
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 05 July 2023
Sec. Neurological Biomarkers
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1172488
 Huaping Du1†
Huaping Du1† Tingting Guo1†
Tingting Guo1† Huan Ye1
Huan Ye1 Yingshi Bao1
Yingshi Bao1 Zhuoyin Qiu1
Zhuoyin Qiu1 Yaming Sun2
Yaming Sun2 Shoujiang You3
Shoujiang You3 Yuan Liu1
Yuan Liu1 Yuan Xu1
Yuan Xu1 Chunqing Zhang3*
Chunqing Zhang3* Chunfang Qiu1*
Chunfang Qiu1*Purpose: An elevated concentration of phosphorus is associated with an increased risk of atherosclerosis and cardiovascular diseases. Common carotid artery intima–media thickness (cIMT) is an imaging marker of atherosclerosis. However, data on the relationship between phosphorus and cIMT in ischemic stroke are scarce. We aimed to evaluate the association between serum phosphorus levels and cIMT in patients who had experienced ischemic stroke.
Patients and methods: A total of 1,450 ischemic stroke patients were enrolled. Participants were divided into four groups (quartiles) according to baseline serum phosphorus level. Carotid atherosclerosis was identified by measurement of cIMT; abnormal cIMT was defined as a maximum cIMT or mean cIMT ≥ 1 mm. Multivariable logistic regression models were used to assess the association between serum phosphorus level and the presence of abnormal cIMT.
Results: In the multivariable adjusted analysis, falling into the highest quartile for serum phosphorus (Q4) was associated with a 2.00-fold increased risk of having abnormal maximum cIMT [adjusted odds ratio (OR) 2.00; 95% confidence interval (CI) 1.44–2.79] and a 1.76-fold increased risk of having abnormal mean cIMT (adjusted OR 1.76; 95% CI 1.22–2.53) in comparison to Q1. Furthermore, the association between serum phosphorus and abnormal cIMT was confirmed in analyses treating serum phosphorus as a continuous variable and in subgroup analyses.
Conclusion: In acute ischemic stroke patients, baseline elevated serum phosphorus level was found to be independently associated with carotid atherosclerosis, as measured by cIMT.
Recent epidemiological data indicate that stroke remains the leading cause of death and disability in Chinese adults. Ischemic stroke, the most common type, accounts for 80% of all strokes. Atherosclerosis is a significant cause of ischemic stroke and is linked to an increased risk of recurrent stroke (1). Common carotid artery intima–media thickness (cIMT) is a recognized subclinical imaging marker of carotid atherosclerosis, with increased cIMT being associated with an elevated risk of cardiovascular disease (CVD), including stroke (2). Limited information is available on the determinants of atherosclerosis in Asian populations, and phosphorus may be an independent determinant; this was investigated in this study.
Serum phosphorus is widely distributed and plays a leading role in many biological processes, including cell signaling, bone metabolism, and muscle activity (3). Elevated phosphorus concentration is associated with an increased risk of CVD and all-cause mortality, as it triggers multiple pathophysiological processes, particularly in individuals with abnormal renal function (4, 5). Moreover, there is a positive association between higher serum phosphorus levels and cIMT, even among asymptomatic young adults (6, 7). Carotid atherosclerosis is a disease commonly associated with aging and is particularly prevalent among ischemic stroke patients. Additionally, an early study found that serum phosphorus levels increase with age (8). Given this evidence, we hypothesized that higher serum phosphorus levels would be associated with carotid artery atherosclerosis in acute ischemic stroke (AIS) patients. Hence, in the present study, we aimed to evaluate the association between serum phosphorus levels and cIMT in patients who had experienced ischemic stroke.
We recruited patients who had experienced AIS or transient ischemic attack (TIA) from 22 hospitals in Suzhou between December of 2013 and May of 2014. A total of 3,720 patients aged ≥ 18 years with a clinical diagnosis of acute ischemic stroke or transient ischemic attack were considered eligible. Acute ischemic stroke was diagnosed by clinical data and computed tomography scan or magnetic resonance imaging of the brain according to the World Health Organization's criteria. Exclusion criteria were as follows: (1) final diagnosis of TIA; (2) time from onset to admission over 7 days; and (3) lack of carotid artery imaging data or serum phosphorus data. Following application of these criteria, a total of 1,450 patients with available data were included in the study (see Supplementary Figure 1 for a flowchart of participant selection).
Demographic and clinical data were collected via a standard questionnaire at admission; these included age, sex, lifestyle risk factors, vascular risk factors (hypertension, diabetes, atrial fibrillation, stroke, and coronary heart disease), and medication use. Information on these factors was also obtained through interviews with patients or members of their immediate family. Hypertension was defined as systolic blood pressure (BP) ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg, or use of antihypertensive medications. Diabetes mellitus was defined as fasting glucose ≥ 7.0 mmol/L (126 mg/dL), non-fasting glucose ≥ 11.1 mmol/L (200 mg/dL), with classic symptoms of hyperglycemia or hyperglycemic crisis and/or use of glucose-lowering drugs. Atrial fibrillation was defined as a history of atrial fibrillation, confirmed by ≥1 electrocardiograms or the presence of arrhythmia during hospitalization. The National Institutes of Health Stroke Scale (NIHSS), administered by trained neurologists, was used to evaluate stroke severity. Stroke was classified according to the Oxfordshire Community Stroke Project (OCSP) categorization by trained neurologists (9).
Blood samples were collected within 24 h of hospital admission. Serum phosphorus and as other laboratory variables were measured via assay at local laboratories.
Carotid ultrasonography examinations were performed by certified sonographers who had received unified training and were unaware of the baseline characteristics and laboratory results of the participants (10). The region of interest for cIMT measurement was the far wall of the bilateral common carotid arteries, proximal to the bifurcation, along a plaque-free segment ≥ 10 mm long on the right and left sides (11). High-resolution B-mode ultrasound systems were used to measure cIMT. In each participating hospital, two sonographers with standardized training evaluated the carotid artery imaging data; discrepancies between their evaluations were resolved by consensus, and the final consistent determination of the two certified sonographers was recorded in each case.
The outcome variables were presence or absence of abnormality of maximum cIMT and abnormality of mean cIMT, defined as maximum cIMT ≥ 1 mm and mean cIMT ≥ 1 mm, respectively (11, 12). Maximum cIMT was defined as the larger of the measurements for the right and left common carotid arteries. Mean cIMT was calculated as the average of the measurements obtained for the right and left common carotid arteries.
All analyses were conducted using the statistical software SPSS, version 17.0. Patients were categorized into four groups based on quartiles of serum phosphorus levels at admission: Q1 (<0.95 mmol/L), Q2 (0.95–1.08 mmol/L), Q3 (1.08–1.20 mmol/L), and Q4 (≥1.20 mmol/L).
Continuous variables are reported in the form mean ± standard deviation (SD) or median [interquartile range (IQR)] and were compared via analysis of variance or the Wilcoxon rank-sum test. Categorical variables are reported in the form frequency (%) and were compared using the chi-square test.
Multivariable logistic regression models were constructed to evaluate the association of serum phosphorus level with abnormal maximum cIMT and abnormal mean cIMT. Odds ratios (ORs) and 95% confidence interval (CIs) were calculated for each group, taking the group with the lowest serum phosphorus (Q1) as the reference. The covariates included in the multivariable models were selected based on prior knowledge and an association with p < 0.2 in the univariate analysis; these variables included age, sex, diastolic BP, current smoking status, alcohol consumption, history of hypertension, history of diabetes mellitus, history of atrial fibrillation, history of stroke, baseline NIHSS score, stroke syndrome, lipid-lowering medications, antiplatelet therapy, triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), fasting plasma glucose (FPG), and estimated glomerular filtration rate (eGFR). To assess the robustness of the association between serum phosphorus level and abnormal cIMT, we also performed an analysis with serum phosphorus treated as a continuous variable. Subgroup analyses were conducted in the form of multivariate adjusted models stratified by age (≥70 years old vs. <70 years old), sex, cigarette smoking status, history of hypertension, and history of diabetes. All statistical tests were two-sided, and P-values < 0.05 were considered to represent statistical significance.
Data from a total of 1,450 acute ischemic stroke patients (872 men, 578 women) were analyzed. The mean age was 68.9 (12.2) years. The baseline characteristics of patients by serum phosphorus quartile are presented in Table 1. In comparison to participants with lower serum phosphorus levels, those with higher serum phosphorus levels were more likely to be younger, to be female, and to have experienced a longer delay from onset of symptoms to hospital admission; they also had a higher prevalence of prior diabetes mellitus, and were more likely to be receiving antihyperglycemic treatment. Patients with higher phosphorus levels also had higher levels of TG. Supplementary Table 1 shows the baseline characteristics of participants with and without abnormal maximum cIMT. Compared to those without abnormal maximum cIMT, patients with abnormal maximum cIMT tended to be older, were more likely to be male, and tended to have higher levels of LDL-C and phosphorus and a lower eGFR.
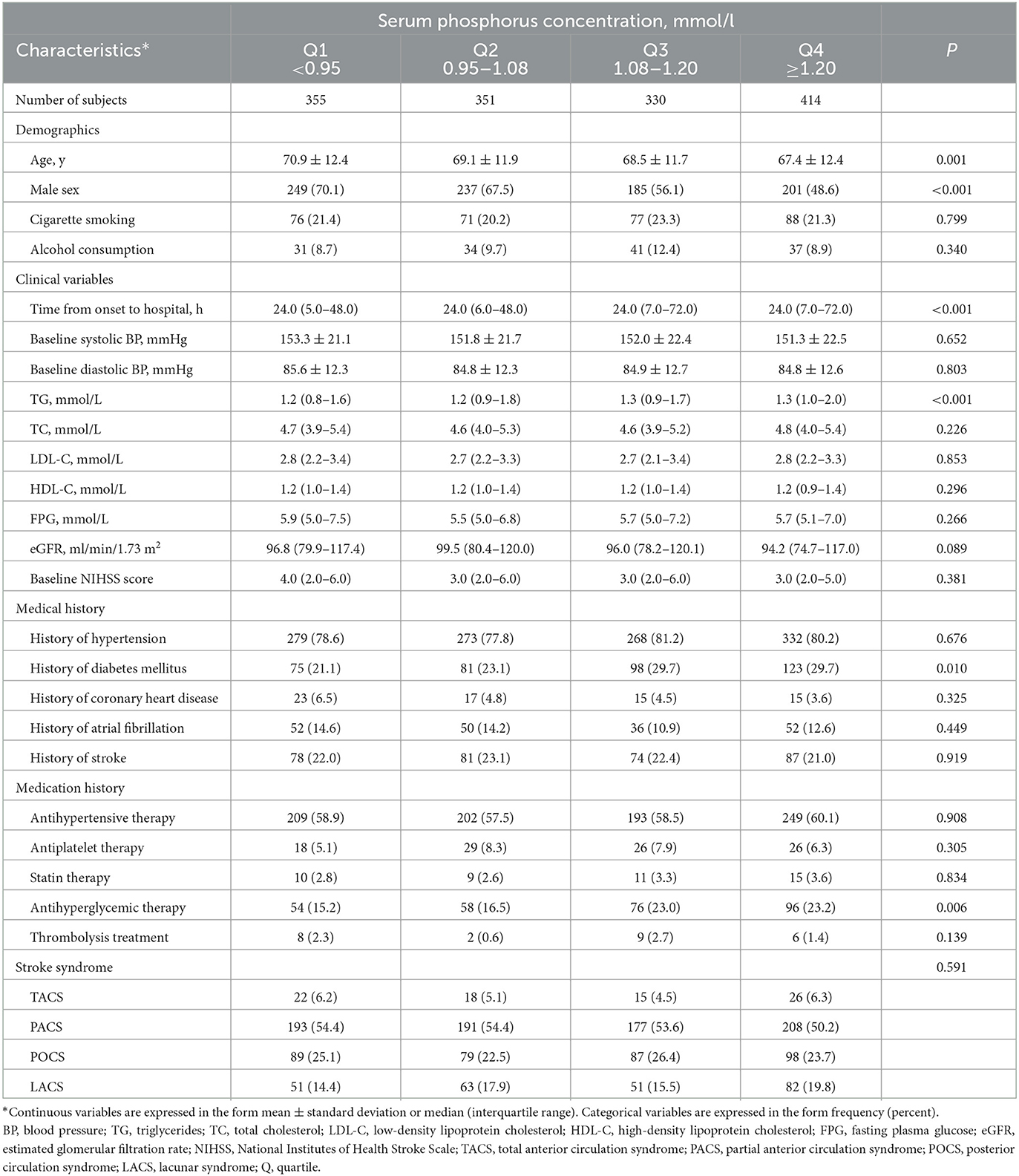
Table 1. Baseline characteristics of 1,450 acute ischemic stroke patients according to serum phosphorus quartile.
There were 538 patients (37.1%) with abnormal maximum cIMT. The relationship between serum phosphorus level and presence of abnormal maximum cIMT according to various models is shown in Table 2. In Model 1, adjusted for age and sex, the rate of abnormal maximum cIMT was significantly higher among participants who fell into the highest quartile in terms of phosphorus level at admission (≥1.20 mmol/L) compared with those who fell into the lowest quartile (<0.95 mmol/L) (OR 1.89; 95% CI 1.38–2.60). After further adjustments for TG, TC, LDL-C, NIHSS score, medical history, and other potential confounding factors in Model 3, the OR (95% CI) for abnormal maximum cIMT among patients falling into the highest quartile for phosphorus at admission as compared to those falling into the lowest quartile was 2.00 (95% CI 1.44–2.79) (Table 2; Figure 1A). Moreover, serum phosphorus was independently associated with increased odds of abnormal maximum cIMT after adjusting for possible confounding factors when serum phosphorus was treated as a continuous variable (OR 3.32; 95% CI 1.84–6.01) (Table 2).
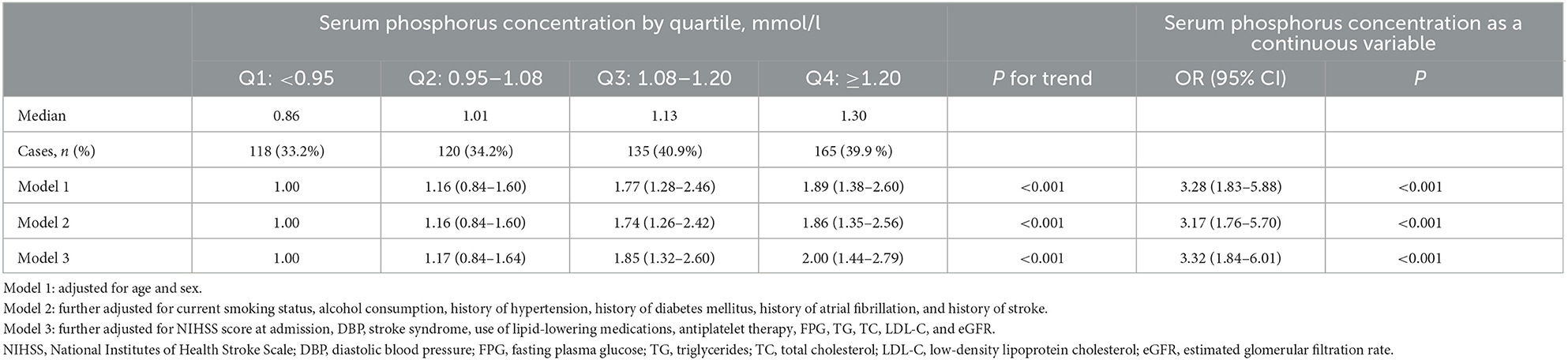
Table 2. Odds ratios and 95% confidence intervals for abnormal maximum cIMT among acute ischemic stroke patients according to serum phosphorus level.
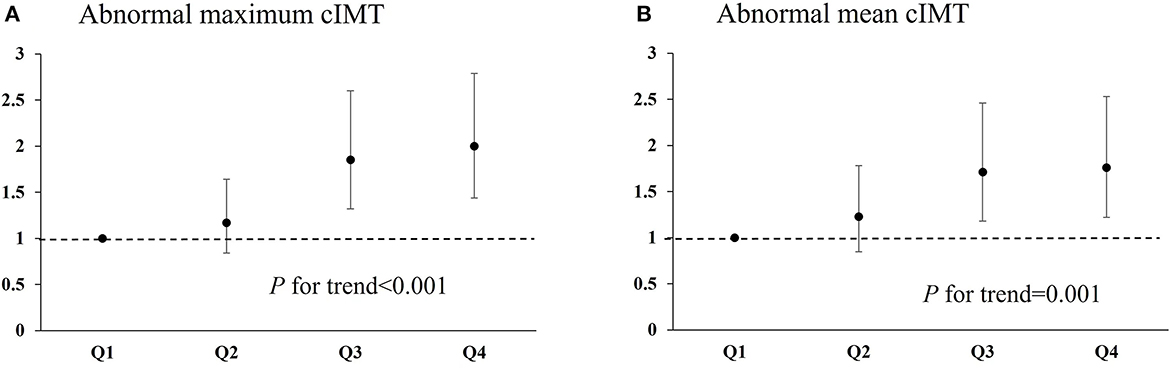
Figure 1. Relationship of serum phosphorus with abnormal maximum cIMT and abnormal mean cIMT in ischemic stroke patients. (A) Odds ratio for abnormal maximum cIMT among patients falling into each quartile for serum phosphorus level. (B) Odds ratio for abnormal mean cIMT among patients falling into each quartile for serum phosphorus level. cIMT, carotid artery intima–media thickness.
There were 418 patients (28.8%) with abnormal mean cIMT. The relationship between serum phosphorus level and presence of abnormal mean cIMT according to various models is presented in Table 3. In Model 1, adjusted for age and sex, the OR (95% CI) for abnormal mean cIMT among participants who fell into the highest quartile for serum phosphorus, compared with those who fell into the lowest quartile, was 1.79 (95% CI 1.27–2.51). Moreover, the relationship between falling into the highest quartile for serum phosphorus and having abnormal mean cIMT (OR 1.76; 95% CI 1.22–2.53) was still significant after further adjustment for TG, TC, LDL-C, NIHSS score, medical history, and other potential confounding factors in Model 3 (Table 3; Figure 1B). In addition, the association between serum phosphorus level and odds of abnormal mean cIMT remained significant (OR 2.37; 95% CI 1.24–4.51) when serum phosphorus was treated as a continuous variable (Table 3).
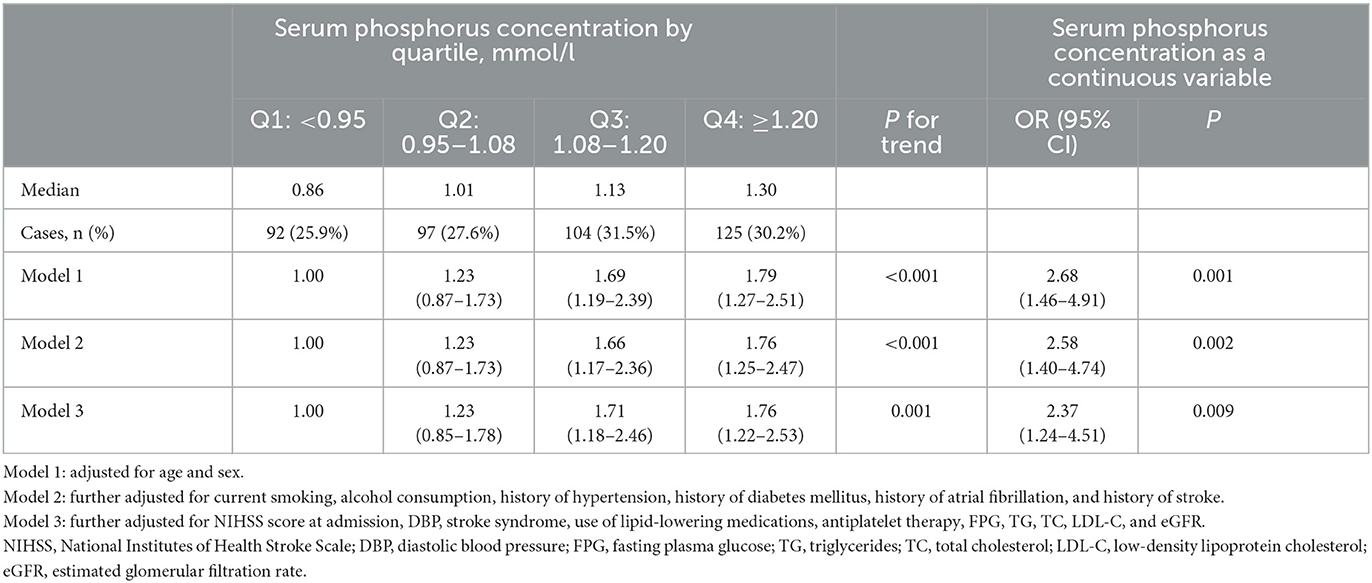
Table 3. Odds ratios and 95% confidence intervals for abnormal mean cIMT among acute ischemic stroke patients according to serum phosphorus level.
Significant associations between admission serum phosphorus level and presence of abnormal maximum cIMT were observed in most subgroups (Table 4). However, no significant interactions were observed between phosphorus levels at admission and subgroup variables in terms of their association with presence of abnormal maximum cIMT (P for interaction > 0.05 in all cases).
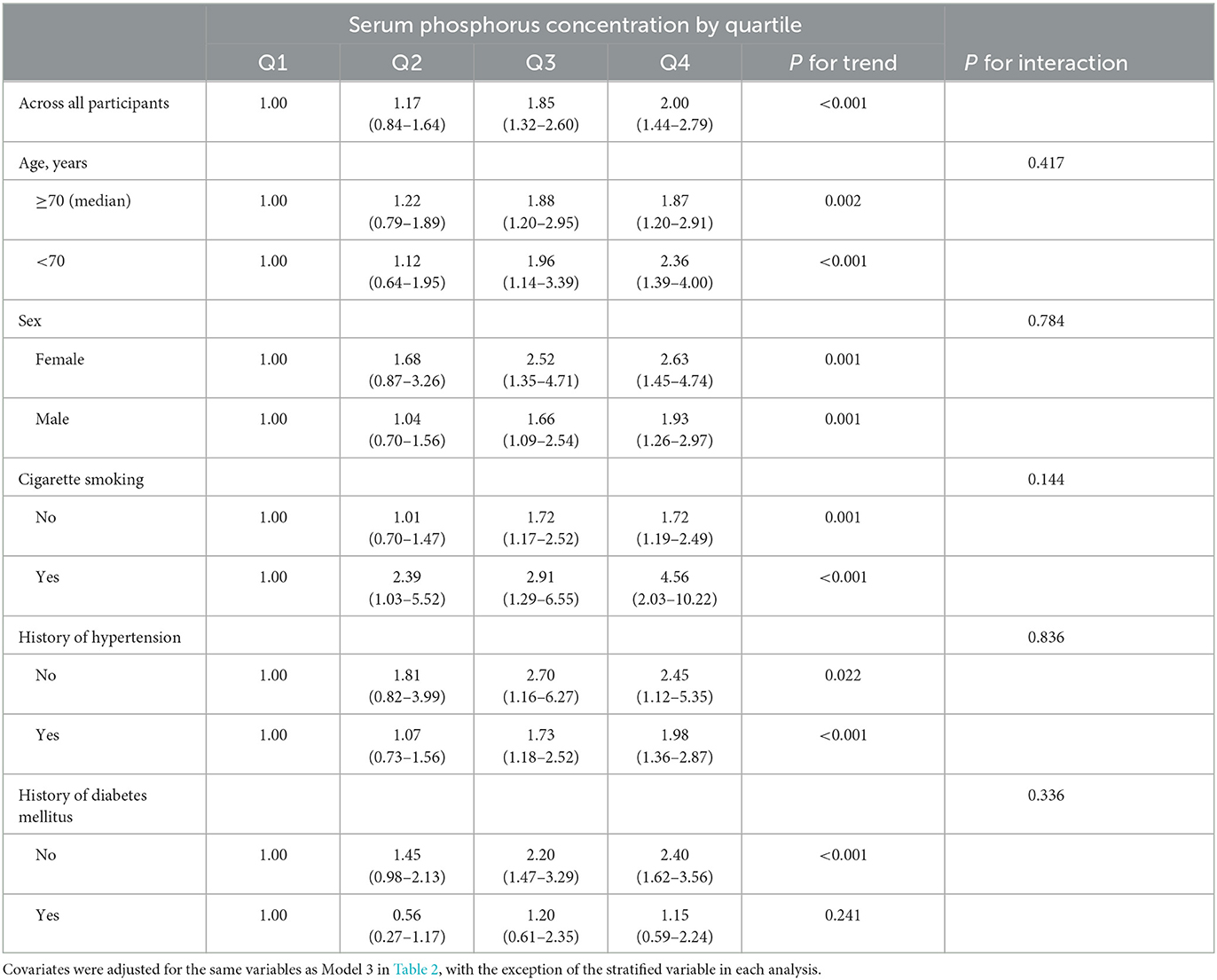
Table 4. Odds ratios and 95% confidence intervals for abnormal maximum cIMT according to serum phosphorus quartile: subgroup analyses.
In this multicenter population-based study, we have demonstrated that serum phosphorus levels at the time of hospital admission are associated with abnormal cIMT in patients with acute AIS. We found that the AIS patients with the highest levels of serum phosphorus appeared to have a 2.00-fold increased risk of abnormal maximum cIMT and a 1.76-fold increased risk of abnormal mean cIMT in comparison to those with the lowest serum phosphorus levels. Significant associations between serum phosphorus levels at admission and prevalence of abnormal cIMT were observed in most subgroups and in analyses in which serum phosphorus was treated as a continuous variable.
cIMT is a widely recognized marker of carotid atherosclerosis and has been shown to be a predictor of cardiovascular events, including stroke (12). A prespecified post-hoc analysis from the J-STARS (Japan Statin Treatment Against Recurrent Stroke) study indicated that participants in the highest cIMT quartile (≥0.931 mm) exhibited a higher incidence of atherothrombotic brain infarction compared to those in the lowest quartile (<0.812 mm) (13). A community-based prospective study from Japan also indicated that maximum cIMT was positively associated with risk of lacunar infarction, but not with non-lacunar infarction (14). In addition, a recent analysis as part of the CSPPT (China Stroke Primary Prevention Trial) revealed that elevated mean cIMT was positively correlated with risk of first ischemic stroke among individuals with hypertension, particularly among those with higher mean arterial pressure or diastolic blood pressure (15). A meta-analysis of 119 clinical trials involving 100,667 patients found that the extent to which cIMT progression was reduced by the effects of various interventions was predictive of degree of stroke risk reduction (16). Moreover, long-term treatment with statins has been shown to decrease cIMT, which may prevent recurrent stroke or cardiovascular events in patients who have experienced stroke (13, 17). However, early meta-analyses have not found conclusive evidence to support cIMT as an independent predictor of future cardiovascular events (18, 19). The variations observed among different studies in terms of their findings may be attributed to the lack of precision and reproducibility in early ultrasound examination techniques (20).
The available evidence indicates that an elevated phosphorus concentration is associated with abnormal cIMT. A recent study from South Korea indicated that serum phosphorus concentration is significantly associated with cIMT in asymptomatic menopausal women (21). In addition, a population-based cohort study of 13,340 participants without cardiovascular or renal disease demonstrated that serum phosphorus level is positively associated with cIMT in men (22). However, a study of 134 pediatric patients with chronic kidney disease found that phosphorus levels are negatively correlated with cIMT in this group (23). The disparity in findings could potentially be attributed to variations in the levels and distribution of levels of serum phosphorus across different age groups. In the present study of 1,450 AIS patients, 37.1% of whom had abnormal maximum cIMT, a significant association was observed between higher serum phosphorus level and presence of abnormal cIMT.
The exact mechanisms underlying the association between phosphorus and cIMT remain unclear. Several hypotheses have been proposed. First, thickening of the intima–media complex is associated with the calcification of vascular smooth muscle cells and the amount of extracellular matrix (24). Previous studies have indicated that elevated phosphorus levels are significantly associated with arterial calcification (25–27) and upregulation of the levels of matrix metalloproteinases (28, 29), which are reported to be associated with an increase in cIMT. Second, hyperphosphatemia may promote endothelial dysfunction, and reduced endothelial function has also been linked to an increase in cIMT (30, 31). Third, elevated phosphorus levels have also been found to be associated with increased oxidative stress, endoplasmic reticulum stress, and inflammatory response (32–34), all of which are known to be associated with elevated cIMT. Further studies are needed to elucidate the mechanism by which phosphorus promotes carotid intima–media thickening and atherosclerosis.
Our findings may have relevant implications for clinical practice. First, our results in AIS patients, together with the findings of a previous study in the general population (22), provide further evidence of the association between serum phosphorus and atherosclerosis. Second, our findings may help to explain the relationship between serum phosphorus and cardiovascular events through the pathogenesis of carotid atherosclerosis. Third, our data suggest that serum phosphorus could be a useful biomarker for carotid atherosclerosis that could be measured routinely as part of blood biochemistry investigations in AIS patients.
We recognize that this study has a number of limitations. First, with a cross-sectional study, it is difficult to demonstrate a causal link between phosphorus and cIMT, and we could not assess the prognostic value of serum phosphorus in terms of stroke recurrence due to a lack of follow-up data. Furthermore, phosphorus level is affected by various factors, such as thyroid disease and dietary intake, which also affect the occurrence of atherosclerosis. Unfortunately, we did not collect data on prior thyroid disease or dietary phosphorus intake. Additionally, some patients were excluded due to a lack of carotid artery imaging data or serum phosphorus data, which may have caused selection bias. Finally, we did not collect data on carotid artery stenosis; therefore, we were not able to further evaluate the association between serum phosphorus and carotid artery stenosis.
Our results showed that an elevated serum phosphorus level at admission was positively correlated with cIMT among ischemic stroke patients, even after adjusting for traditional potential risk factors. Future prospective and randomization studies are needed to verify our findings in acute ischemic stroke patients as well as other populations.
The data that support the findings of this study are available on request from the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University, as well as Ethical Committees at the participating hospitals. Written consent was obtained from all study participants or their immediate family members. The patients/participants provided their written informed consent to participate in this study.
CQ, CZ, and YX contributed to conception and design of the study. YL and SY organized the database. SY performed the statistical analysis. HD wrote the first draft of the manuscript. TG, HY, YB, ZQ, and YS wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This research was supported by the Suzhou Science and Technology Project (SYSD2020044, SKY2022082) and the Wujiang Youth Science and Technology Project (wwk202102).
We thank the study participants, their relatives, and the clinical staff for their support and contributions to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1172488/full#supplementary-material
1. Kim SJ, Schneider DJ, Feldmann E, Liebeskind DS. Intracranial atherosclerosis: review of imaging features and advances in diagnostics. Int J Stroke. (2022) 17:599–607. doi: 10.1177/17474930211066427
2. Qian S, You S, Sun Y, Wu Q, Wang X, Tang W, et al. Remnant cholesterol and common carotid artery intima-media thickness in patients with ischemic stroke. Circ Cardiovasc Imag. (2021) 14:e10953. doi: 10.1161/CIRCIMAGING.120.010953
3. Papadopoulou A, Bountouvi E, Karachaliou FE. The molecular basis of calcium and phosphorus inherited metabolic disorders. Genes Basel. (2021) 12:734. doi: 10.3390/genes12050734
4. Merhi B, Shireman T, Carpenter MA, Kusek JW, Jacques P, Pfeffer M, et al. Serum phosphorus and risk of cardiovascular disease, all-cause mortality, or graft failure in kidney transplant recipients: an ancillary study of the FAVORIT trial cohort. Am J Kidney Dis. (2017) 70:377–85. doi: 10.1053/j.ajkd.2017.04.014
5. Doshi SM, Wish JB. Past, present, and future of phosphate management. Kidney Int Rep. (2022) 7:688–98. doi: 10.1016/j.ekir.2022.01.1055
6. Hisamatsu T, Miura K, Fujiyoshi A, Kadota A, Miyagawa N, Satoh A, et al. Serum magnesium, phosphorus, and calcium levels and subclinical calcific aortic valve disease: a population-based study. Atherosclerosis. (2018) 273:145–52. doi: 10.1016/j.atherosclerosis.2018.03.035
7. Ruan L, Chen W, Srinivasan SR, Xu J, Toprak A, Berenson GS. Relation of serum phosphorus levels to carotid intima-media thickness in asymptomatic young adults (from the Bogalusa Heart Study). Am J Cardiol. (2010) 106:793–7. doi: 10.1016/j.amjcard.2010.05.004
8. Jhuang YH, Kao TW, Peng TC, Chen WL, Chang PK, Wu LW. Serum phosphorus as a risk factor of metabolic syndrome in the elderly in Taiwan: a large-population cohort study. Nutrients. (2019) 11:2340. doi: 10.3390/nu11102340
9. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. (1991) 337:1521–6. doi: 10.1016/0140-6736(91)93206-O
10. Miao M, Zhou G, Bao A, Sun Y, Du H, Song L, et al. Triglyceride-glucose index and common carotid artery intima-media thickness in patients with ischemic stroke. Cardiovasc Diabetol. (2022) 21:43. doi: 10.1186/s12933-022-01472-1
11. Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. (1997) 146:483–94. doi: 10.1093/oxfordjournals.aje.a009302
12. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. Jacc-Cardiovasc Imag. (2014) 7:1025–38. doi: 10.1016/j.jcmg.2013.11.014
13. Wada S, Koga M, Minematsu K, Toyoda K, Suzuki R, Kagimura T, et al. Baseline carotid intima-media thickness and stroke recurrence during secondary prevention with pravastatin. Stroke. (2019) 50:1586–9. doi: 10.1161/STROKEAHA.119.024968
14. Shimoda S, Kitamura A, Imano H, Cui R, Muraki I, Yamagishi K, et al. Associations of carotid intima-media thickness and plaque heterogeneity with the risks of stroke subtypes and coronary artery disease in the Japanese general population: the circulatory risk in communities study. J Am Heart Assoc. (2020) 9:e17020. doi: 10.1161/JAHA.120.017020
15. Sun P, Liu L, Liu C, Zhang Y, Yang Y, Qin X, et al. Carotid intima-media thickness and the risk of first stroke in patients with hypertension. Stroke. (2020) 51:379–86. doi: 10.1161/STROKEAHA.119.026587
16. Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation. (2020) 142:621–42. doi: 10.1161/CIRCULATIONAHA.120.046361
17. Khazaei M, Khosravi M, Mazaheri S, Mazdeh M, Ghiasian M, Taheri M, et al. The effect of atorvastatin on the common carotid artery intima-media thickness in patients with ischemic stroke. Acta Clin Croat. (2020) 59:223–6. doi: 10.20471/acc.2020.59.02.04
18. Eikendal AL, Groenewegen KA, Anderson TJ, Britton AR, Engstrom G, Evans GW, et al. Common carotid intima-media thickness relates to cardiovascular events in adults aged <45 years. Hypertension. (2015) 65:707–13. doi: 10.1161/HYPERTENSIONAHA.114.04658
19. Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. (2012) 379:2053–62. doi: 10.1016/S0140-6736(12)60441-3
20. Plasencia MJ, Garcia SJ. Is manual ultrasonographic measurement of carotid intima-media thickness a reproducible cardiovascular biomarker? Radiologia Madrid. (2017) 59:478–86. doi: 10.1016/j.rx.2017.07.001
21. Kim H, Kim JS, Cho S, Kim JY. Serum phosphorus levels are associated with carotid intima-media thickness in asymptomatic postmenopausal women. Menopause. (2020) 27:1042–6. doi: 10.1097/GME.0000000000001567
22. Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, et al. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis. (2008) 199:424–31. doi: 10.1016/j.atherosclerosis.2007.11.004
23. Campos-Obando N, Bosman A, Kavousi M, Medina-Gomez C, van der Eerden B, Bos D, et al. Genetic evidence for a causal role of serum phosphate in coronary artery calcification: the Rotterdam study. J Am Heart Assoc. (2022) 11:e23024. doi: 10.1161/JAHA.121.023024
24. Chistiakov DA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Calcifying matrix vesicles and atherosclerosis. Biomed Res Int. (2017) 2017:7463590. doi: 10.1155/2017/7463590
25. Wang XR, Yuan L, Shi R, Li H, Wang DG, Wu YG. Predictors of coronary artery calcification and its association with cardiovascular events in patients with chronic kidney disease. Renal Failure. (2021) 43:1172–9. doi: 10.1080/0886022X.2021.1953529
26. Sheridan K, Logomarsino JV. Effects of serum phosphorus on vascular calcification in a healthy, adult population: a systematic review. J Vasc Nurs. (2017) 35:157–69. doi: 10.1016/j.jvn.2017.01.003
27. Voelkl J, Lang F, Eckardt KU, Amann K, Kuro-O M, Pasch A, et al. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol Life Sci. (2019) 76:2077–91. doi: 10.1007/s00018-019-03054-z
28. Chen NX, O'Neill KD, Chen X, Kiattisunthorn K, Gattone VH, Moe SM. Activation of arterial matrix metalloproteinases leads to vascular calcification in chronic kidney disease. Am J Nephrol. (2011) 34:211–9. doi: 10.1159/000330175
29. Lin X, Shan SK, Xu F, Zhong JY, Wu F, Duan JY, et al. The crosstalk between endothelial cells and vascular smooth muscle cells aggravates high phosphorus-induced arterial calcification. Cell Death Dis. (2022) 13:650. doi: 10.1038/s41419-022-05064-5
30. Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. (2009) 20:1504–12. doi: 10.1681/ASN.2008101106
31. Miller VM, Lahr BD, Bailey KR, Hodis HN, Mulvagh SL, Jayachandran M. Specific cell-derived microvesicles: linking endothelial function to carotid artery intima-media thickness in low cardiovascular risk menopausal women. Atherosclerosis. (2016) 246:21–8. doi: 10.1016/j.atherosclerosis.2015.12.030
32. Chen Y, Zhao X, Wu H. Arterial stiffness: a focus on vascular calcification and its link to bone mineralization. Arterioscl Throm Vas. (2020) 40:1078–93. doi: 10.1161/ATVBAHA.120.313131
33. Pang H, Xiao L, Lu Z, Chen H, Shang Z, Jiang N, et al. Targeting androgen receptor in macrophages inhibits phosphate-induced vascular smooth muscle cell calcification by decreasing IL-6 expression. Vasc Pharmacol. (2020) 130:106681. doi: 10.1016/j.vph.2020.106681
Keywords: phosphorus, atherosclerosis, carotid artery intima–media thickness, acute ischemic stroke, predictor
Citation: Du H, Guo T, Ye H, Bao Y, Qiu Z, Sun Y, You S, Liu Y, Xu Y, Zhang C and Qiu C (2023) The association between serum phosphorus and common carotid artery intima–media thickness in ischemic stroke patients. Front. Neurol. 14:1172488. doi: 10.3389/fneur.2023.1172488
Received: 03 March 2023; Accepted: 16 June 2023;
Published: 05 July 2023.
Edited by:
Wei Xu, University of South China, ChinaReviewed by:
Francesco Locatelli, Alessandro Manzoni Hospital, ItalyCopyright © 2023 Du, Guo, Ye, Bao, Qiu, Sun, You, Liu, Xu, Zhang and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunfang Qiu, NTYzMTMyNjg0QHFxLmNvbQ==; Chunqing Zhang, MzA5NDgzMDAyQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.