
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 20 June 2023
Sec. Neuroepidemiology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1188137
This article is part of the Research TopicPublic Health in the Context of Life-Limiting Illnesses: Patient-Centered Care in Advanced and Life-Limiting IllnessesView all 18 articles
Introduction: Epidemiological data on Bell's palsy are vital for elucidating disease prevalence and enhancing therapeutic options. Our objective was to explore the prevalence and possible risk factors associated with Bell's palsy recurrence in the Clinical Center of the University of Debrecen service area. Secondary data analysis was performed using hospital discharge data, including patient information and comorbidities.
Methods: Data was obtained from the Clinical Center of the University of Debrecen, on Bell's palsy patients who were treated at the hospital between January 1, 2015 and December 31, 2021. Multiple logistic regression analysis was used to examine the factors associated with Bell's palsy recurrence.
Results: Of the 613 patients analyzed, 5.87% had recurrent paralysis, and the median time interval between episodes was 315 days. Hypertension was significantly associated with Bell's palsy recurrence. Moreover, seasonal distribution analysis revealed that the number of Bell's palsy episodes was higher in colder seasons, with spring and winter having a significantly higher number of episodes than summer and autumn.
Discussion: This study provides insights into the prevalence and associated risk factors of Bell's palsy recurrence, which could aid in its management and help reduce the long-term consequences of the disease. Further research is necessary to determine the precise mechanisms underlying these findings.
Despite the fact that Bell's palsy is a widespread condition, the epidemiological data on the disease are scarce (1). Bell's palsy can be described as an idiopathic, rapid-onset facial nerve paralysis. It is the most prevalent cause of facial paralysis, and it is defined by the sudden development of lower motor neuron weakening in the facial nerve with no apparent explanation (2).
Infectious, immunological and ischemic processes are all implicated in the pathogenesis of Bell's palsy (3). The most likely explanation is still the viral one, which suggests that the neurotropic herpes simplex (HSV-1, HSV-2) and Varicella zoster viruses reactivated in the geniculate ganglion (4, 5). In addition, other viral infections and autoimmune disease has also been suggested as potential pathomechanisms (6).
In different groups, the yearly incidence of Bell's palsy has been found to range from 11 to 40/100,000 people (7), with a one-in-sixty chance of developing the disease in one's lifetime. In around 71% of untreated instances, the disease resolves completely (8, 9).
About 30% of patients are expected to experience long-term consequence, including incomplete eye closure, crocodile tears, oral incompetence during eating and drinking, articulation problems, muscular contracture, synkinesis, and facial discomfort. Facial palsy can negatively affect psychological wellbeing, quality of life and cause functional and aesthetic deficits (10). Patients with face paresis could experience lower social functioning, since verbal communication and the expression of emotions are impaired by loss of facial function (11). The prevalence of anxiety and depression are higher among them (12), and psychosocial dysfunctions related to facial palsy are more common in women (13).
The variables linked to the occurrence of Bell's palsy, such as age, gender, season, pregnancy, and diabetes mellitus, have been subjected to much debate (9, 10, 14).
The aim of this research was to explore the possible influencing factors and to estimate the prevalence of the reoccurrence of Bell's palsy in the service area of the Clinical Center of University of Debrecen. Moreover, we also aimed to describe the seasonality of the disease.
Based on the information from the Clinical Center of University of Debrecen, secondary data analysis was carried out using hospital discharge data (including all outpatient and inpatient medical records). Bell's palsy cases were identified by ICD-10 codes (G51.0). The data contained detailed information (age, gender, risk factors, date of admission and discharge, comorbidities, side of the paralysis) on Bell's palsy patients treated at the institution between January 1, 2015, and December 31, 2021. To identify reoccurrence of cases, we defined a minimum 90 days interval between the last and the next admission of patients (15).
The occurrence of all patients was investigated according to the months of the admission. To investigate seasonal variations in the occurrence of BP, months was classified as follows: March to May as spring, June to August as summer, September to November as autumn, and December to February as winter.
The total sample consisted of 650 patients. Patients with missing information on the side of paralysis (n = 37) were excluded, thus the final sample consisted of 613 patients with BP.
Text mining tools were also used to assure the diagnoses of International Classification of Diseases (ICD) codes based on free text analysis. Proportions with 95% confidence intervals were calculated for episodes in seasons. Multiple logistic regression model was used to explore the factors associated with the occurrence of Bell's palsy. Odds ratio (OR) with the corresponding 95% confidence intervals were calculated to determine the strength of association. Stata v17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.) was used for statistical analysis; p < 0.05 indicated statistical significance.
The study was approved by the Regional Ethical Committee of University of Debrecen [5678-2021].
Of the 613 patients 51.71% were male (n = 317) and 48.29% were female (n = 296). The age of the studied patients varied between 1 and 96 years, the mean age (±SD) was 43.02 ± 22.96 years.
We analyzed seasonal distribution of Bell's palsy based on episodes of care for the 613 people within the investigated period (another episode was considered separate if it occurred more than 90 days after the previous one) (15). Figure 1 shows the number of episodes in relation to seasonality. The highest number of episodes occurred in the colder seasons. The number of episodes were significantly higher in spring and winter compared to the warmer seasons. Although, there was no significant difference between seasons regarding recurrence, the highest peak was observed in autumn (Figure 2).
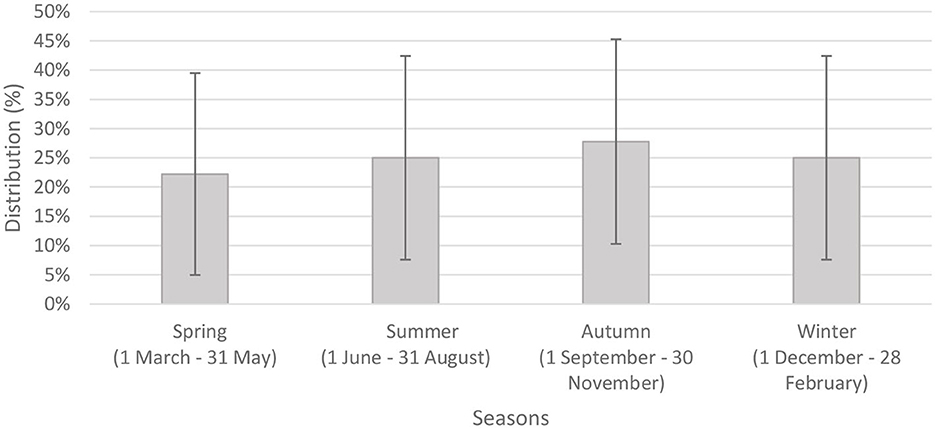
Figure 2. Seasonal distribution of recurrent episodes of care with 95% confidence intervals (n = 36).
Thirty-six patients (5.87%) had recurrent paralysis 90 days after last admission. The median time interval between the last and next episode was 315 days.
Table 1 shows the baseline characteristics and most prevalent comorbidities occurred among patients with recurrent paresis and seasons at occurrence. Out of all the non-recurrent cases, 8.15% had non-insulin-dependent diabetes mellitus (NIDDM), 5.20% had hypertension, 1.04% had hyperlipidemia, and 0.87% had headache. On the other hand, among the recurrent cases, 11.11% had NIDDM, 16.67% had hypertension, 5.56% had hyperlipidemia, and 2.78% had headache.
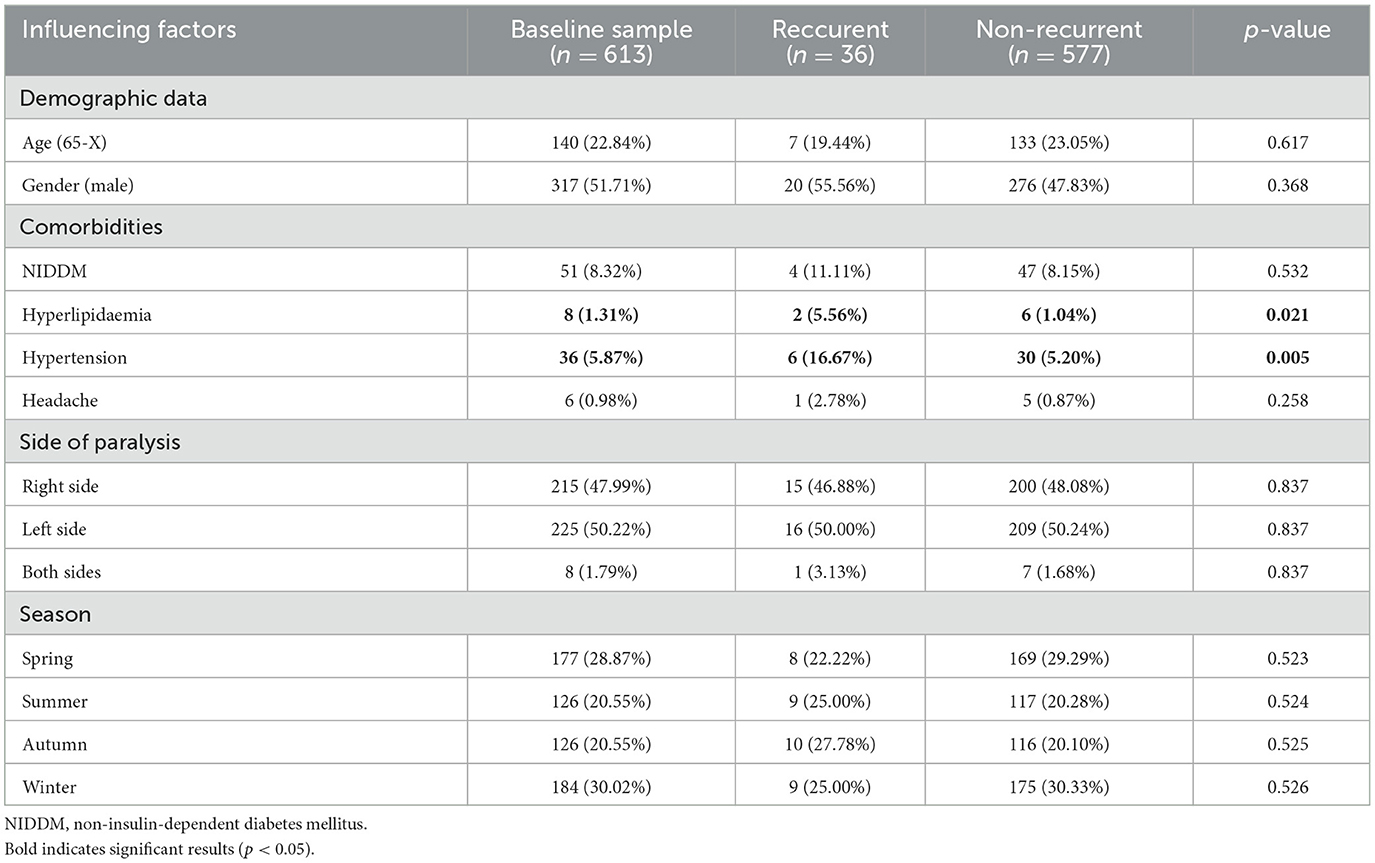
Table 1. Baseline characteristics and prevalence of most common comorbidities and seasons for recurrence of Bell's palsy.
Table 2 shows the associated factors with recurrence in a multiple logistic regression model. The results showed that the age group was not significantly associated with Bell's palsy recurrence (OR = 0.99, p = 0.095, 95% CI: 0.078–1.01). Gender was also not significant (OR = 1.45, p = 0.334, 95% CI: 0.68–3.11). However, hypertension was found to be a significant predictor of Bell's palsy recurrence (OR = 4.16, p = 0.007, 95% CI: 1.47–11.79). The analysis also revealed that the season was a significant predictor of Bell's palsy recurrence, where autumn compared with spring (OR = 3.16, p = 0.031, 95% CI: 1.11–8.97) and dyslipidemia (OR = 7.95, p = 0.024, 95% CI: 1.31–48.17) were significantly associated with higher odds of recurrence.
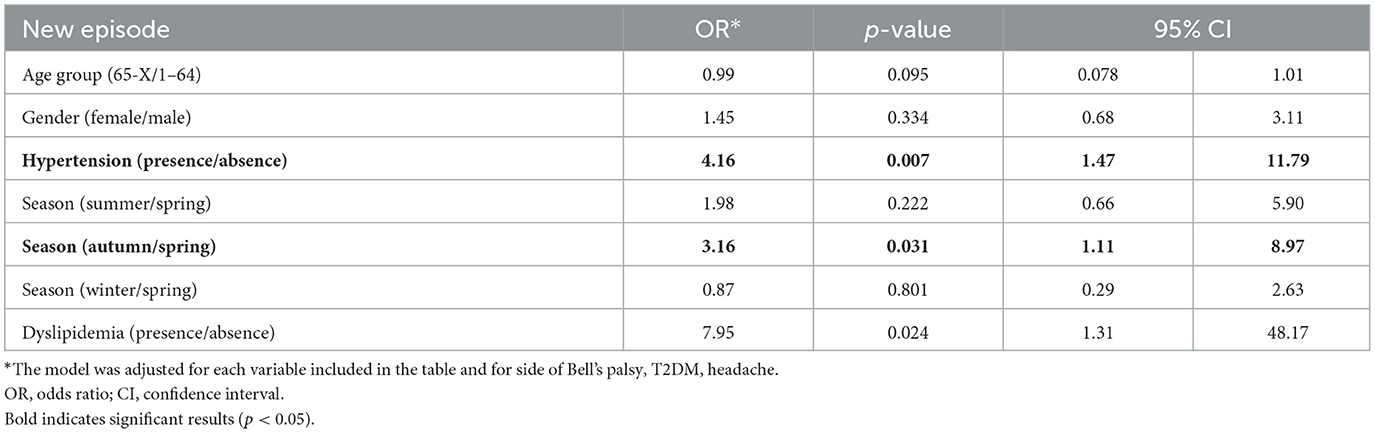
Table 2. Multiple logistic regression model of possible influencing factors on Bell's palsy recurrence.
In summary, age and gender were not found to be a significant factor in the model. In the present research, the mean age (±SD) was 43.02 ± 22.96 years, and 51.71% of the patients were male, which showed similar pattern to the existing literature regarding demographic composition of patients with Bell's palsy (16). According to research, the incidence of Bell's palsy is higher in adults aged 20 to 50 (9, 17–19), whereas others indicated that those aged 60 and older are more affected (20–24).
In this study, recurrent Bell's palsy was observed in 36 cases (5.87%), which was occurred mostly within 1 year after the last admission. The recurrent Bell's palsy, which can develop on either the ipsilateral or contralateral side of the first episode, is more likely to occur in the first 2 years from the onset (25). Findings of different studies are consistent with our findings, as they suggest a recurrence rate between 2.6 and 15.2% of individuals, who have already experienced an initial episode, are affected by recurrent facial palsy (26).
Our results showed that some factors were found to be associated with the recurrence such as hyperlipidemia, hypertension and seasonal factors (specifically autumn), while age, gender and NIDDM were not significant predictors.
In our study, only hypertension showed significant association with recurrence. Patients with Bell's palsy had a higher rate of arterial hypertension (27). The delicate balance of pressure systems inside the facial canal is disrupted by blood pressure variations, particularly diastolic hypertension, resulting in impaired intrafunicular circulation and, as a result, nerve injury (27).
According to the literature, diabetes mellitus, as well as hypertension, dyslipidemia, and the combination of all three comorbidities (diabetes mellitus, hypertension, and dyslipidemia) in a single patient, may affect the initial severity of blood pressure (3).
The influence of diabetes mellitus, hypertension, and dyslipidemia on the result of the facial nerve in Bell's palsy is still the subject of an ongoing debate. Some authors have established in more recent research that there is no link between diabetes mellitus, hypertension, dyslipidemia, and eventual recovery from Bell's palsy (1).
According to our results, Bell's palsy has been observed to occur more likely in spring and winter, while reoccurrence showed highest peak in autumn. Others also found that a higher incidence occurs during the cold seasons (28, 29). The association between seasons and the disease can be explained by several reasons. Kim and Park (30) presented that although low temperature and humidity are related to the onset of Bell's palsy, a marked drop in temperature (autumn) has a greater impact on the occurrence of BP than the actual low temperature (winter). Several studies have also shown associations between temperature differences and increased probability of Bell's palsy occurrence (2). Low temperature, extreme wind chill factors, and unexpected shift in atmospheric pressure have all been associated with an increased risk of Bell's palsy (30, 31). As noted earlier, the consequences of seasons and meteorological circumstances differ by climate and by techniques of data gathering and statistics, underlining the need for further well-designed studies from diverse climatic zones (30).
The specific etiology of Bell's palsy needs to be determined in order to develop targeted treatment strategies (2). Immediate treatment and referral to specialist is essential to increase the chance of full recovery (14). However, patient's self-perception of facial appearance is also necessary to evaluate the success of surgical and non-surgical interventions (32). Facial palsy has substantial impact on a individuals' life, thus measuring impairment and disability is particularly important. Several instruments have been developed to measure the impact of facial dysfunction on quality of life in facial palsy patients. The most widely used patient-reported measures are Facial Clinimetric Evaluation (FaCE) (33) scale and the Facial Disability Index (FDI) (34), which measure both physical and psychosocial function associated with facial palsy (13). Since the disease has significant psychosocial consequences, psychological and social aspects of facial palsy should be also considered during the treatment of patients in order to achieve a better quality of life.
The strength of this study is that the database is representative to the Eastern Hungarian population, and we were able to investigate a time period. The study has some limitations. First, although the studied population is large, extrapolation of results should be made with caution, because our study is not representative for the whole Hungarian population. We used administrative database containing hospital discharge data, in which several lifestyle and socioeconomic factors could be underestimated; therefore, these factors were not involved in this secondary data analyses.
Our findings highlight the importance of considering factors (gender, hypertension, season) when managing patients with Bell's palsy. Bell's palsy was more frequently observed throughout the winter and spring seasons, while the peak of reoccurrence was in autumn.
There has been a lot of debate over whether underlying comorbidities like diabetes and hypertension have a role in the recurrence of Bell's palsy.
Further research is needed to better understand the underlying mechanisms and etiology of disease to prevent recurrent Bell's palsy and improve the patients' quality of life.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Study design, manuscript drafting, data collection, and analysis and interpretation: EV and AN. Critical revision of the manuscript: UB, IS, LH, and NK. All authors approval of the final version for publication.
We are grateful for the assistance and facilitation provided by the clinical practitioners during the data collection process.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lee JS, Kim YH. Epidemiological trends of Bell's palsy treated with steroids in Korea between 2008 and 2018. Muscle Nerve. (2021) 63:845–51. doi: 10.1002/mus.27213
2. Zhang W, Xu L, Luo T, Wu F, Zhao B, Li X. The etiology of Bell's palsy: a review. J Neurol. (2020) 267:1896–905. doi: 10.1007/s00415-019-09282-4
3. Psillas G, Dimas GG, Sarafidou A, Didangelos T, Perifanis V, Kaiafa G, et al. Evaluation of effects of diabetes mellitus, hypercholesterolemia and hypertension on Bell's palsy. J Clin Med. (2021) 10:2357. doi: 10.3390/jcm10112357
4. Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. (1996) 124:27–30. doi: 10.7326/0003-4819-124-1_Part_1-199601010-00005
5. Steiner I. Human herpes viruses latent infection in the nervous system. Immunol Rev. (1996) 152:157–73. doi: 10.1111/j.1600-065X.1996.tb00915.x
6. Greco A, Gallo A, Fusconi M, Marinelli C, Macri GF, de Vincentiis M. Bell's palsy and autoimmunity. Autoimmun Rev. (2012) 12:323–8. doi: 10.1016/j.autrev.2012.05.008
7. De Diego-Sastre JI, Prim-Espada MP, Fernández-García F. [The epidemiology of Bell's palsy]. Rev Neurol. (2005) 41:287–90. doi: 10.33588/rn.4105.2004593
8. Somasundara D, Sullivan F. Management of Bell's palsy. Aust Prescr. (2016) 40:94–6. doi: 10.18773/austprescr.2017.030
9. Peitersen E. Bell's palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. (2002) 122:4–30. doi: 10.1080/000164802320401694
10. Eviston TJ, Croxson GR, Kennedy PGE, Hadlock T, Krishnan AV. Bell's palsy: aetiology, clinical features and multidisciplinary care. J Neurol Neurosurg Psychiatry. (2015) 86:1356–61. doi: 10.1136/jnnp-2014-309563
11. Coulson SE. O'dwyer NJ, Adams RD, Croxson GR. Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol. (2004) 25:1014–9. doi: 10.1097/00129492-200411000-00026
12. Hotton M, Huggons E, Hamlet C, Shore D, Johnson D, Norris JH, et al. The psychosocial impact of facial palsy: a systematic review. Br J Health Psychol. (2020) 25:695–727. doi: 10.1111/bjhp.12440
13. Bylund N, Hultcrantz M, Jonsson L, Marsk E. Quality of life in Bell's palsy: correlation with sunnybrook and house-brackmann over time. Laryngoscope. (2021) 131:E612–8. doi: 10.1002/lary.28751
14. Holland NJ, Weiner GM. Recent developments in Bell's palsy. BMJ. (2004) 329:553–7. doi: 10.1136/bmj.329.7465.553
15. Steinhäuser J, Volk GF, Thielker J, Geitner M, Kuttenreich AM, Klingner CM, et al. Multidisciplinary care of patients with facial palsy: treatment of 1220 patients in a german facial nerve center. JCM. (2022) 11:427. doi: 10.3390/jcm11020427
16. Zhao H, Zhang X, Tang Y, Zhu J, Wang XH, Li ST. Bell's palsy: clinical analysis of 372 cases and review of related literature. Eur Neurol. (2017) 77:168–72. doi: 10.1159/000455073
17. Campbell KE. Effects of climate, latitude, and season on the incidence of Bell's palsy in the US Armed Forces, October 1997 to September 1999. Am J Epidemiol. (2002) 156:32–9. doi: 10.1093/aje/kwf009
18. El Tallawy HNA, Farghaly WMA, Rageh TA, Shehata GA, Metwaly NA, Abo Elftoh N, et al. Epidemiology of major neurological disorders project in Al Kharga district, New Valley, Egypt. Neuroepidemiology. (2010) 35:291–7.
19. Khedr EM, Fawi G, Abbas MAA, El-Fetoh NA, Zaki AF, Gamea A. Prevalence of Bell's palsy in Qena Governorate, Egypt. Neurol Res. (2016) 38:663–8. doi: 10.1080/01616412.2016.1190121
20. Savettieri G, Salemi G, Rocca WA, Meneghini F, Santangelo R, Morgante L, et al. Incidence and lifetime prevalence of Bell's palsy in two Sicilian municipalities. Acta Neurol Scand. (1996) 94:71–5. doi: 10.1111/j.1600-0404.1996.tb00043.x
21. Chang YS, Choi JE, Kim SW, Baek SY, Cho YS. Prevalence and associated factors of facial palsy and lifestyle characteristics: data from the Korean National Health and Nutrition Examination Survey 2010–2012. BMJ Open. (2016) 6:e012628. doi: 10.1136/bmjopen-2016-012628
22. Brandenburg NA, Annegers JF. Incidence and risk factors for Bell's Palsy in Laredo, Texas: 1974–1982. Neuroepidemiology. (1993) 12:313–25. doi: 10.1159/000110333
23. Nicoletti A, Sofia V, Bartoloni A, Bartalesi F, Marletta C, Lo Bartolo ML, et al. Lifetime prevalence of Bell's palsy in rural bolivia: a door-to-door survey. Neuroepidemiology. (2002) 21:100–4. doi: 10.1159/000048624
24. Rowlands S, Hooper R, Hughes R, Burney P. The epidemiology and treatment of Bell's palsy in the UK. Eur J Neurol. (2002) 9:63–7. doi: 10.1046/j.1468-1331.2002.00343.x
25. Cirpaciu D, Goanta C, Cirpaciu M. Recurrences of Bell's palsy. J Med Life. (2014) 7(Spec Iss 3):68–77.
26. Mancini P, Bottaro V, Capitani F, De Soccio G, Prosperini L, Restaino P, et al. Recurrent Bell's palsy: outcomes and correlation with clinical comorbidities. Acta Otorhinolaryngol Ital. (2019) 39:316–21. doi: 10.14639/0392-100X-2415
27. Paolino E, Granieri E, Tola MR, Panarelli MA, Carreras M. Predisposing factors in Bell's palsy: a case-control study. J Neurol. (1985) 232:363–5. doi: 10.1007/BF00313837
28. Spengos K, Sameli S, Stouraitis G, Kolias A, Koulouri O, Kokkinos Z, et al. Seasonal variation of Bell's palsy in Athens, Greece - a hospital-based retrospective evaluation over fifteen years. Eur Neurol. (2006) 55:84–8. doi: 10.1159/000092779
29. Hsieh RL, Wang LY, Lee WC. Correlation between the incidence and severity of Bell's palsy and seasonal variations in Taiwan. Int J Neurosci. (2013) 123:459–64. doi: 10.3109/00207454.2013.763804
30. Kim MH, Park SY. Population-based study and a scoping review for the epidemiology and seasonality in and effect of weather on Bell's palsy. Sci Rep. (2021) 11:16941. doi: 10.1038/s41598-021-96422-4
31. Kokotis P, Katsavos S. Effects of wind chill factor, temperature and other meteorological parameters on the incidence of Bell's palsy: results based on a retrospective, 7-year long, greek population study. Neuroepidemiology. (2015) 45:44–9. doi: 10.1159/000433542
32. Ho AL, Scott AM, Klassen AF, Cano SJ, Pusic AL, Van Laeken N. Measuring quality of life and patient satisfaction in facial paralysis patients: a systematic review of patient-reported outcome measures. Plast Reconstr Surg. (2012) 130:91–9. doi: 10.1097/PRS.0b013e318254b08d
33. Kahn JB, Gliklich RE, Boyev KP, Stewart MG, Metson RB, McKenna MJ. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. Laryngoscope. (2001) 111:387–98. doi: 10.1097/00005537-200103000-00005
Keywords: facial nerve paralysis, Bell's palsy, recurrence, seasonality, epidemiology
Citation: Varga E, Battamir U, Szegedi I, Hudák L, Kovács N and Nagy AC (2023) Seasonal patterns in the epidemiology of Bell's palsy in Hungary. Front. Neurol. 14:1188137. doi: 10.3389/fneur.2023.1188137
Received: 16 March 2023; Accepted: 06 June 2023;
Published: 20 June 2023.
Edited by:
Mevhibe Hocaoglu, King's College London, United KingdomReviewed by:
Tsutomu Nakashima, Nagoya University, JapanCopyright © 2023 Varga, Battamir, Szegedi, Hudák, Kovács and Nagy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Attila Csaba Nagy, YXR0aWxhbmFneUBtZWQudW5pZGViLmh1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.