
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 28 June 2023
Sec. Neuro-Ophthalmology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1163615
 Rong Yan1
Rong Yan1 Yu Mao2
Yu Mao2 Huiyang Zeng3
Huiyang Zeng3 Qian Liu3
Qian Liu3 Hanqiu Jiang1
Hanqiu Jiang1 Jingting Peng1
Jingting Peng1 Qingling Yang1
Qingling Yang1 Shilei Cui1
Shilei Cui1 Lei Liu1
Lei Liu1 Yanjun Guo1
Yanjun Guo1 Jiawei Wang1*
Jiawei Wang1*Objective: Collapsin response mediator protein 5-associated optic neuropathy (CRMP5-ON) is a rare entity of autoimmune optic neuropathy. This study aimed to review the neuro-ophthalmic findings and outcomes in a series of patients with CRMP5-ON to further characterize its clinical phenotype, radiologic clues, and outcomes.
Methods: This was a retrospective case series and a single-center medical chart review of all patients with CRPM5-seropositive ON at the Department of Neurology, Beijing Tongren Hospital, from December 1, 2020, to March 31, 2023. The main outcome measures were neuro-ophthalmic manifestations, radiologic characteristics, and clinical outcomes of CRMP5-ON; coexisting neural autoantibody, paraneoplastic associations, and the impact of immunosuppressant therapy.
Results: Five patients were identified. Four (80%) were female, and the average age at onset was 59.4 years (range 53–69 years), with an average follow-up of 15.3 months (range 1.4–28.7 months). The average best-corrected visual acuity (BCVA) at nadir was 20/120 (range 20/20 to count fingers). Seven of ten affected eyes (70%) showed diffuse defects of the central field. Painless bilateral involvement and optic disk edema occurred in 100% of patients, combined with vitritis, uveitis, or retinitis in four (80%). Four patients (80%) had MRI abnormalities along the optic nerve (one patient with optic nerve enhancement and three patients had optic nerve sheath enhancement or peribulbar fat enhancement). Three patients (60%) had optic neuropathy with other neurologic symptoms. Four patients (80%) had confirmed cancer (two were small-cell lung carcinoma, one was papillary thyroid carcinoma and another was thymoma and invasive pulmonary adenocarcinoma). All cancers were identified after the presentation of the optic neuropathy. The intervention included IVIG, IVMP, surgery and chemotherapy. The average BCVA at the last follow-up was 20/50 (range 20/20 to count fingers). Three patients had surgery during the initial hospitalization, and were stable during the follow-up. Among two patients who received IVMP, both had improvement after treatment, although one patient had worsening non-ocular neurologic symptoms during the steroid taper.
Conclusion: CRMP5-ON presented with optic disc edema, often bilateral involved and combined with vitreitis, retinitis, or uveitis. CRMP5-ON can present with MRI optic nerve or perineural optic nerve enhancement, especially in the optic nerve sheath. CRMP5-ON is closely related to paraneoplastic neurologic syndrome. Cancer screening and intervention are crucial to prognosis.
Currently, optic neuritis has entered the era of biomarkers (1). Patients with optic neuropathy have three clinically validated and specific autoantibodies, binding to aquaporin 4, myelin oligodendrocyte glycoprotein (MOG), and collapsin response mediator protein 5 (CRMP5), which are considered helpful in diagnosing and classifying optic neuritis (2). Recently, Petzold et al. provided a consensus view on the diagnosis and classification of optic neuritis (2). They proposed a new classification of autoimmune optic neuritis, including MS-associated optic neuritis (MS-ON), AQP4 antibody–associated optic neuritis (AQP4-ON), MOG antibody–associated optic neuritis (MOG-ON), and CRMP5 antibody–associated optic neuritis (CRMP5-ON). Compared with typical optic neuritis (usually MS-ON), atypical optic neuritis often presents as bilateral simultaneous or rapidly sequential involvement,severe optic disk swelling, painless and severe visual loss. Some patients have a family or neoplastic history,needing prolonged immunosuppressive regimens (3).
The collapsin response mediator protein (CRMP) family comprises five cytosolic phosphoproteins strongly expressed in the developing nervous system. CRMP5 is mainly expressed in oligodendrocytes, olfactory bulbs, olfactory epithelium, retina, hippocampal dentate gyrus, peripheral nerve axons, and sensory neurons (4). In 1996, Honnorat et al. found that the CV2 antibody was specific for oligodendrocytes and considered that the target antigen was CRMP3 (66 kDa); they were the first to clarify the relationship between CV2 and paraneoplastic neurologic syndrome (PNS) (5). In 2001, Mayo Clinic reported 116 CRMP5-seropositive patients (7% with optic neuropathy). The CV2 antibody was found to be actually specific for CRMP5 (62 kDa) and considered a biomarker of lung cancer and thymoma-related paraneoplastic syndrome (6). CRMP5-ON, as a kind of paraneoplastic optic neuropathy (PON), is associated with cancer and PNS, which is rarer than AQP4-ON and MOG-ON clinically. As previously reported, CRMP5-ON typically presents with painless, bilateral, and subacute progressive vision loss, almost always combined with vitritis, retinitis, or both, but it rarely causes enhancement of the optic nerve on MRI (7, 8). Here we reported a cohort of patients with CRMP5-ON in China with different MRI features of the optic nerve to further characterize this disease.
This study was approved by the ethics committee of Beijing Tongren Hospital, and informed consent was exempted because this study was a retrospective case series. We reviewed all the clinical records of patients with optic neuropathy identified as seropositive for the CRMP5 antibody from the database of the Beijing Tongren Hospital (December 1, 2020, to August 31, 2022). These patients were diagnosed with CRMP5 antibody–associated optic neuropathy (CRMP5-ON) according to the 2022 diagnosis and classification of optic neuritis (2), combined with the clinical manifestations and laboratory examination at baseline. The diagnostic criteria of PNS were followed by the updated diagnostic criteria for PNS and PNS-CARE score (9). The findings of demographic, clinical, pathologic, and neuro-ophthalmology examinations were tabulated, which included best-corrected visual acuity (BCVA) at presentation and final follow-up (with Snellen conversion to the logarithm of the minimum angle of resolution for analysis), poor VAs ≤20/400 (such as “count fingers” or “hand movements”) is defined as 1.3 LogMAR for analysis. Humphrey visual field,optical coherence tomography (OCT), fluorescein fundus angiography (FFA), and magnetic resonance imaging (MRI) of the brain and optic nerve. The MRI and positron emission tomography (PET-CT) of the spinal cord were partially obtained.
The blood tests included antinuclear antibody, anti-double-stranded DNA, anti-Sm, Sjogren syndrome A or Sjogren syndrome B, anticardiolipin (ACL and β2-GPI), antineutrophil cytoplasmic antibodies, rheumatoid factor, and human leucocyte antigen B27 phenotype. The cerebrospinal fluid (CSF) samples were used for the routine testing of white cell counts, total protein levels, concentrations of immunoglobulin G (IgG), and cytopathology. The serum AQP4 antibody and MOG antibody were detected using the cell-based assay. The PNS antibody examinations in serum, including ANNA-1/anti-Hu, PCA-1/Yo, ANNA-2/Ri, Ma2, CRMP5, amphiphysin, recoverin, and SOX1 antibodies, were performed using Western blot assay. Autoimmune encephalitis antibodies were detected using indirect immunofluorescence testing, including anti-N-methyl-D-aspartate receptor, leucine-rich glioma inactivating 1 protein, contact protein–associated protein (Caspr2), α-amino-3-hydroxy-5-methyl-4-isoxalpropionic acid receptor, and γ-aminobutyric acid type B receptor antibody. The serum of two patients with retinitis were sent to the Beijing Institute of Ophthalmology, Beijing Ophthalmology & Visual Sciences Key Laboratory, for the detection of anti-retinal antibodies (α-enolase, CRMP5, recoverin and carbonic anhydraseII etc.) using Western blot assay before treatment.
A total of five patients (four female and one male) diagnosed with CRMP5-ON were identified. The age ranged from 53 to 69 years (average 59.4 years). Table 1 summarizes the visual manifestations, neurological accompaniments, imaging and spinal fluid abnormalities, coexisting neuronal autoantibodies, type of cancer, and clinical course. Patient 1 had a history of colon polypectomy, patient 2 was a smoker with a history of Brucella infection, patient 4 had a history of deep vein thrombosis (DVT), and patient 5 had a history of Hepatitis C and atopic dermatitis. The duration of vision loss at nadir was 7–10 days in three patients and 2 months in two patients. Active cancer was confirmed in four patients (80%) histologically: two had small-cell lung carcinoma, one had papillary thyroid carcinoma, and another was thymoma and invasive pulmonary adenocarcinoma. Patient 4 who lacked the confirmation of cancer showed elevated serum levels of squamous cell carcinoma–associated antigen and had a history of DVT. All cancers were diagnosed after the presentation of the optic neuropathy, and the average time from optic neuropathy to cancer diagnosis was 5.8 months (ranging 1–12 months).
All patients were bilateral involvement without pain: two were simultaneous and three were sequential. Seven of 10 affected eyes (70%) showed a diffuse defect of the central field, and the remaining had peripheral field defect, quadrant field defect, and blind spot enlargement, respectively. The average BCVA at nadir was 20/120 (range from 20/20 to count fingers). All patients had optic disk edema, four of five (80%) with associated vitritis, retinitis, or uveitis, only one (patient 4) had isolated optic disc edema without other ocular findings (Table 1). Patient 1 had bilateral vitritis and uveitis (Figures 1A,B). Patient 2 had bilateral vitritis, retinitis and right eye choroidal neovascularization (CNV; Figures 1C,D). Patient 5 had bilateral vitritis and retinitis (Figures 1E,F).
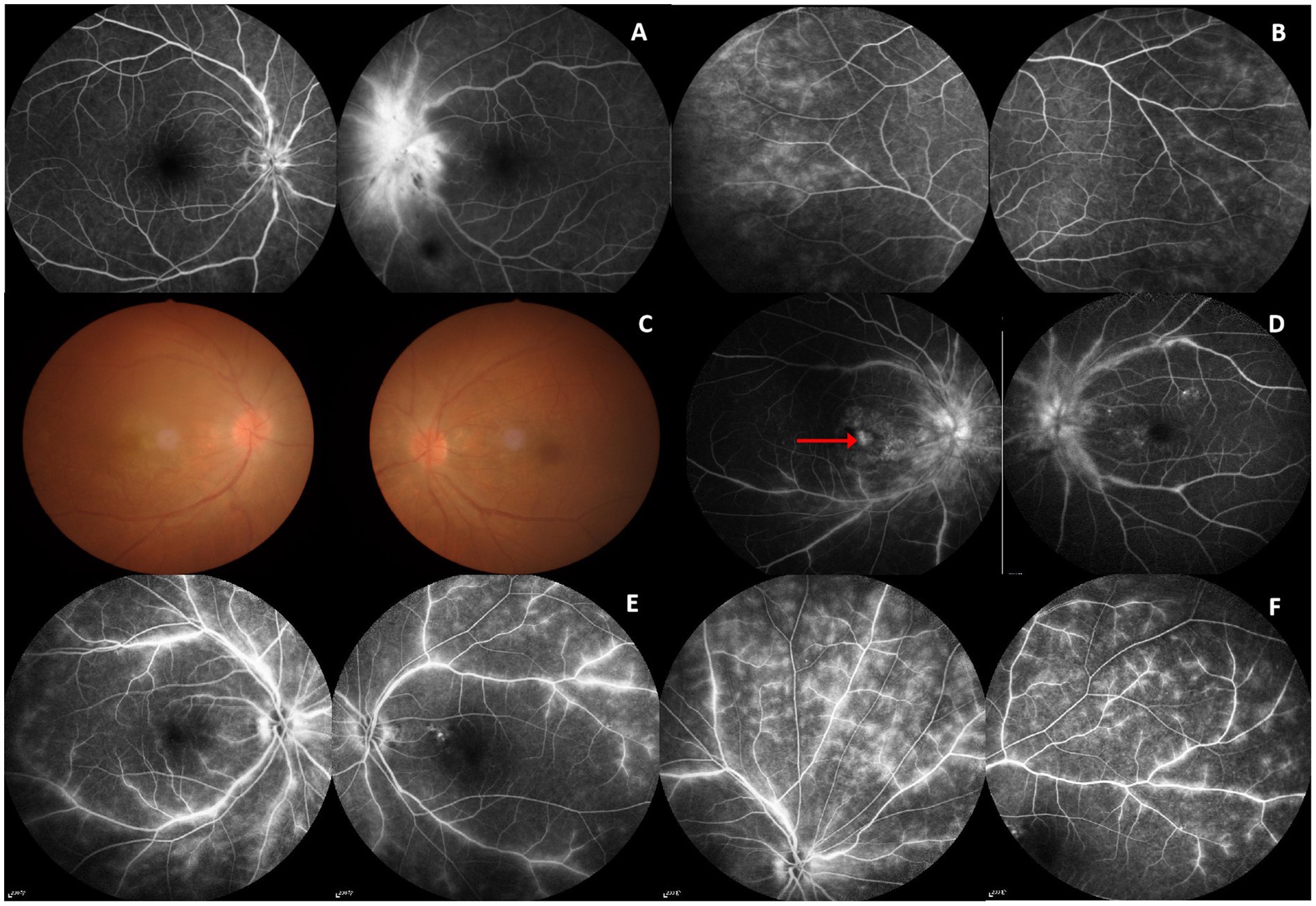
Figure 1. FFA and fundus image. Patient 1 (F, 59 years): (A) FFA: mild disk edema in the right eye and disk edema in the left eye with small hemorrhage. (B) FFA: Bilateral retinal telangiectasia and fluorescence leakage. Patient 2 (M, 69 years): (C) Fundus image: Bilateral disk edema and vitreous opacity. (D) FFA: Bilateral disk edema, retinal vascular staining, and leakage; late frame of the right eye showing hyperfluorescence in the macular area caused by leakage from choroidal neovascularization (CNV; red arrowed). Patient 5 (F,61 years): (E,F) FFA: Bilateral hyperfluorescence and leakage of the optic disc and retinal.
Three patients (60%) had other neurological symptoms or signs during their illness (Table 1). One had painful polyradiculoneuropathy 2 months before ON, one had painful polyradiculoneuropathy 6 months before ON and then myelopathy and cerebellar ataxia 4 months after ON, and one had area postrema syndrome 3 months before ON and myelopathy 1 month before ON.
Four of five (80%) patients had optic nerve or perineural optic nerve enhancement. Only one (patient 2) with MRI performed 1 year after initial visual loss showed bilateral optic nerve atrophy and thinning without T2 lesions or enhancement. The others had MRI within 9 months since visual loss presented with enhancement (patient 1 had left bulbar fascia (retro-ocular orbital fat) enhancement and T2 lesion on the left side of optic chiasma without corresponding enhancement; patient 3 and 4 had bilateral longitudinally optic nerve sheath enhancement; patient 5 had left optic nerve enhancement). Two patients had myelopathy. Spinal cord MRI showed T2 lesions of the involved spinal cord with T1 mild enhancement. Patient 5 was found a meningioma in the left cerebellar (Figures 2A–H). Of the five patients, two completed the PET-CT examination: patient 1 showed a hypermetabolic mass in the left hilum of the lung; patient 3 showed a hypermetabolic nodule in the left lobe of the thyroid and a local metabolic increase in the left cerebellar hemisphere and cervical spinal cord during the follow up.
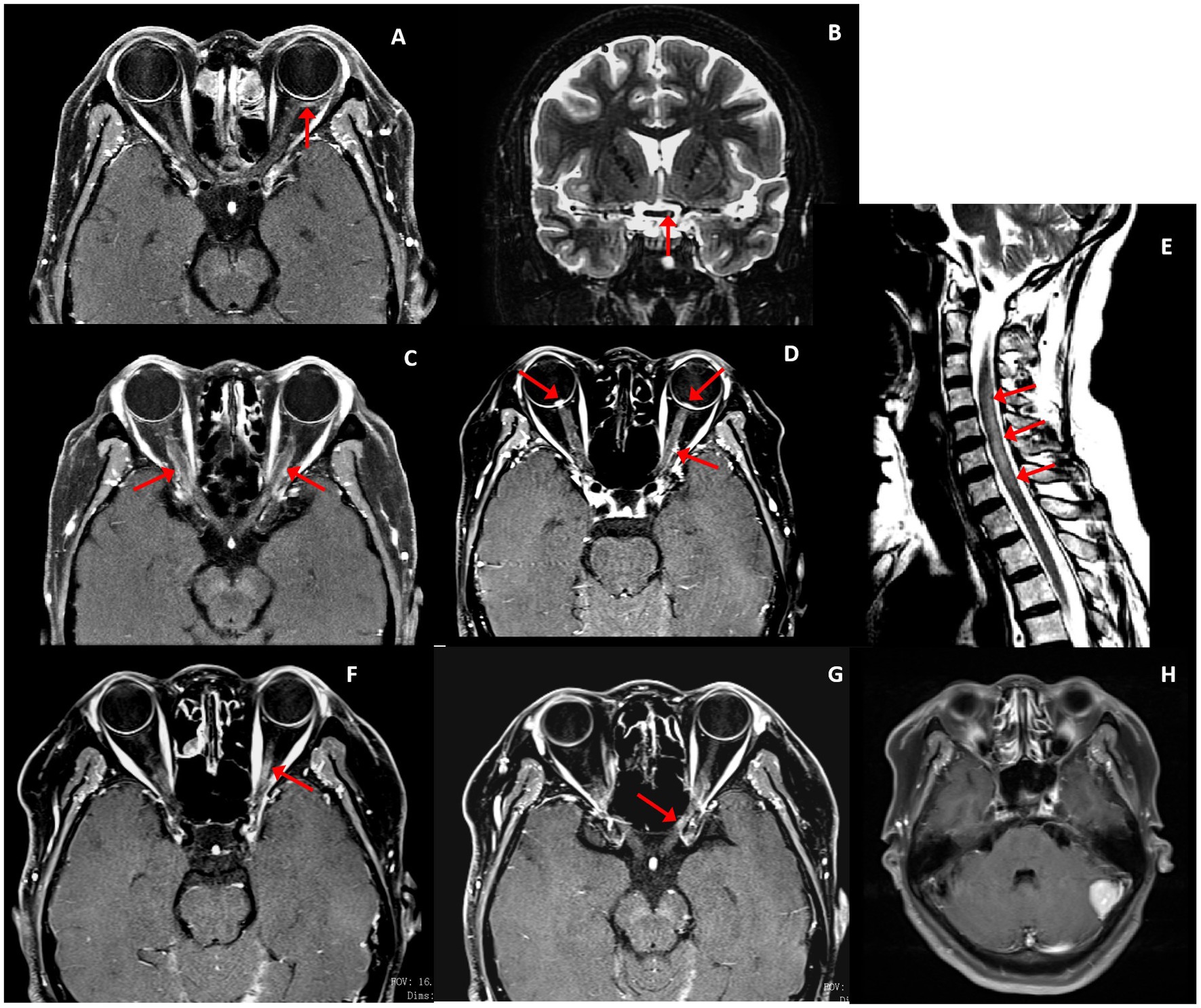
Figure 2. Optic nerve and spinal cord MRI (red arrowed). Patient 1 (F, 59 years): (A) Post-gadolinium axial T1 MRI demonstrated left bulbar fascia mild enhancement. (B) T2 lesion on the left side of the optic chiasma. Patient 4 (F, 55 years): (C) Post-gadolinium axial T1 MRI demonstrated bilateral optic nerve sheath mild enhancement. Patient 3 (F,53 years): (D) Bilateral optic nerve sheath mild enhancement and optic disk enhancement. (E) C2–7 T2 lesions. Patient 5 (F,61 years): (F,G) Left optic nerve enhancement. (H) Left cerebellar meningioma.
All of the serum samples were negative for the AQP4 and MOG antibodies, and no one had abnormal serum autoimmune antibodies. The coexisting neural autoantibodies included antineuronal nuclear-1 (ANNA-1/anti-Hu; 2, 40%), SOX1 (1, 20%), α-enolase (1, 20%), and α-enolase with carbonic anhydraseII (1, 20%). The cerebrospinal fluid (CSF) demonstrated an elevation of white blood cell (WBC) count in two patients (50%) and an elevation of protein and intrathecal IgG synthesis in three patients (75%). The CSF pressure and cytology were initially normal. During the follow-up after an outpatient visit, one patient developed myelopathy and cerebellar ataxia. The CSF pressure and the synthesis rates of protein and intrathecal IgG were higher than earlier, and a few degenerative cells were found on cytological examination.
The average follow-up time was 15.3 months, ranging from 1.4 to 28.7 months. The average BCVA at the last follow-up was 20/50 (range 20/20 to count fingers). The visual acuity of patient 1 did not improve after IVIG, then the patient had surgery and chemotherapy. At 28.7 months of follow-up, the patient’s visual acuity was stable, cancer did not recur, and no other neurological symptoms appeared. Patient 2 only had surgery and chemotherapy, he was stable after 8.5 months follow-up. Only two patients (patient 3 and 4) received IVMP, their visual acuity improved after the treatment. But patient 3 developed myelopathy and cerebellar ataxia during the steroid taper. These symptoms were getting stable after she had surgery of thyroid. Patient 4 was followed up to 27.7 months, the dose of oral methylprednisolone was reduced to 2 mg/day. Her symptoms were stable, and no cancer was found yet. Patient 5 only had surgery, and her visual acuity was stable so far (Table 2).
In 2003, Cross et al. reported 13 patients with CRMP5-seropositive optic neuropathy with retinitis and/or vitritis (7). The orbital MRI was infrequently performed in these earlier studies, therefore the actual incidence of optic nerve enhancement was not known. In 2019, Cohen et al. reported 14 patients with CRMP5-IgG-positive optic neuropathy with optic disk edema, often associated with uveitis and/or retinitis, but without optic nerve signal enhancement (8). Within our CRPM5-ON cohort, all patients presented with painless, bilateral involvement and optic disk edema. Only 1 of our patients with optic disc edema did not have accompanying vitritis or retinitis. Most of them had vitritis, retinitis, or uveitis, which were similar to the previously reported findings; so did the demographic characteristics, coexisting neural autoantibodies, and cancer type (7, 8). However, unlike previous studies (7, 8), most of our patients had optic nerve or perineural optic nerve enhancement (Table 3). Enhancement of optic nerves has been reported in CRMP5 optic neuropathy in some case reports (10, 11). Pathologically, perivascular lymphocytic infiltration in the optic nerve is found along with axonal demyelination, and immunohistochemical studies revealed CD8+ T-cells infiltrating the optic nerve (7). Therefore, CRMP5-ON can present with MRI optic nerve or perineural optic nerve enhancement, especially in the optic nerve sheath. Unlike the typical optic nerve enhancement seen in other causes of optic neuritis (MS, MOGAD, and NMOSD), the enhancement of CRMP5-ON is milder. We assumed that early investigation and the advance orbital MRI techniques and protocols used (2) might increase sensitivity of MRI to detect active inflammation within or around the optic nerve.
We detected serum autoantibodies against α-enolase and carbonic anhydrase II in two of our patients, but not recoverin. The α-enolase and carbonic anhydrase II antibody are anti-retinal antibody (ARA), which in combination with clinical manifestations have been proposed to have important diagnostic value for confirmation of autoimmune retinopathy (12). However, a recent study showed these ARA’s can be present in up to 2/3 of normal individuals, and therefore are fairly nonspecific (13). Most likely, the retinopathy in these cases was related to the CRMP5 antibody, which has been shown to cause retinitis in addition to optic neuropathy and vitritis. Patient 5 did not test electroretinography, but her OCT showed loss of the inner segment/outer segment (IS/OS) junction, and FFA revealed hyperfluorescence and leakage of the optic disc and retinal. Combined with clinical manifestations we diagnosed the patient CRPM5-ON with cancer-associated retinopathy (CAR).
In 2021,the PNS-Care panel updated the diagnostic criteria for PNS and developed a scoring system (PNS-Care Score); paraneoplastic retinopathy and optic neuropathy are not included within the spectrum of PNS (9). PNS of CRMP5 had high clinical heterogeneity. Painful axonal asymmetric polyradiculoneuropathy was established as the major CRMP5 autoimmune neuropathy presentation (65%). The other neurologic associations were cerebellar ataxia (21%), myelopathy (19%), optic neuropathy, and/or retinitis (11%) (14). Within our cohort, 60% of patients had other symptoms of nervous system involvement besides the optic nerve: patient 1 had peripheral neuropathy before ON, complicated with SCLC, PNS-Care scored 10; patient 3 had peripheral neuropathy before ON and myelopathy and cerebellar ataxia after ON, complicated with papillary thyroid carcinoma, PNS-Care scored 10; and patient 4 with a history of DVT had area postrema syndrome and myelopathy before ON, no cancer was found with a follow-up of less than 2 years, but the serum level of squamous cell carcinoma–associated antigen was elevated, PNS-Care scored 7. The first two cases met the diagnostic criteria of definite PNS, and the third case was probable PNS. Therefore, CRMP5-ON can be regarded as a clinical manifestation of PNS, and patients with isolated CRMP5-ON need to be alert toward its future progression to PNS. The latter two patients had clinical manifestations that had some overlap with seronegative NMOSD, but the detection of the CRMP5 antibody changed the following intervention strategy. Therefore, patients with some features of NMOSD, MOGAD or MS with an atypical optic neuropathy combined with retinitis or vitritis should be evaluated for CRMP5 antibodies.
No guidelines exist for the treatment of CRMP5-ON. Local or systemic applications of glucocorticoids, plasma exchange, IVIG, and immunosuppressants have been reported. Most of them are combined with surgery and chemoradiotherapy to intervene the primary tumor. In a cohort, 58% of patients died within 5 years, with a median follow-up of 9.5 months (8). In our cohort, all patients survived at the last time of follow-up (ranged 1.4 to 28.7 months). Three patients had surgery during the initial hospitalization, and were stable during the follow-up. Only two patients who received IVMP improved after the treatment, one patient had worsening non-ocular neurologic symptoms during the steroid taper. After the papillary thyroid carcinoma removed by surgery, the patient stabled. Treatment like IVIG and IVMP may be helpful for visual recovery in a short term, but long-term therapy requires intervention for primary cancer. Therefore, early cancer screening is crucial for prognosis.
This study had a few limitations. A single cohort, small sample size, and short follow-up period hindered the evaluation of the prognosis and relapse rate over the course of the disease. Another limitation was the retrospective design, leading to a certain bias in the data collection. However, this study provided more detailed clinical characteristics of the largest group of patients with CRMP5-ON in China to date. Prospective studies with a larger sample size and longer follow-ups are warranted to confirm our findings.
In conclusion, atypical optic neuropathy seronegative for AQP4 and MOG antibodies, combined with vitreitis, retinitis, or uveitis, should prompt serological testing for CRMP5 antibody. CRMP5-ON can present with MRI optic nerve or perineural optic nerve enhancement, especially in the optic nerve sheath, early investigation might increase sensitivity of MRI. CRMP5-ON also has many neurologic manifestations closely related to PNS. Cancer screening and intervention are crucial to prognosis. Further investigation is needed for a comprehensive understanding of this disease.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the ethics committee of Beijing Tongren Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
RY, YM, and LL contributed to conception and design of the study. RY, YM, HZ, QL, HJ, JP, QY, and SC organized the database. RY performed the statistical analysis and wrote the first draft of the manuscript. YG and JW revised sections of the manuscript. All authors contributed to the article and approved the submitted version.
Early diagnosis and prognostic factors of MOG antibody positive demyelinating optic neuropathy, Capital Health Development Scientific Research Project (2020-2-2056, from 2020-07-01 to 2023-06-30). Study on the mechanism of PD-1/PD-L1 and SOX1 in paraneoplastic associated anti-GABABR encephalitis, The National Natural Science Foundation of China (82271384, from 2023-01 to 2026-12).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen, JJ, Pittock, SJ, Flanagan, EP, Lennon, VA, and Bhatti, MT. Optic neuritis in the era of biomarkers. Surv Ophthalmol. (2020) 65:12–7. doi: 10.1016/j.survophthal.2019.08.001
2. Petzold, A, Fraser, CL, Abegg, M, Alroughani, R, Alshowaeir, D, Alvarenga, R, et al. Diagnosis and classification of optic neuritis. Lancet Neurol. (2022) 21:1120–34. doi: 10.1016/S1474-4422(22)00200-9
3. Toosy, AT, Mason, DF, and Miller, DH. Optic neuritis. Lancet Neurol. (2014) 13:83–99. doi: 10.1016/S1474-4422(13)70259-X
4. Charrier, E, Reibel, S, Rogemond, V, Aguera, M, Thomasset, N, and Honnorat, J. Collapsin response mediator proteins (CRMPs): involvement in nervous system development and adult neurodegenerative disorders. Mol Neurobiol. (2003) 28:51–64. doi: 10.1385/MN:28:1:51
5. Honnorat, J, Antoine, JC, Derrington, E, Aguera, M, and Belin, MF. Antibodies to a subpopulation of glial cells and a 66 kDa developmental protein in patients with paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. (1996) 61:270–8. doi: 10.1136/jnnp.61.3.270
6. Yu, Z, Kryzer, TJ, Griesmann, GE, Kim, K, Benarroch, EE, and Lennon, VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. (2001) 49:146–54. doi: 10.1002/1531-8249(20010201)49:2<146::AID-ANA34>3.0.CO;2-E
7. Cross, SA, Salomao, DR, Parisi, JE, Kryzer, TJ, Bradley, EA, Mines, JA, et al. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann Neurol. (2003) 54:38–50. doi: 10.1002/ana.10587
8. Cohen, DA, Bhatti, MT, Pulido, JS, Lennon, VA, Dubey, D, Flanagan, EP, et al. Collapsin response-mediator protein 5-associated retinitis, vitritis, and optic disc edema. Ophthalmology. (2020) 127:221–9. doi: 10.1016/j.ophtha.2019.09.012
9. Graus, F, Vogrig, A, Muñiz-Castrillo, S, Antoine, JCG, Desestret, V, Dubey, D, et al. Updated diagnostic criteria for Paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1014. doi: 10.1212/NXI.0000000000001014
10. Xu, Q, du, W, Zhou, H, Zhang, X, Liu, H, Song, H, et al. Distinct clinical characteristics of paraneoplastic optic neuropathy. Br J Ophthalmol. (2019) 103:797–801. doi: 10.1136/bjophthalmol-2018-312046
11. Sheorajpanday, R, Slabbynck, H, Van De Sompel, W, Galdermans, D, Neetens, I, and Deyn, D. Small cell lung carcinoma presenting as collapsin response-mediating protein (CRMP) -5 paraneoplastic optic neuropathy. J Neuroophthalmol. (2006) 26:168–72. doi: 10.1097/01.wno.0000235578.80051.0e
12. Zeng, HY, Liu, Q, Peng, XY, Cao, K, Jin, SS, and Xu, K. Detection of serum anti-retinal antibodies in the Chinese patients with presumed autoimmune retinopathy. Graefes Arch Clin Exp Ophthalmol. (2019) 257:1759–64. doi: 10.1007/s00417-019-04359-2
13. Chen, JJ, McKeon, A, Greenwood, TM, Flanagan, EP, Bhatti, MT, Dubey, D, et al. Clinical utility of antiretinal antibody testing. JAMA Ophthalmol. (2021) 139:658–62. doi: 10.1001/jamaophthalmol.2021.0651
Keywords: anti-CRMP5 autoantibody, magnetic resonance imaging, optic neuritis, paraneoplastic neurologic syndromes, autoimmune optic neuropathy
Citation: Yan R, Mao Y, Zeng H, Liu Q, Jiang H, Peng J, Yang Q, Cui S, Liu L, Guo Y and Wang J (2023) Collapsin response mediator protein 5-associated optic neuropathy: clinical characteristics, radiologic clues, and outcomes. Front. Neurol. 14:1163615. doi: 10.3389/fneur.2023.1163615
Received: 11 February 2023; Accepted: 13 June 2023;
Published: 28 June 2023.
Edited by:
John Jing-Wei Chen, Mayo Clinic, United StatesReviewed by:
Devon Cohen, Cleveland Clinic, United StatesCopyright © 2023 Yan, Mao, Zeng, Liu, Jiang, Peng, Yang, Cui, Liu, Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiawei Wang, d2FuZ2p3Y3FAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.