- 1Department of Neurology II, Affiliated Hospital of Weifang Medical University, Weifang, China
- 2Surgical Department, Affiliated Hospital of Weifang Medical University, Weifang, China
Background: Epilepsy is one of the most common neurological diseases, affecting people of any age. Although the treatments of epilepsy are more and more diverse, the uncertainty regarding efficacy and adverse events still exists, especially in the control of childhood epilepsy.
Methods: We performed a systematic review and meta- analysis following the Cochrane Handbook and preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. Four databases including PubMed, Embase, Web of Science and Cochrane library were searched. Studies reporting the use of brivaracetam monotherapy or adjuvant therapy in children (aged ≤18 years) were eligible for inclusion. Each stage of the review was conducted by two authors independently. Random-effects models were used to combine effect sizes for the estimation of efficacy and safety.
Results: A total of 1884 articles were retrieved, and finally 9 articles were included, enrolling 503 children with epilepsy. The retention rate of BRV treatment was 78% (95% CI: 0.64–0.91), the responder rate (reduction of seizure frequency ≥ 50%) was 35% (95% CI: 0.24–0.47), the freedom seizure rate (no seizure) was 18% (95% CI: 0.10–0.25), and the incidence rate of any treatment-emergent adverse events (TEAE) was 39% (95% CI: 0.09–0.68). The most common TEAE was somnolence, which had an incidence rate of 9% (95% CI: 0.07–0.12). And the incidence rate of mental or behavioral disorders was 12% (95% CI: 0.06–0.17).
Conclusion: Our systematic review and meta-analysis showed that BRV seemed to be safe and effective in the treatment of childhood epilepsy.
1. Introduction
Epilepsy is a common pediatric neurological disease, and the prevalence of epilepsy in children is 0.5–1% (1). The sequelae of childhood epilepsy including mental disorders and behavioral problems will have a negative impact on adults’ education and employment (2, 3). Therefore, the social outcome of epilepsy set in childhood period is worse than that of contemporary adults’ epilepsy. Controlling epilepsy in childhood, and effectively achieving remission of epilepsy can greatly improve social outcomes and reduce economic burden (1, 3). However, the existence of various types of childhood epilepsy, poor compliance, long treatment time, adverse reactions, complications and other factors make the diagnosis, treatment and related clinical trials of pediatric epilepsy more difficult. Treatments for epilepsy include
Brivaracetam (BRV), a new antiepileptic drug, is a highly selective ligand of synaptic vesicle protein 2A (SV2A), which has a structure similar to levetiracetam (LEV). It was reported that the affinity of BRV for SV2A is about 15–30 times that of LEV (11). In 2016, BRV was approved by the FDA and EMA to assist in the treatment of focal epilepsy in adults (≥16 years old) (12). In 2018, the United States approved epilepsy patients aged ≥4 years as the applicable population for BRV as adjunctive therapy and monotherapy (13). Pre-clinical studies and clinical data reported that BRV can be made into conventional tablets and oral liquids easily accepted by children. Given that BRV has lipid solubility, it can swiftly cross the blood–brain barrier and subsequently cause a rapid onset by occupying SV2A via intravenous administration, making it an attractive medicine for emergencies such as status epilepticus (11, 14, 15). Additionally, BRV may be effective for focal epilepsy, generalized epilepsy and epilepsy syndrome (11).
Clinical studies on the efficacy and safety of BRV have been carried out, especially in adults with epilepsy. Relevant adults’ data and results can be extrapolated to childhood epilepsy. Manuel (16) has summarized phase IIb (NCT00175929, NCT00175825), phase III/III b (NCT00490035, NCT00464269, NCT00504881, and NCT01261325), LFFU (NCT00175916, NCT00150800, and NCT01339559) trials involving a total of 2,186 adults with partial-onset seizures (POS) that received adjuvant BRV (50–200 mg/d) treatment. These trials reported that the retention rate at 6, 12, 24 months was 91, 79.8, and 68.1% respectively, and the freedom seizure rate was 4.9, 4.2, and 3.0%, respectively. The median reduction of POS frequency during the treatment period was 48.8%, and the response rate was 48.7%. It was also reported that TEAE incidence rate was 84.5%. These results indicate that the use of adjuvant BRV in adult epilepsy has good long-term safety and efficacy. A retrospective cohort study in 2018 reported the use of BRV’s acute intravenous treatment for hereditary generalized epilepsy (GGE) and status epilepticus (SE). The 3-month retention rate was 82.4%, and the response rate was 36%. 26% of patients had somnolence, ataxia, and psychological or behavioral disorders, and the adverse behavioral events were lower than LEV. Therefore, it was considered feasible to convert from LEV to BRV immediately, and to provide a reference for childhood epilepsy (17). Pellock believed that the efficacy of
The purpose of our systematic review and meta-analysis is to analyze relevant studies, to explore the efficacy and safety of BRV in children with epilepsy. This study is the first one to conduct a meta-analysis of all literature involving the use of BRV for children with all types of seizures, aiming to verify the outcomes of BRV in children. We hope that the finding of the review will provide a reference for the clinical application for children epilepsy.
2. Methods
2.1. Literature search strategy
This systematic review and meta-analysis had been prospectively registered in PROSPERO under the registration number of CRD42022368200. Two independent reviewers searched the English literature from PubMed, Embase, Web of science and Cochrane until October 2022 respectively, and conducted a systematic review and meta-analysis of the single-arm and observational retrospective studies. The retrieval was comprehensively conducted by combining mesh words “brivaracetam” and “epilepsy” with corresponding free words. See Supplementary Table S1 for the literature retrieval strategy of the electronic databases.
2.2. Inclusion and exclusion criteria
We implemented the emission standards of this study according to the following “PICOS” principles.
2.2.1. Participants/population
Inclusion criteria included: (1) According to the international definition of children, the patients who were under 18 years old were eligible; (2) All types of epilepsy that met the diagnostic criteria of epilepsy of the International League Against Epilepsy (ILAE) (1).
The exclusion criteria was: (1) Patients>18 years old. (2) Epilepsy caused by brain tumor, progressive encephalopathy or other progressive neurodegenerative diseases.
2.2.2. Interventions
Inclusion criteria: (1) All BRV studies, whether BRV was administered alone or as an adjunctive treatment combined with other
2.2.3. Types of studies to be included
Inclusion: (1) The literatures published in English were included, and the eligibility was not limit by country, gender or setting. (2) The follow-up time was sufficient to reflect the outcome indicators of the study.
Literature such as reviews, conference abstracts, non-English, unrelated case reports, animal experiments, literature that had inconformity of target population, literature that had unobtainable full text, and from which we were unable to extract effective outcome indicators were excluded (22).
2.3. Outcomes
The main outcome of a randomized controlled trials of adjuvant BRV treatment for adult epilepsy was the frequency of weekly POS seizures in the treatment group compared to the placebo group during treatment. However, the studies we included were single-arm trials and retrospective studies, with the retention rate as the primary measure, summarizing the efficacy and tolerability of
Secondary outcomes were responder rate (defined as the proportion of children whose seizure frequency was reduced by ≥50% compared with the baseline period), seizure freedom rate (the proportion of children who had no seizure compared with the number of seizures in the baseline period), the incidence of any TEAE after receiving BRV, and the incidence of the common adverse event (somnolence and mental/behavioral disorders) in children (23).
2.4. Data extraction
The data of each study included were extracted by two reviewers independently, and the disputed parts were determined through discussion with the third reviewer. The extracted data included first author, publication year, country, type of study design, intervention, dose, age, sample size, sex ratio, average age of initial seizure, follow-up time, and outcome indicators (such as the proportion of children who did not withdraw from the trial, the number of participants with positive reaction, epilepsy freedom, side effects, somnolence and mental/behavioral disorders). For missing data, we contacted the author to ensure the integrity of the data, if necessary.
2.5. Risk of bias and quality assessment
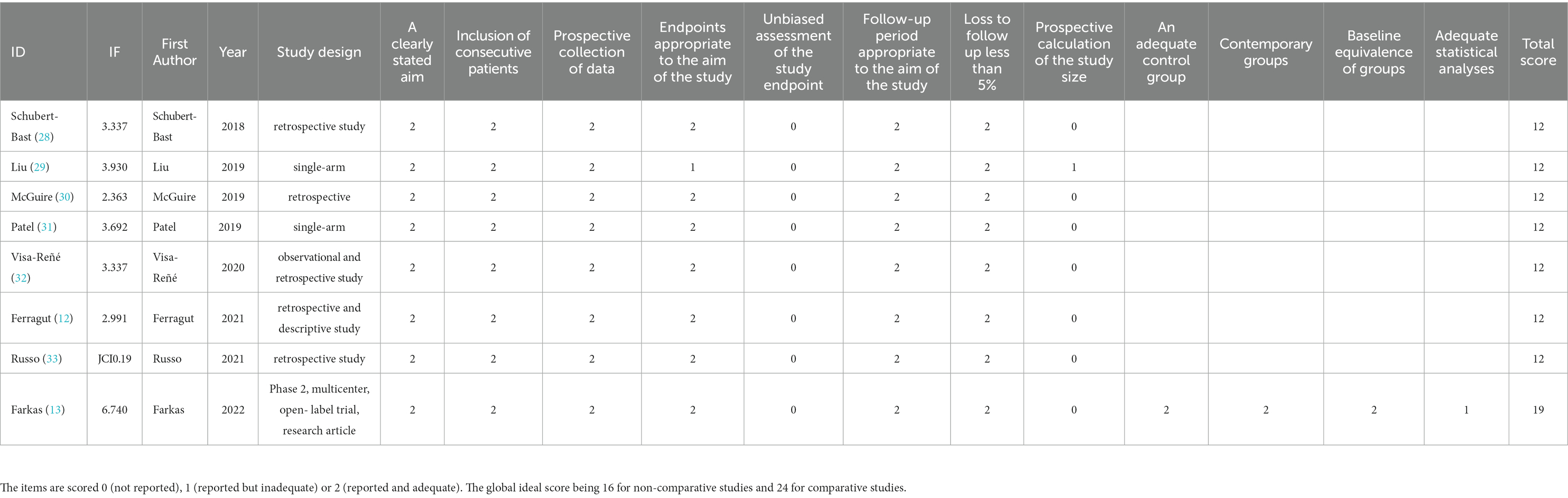
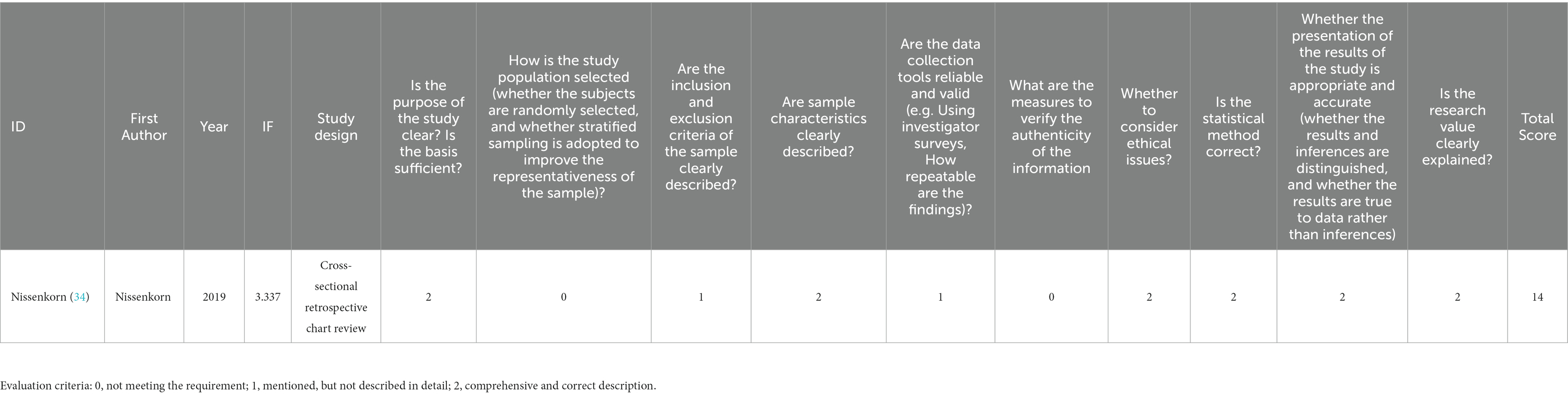
The quality assessment and bias risk assessment were conducted by two reviewers independently, and a third reviewer was consulted to solve the disagreements. Evidence-based medicine suggests using methodological index for non-randomized studies (MINORS) scale to evaluate the bias risk of single-arm researches. The first 8 items of research evaluation in the non-control group have a maximum score of 16 points, while 12 items in the control group have 24 points in total. Conventionally, studies with a score ≥ 12 points means that the quality is good and can be admitted into the analysis (24). The cross-sectional studies can be evaluated according to the Joanna Briggs Institute (JBI) manual, with a maximum score of 20 points. The study with a score of more than 14 points is of good quality (25, 26).
2.6. Statistical analysis and publication bias
The sample size data were analyzed by intention to treat (ITT). We adopted STATA (version 15.1) for statistical analysis and publication bias assessment. We visually checked the forest map to estimate the degree of heterogeneity, and used Cochran’s Q test I2 to assess the heterogeneity (27). I2 < 50% was considered as low heterogeneity, and fixed effects model was used. If I2 > 50% or p > 0.1, it was considered that there was significant heterogeneity, and a random effects model was used to evaluate the combined effect amount. For the secondary variables, the aggregate effect was described by the single rate (p-value) of 95% confidence interval (95% CI).
According to the Cochrane Intervention System Evaluation Manual, a funnel chart was drawn for at least 10 studies to check the symmetry (21). Due to the small number of included articles (less than 10 articles), it was not suitable to draw a funnel chart to evaluate publication bias. Egger’s test was used to evaluate the publication bias quantitatively, and p > 0.05 indicated that there was no significant publication bias.
2.7. Subgroup
If the heterogeneity is significant and the number of literatures is sufficient, we plan on performing a subgroup analysis to assess the possible source of heterogeneity. Subgroup analysis would be conducted by different research types, country, age stratification, sex ratio, race, intervention drug dose and follow-up time, to reduce heterogeneity.
2.8. Sensitivity analyses
We conducted a sensitivity analysis to assess the stability of the outcome indicators included in the study.
3. Results
3.1. Study selection
A total of 1884 documents were obtained through electronic database retrieval, and 1,096 documents remained after removing duplicate documents. Then, 1,057 documents were excluded by screening the titles and abstracts of the literatures and 39 full texts had to be reviewed. Thirty articles were excluded because they did not meet the inclusion criteria (1 article failed to obtain the full text, 21 articles did not have a target population, 3 articles had no extractable data, 2 were review articles, 2 articles for other reasons), and finally 9 articles were included (see Figure 1 for the selection process).
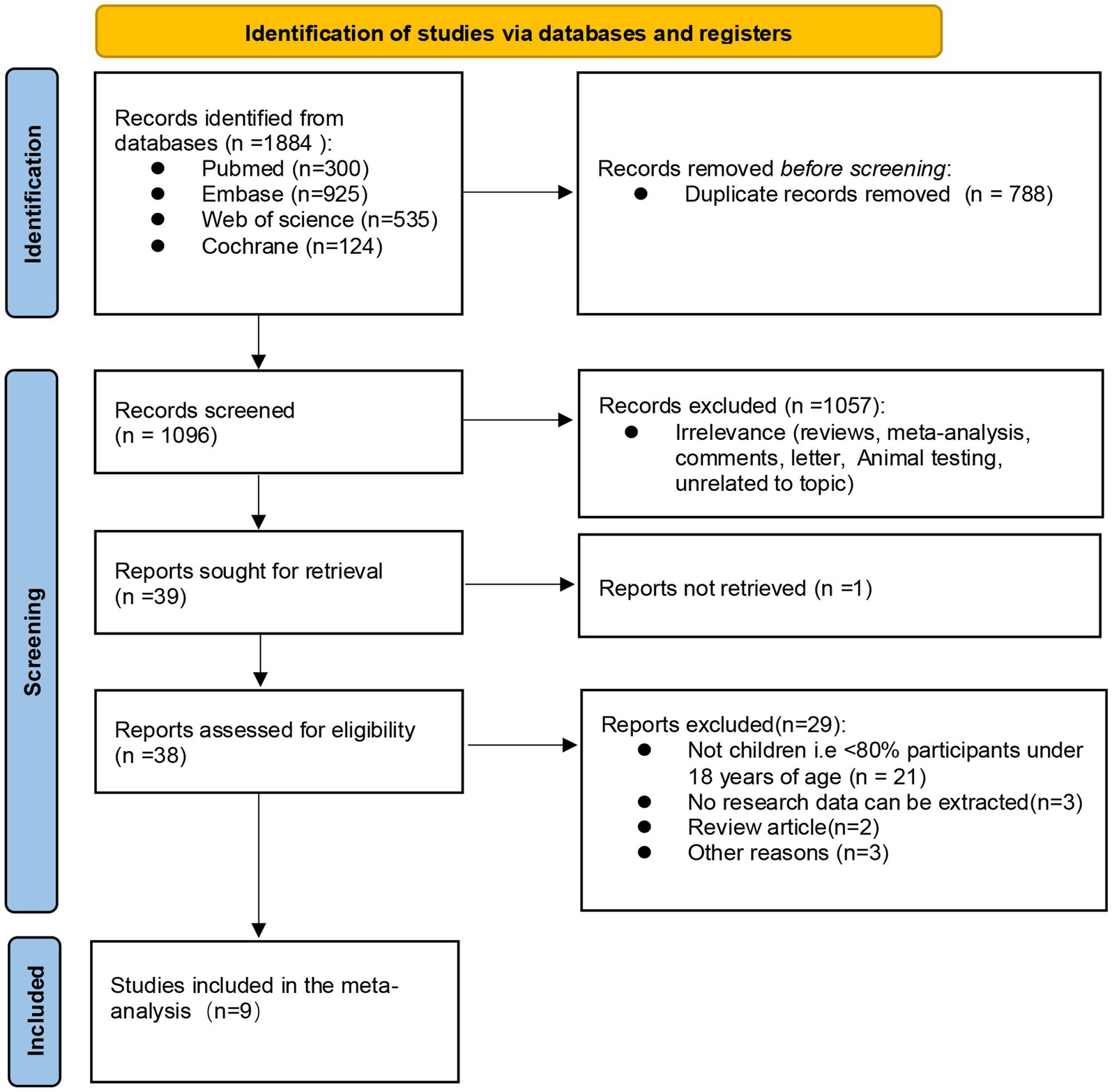
Figure 1. PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow diagram, the final 9 studies were included after inclusion and exclusion criteria.
3.2. Study characteristics and assessments of risk of bias
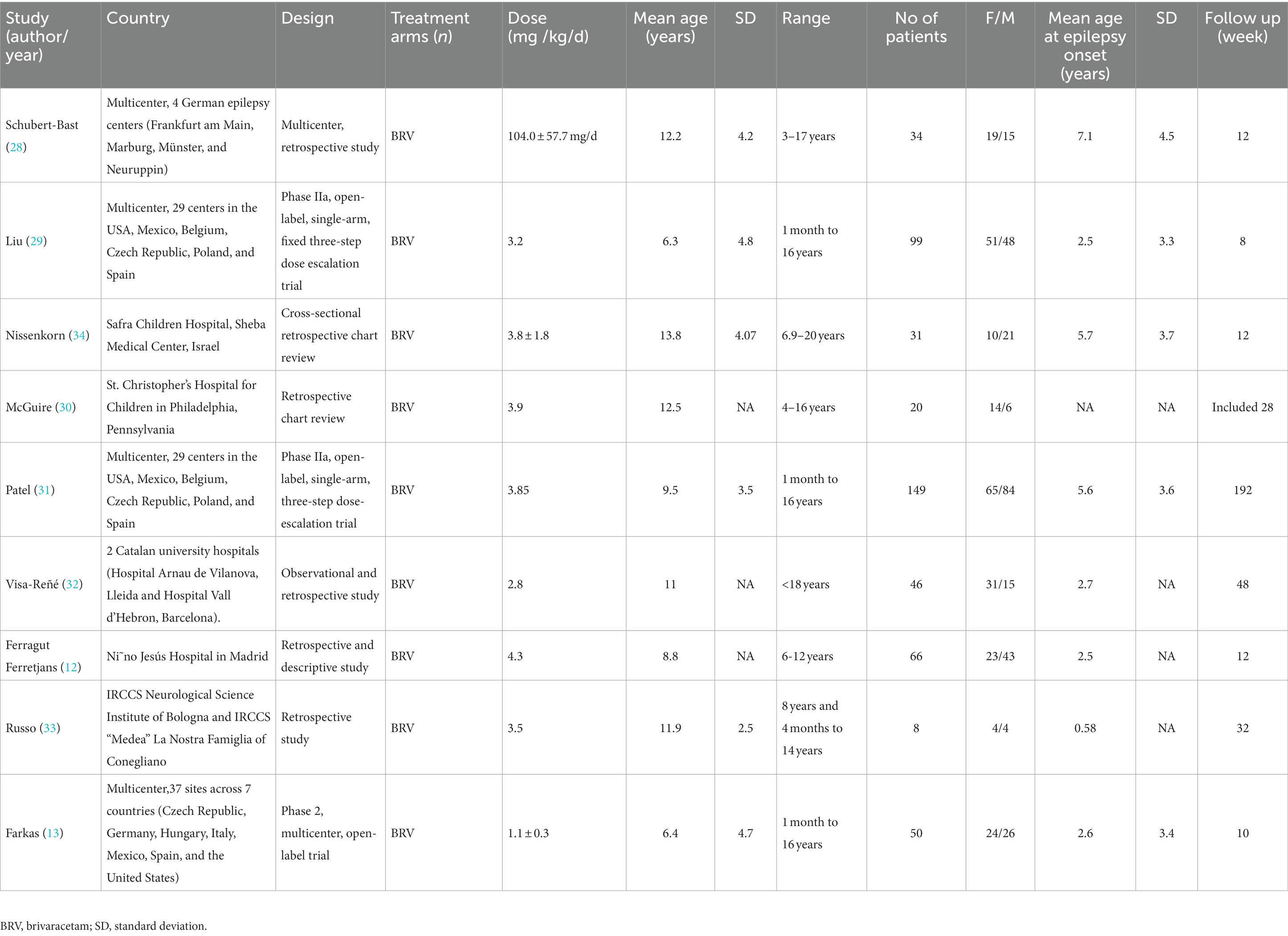
This study includes eight single-arm studies (12, 13, 28–33) and one cross-sectional study (34). In these nine studies, there were four multicenter studies (carried out in several countries), two trials in two hospitals, and three single-center studies, including in total 503 children aged <18 years, with the proportion of men and women accounting for 52.1 and 47.9%, respectively. The study characteristics of the included literature, and the baseline characteristics of the participants, are shown in Table 1 and Supplementary Tables S2, S3. The quality evaluation of the single-arm studies was conducted according to the MINORS scale, while the cross-sectional study used the JBI scale to assess the risk of bias (see Tables 2, 3 for the bias risk scores of the included literatures), which indicated that nine studies could be included in this meta-analysis.
3.3. Outcomes
3.3.1. Primary outcomes
3.3.1.1. Retention rate
A meta-analysis was conducted on the retention rate of six articles in this study. The retention rate of 4 reports was>90%, of which 2 reports was 100%. After heterogeneity test, I2 = 93.1% and p < 0.05 of Q-test, indicating that there was significant heterogeneity between the selected literatures in this study. Based on the random effect model, the total effect amount was 0.78, and the 95% confidence interval was 0.64–0.91, which is statistically significant. The results suggest that the retention rate of BRV treatment for childhood epilepsy is 78% (see Figure 2A).
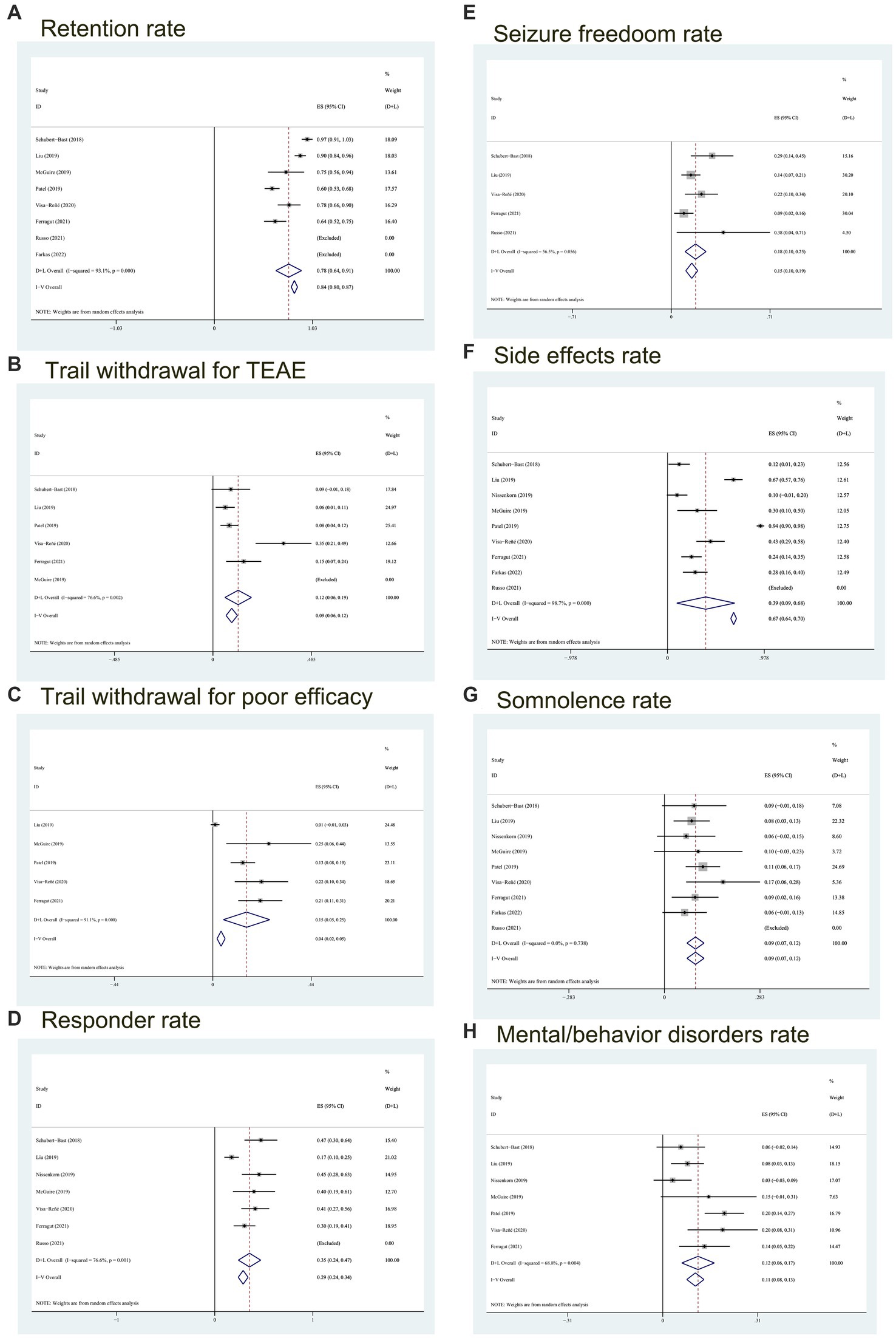
Figure 2. Forest plot of outcomes. (A) The retention rate; (B) trial withdrawal probability for TEAE; (C) probability of poor efficacy leading to discontinuation of the BRV; (D) the responder rate; (E) probability of seizure freedom; (F) the side effects rate; (G) the somnolence rate; (H) the mental/behavior disorders rate. CI, confidence interval; Heterogeneity (I2).
In addition, according to the analysis of the rate of withdrawal from treatment due to TEAE and poor efficacy, we used the random effects model to conclude that the proportion of withdrawal from treatment due to TEAE was 12% (95% CI:0.06–0.19) (see Figure 2B), and the quit rate because of poor efficacy was 15% (95% CI, 0.05–0.25) (see Figure 2C).
3.3.2. Secondary outcomes
3.3.2.1. Responder rate
We analyzed seven trials including 304 children. The meta-analysis results I2 = 76.6% (p < 0.05) showed that there was significant heterogeneity, and the responder rate was 35% (95% CI: 0.24–0.47) (see Figure 2D).
3.3.2.2. Seizure freedom
5 studies reported on the proportion of children without seizures in the follow-up period compared with the baseline period, involving 47 children with freedom seizures during BRV treatment. Heterogeneity test results (I2 = 56.5%, p = 0.056) showed that the studies were of high heterogeneity resulting in the selection of the random effects model. And referring to Figure 2E, the total freedom seizure rate was 18% (95% CI: 0.10–0.25).
3.3.2.3. Side effects
We analyzed the TEAE of BRV in the treatment of childhood epilepsy from two aspects: the incidence of any TEAE and the occurrence of the most common TEAE. Adverse reactions after BRV treatment include lethargy, loss of appetite, behavioral disorders (such as aggression, irritability, impulsion), etc. Among them, somnolence is the most common adverse event.
All 9 studies reported on the proportion of children with any adverse reactions, and the combined incidence was 39% (95% CI: 0.09–0.68, p = 0) according to the random effects model (see Figure 2F). It can be seen from Figure 2 that the occurrence of adverse reactions has a large heterogeneity (I2 = 98.7%), which may be caused by the combination of several
Eight studies enrolling 49 patients who suffer from somnolence reported that heterogeneity (with I2 = 0%, p = 0.738 > 0.1) (see Figure 2G) was low, so the analysis was conducted with the fixed effects model. It was observed that the combined incidence of somnolence was 0.09 (95% CI: 0.07–0.12). The results of this meta-analysis showed that 9% of children had somnolence.
Mental/behavioral adverse reactions are also common side effects of BRV. Neurobehavioral disorders, including behavioral adverse reactions (e.g., depression, aggression, irritability), psychosis comorbidity, and cognitive disturbance, were reported in 7 studies using BRV for epilepsy in children. The Figure 2H showed moderate heterogeneity (I2 = 68.6%, p = 0.004) between studies, so a random effect model was used. As can be seen from Figure 2H, the total effect size of mental or behavioral disorders was 0.12 (95% CI:0.06–0.17), which means that the incidence of neurobehavioral adverse reactions was 12%.
3.3.3. Subgroup
Due to the insufficient number of articles reporting relevant indicators, there was only one article in the subgroups. Considering that reason, we did not carry out the proposed subgroup analysis.
3.4. Publication bias
The publication bias was evaluated by Egger’s test. The results of the Egger’s test, p-value, about retention rate, responder rate, seizure freedom rate, somnolence rate and the incidence rate of mental or behavioral disorders were p = 0.063, 0.563, 0.973, 0.777, 0.226 > 0.05 respectively, indicating no significant publication bias. The Egger’s test of side effect rate showed that the p-value was 0.001 < 0.05, indicating that there was a significant publication deviation. It may be that the study with insignificant statistical effect and insufficient sample size due to obvious withdrawal from BRV treatment was not published.
3.5. Sensitivity analyses
In our analysis, excluding any article did not affect the overall outcome of the article. Therefore, all sensitivity analyses associated with the meta-analysis performed in this study suggested stable results (please refer to Figure 3).
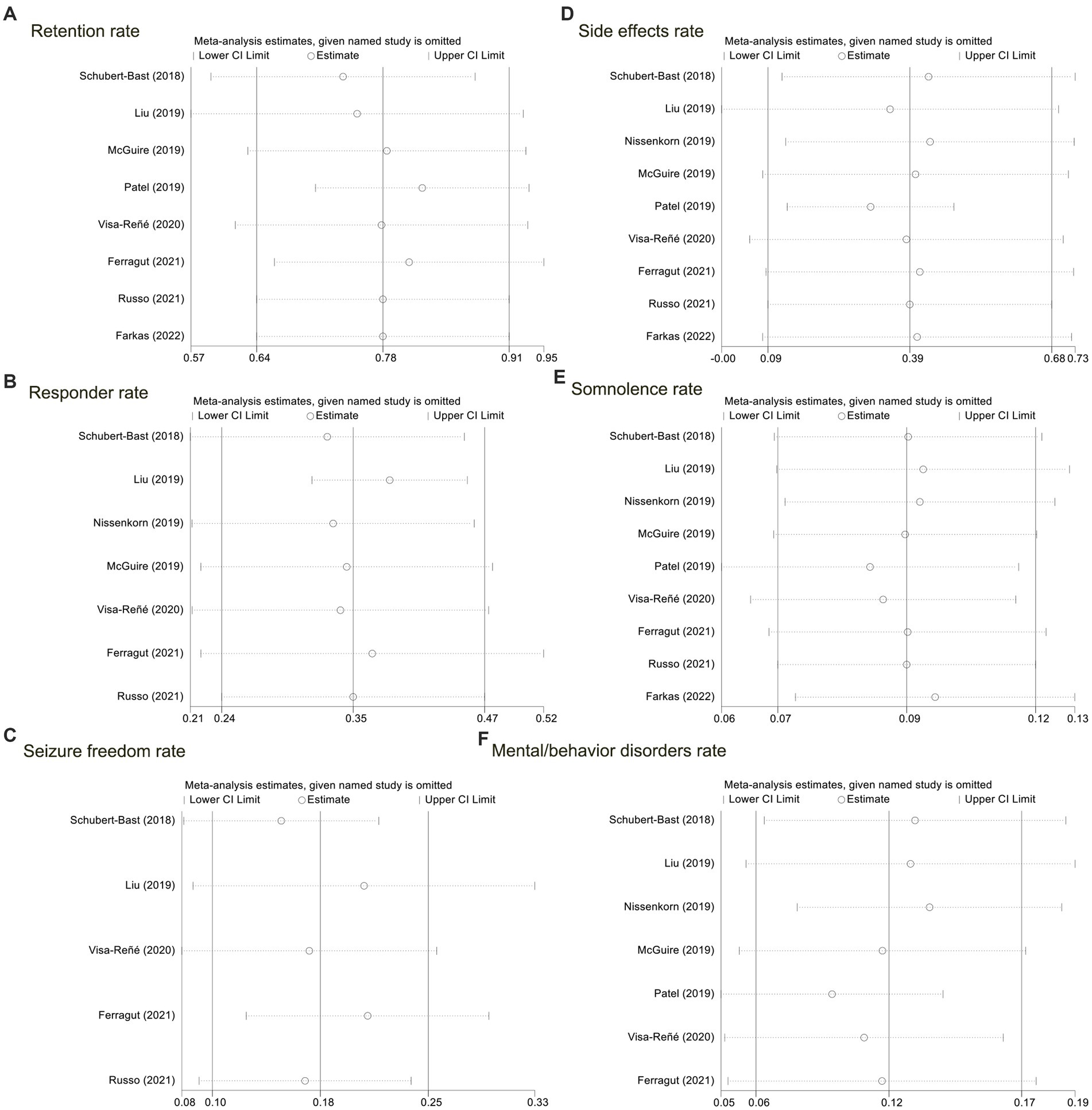
Figure 3. Sensitivity analyses for the nine included studies. (A) The retention rate; (B) the responder rate; (C) probability of seizure freedom; (D) the side effects rate; (E) the somnolence rate; (F) the mental/behavior disorders rate.
4. Discussion
Our systematic review and meta-analysis is the first to evaluate and analyze the safety and effectiveness of BRV, as a single drug or adjuvant drug, in the treatment of childhood epilepsy systematically. In our study, we analyzed nine articles including three open-label trials and six retrospective studies, in which 503 children were involved. The findings indicate that BRV is beneficial for the majority of types of epilepsy and is generally well tolerated and safe. The main findings of our study were that the retention rate of BRV treatment in childhood epilepsy is reasonable. There were three main reasons for withdrawal from BRV treatment: adverse reactions, poor efficacy, and patients’ willingness. Actually, in included studies, most patients terminated the treatment due to adverse reactions and insignificant efficacy. Therefore, the retention rate of BRV is a summary of ASM’s feature, which can comprehensively assess safety and efficacy relatively. This meta-analysis showed that the retention rate of children participating in the BRV treatment trial was 78%, which is consistent with the retention rate at 12 months of the adult trial in Manuel’s study (14). In the cases of drug withdrawal, adverse reactions (12%) and poor efficacy (15%) accounted for the majority.
The long-term prognosis of childhood epilepsy depends on remission, recurrence, seizure freedom, neurological impairment, medication, etc. (35). So far, a large number of clinical trials have reported effective response rate of BRV in the treatment of adult epilepsy. A phase IIb study (N01193; NCT00175825) that enrolled adult patients with refractory POS treated by BRV showed that the responder rates in the 5 mg/d, 20 mg/d, 50 mg/d BRV groups during treatment were 32.0, 44.2, and 55.8% separately (36). In another phase IIb study (N01114; NCT00175929), the responder rate of auxiliary BRV treatment is 30.8–39.6% (37). Phase III experiment (NO: 01253; NCT00464269) using fixed dose scheme found that the responder rate of BRV (5, 20, 50 mg/d) after treatment was 32.7% (38). In phase III study (NO: 1358; NCT01261325), the responder rate of 100 mg/d, 200 mg/d BRV group were 38.9, 37.8%, respectively, (39). Our meta-analysis showed that the response rate of epileptic seizure reduction ≥50% in the random effects model was 35%, which was generally consistent with the response rate of adult POS treated with adjuvant BRV.
In addition, in view of the long-term and extensive impact of childhood seizures, the aim of epilepsy treatment is to eliminate seizures (40). Studies have proved that early realization of epilepsy control and seizure freedom is conducive to early surgical intervention during long-term absence of seizures or cessation of
The adverse reactions of
Common treatment-emergent adverse events such as sedation or somnolence, decreased appetite or increased appetite, irritability, and bad behavior (such as irritability, aggression, etc.) can also occur after the use of BRV as an adjuvant treatment or after the conversion from LEV to BRV. Among them, somnolence is the most common adverse event. The occurrence of adverse behavior events with BRV is less than that after LEV treatment. Epileptiform discharges during the interictal period of epilepsy may disrupt sleep homeostasis at the local or systemic level. In addition, antiepileptic drugs may cause adverse reactions such as sleep disorders in patients with epilepsy (44). Our systematic review and meta-analysis showed that children with epilepsy have relatively good tolerance to BRV treatment. The incidence rate of TEAE in the 9 studies was 39%, most of which were mild to moderate. Somnolence was with an occurrence rate of 9% and mental disorders or bad behavior occurred in 12 percent of cases Remarkably, the incidence of severe TEAE was extremely low, and the deaths were not considered to be caused by BRV treatment. These safety results are consistent with the safety of adult patients receiving BRV treatment. Most adverse events (such as drowsiness, sedation and fatigue) will improve with the decrease of dosage, which can be solved by fractional administration or controlled release agent to increase tolerance (34). Therefore, most of the TEAE of BRV, in the treatment of childhood epilepsy, can be alleviated after adjustment to increase tolerance. Of course, this part of evidence needs to be further tested and studied.
This study has some limitations. Firstly, data on secondary outcomes were unavailable or missing in some studies. Secondly, although this study supports the effectiveness and safety of BRV in the treatment of epilepsy of all seizure types in children, it does not involve separate studies on specific epilepsy types (such as focal epilepsy, generalized epilepsy and epilepsy of unknown etiology according to ILAE) (45). Thirdly, due to the limitations of
5. Conclusion
The systematic review and meta-analysis has proved that BRV is effective and safe in the treatment of childhood epilepsy. Compared with levetiracetam, it has no significant difference in efficacy, and even fewer adverse reactions. Of course, the determination of efficacy and safety of BRV has a greater clinical application prospect for children with drug resistance after long-term use of LEV, to treat epilepsy. The study on BRV treatment for children with epilepsy requires to expand the sample size validation. Similarly, the efficacy, safety and whether BRV is superior to other
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TS, LF, and YX: data curation, methodology, and formal analysis. MP, JG, and XZ: software. YW: funding acquisition. TS: writing—original draft. YW: writing—review and editing. TS, LF, YX, MP, JG, XZ, and YW: conceptualization. All authors commented on previous versions of the manuscript, read and approved the final manuscript.
Funding
This work was supported by Yuandu Scholars and Weifang Key Laboratory, and the Clinical Research Center of Affiliated Hospital of Weifang Medical University (No. 2022WYFYLCYJ02).
Acknowledgments
We recognize effort and great work from all team members and appreciate project coordinators, investigators, and data operators.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1170780/full#supplementary-material
References
1. Aaberg, KM , Gunnes, N , Bakken, IJ , Lund Søraas, C , Berntsen, A , Magnus, P, et al. Incidence and prevalence of childhood epilepsy: a Nationwide cohort study. Pediatrics. (2017) 139:e20163908. doi: 10.1542/peds.2016-3908
2. Berg, AT , Baca, CB , Rychlik, K , Vickrey, BG , Caplan, R , Testa, FM, et al. Determinants of social outcomes in adults with childhood-onset epilepsy. Pediatrics. (2016) 137:e20153944. doi: 10.1542/peds.2015-3944
3. Jennum, P , Christensen, J , Ibsen, R , and Kjellberg, J . Long-term socioeconomic consequences and health care costs of childhood and adolescent-onset epilepsy. Epilepsia. (2016) 57:1078–85. doi: 10.1111/epi.13421
4. Perucca, P , Scheffer, IE , and Kiley, M . The management of epilepsy in children and adults. Med J Aust. (2018) 208:226–33. doi: 10.5694/mja17.00951
5. Elterman, RD , Glauser, TA , Wyllie, E , Reife, R , Wu, SC , and Pledger, G . A double-blind, randomized trial of topiramate as adjunctive therapy for partial-onset seizures in children. Topiramate YP Study Group Neurol. (1999) 52:1338–44. doi: 10.1212/WNL.52.7.1338
6. Glauser, TA , Ayala, R , Elterman, RD , Mitchell, WG , Van Orman, CB , Gauer, LJ, et al. Double-blind placebo-controlled trial of adjunctive levetiracetam in pediatric partial seizures. Neurology. (2006) 66:1654–60. doi: 10.1212/01.wnl.0000217916.00225.3a
7. Levisohn, PM , Mintz, M , Hunter, SJ , Yang, H , and Jones, J . Neurocognitive effects of adjunctive levetiracetam in children with partial-onset seizures: a randomized, double-blind, placebo-controlled, noninferiority trial. Epilepsia. (2009) 50:2377–89. doi: 10.1111/j.1528-1167.2009.02197.x
8. Glauser, TA , Nigro, M , Sachdeo, R , Pasteris, LA , Weinstein, S , Abou-Khalil, B, et al. Adjunctive therapy with oxcarbazepine in children with partial seizures. Oxcarbazepine Pediatric Study Group Neurol. (2000) 54:2237–44. doi: 10.1212/WNL.54.12.2237
9. Piña-Garza, JE , Espinoza, R , Nordli, D , Bennett, DA , Spirito, S , Stites, TE, et al. Oxcarbazepine adjunctive therapy in infants and young children with partial seizures. Neurology. (2005) 65:1370–5. doi: 10.1212/01.wnl.0000186800.18456.72
10. Guerrini, R , Rosati, A , Segieth, J , Pellacani, S , Bradshaw, K , and Giorgi, L . A randomized phase III trial of adjunctive zonisamide in pediatric patients with partial epilepsy. Epilepsia. (2013) 54:1473–80. doi: 10.1111/epi.12233
11. Russo, E , Citraro, R , and Mula, M . The preclinical discovery and development of brivaracetam for the treatment of focal epilepsy. Expert Opin Drug Discovery. (2017) 12:1169–78. doi: 10.1080/17460441.2017.1366985
12. Ferragut Ferretjans, F , Soto Insuga, V , Bernardino Cuesta, B , Cantarín Extremera, V , Duat Rodriguez, A , Legido, MJ, et al. Efficacy of Brivaracetam in children with epilepsy. Epilepsy Res. (2021) 177:106757. doi: 10.1016/j.eplepsyres.2021.106757
13. Farkas, MK , Kang, H , Fogarasi, A , Bozorg, A , James, GD , Krauwinkel, W, et al. Pharmacokinetics, safety, and tolerability of intravenous brivaracetam in pediatric patients with epilepsy: an open-label trial. Epilepsia. (2022) 63:855–64. doi: 10.1111/epi.17187
14. Santamarina, E , Parejo Carbonell, B , Sala, J , Gutiérrez-Viedma, Á , Miró, J , Asensio, M, et al. Use of intravenous brivaracetam in status epilepticus: a multicenter registry. Epilepsia. (2019) 60:1593–601. doi: 10.1111/epi.16094
15. Nicolas, JM , Hannestad, J , Holden, D , Kervyn, S , Nabulsi, N , Tytgat, D, et al. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia. (2016) 57:201–9. doi: 10.1111/epi.13267
16. Toledo, M , Whitesides, J , Schiemann, J , Johnson, ME , Eckhardt, K , McDonough, B, et al. Safety, tolerability, and seizure control during long-term treatment with adjunctive brivaracetam for partial-onset seizures. Epilepsia. (2016) 57:1139–51. doi: 10.1111/epi.13416
17. Strzelczyk, A , Kay, L , Bauer, S , Immisch, I , Klein, KM , Knake, S, et al. Use of brivaracetam in genetic generalized epilepsies and for acute, intravenous treatment of absence status epilepticus. Epilepsia. (2018) 59:1549–56. doi: 10.1111/epi.14476
18. Pellock, JM , Arzimanoglou, A , D'Cruz, O , Holmes, GL , Nordli, D , and Shinnar, S . Extrapolating evidence of antiepileptic drug efficacy in adults to children ≥2 years of age with focal seizures: the case for disease similarity. Epilepsia. (2017) 58:1686–96. doi: 10.1111/epi.13859
19. Schoemaker, R , Wade, JR , and Stockis, A . Extrapolation of a Brivaracetam exposure-response model from adults to children with focal seizures. Clin Pharmacokinet. (2018) 57:843–54. doi: 10.1007/s40262-017-0597-2
20. Wood, MD , and Gillard, M . Evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia. (2017) 58:255–62. doi: 10.1111/epi.13638
21. Wood, MD , Sands, ZA , Vandenplas, C , and Gillard, M . Further evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia. (2018) 59:e147–51. doi: 10.1111/epi.14532
22. Zhang, Z , Yang, L , Han, W , Wu, Y , Zhang, L , Gao, C, et al. Machine learning prediction models for gestational diabetes mellitus: Meta-analysis. J Med Internet Res. (2022) 24:e26634. doi: 10.2196/26634
23. Lezaic, N , Gore, G , Josephson, CB , Wiebe, S , Jetté, N , and Keezer, MR . The medical treatment of epilepsy in the elderly: a systematic review and meta-analysis. Epilepsia. (2019) 60:1325–40. doi: 10.1111/epi.16068
24. Slim, K , Nini, E , Forestier, D , Kwiatkowski, F , Panis, Y , and Chipponi, J . Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
25. Di Toro, F , Gjoka, M , Di Lorenzo, G , De Santo, D , De Seta, F , Maso, G, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect. (2021) 27:36–46. doi: 10.1016/j.cmi.2020.10.007
26. Grønkjær, LL , and Lauridsen, MM . Quality of life and unmet needs in patients with chronic liver disease: a mixed-method systematic review. JHEP Rep. (2021) 3:100370. doi: 10.1016/j.jhepr.2021.100370
27. Arya, S , Schwartz, TA , and Ghaferi, AA . Practical guide to Meta-analysis. JAMA Surg. (2020) 155:430–1. doi: 10.1001/jamasurg.2019.4523
28. Schubert-Bast, S , Willems, LM , Kurlemann, G , Knake, S , Müller-Schlüter, K , Rosenow, F, et al. Postmarketing experience with brivaracetam in the treatment of focal epilepsy in children and adolescents. Epilepsy Behav. (2018) 89:89–93. doi: 10.1016/j.yebeh.2018.10.018
29. Liu, E , Dilley, D , McDonough, B , Stockis, A , and Daniels, T . Safety and tolerability of adjunctive Brivaracetam in pediatric patients < 16 years with epilepsy: an open-label trial. Paediatr Drugs. (2019) 21:291–301. doi: 10.1007/s40272-019-00332-y
30. McGuire, S , Silva, G , Lal, D , Khurana, DS , Legido, A , Hasbani, D, et al. Safety and efficacy of Brivaracetam in pediatric refractory epilepsy: a single-center clinical experience. J Child Neurol. (2020) 35:102–5. doi: 10.1177/0883073819879276
31. Patel, AD , Badalamenti, V , Gasalla, T , Elmoufti, S , and Elshoff, JP . Safety and tolerability of adjunctive brivaracetam in children with focal seizures: interim analysis of pooled data from two open-label trials. Eur J Paediatr Neurol. (2020) 25:68–76. doi: 10.1016/j.ejpn.2019.11.007
32. Visa-Reñé, N , Raspall-Chaure, M , Paredes-Carmona, F , Coromina, JS , and Macaya-Ruiz, A . Clinical experience with brivaracetam in a series of 46 children. Epilepsy Behav. (2020) 107:107067. doi: 10.1016/j.yebeh.2020.107067
33. Russo, A , Cuteri, V , Bansal, L , Bonanni, P , Danieli, A , Pini, A, et al. Brivaracetam in treating epileptic encephalopathy and refractory focal epilepsies in patients under 14 years of age. Iran. J Child Neurol. (2021) 15:95–104. doi: 10.22037/ijcn.v15i4.29819
34. Nissenkorn, A , Tzadok, M , Bar-Yosef, O , and Ben-Zeev, B . Treatment with brivaracetam in children - the experience of a pediatric epilepsy center. Epilepsy Behav. (2019) 101:106541. doi: 10.1016/j.yebeh.2019.106541
35. Brorson, LO , Eriksson, M , Blomberg, K , and Stenninger, E . Fifty years' follow-up of childhood epilepsy: medical outcome, morbidity, and medication. Epilepsia. (2019) 60:381–92. doi: 10.1111/epi.14643
36. French, JA , Costantini, C , Brodsky, A , and von Rosenstiel, P . Adjunctive brivaracetam for refractory partial-onset seizures: a randomized, controlled trial. Neurology. (2010) 75:519–25. doi: 10.1212/WNL.0b013e3181ec7f7f
37. Van Paesschen, W , Hirsch, E , Johnson, M , Falter, U , and von Rosenstiel, P . Efficacy and tolerability of adjunctive brivaracetam in adults with uncontrolled partial-onset seizures: a phase IIb, randomized, controlled trial. Epilepsia. (2013) 54:89–97. doi: 10.1111/j.1528-1167.2012.03598.x
38. Biton, V , Berkovic, SF , Abou-Khalil, B , Sperling, MR , Johnson, ME , and Lu, S . Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. (2014) 55:57–66. doi: 10.1111/epi.12433
39. Klein, P , Schiemann, J , Sperling, MR , Whitesides, J , Liang, W , Stalvey, T, et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. (2015) 56:1890–8. doi: 10.1111/epi.13212
40. Donner, EJ . Opportunity gained, opportunity lost: treating pharmacoresistant epilepsy in children. Epilepsia. (2013) 54:16–8. doi: 10.1111/epi.12178
41. Kadish, NE , Bast, T , Reuner, G , Wagner, K , Mayer, H , Schubert-Bast, S, et al. Epilepsy surgery in the first 3 years of life: predictors of seizure freedom and cognitive development. Neurosurgery. (2019) 84:E368–77. doi: 10.1093/neuros/nyy376
42. Perucca, P , and Gilliam, FG . Adverse effects of antiepileptic drugs. Lancet Neurol. (2012) 11:792–802. doi: 10.1016/S1474-4422(12)70153-9
43. Guilfoyle, SM , Follansbee-Junger, K , Smith, AW , Combs, A , Ollier, S , Hater, B, et al. Antiepileptic drug behavioral side effects and baseline hyperactivity in children and adolescents with new onset epilepsy. Epilepsia. (2018) 59:146–54. doi: 10.1111/epi.13946
44. Chan, SY . Sleep architecture and homeostasis in children with epilepsy: a neurodevelopmental perspective. Dev Med Child Neurol. (2020) 62:426–33. doi: 10.1111/dmcn.14437
45. Fisher, RS , Cross, JH , French, JA , Higurashi, N , Hirsch, E , Jansen, FE, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. (2017) 58:522–30. doi: 10.1111/epi.13670
Keywords: brivaracetam, children epilepsy, seizures, the retention rate, adverse events
Citation: Song T, Feng L, Xia Y, Pang M, Geng J, Zhang X and Wang Y (2023) Safety and efficacy of brivaracetam in children epilepsy: a systematic review and meta-analysis. Front. Neurol. 14:1170780. doi: 10.3389/fneur.2023.1170780
Edited by:
Sara Gasparini, Magna Græcia University, ItalyReviewed by:
Antonio Leo, University Magna Graecia of Catanzaro, ItalyErik Taubøll, Oslo University Hospital, Norway
Copyright © 2023 Song, Feng, Xia, Pang, Geng, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanqiang Wang, d2FuZ3lxNkBtYWlsMy5zeXN1LmVkdS5jbg==; d2FuZ3FpYW5nZG9jdG9yQDEyNi5jb20=
 Ting Song1
Ting Song1 Yanqiang Wang
Yanqiang Wang