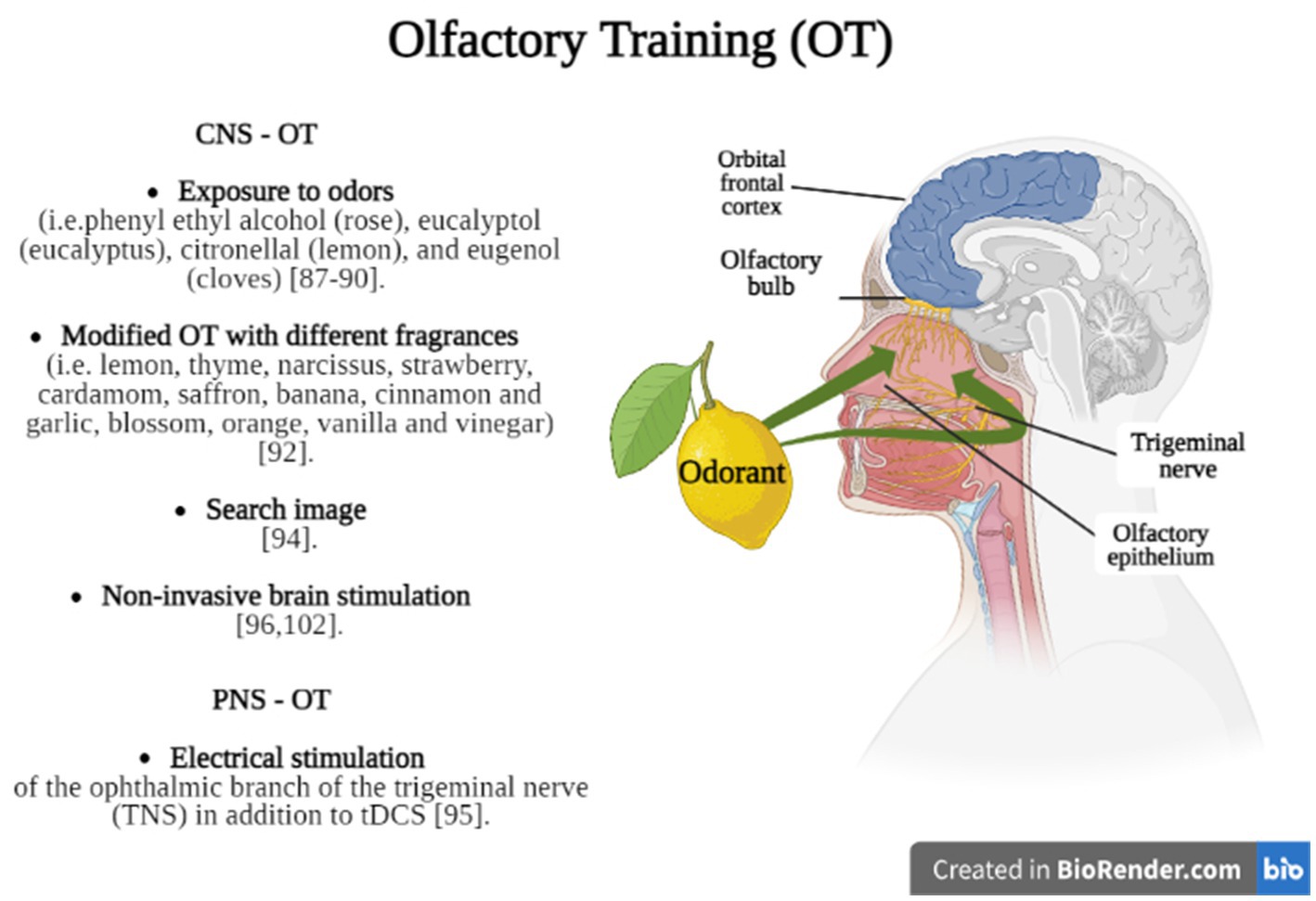
Figure 2. Briefly shows the main OT methods divided into techniques which stimulate olfactory function through CNS (CNS – OT) or PNS (PNS – OT) stimulation. Created in BioRender.com.
Recently, a meta-analysis (86) found that OT was effective in 36.31% of PTOD patients who achieved clinically significant results after 8 months of training, while 27% of patients experienced spontaneous recovery of olfaction. In fact, OT could promote olfactory function through top-down (central) rather than bottom-up (peripheral) processes, as confirmed by Pellegrino et al. (87) and Konstantinidis et al. (88) applied to their patients a systematic OT for sixteen weeks, twice daily (in the morning and in the evening) and using four different odors, including phenyl ethyl alcohol (rose), eucalyptol (eucalyptus), citronellal (lemon), and eugenol (cloves); each odor was administered for ten seconds, with the same time interval of ten seconds between smells. They found that OT increased the identification and discrimination of olfactory functions in TBI patients, with positive effects also in cognitive functions. However, Jiang et al. (89) found that the administration of phenyl ethyl alcohol during OT produced improvements in olfactory thresholds in 23% of patients affected by post-traumatic anosmia but did not improve the odor identification ability. Using the Konstantinidis’s protocol (88), some authors suggested that the training is based on modulation of the regeneration processes linked to the repeated exposure to an odor, involving olfactory bulb and brain connectivity (90, 91).
Rezaeyan et al. (92), indeed, introduced a modified OT (Figure 1) stimulating the olfactory receptors with a variety of odorants over a certain period. The olfactory training was performed using four different packages including: (1) rose, lemon, thyme, and eucalyptus for the first month; (2) narcissus, strawberry, cardamom, and peppermint for the second month; (3) saffron, banana, cinnamon and garlic for the third month; (4) blossom, orange, vanilla and vinegar for the fourth month and led to positive results. It seems that the effectiveness of both OT, traditional and modified, depends on improved cognitive processing of olfactory information and increased attention paid to odors, supporting greater involvement of the CNS (93).
Laing et al. have described a rehabilitative method founded on the paradigm employed by Zelano et al., in which a neural representation of an odor is recalled from olfactory memory. In fact, the visual sighting of a food and imaging the food odor could activate the posterior piriform cortex that may allow a subject to perceive and identify the odor using central mechanisms only (94). Nevertheless, the effects of OT on neural structural changes due to the close connection between brain structure and olfactory function remains an unsolved question (95). For these reasons, more research is needed to clarify the optimal odor concentration, training duration, frequency, and the most suitable population for OT to better understand mechanisms underlying the recovery processes.
The olfactory nerve can also play a key role in such plastic and regenerative processes (96). Indeed, it has been shown that neurostimulation (about thirty minutes), delivered to the ophthalmic branch of the trigeminal nerve through trigeminal nerve stimulation and transcranial direct current stimulation, significantly improved the olfactory performance to guaiacol, an odorant involved in the activation of intranasal trigeminal circuit. In this way, both methods may induce persistent modulation changes through a direct activation of the trigeminal nerve. On the other hand, these neuromodulator effects may be driven via activation of distal secondary olfactory cortex structures, such as the orbitofrontal cortex which is highly associated with processing of odor learning and memory. In fact, non-invasive brain stimulation (NIBS) is considered an emerging and promising approach to induce long-lasting neuroplastic changes through electrical and/or magnetic energy. According to Hara et al. (97), the combined use of NIBS with rehabilitation could enhance a positive synergic effect, promoting not only modulation of neural connections, but also functional re-learning in post-TBI patients. However, the use of NIBS in the treatment of PTOD is an issue that deserves to be investigated.
Olfactory dysfunction is an underestimated and challenging issue in TBI that worsens patients’ QoL, not only in eating and enjoyment of food, but also in hazard avoidance (gas leaks, smoke detection, chemical vapors, and rotten food). Our review suggests that PTOD is commonly associated with cognitive and neuropsychiatric sequelae in TBI patients due to OFC damage, and some authors reported that OD is also linked to the neurodegenerative pathology. Then, it could be considered as a clinical marker of neurodegeneration likewise other more direct clinical, biological and neuroimaging markers. For this reason, an early assessment of olfactive function should be implemented in clinical practice, especially when dealing with TBI, and this is why a comprehensive review on this issue is of utmost importance. In fact, it seems that olfactory testing, especially in the acute phase, could be useful as a screening tool for long-term outcomes, including mood symptoms. According to Logan et al., there is a bidirectional correlation between OD and depression, due to reduced input to the olfactory bulb and the consequential lower levels of neurotransmitter concentration, leading to the potential disturbance of emotional functioning (98).
PTOD is also related to other neuropsychiatric sequelae, including anxiety which affects odor thresholds, identification and discrimination of different smells. It seems that the amygdala and the OFC are both involved in anxious states and in the olfactory functioning (99). OD is also associated with impulsivity probably because OFC, is involved in both olfactory neural pathways and the regulation and inhibition of behavior (100).
Moreover, it has been shown that detection of olfactory abnormalities may be related to affect and empathy deficits (19), and this could be due to a common dopaminergic pathway dysfunction. Then, addressing OD and the related cognitive/behavioral dysfunction may be of help in better manage the rehabilitation of patients with TBI. Clinicians have a wide range of PTOD assessment methods, both subjective and objective, although it is not always easy to administer the right test or measure. The Sniffin’ Test, UPSIT and CCCRC are the most used in clinical practice for their rapidity and cost convenience. Despite their short duration, there are some limitations related to learning effects due to repeated testing, and the low resolution in terms of detecting changes. In detail, the CCCRC and the BAST-24 provide verbal odor identification which is strictly dependent on language function and cognition (101). This is why patients are exposed to a pre-selected list of odor descriptors without which there would be no reliable clinical results. However, the BAST-24 is particularly useful to detect not only olfactory changes, but also neurobehavioral disorders (i.e., eating) (100).
OERPs are instead more accurate to detect changes in olfactory function, despite their limited availability in standard health care and the high cost of administration. Nevertheless, objective examinations are particularly useful when level of cognition is too impaired or when subjects may exaggerate the smell deficit for a secondary gain or for other medico-legal reasons. In addition, OERPs allow to understand the site of damage: loss of smell without loss of OERPs suggest peripheral nerve lesions, while a reduction/absence of OERPs indicates damage to central olfactory system (72). Another important issue to consider is the best way to manage PTOD, although it is still an underestimated problem. Currently, evidence supports the use of topical corticosteroids that allow neuronal recovery following olfactory nerve transection through the reduction of the inflammatory reaction and decrease of glial scar formation (101). This may explain why corticosteroids combined with OT are more effective (102). Other medications to treat olfactory loss include supplementation with alpha-lipoic acid, vitamin A and omega-3 for their neurodegenerative potential and antioxidant properties. Other promising treatments are related to the administration of the experimental N-acetylcysteine (100 mg/kg twice daily) after acute olfactory neuronal injury in animal models, since it reduces neural loss in the olfactory bulb (103). In fact, the neuroprotective effect of this medication could provide clinical benefit also in the TBI population. OT has been introduced in patients’ care despite the lack of specific recommendations; moreover, its role in stimulating central or peripheral components of the olfactory system is mostly unknown. For this reason, the real effectiveness of OT remains a challenge, although it could be considered a good option to manage this growing and important problem. According to Turner et al. (104), a higher quality of evidence is needed with respect to patient populations, protocols, and outcome measures. Recently, researchers have studied the role of emerging approaches, including the use of NIBS that could boost neuroplasticity, further potentiating the OT after-effects (105–107). However, the lack of conclusive evidence does not allow to recommend this therapeutic approach in terms of efficacy. Another treatment for olfactory dysfunction is the use of platelet-rich-plasma which is derived from blood’s patient with pro-regenerative properties (108). Nevertheless, larger studies are needed to understand if it can be adaptable also in TBI patients. Finally, the traditional Chinese acupuncture (109), used for various medical conditions, was proven effective in post-viral infection patients who were refractory to other treatments, including OT, oral steroids and supplementation. Although the reporting information in this review followed the PRISMA guidelines to reduce bias, there are some limitations to acknowledge. Since we included only English papers, some studies may have been excluded based on the language criteria. In addition, we did not provide any statistical analysis for each study included because of our intention was to describe the most used tools and methods to assess olfactory function in PTOD and its rehabilitative approach, since an international consensus about a gold-standard does not exist yet.
In conclusion, an early assessment of olfactory sense, considering also its correlation with cognitive functioning, is recommended in clinical practice and especially in the rehabilitation of patients with TBI. Although no clear evidence exists on the best treatment option, OT could be considered a valuable and effective tool to promote neuroplastic processes and improve OD following TBI. Further research is needed to investigate the promising role of OT coupled to other emerging training methods in the management of patients with TBI and olfactory loss and/or alterations.
MB, RC, and RL: conceptualization and investigation. MB and RL: methodology, data curation, and writing—original draft preparation. MB and CR: software. RL, MB, CR, AQ, and RC: validation and visualization. AQ: resources and funding acquisition. RC: writing—review and editing and supervision. All authors have read and agreed to the published version of the manuscript.
This study was supported by Current Research funds 2023, Ministry of Health, Italy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Frasnelli, J, Laguë-Beauvais, M, LeBlanc, J, Alturki, AY, Champoux, MC, Couturier, C, et al. Olfactory function in acute traumatic brain injury. Clin Neurol Neurosurg. (2016) 140:68–72. doi: 10.1016/j.clineuro.2015.11.013
2. Costanzo, RM, and Miwa, T. Post traumatic olfactory loss. Adv Otorhinolaryngol. (2006) 63:99–107. doi: 10.1159/000093753
3. Bratt, M, Skandsen, T, Hummel, T, Moen, KG, Vik, A, Nordgård, S, et al. Frequency and prognostic factors of olfactory dysfunction after traumatic brain injury. Brain Inj. (2018) 32:1021–7. doi: 10.1080/02699052.2018.1469043
4. Howell, J, Costanzo, RM, and Reiter, ER. Head trauma and olfactory function. World J Otorhinolaryngol Head Neck Surg. (2018) 4:39–45. doi: 10.1016/j.wjorl.2018.02.001
5. Costanzo, RM, Reiter, RJ, and Yelverton, JC. Smell and taste In: ND Zasler, DI Katz, and RD Zafonte, editors. Brain injury medicine: Principles and practice. New York, NY: Demos (2012). 794–808.
6. Marin, C, Langdon, C, Alobid, I, and Mullol, J. Olfactory dysfunction in traumatic brain injury: the role of neurogenesis. Curr Allergy Asthma Rep. (2020) 20:55. doi: 10.1007/s11882-020-00949-x
7. Wood, RL, and Worthington, A. Neurobehavioral abnormalities associated with executive dysfunction after traumatic brain injury. Front Behav Neurosci. (2017) 11:195. doi: 10.3389/fnbeh.2017.00195
8. Langdon, C, Laxe, S, Lehrer, E, Berenguer, J, Alobid, I, Quintó, L, et al. Loss of smell in patients with traumatic brain injury is associated with neuropsychiatric behavioral alterations. Brain Inj. (2021) 35:1418–24. doi: 10.1080/02699052.2021.1972447
9. Ahmedy, F, Mazlan, M, Danaee, M, and Abu Bakar, MZ. Post-traumatic brain injury olfactory dysfunction: factors influencing quality of life. Eur Arch Otorhinolaryngol. (2020) 277:1343–51. doi: 10.1007/s00405-020-05823-0
10. Drummond, M, Douglas, J, and Olver, J. 'If I haven't got any smell … I'm out of work': consequences of olfactory impairment following traumatic brain injury. Brain Inj. (2013) 27:332–45. doi: 10.3109/02699052.2012.750743
11. Schofield, PW, and Doty, RL. The influence of head injury on olfactory and gustatory function. Handb Clin Neurol. (2019) 164:409–29. doi: 10.1016/B978-0-444-63855-7.00023-X
12. Hähner, A, and Hummel, T. Classic Phantosmia. Dtsch Arztebl Int. (2020) 117:689. doi: 10.3238/arztebl.2020.0689a
13. Caminiti, F, Ciurleo, R, Bramanti, P, and Marino, S. Persistent anosmia in a traumatic brain injury patient: role of orbitofrontal cortex. Brain Inj. (2013) 27:1715–8. doi: 10.3109/02699052.2013.823667
14. Singh, R, Humphries, T, Mason, S, Lecky, F, Dawson, J, and Sinha, S. The incidence of anosmia after traumatic brain injury: the SHEFBIT cohort. Brain Inj. (2018) 32:1122–8. doi: 10.1080/02699052.2018.1483028
15. Sigurdardottir, S, Andelic, N, Skandsen, T, Anke, A, Roe, C, Holthe, OO, et al. Olfactory identification and its relationship to executive functions, memory, and disability one year after severe traumatic brain injury. Neuropsychology. (2016) 30:98–108. doi: 10.1037/neu0000206
16. Alderfer, BS, Arciniegas, D, and Silver, JM. Treatment of depression following traumatic brain injury. J Head Trauma Rehabil. (2005) 20:544–62. doi: 10.1097/00001199-200511000-00006
17. Rolls, ET, Cheng, W, and Feng, J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. (2020) 2:fcaa196. doi: 10.1093/braincomms/fcaa196
18. Osborne-Crowley, K, and McDonald, S. Hyposmia, not emotion perception, is associated with psychosocial outcome after severe traumatic brain injury. Neuropsychology. (2016) 30:820–9. doi: 10.1037/neu0000293
19. Neumann, D, Zupan, B, Babbage, DR, Radnovich, AJ, Tomita, M, Hammond, F, et al. Affect recognition, empathy, and dysosmia after traumatic brain injury. Arch Phys Med Rehabil. (2012) 93:1414–20. doi: 10.1016/j.apmr.2012.03.009
20. Lecuyer Giguere, F, Jobin, B, Robert, J, Bastien, L, Giguère, JF, De Beaumont, L, et al. Early parosmia signs and affective states predicts depression and anxiety symptoms six months after a mild traumatic brain injury. Chem Senses. (2020):bjaa037. doi: 10.1093/chemse/bjaa037
21. Ciurleo, R, De Salvo, S, Bonanno, L, Marino, S, Bramanti, P, and Caminiti, F. Parosmia and neurological disorders: a neglected association. Front Neurol. (2020) 11:543275. doi: 10.3389/fneur.2020.543275
22. Terpstra, AR, Girard, TA, Colella, B, and Green, REA. Higher anxiety symptoms predict progressive hippocampal atrophy in the chronic stages of moderate to severe traumatic brain injury. Neurorehabil Neural Repair. (2017) 31:1063–71. doi: 10.1177/1545968317736817
23. Hudak, A, Warner, M, Marquez de la Plata, C, Moore, C, Harper, C, and Diaz-Arrastia, R. Brain morphometry changes and depressive symptoms after traumatic brain injury. Psychiatry Res. (2011) 191:160–5. doi: 10.1016/j.pscychresns.2010.10.003
24. Bainbridge, KE, Byrd-Clark, D, and Leopold, D. Factors associated with phantom odor perception among US adults: findings from the National Health and nutrition examination survey. JAMA. Otolaryngol Head Neck Surg. (2018) 144:807–14. doi: 10.1001/jamaoto.2018.1446
25. Landis, BN, Reden, J, and Haehner, A. Idiopathic phantosmia: outcome and clinical significance. ORL J Otorhinolaryngol Relat Spec. (2010) 72:252–5. doi: 10.1159/000317024
26. Leopold, D . Distortion of olfactory perception: diagnosis and treatment. Chem Senses. (2002) 27:611–5. doi: 10.1093/chemse/27.7.611
27. Jafari, A, and Holbrook, EH. Therapies for olfactory dysfunction – an update. Curr Allergy Asthma Rep. (2022) 22:21–8. doi: 10.1007/s11882-022-01028-z
28. Helman, SN, Adler, J, Jafari, A, Bennett, S, Vuncannon, JR, Cozart, AC, et al. Treatment strategies for postviral olfactory dysfunction: a systematic review. Allergy Asthma Proc. (2022) 43:96–105. doi: 10.2500/aap.2022.43.210107
29. Welge-Lüssen, A, Hilgenfeld, A, Meusel, T, and Hummel, T. Longterm follow-up of posttraumatic olfactory disorders. Rhinology. (2012, 2012) 50:67–72. doi: 10.4193/Rhino11.141
30. AbdelBari Mattar, M, and El Adle, H. Prognostic factors for olfactory dysfunction in adult mild head trauma. World Neurosurg. (2020) 141:e545–52. doi: 10.1016/j.wneu.2020.05.232
31. Sorokowska, A, Drechsler, E, Karwowski, M, and Hummel, T. Effects of olfactory training: a meta-analysis. Rhinology. (2017) 55:17–26. doi: 10.4193/Rhino16.195
32. Konstantinidis, I, Tsakiropoulou, E, Bekiaridou, P, Kazantzidou, C, and Constantinidis, J. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope. (2013) 123:E85–90. doi: 10.1002/lary.24390
33. Su, B, Bleier, B, Wei, Y, and Wu, D. Clinical implications of psychophysical olfactory testing: assessment, diagnosis, and treatment outcome. Front Neuro Sci. (2021) 15:646956. doi: 10.3389/fnins.2021.646956
34. Limphaibool, N, Iwanowski, P, Kozubski, W, Swidziński, T, Frankowska, A, Kamińska, I, et al. Subjective and objective assessments of post-traumatic olfactory dysfunction. Front Neurol. (2020) 11:970. doi: 10.3389/fneur.2020.00970
35. Çelik, C, Güler, H, and Pehlivan, M. Medicolegal aspect of loss of smell and olfactory event-related potentials. Egypt J Forensic Sci. (2022) 12:47. doi: 10.1186/s41935-022-00306-1
36. Červený, K, Janoušková, K, Vaněčková, K, Zavázalová, Š, Funda, D, Astl, J, et al. Olfactory evaluation in clinical medical practice. J Clin Med. (2022) 11:6628. doi: 10.3390/jcm11226628
37. Hummel, T, Whitcroft, KL, Andrews, P, Altundag, A, Cinghi, C, Costanzo, RM, et al. Position paper on olfactory dysfunction. Rhinol Suppl. (2017) 54:1–30. doi: 10.4193/Rhino16.248
38. Howell, J, Costanzo, RM, and Reiter, ER. Head trauma and olfactory function. World J Otorhinolaryngol Head Neck Surg. (2018) 4:39–45. doi: 10.1016/j.wjorl.2018.02.001
39. Haehner, A, Mayer, AM, Landis, BN, Pournaras, I, Lill, K, Gudziol, V, et al. High test-retest reliability of the extended version of the "Sniffin' sticks" test. Chem Senses. (2009) 34:705–11. doi: 10.1093/chemse/bjp057
40. Delgado-Losada, ML, Delgado-Lima, AH, and Bouhaben, J. Spanish validation for olfactory function testing using the Sniffin' sticks olfactory test: threshold, discrimination, and identification. Brain Sci. (2020):10. doi: 10.3390/brainsci10120943
41. Suzuki, Y, Yamamoto, S, Umegaki, H, Onishi, J, Mogi, N, Fujishiro, H, et al. Smell identification test as an indicator for cognitive impairment in Alzheimer's disease. Int J Geriatr Psychiatry. (2004) 19:727–33. doi: 10.1002/gps.1161
42. Menon, C, Westervelt, HJ, Jahn, DR, Dressel, JA, and O'Bryant, SE. Normative performance on the brief smell identification test (BSIT) in a multi-ethnic bilingual cohort: a project FRONTIER study. Clin Neuropsychol. (2013) 27:946–61. doi: 10.1080/13854046.2013.796406
43. Picillo, M, Pellecchia, MT, Erro, R, Amboni, M, Vitale, C, Iavarone, A, et al. The use of University of Pennsylvania Smell Identification Test in the diagnosis of Parkinson's disease in Italy. Neurol Sci. (2014) 35:379–83. doi: 10.1007/s10072-013-1522-6
44. Doty, RL, Frye, RE, and Agrawal, U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys. (1989) 45:381–4. doi: 10.3758/BF03210709
45. Doty, RL, Shaman, P, and Dann, M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. (1984) 32:e502. doi: 10.1016/0031-9384(84)90269-5
46. Morley, JF, Cohen, A, Silveira-Moriyama, L, Lees, AJ, Williams, DR, Katzenschlager, R, et al. Optimizing olfactory testing for the diagnosis of Parkinson's disease: item analysis of the university of Pennsylvania smell identification test. NPJ Parkinson Dis. (2018) 4:2. doi: 10.1038/s41531-017-0039-8
47. Cardesín, A, Alobid, I, Benítez, P, Sierra, E, de Haro, J, Bernal-Sprekelsen, M, et al. Barcelona smell test – 24 (BAST-24): validation and smell characteristics in the healthy Spanish population. Rhinology. (2006) 44:83–9.
48. Langdon, C, Lehrer, E, Berenguer, J, Laxe, S, and Alobid, I. Olfactory training in post-traumatic smell impairment: mild improvement in threshold performances: results from a randomized controlled trial. J Neurotrauma. (2018) 35:2641–52. doi: 10.1089/neu.2017.5230
49. Toledano, A, González, E, Onrubia, TJ, Herráiz, C, Mate, MA, García, M, et al. Test de olfato de Connecticut (CCCRC): valores en voluntarios sanos [the Connecticut Chemosensorial clinical research center olfaction test: values in healthy volunteers]. Acta Otorrinolaringol Espanola. (2003) 54:678–85. doi: 10.1016/S0001-6519(03)78467-2
50. Denzer, MY, Gailer, S, Kern, DW, Schumm, LP, Thuerauf, N, Kornhuber, J, et al. Quantitative validation of the n-butanol Sniffin' sticks threshold pens. Chemosens Percept. (2014) 7:91–101. doi: 10.1007/s12078-014-9168-1. Epub 2014 Apr 29
51. Schäfer, L, Schriever, VA, and Croy, I. Human olfactory dysfunction: causes and consequences. Cell Tissue Res. (2021) 383:569–79. doi: 10.1007/s00441-020-03381-9
52. Brattoli, M, de Gennaro, G, de Pinto, V, Loiotile, AD, Lovascio, S, and Penza, M. Odour detection methods: olfactometry and chemical sensors. Sensors (Basel). (2011) 11:5290–322. doi: 10.3390/s110505290
53. Gillett, S, Hopkins, C, Slack, R, and Browne, JP. A pilot study of the SNOT 22 score in adults with no sinonasal disease. Clin Otolaryngol. (2009) 34:467–9. doi: 10.1111/j.1749-4486.2009.01975.x
54. La Mantia, I, Ragusa, M, Grigaliute, E, Cocuzza, S, Radulesco, T, Calvo-Henriquez, C, et al. Sensibility, specificity, and accuracy of the Sinonasal outcome test 8 (SNOT-8) in patients with chronic rhinosinusitis (CRS): a cross-sectional cohort study. Eur Arch Otorhinolaryngol. (2023) 280:3259–64. doi: 10.1007/s00405-023-07855-8
55. Nowicki, S, and Duke, MP. Individual differences in the nonverbal communication of affect: the diagnostic analysis of nonverbal accuracy scale. J Nonverb Behav. (1994) 18:9–35. doi: 10.1007/BF02169077
56. Booth, AJ, Rodgers, JD, Volker, MA, Lopata, C, and Thomeer, ML. Psychometric characteristics of the DANVA-2 in high-functioning children with ASD. J Autism Dev Disord. (2019) 49:4147–58. doi: 10.1007/s10803-019-04130-w
57. Zupan, B, Neumann, D, Babbage, DR, and Willer, B. Exploration of a new tool for assessing emotional inferencing after traumatic brain injury. Brain Inj. (2015) 29:877–87. doi: 10.3109/02699052.2015.1011233
58. Nowicki, S Jr, and Carton, J. The measurement of emotional intensity from facial expressions: the DANVA FACES 2. J Soc Psychol. (1993) 133:749–50. doi: 10.1080/00224545.1993.9713934
59. Davis, MH . A multidimensional approach to individual differences in empathy. JSAS Catalog Select Doc Psychol. (1980) 10:85.
60. Davis, MH . Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. (1983) 44:113–26. doi: 10.1037/0022-3514.44.1.113
61. Invitto, S, Piraino, G, Ciccarese, V, Carmillo, L, Caggiula, M, Trianni, G, et al. Potential role of OERP as an early marker of mild cognitive impairment. Front Aging Neurosci. (2018) 10:272. doi: 10.3389/fnagi.2018.00272
62. Humphries, T, and Singh, R. Assessment of olfactory function after traumatic brain injury: comparison of single odour tool with detailed assessment tool. Brain Inj. (2018) 32:557–62. doi: 10.1080/02699052.2018.1434237
63. Hummel, T, Sekinger, B, Wolf, S, Pauli, E, and Kobal, G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. (1997) 22:39–52. doi: 10.1093/chemse/22.1.39
64. Doty, RL . Olfactory dysfunction and its measurement in the clinic. World J Otorhinolaryngol Head Neck Surg. (2015) 1:28–33. doi: 10.1016/j.wjorl.2015.09.007
65. Saltagi, AK, Saltagi, MZ, Nag, AK, Wu, AW, Higgins, TS, and Knisely, A. Diagnosis of anosmia and Hyposmia: a systematic review. Allergy Rhinol. (2021) 12:21526567211026568. doi: 10.1177/21526567211026568
66. Renner, B, and Schreiber, K. Olfactory and trigeminal interaction of menthol and nicotine in humans. Exp Brain Res. (2012) 219:13–26. doi: 10.1007/s00221-012-3063-2
67. Schofield, PW, Moore, TM, and Gardner, A. Traumatic brain injury and olfaction: a systematic review. Front Neurol. (2014) 5:5. doi: 10.3389/fneur.2014.00005
68. Sattin, D, Bruzzone, MG, Ferraro, S, Nigri, A, Leonardi, M, and Guido, D. Coma research center, Fondazione IRCCS Istituto Neurologico “Carlo Besta”, Milan, Italy. Olfactory discrimination in disorders of consciousness: a new sniff protocol. Brain Behav. (2019) 9:e01273. doi: 10.1002/brb3.1273
69. Frank, RA, Dulay, MF, and Gesteland, RC. Assessment of the sniff magnitude test as a clinical test of olfactory function. Physiol Behav. (2003) 78:195–204. doi: 10.1016/S0031-9384(02)00965-4
70. Kobal, G, Palisch, K, Wolf, SR, Meyer, ED, Hüttenbrink, KB, Roscher, S, et al. A threshold-like measure for the assessment of olfactory sensitivity: the "random" procedure. Eur Arch Otorhinolaryngol. (2001) 258:168–72. doi: 10.1007/s004050100328
71. Murata, Y, Okutani, F, Nakahira, M, Ushida, T, Ikemoto, T, Yokoe, I, et al. Effects of olfactory stimulation with isovaleric acid on brain activation in informed and naı¨ve conditions: a functional MRI study. Auris Nasus Larynx. (2007) 34:465–9. doi: 10.1016/j.anl.2007.01.014
72. Gudziol, H, and Guntinas-Lichius, O. Electrophysiologic assessment of olfactory and gustatory function. Handb Clin Neurol. (2019) 164:247–62. doi: 10.1016/B978-0-444-63855-7.00016-2
73. Caminiti, F, De Salvo, S, De Cola, MC, Russo, M, Bramanti, P, and Marino, S. Detection of olfactory dysfunction using olfactory event related potentials in young patients with multiple sclerosis. PLoS One. (2014) 9:e103151. doi: 10.1371/journal.pone.0103151
74. Murphy, C, Schubert, CR, Cruickshanks, KJ, Klein, BE, Klein, R, and Nondahl, DM. Prevalence of olfactory impairment in older adults. JAMA. (2002) 288:2307–12. doi: 10.1001/jama.288.18.2307
75. Huttenbrink, KB, Hummel, T, Berg, D, Gasser, T, and Hähner, A. Olfactory dysfunction: common in later life and early warning of neurodegenerative disease. Dtsch Arztebl Int. (2013) 110:1. doi: 10.3238/arztebl.2013.0001
76. Marin, C, Vilas, D, Langdon, C, Alobid, I, López-Chacón, M, Haehner, A, et al. Olfactory dysfunction in neurodegenerative diseases. Curr Allergy Asthma Rep. (2018) 18:42. doi: 10.1007/s11882-018-0796-4
77. Haehner, A, Boesveldt, S, Berendse, HW, Mackay-Sim, A, Fleischmann, J, Silburn, PA, et al. Hummel T prevalence of smell loss in Parkinson's disease a multicenter study. Parkinsonism Relat Disord. (2009) 15:490–4. doi: 10.1016/j.parkreldis.2008.12.005
78. Doty, RL, and Kamath, V. The influences of age on olfaction: a review. Front Psychol. (2014) 5:20. doi: 10.3389/fpsyg.2014.00020
79. Mobley, AS, Rodriguez-Gil, DJIF, and Greer, CA. Aging in the olfactory system. Trends Neurosci. (2013) 37:77–84. doi: 10.1016/j.tins.2013.11.004
80. Croy, I, and Hummel, T. Olfaction as a marker for depression. J Neurol. (2017) 264:631–8. doi: 10.1007/s00415-016-8227-8
81. Hummel, T, and Podlesek, D. Clinical assessment of olfactory function. Chem Senses. (2021) 46:bjab053. doi: 10.1093/chemse/bjab053
82. Shiga, H, Taki, J, Washiyama, K, Yamamoto, J, Kinase, S, Okuda, K, et al. Miwa T Assessment of olfactory nerve by SPECT-MRI image with nasal thallium-201 administration in patients with olfactory impairments in comparison to healthy volunteers. PLoS One. (2013) 8:e57671. doi: 10.1371/journal.pone.0057671
83. Jafari, A, and Holbrook, EH. Therapies for olfactory dysfunction – an update. Curr Allergy Asthma Rep. (2022) 22:21–8. doi: 10.1007/s11882-022-01028-z
84. Eliyan, Y, Wroblewski, KE, McClintock, MK, and Pinto, JM. Olfactory dysfunction predicts the development of depression in older US adults. Chem Senses. (2020) 46:bjaa075. doi: 10.1093/chemse/bjaa075
85. Haehner, A, Hummel, T, and Reichmann, H. Olfactory loss in Parkinson's disease. Parkinson Dis. (2011):450939. doi: 10.4061/2011/450939
86. Huang, T, Wei, Y, and Wu, D. Effects of olfactory training on posttraumatic olfactory dysfunction: a systematic review and meta-analysis. Int Forum Allergy Rhinol. (2021) 11:1102–12. doi: 10.1002/alr.22758
87. Pellegrino, R, Han, P, Reither, N, and Hummel, T. Effectiveness of olfactory training on different severities of posttraumatic loss of smell. Laryngoscope. (2019) 129:1737–43. doi: 10.1002/lary.27832
88. Konstantinidis, I, Tsakiropoulou, E, Bekiaridou, P, Kazantzidou, C, and Constantinidis, J. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope. (2013) 123:E85–90. doi: 10.1002/lary.24390
89. Jiang, RS, Twu, CW, and Liang, KL. The effect of olfactory training on odor identification in patients with traumatic anosmia. Int Forum Allergy Rhinol. (2019) 9:1244–51. doi: 10.1002/alr.22409
90. Altundag, A, Saatci, O, Kandemirli, SG, Sanli, D, Duz, OA, Sanli, AN, et al. Imaging features to predict response to olfactory training in post-traumatic olfactory dysfunction. Laryngoscope. (2021) 131:E2243–50. doi: 10.1002/lary.29392
91. Chang, Y . Reorganization and plastic changes of the human brain associated with skill learning and expertise. Front Hum Neurosci. (2014) 8:35. doi: 10.3389/fnhum.2014.00035
92. Rezaeyan, A, Asadi, S, Kamrava, SK, Khoei, S, and Zare-Sadeghi, A. Reorganizing brain structure through olfactory training in post-traumatic smell impairment: an MRI study. J Neuroradiol. (2022) 49:333–42. doi: 10.1016/j.neurad.2021.04.035
93. Hu, B, Zhang, J, Gong, M, Deng, Y, Cao, Y, and Xiang, Y. Ye D Research Progress of olfactory nerve regeneration mechanism and olfactory training. Ther Clin Risk Manag. (2022) 18:185–95. doi: 10.2147/TCRM.S354695
94. Laing, DG, Epps, A, and Jinks, AL. Chemosensory loss during a traumatic brain injury suggests a central pathway for the rehabilitation of anosmia. Chem Senses. (2021) 46:bjab016. doi: 10.1093/chemse/bjab016
95. Badran, BW, Gruber, EM, O'Leary, GH, Austelle, CW, Huffman, SM, Kahn, AT, et al. Electrical stimulation of the trigeminal nerve improves olfaction in healthy individuals: a randomized, double-blind, sham-controlled trial. Brain Stimul. (2022) 15:761–8. doi: 10.1016/j.brs.2022.05.005
96. Henkin, RI, Potolicchio, SJ, and Levy, LM. Improvement in smell and taste dysfunction after repetitive transcranial magnetic stimulation. Am J Otolaryngol. (2011) 32:38–46. doi: 10.1016/j.amjoto.2009.10.001
97. Hara, T, Shanmugalingam, A, McIntyre, A, and Burhan, AM. The effect of non-invasive brain stimulation (NIBS) on executive functioning, attention and memory in rehabilitation patients with traumatic brain injury: a systematic review. Diagnostics. (2021) 11:627. doi: 10.3390/diagnostics11040627
98. Logan, M, Kapoor, S, Peterson, L, Oliveira, M, and Han, DY. Mechanism of olfactory deficit in neurotrauma and its related affective distress: a narrative review. World J Psychiatry. (2021) 11:1259–66. doi: 10.5498/wjp.v11.i12.1259
99. Marin, C, Alobid, I, Fuentes, M, López-Chacón, M, and Mullol, J. Olfactory dysfunction in mental illness. Curr Allergy Asthma Rep. (2023) 23:153–64. doi: 10.1007/s11882-023-01068-z
100. Herman, AM, Critchley, H, and Duka, T. Decreased olfactory discrimination is associated with impulsivity in healthy volunteers. Sci Rep. (2018) 8:15584. doi: 10.1038/s41598-018-34056-9
101. Bratt, M, Moen, KG, Nordgård, S, Helvik, AS, and Skandsen, T. Treatment of posttraumatic olfactory dysfunction with corticosteroids and olfactory training. Acta Otolaryngol. (2020) 140:753–9. doi: 10.1080/00016489.2020.1767301
102. Vestito, L, Mori, L, Trompetto, C, Bagnasco, D, Canevari, RF, Ponzano, M, et al. Impact of tDCS on persistent COVID-19 olfactory dysfunction: a double-blind sham-controlled study. J Neurol Neurosurg Psychiatry. (2023) 94:87–8. doi: 10.1136/jnnp-2022-329162
103. Jafari, A, and Holbrook, EH. Therapies for olfactory dysfunction – an update. Curr Allergy Asthma Rep. (2022) 22:21–8. doi: 10.1007/s11882-022-01028-z
104. Turner, JH . Olfactory training: what is the evidence? Int Forum Allergy Rhinol. (2020) 10:1199–200. doi: 10.1002/alr.22681
105. Hedner, M, Larsson, M, Arnold, N, Zucco, G, and Hummel, T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. (2010) 32:1062–7. doi: 10.1080/13803391003683070
106. Pekala, K, Chandra, RK, and Turner, JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. Int ForumAllergy Rhinol. (2016) 6:299–307. doi: 10.1002/alr.21669
107. Goncalves, S, and Goldstein, BJ. Acute N-acetylcysteine administration ameliorates loss of olfactory neurons following experimental injury in vivo. Anat Rec. (2020) 303:626–33. doi: 10.1002/ar.24066
108. Yan, CH, Mundy, DC, and Patel, ZM. The use of platelet-rich plasma in treatment of olfactory dysfunction: a pilot study. Laryngoscope Investig Otolaryngol. (2020) 5:187–93. doi: 10.1002/lio2.357
Keywords: post-traumatic olfactory dysfunction, neurorehabilitation, traumatic brain injury, olfactory training, olfactory assessment
Citation: De Luca R, Bonanno M, Rifici C, Quartarone A and Calabrò RS (2023) Post-traumatic olfactory dysfunction: a scoping review of assessment and rehabilitation approaches. Front. Neurol. 14:1193406. doi: 10.3389/fneur.2023.1193406
Received: 24 March 2023; Accepted: 27 June 2023;
Published: 13 July 2023.
Edited by:
Jian Shi, Central South University, ChinaReviewed by:
Antonino F. Germanò, University of Messina, ItalyCopyright © 2023 De Luca, Bonanno, Rifici, Quartarone and Calabrò. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mirjam Bonanno, bWlyamFtLmJvbmFubm9AaXJjY3NtZS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.