
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 09 June 2023
Sec. Neurocritical and Neurohospitalist Care
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1179243
Importance: Identifying biomarkers that, at hospital admission, predict subsequent delirium will help to focus our clinical efforts on prevention and management.
Objective: The study aimed to investigate biomarkers at hospital admission that may be associated with delirium during hospitalization.
Data sources: A librarian at the Fraser Health Authority Health Sciences Library performed searches from 28 June 2021 to 9 July 2021, using the following sources: Medline, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, and the Database of Abstracts of Reviews and Effects.
Study selection: The inclusion criteria were articles in English that investigated the link between serum concentration of biomarkers at hospital admission and delirium during hospitalization. Exclusion criteria were single case reports, case series, comments, editorials, letters to the editor, articles that were not relevant to the review objective, and articles concerning pediatrics. After excluding duplicates, 55 studies were included.
Data extraction and synthesis: This meta-analysis followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) protocol. Independent extraction, with the consensus of multiple reviewers, was used to determine the final studies included. The weight and heterogeneity of the manuscripts were calculated using inverse covariance with a random-effects model.
Main outcome(s) and measure(s): Differences in mean serum concentration of biomarkers at hospital admission between patients who did and did not develop delirium during hospitalization.
Results: Our search found evidence that patients who developed delirium during hospitalization had, at hospital admission, significantly greater concentrations of certain inflammatory biomarkers and one blood–brain barrier leakage marker than patients who did not develop delirium during hospitalization (differences in the mean: cortisol: 3.36 ng/ml, p < 0.0001; CRP: 41.39 mg/L, p < 0.00001; IL-6: 24.05 pg/ml, p < 0.00001; S100β 0.07 ng/ml, p < 0.00001). These differences were independent of other confounding variables such as the patient's severity of illness. A significantly lower serum concentration, at hospital admission, of acetylcholinesterase (difference in the means −0.86 U/ml, p = 0.004) was also associated with an increased vulnerability to developing delirium during hospitalization.
Conclusion and relevance: Our meta-analysis supports the hypothesis that patients with hypothalamic-pituitary axis dysfunction, increased blood–brain barrier permeability, and chronic overload of the cholinergic system, at hospital admission, are more vulnerable to developing delirium during hospitalization.
Delirium is characterized by acute and transient changes in cognitive function, especially in attention (1, 2). Patients who develop delirium during hospitalization have a 3-fold higher 1-year mortality and an approximately 20-day greater length of stay than patients who do not develop delirium during hospitalization (3, 4). Although delirium is common during hospitalization, having an incidence range of 10–60%, its etiology is still not well understood (5, 6). Direct brain insults, chronic overactivation of the immunologic system, and overload of the cholinergic system have been proposed as causes of increased individual susceptibility to delirium during hospitalization (1).
Many factors may trigger delirium during hospitalization (1). It has been hypothesized that an acute overactivation of the immunologic system might affect neuronal synapses, leading to acute cognitive impairment and/or delirium (1). Patient susceptibility to developing delirium is therefore attributed, in part, to how the immunologic system responds to inflammation and stress (1, 7). Chronic inflammation has been shown to prime microglia activity and to increase blood–brain barrier (BBB) permeability, increasing the likelihood of overactivation of the immunologic system in the brain during acute inflammation and stress (1, 7). An inappropriate immunologic response to inflammation may result in an acute neurotransmitter imbalance, potentially manifesting as delirium (1, 7). Hypothalamic-pituitary axis (HPA) dysfunction has also been associated with chronic inflammation and inappropriate immunologic response during inflammation and stress (1, 7). HPA dysfunction is also linked to neurotransmitter imbalance and has been associated with decreased activity in the prefrontal cortex, resulting in disturbance of attention, memory, and executive functions (1, 7). While any one of these mechanisms might trigger delirium, all of them can coexist in a single patient (1). Many studies have tried to demonstrate an association between delirium and the presence of biomarkers in the serum (8–10). These biomarkers reflect different potential precipitants of delirium and may not only predict delirium but also give clues as to the likely mechanism.
Although the risk factors for delirium have been well documented, few studies have focused on the pathophysiology of delirium. We conducted a systematic review and meta-analysis on the association of serological biomarkers at hospital admission with the development of delirium during hospitalization. The primary objective was to summarize the current findings in the literature regarding serum concentration of biomarkers at hospital admission as predictors of the development of delirium during hospitalization. The second objective was to identify possible mechanisms for delirium. The third objective was to identify gaps in the literature.
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) protocol (Diagram 1). A librarian at the Health Sciences Library at Fraser Health Authority, Royal Columbian Hospital (New Westminster, Canada), performed the searches. Searches were conducted using the following sources: Medline (1946-present), EMBASE (1974-present), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, and the Database of Abstracts of Reviews and Effects (DARE).
We developed search strategies based on the search interface to ensure an appropriate balance between search sensitivity and specificity. Articles were limited to published prospective and retrospective studies. Keyword, adjacency, wildcard, and subject-heading searching were employed in all search strategies to maximize the sensitivity of the search, while publication limits, specific clinical terms, and their variants were used to increase the specificity of the search results. Inclusion criteria were articles in English that investigated links between serum concentration of biomarkers at hospital admission and delirium during hospitalization. Exclusion criteria were single case reports, case series, comments, editorials, letters to the editor, articles that were not relevant to the review objective, and articles concerning pediatrics.
Formal searches for articles were conducted, using pre-established keywords, from 28 June 2021 to 9 July 2021 (Supplementary Table S1). A pre-screening review was conducted by the first author (TB) to eliminate articles that did not satisfy the inclusion criteria. Articles not eliminated during the pre-screening review then underwent an in-depth review. Each included manuscript was fully and independently evaluated by two out of the three reviewers (TB, ER, and MN). Articles were divided into three groups with a similar number of articles. Each group of articles was reviewed by one of the three unique pairings of reviewers, resulting in each reviewer assessing the risk of bias and quality of approximately two-thirds of the articles. The revised National Heart, Lung, and Blood Institute score system was used to assess the risk of bias. Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was used to assess the quality of the articles. In the event of significant score discrepancies between the first two reviewers, the third reviewer independently evaluated the study and served as the adjudicator.
The reviewers independently identified the biomarkers that were investigated in each study. All types of biomarkers regardless of their biochemical specificity and the methodology applied to measure them were included in the study. However, only biomarkers that appeared in four or more studies were included in the meta-analysis. Studies that investigated surgical and non-surgical populations were included in the study. Differences between the means in serum concentration at hospital admission between patients who did and did not develop delirium during hospitalization were our primary outcome for the meta-analysis. Only the serum concentration at hospital admission was used to conduct the meta-analysis although studies reported serum concentrations at other timelines such as post-surgery and on day 1 or day 2 after hospital admission, for example. The weights and heterogeneity of the manuscripts were calculated using inverse covariance with a random-effects model. A p-value of < 0.1 for the chi-square test was considered to be statistically significant. Heterogeneity was evaluated using the Higgins metric (I2), where I2 > 75% was considered as having significant heterogeneity, I2 of 40–74% was considered moderate heterogeneity, and I2 < 39% was considered no heterogeneity. If high heterogeneity was found, a subgroup analysis was performed to investigate the cause of high heterogeneity. All statistical analyses were performed using the Review Manager software [RevMan, Version 5.4.1, The Cochrane Collaboration, (11)].
The formal searches identified 1, 265 manuscripts, of which 65 were selected after applying the inclusion and exclusion criteria. Fifty-five manuscripts were included as part of the systematic review after excluding duplicates (Table 1). All studies selected were observational/cohort, except one study that was interventional. Our assessment showed that all studies had a low risk of selection bias. The risk of performance bias (inadvertently introducing differences between groups) was assessed as high in 55%, unclear in 12%, and low in 33% of the studies. The risk of detection bias (difference in how to measure outcomes between groups) was assessed as high in 25%, and low in 75% of the studies. The risk of attribution bias (the tendency to favor one group as a result of individual beliefs) was assessed as low for all studies. The risk of reporting bias was high in 36% and low in 64% of the studies. For the 55 studies selected, quality assessment scored 15 (27%) as “good,” 36 (65%) as “fair,” and 4 (7%) as “poor.” The most common population studied was post-orthopedic surgical patients (19 publications), followed by cardiovascular surgical patients (14), ICU patients (12), and general-ward patients (10).
Sixty-seven biomarkers were investigated within the 55 studies selected (Supplementary Table S2). Twenty-two studies investigated the association of IL-6 and delirium, which made it the most investigated biomarker. The second-most investigated biomarker was C-reactive protein (CRP) with 18 studies. S100β was the third-most investigated biomarker, with 12 studies, followed by cortisol, with 10 studies.
Ten biomarkers were investigated in four or more studies and were included in the meta-analysis. Analyses of NSE, TNFα, IL-1β, IL-8, and IL-10 serum concentrations at hospital admission showed no statistically significant differences when comparing patients who did and did not develop delirium during hospitalization. These are reported in the Supplemental material. Analyses of the other five biomarkers [cortisol, CRP, IL-6, S100 β and acetylcholinesterase (AchE)] are summarized below.
Twenty-two studies conducted multi-regression analyses to investigate the possible independence of the variable of interest (Table 2). The most common confounding variables included in the multi-regression analyses were age, sex, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, intubation, living alone, physical restraint, alcohol consumption, smoking, type of medical condition, hospital length of stay before ICU admission, American Society of Anesthesiologists Preoperative score (ASA score), type of surgery, sequential organ failure assessment (SOFA) score, and the existence of cognitive impairment pre-hospitalization. The most common delirium assessment tool used in the studies was the confusion assessment method-intensive care unit (CAM-ICU). Fifty-one out of fifty-five studies used CAM-ICU to assess delirium (Table 1). The most common cognitive test used to assess cognitive impairment was the Mini-Mental State Examination with 15 studies, followed by the Informant Questionnaire on Cognitive Decline with seven studies. Cognition was assessed as part of the exclusion criteria before study enrollment in 16 studies, excluding patients who had lower cognitive scores. In six studies, a cognitive test was included to investigate whether lower cognitive scores would be associated with a greater risk of delirium.
Twenty-two studies investigated the association between IL-6 serum concentration at hospital admission and delirium during hospitalization (8, 13, 14, 16–18, 20, 21, 23, 25, 31, 37, 39, 42, 47, 48, 51, 52, 54, 57, 58, 60). Mean IL-6 serum concentrations at hospital admission were reported in 17 studies, with a difference between the means of 24.05 pg/ml greater in patients who developed delirium than in those who did not (8, 14, 17, 20, 21, 23, 25, 31, 37, 39, 47, 48, 51, 54, 57, 58, 60). High heterogeneity was observed between these 17 studies, with a p-value of < 0.00001 for chi-square, I2 of 98%, and p < 0.00001 for the total overall effect (Figure 1). The standard mean difference between patients who developed delirium and those who did not was 1.18 pg/ml, with a 95% CI [0.73, 1.63] with a p-value of < 0.0001 for chi-square, I2 of 95%, and p < 0.0001 for the total overall effect. A subgroup analysis identified that 5 of the 22 studies found IL-6 serum concentration at hospital admission to be an independent variable for an increased likelihood of developing delirium, with a difference between the means of 29.66 pg/ml greater in patients who developed delirium than in those who did not (25, 34, 57, 60, 67). High heterogeneity was also observed between studies reporting mean serum concentration of IL-6 that controlled for illness, with p < 0.00001 for chi-square, I2 of 94%, and p < 0.0006 for the total overall effect. A subgroup analysis that included exclusively post-surgical patients still showed high heterogeneity between the studies and a mean difference in serum concentration of IL-6 at hospital admission between patients who developed delirium and those who did not was 29.04 pg/ml, p = 0.0001 for chi-square, I2 of 82%, and p = 0.0002 for the total overall effect (Supplementary Figure S1) (17, 48, 52, 58, 60).
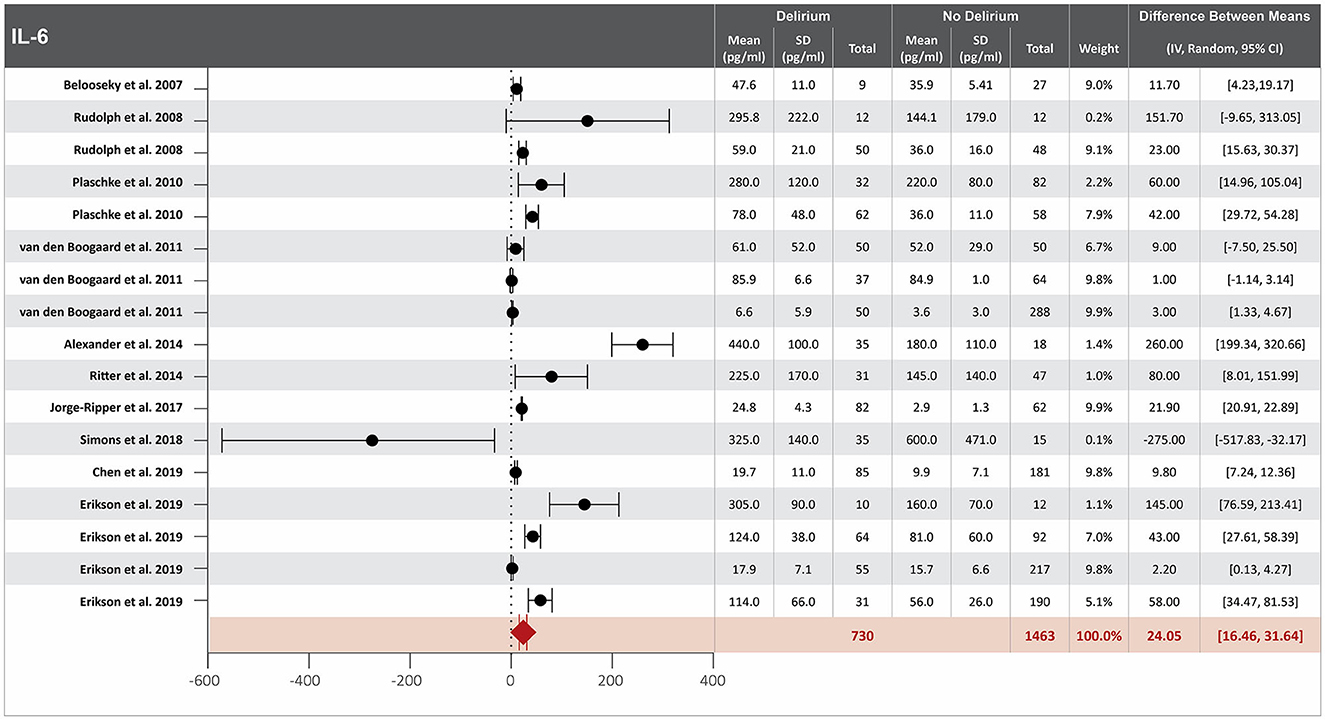
Figure 1. Forest plot showing the difference in mean serum concentration of interleukin-6 (IL-6) at hospital admission between patients who did and did not develop delirium during hospitalization. The difference between the means was 24.05 pg/ml and the heterogeneity between the studies with a Tau2 of 151.47, Chi2 of 832.4, a df of 16 with a p < 0.00001, and I2 98%, Z of 6.21 with a p < 0.00001 for total overall effect.
Eighteen studies investigated the association between CRP serum concentration at hospital admission and delirium during hospitalization (10, 13, 17, 19, 20, 23, 25, 35, 38, 41, 46, 47, 49, 50, 55, 57, 63, 65). Mean CRP serum concentrations at hospital admission were reported in 15 studies, with a difference of 41.39 mg/L between the means that is greater in patients who developed delirium than in those who did not (10, 13, 17, 19, 20, 23, 25, 35, 38, 41, 49, 50, 55, 57, 65). High heterogeneity was observed between these 15 studies, with a p-value of < 0.00001 for chi-square, I2 of 98%, and p < 0.00001 for the total overall effect (Figure 2). The standard mean difference between patients who developed delirium and those who did not was 1.58 mg/L, with a 95% CI [0.79, 2.37] with a p-value of < 0.0001 for chi-square, I2 of 98%, and p < 0.0001 for the total overall effect. A subgroup analysis identified that nine of the eighteen studies found CRP serum concentration at hospital admission to be an independent variable for increased likelihood of delirium, with a difference of 46.65 mg/L between the means that is greater in patients who developed delirium than in those who did not (10, 17, 19, 20, 35, 41, 49, 50, 65). High heterogeneity was also observed between studies reporting mean CRP serum concentration that controlled for illness, with a p-value of < 0.00001 for chi-square, I2 of 98%, and a p-value of < 0.00001 for the total overall effect. A subgroup analysis that included exclusively post-surgical patients showed moderate heterogeneity between the studies and a mean difference in serum concentration of CRP at hospital admission between patients who developed delirium and those who did not was 11.93 mg/L, with a p-value of 0.03 for chi-square, I2 of 67%, and a p-value of 0.0006 for the total overall effect (Supplementary Figure S2) (20, 46, 47, 49, 55).
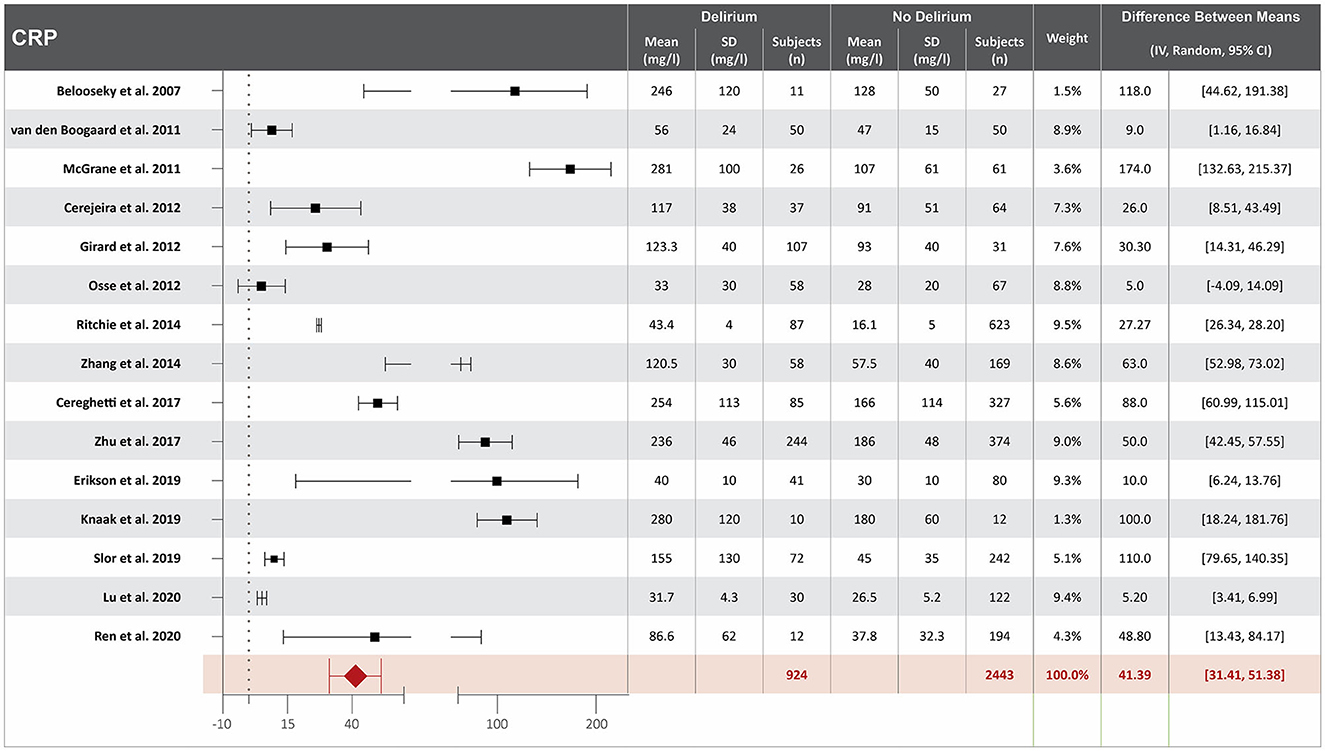
Figure 2. Forest plot showing the difference in mean serum concentration of C-reactive protein (CRP) at hospital admission between patients who did and did not develop delirium during hospitalization. The difference between the means was 41.39/mg/l and the heterogeneity between the studies with a Tau2 of 273.96, Chi2 of 759.6, a df of 14 with a p < 0.00001, and I2 98%, Z of 8.13 with a p < 0.00001 for total overall effect.
Twelve studies investigated the association between S100β serum concentration at hospital admission and delirium during hospitalization (8, 21, 25, 28, 31, 34, 36, 59, 61, 63, 64, 66). Mean S100 β serum concentrations at hospital admission were reported in eight studies, with a difference of 0.07 ng/ml between the means that is greater in patients who developed delirium than in those who did not. High heterogeneity was observed between these eight studies, with a p-value of < 0.00001 for chi-square, I2 of 96%, and a p-value of < 0.00001 for the total overall effect (Figure 3) (25, 28, 31, 36, 59, 61, 64, 66). The standard mean difference between patients who developed delirium and those who did not was 1.30 ng/ml, with a 95% CI [0.70, 1.89] with a p-value of < 0.0001 for chi-square, I2 of 95%, and a p-value of < 0.0001 for the total overall effect. A subgroup analysis identified that eight of the twelve studies found S100β serum concentration at hospital admission to be an independent variable for increased likelihood of delirium, with a difference between the means of 0.05 ng/ml greater in patients who developed delirium than in those who did not (25, 28, 31, 36, 59, 61, 64, 66). High heterogeneity was also observed between studies reporting mean S100 β serum concentration that controlled for illness, with a p-value of < 0.00001 for chi-square, I2 of 97%, and a p-value of < 0.0001 for the total overall effect. A subgroup analysis that included exclusively post-surgical patients showed no heterogeneity between the studies and a mean difference in serum concentration of S100β at hospital admission between patients who developed delirium and those who did not was 0.04 ng/ml, a p-value of 0.76 for chi-square, I2 of 0%, and a p-value of < 0.00001 for the total overall effect (Supplementary Figure S3) (36, 59, 61).
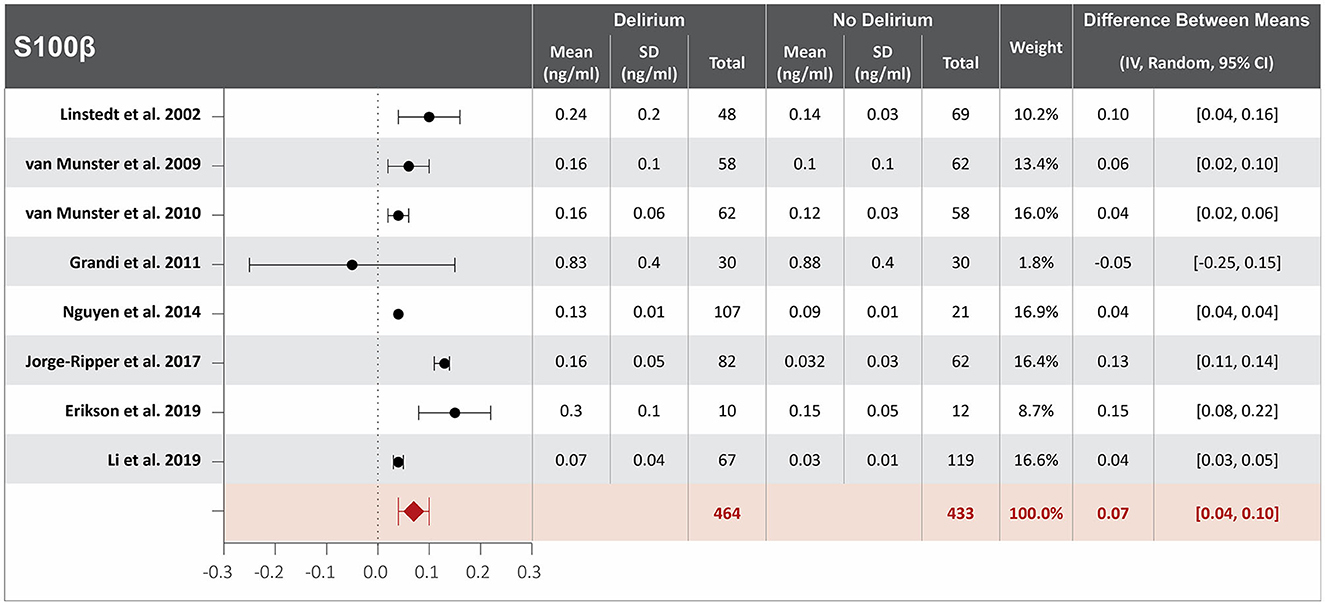
Figure 3. Forest plot showing the difference in mean serum concentration of calcium-binding protein S-100β at hospital admission between patients who did and did not develop delirium during hospitalization. The difference between the means was 0.07 ng/ml and the heterogeneity between the studies with a Tau2 of 0.00, Chi2 of 169.99, a df of 7 with a p < 0.00001, and I2 96%, Z of 4.84 with a p < 0.00001 for total overall effect.
Ten studies investigated the association between cortisol serum concentration at hospital admission and delirium during hospitalization (20, 22, 32, 33, 40, 43, 48, 57, 60, 64). Mean cortisol serum concentrations at hospital admission were reported in eight studies, with a difference of 3.36 ng/ml between the means that is greater in patients who developed delirium than in those who did not (20, 22, 32, 40, 48, 57, 60, 64). High heterogeneity was observed between studies that reported mean serum concentration of cortisol, with a p-value of < 0.00001 for chi-square, I2 of 93%, and a p-value of < 0.0001 for the total overall effect (Figure 4). The standard means difference between patients who developed delirium and those who did not was 1.55 ng/ml, with a 95% CI [0.62, 2.48] with a p-value of < 0.0001 for chi-square, I2 of 97%, and a p-value of p = 0.001 for the total overall effect. A subgroup analysis identified that four of the ten studies found cortisol serum concentration at hospital admission to be an independent variable for increased likelihood of delirium, with a difference of 3.32 ng/ml between the means that is greater in patients who developed delirium than in those who did not (22, 40, 43, 48). High heterogeneity was also observed between studies reporting mean cortisol serum concentration that controlled for illness, with a p-value of < 0.00001 for chi-square, I2 of 91%, and a p-value of < 0.0001 for the total overall effect. A subgroup analysis that included exclusively post-surgical patients showed moderate heterogeneity between the studies and a mean difference in serum concentration of cortisol at hospital admission between patients who developed delirium and those who did not was 2.02 ng/ml, a p-value of 0.12 for chi-square, I2 of 52%, and a p-value of 0.006 for the total overall effect (Supplementary Figure S4) (32, 40, 60).
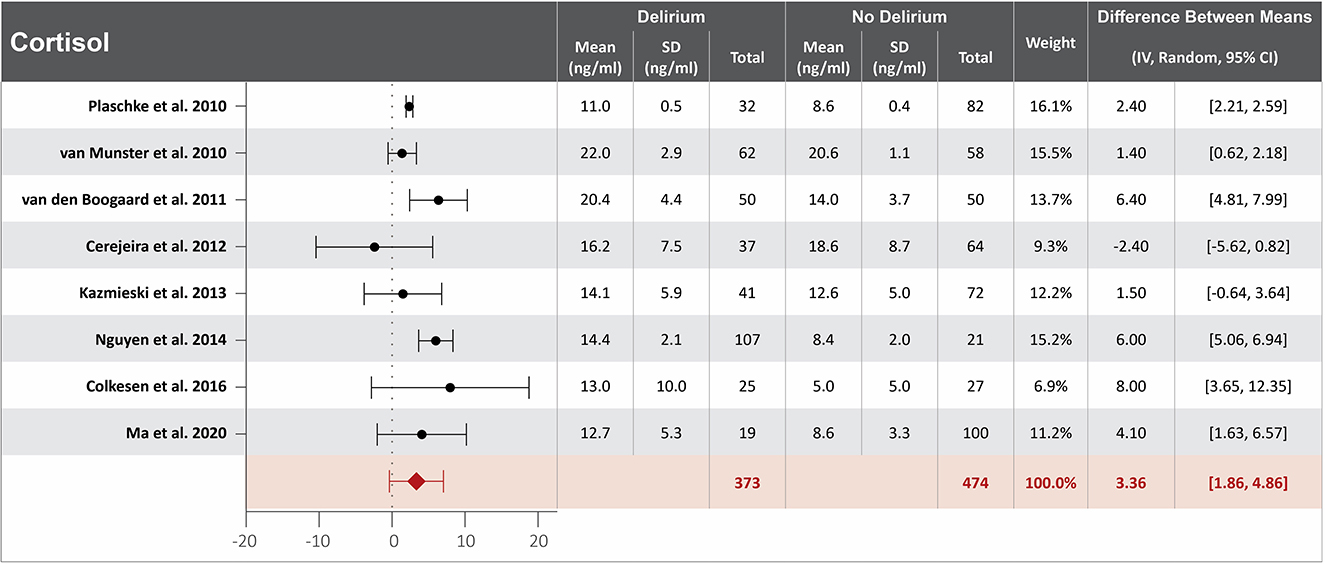
Figure 4. Forest plot showing the difference in mean serum concentration of cortisol at hospital admission between patients who did and did not develop delirium during hospitalization. The difference between the means was 3.36 ng/ml and the heterogeneity between the studies with a Tau2 of 3.62, Chi2 of 102.02, a df of 7 with a p < 0.00001, and I2 93%, Z of 4.39 with a p < 0.00001 for total overall effect.
Seven studies investigated the association between acetylcholinesterase (AchE) serum concentration at hospital admission and delirium during hospitalization (12, 20, 29, 40, 44, 56, 62). Mean AchE serum concentrations at hospital admission were reported in all seven studies, with a difference of 0.86 U/ml between the means that is lower in patients who developed delirium than in those who did not. High heterogeneity was observed between these seven studies, with a p-value of < 0.0001 for chi-square, I2 of 98%, and a p-value of 0.04 for the total overall effect (Figure 5) (12, 20, 29, 40, 44, 56, 62). The standard means difference between patients who developed delirium and those who did not was −0.50 U/ml, with a 95% CI [−1.18, 0.19], a p-value of < 0.0001 for chi-square, I2 of 96%, and a p-value of 0.16 for the total overall effect. A subgroup analysis identified that three of the seven studies found AchE serum concentration at hospital admission to be an independent variable for identifying those patients with an increased likelihood of developing delirium, with a difference of 1.4 U/ml between the means, which was lower in patients who developed delirium than in those who did not (12, 20, 40). High heterogeneity was also observed between studies reporting mean AchE serum concentration that controlled for illness, with a p-value of < 0.00001 for chi-square, I2 of 98%, and a p-value of < 0.02 for the total overall effect. A subgroup analysis that included exclusively post-surgical patients still showed high heterogeneity between the studies and a mean difference in serum concentration of cortisol at hospital admission between patients who developed delirium and those who did not was −0.26. U/ml, a p-value of < 0.000 1 for chi-square, I2 of 99%, and a p-value of < 0.0001 for the total overall effect (Supplementary Figure S5) (12, 20, 40, 44, 62).
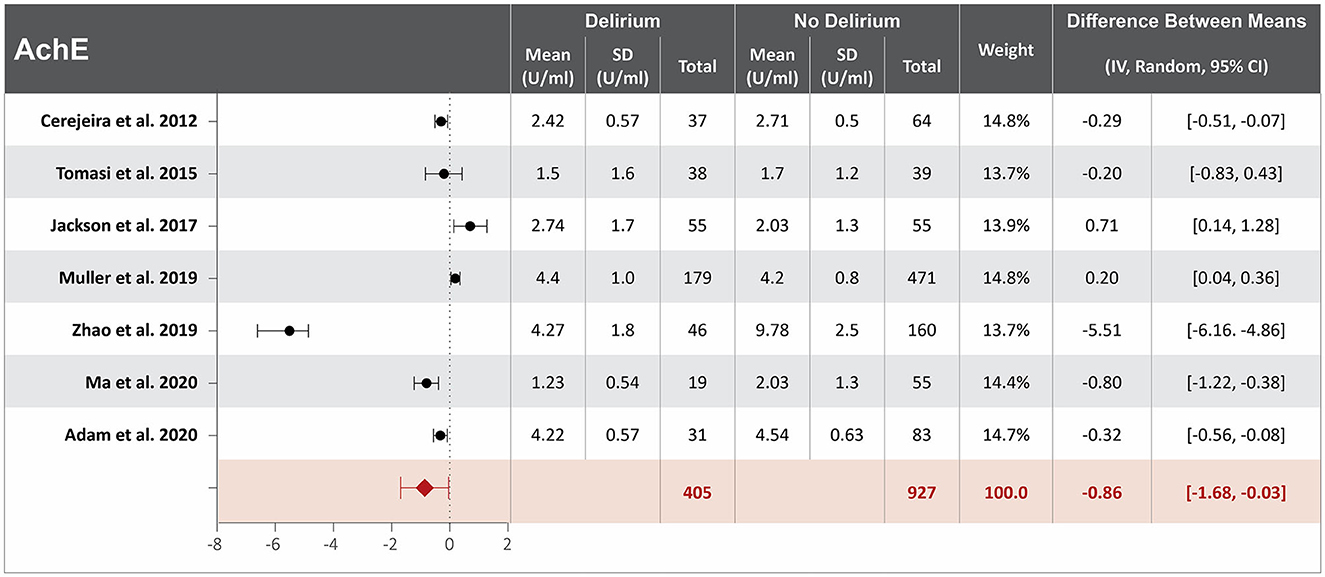
Figure 5. Forest plot showing the difference in mean serum concentration of acetylcholinesterase at hospital admission between patients who did and did not develop delirium during hospitalization. The difference between the means was −0.86 U/ml and the heterogeneity between the studies with a Tau2 of 1.19, Chi2 of 299.7, a df of 6 with a p < 0.00001, and I2 98%, Z of 2.03 with a p = 0.04 for total overall effect.
Our meta-analysis found evidence that serum concentrations, at hospital admission, of three inflammatory proteins (cortisol, CRP, and IL-6) and one biomarker for blood-brain barrier leakage (S100β) were higher in patients who subsequently developed delirium during hospitalization in comparison to those who did not. Moreover, our systematic review showed that lower serum concentration of acetylcholinesterase at hospital admission was possibly associated with an increased likelihood of developing delirium during hospitalization.
Delirium is a complex syndrome that may have multiple triggering factors (1, 8, 9). It is an enormous challenge to isolate variables that can trigger delirium. It has been demonstrated that delirium is associated with systemic inflammation, neuroinflammation, and consequently, increased BBB permeability (1, 8, 9, 68, 69). Preclinical experiments have shown that systemic inflammation, characterized by an increased serum concentration of IL-6, induces neuroinflammation and BBB breakdown (45, 70, 71). Moreover, neuroinflammation has been demonstrated to lead to neurotransmitter imbalance and possibly cognitive dysfunction (72). For instance, dopamine overexpression is associated with microglia priming and neuronal apoptosis, which are associated with working-memory dysfunction (72).
Many factors can trigger neuroinflammation, and systemic inflammation is one of those factors (72). C-reactive protein is considered a systemic inflammatory biomarker that has been linked to increased BBB permeability (65). Our systematic review identified nine studies that showed elevated CRP serum concentrations at hospital admission as a predictor of delirium. For example, Zhang et al. (65) investigated 223 ICU patients, showing that those who developed delirium had higher CRP serum concentration compared to those who did not develop delirium during hospitalization (120.5 vs. 57.5 mg/L) (65). Moreover, the same study showed that after adjusting for confounding variables, including age, sex, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, intubation, living alone, physical restraint, alcohol consumption, smoking, type of medical condition, and hospital length of stay before ICU admission, in a logistic regression model, elevated CRP remained an independent predictor of delirium (odds ratio, 1.07; 95% confidence interval, 1.01–1.15) (65). Our systematic review identified 17 studies that showed elevated IL-6 serum concentrations as a predictor of delirium. For instance, Erikson et al. (65) found that patients who developed delirium during hospitalization had greater IL-6 serum concentration compared to those who did not, 138.3 pg/ml (28.0–297.7) vs. 53.6 pg/ml (109.3–505.0), respectively (65). Thus, there is a strong signal that elevated serum concentration of inflammatory markers may be a predisposing factor to increase patient vulnerability to developing delirium during hospitalization. This is supported by greater serum concentrations of CRP and IL-6 in patients who developed delirium during hospitalization (65, 73, 74).
Preclinical and clinical studies have shown that increased BBB permeability is associated with greater S100β serum concentration (75). Increased BBB permeability facilitates the entry of inflammatory proteins into the central nervous system, promoting neuroinflammation (75). It is reasonable to argue that the brains of patients who have increased BBB permeability are more vulnerable to the effects of systemic inflammation when compared to patients with intact BBBs. For these vulnerable patients, events that normally would not trigger neuroinflammation, and consequently cognitive dysfunction, might initiate neuroinflammation resulting in cognitive dysfunction due to this increased BBB permeability. Erikson et al. (65) showed a positive, linear, and moderate correlation between S100β and IL-6 serum concentrations (r = 0.489, p = 0.021) which supports the argument of a link between systemic inflammation and BBB permeability (65). Our systematic review identified 12 studies that investigated S100β serum concentration as a predictor for delirium during hospitalization, eight of which controlled for illness, and six out of these eight controlled for cognitive impairment before hospitalization (8, 21, 25, 28, 31, 34, 36, 59, 61, 63, 64, 66). For example, van Munster et al. (60) investigated 120 patients, showing that S100β serum concentration was an independent variable for predicting delirium after a multi-regression analysis, controlling for illness, and even including pre-existing cognitive impairment (60). Moreover, a subgroup analysis identified that surgical patients who developed delirium during hospitalization had a mean difference in S100β serum concentration at hospital admission of 0.04 ng/ml greater than surgical patients who did not develop delirium during hospitalization.
Hypothalamic-pituitary axis (HPA) dysfunction is another factor that has been proposed to increase patients' vulnerability to developing delirium during hospitalization (1). Persistent high serum concentration of cortisol has been proposed as indicators of HPA dysfunction, both of which make patients more vulnerable to systemic inflammation due to the inability of the body to properly respond to inflammation (1). Moreover, HPA dysfunction has been shown to be associated with changes in the brain's neurophysiology and consequently in the homeostasis of dopamine (1). Our systematic review showed that higher serum concentrations of cortisol and lower serum concentrations of acetylcholinesterase at hospital admission were each associated with a greater likelihood of developing delirium during hospitalization. For instance, Ma et al. (40) investigated 119 patients, showing that after isolating patient illness severity, patients who developed delirium during hospitalization had higher serum concentrations of cortisol and lower serum concentrations of acetylcholinesterase compared to patients who did not develop delirium during hospitalization (40).
Greater serum concentrations of IL-6, CRP, and cortisol can be used as markers of systemic inflammation (22, 32, 33, 43, 60, 64, 76). Chronic inflammation has been linked to BBB dysfunction (70, 71, 75, 77). It is unknown whether greater S100β serum concentration could be a result of chronic inflammation, increasing patients' vulnerability to develop delirium, or greater S100β serum concentration might be the cause for increased vulnerability to delirium (24, 46, 54). It is also unknown whether chronic inflammation could also modulate acetylcholinesterase activity.
Studies have shown an association between age, chronic use of anticholinergic drugs, and lower serum concentration of acetylcholinesterase (1, 78, 79). The cholinergic system is vital to modulate the neurotransmitter balance in the brain (78). Thus, it has been argued that an impaired cholinergic system, leading to neurotransmitter unbalance might increase the patient's vulnerability to developing delirium. It has been shown that the inhibition of the postsynaptic cholinergic receptors is associated with delirium-like symptoms such as confusion, acute cognitive impairment, and lack of attention (1). Another reason for a decline in acetylcholinergic receptors concentration is aging, which also could result in a reduction of the acetylcholinesterase activity leading to greater vulnerability to developing delirium in this population (1, 78, 79). It is important to highlight that studies that control for age and cognitive impairment pre-hospitalization have shown an association between lower serum concentration of acetylcholinesterase and greater risk for delirium during hospitalization (1, 29, 40, 56). However, these studies have not controlled for the use of anticholinergic medications pre-hospitalization. Combining all the data together, it can be hypothesized that greater vulnerability to developing delirium during hospitalization might be an effect of chronic inflammation, lower acetylcholinergic activity, and BBB dysfunction, and all of these factors might be potentiated by aging.
More studies should focus on neuroinflammation and delirium, investigating neuroinflammation as a potentiator of delirium. For example, in our search only three studies investigated the association of biomarkers for microglia priming (neopterin) and delirium, indicating that more studies focusing on microglia-priming markers are needed (24, 46, 54).
Moreover, more studies focusing on the pathophysiology of delirium to identify any common pathways to developing delirium during hospitalization should also be conducted. For instance, studies should investigate chronic inflammation and possible modulation of acetylcholinesterase activity. Finally, a prospective study that tests the predictive ability of a test panel comprised of the identified biomarkers and their respective thresholds to predict delirium would be truly important for this research field.
Our research has some limitations. First, most of the studies selected by our systematic review showed high heterogeneity, demonstrating high variability in any signals indicating that inflammatory markers, BBB permeability, HPA dysfunction, and cholinergic burden at hospital admission might be associated with a greater likelihood of developing delirium during hospitalization. Another limitation is the high heterogenicity of the population analyzed since we have pooled critically ill patients, general-ward patients, and post-surgical procedure patients into the same analysis. In a highly variable population, the effect of inflammation and neurotransmitter imbalance pre-hospitalization could be an important confounder affecting the serum concentrations of the biomarkers analyzed. Despite that, our meta-analysis found an association between elevated mean serum concentrations of inflammatory markers and neurotransmitter imbalances at hospital admission and the development of delirium during hospitalization. The consistency of these results across a highly variable population increases the translatability of our findings because, in a real clinical scenario, patients have a high variability of diseases and conditions. More studies focusing on biomarkers for neurotransmitter imbalances before hospitalization and the likelihood of developing delirium during hospitalization are needed, such as but not limited to, the overexpression of dopamine. The findings of this systematic review and meta-analysis should be interpreted within the context of the included studies.
Our meta-analysis indicates that greater serum concentrations of pro-inflammatory proteins (cortisol, C-reactive protein, and IL-6) at hospital admission are associated with a greater likelihood of patients developing delirium when compared to patients with lower serum concentrations of pro-inflammatory proteins at hospital admission, and this is independent of the severity of illness. Additionally, a greater serum concentration of S100β at hospital admission is associated with a greater likelihood to develop delirium during hospitalization. A lower serum concentration of acetylcholinesterase was also associated with an increased vulnerability to developing delirium during hospitalization. Our meta-analysis found a signal that patients with hypothalamic-pituitary axis dysfunction, increased blood-brain barrier permeability, and chronic overload of the cholinergic system, at hospital admission, are more vulnerable to developing delirium during hospitalization. Gaps in the literature were identified, such as the small number of studies investigating biomarkers for neurotransmitter imbalance and microglia priming pre-hospitalization.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
TB was responsible for the hypothesis generation and conception of this study. TB and SR contributed to the study design and data interpretation. TB, ER, and SR were responsible for writing the article. TB, ER, and MN performed data acquisition and conducted data analysis. All authors have agreed with the final version of the manuscript before submission.
Funding was provided by the Lungpacer Medical Inc., Royal Columbian Hospital Foundation, TB Vets Foundation, BC Lung Foundation, and MITACS.
TB was employed by the company Lungpacer Medical Inc. ER, MN, and SR are paid consultants for Lungpacer Medical Inc.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1179243/full#supplementary-material
1. Cunningham C, MacLullich AMJ. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun. (2013) 28:1–13. doi: 10.1016/j.bbi.2012.07.012
2. Bassi TG, Rohrs EC, Reynolds SC. Systematic review of cognitive impairment and brain insult after mechanical ventilation. Crit Care. (2021) 25:99. doi: 10.1186/s13054-021-03521-9
3. Kiely DK, Marcantonio ER, Inouye SK, Shaffer ML, Bergmann MA, Yang FM, et al. Persistent delirium predicts greater mortality. J Am Geriatr Soc. (2009) 57:55–61. doi: 10.1111/j.1532-5415.2008.02092.x
4. da Silva Machado AS, Alves MRT, Vieira DN, et al. Occurrence of Delirium and Length of Stay of Patients in the Intensive Care Unit. J Biosci Med. (2021) 09:1–9. doi: 10.4236/jbm.2021.98001
5. Sánchez-Hurtado LA, Hernández-Sánchez N, Moral-Armengol MD, Guevara-García H, García-Guillén FJ, Herrera-Gómez Á, et al. Incidence of delirium in critically ill cancer patients. Pain Res Manag. (2018) 2018:275. doi: 10.1155/2018/4193275
6. Vasilevskis EE, Chandrasekhar R, Holtze CH, Graves J, Speroff T, Girard TD, et al. The cost of ICU delirium and coma in the intensive care unit patient. Med Care. (2018) 56:890–7. doi: 10.1097/MLR.0000000000000975
7. van Munster BC. Delirium: a synthesis of current knowledge clinical medicine. J Royal College Phys London. (2014) 14:192–5. doi: 10.7861/clinmedicine.14-2-192
8. McNeil JB, Hughes CG, Girard T, Ware LB, Ely EW, Chandrasekhar R, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS ONE. (2019) 14:12. doi: 10.1371/journal.pone.0226412
9. Girard TD, Self WH, Edwards KM. Long-term cognitive impairment after hospitalization for community-acquired pneumonia: a prospective cohort study. J Gen Intern Med. (2018) 33:929–35. doi: 10.1007/s11606-017-4301-x
10. Girard TD, Ware LB, Bernard GR, Pandharipande PP, Thompson JL, Shintani AK, et al. Associations of markers of inflammation and coagulation with delirium during critical illness. Intensive Care Med. (2012) 38:1965–73. doi: 10.1007/s00134-012-2678-x
11. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane (2022). Available online at: www.training.cochrane.org/handbook
12. Adam EH, Haas V, Lindau S, Zacharowski K, Scheller B. Cholinesterase alterations in delirium after cardiosurgery: A German monocentric prospective study. BMJ Open. (2020) 10:1212. doi: 10.1136/bmjopen-2019-031212
13. Adamis D, Treloar A, Martin FC, Gregson N, Hamilton G, Macdonald AJD, et al. and cytokines as biological markers for recovery of prevalent delirium in elderly medical inpatients. Int J Geriatr Psychiatry. (2007) 22:688–94. doi: 10.1002/gps.1732
14. Alexander SA, Ren D, Gunn SR, et al. Interleukin 6 and apolipoprotein e as predictors of acute brain dysfunction and survival in critical care patients. Am J Critical Care. (2014) 23:49–57. doi: 10.4037/ajcc2014578
15. Anderson BJ, Reilly JP, Shashaty MGS, Palakshapa JA, Wysoczanski A, Dunn TG, et al. Admission plasma levels of the neuronal injury marker neuron-specific enolase are associated with mortality and delirium in sepsis. J Crit Care. (2016) 36:18–23. doi: 10.1016/j.jcrc.2016.06.012
16. Ballweg F. Association between plasma tau and postoperative deliriumincidence and severity: a prospective observational study. Br J Anaesth. (2021) 126:458–66. doi: 10.1016/j.bja.2020.08.061
17. Belooseky Y, Hendel D, Weiss A, Hershkovitz A, Grinblat J, Pirotsky A, et al. Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. J Gerontol Med Sci. (2007). 62A:420–6. doi: 10.1093/gerona/62.4.420
18. Casey CP, Lindroth H, Mohanty R, Farahbakhsh Z, Ballweg T, Twadell S, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. (2020) 143:47–54. doi: 10.1093/brain/awz354
19. Cereghetti C, Siegemund M, Schaedelin S. Independent predictors of the duration and overall burden of postoperative delirium after cardiac surgery in adults: an observational cohort study. J Cardiothorac Vasc Anesth. (2017) 31:1966–73. doi: 10.1053/j.jvca.2017.03.042
20. Cerejeira JMS, Nogueira V, Luís P, Vaz-Serra A, Mukaetova-Ladinska EB. The cholinergic system and inflammation: Common pathways in delirium pathophysiology. J Am Geriatr Soc. (2012) 60:669–75. doi: 10.1111/j.1532-5415.2011.03883.x
21. Chen Y, Lu S, Wu Y, Shen Y, Zhao H, Ding S, et al. Change in serum level of interleukin 6 and delirium after coronary artery bypass graft. Am J Critical Care. (2019) 28:462–70. doi: 10.4037/ajcc2019976
22. Colkesen Y, Giray S, Ozenli Y, Sezgin N, Coskun I. Relation of serum cortisol to delirium occurring after acute coronary syndromes. Am J Emerg Med. (2013) 31:161–5. doi: 10.1016/j.ajem.2012.07.001
23. de Rooij SE, van Munster BC, Korevaar JC, Levi M. Cytokines and acute phase response in delirium. J Psychosom Res. (2007) 62:521–5. doi: 10.1016/j.jpsychores.2006.11.013
24. Egberts A, Osse RJ, Fekkes D, Tulen JHM, van der Cammen TJM, Mattace-Raso FUS. Differences in potential biomarkers of delirium between acutely ill medical and elective cardiac surgery patients. Clin Interv Aging. (2019) 14:271–81. doi: 10.2147/CIA.S193605
25. Erikson K, Ala-Kokko TI, Koskenkari J, Liisanantti JH, Kamakura R, Herzig KH, Syrjälä H. Elevated serum S-100β in patients with septic shock is associated with delirium. Acta Anaesthesiol Scand. (2019) 63:69–73. doi: 10.1111/aas.13228
26. Fong TG, Vasunilashorn SM, Ngo L, Libermann TA, Dillon ST, Schmitt EM, et al. Association of plasma neurofilament light with postoperative delirium. Ann Neurol. (2020) 88:984–94. doi: 10.1002/ana.25889
27. Gao F, Zhang Q, Li Y, Tai Y, Xin X, Wang X, et al. Transcutaneous electrical acupoint stimulation for prevention of postoperative delirium in geriatric patients with silent lacunar infarction: a preliminary study. Clin Interv Aging. (2018) 13:2127–34. doi: 10.2147/CIA.S183698
28. Grandi C, Tomasi CD, Fernandes K. Brain-derived neurotrophic factor and neuron-specific enolase, but not S100β, levels are associated to the occurrence of delirium in intensive care unit patients. J Crit Care. (2011) 26:133–7. doi: 10.1016/j.jcrc.2010.10.006
29. Jackson TA, Moorey HC, Sheehan B, Maclullich AMJ, Gladman JR, Lord JM. Acetylcholinesterase Activity Measurement and Clinical Features of Delirium. Dement Geriatr Cogn Disord. (2017) 43:29–37. doi: 10.1159/000452832
30. Jones EL, Gauge N, Nilsen OB, Lowery D, Wesnes K, Katsaiti E, et al. Analysis of neuron-specific enolase and S100B as biomarkers of cognitive decline following surgery in older people. Dement Geriatr Cogn Disord. (2012) 34(5-6):307–11. doi: 10.1159/000345538
31. Jorge-Ripper C, Alemán MR, Ros R, et al. Prognostic value of acute delirium recovery in older adults. Geriatr Gerontol Int. (2017) 17:1161–7. doi: 10.1111/ggi.12842
32. Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Cortisol levels and neuropsychiatric diagnosis as markers of postoperative delirium: A prospective cohort study. Crit Care. (2013) 17:2. doi: 10.1186/cc12548
33. Kazmierski J, Banys A, Latek J. Mild cognitive impairment with associated inflammatory and cortisol alterations as independent risk factor for postoperative delirium. Dement Geriatr Cogn Disord. (2014) 38:65–78. doi: 10.1159/000357454
34. Khan BA, Farber MO, Campbell N. S100 calcium binding protein B as a biomarker of delirium duration in the intensive care unit - An exploratory analysis. Int J Gen Med. (2013) 6:855–61. doi: 10.2147/IJGM.S51004
35. Knaak C, Vorderwülbecke G, Spies C. C-reactive protein for risk prediction of post-operative delirium and post-operative neurocognitive disorder. Acta Anaesthesiol Scand. (2019) 63:1282–9. doi: 10.1111/aas.13441
36. Li QH, Yu L, Yu ZW. Relation of postoperative serum S100A12 levels to delirium and cognitive dysfunction occurring after hip fracture surgery in elderly patients. Brain Behav. (2019) 9:1. doi: 10.1002/brb3.1176
37. Liu P, Li Y, Wang X, Zou X, Zhang D, Wang D, et al. High serum interleukin-6 level is associated with increased risk of delirium in elderly patients after noncardiac surgery: a prospective cohort study. Chin Med J. (2013) 126:3621–7.
38. Lu GW, Chou YE, Jin WL, Su XB. Usefulness of postoperative serum translocator protein as a predictive marker for delirium after breast cancer surgery in elderly women. Journal of International Medical Research. 2020;48(6). doi: 10.1177/0300060520910044
39. Lv XC, Lin Y, Wu Q. Plasma interleukin-6 is a potential predictive biomarker for postoperative delirium among acute type a aortic dissection patients treated with open surgical repair. J Cardiothorac Surg. (2021) 16:74. doi: 10.1186/s13019-021-01529-4
40. Ma J, Fan M, Wang Z. Age, preoperative higher serum cortisol levels, and lower serum acetylcholine levels predict delirium after percutaneous coronary intervention in acute coronary syndrome patients accompanied with renal dysfunction. Indian J Psychiatry. (2020) 62:172–7. doi: 10.4103/psychiatry.IndianJPsychiatry_37_19
41. McGrane S, Girard TD, Thompson JL, et al. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care. (2011) 15:70. doi: 10.1186/cc10070
42. McKay TB, Rhee J, Colon K, Adelsberger K, Turco I, Mueller A, et al. Preliminary Study of Serum Biomarkers Associated With Delirium After Major Cardiac Surgery. J Cardiothorac Vasc Anesth Published online. (2021). doi: 10.1053/j.jvca.2021.05.002
43. Mu DL, Wang DX, Li LH. High serum cortisol level is associated with increased risk of delirium after coronary artery bypass graft surgery: a prospective cohort study. Crit Care. (2010) 14:393. doi: 10.1186/cc9393
44. Muller A, Olbert M, Heymann A. Relevance of peripheral cholinesterase activity on postoperative delirium in adult surgical patients (CESARO): A prospective observational cohort study. Eur J Anaesthesiol. (2019) 36:114–22. doi: 10.1097/EJA.0000000000000888
45. McPherson CA, Aoyama M, Harry GJ. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: Differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav Immun. (2011) 25:850–62. doi: 10.1016/j.bbi.2010.09.003
46. Osse RJ, Fekkes D, Tulen JHM. High preoperative plasma neopterin predicts delirium after cardiac surgery in older adults. J Am Geriatr Soc. (2012) 60:661–8. doi: 10.1111/j.1532-5415.2011.03885.x
47. Peng J, Wu G, Chen J, Chen H. Preoperative C-reactive protein/albumin ratio, a risk factor for postoperative delirium in elderly patients after total joint arthroplasty. J. Arthroplasty. (2019) 34:2601–5. doi: 10.1016/j.arth.2019.06.042
48. Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. (2010) 36:2081–9. doi: 10.1007/s00134-010-2004-4
49. Ren Q, Wen YZ, Wang J. Elevated level of serum c-reactive protein predicts postoperative delirium among patients receiving cervical or lumbar surgery. Biomed Res Int. (2020) 2020:148. doi: 10.1155/2020/5480148
50. Ritchie CW, Newman TH, Leurent B, Sampson EL. The association between C-reactive protein and delirium in 710 acute elderly hospital admissions. Int Psychogeriatr. (2014) 26:717–24. doi: 10.1017/S1041610213002433
51. Ritter C, Tomasi CD, Dal-Pizzol F, Pinto BB, Dyson A, de Miranda AS, et al. Inflammation biomarkers and delirium in critically ill patients. Crit Care. (2014) 18: 887. doi: 10.1186/cc13887
52. Rudolph JL, Ramlawi B, Kuchel GA, McElhaney JE, Xie D, Sellke FW, et al. Chemokines are associated with delirium after cardiac surgery. J Gerontol Med Sci. (2008) 63A:184–9. (2008). doi: 10.1093/gerona/63.2.184
53. Saller T, Petzold A, Zetterberg H, Kuhle J, Chappell D, von Dossow V, et al. A case series on the value of tau and neurofilament protein levels to predict and detect delirium in cardiac surgery patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2019) 163:241–6. doi: 10.5507/bp.2019.043
54. Simons KS, van den Boogaard M, Hendriksen E, Gerretsen J, van der Hoeven JG, Pickkers P, et al. Temporal biomarker profiles and their association with icu acquired delirium: a cohort study. Crit Care. (2018) 22:5. doi: 10.1186/s13054-018-2054-5
55. Slor CJ, Witlox J, Adamis D. The trajectory of C-reactive protein serum levels in older hip fracture patients with postoperative delirium. Int J Geriatr Psychiatry. (2019) 34:1438–46. doi: 10.1002/gps.5139
56. Tomasi CD, Salluh J, Soares M. Baseline acetylcholinesterase activity and serotonin plasma levels are not associated with delirium in critically ill patients. Rev Bras Ter Intensiva. (2015) 27:170–7. doi: 10.5935/0103-507X.20150029
57. van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. (2011) 15:R297. doi: 10.1186/cc10598
58. Van Munster BC, Korevaar JC, Zwinderman AH, Levi M, Wiersinga WJ, De Rooij SE. Time-course of cytokines during delirium in elderly patients with hip fractures. J Am Geriatr Soc. (2008) 56:1704–9. doi: 10.1111/j.1532-5415.2008.01851.x
59. van Munster BC, Korse CM, de Rooij SE, Bonfrer JM, Zwinderman AH, Korevaar JC. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC Neurol. (2009) 9:21. doi: 10.1186/1471-2377-9-21
60. van Munster BC, Bisschop PH, Zwinderman AH, et al. Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn. (2010) 74:18–23. doi: 10.1016/j.bandc.2010.05.010
61. Van Munster BC, Korevaar JC, Korse CM, Bonfrer JM, Zwinderman AH, De Rooij SE. Serum S100B in elderly patients with and without delirium. Int J Geriatr Psychiatry. (2010) 25:234–9. doi: 10.1002/gps.2326
62. Zhao B, Ni Y, Tian X. Low plasma cholinesterase activity is associated with postoperative delirium after noncardiac surgery in elderly patients: aprospective observational study. Psychosomatics. (2019) 60:190–6. doi: 10.1016/j.psym.2018.06.014
63. Zhu Y, Hu W, Zhu ML, Yin T, Su J, Wang JR. Serum galectin-3 levels and delirium among postpartum intensive care unit women. Brain Behav. (2017) 7:773. doi: 10.1002/brb3.773
64. Nguyen DN, Huyghens L, Zhang H, Schiettecatte J, Smitz J, Vincent JL. Cortisol is an associated-risk factor of brain dysfunction in patients with severe sepsis and septic shock. Biomed Res Int. (2014) 2014:742. doi: 10.1155/2014/712742
65. Zhang Z, Pan L, Deng H, Ni H, Xu X. Prediction of delirium in critically ill patients with elevated C-reactive protein. J Crit Care. (2014) 29:88–92. doi: 10.1016/j.jcrc.2013.09.002
66. Linstedt U, Meyer O, Kropp P, Berkau A, Tapp E, Zenz M. Serum concentration of S-100 protein in assessment of cognitive dysfunction after general anesthesia in different types of surgery. Acta Anaesthesiol Scand. (2002) 46:384–9. doi: 10.1034/j.1399-6576.2002.460409.x
67. Li YC Xi CH, An YF, Dong WH, Zhou M. Perioperative inflammatory response and protein S-100β concentrations? Relationship with post-operative cognitive dysfunction in elderly patients. Acta Anaesthesiol Scand. (2012) 56:595–600. doi: 10.1111/j.1399-6576.2011.02616.x
68. Pandharipande P, Jackson J, Ely EW. Delirium: Acute cognitive dysfunction in the critically ill. Curr Opin Crit Care. (2005) 11:360–8. doi: 10.1097/01.ccx.0000170503.76528.4b
69. Morandi A, Rogers BP, Gunther ML, Merkle K, Pandharipande P, Girard TD. The relationship between delirium, white matter integrity, and cognitive impairment in intensive care unit Survivors as determined by diffusion tensor imaging. Crit Care Med. (2013) 40:2182–9. doi: 10.1097/CCM.0b013e318250acdc
70. Koh SXT, Lee JKW. S100B as a marker for brain damage and blood-brain barrier disruption following exercise. Sports Med. (2014) 44:369–85. doi: 10.1007/s40279-013-0119-9
71. Daniels BP, Klein RS. Knocking on closed doors: host interferons dynamically regulate blood-brain barrier function during viral infections of the central nervous system. PLoS Pathog. (2015) 11:5096. doi: 10.1371/journal.ppat.1005096
72. Giordano G, Pugliese F, Bilotta F. Neuroinflammation, neuronal damage or cognitive impairment associated with mechanical ventilation: a systematic review of evidence from animal studies. J Crit Care. (2021) 62:246–55. doi: 10.1016/j.jcrc.2020.12.017
73. Anwar F, Sparrow NA, Rashid MH, et al. Systemic interleukin-6 inhibition ameliorates acute neuropsychiatric phenotypes in a murine model of acute lung injury. Crit Care. (2022) 26:274. doi: 10.1186/s13054-022-04159-x
74. Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. (2012) 8:1254–66. doi: 10.7150/ijbs.4679
75. Marchi N, Rasmussen P, Janigro D. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor Neurol Neurosci. (2003) 21:109–21.
76. Troubat R, Barone P, Leman S. Neuroinflammation and depression: A review. Eur J Neurosci. (2021) 53:151–71. doi: 10.1111/ejn.14720
77. Blyth BJ, Farhavar A, Gee. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J Neurotrauma. (2009) 26:1497–507. doi: 10.1089/neu.2008.0738
78. van Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, de Rooij SEJA. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res. (2011) 14:615–22. doi: 10.1089/rej.2011.1185
Keywords: inflammation, hypothalamic-pituitary axis dysfunction, delirium, biomarkers, cholinergic burden
Citation: Bassi T, Rohrs E, Nicholas M and Reynolds S (2023) Meta-analysis of serological biomarkers at hospital admission for the likelihood of developing delirium during hospitalization. Front. Neurol. 14:1179243. doi: 10.3389/fneur.2023.1179243
Received: 03 March 2023; Accepted: 11 May 2023;
Published: 09 June 2023.
Edited by:
Prem Kandiah, Emory University Hospital, United StatesReviewed by:
Jakub Kazmierski, Medical University of Lodz, PolandCopyright © 2023 Bassi, Rohrs, Nicholas and Reynolds. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thiago Bassi, dGJhc3NpQGx1bmdwYWNlci5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.