- 1Department of Neurosurgery, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, China
- 2Institute of Neuroscience, Nanchang University, Nanchang, Jiangxi Province, China
Background: Primary extranodal mucosa-associated lymphoid tissue (MALT) lymphoma in the sellar region is a rare indolent B-cell lymphoma.
Case presentation: A newly diagnosed patient with MALT lymphoma originating from the pituitary stalk is reported. A space-occupying lesion in the sellar region was found in a 24 year-old man who had no clinical symptoms except for those relating to a sex hormone disorder (rising estrogen and falling androgen) identified during a pre-employment physical examination. MALT lymphoma was diagnosed pathologically. Radiotherapy and chemotherapy were proposed after surgery. However, the patient selected androgen replacement therapy only rather than chemoradiotherapy. Over the next 3 months, no visual disturbance, headache, cranial nerve abnormality, or other symptoms occurred.
Conclusion: Primary sellar region MALT lymphoma is an extremely rare disease. The differential diagnosis of sellar and parasellar masses should include primary sellar region MALT lymphoma. Early detection and treatment of this lymphoma can effectively improve the prognosis.
1. Introduction
Marginal zone lymphoma (MZL) is a low-grade, non-Hodgkin lymphoma. The World Health Organization divides it into three types: extranodal marginal zone B cell lymphoma (EMZL), intranodal lymphoma, and intrasplenic lymphoma (1). EMZL is an indolent lymphoma that can appear at any extranodal location. Isaacson and Wright initially defined EMZL as a low-grade lymphoma of the gastrointestinal tract (2). However, according to the location, EMZL can be divided into gastric and non-gastric mucosa-associated lymphoid tissue (MALT) lymphoma. The latter frequently appears in ocular appendages, skin, thyroid, lungs, salivary glands, and breasts (3) but is extremely rare in the sellar region; most central nervous system (CNS) cases occur in the dura or the brain parenchyma (4–8). A case of MALT lymphoma originating from the pituitary stalk is reported here, and the literature was searched for case studies to review how to diagnose and treat MALT lymphoma in the sellar region.
2. Case presentation
2.1. Medical history
Sparse body hair and pale skin were found in a 24 year-old male patient, which were considered to be the result of a decrease in male hormones caused by pituitary dysfunction. This decrease in male hormones has been identified in a pre-employment physical examination 2 months earlier. Brain magnetic resonance imaging (MRI) was performed and hormone levels were assessed. The patient had no dizziness, headache, blurred vision, or other symptoms, and no history of hypertension, diabetes, or HIV. Physical examination on admission showed that body temperature was 36.6°C, heart rate was 74 beats/min, breathing rate was 20 beats/min, and blood pressure was 117/74 mmHg. The patient had normal development. Systemic superficial lymph nodes were negative. Binocular eye movement and light reflex were normal. Physiological reflexes were normal, and pathological reflexes were not elicited.
2.2. Laboratory examination
After admission, biochemical index tests, routine urine tests, and routine stool tests were conducted multiple times. The red blood cell count was 3.93 × 1012/L (normal range: 3–5.8 × 1012/L) and hemoglobin was 114 g/L (normal range: 130–175 g/L). Estradiol was 52.02 pg./mL (normal range: 0–39.8 pg./mL), prolactin was 28.47 ng/mL (normal range: 2.1–17.7 ng/mL), luteinizing hormone was <0.07 mIU/mL (normal range: 1.5–9.3 mIU/mL), follicle-stimulating hormone was <0.30 mIU/mL (normal range: 1.4–18.1 mIU/mL), progesterone was <0.21 ng/mL (normal range: 0.28–1.22 ng/mL), testosterone was 7.35 ng/dL (normal range: 123.06–813.8 ng/dL), total triiodothyronine was 0.43 ng/mL (normal range: 0.6–1.81 ng/mL), total thyroxine was 3.5 μg/dL (normal range: 3.2–12.6 μg/dL), thyroid-stimulating hormone was 2.623 mIU/L (normal range: 0.55–4.78 mIU/L), and serum cortisol (at 8:00 AM) was 12.67 μg/dL (normal range: 4.3–22.4 μg/dL). There were no signs or symptoms related to the posterior pituitary.
2.3. Imaging
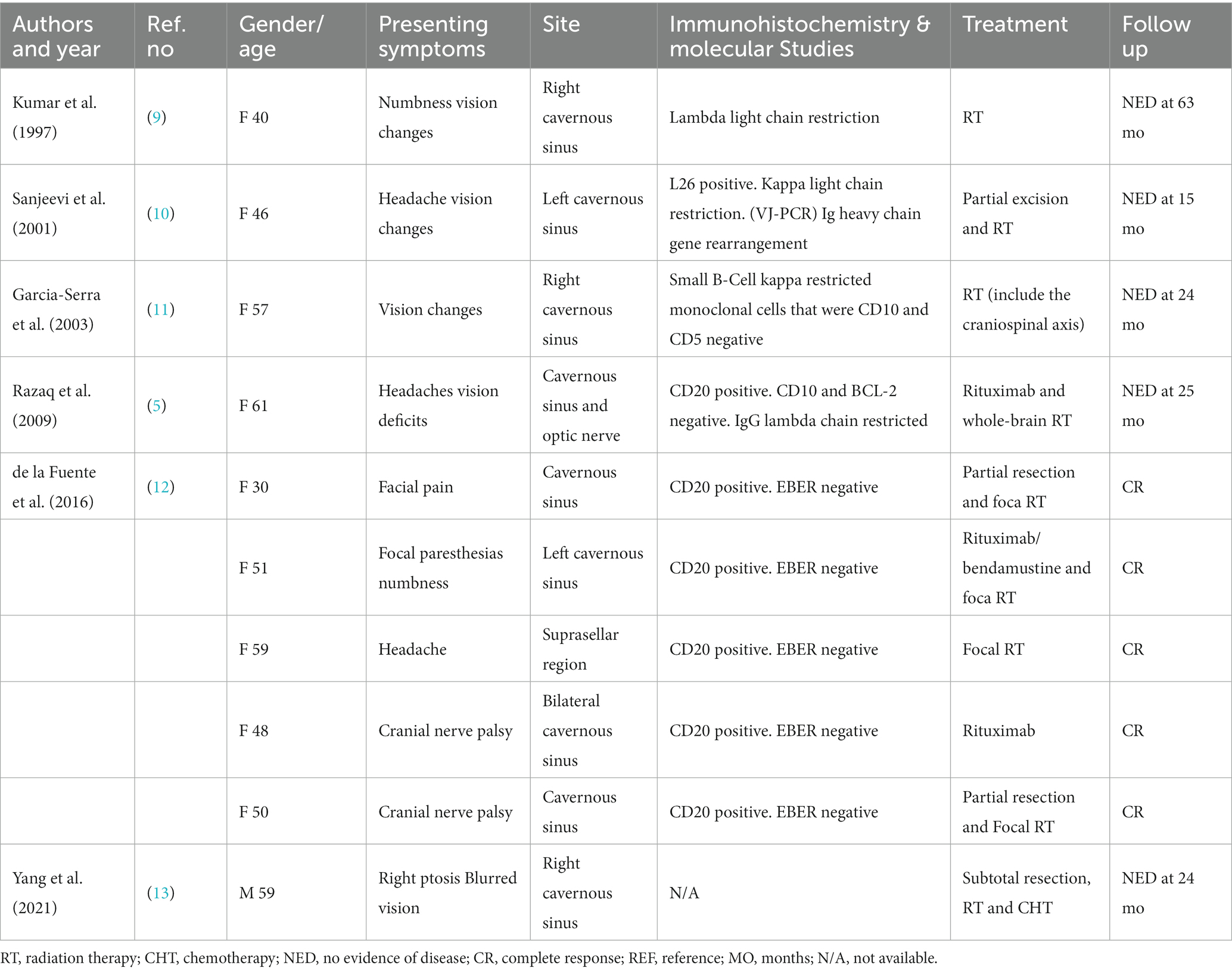
On a contrast-enhanced MRI scan of the head, a pituitary stalk tumor exhibited isointense signals on T1- and T2-weighted images, and homogeneous contrast enhancement was observed (Figure 1). The lesion size was about 12 × 15 × 16 mm. Coronal and sagittal images showed that the tumor and the normal pituitary gland were bounded by the diaphragma sellae. The optic chiasm was slightly compressed, and the bottom of the sella sank to the left. The cavernous sinus structure on each side was clear. The eye sockets and nasopharynx were normal. The mucosae of the ethmoid sinus and left maxillary sinus were thickened. The initial diagnosis was lymphocytic hypophysitis.
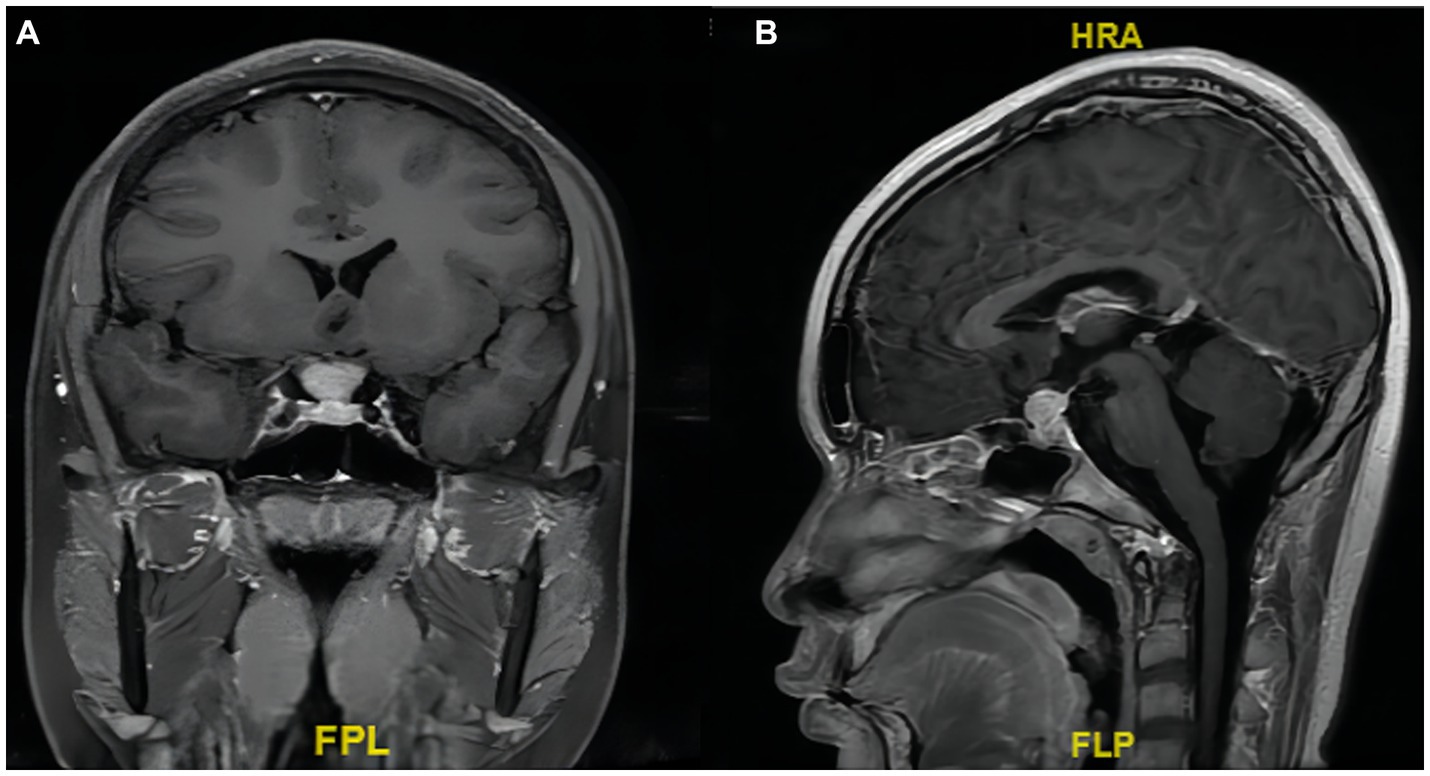
Figure 1. Preoperative enhanced T1-weighted MRI scan showing an enlarged pituitary stalk. (A) Coronal and (B) sagittal images showing that the tumor and the normal pituitary gland are bounded by the diaphragma sellae. The tumor size is about 12 × 15 × 16 mm.
2.4. Treatment
After admission for preoperative examination, the patient underwent endoscopic transnasal mass exploration. During the operation, the sellar dura was opened and normal pituitary tissue was found. The tuberculum sellae was abraded to expand the approach, and the brain was explored. This showed that the pituitary stalk had grown expansively and the blood supply was abundant (Figure 2). The tumor texture was hard. It was light pink, and it was difficult to find normal pituitary stalk tissue. The tumor was partially resected for pathological examination. The bottom of the sella was reconstructed with artificial meninges and mucosal flaps. Transient diabetes insipidus occurred after the operation, but there was no cerebrospinal fluid leakage or intracranial infection.

Figure 2. During the operation, we saw that the enlarged pituitary stalk had an abundant blood supply (arrow).
Pathological examination of the surgical specimens showed that the lymphoid tissue proliferated in the mass, and the cells in the focal area were mildly heterogeneous. Cell immunohistochemistry indicated that the surgical specimens were positive for CD20, PAX5, CD79α, CD3, CD5, CD23, CD21, Bcl-2, K, and λ. CK, CD56, Syn, CgA, SALL4, S-100, Bcl-6, CyclinD1, Langerin, CD1α, Mum-1, MPO, CD117, and EBER were negative. The Ki-67 index of cell proliferation was 40%. The pathological diagnosis was extranodal MALT of the sellar region, which is an MZL (Figure 3).
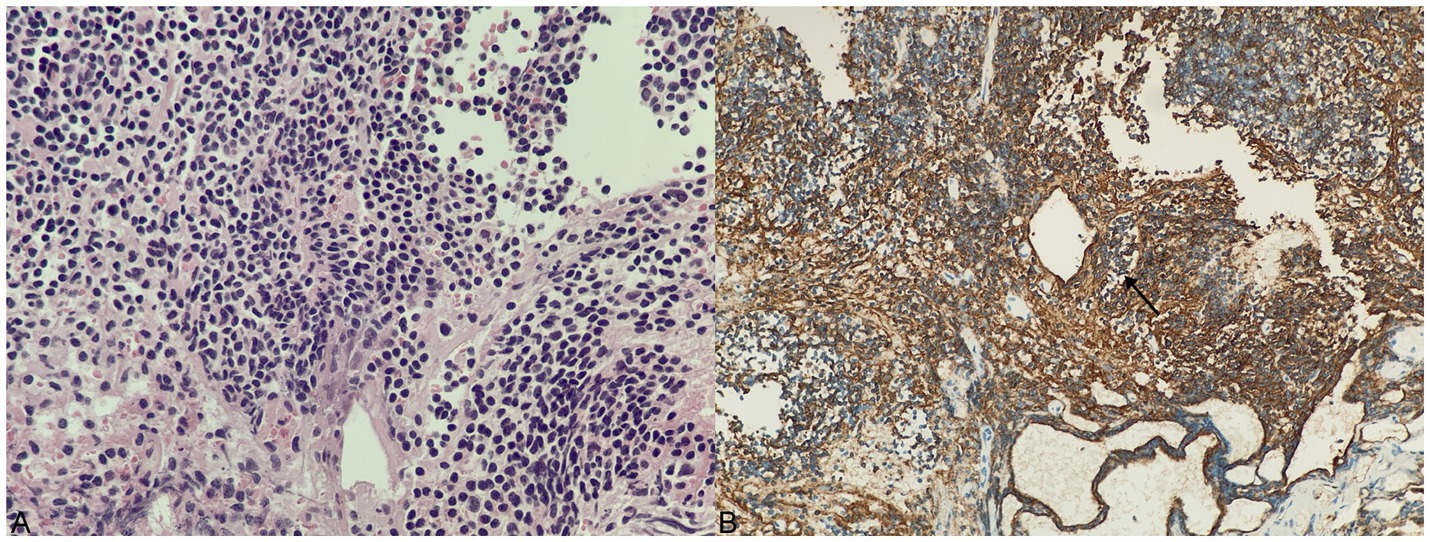
Figure 3. Histology and immunohistochemical staining of tumor. (A) H&E staining, ×400. (B) Substantial CD20-positive B lymphocytes (arrow shows that the cell membrane of a CD20-positive B lymphocyte is tan).
The patient and family members refused radiotherapy and chemotherapy. Based on the pituitary and adrenal cortex function test results, androgen replacement therapy was given. At the follow-up 3 months later, a pituitary MRI showed that the tumor did not grow after surgery (Figure 4; the patient and his family selected only unenhanced MRI). The testosterone level was normalized (151.67 ng/dL; normal range: 123.06–813.8 ng/dL). The patient did not have symptoms such as vision defects, headache, or cranial nerve abnormalities. He was restored to a healthy life.
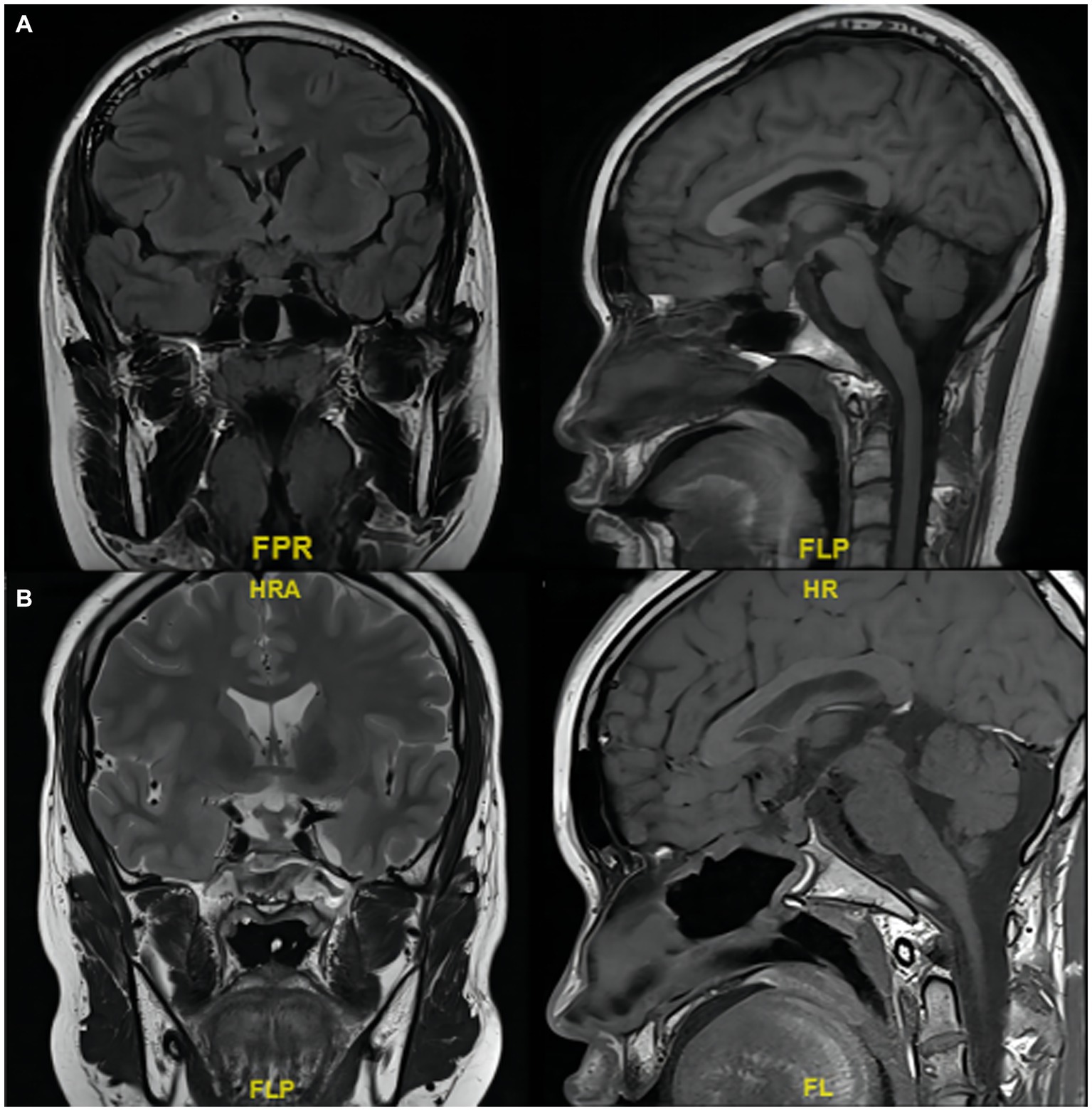
Figure 4. MRI scans of the tumor in the sellar region involving the pituitary stalk. (A) Before and (B) 3 months after partial resection.
3. Discussion
Using the PubMed database, we conducted a retrospective analysis of case reports on MALT lymphoma in the sellar region from 1997 to 2022. Table 1 provides a detailed clinical summary of the ten identified patients. The median age was 50 years (range: 24–61 years). Nine were women (90%). The most common clinical symptoms were vision defects (50%) and headaches (30%). In terms of treatment, three patients (30%) received radiotherapy only, one (10%) received chemotherapy only, three (30%) received surgery and radiotherapy, two (20%) received radiotherapy and chemotherapy, and one (10%) received surgery, radiotherapy, and chemotherapy. Despite the varying treatments, the prognoses were good, indicating that MALT lymphoma is less malignant.
Patients with strong immunity rarely suffer from primary CNS lymphoma (PCNSL), which accounts for 4% of intracranial tumors and 4%–6% of extranodal lymphomas (14). According to epidemiological analysis, primary pituitary lymphoma (PPL) is more likely to occur in individuals aged 50–60, and the number of females is slightly higher than that of males (15). Most PPL is diffused large B-cell lymphoma (DLBCL), while MALT lymphoma is rare. MALT lymphoma is an indolent tumor that spreads slowly, and most patients have a good prognosis. It often occurs in the gastrointestinal tract, eye appendages, skin, thyroid, lungs, salivary glands, breasts, etc., while it is extremely rare in the sellar region.
The specific pathogenesis of PPL is still unclear. Some researchers believe that when normal lymphocytes enter the CNS during inflammation, the tumor is transformed, leading to PPL, or the normal lymphoid tissues in the CNS are transformed, leading to PPL (15). Inflammation and autoimmune diseases will cause chronic irritation, which may eventually lead to MALT lymphoma (16). It is worth studying whether the tumor will subside if the initial cause is eliminated.
Pathology and immunohistochemistry remain the gold standards for diagnosing PPL (5). Endoscopic transnasal transsphenoidal biopsy and stereotactic biopsy are two ways to diagnose PPL. However, it should be noted that corticosteroids, which can significantly shrink tumors, should be avoided before stereotactic biopsy. The MRI characteristic of PPL is equal or low signals on T1-and T2-weighted images (17). The T2-weighted image signal is not high as the tumor cells are dense and the nucleo-plasma ratio is high. This characteristic makes it easier to distinguish PPL from other sellar tumors (18). Nonetheless, MRI scans of patients have been reported to be varied and nonspecific. Misdiagnosis is likely to occur. The clinical symptoms of PPL are also nonspecific. Most patients with PPL often have headaches, cranial nerve abnormalities, and pituitary insufficiency (19). Headaches may occur as the sellar bone erodes, the diaphragma sellae extends, or ventriculomegaly occurs (20). Except for the sex hormone secretion disorder, no other symptoms or signs were found in the patient. In summary, it is difficult to diagnose PPL clinically based on symptoms and signs (including MRI scans).
PPL should be managed based on the protocol for treating PCNSL, including surgery, chemotherapy, and/or radiotherapy (15, 21). As PPL is invasive and multifocal, the lesion cannot be completely removed by surgery. The main purpose of the surgery is to relieve local compression and confirm the diagnosis by biopsy. However, Weller et al. confirmed that the progression-free and overall survival of patients undergoing complete or subtotal resection are higher than those undergoing biopsy (22). Comprehensive treatment with chemotherapy as the main treatment and radiotherapy as the auxiliary treatment is effective for PCNSL. High-dose methotrexate-based systemic chemotherapy improves the survival rate of PCNSL patients (23–26). Additionally, combination treatment involving rituximab/temozolomide±interferon-beta is also effective for patients with relapsing and refractory PCNSL (23).
In the current case, the tumor in the sellar region originated from the pituitary stalk. Blindly removing the tumor would cut off the connection between the pituitary and hypothalamus, which would eventually lead to a hormone disorder that would necessitate lifelong hormone replacement therapy. Therefore, we performed partial resection and then performed pathological examination to confirm the diagnosis. After the diagnosis, the patient and family members refused radiotherapy and chemotherapy. They only agreed to androgen therapy for the sex hormone disorder.
4. Conclusion
Primary sellar region MALT lymphoma is a rare illness. Identification of more cases is required to study its pathogenesis and formulate better treatment plans. In the current case, the patient did not undergo radiotherapy or chemotherapy after partial resection. Close follow-up is needed, and the next treatment plan will be formulated based on the patient’s condition. In summary, MRI scans and clinical symptoms of sellar region MALT lymphoma lack specificity. Neurosurgeons should pay attention to this rare disease in the differential diagnosis of sellar disease, as timely diagnosis and treatment can significantly improve the patient’s prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SC was responsible for methodology, data curation, resources, formal analysis, and writing – original draft. JX, PC, and HL were responsible for supervision and writing – review and editing. ZC was responsible for ensuring that the descriptions are accurate and agreed on by all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Major Research Projects of the Chinese Academy of Traditional Chinese Medicine (grant no. ZZ15-WT-04); the Science and Technology Research Project of Jiangxi Provincial Department of Education (grant no. GJJ200133); and the Science and Technology Plan of Jiangxi Provincial Health Commission (grant no. 202130447).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1193391/full#supplementary-material
References
1. Jakic-Razumovic, J, and Aurer, I. The World Health Organization classification of lymphomas. Croat Med J. (2002) 43:527–34. doi: 10.1097/00000441-200210000-00011
2. Isaacson, P, and Wright, DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. (1983) 52:1410–6. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3
3. Defrancesco, I, and Arcaini, L. Overview on the management of non-gastric MALT lymphomas. Best Pract Res Clin Haematol. (2018) 31:57–64. doi: 10.1016/j.beha.2017.11.001
4. Nomani, L, Cotta, CV, Hsi, ED, Ferry, JA, and Cook, JR. Extranodal marginal zone lymphoma of the central nervous system includes parenchymal-based cases with characteristic features. Am J Clin Pathol. (2020) 154:124–32. doi: 10.1093/ajcp/aqaa032
5. Razaq, W, Goel, A, Amin, A, and Grossbard, ML. Primary central nervous system mucosa-associated lymphoid tissue lymphoma: case report and literature review. Clin Lymphoma Myeloma. (2009) 9:E5–9. doi: 10.3816/CLM.2009.n.052
6. Jesionek-Kupnicka, D, Smolewski, P, Kupnicki, P, Pluciennik, E, Zawlik, I, Papierz, W, et al. Primary extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type in the central nervous system (MZL CNS) presented as traumatic subdural hematoma and subarachnoid bleeding – case report. Clin Neuropathol. (2013) 32:384–92. doi: 10.5414/NP300579
7. Choi, JY, Chung, JH, Park, YJ, Jung, GY, Yoon, TW, Kim, YJ, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated tissue type involving the dura. Cancer Res Treat. (2016) 48:859–63. doi: 10.4143/crt.2014.334
8. Zhao, YR, Hu, RH, Wu, R, and Xu, JK. Primary mucosa-associated lymphoid tissue lymphoma in the midbrain: a case report. World J Clin Cases. (2021) 9:6566–74. doi: 10.12998/wjcc.v9.i22.6566
9. Kumar, S, Kumar, D, Kaldjian, EP, Bauserman, S, Raffeld, M, and Jaffe, ES. Primary low-grade B-cell lymphoma of the dura: a mucosa associated lymphoid tissue-type lymphoma. Am J Surg Pathol. (1997) 21:81–7. doi: 10.1097/00000478-199701000-00009
10. Sanjeevi, A, Krishnan, J, Bailey, PR, and Catlett, J. Extranodal marginal zone B-cell lymphoma of malt type involving the cavernous sinus. Leuk Lymphoma. (2001) 42:1133–7. doi: 10.3109/10428190109097736
11. Garcia-Serra, A, Price Mendenhall, N, Hinerman, RW, Lynch, JW, Braylan, RC, and Mancuso, AA. Management of neurotropic low-grade B-cell lymphoma: report of two cases. Head Neck. (2003) 25:972–6. doi: 10.1002/hed.10311
12. de la Fuente, MI, Haggiagi, A, Moul, A, Young, RJ, Sidani, C, Markoe, A, et al. Marginal zone dural lymphoma: the memorial Sloan Kettering cancer Center and University of Miami experiences. Leuk Lymphoma. (2017) 58:882–8. doi: 10.1080/10428194.2016.1218006
13. Yang, CC, Chen, TY, Tsui, YK, and Ko, CC. Primary marginal zone B-cell lymphoma of the cavernous sinus: a case report and review of the literature. BMC Med Imaging. (2021) 21:25. doi: 10.1186/s12880-021-00556-w
14. Grommes, C, and DeAngelis, LM. Primary CNS lymphoma. J Clin Oncol. (2017) 35:2410–8. doi: 10.1200/JCO.2017.72.7602
15. Tarabay, A, Cossu, G, Berhouma, M, Levivier, M, Daniel, RT, and Messerer, M. Primary pituitary lymphoma: an update of the literature. J Neuro-Oncol. (2016) 130:383–95. doi: 10.1007/s11060-016-2249-z
16. Joshi, M, Sheikh, H, Abbi, K, Long, S, Sharma, K, Tulchinsky, M, et al. Marginal zone lymphoma: old, new, targeted, and epigenetic therapies. Ther Adv Hematol. (2012) 3:275–90. doi: 10.1177/2040620712453595
17. Erdag, N, Bhorade, RM, Alberico, RA, Yousuf, N, and Patel, MR. Primary lymphoma of the central nervous system: typical and atypical CT and MR imaging appearances. AJR Am J Roentgenol. (2001) 176:1319–26. doi: 10.2214/ajr.176.5.1761319
18. Rudnik, A, Larysz, D, Blamek, S, Larysz, P, Bierzyńska-Macyszyn, G, Właszczuk, P, et al. Primary pituitary lymphoma. Folia Neuropathol. (2007) 45:144–8. doi: 10.1080/15513810701697005
19. Landman, RE, Wardlaw, SL, McConnell, RJ, Khandji, AG, Bruce, JN, and Freda, PU. Pituitary lymphoma presenting as fever of unknown origin. J Clin Endocrinol Metab. (2001) 86:1470–6. doi: 10.1210/jcem.86.4.7389
20. Giustina, A, Gola, M, Doga, M, and Rosei, EA. Clinical review 136: primary lymphoma of the pituitary: an emerging clinical entity. J Clin Endocrinol Metab. (2001) 86:4567–75. doi: 10.1210/jcem.86.10.7909
21. Zhang, Y, Ma, L, Liu, J, Zhu, H, Lu, L, Deng, K, et al. Case report: identification of potential prognosis-related TP53 mutation and BCL6-LPP fusion in primary pituitary lymphoma by next generation sequencing: two cases. Front Endocrinol (Lausanne). (2021) 12:673908. doi: 10.3389/fendo.2021.673908
22. Weller, M, Martus, P, Roth, P, Thiel, E, and Korfel, A, German PCNSL Study Group. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro-Oncology. (2012) 14:1481–4. doi: 10.1093/neuonc/nos159
23. Murakami, M, Fujimaki, T, Asano, S, Nakaguchi, H, Yamada, SM, Hoya, K, et al. Combination therapy with rituximab and temozolomide for recurrent and refractory primary central nervous system lymphoma. Yonsei Med J. (2011) 52:1031–4. doi: 10.3349/ymj.2011.52.6.1031
24. von Baumgarten, L, Illerhaus, G, Korfel, A, Schlegel, U, Deckert, M, and Dreyling, M. The diagnosis and treatment of primary CNS lymphoma. Dtsch Arztebl Int. (2018) 115:419–26. doi: 10.3238/arztebl.2018.0419
25. Yasuda, M, Akiyama, N, Miyamoto, S, Warabi, M, Takahama, Y, Kitamura, M, et al. Primary sellar lymphoma: intravascular large B-cell lymphoma diagnosed as a double cancer and improved with chemotherapy, and literature review of primary parasellar lymphoma. Pituitary. (2010) 13:39–47. doi: 10.1007/s11102-009-0196-9
Keywords: primary CNS lymphoma, MALT lymphoma, pituitary stalk mass, surgery, hormone replacement therapy
Citation: Cai S, Xiao J, Chen P, Luo H and Cheng Z (2023) Primary pituitary stalk mucosa-associated lymphoid tissue lymphoma: a case report and literature review. Front. Neurol. 14:1193391. doi: 10.3389/fneur.2023.1193391
Edited by:
Mark S. Shiroishi, University of Southern California, United StatesReviewed by:
Huaping Xie, Huazhong University of Science and Technology, ChinaAntonio Todisco, Campus Bio-Medico University, Italy
Copyright © 2023 Cai, Xiao, Chen, Luo and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zujue Cheng, anVlanVlQDEyNi5jb20=
 Shihao Cai
Shihao Cai Juexian Xiao1,2
Juexian Xiao1,2 Peng Chen
Peng Chen Haitao Luo
Haitao Luo Zujue Cheng
Zujue Cheng