- 1Department of Orthodontics, Pomeranian Medical University, Szczecin, Poland
- 2Department of Neurosciences, Reproductive and Odontostomatological Sciences, University of Naples “Federico II”, Napoli, Italy
- 3School of Dentistry, College of Dental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
Background: Temporomandibular disorders (TMD) are a set of painful conditions affecting the orofacial region that are prevalent and constitute the most frequent type of non-dental pain complaint in the maxillofacial area. Pain-related TMD (TMD-P) is characterized by ongoing pain in the masticatory muscles, the temporomandibular joint, or surrounding structures. Due to the multiple factors that contribute to the development of this condition, it can be challenging to accurately diagnose. One of the useful method for assessing patients with TMD-P is surface electromyography (sEMG). The aim of this systematic review was to provide a comprehensive overview of the current scientific literature on the evaluation of masticatory muscle activity (MMA) in individuals diagnosed with TMD-P, through the utilization of sEMG.
Methods: To gather relevant information, electronic databases such as PubMed, Web of Science, Scopus, and Embase were searched using specific keywords including: “pain” AND (“temporomandibular disorder*” OR “temporomandibular dysfunction*”) AND “surface electromyography” AND “masticatory muscle activity.” The inclusion criteria were studies assessing MMA in patients with TMD-P using sEMG. The Effective Public Health Practice Project (EPHPP) Quality Assessment Tool for Quantitative Studies was utilized to evaluate the quality of the studies that were included in the review.
Results: The search strategy identified 450 potential articles. Fourteen papers met the inclusion criteria. Global quality rating for significant part of the articles was weak. Most studies showed greater sEMG activity of the masseter (MM) and temporal anterior (TA) muscles at rest in TMD-P subjects than in the asymptomatic controls, while the MM and TA muscles were less active in the pain-related TMD group compared to the non-TMD group during maximal voluntary clenching (MVC).
Conclusion: There were differences in MMA in the TMD-pain population compared to a healthy control group during various tasks. The diagnostic efficacy of surface electromyography in assessing individuals with TMD-P remains unclear.
1. Introduction
Temporomandibular disorders (TMD) are a set of painful conditions that are prevalent and constitute the most frequent type of non-dental pain complaint in the maxillofacial area (1, 2). These conditions are linked to various clinical scenarios that impact the stomatognathic system, which primarily involves the masticatory muscles, the temporomandibular joint (TMJ), and other related structures. TMDs can be manifested by tenderness or pain in the muscles and joints, joint noise, and deviation in mandibular movements (3–7). Pain-related TMD (TMD-P) is characterized by ongoing pain in the muscles of the mandible, the temporomandibular joint, or surrounding structures. This pain can be persistent, recurrent, or chronic (4–6). The temporomandibular pain appears to be relatively common; this condition predominantly affects young and middle-aged adults, as opposed to children or older individuals. Furthermore, it is more prevalent in women, occurring approximately twice as frequently as it does in men (8–11).
The Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) is a highly sophisticated and valuable diagnostic instrument that provides both clinical and research criteria for the accurate evaluation of TMD in both pediatric and adult populations. The RDC/TMD were gradually replaced by the updated Diagnostic Criteria for TMD (DC/TMD) (12, 13). It should be noted that the multifactorial etiology of TMDs can be challenging to accurately diagnose. Therefore, it is crucial to have reliable and effective tools and measures in place to ensure proper evaluation (14–18). In the assessment of TMD, certain instruments can offer valuable quantitative data that may prove useful in a clinical setting (16, 17). Among these instruments, surface electromyography (sEMG) has been widely utilized as a non-invasive tool to evaluate patients with TMD, as well as to analyze the electrophysiological behavior of muscles (6, 18–23). The advantages of using sEMG include its ease of use, accessibility, and non-invasive nature. However, it should be emphasized that sEMG is sensitive to impedance imbalances, which may affect the accuracy and reliability of electromyographic (EMG) assessments (18, 19).
In a recent systematic review Dinsdale et al. (24) studied muscle activity using sEMG in adults with persistent TMD compared to healthy controls. They found that in TMD there are changes in masticatory muscle activity (MMA) that are both task-specific and muscle-specific. However, it is also important what differences in MMA exist between pain-related TMD and asymptomatic healthy controls. Research indicates that subjects diagnosed with TMD-P may modify the activation of their masticatory muscles due to sensorimotor interactions. Pain can alter the formation of action potentials and, perhaps, electromyographic activity (25, 26). Additionally, the presence of pain can result in increased variability in EMG signals, which can undermine the accuracy of sEMG assessments (17). Given these limitations, it is important to note that the use of sEMG as a means of evaluating individuals with TMD, particularly pain-related TMD, remains a subject of debate due to the significant variability in results reported in the literature. As such, the diagnostic efficacy of surface electromyography in assessing this condition has yet to be definitively established (17, 18, 27–29).
The aim of this systematic review was to provide a comprehensive overview of the current scientific literature on the evaluation of masticatory muscle activity (MMA) in individuals diagnosed with TMD-P, through the utilization of sEMG. This paper also aimed to summarize the literature on the diagnostic value of sEMG in diagnosing patients with TMD-P.
2. Methods
2.1. Search strategy
The systematic review adhered to the guidelines outlined in the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) (30).
According to PICO (31), the framework for this systematic review is as follows: Population (P): patients with pain-related temporomandibular disorders; Intervention (I): surface electromyography; Comparison (C): pain-related TMD vs. asymptomatic non-TMD patients; Outcomes (O): changes in masticatory muscle activity and diagnostic utility of sEMG in identifying TMD-P patients. The PICO question was: “Does the masticatory muscle electromyographical (EMG) activity in TMD-P patients differ from that in the healthy non-TMD population? and “Is the surface electromyography useful in differentiating between patients with pain-related TMD and asymptomatic patients?” To gather relevant information, electronic databases such as PubMed, Web of Science, Scopus, and Embase were thoroughly searched using specific keywords including: “pain” AND (“temporomandibular disorder*” OR “temporomandibular dysfunction*”) AND “surface electromyography” AND “masticatory muscle activity.”
The literature search was carried out by two independent reviewers (L.S.S. and M.S.D.), who examined all publications without imposing any temporal restrictions. The final search was performed on December 31, 2022, and encompassed all language versions of the publications.
2.2. Eligibility criteria
The inclusion criteria for this review were as follows:
- Study type: observational/interventional studies on the assessment of MMA in patients with TMD-P by surface electromyography, English language;
- Outcome of interest: masticatory muscle activity assessed by sEMG;
- Object of the study: (a) comparison of MMA in TMD-P patients with a healthy population without TMD and (b) evaluation of the diagnostic efficiency of sEMG in diagnosing patients with TMD-P;
- Participants: human subjects.
The exclusion criteria were as follows: ineligible study design; ineligible outcome measure; ineligible population, e.g., studies on TMD patients not related to pain, studies on patients with congenital craniofacial malformations; case reports, reviews, animal studies.
2.3. Data extraction
After removing duplicates, all titles and abstracts were read by the first (L.S.S.) and reviewed by the second (M.S.D.) author to identify potentially eligible studies. Subsequently, the full texts of the selected works were scrutinized, and the papers were either qualified or excluded based on the predetermined inclusion and exclusion criteria. Only papers comparing MMA in patients with TMD-P with respect to healthy patients without TMD were included. All ambiguities were resolved through discussions between investigators. The Cohen’s Kappa statistic was performed to measure the agreement between the two authors. The review process involved gathering information related to various aspects of the studies, including study design, participant characteristics, diagnostic criteria employed to diagnose and categorize TMD, outcome measures, such as measurement tools, procedures, and data analysis, as well as principal findings. EMG results were collected from each study by a single reviewer (L.S.S.) and documented in an Excel spreadsheet.
2.4. Quality assessment
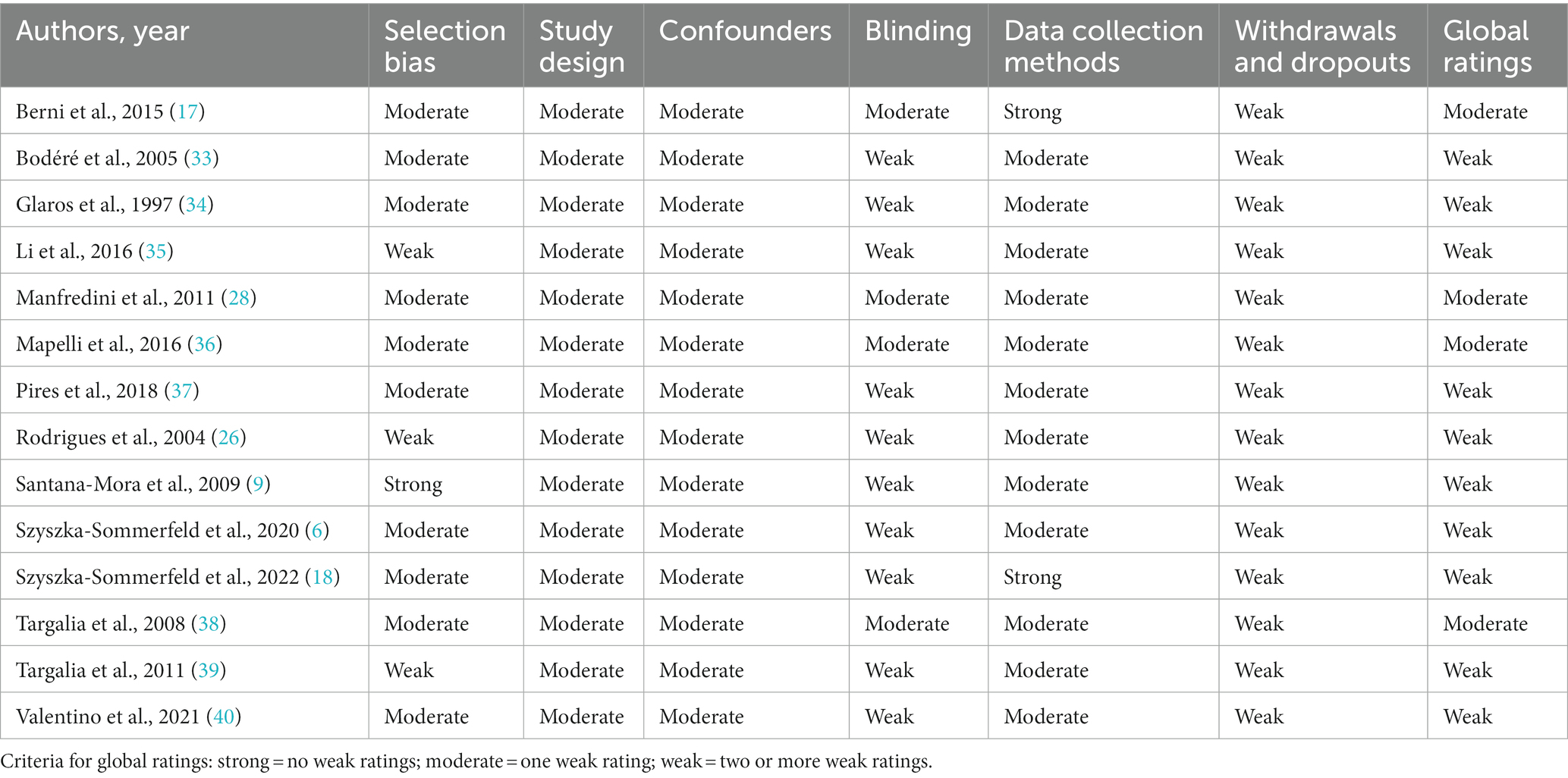
The Effective Public Health Practice Project (EPHPP, McMaster University, Ontario, Canada) Quality Assessment Tool for Quantitative Studies was utilized to evaluate the quality of the studies that were included in the review (32). This tool assesses various components, such as study design, selection bias, confounders, blinding, data collection methods, and withdrawals, and provides an overall rating of the study as either “strong,” “moderate,” or “weak.” The quality assessment was performed independently by two authors (L.S.S and M.S.D). All ambiguities were resolved through discussions between reviewers. The Cohen’s Kappa coefficient for the agreement between the authors was calculated.
3. Results
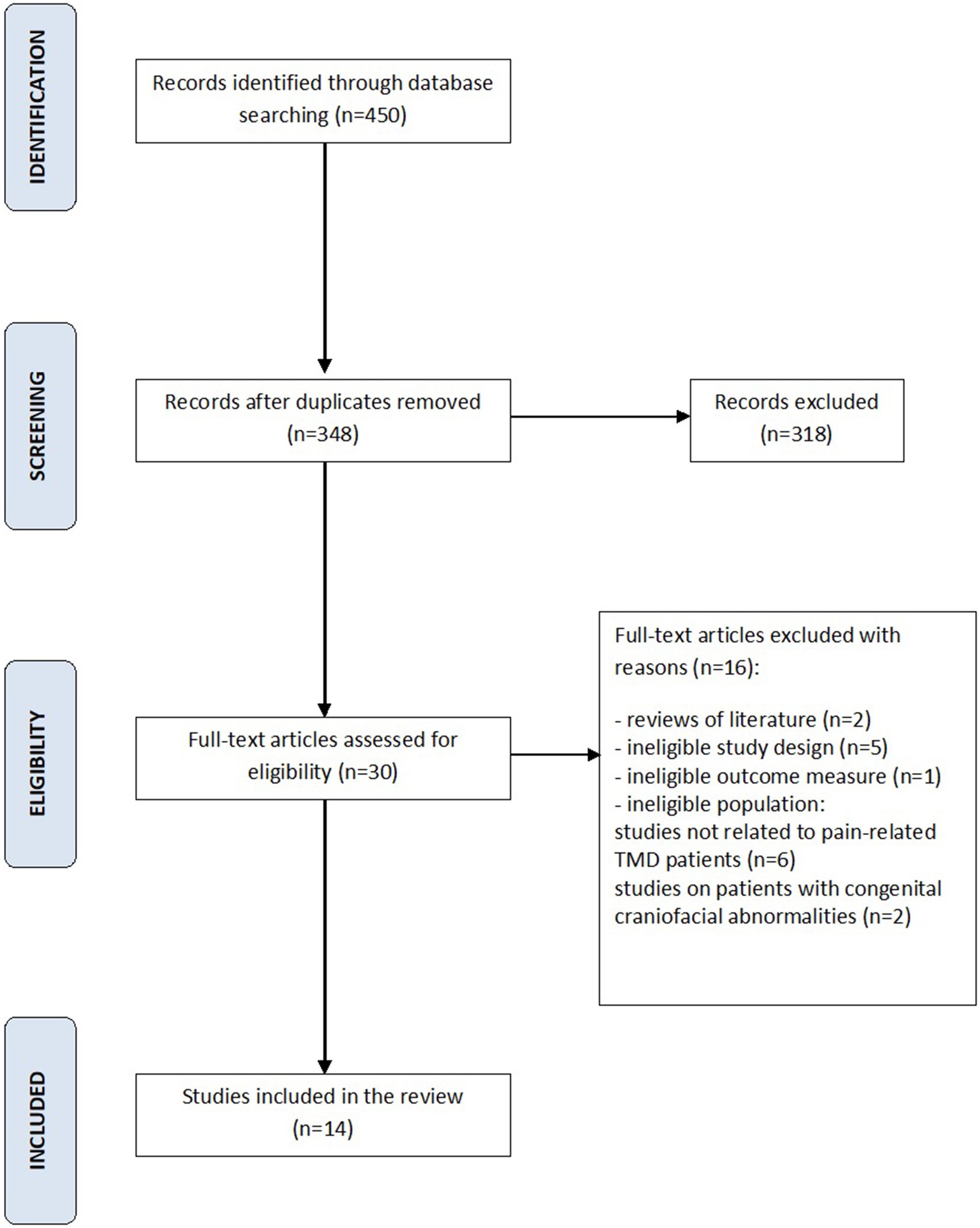
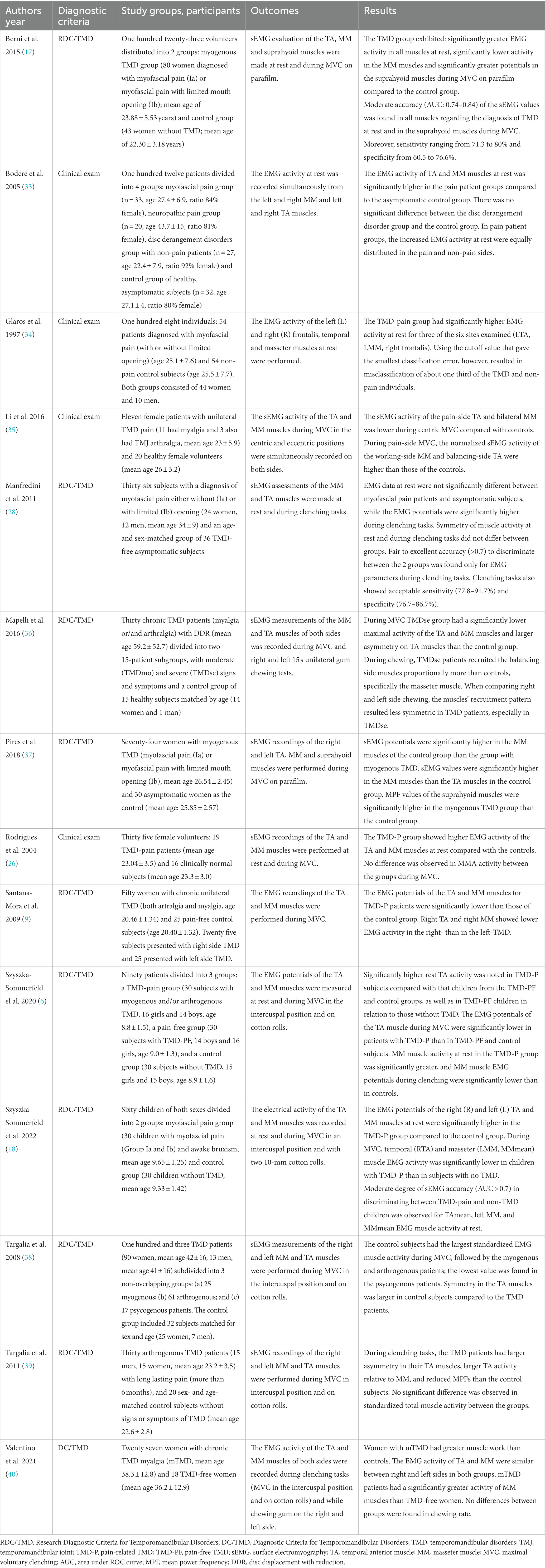
Following search strategy 450 potential articles were identified: 225 from PubMed, 97 from Web of science, 65 from Scopus, and 63 from Embase. After removing of 102 duplicates, 348 articles were analyzed. As a result of title and abstract screening, 318 papers were excluded based on the predetermined inclusion and exclusion criteria. Of the remaining 30 articles, 16 were excluded because they were literature reviews, studies of ineligible study design, studies of ineligible outcome measure, or ineligible population, such as patients with congenital craniofacial anomalies, patients with TMD without pain. Finally, 14 papers were included in the review. The entire process was schematized in the Prisma Flow Diagram (Figure 1, Flow diagram). The Kappa value between two reviewers was calculated as 0.97. Table 1 displays the principal features of each study that was included in the review.
The quality assessment results for each study are summarized in Table 2. The Cohen’s Kappa coefficient for the agreement between the authors was calculated as 0.92. Global quality rating using the EPHPP Quality Assessment Tool for Quantitative Studies for most of the studies (n = 10) was weak.
Two of the included articles focused on evaluating MMA in children and adolescents with TMD-P (6, 18). Adults were included in remaining 12 studies.
The Research Diagnostic Criteria for Temporomandibular Disorders were the most frequently used criteria to diagnose and categorize TMD (n = 9), followed by clinical examination (n = 4), and the DC/TMD (n = 1). Within these criteria, muscle disorders (myofascial pain, myalgia) were the most frequently studied subgroup (n = 7) (17, 18, 26, 28, 34, 37, 40), followed by mixed TMD (n = 6) - myalgia and arthralgia diagnoses (6, 9, 35, 36); myogenous, arthrogenous and psycogenous pain groups (38); myalgia and neuropatic pain groups (33). Arthrogenous TMD were the least reported (n = 1) (39).
Masticatory muscle activity was reported using sEMG under different conditions: (a) resting position (b) clenching, including MVC (maximal voluntary clenching) in the intercuspal position and MVC on cotton rolls or parafilm (c) and chewing. The masseter (MM) and temporal anterior (TA) muscles were assessed in all studies analyzed, while the suprahyoid muscles were assessed in 2 studies.
Out of the 14 studies that were reviewed, 13 studies conducted the processing and analysis of the EMG signal based on the time-domain. One study, however, performed both time-domain and frequency-domain analyses (37).
Masseter and temporal muscle activity at rest was assessed in 7 studies. In 6 of them reported significantly greater MM and TA activity in the TMD-P group compared to a healthy non-TMD group (6, 17, 18, 26, 33, 34). In contrast, one study found no significant differences in the MM and TA electromyographical activity between myofascial pain subjects and asymptomatic controls (28).
Masseter muscle EMG activity during MVC was measured in 12 studies. Of these, 8 reported significantly lower MM activity in the TMD-P group compared to asymptomatic non-TMD patients (6, 9, 17, 18, 35–38) and 2 reported higher MM electrical activity in the pain-related TMD group (28, 40). Two studies found no significant differences in the MM electrical potentials between pain-related TMD patients and asymptomatic control subjects (26, 39). Twelve studies examined TA muscle electrical activity during MVC. Most of them (n = 6) showed lower EMG potentials in the pain-related TMD group compared to the control group (6, 9, 18, 35, 36, 38) and one observed higher EMG activity of the TA muscles in the TMD-pain group (28). Five studies found no significant differences in the temporal muscle EMG activity during MVC between TMD-pain patients and asymptomatic controls (17, 26, 37, 39, 40).
Two studies examined masseter and temporal muscle electrical potentials during chewing (36, 40). One study found no differences in the MM and TA activity between the TMD-P group and the control group (40). A single study observed that individuals with TMDse exhibited greater recruitment of muscles on the balancing side during chewing, particularly in the masseter muscle, when compared to control subjects (36).
The EMG activity of the suprahyoid muscles was measured in 2 studies (17, 38). The results showed significantly greater EMG activity in TMD-P patients during MVC on parafilm in comparison to the control group.
Four studies evaluated the diagnostic efficiency of surface electromyography in assessing patients with TMD-P (17, 18, 28, 34). In one study, sEMG was found to have moderate accuracy in differentiating between children with pain-related TMD and those without TMD, specifically for MMmean, left MM and TAmean normalized EMG activity at rest (18). Another two studies investigated the diagnostic utility of surface electromyography based on raw EMG values for the diagnosis of adults with myofascial pain (17, 28). One study reported moderate accuracy of sEMG in diagnosing TMD-P and healthy non-TMD individuals at rest. The authors stated that the surface electromyography is a valuable additional tool for diagnosis of TMD patients with myofascial pain of the masticatory muscles (17). In contrast, one study found that fair to excellent accuracy in discrimination between patients diagnosed with myofascial pain and non-TMD controls was obtained only for EMG values during teeth clenching tasks. However, the authors warned against the potential risk of overdiagnosis and overtreatment when using sEMG as a diagnostic tool for myogenous TMD (28). In addition, another study showed that the EMG data at rest provided little support for precisely distinguishing between TMD-pain patients and asymptomatic controls (34).
4. Discussion
This systematic review presents relevant findings on masticatory muscle activity during various tasks in individuals with pain-related TMD, as well as the diagnostic utility of sEMG in assessing patients with TMD-P. Fourteen studies examining the MMA in the rest position, during MVC and chewing were included in the review.
It was shown that electromyographical activity of the masticatory muscles differed between the TMD-pain and non-TMD groups. In addition, the direction of changes in MMA varied depending on the task studied. During the evaluation of pain-related TMD subjects using sEMG, the tasks that were most frequently analyzed included resting muscle activity and muscle activity during MVC. Most studies showed that the EMG activity of the MM and TA muscles at rest in TMD-P subjects was higher than in the asymptomatic controls (6, 17, 18, 26, 33, 34), while the MM and TA muscles were less active in pain-related subjects than in the control group during MVC (6, 9, 18, 35, 36, 38). This observation suggests that the presence of pain may result in reduction in muscle activity and restriction of movement patterns as a protective mechanism to prevent injury (41, 42).
The symmetry of muscle activity between sides is another crucial factor to consider. A number of studies included in this systematic review found a higher level of asymmetry in pain-related TMD groups compared with healthy, pain-free control groups (36, 38, 39), while others found no difference in the symmetry of muscle activity between TMD-P patients and asymptomatic controls (28).
Clinically, it is crucial to note that alterations in the pattern of muscle activity in individuals with TMD-P can impact muscle fatigue and, therefore, muscle function (43). Muscle activity testing should include examination of specific muscle groups in certain tasks to properly recognize abnormalities. Normalizing muscle activity and improving muscle function are essential aspects of developing effective treatment protocols for TMD-P patients.
sEMG is a widely used non-invasive technique that has found application in the diagnosis of patients with general muscle disorders, neuromuscular diseases or diseases affecting neuromuscular performance (18). In dentistry, sEMG plays an important role in the assessment of painful and non-painful conditions of TMDs, dystonia, head and neck muscle diseases, cranial nerve lesions, as well as seizures and sleep disorders (44). Adequate quality of the EMG assessment and reporting is essential to ensure reliable evaluation of patients with TMD. It should be noted that one of the main disadvantages of surface electromyography is its sensitive to impedance imbalances, which may reduce the accuracy of the EMG recordings (19, 27). Problems with the reproducibility of sEMG related to technical artifacts (instrumental noise), anatomical variations such as facial type, age, gender, thickness of subcutaneous fat, as well as muscle cross-talk, may hinder its clinical validity (4). The reproducibility of sEMG is also debatable due to the different inter-electrode distances and their various positioning over muscles. Therefore, special attention should be paid to establishing a fixed inter-electrode distances and creating a standardized protocol for surface electrode placement. In this context, it should be emphasized that the precision of EMG outcomes can be greatly influenced by a number of factors, including technical aspects, such as positioning of electrodes, signal processing, as well as the particular hardware and software used (19, 27). In the included studies, EMG recordings of the subjects were performed using a variety of EMG devices with different technical parameters, including: the DAB-Bluetooth Instrument (Zebris Medical GmbH, Germany) (6, 18), the BIO-EMG 1000 electromyograph (Lynx Tecnologia Eletrônica Ltda, São Paulo, SP, Brazil) (17, 26, 37), the K6 Diagnostic System (Myotronics Inc., Seattle, WA, USA) (28), the computerized instrument (Freely, De Götzen srl; Legnano, Milano, Italy) (38, 39), the Nicolet Viking Select electrodiagnostic system (Nicolet Biomedical, Madison, WI, USA) (9, 33), the EA-1, J&J Instruments (Poulsboro, WA, USA) (34), the EMG system (TMJOINT, BTS, SpA, Garbagnate Milanese, Italy) (36, 40), the BioEMG III with a BioPak Measurement System, version 6.0 (Bioresearch Associates, Inc., Milwaukee, WI, USA) (35). Prior to the electrodes placement, the patient’s skin surface was cleaned to reduce impedance (6, 9, 17, 18, 34, 36, 37, 40). Different types of surface electrodes were placed over the muscle belly of the masseter, anterior temporal and suprahyoid muscles in specific positions, and then all experimental tasks were performed. During the EMG recordings, the subjects sat in a chair with natural head position. To evaluate the EMG data, the researchers used various parameters to analyze the electromyographic signal in TMD-P subjects. Data processing was performed in the amplitude domain with normalized (6, 18, 35, 36, 38–40) or non-normalized data (9, 17, 26, 28, 33, 34) and/or in the frequency domain (37). In this context, it should be noted that the normalization process is essential to ensure intercomparisons and further data analysis. Normalized EMG data will offer insight into the impact of occlusion on neuromuscular activity, while disregarding individual variations such as anatomical variances, physiological and psychological state, and others. To standardize the interpretation of muscle electrical potentials, normalization techniques are used to compare them with reference values obtained during standardization recordings. In the standardization recording, among the various protocols, MVC on two cotton rolls positioned on mandibular molars is now commonly used (6, 18, 21, 36, 38–40, 45).
It should also be emphasized that several restraints of sEMG, including its time-consuming nature, the need of specialized equipment, and the need of proper training and calibration of the examiner may limit the use of sEMG in clinical settings. However, the advantages of sEMG such as its ease of use, availability, and non-invasive and painless nature, partially compensate for the aforementioned limitations (19). For these reasons, the method can also be used in children and adolescents (6, 18).
In light of the above, it is also important to evaluate the diagnostic utility of surface electromyography in the diagnosis of pain-related TMD. The utilization of sEMG as a tool for evaluating patients with TMD-pain diagnosis versus RDC/TMD as the gold standard is debatable due to the significant variability in results (17, 18, 27, 28). While some authors have suggested that sEMG can be used as an additional diagnostic tool for identifying TMD pain (17, 18), others have argued that electromyography may not be useful for this purpose (28, 34). These findings encourage the search for other measurable instrumental diagnostic methods that allow objective and quantitative analysis of masticatory muscle function and may be useful in the assessment of TMD-P, such as thermography, kinesiography or pressure algometry (28, 46–52). The advantages of thermography, including its non-invasiveness, lack of ionizing radiation, and relatively low cost, are sufficient to recommend its use among the supplementary tools employed in TMD diagnosis. However, the validity of the use of thermography in the evaluation of TMD is still under investigation (46, 47, 50). Some studies have confirmed the diagnostic utility of thermography in identifying patients with TMD (46, 51), while other researches have observed low accuracy of infrared thermography analysis in differentiating between subjects with myogenous TMD and asymptomatic controls (50). An important limitation of using this method in clinical practice is that there is no standardized protocol for measuring masticatory muscle temperature using infrared thermography. Standardization of all protocols requires ensuring that all possible thermal changes related to the image acquisition room and patient habits do not interfere with the acquisition of data. Therefore, the need of control the measurement conditions, as well as problems with complete objectification of the results may limit the use of thermography in the assessment of TMD (47). The diagnostic utility of kinesiography has also been questioned. Low accuracy using kinesiography recordings in identifying subjects with myofascial pain in the masticatory muscles has been reported (28). The lack of normative values on which to base discriminatory power between TMD patients and asymptomatic subjects is a major limitation to conclusively assessing the validity and application of this method in a clinical practice. Similarly, pressure algometry is a non-invasive and easy to use method to assess TMD, but because of the specific nature of the examination, this technique is also dependent on a number of factors which may limited its application. A key element is maintaining consistent test conditions. Among the main factors that are particularly important in this regard are the invariability of the position of the algometer in relation to the structures under examination, the dynamics of the pressure exerted, the area to which pressure is applied, and the differences between algometers (48). While some authors have confirmed the high diagnostic value of pressure algometry in differentiating TMD cases from controls (48, 52), others report low accuracy of pressure algometry in diagnosis of myofascial pain of the masticatory muscles (49).
This systematic review presents some limitations that should be acknowledged: (a) most of the included studies were of weak quality according to the EPHPP tool; (b) only four articles included in this review focused on the diagnostic utility in differentiating between TMD-P and asymptomatic control subjects; (c) the use of different EMG devices and various parameters to analyze the EMG signal may affect the results among the included studies; (d) we should also be aware that the differences in study groups characteristics such as gender, age, TMD subgroup may affect the EMG results; (e) as the reliability and validity of sEMG largely depends on biological, instrumental and technical factors, future studies need to investigate the quality of EMG testing and reporting procedures using a standardized framework.
5. Conclusion
This systematic review comprehensively examines changes in masticatory muscle activity in pain-related TMD subjects using surface electromyography. Differences were found in masticatory muscle activity in the TMD-pain population compared to a healthy control group during various tasks. The diagnostic efficacy of surface electromyography in assessing individuals with pain-related TMD remains unclear.
Author contributions
LS-S conception and design of the study, manuscript writing, and realization of figures and tables. LS-S and MS-D literature review and article selection. GS, MS-D, and KW participation in the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Orzeszek, S, Waliszewska-Prosol, M, Ettlin, D, Seweryn, P, Straburzynski, M, Martelletti, P, et al. Efficiency of occlusal splint therapy on orofacial muscle pain reduction: a systematic review. BMC Oral Health. (2023) 23:180. doi: 10.1186/s12903-023-02897-0
2. Li, DTS, and Leung, YY. Temporomandibular disorders: current concepts and controversies in diagnosis and management. Diagnostics (Basel). (2021) 11:459. doi: 10.3390/diagnostics11030459
3. Scrivani, SJ, Keith, DA, and Kaban, LB. Temporomandibular disorders. N Engl J Med. (2008) 359:2693–705. doi: 10.1056/NEJMra0802472
4. Dworkin, SF, and LeResche, L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomand Disord. (1992) 6:301–55.
5. De Leeuw, R, and Klasser, GD. Orofacial Pain - Guidelines for Assessment, Diagnosis and Management. Chicago: Quintessence (2013).
6. Szyszka-Sommerfeld, L, Machoy, M, Lipski, M, and Woźniak, K. Electromyography as a means of assessing masticatory muscle activity in patients with pain-related temporomandibular disorders. Pain Res Manag. (2020) 2020:9750915. doi: 10.1155/2020/9750915
7. Khawaja, SN, McCall, W Jr, Dunford, R, Nickel, JC, Iwasaki, LR, Crow, HC, et al. Infield masticatory muscle activity in subjects with pain-related temporomandibular disorders diagnoses. Orthod Craniofac Res. (2015) 18:137–45. doi: 10.1111/ocr.12077
8. Alkhubaizi, Q, Khalaf, ME, and Faridoun, A. Prevalence of temporomandibular disorder-related pain among adults seeking dental care: a cross-sectional study. Int J Dent. (2022) 2022:3186069. doi: 10.1155/2022/3186069
9. Santana-Mora, U, Cudeiro, J, Mora-Bermúdez, MJ, Rilo-Pousa, B, Ferreira-Pinho, JC, Otero-Cepeda, JL, et al. Changes in EMG activity during clenching in chronic pain patients with unilateral temporomandibular disorders. J Electromyogr Kinesiol. (2009) 19:e543–9. doi: 10.1016/j.jelekin.2008.10.002
10. LeResche, L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. (1997) 8:291–305. doi: 10.1177/10454411970080030401
11. Valesan, LF, Da-Cas, CD, Réus, JC, Denardin, ACS, Garanhani, RR, Bonotto, D, et al. Prevalence of temporomandibular joint disorders: a systematic review and meta-analysis. Clin Oral Investig. (2021) 25:441–53. doi: 10.1007/s00784-020-03710-w
12. Schiffman, E, Ohrbach, R, Truelove, E, Look, J, Anderson, G, Goulet, JP, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network* and orofacial pain special interest Groupdagger. J Oral Facial Pain Headache. (2014) 28:6–27. doi: 10.11607/jop.1151
13. Wieckiewicz, M, Grychowska, N, Wojciechowski, K, Pelc, A, Augustyniak, M, Sleboda, A, et al. Prevalence and correlation between TMD based on RDC/TMD diagnoses, oral parafunctions and psychoemotional stress in polish university students. Biomed Res Int. (2014) 2014:472346. doi: 10.1155/2014/472346
14. Wieckiewicz, M, Paradowska-Stolarz, A, and Wieckiewicz, W. Psychosocial aspects of bruxism: the paramount factor influencing teeth grinding. Biomed Res Int. (2014) 2014:469187. doi: 10.1155/2014/469187
15. Medllicott, MS, and Harris, SR. A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorder. Phys Ther. (2006) 86:955–73. doi: 10.1093/ptj/86.7.955
16. Gonzalez, YM, Greener, CS, and Mohl, ND. Technological devices in the diagnosis of temporomandibular disorders. Oral Maxillofac Surg Clin North Am. (2008) 20:211–20. doi: 10.1016/j.coms.2007.12.006
17. Berni, KC, Dibai-Filho, AV, Pires, PF, and Rodrigues-Bigaton, D. Accuracy of the surface electromyography RMS processing for the diagnosis of myogenous temporomandibular disorder. J Electromyogr Kinesiol. (2015) 25:596–602. doi: 10.1016/j.jelekin.2015.05.004
18. Szyszka-Sommerfeld, L, Sycińska-Dziarnowska, M, Budzyńska, A, and Woźniak, K. Accuracy of surface electromyography in the diagnosis of pain-related temporomandibular disorders in children with awake bruxism. J Clin Med. (2022) 11:1323. doi: 10.3390/jcm11051323
19. Woźniak, K, Piątkowska, D, Lipski, M, and Mehr, K. Surface electromyography in orthodontics - a literature review. Med Sci Monit. (2013) 19:416–23. doi: 10.12659/MSM.883927
20. Al-Saleh, MA, Armijo-Olivo, S, Flores-Mir, C, and Thie, NM. Electromyography in diagnosing temporomandibular disorders. J Am Dent Assoc. (2012) 143:351–62. doi: 10.14219/jada.archive.2012.0177
21. De Felício, CM, Ferreira, CL, Medeiros, AP, Rodriques Da Silva, MA, Targalia, GM, and Sforza, C. Electromyographic indices, orofacial myofunctional status and temporomandibular disorders severity: a correlation study. J Electromyogr Kinesiol. (2012) 22:266–72. doi: 10.1016/j.jelekin.2011.11.013
22. Ferrario, VF, Serrao, G, Dellavia, C, Caruso, E, and Sforza, C. Relationship between the number of occlusal contacts and masticatory muscle activity in healthy young adults. Cranio. (2002) 20:91–8. doi: 10.1080/08869634.2002.11746196
23. Ferrario, VF, Tartaglia, GM, Galletta, A, Grassi, GP, and Sforza, C. The influence of occlusion on jaw and neck muscle activity: a surface EMG study in healthy young adults. J Oral Rehabil. (2006) 33:341–8. doi: 10.1111/j.1365-2842.2005.01558.x
24. Dinsdale, A, Liang, Z, Thomas, L, and Treleaven, J. Is jaw muscle activity impaired in adults with persistent temporomandibular disorders? A systematic review and meta analysis. J Oral Rehabil. (2021) 48:487–516. doi: 10.1111/joor.13139
25. Nielsen, L, McNeill, C, Danzig, W, Goldman, S, Levy, J, and Miller, AJ. Adaptation of craniofacial muscles in subjects with craniomandibular disorders. Am J Orthod Dentofac Orthop. (1990) 97:20–34. doi: 10.1016/S0889-5406(05)81705-6
26. Rodrigues, D, Siriani, AO, and Berzin, F. Effect of conventional TENS on pain and electromyographic activity of masticatory muscles in TMD patients. Br Oral Res. (2004) 18:290–5. doi: 10.1590/s1806-83242004000400003
27. Szyszka-Sommerfeld, L, Machoy, M, Lipski, M, and Woźniak, K. The diagnostic value of electromyography in identifying patients with pain-related temporomandibular disorders. Front Neurol. (2019) 10:180. doi: 10.3389/fneur.2019.00180
28. Manfredini, D, Cocilovo, F, Favero, L, Ferronato, G, Tonello, S, and Guarda-Nardini, L. Surface electromyography of jaw muscles and kinesiographic recordings: diagnostic accuracy for myofascial pain. J Oral Rehabil. (2011) 38:791–9. doi: 10.1111/j.1365-2842.2011.02218.x
29. Santana-Mora, U, López-Ratón, M, Mora, MJ, Cadarso-Suárez, C, López-Cedrún, J, and Santana-Penín, U. Surface raw electromyography has a moderate discriminatory capacity for differentiating between healthy individuals and those with TMD: a diagnostic study. J Electromyogr Kinesiol. (2014) 24:332–40. doi: 10.1016/j.jelekin.2014.03.001
30. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
31. Sackett, DL, Strauss, SE, Richardson, WS, Rosenberg, W, and Haynes, BR. Evidence Based Medicine: How to Practice and Teach EBM. 2nd ed. Philadelphia: Elsevier Churchill Livingstone (2000).
32. Thomas, BH, Ciliska, D, Dobbins, M, and Micucci, S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid-Based Nurs. (2004) 1:176–84. doi: 10.1111/j.1524-475X.2004.04006.x
33. Bodéré, C, Hack Téa, S, Giroux-Metges, MA, and Woda, A. Activity of masticatory muscles in subjects with different orofacial pain conditions. Pain. (2005) 116:33–41. doi: 10.1016/j.pain.2005.03.011
34. Glaros, AG, Glass, EG, and Brockman, D. Electromyographic data from TMD patients with myofascial pain and from matched control subjects: evidence for statistical, not clinical, significance. J Orofac Pain. (1997) 11:125–9.
35. Li, BY, Zhou, LJ, Guo, SX, Zhang, Y, Lu, L, and Wang, MQ. An investigation on the simultaneously recorded occlusion contact and surface electromyographic activity for patients with unilateral temporomandibular disorders pain. J Electromyogr Kinesiol. (2016) 28:199–207. doi: 10.1016/j.jelekin.2015.11.002
36. Mapelli, A, Zanandréa Machado, BC, Giglio, LD, Sforza, C, and De Felício, CM. Reorganization of muscle activity in patients with chronic temporomandibular disorders. Arch Oral Biol. (2016) 72:164–71. doi: 10.1016/j.archoralbio.2016.08.022
37. Pires, PF, and Rodrigues-Bigaton, D. Evaluation of integral electromyographic values and median power frequency values in women with myogenous temporomandibular disorder and asymptomatic controls. J Bodyw Mov Ther. (2018) 22:720–6. doi: 10.1016/j.jbmt.2017.09.001
38. Tartaglia, GM, Rodrigues, M, da Silva, MA, Bottini, S, Sforza, C, and Ferrario, VF. Masticatory muscle activity during maximum voluntary clench in different research diagnostic criteria for temporomandibular disorders (RDC/TMD) groups. Man Ther. (2008) 13:434–40. doi: 10.1016/j.math.2007.05.011
39. Tartaglia, GM, Lodetti, G, Paiva, G, De Felicio, CM, and Sforza, C. Surface electromyographic assessment of patients with long lasting temporomandibular joint disorder pain. J Electromyogr Kinesiol. (2011) 21:659–64. doi: 10.1016/j.jelekin.2011.03.003
40. Valentino, R, Cioffi, I, Vollaro, S, Cimino, R, Baiano, R, and Michelotti, A. Jaw muscle activity patterns in women with chronic TMD myalgia during standardized clenching and chewing tasks. Cranio. (2021) 39:157–63. doi: 10.1080/08869634.2019.1589703
41. Nickel, JC, Iwasaki, LR, Walker, RD, McLachlan, KR, and McCall, WD Jr. Human masticatory muscle forces during static biting. J Dent Res. (2003) 82:212–7. doi: 10.1177/154405910308200312
42. Sessle, BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. (2000) 11:57–91. doi: 10.1177/10454411000110010401
43. Ries, LGK, Graciosa, MD, Soares, LP, Sperandio, FF, Santos, GM, Degan, VV, et al. Effect of time of contraction and rest on the masseter and anterior temporal muscles activity in subjects with temporomandibular disorder. Codas. (2016) 28:155–62. doi: 10.1590/2317-1782/201620150112
44. Nishi, SE, Basri, R, and Alam, MK. Uses of electromyography in dentistry: an overview with Meta-analysis. Eur J Dent. (2016) 10:419–25. doi: 10.4103/1305-7456.184156
45. Ferrario, VF, Sforza, C, Colombo, A, and Ciusa, V. An electromyographic investigation of masticatory muscles symmetry in normo-occlusion subjects. J Oral Rehabil. (2000) 27:33–40. doi: 10.1046/j.1365-2842.2000.00490.x
46. Woźniak, K, Szyszka-Sommerfeld, L, Trybek, G, and Piątkowska, D. Assessment of the sensitivity, specificity, and accuracy of thermography in identifying patients with TMD. Med Sci Monit. (2015) 21:1485–93. doi: 10.12659/MSM.893863
47. Machoy, M, Szyszka-Sommerfeld, L, Rahnama, M, Koprowski, R, Wilczyński, S, and Woźniak, K. Diagnosis of temporomandibular disorders using Thermovision imaging. Pain Res Manag. (2020) 2020:1–8. doi: 10.1155/2020/5481365
48. Więckiewicz, W, Woźniak, K, Piątkowska, D, Szyszka-Sommerfeld, L, and Lipski, M. The diagnostic value of pressure algometry for temporomandibular disorders. Biomed Res Int. (2015) 2015:575038. doi: 10.1155/2015/575038
49. Farella, M, Michelotti, A, Steenks, MH, Romeo, R, Cimino, R, and Bosman, F. The diagnostic value of pressure algometry in myofascial pain of the jaw muscles. J Oral Rehabil. (2000) 27:9–14. doi: 10.1046/j.1365-2842.2000.00526.x
50. Dibai Filho, AV, Packer, AC, Costa, AC, and Rodrigues-Bigaton, D. Accuracy of infrared thermography of the masticatory muscles for the diagnosis of myogenous temporomandibular disorder. J Manip Physiol Ther. (2013) 36:245–52. doi: 10.1016/j.jmpt.2013.04.007
51. McBeth, SB, and Gratt, BM. Thermographic assessment of temporomandibular disorders symptomology during orthodontic treatment. Am J Orthod Dentofac Orthop. (1996) 109:481–8. doi: 10.1016/S0889-5406(96)70132-4
Keywords: orofacial pain, temporomandibular disorders, pain-related temporomandibular disorders, surface electromyography, masticatory muscle activity
Citation: Szyszka-Sommerfeld L, Sycińska-Dziarnowska M, Spagnuolo G and Woźniak K (2023) Surface electromyography in the assessment of masticatory muscle activity in patients with pain-related temporomandibular disorders: a systematic review. Front. Neurol. 14:1184036. doi: 10.3389/fneur.2023.1184036
Edited by:
Mieszko Wieckiewicz, Wroclaw Medical University, PolandReviewed by:
Marta Waliszewska-Prosół, Wroclaw Medical University, PolandHelena Martynowicz, Wroclaw Medical University, Poland
Copyright © 2023 Szyszka-Sommerfeld, Sycińska-Dziarnowska, Spagnuolo and Woźniak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liliana Szyszka-Sommerfeld, liliana.szyszka@gmail.com
 Liliana Szyszka-Sommerfeld
Liliana Szyszka-Sommerfeld