
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 14 June 2023
Sec. Dementia and Neurodegenerative Diseases
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1168904
 Lu Zhou1†
Lu Zhou1† Yun Tian1,2,3†
Yun Tian1,2,3† Sizhe Zhang1
Sizhe Zhang1 Bin Jiao1,3,4
Bin Jiao1,3,4 Xinxin Liao2
Xinxin Liao2 Yafang Zhou2
Yafang Zhou2 Qiao Xiao5
Qiao Xiao5 Jin Xue5
Jin Xue5 Ranhui Duan5
Ranhui Duan5 Beisha Tang1,3,4,6
Beisha Tang1,3,4,6 Lu Shen1,3,4,6,7,8*
Lu Shen1,3,4,6,7,8*Background: This study aimed to investigate the features of autonomic dysfunction (AutD) in a large cohort of patients with neuronal intranuclear inclusion disease (NIID).
Methods: A total of 122 patients with NIID and 122 controls were enrolled. All participants completed the Scales for Outcomes in Parkinson’s Disease-Autonomic Questionnaire (SCOPA-AUT) and genetic screening for GGC expanded repeats within the NOTCH2NLC gene. All patients underwent neuropsychological and clinical assessments. SCOPA-AUT was performed to compare AutD between patients and controls. The associations between AutD and disease-related characteristics of NIID were studied.
Results: 94.26% of patients had AutD. Compared with controls, patients had more severe AutD in total SCOPA-AUT, gastrointestinal, urinary, cardiovascular, thermoregulatory, pupillomotor and sexual domains (all p < 0.05). The area under the curve (AUC) value for the total SCOPA-AUT (AUC = 0.846, sensitivity = 69.7%, specificity = 85.2%, cutoff value = 4.5) was high in differentiating AtuD of patients with NIID from controls. The total SCOPA-AUT was significantly and positively associated with age (r = 0.185, p = 0.041), disease duration (r = 0.207, p = 0.022), Neuropsychiatric Inventory (NPI) (r = 0.446, p < 0.01), and Activities of Daily Living (ADL) (r = 0.390, p < 0.01). Patients with onset-of-AutD had higher SCOPA-AUT scores than patients without onset-of-AutD (p < 0.001), especially in the urinary system (p < 0.001) and male sexual dysfunction (p < 0.05).
Conclusion: SCOPA-AUT can be used as a diagnostic and quantitative tool for autonomic dysfunction in NIID. The high prevalence of AutD in patients suggests that NIID diagnosis should be considered in patients with AutD, especially in those with unexplained AutD alone. AutD in patients is related to age, disease duration, impairment of daily living ability, and psychiatric symptoms.
Neuronal intranuclear inclusion disease (NIID) is a rare neurodegenerative disease that is characterized by intranuclear inclusions in the central and peripheral nervous systems and in other organs (1–3). In 2019, an expansion of GGC repeats located in the 5′ UTR of the NOTCH2NLC gene was found to be associated with individuals with NIID, mostly in people of Asian origin (4–7). Clinically, NIID symptoms are highly heterogeneous, with various dysfunctions of the central and peripheral nervous systems, including progressive dementia and cognitive impairment, parkinsonism, stroke-like episodes, encephalitic episodes, cerebellar ataxia, sensory disturbance, peripheral neuropathy, and autonomic dysfunction (3, 7–9).
Many patients with NIID, especially those of increased age, usually show autonomic dysfunction (AutD), such as urinary incontinence, miosis, vomiting, gastrointestinal dysfunction, orthostatic hypotension, arrhythmia, and sexual dysfunction (3, 7, 8, 10–12). Sone et al. (3) reported that the prevalence of bladder dysfunction (33.3% in sporadic cases and 62.5% in familial cases), miosis (94.4% in sporadic cases and 63.6% in familial cases), vomiting (15.8% in sporadic cases and 31.6% in familial cases), and syncope (8.1% in sporadic cases and 0% in familial cases) was high in a Japanese cohort. Our group has also found that AutD was frequently observed in 64.0% (158/247) of patients with NIID in a large Chinese cohort, and bladder dysfunction (48.4%), miosis (20.8%), emesis (14.6%), and orthostatic hypotension (13.5%) were the most common AutDs in that study (13). Interestingly, AutD has been observed in some patients as their only symptom for many years, indicating that AutD may be an optional diagnostic biomarker to identify NIID (14). However, the prevalence and detailed clinical features of AutD in patients with NIID have rarely been studied.
The Scales for Outcomes in Parkinson’s Disease-Autonomic Questionnaire (SCOPA-AUT) (15) has 23 items in six regions. It is a reliable and valid questionnaire that encompasses the full spectrum of autonomic problems (16) and is widely used to assess autonomic symptoms in patients with Parkinson’s disease (PD) (17, 18) and other neurodegenerative diseases (19–21).
This study aimed to use SCOPA-AUT to investigate the prevalence and clinical characteristics of AutD in patients with NIID and the correlations between AutD and other NIID symptoms in a large cohort.
A total of 122 patients with genetically confirmed NIID were enrolled between January 2018 and December 2021. All the patients were evaluated by at least two neurologists. NIID was diagnosed based on clinical features and abnormal GGC repeats (>65) within the NOTCH2NLC. One hundred and twenty-two age- and sex-matched healthy controls were enrolled, and genetic tests were performed to exclude NIID. This study was approved by the Ethics Committee of the Xiangya Hospital, Central South University. Written informed consent was obtained from all the participants at enrollment.
Clinical manifestation data were collected during the clinical examination interviews. The SCOPA-AUT was used to assess AutD in all participants. For patients, the Mini-Mental State Examination (MMSE) (22), Montreal Cognitive Assessment Scale (MoCA) (23) and Frontal Assessment Battery (FAB) (24) were used to screen for cognitive impairments; the Neuropsychiatric Inventory (NPI) (25) was used to assess mental problems; and the Activities of Daily Living scale (ADL) (26) was used to assess activities of daily living. Repeat-primed PCR (RP-PCR) and GC-rich PCR (GC-PCR) assays were performed to confirm GGC expanded repeats within NOTCH2NLC as previously described (7).
AutD was defined as a score ≥1 in SCOPA-AUT. A score of zero was regarded as normal autonomic function.
Descriptive summaries are reported as medians (interquartile range, IQR) for continuous variables and percentages for categorical variables. Mann–Whitney U tests and chi-square tests were performed for variance analysis. Multivariate linear regression analyses were used to adjust for sex and/or age. A receiver operating characteristic (ROC) curve was generated to assess the prediction accuracy of the SCOPA-AUT for NIID. Spearman’s rank test was used for correlation analysis. Differences with p < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 26.0 (IBM SPSS, Inc., Chicago, IL, United States).
A total of 122 patients with NIID and 122 control participants were enrolled in this study. Patients and controls had similar sex proportion (male: female total ratio of 58/64 vs. 55/67, p = 0.700). The median (IQR) age was 65 (59–69) years in the patients with NIID and 65 (63–68) years in the control participants (p = 0.434). The median expansion of NOTCH2NLC was 116.5 (100.5–139.5) in the NIID group and 19 (16–21) in the control group (p < 0.001). Patients with NIID had a median (IQR) disease duration of 5 (2–10) years and a median (IQR) age at onset of 60 (52–64) years. The manifestations of patients with NIID were summarized as five symptom categories: cognitive impairment (49.18%), muscle weakness (34.43%), movement disorders(56.56%), paroxysmal symptom (including disturbance of consciousness, encephalitic episodes, stroke-like episodes, generalized convulsions and chronic headache, 72.95%), and autonomic dysfunction (70.49%). 86/122 patients with NIID complained of autonomic dysfunction. 18 patients had undergone cystostomy or long-term indwelling catheter insertion. Detailed information is shown in Table 1.
In patients with NIID, 94.26% (115/122) had AutD, and the most frequently affected domain in NIID with AutD was the urinary (98/122, 80.33%), followed by the gastrointestinal (89/122, 72.95%), thermoregulatory (56/122, 45.9%), cardiovascular (54/122, 44.26%), pupillomotor dysfunction (31/122, 25.41%), and sexual (14/122, 11.48%) domains. The median SCOPA-AUT score was 11.00 (4.00–18.00). Compared with the control participants, patients with NIID displayed more severe AutD in the total SCOPA-AUT, gastrointestinal, urinary, cardiovascular, thermoregulatory, and pupillomotor domains (all p < 0.01). There were significant difference in the sexual domain (adjusted p = 0.043) and sexual domain of men (adjusted p = 0.010) between the NIID and control groups. The details are presented in Table 2. Given that females may tend to refuse admitting sexual problems on the questionnaire, the statistical analysis of the female sexual domain may be biased.
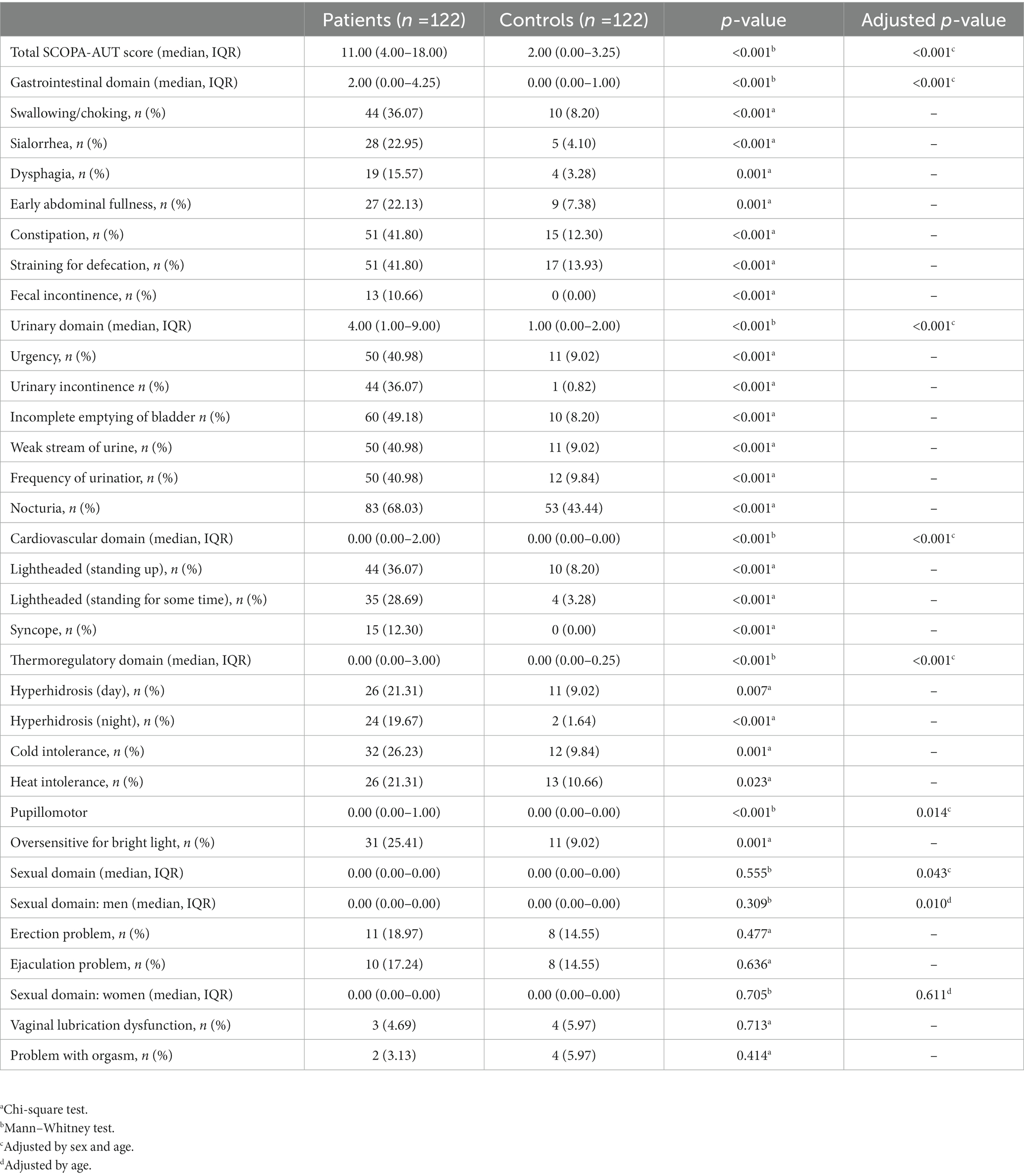
Table 2. Autonomic symptom severity and frequency (% with an item score ≥1) in the study population.
The most frequently involved symptoms in patients with NIID were nocturia (68.03%), incomplete emptying of bladder (49.18%), constipation (41.80%), straining for defecation (40.98%), and urinary urgency (40.98%). The least frequently involved symptoms were problem with orgasm (3.13%), dysfunction of vaginal lubrication (4.69%), fecal incontinence (10.66%), syncope (12.30%), and dysphagia (15.57%). The largest differences between patients with NIID and control participants were the frequency of incomplete emptying of bladder (49.18% vs. 8.20%), urinary incontinence (36.07% vs. 0.82%), urinary urgency (40.98% vs. 9.02%), weak stream of urine (40.98% vs. 9.02%), and lightheadedness upon standing (36.07% vs. 8.20%) (Table 2). There were statistically significant differences (all p < 0.05) (Table 2) between patients with NIID and control participants in all symptoms, except sexual symptoms.
To detect the diagnostic accuracy of SCOPA-AUT for AutD in NIID, we performed ROC curve analysis. As presented in Table 3, the area under the curve (AUC) values were the highest for the total SCOPA-AUT (AUC = 0.846, sensitivity =69.7%, specificity = 85.2%, cutoff value = 4.5), gastrointestinal domain (AUC = 0.765, sensitivity = 73.0%, specificity = 68.9%, cutoff value = 0.5), and urinary domain (AUC = 0.776, sensitivity = 59.8%, specificity = 91.8%, cutoff value = 2.5). The ROC curves are shown in Figure 1. The AUC values for the other domains were <0.7. The results suggest that SCOPA-AUT, gastrointestinal and urinary symptoms, showed high diagnostic performance in differentiating AutD of patients with NIID from healthy controls and could be a diagnostic and quantitative tool for AutD in NIID.
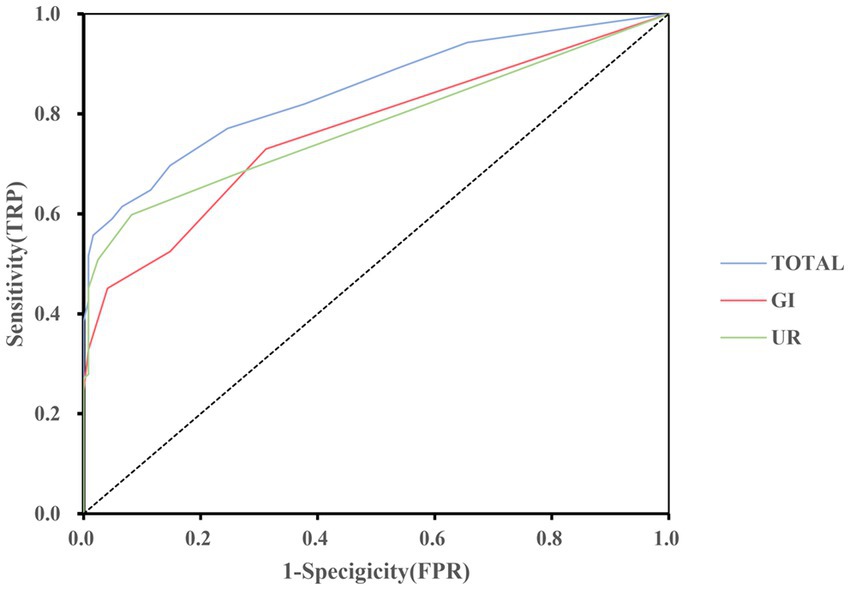
Figure 1. Receiver operating characteristic curve of the SCOPA-AUT and domains to diagnosis for AutD in NIID. GI, gastrointestinal functioning; UR, urinary functioning.
The presence and severity of AutD may be affected by diverse clinical factors, as some autonomic phenotypes may occur simultaneously with other clinical symptoms. Spearman analysis was used to thoroughly investigate the correlation between SCOPA-AUT and other clinical manifestations. The total SCOPA-AUT score was significantly and positively associated with age (r = 0.185, p = 0.041), disease duration (r = 0.207, p = 0.022), NPI (r = 0.446, p < 0.01), and ADL (r = 0.390, p < 0.01) in patients with NIID. Meanwhile, the total SCOPA-AUT score was not correlated with age of onset, expanded repeat sizes within the NOTCH2NLC gene, MMSE, MoCA, or FAB (p > 0.05). The details are presented in Table 4.
Eighteen of 122 (14.75%) patients with NIID had AutD as the first symptom, a condition referred to as onset-with-AutD NIID. All patients started with urinary symptoms of unknown origin, and the relevant symptomatic treatment was ineffective. In severe cases, cystostomy or long-term indwelling catheter insertion was performed before the appearance of other symptoms. The median SCOPA-AUT score of the patients with onset-with-AutD NIID was 19.00 (15.00–24.00). Other patients (85.25%) who did not present with AutD as their initial symptoms were referred to as patients with onset-without-AutD NIID. The median SCOPA-AUT score in this subgroup was 9.50 (3.00–16.00). Patients with onset-with-AutD had more severe SCOPA-AUT scores than patients with NIID in the onset-without-AutD group (p < 0.001), and the two groups also differed in urinary system (p < 0.001) and male sexual dysfunction (adjusted p = 0.026), while there were no differences in other domains. There were no differences in age (p = 0.894), sex (p = 0.821), age of onset (p = 0.734), duration of disease (p = 0.367), and expanded repeat sizes within NOTCH2NLC (p = 0.883) between the two groups. Detailed information is shown in Table 5.
By comparing the differences in autonomic symptoms between the two groups, we found that the symptoms of urinary urgency, urinary incontinence, incomplete emptying of bladder, weak stream of urine, frequency of urination, nocturia, and ejaculation problems were more frequently seen in the subgroup that began with autonomic dysfunction (p < 0.05, adjusted p < 0.05) (Table 5). These results suggest that patients with an early onset of unexplained urinary symptoms or male sexual dysfunction should be considered for NIID diagnosis.
NIID is a rare disease with variable clinical manifestations, and further investigation is required to explore the clinical spectrum of this disease. In this study, we recruited a large cohort of patients with NIID and performed comprehensive clinical evaluation of autonomic dysfunction. We found that patients with NIID usually presented with autonomic symptoms, particularly in the urinary, gastrointestinal, thermoregulatory, and cardiovascular domains.
The most prominent complaints in patients with NIID were urinary system and gastrointestinal tract dysfunction, including nocturia, incomplete emptying of bladder, constipation, straining for defecation, and frequency of urination. The rate of these symptoms differed from other reported studies (3). The prevelance of miosis in this study was lower than that in the Japanese cohort, possibly because our patients were diagnosed at an early stage of the disease through genetic testing. However, the sample size and application of systematic questionnaires could also lead to these differences. Compared with age- and sex-matched controls, patients with NIID experienced significantly more problems with urinary, gastrointestinal, thermoregulatory, cardiovascular, pupillomotor, and male sexual dyfunction. However, there was no significant difference in female sexual dysfunction, which may be related to the fact that females may tend to deny sexual problems, and the statistical analysis of the sexual domain may be biased.
Compared with previous study (20), patients with MSA had higher scores in total SCOPA-AUT, urinary domain and sexual domain, while the patients with NIID of this study had higher score in the digestive system. These finding suggest that the severity and involved domain of autonomic dysfunction differed between NIID and other neurodegenerative diseases.
The manifestations of AutD in NIID have been reported in many cases (12, 27, 28), and the mechanism may be related to the formation of neuronal intranuclear inclusions in the autonomic nerves or the loss of autonomic neurons. Sone et al. (10) reported that eosinophilic inclusion bodies were widespread, especially in sympathetic and myenteric ganglion neurons, dorsal root ganglion neurons, and spinal motor neurons in the autopsy of two patients with NIID with peripheral neuropathy and AutD. In an autopsy of a 21 years-old female patient, Sung et al. (29) found that the Onuf’s nucleus and the intermediolateral nucleus of sacral autonomic neurons were largely lost. Two studies also revealed that many intranuclear inclusions were located in the hypothalamus and autonomic nerve centers (10, 30).
In the present study, we first applied the SCOPA-AUT questionnaire to evaluate autonomic symptoms in patients with NIID. The SCOPA-AUT is comprehensive and easy to operate. The AUCs of SCOPA-AUT were >0.7 in total score, gastrointestinal domain, and urinary domain, suggesting that the SCOPA-AUT could be used as a diagnostic and quantitative tool for autonomic dysfunction in NIID.
We also discovered that AutD in patients with NIID was positively correlated with disease duration and age, indicating that autonomic dysfunction in patients with NIID gradually increased with disease progression. Simultaneously, the study revealed that AutD was associated with impaired activities of daily living and mental symptoms in patients with NIID. This may be due to autonomic nervous dysfunctions, such as urinary incontinence and dysphagia, which usually lead to inconvenience in daily life and affect daily function. Although it has been reported that psychiatric symptoms, such as depression, have an important impact on autonomic problems in patients with PD (31), this study was only a cross-sectional study, and we were not able to draw a causal relationship between the symptoms through this result.
Additionally, we found that 18/122 (14.75%) patients with NIID with AutD as onset symptoms showed significantly higher scores in the total SCOPA-AUT, urinary domain, and sexual domain (p < 0.05), compared with those of patients with NIID with the onset without AutD, and there were no significant differences in age and disease duration between these two groups. This suggests that onset-with-AutD NIID may suffer more severe autonomic dysfunction than those of onset-without-AutD NIID. We also observed that the symptoms of urinary urgency, urinary incontinence, incomplete emptying of bladder, weak stream of urine, frequency of urination, nocturia, and ejaculation problems, were more frequently seen in the onset-with-AutD subgroup than in the onset-without-AutD subgroup (all p < 0.05). Eighteen patients with NIID started with urinary symptoms of unknown origin and the relevant symptomatic treatment was ineffective. In severe cases, cystostomy or long-term indwelling catheter insertion was performed before the appearance of other symptoms. These findings indicate that autonomic symptoms may occur before the onset of other NIID symptoms, as previously reported (14). The high prevalence of AutD suggests that NIID should be considered in patients with AutD, especially in those with unexplained AutD alone.
This study had limitations, as it only referred to autonomic nervous dysfunction in patients with adult-onset NIID, and further longitudinal studies are needed to elucidate its mechanism and changes during disease duration. This study also did not combine the corresponding autonomic nerve function examination, due to the fact that some patients were in an acute stage of the disease and could not complete objective test for autonomic dysfunction such as Quantitative Sudomotor Axon Reflex Tests (ex: QSART, QSWEAT) or urodynamic studies. In addition, many patients had undergone cystostomy or long-term indwelling catheter insertion in the early stage of the disease, which made it difficult to carry out relevant auxiliary examinations. Therefore, only 14 patients underwent bladder residual urine volume examination in this study, of which 10 patients showed increased bladder residual urine volume. For patients with NIID, objective examination of autonomic nervous function is necessary, and further studies with larger sample size are needed to explore the autonomic dysfunction in NIID. Finally, SCOPA-AUT had its own limitation in assessing autonomic dysfunction of NIID. For instance, episodic vomiting, which was frequently observed in many patients with NIID (3, 13), was not included in SCOPA-AUT.
In conclusion, SCOPA-AUT can be used for evaluation of autonomic dysfunction in NIID. The high prevalence of AutD in patients with NIID suggests that NIID diagnosis should be considered in patients with AutD, especially in those with unexplained AutD alone. In addition, AutD in patients with NIID is related to age, disease duration, impairment of daily living ability, and psychiatric symptoms.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Xiangya Hospital, Central South University. The patients/participants provided their written informed consent to participate in this study.
LS and BT original idea and revision of draft. LZ draft of the paper and participants data collection. YT draft of the paper and statistical analyses. SZ statistical analyses. BJ, XL, and YZ patients clinical diagnosis. QX, JX, and RD gene mutation detection. All authors contributed to the article and approved the submitted version.
This study supported by the National Key R&D Program of China (nos. 2020YFC2008500 and 2022ZD0213700), the National Natural Science Foundation of China (nos. 81971029, 81671075 and 82071216, 81901171), and Hunan Innovative Province Construction Project (no. 2019SK2335).
The authors want to thank all the patients and participants involved in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lindenberg, R, Rubinstein, LJ, Herman, MM, and Haydon, GB. A light and electron microscopy study of an unusual widespread nuclear inclusion body disease. A possible residuum of an old herpesvirus infection. Acta Neuropathol. (1968) 10:54–73. doi: 10.1007/BF00690510
2. Munoz-Garcia, D, and Ludwin, SK. Adult-onset neuronal intranuclear hyaline inclusion disease. Neurology. (1986) 36:785–90. doi: 10.1212/WNL.36.6.785
3. Sone, J, Mori, K, Inagaki, T, Katsumata, R, Takagi, S, Yokoi, S, et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain. (2016) 139:3170–86. doi: 10.1093/brain/aww249
4. Deng, J, Gu, M, Miao, Y, Yao, S, Zhu, M, Fang, P, et al. Long-read sequencing identified repeat expansions in the 5’UTR of the NOTCH2NLC gene from Chinese patients with neuronal intranuclear inclusion disease. J Med Genet. (2019) 56:758–64. doi: 10.1136/jmedgenet-2019-106268
5. Ishiura, H, Shibata, S, Yoshimura, J, Suzuki, Y, Qu, W, Doi, K, et al. Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nat Genet. (2019) 51:1222–32. doi: 10.1038/s41588-019-0458-z
6. Sone, J, Mitsuhashi, S, Fujita, A, Mizuguchi, T, Hamanaka, K, Mori, K, et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet. (2019) 51:1215–21. doi: 10.1038/s41588-019-0459-y
7. Tian, Y, Wang, JL, Huang, W, Zeng, S, Jiao, B, Liu, Z, et al. Expansion of human-specific GGC repeat in neuronal intranuclear inclusion disease-related disorders. Am J Hum Genet. (2019) 105:166–76. doi: 10.1016/j.ajhg.2019.05.013
8. Takahashi-Fujigasaki, J. Neuronal intranuclear hyaline inclusion disease. Neuropathology. (2003) 23:351–9. doi: 10.1046/j.1440-1789.2003.00524.x
9. Takahashi-Fujigasaki, J, Nakano, Y, Uchino, A, and Murayama, S. Adult-onset neuronal intranuclear hyaline inclusion disease is not rare in older adults. Geriatr Gerontol Int. (2016) 16:51–6. doi: 10.1111/ggi.12725
10. Sone, J, Hishikawa, N, Koike, H, Hattori, N, Hirayama, M, Nagamatsu, M, et al. Neuronal intranuclear hyaline inclusion disease showing motor-sensory and autonomic neuropathy. Neurology. (2005) 65:1538–43. doi: 10.1212/01.wnl.0000184490.22527.90
11. Wang, Y, Wang, B, Wang, L, Yao, S, Zhao, J, Zhong, S, et al. Diagnostic indicators for adult-onset neuronal intranuclear inclusion disease. Clin Neuropathol. (2020) 39:7–18. doi: 10.5414/NP301203
12. Zannolli, R, Gilman, S, Rossi, S, Volpi, N, Bernini, A, Galluzzi, P, et al. Hereditary neuronal intranuclear inclusion disease with autonomic failure and cerebellar degeneration. Arch Neurol. (2002) 59:1319–26. doi: 10.1001/archneur.59.8.1319
13. Tian, Y, Zhou, L, Gao, J, Jiao, B, Zhang, S, Xiao, Q, et al. Clinical features of NOTCH2NLC-related neuronal intranuclear inclusion disease. J Neurol Neurosurg Psychiatry. (2022) 93:1289–98. doi: 10.1136/jnnp-2022-329772
14. Nakamura, M, Ueki, S, Kubo, M, Yagi, H, Sasaki, R, Okada, Y, et al. Two cases of sporadic adult-onset neuronal intranuclear inclusion disease preceded by urinary disturbance for many years. J Neurol Sci. (2018) 392:89–93. doi: 10.1016/j.jns.2018.07.012
15. Visser, M, Marinus, J, Stiggelbout, AM, and Van Hilten, JJ. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord. (2004) 19:1306–12. doi: 10.1002/mds.20153
16. Rodriguez-Blazquez, C, Forjaz, MJ, Frades-Payo, B, De Pedro-Cuesta, J, and Martinez-Martin, P, Longitudinal Parkinson’s Disease Patient Study, Estudio Longitudinal de Pacients con Enfermedad da Parkinson Group. Independent validation of the scales for outcomes in Parkinson’s disease-autonomic (SCOPA-AUT). Eur J Neurol. (2010) 17:194–201. doi: 10.1111/j.1468-1331.2009.02788.x
17. Verbaan, D, Marinus, J, Visser, M, Van Rooden, SM, Stiggelbout, AM, and van Hilten, JJ. Patient-reported autonomic symptoms in Parkinson disease. Neurology. (2007) 69:333–41. doi: 10.1212/01.wnl.0000266593.50534.e8
18. Zhou, Z, Zhou, X, Zhou, X, Xiang, Y, Zhu, L, Qin, L, et al. Characteristics of autonomic dysfunction in Parkinson’s disease: a large Chinese multicenter cohort study. Front Aging Neurosci. (2021) 13:761044. doi: 10.3389/fnagi.2021.761044
19. Aziz, NA, Anguelova, GV, Marinus, J, Van Dijk, JG, and Roos, RA. Autonomic symptoms in patients and pre-manifest mutation carriers of Huntington’s disease. Eur J Neurol. (2010) 17:1068–74. doi: 10.1111/j.1468-1331.2010.02973.x
20. Damon-Perriere, N, Foubert-Samier, A, De Cock, VC, Gerdelat-Mas, A, Debs, R, Pavy-Le Traon, A, et al. Assessment of the SCOPA-AUT questionnaire in multiple system atrophy: relation to UMSARS scores and progression over time. Parkinsonism Relat Disord. (2012) 18:612–5. doi: 10.1016/j.parkreldis.2011.12.009
21. Del Pino, R, Murueta-Goyena, A, Acera, M, Carmona-Abellan, M, Tijero, B, Lucas-Jimenez, O, et al. Autonomic dysfunction is associated with neuropsychological impairment in Lewy body disease. J Neurol. (2020) 267:1941–51. doi: 10.1007/s00415-020-09783-7
22. Folstein, MF, Folstein, SE, and Mchugh, PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
23. Nasreddine, ZS, Phillips, NA, Bedirian, V, Charbonneau, S, Whitehead, V, Collin, I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
24. Dubois, B, Slachevsky, A, Litvan, I, and Pillon, B. The FAB: a frontal assessment battery at bedside. Neurology. (2000) 55:1621–6. doi: 10.1212/WNL.55.11.1621
25. Cummings, JL, Mega, M, Gray, K, Rosenberg-Thompson, S, Carusi, DA, and Gornbein, J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. (1994) 44:2308–14. doi: 10.1212/WNL.44.12.2308
26. Bucks, RS, Ashworth, DL, Wilcock, GK, and Siegfried, K. Assessment of activities of daily living in dementia: development of the Bristol activities of daily living scale. Age Ageing. (1996) 25:113–20. doi: 10.1093/ageing/25.2.113
27. Barnett, JL, Mcdonnell, WM, Appelman, HD, and Dobbins, WO. Familial visceral neuropathy with neuronal intranuclear inclusions: diagnosis by rectal biopsy. Gastroenterology. (1992) 102:684–91. doi: 10.1016/0016-5085(92)90121-E
28. Kulikova-Schupak, R, Knupp, KG, Pascual, JM, Chin, SS, Kairam, R, and Patterson, MC. Rectal biopsy in the diagnosis of neuronal intranuclear hyaline inclusion disease. J Child Neurol. (2004) 19:59–62. doi: 10.1177/08830738040190010707
29. Sung, JH, Ramirez-Lassepas, M, Mastri, AR, and Larkin, SM. An unusual degenerative disorder of neurons associated with a novel intranuclear hyaline inclusion (neuronal intranuclear hyaline inclusion disease). A clinicopathological study of a case. J Neuropathol Exp Neurol. (1980) 39:107–30. doi: 10.1097/00005072-198003000-00001
30. Michaud, J, and Gilbert, JJ. Multiple system atrophy with neuronal intranuclear hyaline inclusions. Report of a new case with light and electron microscopic studies. Acta Neuropathol. (1981) 54:113–9. doi: 10.1007/BF00689403
Keywords: neuronal intranuclear inclusion disease, autonomic dysfunction, SCOPA-AUT, assessment, autonomic symptoms
Citation: Zhou L, Tian Y, Zhang S, Jiao B, Liao X, Zhou Y, Xiao Q, Xue J, Duan R, Tang B and Shen L (2023) Characteristics of autonomic dysfunction in neuronal intranuclear inclusion disease. Front. Neurol. 14:1168904. doi: 10.3389/fneur.2023.1168904
Received: 20 February 2023; Accepted: 22 May 2023;
Published: 14 June 2023.
Edited by:
Marcondes C. França, State University of Campinas, BrazilReviewed by:
Juan Idiaquez, Pontificia Universidad Católica de Chile, ChileCopyright © 2023 Zhou, Tian, Zhang, Jiao, Liao, Zhou, Xiao, Xue, Duan, Tang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Shen, c2hlbmx1QGNzdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.