
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 18 July 2023
Sec. Sleep Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1180393
This article is part of the Research Topic Chronic insomnia: Treatment and management View all 13 articles
Importance: Primary insomnia (PI) has a high global incidence, and effective treatments with fewer side effects are needed. Acupuncture, a treatment used in traditional Chinese medicine, has become increasingly established as a treatment method for PI and is recognized by many physicians and patients. Some evidence has suggested that acupuncture was associated with improvements in objective sleep parameters and might induce changes in some brain regions. Individual studies with limited sample size and low detection thresholds may lead to false positives, and no systematic review of the effects of acupuncture has been conducted in PI.
Objective: The aim of this systematic review and coordinate-based meta-analysis was to summarize the literature on fMRI evaluation of patients with PI treated with acupuncture.
Design: We performed a methodical and comprehensive search of multiple publication databases (from inception to December 2022): Web of Science, PubMed, ScienceDirect, Embase, Wan Fang, China National Knowledge Infrastructure, and Chinese Scientific Journal Database. Bias and quality of studies were evaluated by three researchers. Furthermore, a seed-based D-mapping meta-analysis with permutation of subject images (SDM-PSI) was applied to investigate the central mechanisms behind acupuncture treatment at PI. The International Prospective Registry of Systematic Reviews received the protocol for this study. (PROSPERO: CRD42023400086).
Results: The analysis included 305 patients with PI and 116 healthy controls from 11 studies. SDM-PSI analysis showed that patients with PI exhibited increased amplitudes of regional homogeneity and low-frequency fluctuations in the left superior frontal gyrus (1352 voxels, p = 0.0028), right angular gyrus (14 voxels, p = 0.0457), and cerebellum (12 voxels, p = 0.0446). Acupuncture improved the function of right superior frontal gyrus (1, 404 voxels, p = 0.0123), left inferior frontal gyrus (1068 voxels, p = 0.0088), left inferior temporal gyrus (903 voxels, p = 0.0074), left supramarginal gyrus (888 voxels, p = 0.0113), left precuneus (457 voxels, p = 0.0247), right precuneus (302 voxels, p = 0.0191), left supplementary motor area (82 voxels, p = 0.0354), and right parahippocampal gyrus (28 voxels, p = 0.0379). The brain regions affected by non-acupoint acupuncture were all located in the frontal lobe. The Cochrane risk-of bias tool and MINORS5 were used for quality assessment and the included articles had high performance bias and attrition bias.
Conclusion: This coordinate-based meta-analysis found that acupuncture in patients with PI had significant effects on the default mode network, particularly on the frontal lobe and precuneus, and that non-acupoint acupuncture may provide some benefit to frontal brain region function.
Systematic review registration: PROSPERO: CRD42023400086.
Primary insomnia (PI), typically characterized by problems of falling asleep and staying asleep, occurs in 6–10% of the global population (1). The persistent state of PI not only impairs daytime performance but also increases the risk of several psychiatric disorders, especially depression and anxiety (2). In recent years, several high-quality clinical trials have addressed the treatment of PI (3, 4).
Currently, medications and cognitive behavioral therapy are used to treat insomnia (5). Sleep medications may cause serious side effects, including dependence, cognitive deficits, confusion, and falls, so researchers are exploring other effective options with fewer side effects (6). Acupuncture, a traditional Chinese medicine procedure used in which sterile needles are inserted into specific areas of the body, is often used to treat PI because of its low side effects. This treatment method regulates mood and insomnia symptoms and is consistent with the contemporary biological–psychological– social–medical philosophy. Several systematic reviews have shown that acupuncture was associated with improvements in some objective sleep parameters (total sleep time, sleep efficiency, and number of awakenings) (7), and that treatment of more than 3 weeks could significantly improve symptoms (8). However, the certainty of the evidence was moderate to low.
Recently, with the popularization of neuroimaging methods, more researchers have focused on structural and functional changes in the brains of PI patients. Functional magnetic resonance imaging (fMRI), a technique that assesses hemodynamics using blood oxygen level-dependent signals, is commonly used to study changes in the brain. Assessment of spontaneous neuronal activity in the blood oxygenation level-dependent (BOLD) signal can be achieved by calculating regional intensity at rest using measures such as amplitude of low-frequency fluctuation (ALFF) and regional homogeneity (ReHo) (9, 10). Central nervous system changes in patients with PI tend to focus on brain regions associated with emotional and cognitive functions, and these changes have been linked to the onset, development, and severity of the disorder. For instance, insomnia symptoms in patients with PI may be associated with decreased ReHo levels in the amygdala, cingulate cortex, and frontal lobe, and decreased ALFF levels in the thalamus. These changes may correspond to high levels of introspection, worry, and rumination (11). Several studies have demonstrated that appropriate treatment of PI can reverse structural and functional brain abnormalities in patients. The function of several brain regions was shown to change in patients with PI after cognitive behavioral therapy (12), treatment with benzodiazepine, and cupping (13).
Previous studies on the mechanisms of acupuncture have shown that acupuncture can affect brain activity and have long-lasting and significant effects on neural functions (14). As the number of clinical trials using acupuncture to treat PI continues to increase, a number of studies have confirmed the hypothesis that the activity of specific brain areas was related to sleep and shown that acupuncture could alter these regions (15, 16). However, single studies with small samples and low detection thresholds may lead to false positive results, and there are no systematic reviews of how acupuncture or non-pharmacological treatments affect brain imaging characteristics in PI patients.
The aim of this systematic review and coordinate-based meta-analysis was to summarize the current understanding of regional brain fMRI imaging features in patients with PI treated with acupuncture. To this end, we summarized the coordinates of ReHo and ALFF in qualified studies and used Seed- based D Mapping with Permutation of Subject Images (SDM-PSI) for data processing, summarization, and analysis to systematically summarize the brain imaging features of patients with PI and evaluate the therapeutic effects of acupuncture.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (17) were observed by the conduct of the present study. The International Prospective Registry of Systematic Reviews received the protocol for this study. (PROSPERO: CRD42023400086).
We performed a methodical and comprehensive search of the literature published between the establishment of the following publication databases until December 2022: Web of Science, PubMed, ScienceDirect, Embase, Wan Fang (WF), China National Knowledge Infrastructure (CNKI), and Chinese Scientific Journal Database (VIP). The following search terms were used under the “subject words plus free words” retrieval strategy: (“Acupuncture” OR “Needle” OR “Acupoint”) AND (“fMRI” OR “Magnetic resonance imaging” OR “FC” OR “ROI” OR “ReHo” OR “ALFF” OR “Neuroimaging” OR “Brain”) AND (“Insomnia” OR “Sleep Disorder”). In addition, all reference lists for each article, and all relevant review articles, were manually reviewed to identify additional potentially qualifying studies. The search process was performed individually by two authors (ZSH and CY).
The following inclusion criteria were used to screen all original studies published in English or Chinese:
Types of trials: Randomized controlled trials, non-randomized controlled trials, and observational studies;
Participants: Adult patients who met the diagnostic criterion for primary insomnia from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5);
Intervention: Various forms of acupuncture (characterized by the insertion of a needle into the skin surface of the acupoint, such as manual acupuncture, electroacupuncture, auricular acupuncture and needle warming moxibustion) or acupuncture in combination with other medications;
Outcomes: (1) Reporting an analysis of relevant data (patient data before and after acupuncture, comparisons with healthy subjects or non-acupoint acupuncture groups); (2) fMRI and subjective scales were used as parameters, with whole-brain imaging with function (ALFFs or ReHo) at rest serving as the primary outcome measure; (3) Whole-brain imaging results were displayed in stereotactic coordinates (Talairach or Montreal Neurological Institute [MNI]) in three dimensions (x, y, and z).
The following exclusion criteria were also used: (1) Participants included patients with insomnia comorbid with other conditions; (2) Animal experiments and duplicate work; (3) No available stereotactic peak coordinates; (4) Insufficient data for meta-analysis in the original study or after contacting the authors.
Duplicate studies were eliminated using NoteExpress software (v3.2.0.7276; Aegean Software Corp., Beijing, China). Studies that did not meet the inclusion criteria were then excluded based on the titles and abstracts of the remaining articles. In a final step, a thorough full-text review of potentiallyrelevant studies were performed. Two authors (ZSH and CY) assessed the articles separately, resolving any disagreements by consensus or consultation with a third principal investigator (CHN).
For each study, a graph was created with information on the following: (1) publication details (author, journal, year); (2) basic study details (type of study, comparisons, sample size, scanning instrument, functional data analysis); (3) acupuncture methods (acupuncture type, acupoints, duration of treatment, frequency, duration); (4) clinical outcome measures (Pittsburgh Sleep Quality Index scores before and after treatment); (5) neuroimaging results (peak coordinates [MNI or Talairach] and corresponding statistics (t scores, Z scores). If certain data were missing from the original manuscripts, the corresponding authors were contacted by e-mail for further information. Data entry and extraction were performed by two independent authors (ZSH and CY).
We used risk-of-bias tools developed by the Cochrane Collaboration (18) (https://training.cochrane.org/handbook) to assess the quality of the literature, considering seven factors: creation of random sequences, allocation concealment, blinding of researchers and study participants, blinded assessment of study results, completeness of data, incomplete reporting, and other bias risk. MINORS5 (19) was used to assess the quality of non-randomized controlled trials. Quality assessment was carried out independently by two authors (ZSH and CY). In case of disagreement, a third author (CHN) was consulted.
Imaging data from the eligible articles (ReHo and ALFF) were analyzed using an SDM software package (SDM v6.22; www.sdmproject.com). Differences in brain activity between patients with PI and healthy subjects, before and after acupuncture treatment in patients with PI, and between real and sham acupuncture therapy was compared. Software instructions were strictly followed, and accurate and comprehensive data were expected (20). (1) A text file containing peak positions and effect dimensions (t value or z-score or P-value) was created by extracting the peak data. Talairach coordinates were also converted to MNI coordinates. (2) A new statistical chart was recreated separately for each data set in the standard MNI chart. (3) Mean maps were constructed from the means of the random effects datasets based on sample size, study variance, and between-group heterogeneity. (4) A quantitative meta-analysis was performed to compare fMRI changes between patients with PI and healthy subjects, and between patients with PI in the true acupuncture group before and after treatment. (5) The following default SDM parameters were used: anisotropy = 1, isotropic full width at half maximum = 20, peak Z = 1, and voxel size = 2. (6) MRIcron software was used to display all statistically significant results.
An initial search identified 316 articles, comprising 196 articles in English and 120 in Chinese. After screening the type and content of these articles, 11 studies (9 in Chinese and 2 in English) were finally included. An illustration of the article selection process can be found in Figure 1.
The included studies were published from 2016 to 2021. A total of 116 healthy controls were compared to 305 patients with PI. Table 1 represents essential details of the eligible articles, summarized as follows. (1) Study types: the selected studies included five randomized controlled trials, three unequal group-controlled trials (healthy subjects formed control groups), and three self-controlled trials. (2) Participants: all studies recruited patients diagnosed as PI according to the DSM-5 criteria; three of them recruited healthy control subjects, and demographic data of all included healthy controls were matched with those of patients in each study. (3) Intervention: all interventions in the treatment groups were acupuncture, including manual acupuncture, electroacupuncture, and auricular acupuncture; five studies used non-acupoint acupuncture as control treatment. (4) Age and gender: most of the subjects included in the studies were middle-age and elderly, with significantly more women than men. (5) Acupuncture methods and acupoints: Pure acupuncture was used in five studies, auricular acupuncture in three, electroacupuncture in two, and both acupuncture and fire acupuncture in one; the most frequently chosen acupoint was Shenmen (HT-7), followed by Baihui (GV-20) and Sanyinjiao (SP-6). In addition, acupoints regulating liver and spleen were also used. (6) Outcome measures: ReHo and ALFF were measured by fMRI in all studies; nine studies presented data on alterations in brain regions in PI patients both before and after an acupuncture session, three articles presented comparisons of brain regions between patients with PI and healthy controls, and we also found imaging findings from non-acupoint acupuncture in patients with PI and/or comparisons of brain area changes in four studies.
The Cochrane risk-of bias tool was used for quality assessment, and there were seven entries in the evaluation criteria. In all included randomized controlled trials, allocation concealment and blinding were not discussed, resulting in a high level of performance bias. In five randomized controlled trials, three studies discussed the process of generating random sequences. Some missing data resulted in high attrition bias. We did not detect any other factors leading to bias. Figure 2 illustrates this assessment. Quality assessment of non-randomized controlled trials was performed using MINORS5, which is also shown in Table 2.
We found a significant increase in activity in the left superior frontal gyrus in patients with PI compared to healthy subjects (1352 voxels, p = 0.0028). Signals in the right angular gyrus (14 voxels, p = 0.0457) and cerebellum (12 voxels, p = 0.0446) were also elevated.
According to our analysis, acupuncture resulted in increased signals in some brain regions, with the largest cluster (1, 404 voxels, p = 0.0123) involving the right superior frontal gyrus, followed by the left inferior frontal gyrus (1, 068 voxels, p = 0.0088). Other brain regions affected included left inferior temporal gyrus (903 voxels, p = 0.0074), left supramarginal gyrus (888 voxels, p = 0.0113), left precuneus (457 voxels, p = 0.0247), right precuneus (302 voxels, p = 0.0191), left supplementary motor area (82 voxels, p = 0.0354), and right parahippocampal gyrus (28 voxels, p = 0.0379); all showed an increase in activity after acupuncture.
Non-acupoint acupuncture had an effect in some brain regions, involving few voxels that increased their signal. These brain regions were concentrated in the frontal lobe, including the left superior frontal gyrus (298 voxels, p = 0.0044), left inferior frontal gyrus (227 voxels, p = 0.0088), and right inferior frontal gyrus (122 voxels, p = 0.0404).
Four articles compared different brain regions affected by acupuncture and non-acupoint acupuncture including the left cerebellum (40 voxels, p = 0.0491), right superior frontal gyrus (22 voxels, p = 0.0454), and left precuneus (12 voxels, p = 0.0456). The results of the coordinate meta-analysis are shown in Table 3 and Figures 3–6 in detail.

Figure 5. Brain regions with increased signals before and after non-acupoint acupuncture in patients with PI.
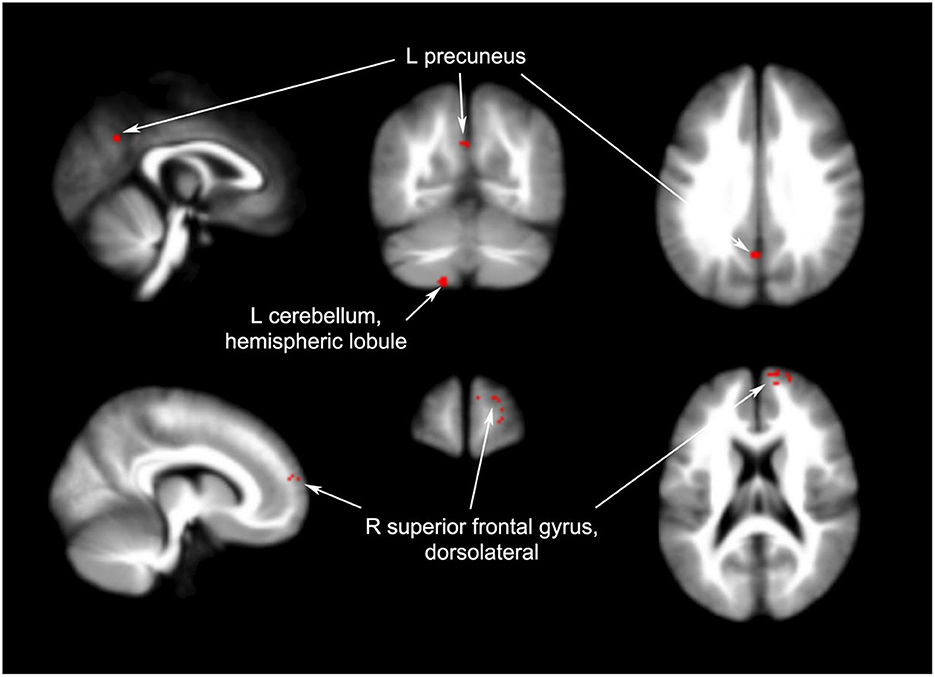
Figure 6. Brain regions with increased signals in acupuncture groups compared to non-acupuncture groups.
Focusing on changes in ReHo and ALFF, we summarized the current state of research and the effects of acupuncture on brain areas in PI patients based on neuroimaging data. In total, 11 fMRI studies on coordinates were pooled using the SDM-PSI meta-analysis method, and we uncovered some meaningful results. One notable finding was that frontal lobe activation appears to be both a key pathological feature of patients with PI and an important mechanism for the effect of acupuncture on insomnia. In addition, the precuneus, supramarginal gyrus, and supplementary motor area in patients with PI showed increased resting-state activity after acupuncture treatment. We also found that most of the brain areas affected by acupuncture were primarily associated with the default mode network (DMN).
One of the accepted theories is that PI is accompanied by some form of unique hyperarousal (30). In accordance with this, fMRI results have indicated increased activity and disinhibition, as well as increased instability of intrinsic activity in PI (31). Summarizing the three studies included here with healthy controls (133 patients with PI vs. 116 healthy controls), patients with PI had higher ALFF and ReHo levels in the left superior frontal gyrus, right angular gyrus, and cerebellum. No brain areas with low ReHo and ALFF levels were found, which was highly in keeping with this theory. This did not seem to be entirely consistent with previous studies (32), possibly due to the small sample size. Unexpectedly, several brain regions with increased ALFF and ReHo levels were also found after both acupuncture and non-acupoint acupuncture treatment, which were associated with a reduction in insomnia symptoms. In conjunction with the associations between hyperarousal in insomnia and neurocognitive deficits (33), we hypothesized that there might be a “spurious active” state in certain brain regions in patients with PI and that acupuncture treatment could actually improve the function of these regions.
The main function of the frontal lobe is to integrate signals from other brain regions, and this area is highly involved in understanding of past and current experiences and developing plans for future actions (34). Previous studies have shown that maturation of the frontal lobe was related to sleep status (35). The present results showed that significant changes in the frontal lobe occurred not only in patients with PI compared to healthy controls, but also when comparing patients with PI before and after treatment, indicating the important role of frontal lobe modifications in the neuropathological changes associated with PI.
The prefrontal lobe is not only involved in linking external and internal environments and identifying episodic memories, but also works with other parts of the brain to maintain resting state network activity (36). Studies have shown that difficulty falling asleep may be related to a lack of reduction in activity in the prefrontal cortex (37) and that people with insomnia showed a slower decrease in relative cerebral metabolic rates of glucose in the medial prefrontal cortex during the transition from wakefulness to non-REM sleep compared to controls (38). The orbitofrontal lobe, located in the prefrontal lobe, may influence the regulation of emotional memories during sleep (39). In the present statistical results, the orbitofrontal lobe showed the most significant difference when comparing patients with PI and healthy individuals, suggesting that this may be a crucial brain region associated with insomnia symptoms.
Two parts of the frontal lobe, the left inferior frontal gyrus (opercular part) and the right superior frontal gyrus (medial), changed significantly before and after acupuncture. The left inferior frontal gyrus has a crucial function in phonological processing and verbal working memory (40). The right superior frontal gyrus has a specific role in translating conflict anticipation into the control of impulsive responses, and its activation is associated with more effective response inhibition and a lower sense of motor urgency (41). It is possible that changes in these two brain regions are responsible for the improved information processing ability after acupuncture in patients with PI.
The frontal lobe was the most frequently changed brain region in patients with PI following non- acupoint acupuncture, but the number of changed voxels in all brain regions was much lower than after acupuncture treatment. The changes occurred in the left superior frontal gyrus (dorsolateral), left inferior frontal gyrus (triangular part), and right inferior frontal gyrus (opercular part). The dorsolateral circuit is thought to control attention, planning, and organization (33). The triangular part of the inferior frontal gyrus serves as a connection point between the DMN, the sensory/somatomotor network, and the dorsal and ventral attention networks (42). The placebo effect of non-acupoint acupuncture could promote the perception of improved sleep in patients with PI and increase their confidence in their sleep quality.
In general, both acupuncture and non-acupoint acupuncture enhanced frontal brain activity in patients with PI. Frontal lobe function is involved in a number of cognitive processes, including problem solving, working memory, planning, and evaluation of goal-oriented activities. Enhancing its function may help alleviate the subjective insomnia and environmental discomfort experienced by people with PI, thereby reducing their pre-sleep worries and rumination (37). Compared with the results of non-acupoint acupuncture, it can be concluded that acupoint acupuncture had a real effect on the function of the frontal lobe function.
The default mode network (DMN) is activated when people are left alone to contemplate and comprises a group of interconnected brain regions involved in cognition (43). The prefrontal cortex, precuneus, angular gyrus, posterior cingulate cortex, and transverse parietal cortex make up the most of the DMN (44). During deep sleep, certain DMN regions dissociate, suggesting that connectivity in this network may support certain states of consciousness (45). Based on the understanding of self-referential stress in patients with PI, one theory is that insomnia may be caused by the disruption or imbalance of DMN activity, and previous studies have confirmed that it appears to be altered or imbalanced in insomnia (46).
In terms of function, the DMN can be divided into two parts: the pre-DMN, which is centered on the prefrontal lobe, and the rear-DMN, which is centered on the precuneus and angular gyrus (47). The pre-DMN is involved in psychological behaviors that are self-referential, while the post-DMN is involved in memory of past experiences (48). The effects of acupuncture on the prefrontal lobe were discussed above and suggested that the function of the pre-DMN was altered by acupuncture. The precuneus, the functional core of the rear-DMN, is a brain area that helps remember events from the past, which is fundamental to our self-esteem and interactions with others (49). The function of the precuneus is bidirectional in relation to sleep status. People with increased blood oxygen level-dependent signals in the bilateral precuneus showed high subjective–objective discrepancy of sleep (50), and the functional connectivity density of the precuneus decreased after sleep deprivation (51). The present study showed that acupuncture affected the function of the left and right precuneus in patients with PI, and the precuneus also appeared in comparisons between acupuncture and non-acupoint acupuncture, suggesting that acupuncture can indeed alter its function. With the exception of the prefrontal lobe and the precuneus as mentioned above, other brain areas affected after acupuncture were the supramarginal gyrus and inferior temporal gyrus, which are also part of the DMN. This seemes to indicate that acupuncture improved the function of the DMN in patients with PI.
Impairments associated with insomnia can be classified into cognitive, self-referential, affective, and sleep–wake promotion domains (52), among which DMN dysfunction primarily affecting cognition and self-referential systems. The present results suggest that acupuncture could act on the DMN to improve self-awareness and perception of the environment and reduce the occurrence of their bad memories in patients with PI, affecting their adaptability to the environment and their perception of sleep.
To our knowledge, the present study was the first to review the results of fMRI brain imaging associated with acupuncture in patients with PI using a coordinate-based meta-analysis. Here, we presented new evidence of neuroplasticity induced by acupuncture in a neurological disorder. Precise inclusion criteria were established to avoid selection bias, and 11 studies were thoroughly read and filtered before being pooled in the final analysis. Ultimately, we identified some meaningful results that provide a foundation and challenges for future scientific research.
However, this meta-analysis has certain limitations. First, we included only 11 studies and did not test for heterogeneity. Because of the limited sample size of the listed studies, there were large differences among the studies in the design of the clinical study protocol, the choice of acupoints, and the methods of analysis, and some results were not reproducible, which significantly affected the reliability of the results. As a result, caution should be exercised in interpreting the results. In most studies, the methods of blinding and concealment of group assignment were not accurately described, therefore the results had some degree of methodological bias. Finally, only publications in Chinese and English languages were used, which may have influenced the results and affected the usefulness and reliability of the present study.
Larger sample numbers and more rigorous randomized controlled trials are needed in future studies to improve reproducibility and accuracy. To fully explore the true therapeutic effect of acupuncture, future clinical trials should standardize sham acupuncture groups and apply more uniform implementation standards for device selection, patient characteristics at baseline, and outcome assessment.
The present coordinate-based meta-analysis found that the impact of acupuncture in patients with PI was mainly concentrated in the DMN, especially in the frontal lobe and precuneus, and non-acupoint acupuncture also showed some benefits in the frontal brain. This work provided a new perspective to clarify the function of brain areas in acupuncture treatment of PI. To ensure the reliability of future clinical data, large-scale and rigorous randomized controlled trials should be conducted.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
SZ, YC, HC, and LZ: concept and design. SZ, YC, and HC: acquisition of data. SZ and YC: drafting of the manuscript. LZ and HS: critical revision of the manuscript. All authors agree to be accountable for all aspects of the work, contributed to the article and approved the submitted version.
This work was supported by the First-class discipline Construction Project of Beijing University of Traditional Chinese Medicine.
We extend our gratitude to all the authors of the included studies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Xie D, Qin H, Dong F, Wang X, Liu C, Xue T, et al. Functional connectivity abnormalities of brain regions with structural deficits in primary insomnia patients. Front Neurosci. (2020) 14:566. doi: 10.3389/fnins.2020.00566
2. Palagini L, Hertenstein E, Riemann D, Nissen C. Sleep, insomnia and mental health. J Sleep Res. (2022) 31:e13628. doi: 10.1111/jsr.13628
3. Wang HX, Wang L, Zhang WR, Xue Q, Peng M, Sun ZC, et al. Effect of transcranial alternating current stimulation for the treatment of chronic insomnia: a randomized, double-blind, parallel-group, placebo-controlled clinical trial. Psychother Psychosom. (2020) 89:38–47. doi: 10.1159/000504609
4. Ji X, Ivers H, Beaulieu-Bonneau S, Morin CM. Complementary and alternative treatments for insomnia/insomnia -depression-anxiety symptom cluster: meta-analysis of English and Chinese literature. Sleep Med Rev. (2021) 58:101445. doi: 10.1016/j.smrv.2021.101445
5. Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. (2017) 26:675–700. doi: 10.1111/jsr.12594
6. Soong C, Burry L, Greco M, Tannenbaum C. Advise non-pharmacological therapy as first line treatment for chronic insomnia. BMJ. (2021) 372:n680. doi: 10.1136/bmj.n680
7. Zhao FY, Fu QQ, Kennedy GA, Conduit R, Zhang WJ, Wu WZ, et al. Can acupuncture improve objective sleep indices in patients with primary insomnia? A systematic review and meta-analysis. Sleep Med. (2021) 80:244–59. doi: 10.1016/j.sleep.2021.01.053
8. Kim SA, Lee SH, Kim JH, van den Noort M, Bosch P, Won T, et al. Efficacy of acupuncture for insomnia: a systematic review and meta-analysis. Am J Chin Med. (2021) 49:1135–50. doi: 10.1142/S0192415X21500543
9. Adhikari BM, Hong LE, Zhao Z, Wang DJJ, Thompson PM, Jahanshad N, et al. Cerebral blood flow and cardiovascular risk effects on resting brain regional homogeneity. Neuroimage. (2022) 262:119555. doi: 10.1016/j.neuroimage.2022.119555
10. Zhao B, Bi Y, Li L, Zhang J, Hong Y, Zhang L, et al. The instant spontaneous neuronal activity modulation of transcutaneous auricular vagus nerve stimulation on patients with primary insomnia. Front Neurosci. (2020) 14:205. doi: 10.3389/fnins.2020.00205
11. Zang SH, Shi HW, Zhang XR, Zhu LH, Zhou L. Advances in imaging studies of central nervous system in primary insomnia. Chin J Neuropsychiatric Disord. (2022) 48:503–7. doi: 10.3969/j.issn.1002-0152.2022.08.011
12. Lee YG, Kim S, Kim N, Choi JW, Park J, Kim SJ, et al. Changes in subcortical resting-state functional connectivity in patients with psychophysiological insomnia after cognitive- behavioral therapy: changes in resting-state FC after CBT for insomnia patients. Neuroimage Clin. (2017) 17:115–23. doi: 10.1016/j.nicl.2017.10.013
13. Feng F, Yu S, Wang Z, Wang J, Park J, Wilson G, et al. Non-pharmacological and pharmacological interventions relieve insomnia symptoms by modulating a shared network: a controlled longitudinal study. Neuroimage Clin. (2019) 22:101745. doi: 10.1016/j.nicl.2019.101745
14. Yin Z, Zhou J, Xia M, Chen Z, Li Y, Zhang X, et al. Acupuncture on mild cognitive impairment: a systematic review of neuroimaging studies. Front Aging Neurosci. (2023) 15:1007436. doi: 10.3389/fnagi.2023.1007436
15. Wang YK Li T, Ha LJ, Lv ZW, Wang FC, Wang ZH, et al. Effectiveness and cerebral responses of multi-points acupuncture for primary insomnia: a preliminary randomized clinical trial and fMRI study. BMC Complement Med Ther. (2020) 20:254. doi: 10.1186/s12906- 020-02969-6
16. Shi XH, Wang YK Li T, Liu HY, Wang XT, Wang ZH, et al. Gender-related difference in altered fractional amplitude of low-frequency fluctuations after electroacupuncture on primary insomnia patients: a resting-state fMRI study. Brain Behav. (2021) 11:e01927. doi: 10.1002/brb3.1927
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3. (updated February 2022). Cochrane. (2022). Available online at: https://www.training.cochrane.org/handbook (accessed March 02, 2022).
19. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies. (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
20. Albajes-Eizagirre A, Solanes A, Fullana MA. Meta-analysis of voxel-based neuroimaging studies using seed-based d mapping with permutation of subject images. (SDM-PSI). JoVE. (2019) 153:23. doi: 10.3791/59841-v
21. Luo WT. Research on the mechanism and efficacy prediction of auricular vagus nerve stimulation in the treatment of primary insomnia based on the characteristics of brain function. Guangzhou Univ Chin Med. (2021) 5:44 doi: 10.27044/d.cnki.gszyu.2021.001044
22. He JK, Wang CY, Fang JL, Gao DQ, Wang JY, Chen LM, et al. Effect of percutaneous auricular acupoint electroacupuncture on primary insomnia and sleepiness symptom based on resting state functional magnetic resonance imaging. Chin J Trad Chin Med. (2021) 36:4637–41.
23. Wang C. Clinical observation and central response characteristics of acupuncture Shenmen combined with Fuliang in the treatment of cardio-renal incompatibility insomnia. Shanghai Univ Chin Med. (2020) 45:58 doi: 10.27320/d.cnki.gszyu.2020.000058
24. Wang Y K. Study on central mechanism of acupuncture intervention in primary insomnia based on ET and fMRI. Jilin Univ. (2020) 5:2236. doi: 10.27162/d.cnki.gjlin.2020.002236
25. Ding JL. Curative effect of acupuncture combined with millifireneedle in treating insomnia with deficiency of heart and spleen and study on fMRI imaging of resting brain. Shanghai Univ Trad Chinese Med. (2019) 5:434. doi: 10.27320/d.cnki.gszyu.2019.000434
26. Zhang F. Acupuncture regulation of hyperarousal in chronic insomnia with deficiency of heart and spleen. Beijing University of Chinese Medicine. (2019).
27. Huang TY. Clinical effect and fMRI immediate response characteristics of “acupuncture delivery” of Neijing in the treatment of insomnia with liver depression and spleen deficiency. Shanghai Univ Trad Chinese Med. (2019) 25:13 doi: 10.27320/d.cnki.gszyu.2019.000013
28. Huang HJ, Wang Y. Evaluation of the effects of acupuncture on brain function in primary insomnia based on whole brain resting state fMRI. J Cardio-Cerebrovasc Dis. (2019) 17:3040–3. doi: 10.12102/j.issn.1672-1349.2019.19.044
29. Xu XY, Wang S, Ge TN. Study on the neural mechanism of acupuncture for primary insomnia patients. Chin J Neuromed. (2016) 15:1251–4. doi: 10.3760/cma.j.issn.1671-8925.2016.12.012
30. Drummond SP, Smith MT, Orff HJ, Chengazi V, Perlis ML. Functional imaging of the sleeping brain: review of findings and implications for the study of insomnia. Sleep Med Rev. (2004) 8:227–42. doi: 10.1016/j.smrv.2003.10.005
31. Li C, Dong M, Yin Y, Hua K, Fu S, Jiang G. Abnormal whole-brain functional connectivity in patients with primary insomnia. Neuropsychiatr Dis Treat. (2017) 13:427–35. doi: 10.2147/NDT.S128811
32. Yang N, Yuan S, Li C, Xiao W, Xie S, Li L, et al. Diagnostic identification of chronic insomnia using ALFF and FC features of resting-state functional MRI and logistic regression approach. Sci Rep. (2023) 13:406. doi: 10.1038/s41598-022-24837-8
33. Khassawneh BY, Bathgate CJ, Tsai SC, Edinger JD. Neurocognitive performance in insomnia disorder: the impact of hyperarousal and short sleep duration. J Sleep Res. (2018) 27:e12747. doi: 10.1111/jsr.12747
34. Burruss JW, Hurley RA, Taber KH, Rauch RA, Norton RE, Hayman LA. Functional neuroanatomy of the frontal lobe circuits. Radiology. (2000) 214:227–30. doi: 10.1148/radiology.214.1.r00ja43227
35. Anastasiades PG, de Vivo L, Bellesi M, Jones MW. Adolescent sleep and the foundations of prefrontal cortical development and dysfunction. Prog Neurobiol. (2022) 218:102338. doi: 10.1016/j.pneurobio.2022.102338
36. Gong H, Sun H, Ma Y, Tan Y, Cui M, Luo M, et al. Prefrontal brain function in patients with chronic insomnia disorder: a pilot functional near-infrared spectroscopy study. Front Neurol. (2022) 13:985988. doi: 10.3389/fneur.2022.985988
37. Dang-Vu TT, Desseilles M, Petit D, Mazza S, Montplaisir J, Maquet P. Neuroimaging in sleep medicine. Sleep Med. (2007) 8:349–72. doi: 10.1016/j.sleep.2007.03.006
38. Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. (2004) 161:2126–8. doi: 10.1176/appi.ajp.161.11.2126
39. Pereira SIR, Tsimpanouli ME, Hutchison I, Schneider J, Anderson IM, McFarquhar M, et al. Cueing emotional memories during slow wave sleep modulates next-day activity in the orbitofrontal cortex and the amygdala. Neuroimage. (2022) 253:119120. doi: 10.1016/j.neuroimage.2022.119120
40. Nixon P, Lazarova J, Hodinott-Hill I, Gough P, Passingham R. The inferior frontal gyrus and phonological processing: an investigation using rTMS. J Cogn Neurosci. (2004) 16:289–300. doi: 10.1162/089892904322984571
41. Hu S, Ide JS, Zhang S, Li CR. The right superior frontal gyrus and individual variation in proactive control of impulsive response. J Neurosci. (2016) 36:12688–96. doi: 10.1523/JNEUROSCI.1175-16.2016
42. Luo L, Xiao M, Luo Y, Yi H, Dong D, Liu Y, et al. Knowing what you feel: Inferior frontal gyrus-based structural and functional neural patterns underpinning adaptive body awareness. J Affect Disord. (2022) 315:224–33. doi: 10.1016/j.jad.2022.07.051
43. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network. Ann NY Acad Sci. (2008) 1124:1–38. doi: 10.1196/annals.1440.011
44. Xia G, Hu Y, Chai F, Wang Y, Liu X. Abnormalities of the default mode network functional connectivity in patients with insomnia disorder. Contrast Media Mol Imaging. (2022) 27:9197858. doi: 10.1155/2022/9197858
45. Tashjian SM, Goldenberg D, Monti MM, Galván A. Sleep quality and adolescent default mode network connectivity. Soc Cogn Affect Neurosci. (2018) 13:290–9. doi: 10.1093/scan/nsy009
46. Marques DR, Gomes AA, Caetano G, Castelo-Branco M. Insomnia disorder and brain's default-mode network. Curr Neurol Neurosci Rep. (2018) 18:45. doi: 10.1007/s11910-018-0861-3
47. Raichle ME. The brain's default mode network. Annu Rev Neurosci. (2015) 38:433–47. doi: 10.1146/annurev-neuro-071013-014030
48. Andrews-Hanna JR, Smallwood J, Spreng R. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. (2014) 1316:29–52. doi: 10.1111/nyas.12360
49. Hebscher M, Meltzer JA, Gilboa A. A causal role for the precuneus in network-wide theta and gamma oscillatory activity during complex memory retrieval. Elife. (2019) 8:e43114. doi: 10.7554/eLife.43114
50. Kim YB, Kim N, Lee JJ, Cho SE, Na KS, Kang SG. Brain reactivity using fMRI to insomnia stimuli in insomnia patients with discrepancy between subjective and objective sleep. Sci Rep. (2021) 11:1592. doi: 10.1038/s41598-021-81219-2
51. Yang L, Lei Y, Wang L, Chen P, Cheng S, Chen S, et al. Abnormal functional connectivity density in sleep-deprived subjects. Brain Imaging Behav. (2018) 12:1650–7. doi: 10.1007/s11682-018-9829-9
Keywords: primary insomnia (PI), acupuncture, functional magnetic resonance imaging, amplitude of low-frequency fluctuations, regional homogeneity, systematic review
Citation: Zang S, Chen Y, Chen H, Shi H and Zhou L (2023) Effects of acupuncture on the brain in primary insomnia: a coordinate-based meta-analysis of fMRI studies. Front. Neurol. 14:1180393. doi: 10.3389/fneur.2023.1180393
Received: 06 March 2023; Accepted: 30 May 2023;
Published: 18 July 2023.
Edited by:
Shazia M. Jamil, Scripps Clinic, United StatesCopyright © 2023 Zang, Chen, Chen, Shi and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhou, WmhvdWxqazcyMTFAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.