
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol., 02 June 2023
Sec. Movement Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1184713
This article is part of the Research TopicAtypical Parkinsonian Syndromes - Novelties in Treatment and DiagnosisView all 4 articles
Background: Parkinsonism and akinetic mutism (AM) following ventriculo-peritoneal shunt (VPS) without underdrainage used to be considered rare, but may be underdiagnosed in daily clinical practice. Although the pathophysiology is still unclear, in several case reports, the parkinsonism and AM after VPS shows responsiveness to dopaminergic treatment.
Case presentation: We report a 19-year-old male that presented with severe parkinsonism and AM after VPS. Meanwhile, 18F-FDG-PET showed a cortical and subcortical hypometabolism. Fortunately, levodopa dramatically improved patient's symptoms and brain hypometabolism. This report provides support for the possibility that dopamine deficiency inhibits brain metabolism, and further elucidates the pathogenesis of parkinsonism and AM.
Conclusion: This report highlights the presentation of a treatable parkinsonism and points out that Levodopa and/or dopamine agonist should be the first choice if the patients develop parkinson-like symptoms after VPS.
Parkinsonism following ventriculo-peritoneal shunt (VPS) has been reported in patients without underdrainage (1–3), but the pathophysiology is unclear. Here, we report a similar case that presented with severe parkinsonism and akinetic mutism (AM) after VPS. Interestingly, Levodopa treatment not only improved the clinical outcomes, but also reversed extensive cortical and subcortical hypometabolism.
A 19-year-old male presented with chronic headache, depression, irritability, and inattention. He was diagnosed with obstructive hydrocephalus caused by aqueduct stenosis and underwent VPS. Post-operation, the patient's headache rapidly relieved and other symptoms improved within a month. 3 months after shunt insertion, the patient was hospitalized with complaints of headache, drowsiness, and intermittent confusion. Head CT scan showed dilation of the lateral ventricles and the third ventricle. After recalibrating the VPS setting and reducing the cerebrospinal fluid (CSF) pressure to 120 mmH2O, the patient's symptoms improved, and the ventricular size returned to normal within a week.
However, 6 months after VPS, the patient was readmitted due to blunted response and dilation of ventricles. This time, the CSF pressure was reduced to 100 mmH2O. Although the enlarged ventricles contracted after a few days, there was no improvement in symptoms. Additionally, progressive aggravation, gradual -hypophonia, -salivation, -eating difficulty, -muscle stiffness and -bradykinesia was observed.
Six months later (i.e., 12 months after VPS), the patient was referred to our center with mutism and immobility. On examination, he had eye movement disorders, severe rigidity of limbs and bradykinesia, hyperreflexia, and clonus of both lower limbs with positive Babinski sign. Head CT scan showed no ventricle enlargement. 18F fluorodeoxyglucose (18F-FDG) PET/MR images showed extensive cortical and subcortical hypometabolic patterns (Figure 1).
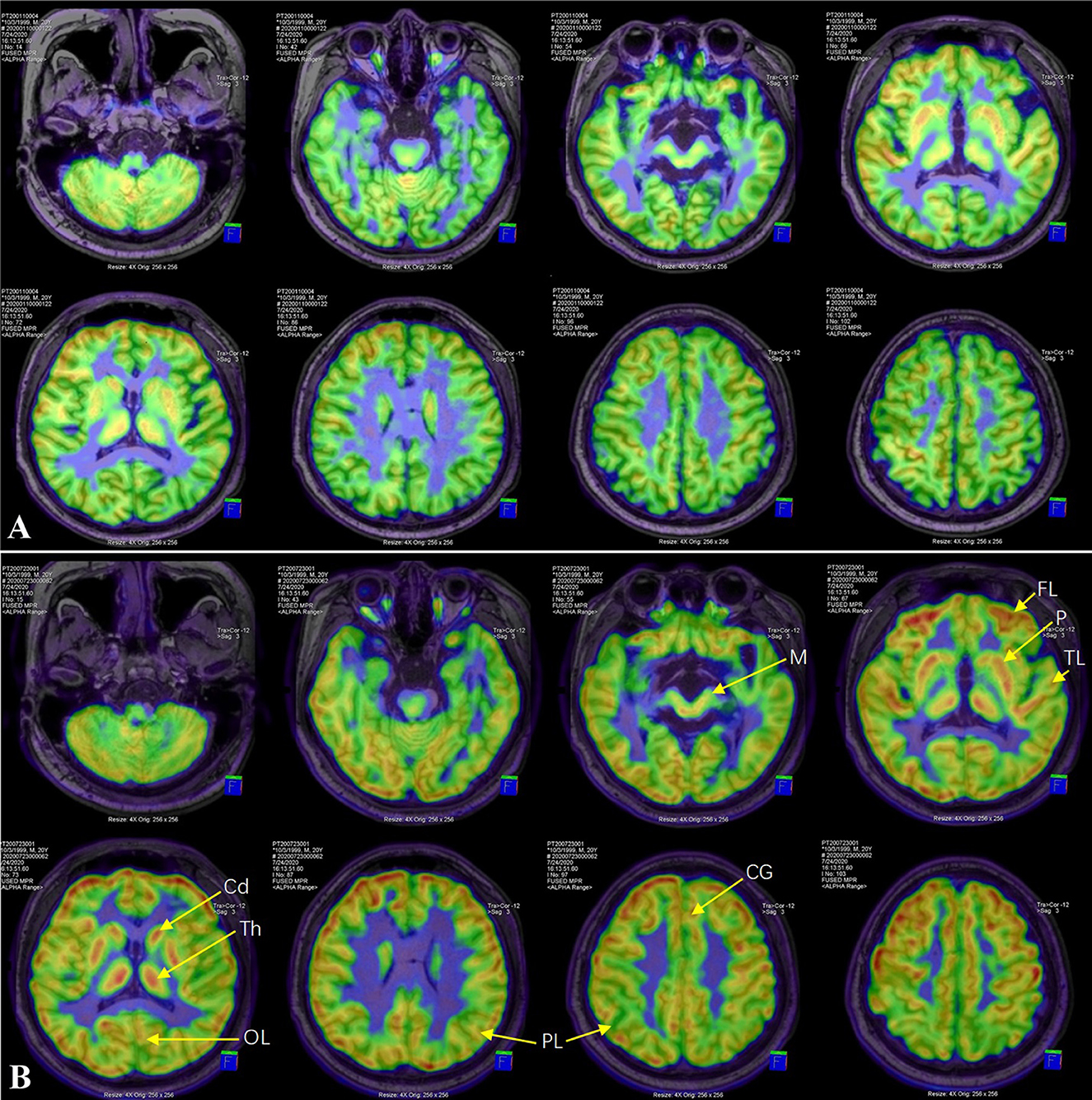
Figure 1. 18F-FDG PET/MR image. Compared with before levodopa treatment (A), FDG-SUVR of all ROIs, including cortex (frontal lobe, parietal lobe, temporal lobe, occipital lobe and cingulate gyrus) and subcortex (putamen, caudate nucleus, thalamus and midbrain), increased significantly after 6 months of treatment (B). FL, frontal lobe; PL, parietal lobe; TL, temporal lobe; OL, occipital lobe; CG, cingulate gyrus; Cd, caudate nucleus; P, putamen; Th, thalamus; M, midbrain.
Severe Parkinsonism and AM after VPS was diagnosed. The therapy of Madopar (levodopa/benserazide) and Pramipexole was initiated. Over the next 2 weeks, the dose of Madopar and Pramipexole slowly increased to 550/137.5 mg/day and 1.125 mg/day, respectively. Nearly a month after initiation of treatment, the patient let out a long-lost weak cry leading to an emotional moment for the family. After that, he began to pronounce monosyllabic words and was able to stand and walk with assistance. A complete recovery of speech and motor skills was observed 2 months later. The patient could not recall the events he had experienced during the period of severe illness. At a 6-month follow-up post-treatment, head MRI scan showed no ventricular dilation. The regions of interest (ROI) of cortex (frontal lobe, parietal lobe, temporal lobe, occipital lobe and cingulate gyrus) and subcortex (putamen, caudate nucleus, thalamus and midbrain) were delineated on 18F-FDG PET/MR images. The uptake value of each ROI was normalized by cerebellum to obtain the standard uptake value ratio of FDG (FDG-SUVR). Compared with before levodopa treatment, FDG-SUVR of all ROIs increased significantly after 6 months of treatment (Table 1). Over the next year, the doses of Madopar and Pramipexole were gradually reduced until they were discontinued. The patient's neurological symptoms have not recurred during the 10-month follow-up.
Aqueduct stenosis is known to obstruct CSF circulation and block the CSF pressure transmission between the supratentorial ventricular system (lateral ventricle and third ventricle) and the infratentorial ventricular system (fourth ventricle and subarachnoid cavity) (4). When VPS is performed, the supratentorial pressure decreases rapidly, while the infratentorial pressure increases relatively. This change in pressure causes the ventral cisterna, its adjacent structures (directly adjacent to the front and bottom of the third ventricle) to be disturbed by the CSF pressure gradient. This is especially true in the midbrain, even contributing to midbrain displacement. It has previously been reported that local pressure to the ventral midbrain, accompanied by shearing and torsion of nigrostriatal projection fibers, causes the global rostral midbrain dysfunction (3, 5, 6). Enlargement of the ventral cisterns (cistern of lamina terminalis, chiasmatic cistern, interpeduncular cistern, and prepontine cistern) and contraction of the third ventricle are revealed by MRI scans in our patient, post VPS (Figures 2A, B) indicating that there had been a change in CSF pressure gradient here. Clinical evidence supporting the above mechanism is that endoscopic third ventriculostomy (ETV) successfully improved parkinsonism following VPS in some patients (5–7). Unlike VPS, ETV is performed at the bottom of the third ventricle to re-establish the connection between the supratentorial and infratentorial ventricle systems, thereby eliminating the transentorial CSF pressure gradient.
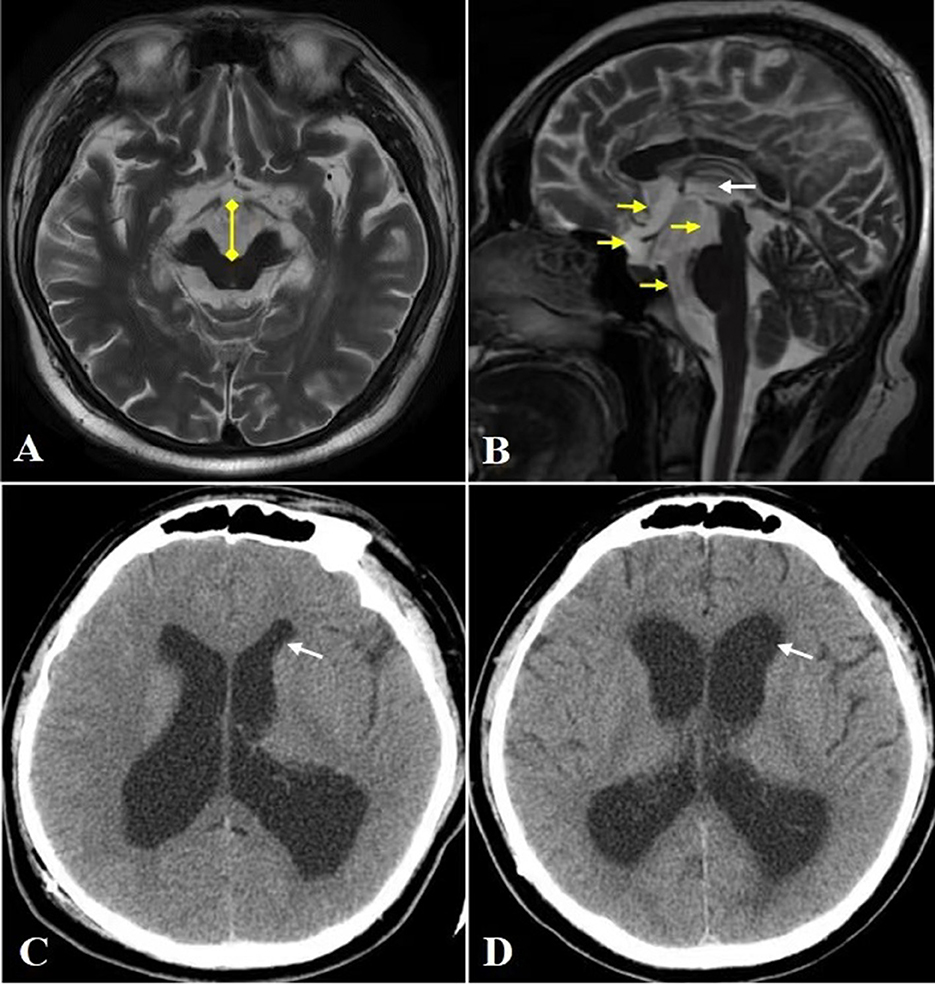
Figure 2. CT and MRI. (A) Head MRI axial view reveals significantly widened interpeduncular cistern (yellow arrow). (B) Head MRI sagittal view reveals marked enlargement of cistern of lamina terminalis, chiasmatic cistern, interpeduncular cistern and prepontine cistern (yellow arrow), and contraction of the third ventricle (white arrow). (C, D) The anterior horn (white arrow) of dilated lateral ventricle before VPS (C) and after VPS (D), the latter shows widening of the anterior horn and Evan's index >0.3, which are characteristics of NPH.
Another possible pathogenesis is that repeated sharp changes in the size of the ventricle led to stretching of the ventricle walls and damage the ascending dopaminergic projection system located in the paraventricular area (8). In addition, with the expansion and contraction of the third ventricle, the CSF pressure fluctuation in the ventral cistern becomes frequent, which further leads to midbrain injury. This makes us have to think about the risk factors of repeated ventricular dilatation as well as how to deal with. Long-term excessive stretching of ventricular wall will result in an irreversible damage to ventricular wall compliance, increasing susceptibility to normal pressure hydrocephalus (NPH) and even low-pressure hydrocephalus (9, 10). In our case, the lateral ventricular dilatation is different before and after VPS, and the latter shows the ventricular dilatation characteristics of NPH (Evan's index >0.3) (Figures 2C, D). Therefore, post VPS, setting a lower pressure range of CSF may be considered for patient safety.
Although the normalization in size of the ventricles, Parkinsonism and AM in the patient had progressively worsened prior to treatment with levodopa. The patient's symptoms improved dramatically after levodopa and pramipexole therapy. Parkinsonism and AM have not recurred during the follow-up of 2 years and 4 months, even 10 months after withdrawal of Levodopa. It is speculated that reversible dysfunction of the presynaptic nigrostriatal dopaminergic pathway caused by the above mechanical factors is the main pathophysiological mechanism in this case, which is also a widely accepted view at present (11–14). According to the pathophysiological mechanism of impaired presynaptic dopaminergic pathway, dopaminergic drugs should play an active therapeutic role. As previously reported in parkinsonism and/or AM after VPS for hydrocephalus, the majority of patients treated with levodopa achieved significant improvement (1–3, 5–7, 10–22). A few patients received dopamine agonist and/or amantadine, and achieved efficacy (15, 18–28). Anticholinergic drugs have also been reported to relieve symptoms (3, 13, 22) (Table 2).
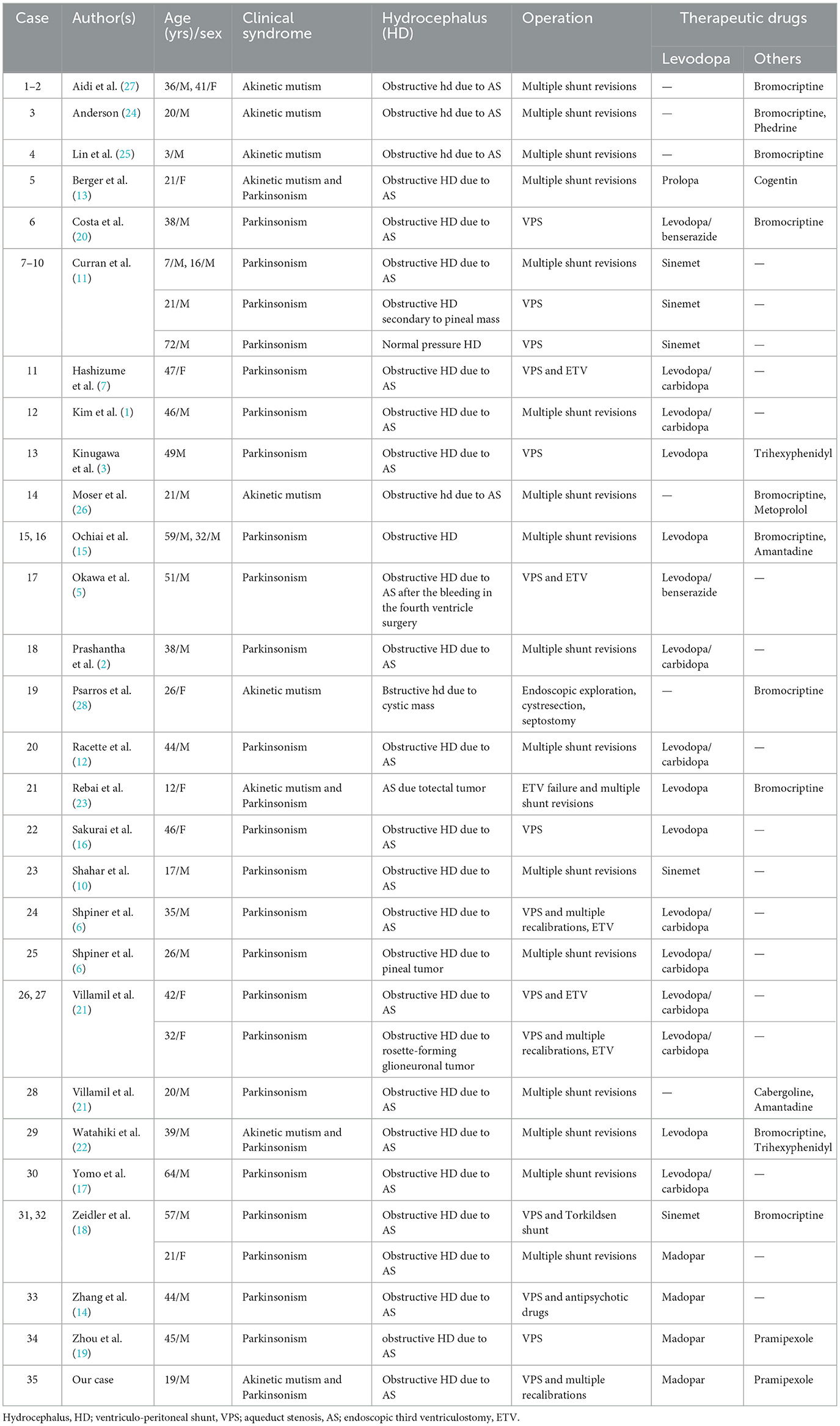
Table 2. Summary of clinical features in patients with Parkinsonism and/or akinetic mutism following hydrocephalus treatment.
Our patient was diagnosed with parkinsonism and AM after VPS and completely relieved after receiving Madopar and Pramipexole treatment, which is similar to most of the cases in Table 2. Notably, unlike previous cases, this report provides support for the possibility that dopamine deficiency inhibits brain glucose metabolism. Before dopaminergic treatment, the patient's brain functions such as sensation, movement, memory, and language were impaired. 18F-FDG PET also recorded extensive cortical and subcortical hypometabolic patterns (Figure 1A). Our treatment constituted of only dopamine supplementation resulting in relief of clinical symptoms and reversal of brain hypometabolism patterns (Figure 1B). Previously, it was reported that ETV improved the symptoms of severe parkinsonism after VPS and restored cerebral cortical flow (7). As mentioned above, ETV can eliminate the damage of CSF pressure gradient to midbrain and contribute to the recovery of nigrostriatal dopaminergic function. Therefore, it seems reasonable to believe that ETV may also function through the dopaminergic pathway. Parkinson's disease is the most representative disease of central dopamine deficiency. The Parkinson's disease related pattern (PDRP) has been validated in multiple independent populations worldwide (29, 30). PDRP is characterized by relatively increased metabolism in the thalamus, putamen/pallidum, pons, cerebellum, and motor cortex and relative decreases in the lateral frontal and parietooccipital areas. However, unlike PDRP, our case shows a broad (whether cortical or subcortical), symmetrical pattern of brain hypometabolism, which is significantly improved after dopaminergic treatment.
In this report, two limitations should be mentioned. Firstly, due to technical limitations, the patient failed to complete the evaluation of presynaptic nigrostriatal dopaminergic function, such as DAT, VMAT2 or 18 F-DOPA PET tests. Secondly, the limitations of the case report itself, that is to say, it is difficult to draw a firm conclusion from only one clinical case, and more accumulation is needed to confirm our findings.
In conclusion, we reported a case of severe parkinsonism and AM with cerebral hypometabolism after VPS, which was dramatically improved by levodopa. The hint for clinical practice is that Levodopa and/or dopamine agonist should be the first choice if the patients develop Parkinson-like symptoms after shunt.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YZ drafted the manuscript and was responsible for patient diagnosis and treatment. PL and JZ performed the PET CT detection and analysis. CL and PS participated in the collection and collation of clinical data. FL and ZJ critically revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kim MJ, Chung SJ, Sung YH, Lee MC, Im JH. Levodopa-responsive Parkinsonism associated with hydrocephalus. Mov Disord. (2006) 21:1279–81. doi: 10.1002/mds.20901
2. Prashantha DK, Netravathi M, Ravishankar S, Panda S, Pal PK. Reversible parkinsonism following ventriculoperitoneal shunt in a patient with obstructive hydrocephalus secondary to intraventricular neurocysticercosis. Clin Neurol Neurosurg. (2008) 110:718–21. doi: 10.1016/j.clineuro.2008.03.008
3. Kinugawa K, Itti E, Lepeintre JF, Mari I, Czernecki V, Heran F, et al. Subacute dopa-responsive Parkinsonism after successful surgical treatment of aqueductal stenosis. Mov Disord. (2009) 24:2438–40. doi: 10.1002/mds.22862
4. Klarica M, Oresković D, Bozić B, Vukić M, Butković V, Bulat M. New experimental model of acute aqueductal blockage in cats: effects on cerebrospinal fluid pressure and the size of brain ventricles. Neuroscience. (2009) 158:1397–405. doi: 10.1016/j.neuroscience.2008.11.041
5. Okawa S, Sanpei Y, Sugawara M, Nakazawa M, Endo T, Ohnishi H. Parkinsonism improved with levodopa after endoscopic third ventriculostomy in shunted hydrocephalus due to aqueductal stenosis. Neurologist. (2015) 20:4–7. doi: 10.1097/NRL.0000000000000035
6. Shpiner DS, Margolesky J, Singer C, Lizarraga KJ. Transtentorial fluctuations and atypical parkinsonism after ventriculo-peritoneal shunting. Can J Neurol Sci. (2021) 48:582–4. doi: 10.1017/cjn.2020.228
7. Hashizume A, Watanabe H, Matsuo K, Katsuno M, Tanaka F, Nagatani T, et al. Endoscopic third ventriculotomy improves parkinsonism following a ventriculo-peritoneal shunt in a patient with non-communicating hydrocephalus secondary to idiopathic aqueduct stenosis. J Neurol Sci. (2011) 309:148–50. doi: 10.1016/j.jns.2011.07.025
8. Akiyama T, Tanizaki Y, Akaji K, Hiraga K, Akiyama T, Takao M, et al. Severe parkinsonism following endoscopic third ventriculostomy for non-communicating hydrocephalus–case report. Neurol Med Chir. (2011) 51:60–3. doi: 10.2176/nmc.51.60
9. Pang D, Altschuler E. Low-pressure hydrocephalic state and viscoelastic alterations in the brain. Neurosurgery. (1994) 35:643–55. doi: 10.1227/00006123-199410000-00010
10. Shahar E, Lambert R, Hwang PA, Hoffman HJ. Obstructive hydrocephalus-induced parkinsonism. I: Decreased basal ganglia regional blood flow. Pediatr Neurol. (1988) 4:117–9. doi: 10.1016/0887-8994(88)90052-5
11. Curran T, Lang AE. Parkinsonian syndromes associated with hydrocephalus: case reports, a review of the literature, and pathophysiological hypotheses. Mov Disord. (1994) 9:508–20. doi: 10.1002/mds.870090503
12. Racette BA, Esper GJ, Antenor J, Black KJ, Burkey A, Moerlein SM, et al. Pathophysiology of parkinsonism due to hydrocephalus. J Neurol Neurosurg Psychiatry. (2004) 75:1617–9. doi: 10.1136/jnnp.2003.028449
13. Berger L, Gauthier S, Leblanc R. Akinetic mutism and parkinsonism associated with obstructive hydrocephalus. Can J Neurol Sci. (1985) 12:255–8. doi: 10.1017/s0317167100047119
14. Zhang Y, Chen BW, Mao W, Wu FY, Zhang Y. Parkinsonism after ventriculoperitoneal shunt for hydrocephalus. BMC Neurol. (2023) 23:38. doi: 10.1186/s12883-023-03064-2
15. Ochiai H, Yamakawa Y, Miyata S, Kawasoe T. L-dopa effective parkinsonism appeared after shunt revision of the aqueductal stenosis: report of two cases. No To Shinkei. (2000) 52:425–9.
16. Sakurai T, Kimura A, Yamada M, Hayashi Y, Tanaka Y, Hozumi I, et al. Rapidly progressive parkinsonism that developed one year after ventriculoperitoneal shunting for idiopathic aqueductal stenosis: a case report. Brain Nerve. (2010) 62:527–31.
17. Yomo S, Hongo K, Kuroyanagi T, Kobayashi S. Parkinsonism and midbrain dysfunction after shunt placement for obstructive hydrocephalus. J Clin Neurosci. (2006) 13:373–8. doi: 10.1016/j.jocn.2005.04.023
18. Zeidler M, Dorman PJ, Ferguson IT, Bateman DE. Parkinsonism associated with obstructive hydrocephalus due to idiopathic aqueductal stenosis. J Neurol Neurosurg Psychiatry. (1998) 64:657–9. doi: 10.1136/jnnp.64.5.657
19. Zhou J, Chen Y, Huang C, Ming Y, Xiang W, Li S, et al. Parkinsonism after chronic subdural haematoma followed by ventriculoperitoneal shunt for obstructive hydrocephalus: a case report. Br J Neurosurg. (2019) 33:302–4. doi: 10.1080/02688697.2017.1344616
20. da Costa AC, Pinheiro Júnior N, Godeiro Junior C, Fernandes ACA, de Queiroz CT, de Moura ACMA, et al. Parkinsonism secondary to ventriculoperitoneal shunt in a patient with hydrocephalus. Surg Neurol Int. (2021) 12:432. doi: 10.25259/SNI_629_2021
21. Villamil F, Varela F, Caffaratti G, Ricciardi M, Cammarota A, Cervio A. Global Rostral Midbrain Syndrome (GRMS) and Corpus callosum infarction in the context of shunt overdrainage. Clin Neurol Neurosurg. (2022) 213:107098. doi: 10.1016/j.clineuro.2021.107098
22. Watahiki Y, Narita S, Kurahashi K, Tanaka T, Matsunaga M. Akinetic mutism from recurrent hydrocephalus: successful treatment with levodopa, bromocriptine and trihexyphenidyl. No To Shinkei. (1987) 39:977–82.
23. Rebai RM, Houissa S, Mustapha ME, Azzouni H, Assaggaf S. Akinetic mutism and parkinsonism after multiple shunt failure: case report and literature review. J Neurol Surg A Cent Eur Neurosurg. (2012) 73:341–6. doi: 10.1055/s-0032-1313632
24. Anderson B. Relief of akinetic mutism from obstructive hydrocephalus using bromocriptine and ephedrine. Case report J Neurosurg. (1992) 76:152–5. doi: 10.3171/jns.1992.76.1.0152
25. Lin KL, Wang HS, Chou ML, Rui TN. Role of cavum septum pellucidum in akinetic mutism of hydrocephalic children. Pediatr Neurol. (1997) 16:156–9. doi: 10.1016/s0887-8994(96)00294-9
26. Moser A, Freyberger HJ, Brückmann H, Kömpf D. Akinetischer Mutismus bei dekompensiertem triventrikulären Hydrozephalus Akinetic mutism in decompensated triventricular hydrocephalus. Fortschr Neurol Psychiatr. (1995) 63:248–51. doi: 10.1055/s-2007-996623
27. Aidi S, Elalaoui-Faris M, Benabdeljlil M, Benomar A, Chaoui M, Chkili T. Mutisme akinétique et pseudo-paralysie supranucléaire progressive secondaires à la dérivation d'une hydrocéphalie obstructive. Effet bénéfique de la bromocriptine: 2 cas Akinetic mutism and progressive supranuclear palsy-like syndrome after the shunt of an obstructive hydrocephalus. Successful treatment with bromocriptine: 2 cases. Rev Neurol. (2000) 156:380–3.
28. Psarros T, Zouros A, Coimbra C. Bromocriptine-responsive akinetic mutism following endoscopy for ventricular neurocysticercosis. Case report and review of the literature. J Neurosurg. (2003) 99:397–401. doi: 10.3171/jns.2003.99.2.0397
29. Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson's disease: test-retest reproducibility. J Cereb Blood Flow Metab. (2007) 27:597–605. doi: 10.1038/sj.jcbfm.9600358
Keywords: parkinsonism, ventriculo-peritoneal shunt, 18F fluorodeoxyglucose, akinetic mutism, levodopa
Citation: Zhang Y, Li P, Zhang J, Li C, Sun P, Li F and Jiao Z (2023) Case report: Levodopa-responsive parkinsonism with akinetic mutism after ventriculo-peritoneal shunt. Front. Neurol. 14:1184713. doi: 10.3389/fneur.2023.1184713
Received: 12 March 2023; Accepted: 12 May 2023;
Published: 02 June 2023.
Edited by:
Tobias Warnecke, University Hospital Münster, GermanyReviewed by:
Piotr Alster, Medical University of Warsaw, PolandCopyright © 2023 Zhang, Li, Zhang, Li, Sun, Li and Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuomin Jiao, NjAxMjQ0QGhyYm11LmVkdS5jbg==; Fujun Li, NTU5NzdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.