- 1Brain Body Mind Laboratory, Division of Physical Therapy, Division of Rehabilitation Science, Department of Rehabilitation Medicine, Medical School, University of Minnesota, Minneapolis, MN, United States
- 2Neurocenter, Luzerner Kantonsspital, Lucerne, Switzerland
- 3ARTORG Center for Biomedical Engineering Research, Gerontechnology and Rehabilitation Group, University of Bern, Bern, Switzerland
- 4Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Introduction: About 77% of adults with stroke have upper limb impairments. Many scales are available to measure the impairment and activity level of the affected limb. However, an observational scale to assess dependency on others in upper limb performance during daily life activities instead of laboratory settings is lacking. Therefore, we developed a new 5-item Upper Limb Lucerne ICF-based Multidisciplinary Observation Scale (UL-LIMOS). As next step in the psychometric analysis, we evaluated the unidimensionality and structural validity of the UL-LIMOS with Rasch Measurement Theory and we calculated a cut-off score for independent arm use in daily life activities at discharge.
Methods: This is a single-center cross-sectional study in adults with (sub) acute stroke. We applied Rasch Measurement Theory (RMT) to analyze the structural validation and unidimensionality of the UL-LIMOS. The outputs provide evidence of unidimensionality, item and person fit, overall fit, differential item functioning (DIF), principal component analysis of residuals (PCAR), person separation reliability (PSR), and residual item correlations (to identify local item dependence). Person mean location, floor and ceiling effects identify proper targeting.
Results: We recruited 407 adults with (sub) acute stroke (median age 63 years, 157 women). All items and persons fit the Rasch model. The PSR of 0.90 indicates that clinicians and researchers can reliably use the scale for individual decision-making. There were small floor (2.70%) and ceiling (13.00%) effects. The average person mean location was 1.32 ± 2.99 logits. There was no DIF. PCAR eigenvalue was 2.46 with 49.23% explained variance. Paired t-tests revealed that 0.89% of person locations were significantly different, confirming unidimensionality. One pair of items (arm and hand use and fine hand use) showed residual item correlations. The ROC’s AUC was 0.90, CI95% = [0.85–0.96] with cut-off score of ≥14/20, and high sensitivity (87%, CI95% = [81%–91%]), specificity (83%, CI95% = [77%–87%]) for independent arm use in daily living at discharge.
Discussion: The new Rasch-based UL-LIMOS is a valid ICF-based observation performance scale at the ICF-activity level, to evaluate dependency during upper limb use in daily life in adults with stroke. Additional psychometric analyses are warranted. The UL-LIMOS would be a valuable addition to the core assessments of adults with (sub) acute stroke.
1. Introduction
About 77% of adults with stroke have upper limb impairments (1). These impairments hinder performing Activities of Daily Living (ADL) independently and result in long-term dependency in 50% of the cases (2, 3). This dependency decreases the quality of life (QoL) (4) and results in an inability to return to work in 40% of working-age adults who have had a stroke (5).
Many upper limb measures evaluate motor recovery after stroke [for an overview (see 6)]. Following the International Classification of Functioning, Disability and Health (ICF) (7), these measures assess either upper limb impairment (e.g., mobility of joints, muscle power/tone/endurance), or assess activities (e.g., grasping a block of wood) in laboratory settings. Additionally, there is a distinction between performance and capacity as qualifiers for the ICF activity domain, with “capacity” being what an individual can do in a standardized environment (e.g., a clinical assessment), and ‘performance’ relating to what the person actually does in his/her/their usual environment. Of all the upper limb capacity measures, the Upper Extremity Subscale of the Fugl-Meyer Motor Assessment (FMA-UE) (8) on the impairment level and the Action Research Arm Test (ARAT) (9) on the activity level were suggested as core upper limb assessments for stroke rehabilitation trials (10) and clinical rehabilitation (11). So far, there is no consensus on scale recommendations for assessing dependency in daily upper limb use (10). The Barthel Index (BI) (12) and the Functional Independence Measure (FIM) (13)—originally designed to evaluate the need for nursing care—are commonly used in clinical rehabilitation and research to assess daily life functioning in general (11, 14), such as feeding, bathing, dressing, and undressing. However, BI (15) and FIM (16) do not focus on specific upper limb use in daily life. Moreover, problematic floor and ceiling effects (>15%) have been reported (15–17). Some patient-reported outcome measures, such as the Motor Activity Log (MAL) (18), or ABILHAND (19), which are administered through semi-structured interviews, were developed to evaluate the stroke individual’s perspective on real-life upper limb performance. Yet, due to the subjective nature of patients’ reports, these measures should not be used with adults with stroke who have moderate to severe cognitive deficits. For this reason, they were not suggested as core measures to assess daily upper limb performance (10). The Actual Amount of Use Test (AAUT) and the Functional ASsessment Test for Upper Limb (FAST-UL) are observational capacity tests, i.e., spontaneous use of the affected arm during predefined tasks in a predefined setting. Therefore, the AAUT and the FAST-UL also do not reflect spontaneous upper limb use in daily life (20, 21).
Others have used accelerometers as a measure of upper limb “performance” in daily life, as defined above, which has the advantage of not being biased by patients’ subjective reports (22, 23). However, accelerometers cannot determine what type of activity was performed. In sum, an observational scale specifically focused on assessing dependency in upper limb use during actual daily life activities (as opposed to testing in a laboratory setting) is lacking.
Recently, we demonstrated that the Lucerne ICF-based Multidisciplinary Observation Scale (LIMOS), a clinician-reported measure, was reliable and valid in evaluating the performance of activities in daily life in adults with acute and subacute stroke (24–27). Clinician-reported measures are measures scored by a health care professional based on observing the patients’ spontaneous behaviors, e.g., during their stay in the hospital or rehabilitation center. It can therefore be used in adults with moderate to severe cognitive impairments after stroke. LIMOS covered several domains (motor, communication, learning and applying knowledge, and domestic life), and showed no problematic floor or ceiling effects (24, 27). Moreover, responsiveness was higher for LIMOS than for BI and FIM (26).
Based on this previous work, and to fill the gap concerning assessing actual arm and hand use in daily life after a stroke (i.e., “performance”), we developed a new, 5-item Upper Limb Lucerne ICF-based Multidisciplinary Observation Scale (UL-LIMOS), which evaluates upper limb use in daily life. The goal of developing this new measure, derived from the reliable and valid LIMOS (24–27), is to obtain a quick evaluation measurement of dependency on others for upper limb use in daily life, for use in the hospital and rehabilitation centers, or in research (28). Therefore, a core team of clinicians (B.O. T.V., T.N.) with expertise in stroke and LIMOS used face validity to select and test the items that ultimately were selected for UL-LIMOS. As a next step in establishing the psychometrics of this scale, we aim to test the structural validity and unidimensionality of the UL-LIMOS, using Rasch measurement Theory. We also aim to use a Receiver Operating Characteristics (ROC) analysis to establish an optimal cut-off score of the UL-LIMOS with high sensitivity and specificity (> 80%) in order to predict a good overall outcome at discharge, meaning the ability to independently use the affected upper limb in daily life activities at rehabilitation discharge.
2. Materials and methods
2.1. Participants
We approached adults with (sub) acute stroke for participation in the study, who were admitted for inpatient neurorehabilitation in the rehabilitation center Neurocenter, Luzerner Kantonsspital, Lucerne, in Switzerland. Stroke diagnosis was based on the European Stroke Organization (ESO) guidelines, which are based on both clinical and Magnetic Resonance Imaging (MRI) criteria. We included adults with a first-ever acute to subacute stroke, up to 6 months post-stroke, showing unilateral ischemic or hemorrhagic supratentorial lesions (29). Adults with stroke were excluded if they had bilateral lesions. There were no other inclusion and exclusion criteria.
The study was conducted in accordance with the principles of the Declaration of Helsinki (2013) and was approved by the local Ethical Committees of the state of Luzern (BASEC-ID 2017-00998). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement (30). The participants gave written informed consent. A family member with power of attorney consented if participants had severe cognitive impairments, preventing them to consent independently.
2.2. Main outcome measures
We acquired demographic (sex, age) and clinical data (type and time after stroke, location of stroke, presence of cognitive deficits (31–33), and more specifically, unilateral neglect (34) and apraxia (35–37) for their impact on motor function) as well as the UL-LIMOS at admission. The UL-LIMOS is composed of 5 items, which are items selected from the more encompassing LIMOS (24–27). The LIMOS addressed the dependency of others during daily activities on several domains, among which motor activities (with 18 items).
Our previous reliability and validity studies on LIMOS demonstrated high internal consistency (coefficient α = 0.98), good test–retest reliability at the item level (moderate to excellent range of kappa between 0.41 and 0.84, except for two items with fair agreement, kappa = 0.32–0.37), and subscale levels (intraclass correlation coefficient r > 0.75, range 0.76–0.95) (24, 27). Inter-rater reliability demonstrated moderate to excellent agreement with kappa values ranging from 0.41 to 0.92 (24, 27) except for 12 items demonstrating fair agreement.
We demonstrated a strong convergent validity between LIMOS motor and FIM motor (r = 0.89), between LIMOS motor and Barthel Index (r = 0.92), between LIMOS motor and FIM mobility (r = 0.90), and between the subscales LIMOS knowledge and FIM cognition (r = 0.81) (24, 27). Correlations between other subscales of the LIMOS (self-care, general tasks, domestic life) and the subscales of the FIM ranged between r = 0.36–0.79 (24, 27). A moderate positive correlation was found between LIMOS cognition and communication subscale and FIM cognition (r = 0.67) (24, 26, 27).
The LIMOS motor subscale, and the applying knowledge and communication subscale were more responsive, expressed by higher effect sizes (ES = 0.65, Standardized Response Mean, SRM = 1.17 and ES = 0.52, SRM = 1.17, respectively) as compared with FIM motor (ES = 0.54, SRM = 0.96) and FIM cognition (ES = 0.41, SRM = 0.88) or Barthel (ES = 0.41, SRM = 0.65) (26).
Rasch-based LIMOS subscales fit the Rasch model after reducing and rescoring items: LIMOS subscales motor (18 items), communication (5 items), applying knowledge/cognition (13 items), and domestic life (5 items) (25).
Regarding UL-LIMOS, the 5 items, used in previous studies (28, 38) are “lifting and carrying objects” (item 1), “fine hand use” (item 2), “hand and arm use” (item 3), “washing oneself” (item 4), and “dressing” (item 5). The items were scored on a 5-point scale (0 to 4) with 0 being “patient is not able to fulfill a task or needs assistance up to 75% (corresponding to “complete”)”; 1 representing “patient is able to fulfill tasks with assistance of 25% to 75% (corresponding to “severe”); 2 being “patient is able to fulfill tasks with assistance less than 25% or under supervision (corresponding to “moderate”)”; 3 representing “patient is able to fulfill tasks independently but needs more time and/or with auxiliary materials, aids (corresponding to “slight”)”; and 4 being “patient is able to fulfill tasks independently (corresponding to “none”).” The 5 items are summed to obtain a total UL-LIMOS score, ranging from 0 representing no use of the upper arm in daily life to 20 representing independent use of the upper limb in daily life (for the manual containing the scoring sheet and instructions, see Supplementary material).
We previously demonstrated a strong positive correlation (r = 0.78) between UL-LIMOS and handgrip strength (assessed with the Jamar dynamometer), and a moderate negative correlation (r = 0.69) with the Catherine Bergego Scale, which quantifies the influence of spatial neglect-related deficits on the ADL (38).
2.3. Statistical analysis
The Rasch Measurement Theory (RMT) was applied to analyze the structural validation and unidimensionality of the new UL-LIMOS. We chose a polytomous partial credit model using the Rasch Unidimensional Measurement Model (RUMM) 2030 software.
A major advantage of RMT is that UL-LIMOS items can be hierarchically ordered from easy to difficult and that ordinal scales are converted to interval scales where person and item distributions are located on the same ruler with logit units. The Rasch prediction model states that a person with a higher level of independence in upper limb use in daily life has a higher probability of scoring higher on items than a person with less independence in upper limb use in daily life.
We followed the RULER guideline recommendations for reporting Rasch-based studies, recently published by Mallinson et al. (39) and Van de Winckel et al. (40). In short, to test these assumptions of the prediction model and aspects of unidimensionality, Chi-square statistics are calculated to evaluate the item and person fit, as well as the overall fit of the scale (39, 40). We evaluated differential item functioning (DIF) for sex (men, women), age (below or equal/above 65 years of age), type of stroke (ischemic, hemorrhagic), neglect (presence, no presence), and apraxia (presence, no presence). The principal component analysis of residuals (PCAR) provides additional information in relation to the unidimensionality of the scale (39–41) with eigenvalues and percentage variance accounted for by each principal component. Further analysis of paired t-tests provides evidence that the scale is unidimensional, if less than 5% significant difference is found when comparing the two subtests based on positive and negative loadings on the first principal component of the PCAR.
The person separation reliability (PSR) identifies the measurement’s precision and indicates whether we can reliably separate high from low person ability at a group (PSR ≥ 0.70) or individual level (PSR > 0.90) (39, 40, 42). Targeting is identified by floor and ceiling effects (>15% considered as problematic), as well as the person mean location relative to the item location, which is by default positioned on the logit scale at 0 logits ±1 standard deviation (39, 40). The scale is well targeted when the person mean location is within 0.5 logits of the item mean (39, 40). We identify local item dependence through residual correlations (43). A correlation of at least 0.2 above the average residual item correlation indicates that this pair of items have more in common with each other than with the whole scale (43). Finally, we calculated sensitivity and specificity and performed a ROC analysis using SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp.
3. Results
We recruited 407 adults with (sub) acute stroke (63.2 ± 16.0 years of age; 157 women). The demographic and clinical details are presented in Table 1. All patients were admitted to the Neurocenter rehabilitation center for inpatient neurorehabilitation between January 2014 and November 2016 (25).
3.1. Rasch-based UL-LIMOS
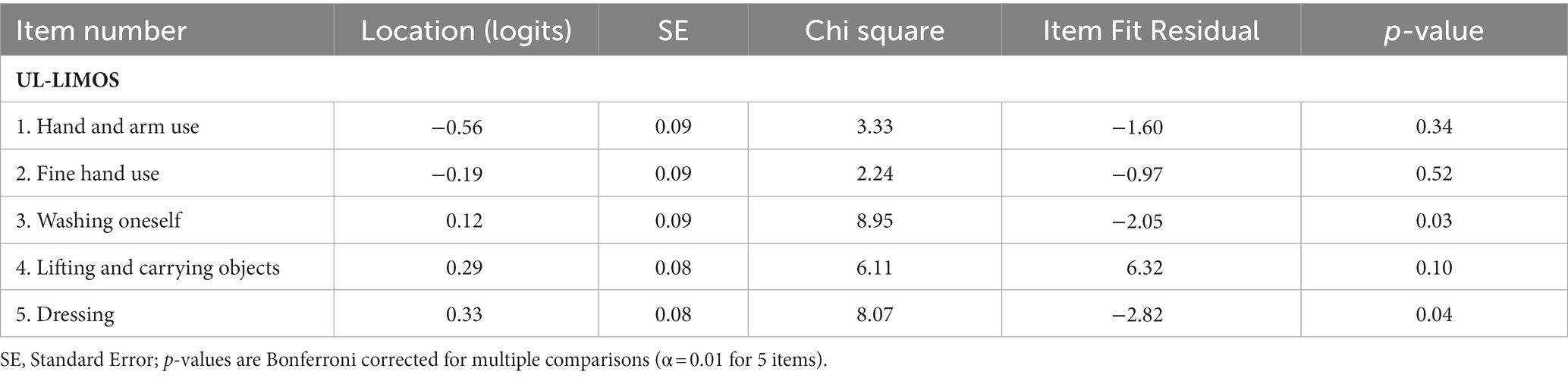
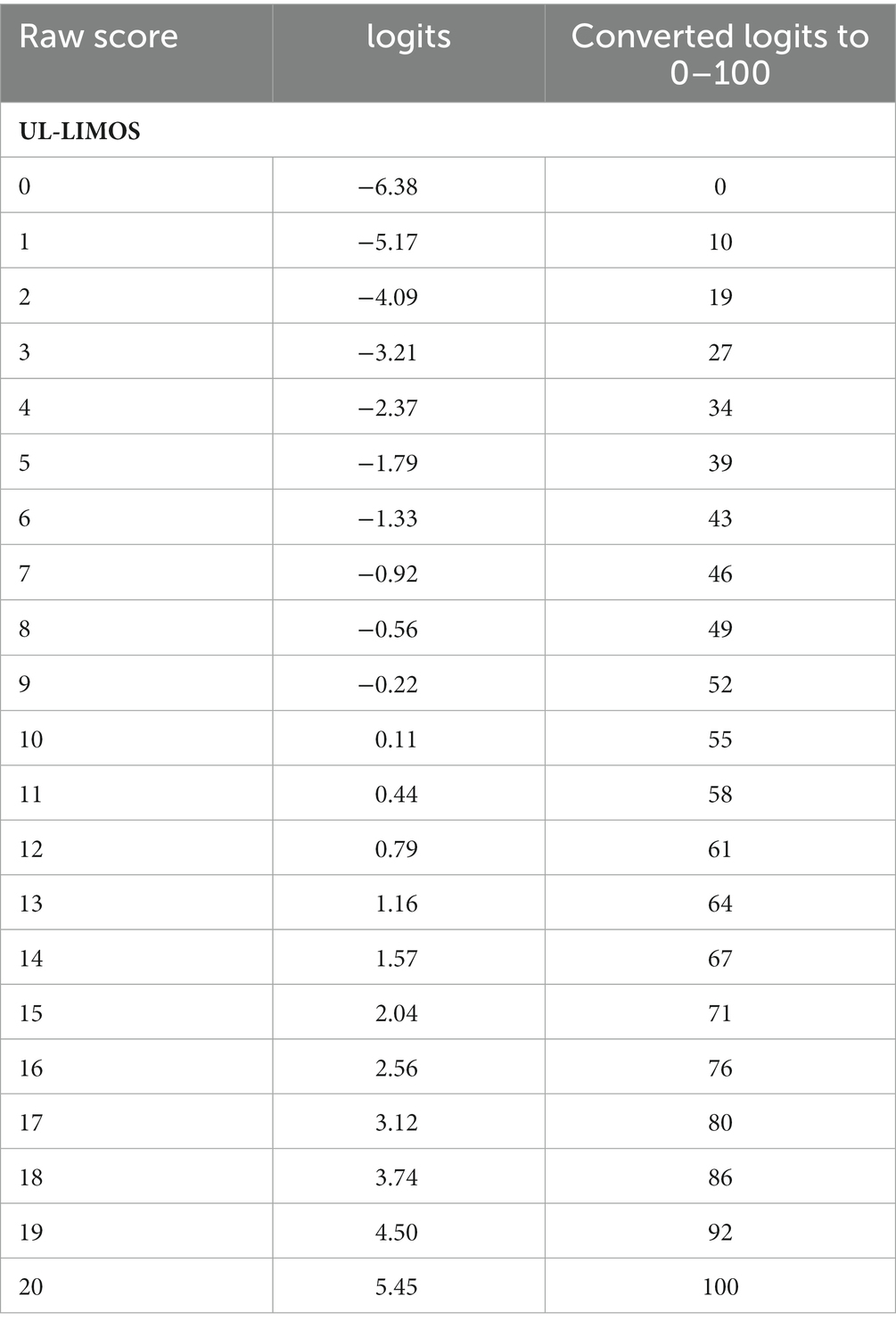
The overall fit, item and person fit, PSR, floor-and ceiling percentages, and PCAR are shown in Table 2. All items and persons fit the model. The UL-LIMOS fit the Rasch model without the need to remove or rescore items. The individual item fit is displayed in Table 3. The threshold map for the Rasch-converted UL-LIMOS is displayed in Figure 1A. This threshold map can be used in the hospital or rehabilitation center for individual patient assessment. The person-item threshold distribution is shown in Figure 1B. The total score of Rasch UL-LIMOS is displayed in Table 4, with the conversion from the original ordinal scores (0 to 20 points) to logits, and logits further converted to a 0 to 100 scoring.
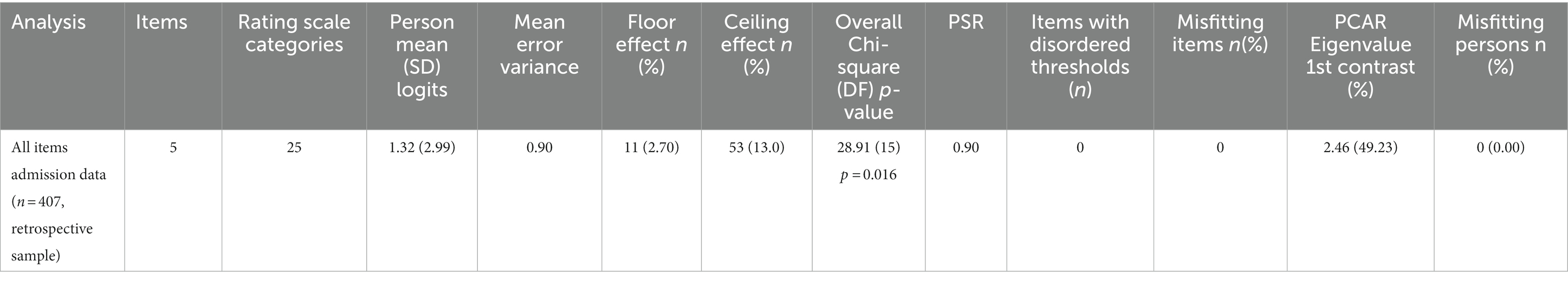
Table 2. The overall fit, item and person fit, and person separation reliability (PSR), floor-and ceiling values and percentages, and principal component analysis of residuals (PCAR).
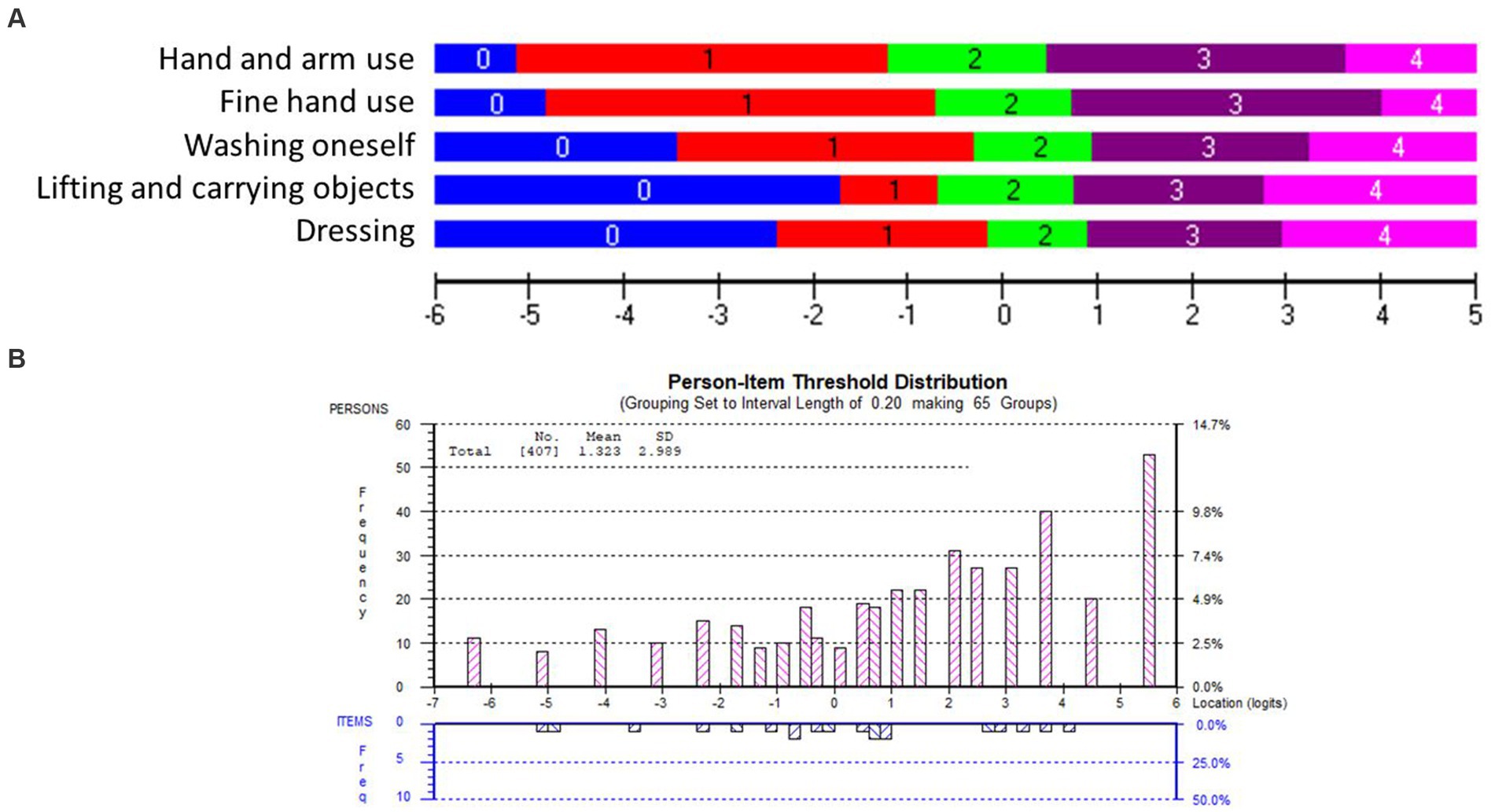
Figure 1. (A) The Rasch-based UL-LIMOS scale: item threshold map. The item threshold map depicts the difficulty of the items from the easiest item at the top to the most difficult item at the bottom along with the scoring categories. These item thresholds are matched on the same logit scale (horizontal black line at the bottom of the picture) as the person’s ability. This is a visual depiction of the interval scale, using the same color coding for each item threshold. This demonstrates that with increasing ability, it is easier to get a higher score on an easy item than on a difficult item. It also shows what score would be expected for each item, based on a person’s ability. (B) The Rasch-based UL-LIMOS scale: person-item threshold distribution. The ability of the persons (top, pink bars) is plotted on the same logit scale as the difficulty of the item thresholds (bottom, blue bars). The histograms depict the frequency of persons at a certain ability level, from a low ability on the left to a high ability on the right side of the ruler. The number of item thresholds is organized in increasing difficulty levels from the easiest on the left to the most difficult item thresholds on the right side of the ruler.
Around 13.00% of participants obtained a maximum score, meaning there was a small but not problematic ceiling effect. There was also a small (2.70%) floor effect. The average person mean location was 1.32 ± 2.99 logits, indicating that the items were too easy for this group of adults with (sub) acute stroke. PSR was 0.90, meaning the scale can reliably distinguish individuals of different ability levels for decision-making in research and in the hospital or rehabilitation center (40). None of the variables had DIF.
The PCAR’s eigenvalue on the first contrast was 2.46 with 49.23% explained variance on the first principal component. Further analysis of paired t-tests revealed that 0.89% of person locations are significantly different when comparing the two subtests formed based on positive (items 1 and 2) and negative loadings (items 3 and 5) on the first principal component, thereby confirming the unidimensionality of the scale.
Only one pair of items (items 1 “lifting and carrying objects” and item 2 “fine hand use”; r = 0.65) was above 0.2 of the average residual item correlations (r = 0.43). Both specifically identify hand use, which could explain why they are more strongly related to each other than to the rest of the items.
The ROC curve had an Area Under the Curve (AUC) of 0.90, CI95% = [0.85–0.96], in predicting independent use of the upper arm during daily life activities at discharge, meaning that the clinician will make an accurate prediction 90% of the time. We found a cut-off score of 14/20 with high sensitivity (87%, CI95% = [81%–91%]) and high specificity (83%, CI95% = [77%–87%]), with 14/20 or higher predicting independent use of the arm in daily living at discharge (Figure 2).
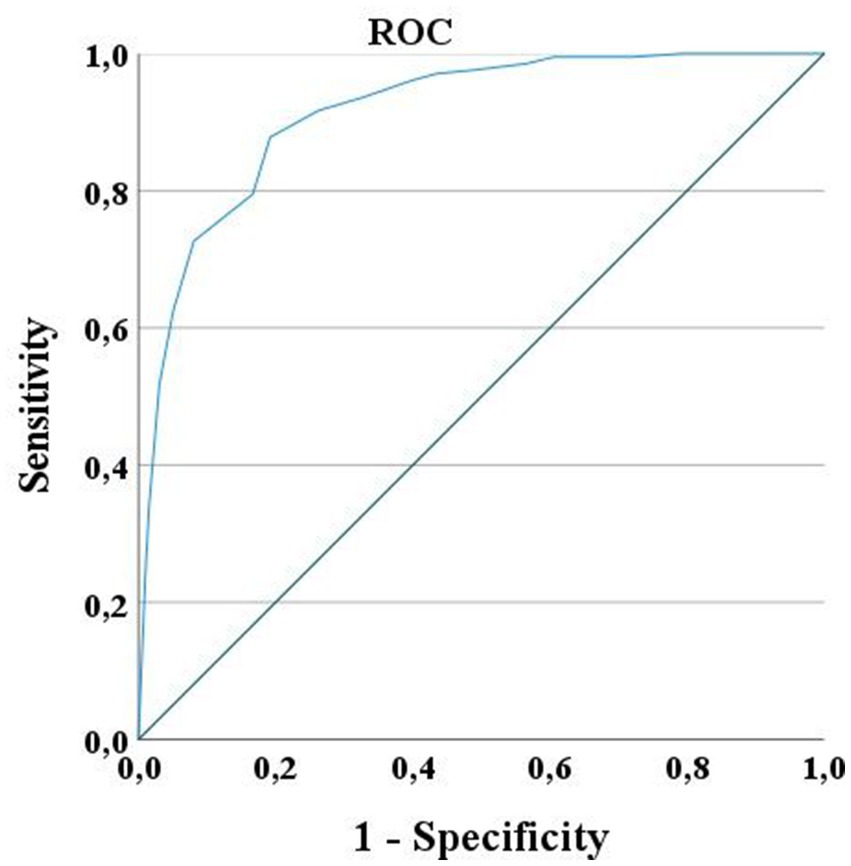
Figure 2. ROC Curve for UL-LIMOS. ROC curve for UL-LIMOS with an AUC of 0.90, CI95% = [0.85–0.96], in predicting independence of affected arm use in daily living at discharge. The best cut-point value of UL-LIMOS to predict good outcome after discharge was 14/20, with 14 or higher predicting the ability to independently using the affected arm during daily activities after discharge.
4. Discussion
This study presents a new valid ICF-based observation performance scale (UL-LIMOS) to evaluate dependency during upper limb use in daily life in adults with stroke at the ICF-activity level. Structural validity of the UL-LIMOS was evaluated with Rasch analysis in 407 adults with (sub) acute stroke, demonstrating that UL-LIMOS fit the model, without problematic floor or ceiling effects or DIF, and with a high PSR of 0.90, which allows clinicians and researchers to use the scale for individual decision-making. Both sensitivity and specificity were above 80%, which is high and above the generally accepted standard of 75% (44). With regard to specificity, 17% of patients were classified as false positives, suggesting that these patients were below cut-off 14 on UL-LIMOS, but were still making good use of their upper limbs. However, this finding is consistent with clinical observations that adults with stroke sometimes find ways and compensatory strategies to make good use of their upper limbs in certain contexts, although this was not expected or observed by the clinicians.
Rasch analysis also provided insight into the hierarchy of difficulty of the five items. As expected, based on the conceptual framework of upper limb movements, fine hand use requires more dependency than arm and hand use. Tasks such as washing and dressing are even more difficult because they require more awareness and interaction with the whole body, and/or require more cognitive motor planning regarding the different motor action sequences to perform the activity. This is reflected within the Rasch-based hierarchical order of the items.
Dressing appeared to be the most difficult item, confirming previous descriptions of dressing as a complex skill that requires several physical motor function skills and cognitive abilities (45). Notably, approximately 50% of adults with stroke still cannot dress independently 6 months post-stroke (46). Cognitive deficits could be an important factor for this dependency, and, among the spectrum of different cognitive factors, spatial neglect has been shown to have a major negative impact on dressing skills in adults with a right hemispheric stroke (45, 47). Upper limb apraxia has been shown to affect dressing in adults with a left hemispheric stroke (38, 39). In our sample, 78% had cognitive deficits as assessed with the MoCA (31–33). Individual testing of specific cognitive functions showed that 39% of the patients had spatial neglect (34), and 35% had moderate to severe apraxia (35–37). This further confirms the negative impact of spatial neglect and apraxia that were previously identified in the literature, on motor actions. We confirm earlier findings in the literature that more adults with right hemispheric stroke exhibit neglect and more adults with a left hemispheric stroke have upper limb apraxia. However, the influence of these cognitive disorders on the use of the upper limb in daily life needs to be studied, and assessed comprehensively, in much more detail in future studies.
Evaluating upper limb motor impairment and/or activity in a structured, laboratory-based setting (“capacity”), such as Fugl-Meyer (8), MESUPES (48), and ARAT (9), and comparing those results to their performance level with UL-LIMOS, measuring reliance on others for upper limb use in daily life, is important because these outcomes may not always line up. Adults with stroke may have the ability to recruit motor units to perform specific motor actions in a laboratory setting but may not be able to generate the necessary motor programs or have the necessary cognitive processing skills to perform tasks in a more unstructured and more complex environment such as is the case with ADLs.
Evaluation scales are often used in the hospital and rehabilitation settings to provide some estimates to patients regarding their recovery potential and which treatments would be most appropriate for them. Therefore, early prediction algorithms have gained much attraction in recent years (49–53). Yet, the current upper limb prediction models test upper limb motor function in a structured laboratory setting (49), which does not reflect actual upper limb use in daily life. In addition, these studies often exclude adults with cognitive deficits post-stroke (50–52, 54). Thus, the predictions are only applicable to a subset of adults with stroke given the high prevalence of cognitive deficits after stroke, including spatial neglect (55) and apraxia (36). Interestingly, Stinear et al., who developed the prediction algorithms PREP and PREP-2, emphasized the importance of including cognitive factors in future prediction paradigms, because these factors influenced upper limb outcomes (50, 53). This stresses the need for new predictive models that consider the evaluation of dependency on others during upper limb use in daily life. The UL-LIMOS, which describes dependency on others during spontaneous upper limb use in daily life, can also be used in patients with neglect and apraxia. Therefore, UL-LIMOS, as well as measures of neglect and apraxia could therefore be valuable factors in future predictive models of upper limb motor recovery after stroke.
Our study has limitations. Adults with stroke were recruited in only one neurorehabilitation center in Switzerland, which could limit the generalizability to other countries with different cultures. Furthermore, targeting could be improved in the future by adding more difficult items to the scale. Other psychometrics such as sensitivity to change need to be performed on the 5-item UL-LIMOS. Lastly, the scale also needs to be validated in adults with chronic stroke to ensure the generalizability of the results.
In conclusion, we present a new 5-item Rasch-based UL-LIMOS scale, which was validated in 407 adults with acute or subacute stroke. We recommend validation of the UL-LIMOS in other countries to account for cultural differences. The UL-LIMOS could also be validated in chronic stroke stages when adults have returned to their home setting. A comparison of the UL-LIMOS data with self-reported measurements or with accelerometers could potentially lead to changes to the existing core datasets recommended for the evaluation of upper limb performance of adults with stroke (10, 11).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted in accordance with the principles of the Declaration of Helsinki (2013) and was approved by the local Ethical Committees of the state of Luzern (BASEC-ID 2017-00998). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement (29). The patients/participants provided their written informed consent to participate in this study. A family member with power of attorney consented if participants had severe cognitive impairments, preventing them to consent independently.
Author contributions
TV, BO, JV, and TN contributed to the conception and design of the study. TV organized the database and wrote sections of the manuscript. AW performed the statistical analysis and the interpretation of the results, and wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Swiss National Science Foundation grants (TN 320030_140696 and 320030_169789) and Innosuisse grant (TV 52272.1 IP-SBM).
Acknowledgments
We thank the neurorehabilitation team involved in the data collection and all participants for their time and investment. We like to express our deep gratitude to Marc Noël for the critical review of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1154322/full#supplementary-material
References
1. Lawrence, ES, Coshall, C, Dundas, R, Stewart, J, Rudd, AG, Howard, R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. (2001) 32:1279–84. doi: 10.1161/01.STR.32.6.1279
2. Veerbeek, JM, Kwakkel, G, van Wegen, EEH, Ket, JCF, and Heymans, MW. Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke. (2011) 42:1482–8. doi: 10.1161/STROKEAHA.110.604090
3. Chausson, N, Olindo, S, Cabre, P, Saint-Vil, M, and Smadja, D. Five-year outcome of a stroke cohort in Martinique, French West Indies: Etude Realisee en Martinique et Centree sur l’Incidence des Accidents vasculaires cerebraux, Part 2. Stroke (2010) 41:594-9. doi: 10.1161/strokeaha.109.573402
4. Schwab-Malek, S, Vatankhah, B, Bogdahn, U, Horn, M, and Audebert, HJ. Depressive symptoms and quality of life after thrombolysis in stroke: the TEMPiS study. J Neurol. (2010) 257:1848–54. doi: 10.1007/s00415-010-5622-4
5. Aarnio, K, Rodríguez-Pardo, J, Siegerink, B, Hardt, J, Broman, J, Tulkki, L, et al. Return to work after ischemic stroke in young adults. Neurology. (2018) 91:e1909–17. doi: 10.1212/wnl.0000000000006510
6. Alt Murphy, M, Resteghini, C, Feys, P, and Lamers, I. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. (2015) 15:29. doi: 10.1186/s12883-015-0292-6
7. World Health Organization. International Classification of Functioning, Disability and Health: ICF World Health Organization (2001). 228 p. Available at: https://apps.who.int/iris/bitstream/handle/10665/42407/9241545429.pdf?sequence=1
8. Fugl-Meyer, AR, Jääskö, L, Leyman, I, Olsson, S, and Steglind, S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. (1975) 7:13–31. doi: 10.2340/1650197771331
9. Lyle, RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. (1981) 4:483–92. doi: 10.1097/00004356-198112000-00001
10. Kwakkel, G, Lannin, NA, Borschmann, K, English, C, Ali, M, Churilov, L, et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based Core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. (2017) 31:784–92. doi: 10.1177/1545968317732662
11. Pohl, J, Held, JPO, Verheyden, G, Murphy, MA, Engelter, S, Flöel, A, et al. Corrigendum: consensus-based Core set of outcome measures for clinical motor rehabilitation after stroke—a Delphi study. Front Neurol. (2021) 12:7935. doi: 10.3389/fneur.2021.697935
13. Keith, RA, Granger, CV, Hamilton, BB, and Sherwin, FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. (1987) 1:6–18.
14. Harvey, RL. Predictors of functional outcome following stroke. Phys Med Rehabil Clin N Am. (2015) 26:583–98. doi: 10.1016/j.pmr.2015.07.002
15. Hsueh, IP, Lee, MM, and Hsieh, CL. Psychometric characteristics of the Barthel activities of daily living index in stroke patients. J Formos Med Assoc. (2001) 100:526–32.
16. Hsueh, I-P, Lin, J-H, Jeng, J-S, and Hsieh, C-L. Comparison of the psychometric characteristics of the functional independence measure, 5 item Barthel index, and 10 item Barthel index in patients with stroke. J Neurol Neurosurg Psychiatry. (2002) 73:188–90. doi: 10.1136/jnnp.73.2.188
17. Duncan, PW, Samsa, GP, Weinberger, M, Goldstein, LB, Bonito, A, Witter, DM, et al. Health status of individuals with mild stroke. Stroke. (1997) 28:740–5. doi: 10.1161/01.STR.28.4.740
18. Taub, E, Miller, NE, Novack, TA, Cook, EW 3rd, Fleming, WC, Nepomuceno, CS, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. (1993) 74:347–54.
19. Penta, M, Tesio, L, Arnould, C, Zancan, A, and Thonnard, JL. The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: Rasch-based validation and relationship to upper limb impairment. Stroke. (2001) 32:1627–34. doi: 10.1161/01.STR.32.7.1627
20. Taub, E, Crago, JE, and Uswatte, G. Constraint-induced movement therapy: a new approach to treatment in physical rehabilitation. Rehabil Psychol. (1998) 43:152–70. doi: 10.1037/0090-5550.43.2.152
21. Gasperini, G, Rota, M, Guanziroli, E, Bissolotti, L, Balestrieri, F, Chisari, C, et al. Development and Rasch validation of an observational assessment tool of upper limb functional impairment in stroke survivors: functional assessment test for upper limb. Arch Phys Med Rehabil. (2023) 104:597–604. doi: 10.1016/j.apmr.2022.10.003
22. Doman, CA, Waddell, KJ, Bailey, RR, Moore, JL, and Lang, CE. Changes in upper-extremity functional capacity and daily performance during outpatient occupational therapy for people with stroke. Am J Occup Ther. (2016) 70:7003290040p1–7003290040p11. doi: 10.5014/ajot.2016.020891
23. Lang, CE, Waddell, KJ, Barth, J, Holleran, CL, Strube, MJ, and Bland, MD. Upper limb performance in daily life approaches plateau around three to six weeks post-stroke. Neurorehabil Neural Repair. (2021) 35:903–14. doi: 10.1177/15459683211041302
24. Ottiger, B, Vanbellingen, T, Gabriel, C, Huberle, E, Koenig-Bruhin, M, Pflugshaupt, T, et al. Correction: validation of the new Lucerne ICF based multidisciplinary observation scale (LIMOS) for stroke patients. PLoS One. (2015) 10:e0134186. doi: 10.1371/journal.pone.0134186
25. Van de Winckel, A, Ottiger, B, Bohlhalter, S, Nyffeler, T, and Vanbellingen, T. Comprehensive ADL outcome measurement after stroke: Rasch validation of the Lucerne ICF-based multidisciplinary observation scale (LIMOS). Arch Phys Med Rehabil. (2019) 100:2314–23. doi: 10.1016/j.apmr.2019.02.012
26. Vanbellingen, T, Ottiger, B, Pflugshaupt, T, Mehrholz, J, Bohlhalter, S, Nef, T, et al. The responsiveness of the Lucerne ICF-based multidisciplinary observation scale: a comparison with the functional Independence measure and the Barthel index. Front Neurol. (2016) 7:152. doi: 10.3389/fneur.2016.00152
27. Ottiger, B, Vanbellingen, T, Gabriel, C, Huberle, E, Koenig-Bruhin, M, Pflugshaupt, T, et al. Validation of the new Lucerne ICF based multidisciplinary observation scale (LIMOS) for stroke patients. PLoS One. (2015) 10:e0130925. doi: 10.1371/journal.pone.0130925
28. Nyffeler, T, Vanbellingen, T, Kaufmann, BC, Pflugshaupt, T, Bauer, D, Frey, J, et al. Theta burst stimulation in neglect after stroke: functional outcome and response variability origins. Brain. (2019) 142:992–1008. doi: 10.1093/brain/awz029
29. Bernhardt, J, Hayward, KS, Kwakkel, G, Ward, NS, Wolf, SL, Borschmann, K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke. (2017) 12:444–50. doi: 10.1177/1747493017711816
30. Vandenbroucke, JP, von Elm, E, Altman, DG, Gøtzsche, PC, Mulrow, CD, Pocock, SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. (2014) 12:1500–24. doi: 10.1016/j.ijsu.2014.07.014
31. Zuo, L, Dong, Y, Zhu, R, Jin, Z, Li, Z, Wang, Y, et al. Screening for cognitive impairment with the Montreal cognitive assessment in Chinese patients with acute mild stroke and transient ischaemic attack: a validation study. BMJ Open. (2016) 6:e011310. doi: 10.1136/bmjopen-2016-011310
32. Feng, Y, Zhang, J, Zhou, Y, Chen, B, and Yin, Y. Concurrent validity of the short version of Montreal cognitive assessment (MoCA) for patients with stroke. Sci Rep. (2021) 11:7204. doi: 10.1038/s41598-021-86615-2
33. Webb, SS, Hobden, G, Roberts, R, Chiu, EG, King, S, and Demeyere, N. Validation of the UK English Oxford cognitive screen-plus in sub-acute and chronic stroke survivors. Eur Stroke J. (2022) 7:476–86. doi: 10.1177/23969873221119940
34. Marques, CLS, de Souza, JT, Gonçalves, MG, da Silva, TR, da Costa, RDM, Modolo, GP, et al. Validation of the Catherine Bergego scale in patients with unilateral spatial neglect after stroke. Dement Neuropsychol. (2019) 13:82–8. doi: 10.1590/1980-57642018dn13-010009
35. Vanbellingen, T, Kersten, B, Van de Winckel, A, Bellion, M, Baronti, F, Müri, R, et al. A new bedside test of gestures in stroke: the apraxia screen of TULIA (AST). J Neurol Neurosurg Psychiatry. (2011) 82:389–92. doi: 10.1136/jnnp.2010.213371
36. Vanbellingen, T, Kersten, B, Van Hemelrijk, B, Van de Winckel, A, Bertschi, M, Müri, R, et al. Comprehensive assessment of gesture production: a new test of upper limb apraxia (TULIA). Eur J Neurol. (2010) 17:59–66. doi: 10.1111/j.1468-1331.2009.02741.x
37. Van Hemelrijk, B, Vanbellingen, T, Van de Winckel, A, De Weerdt, W, and Bohlhalter, S. A new test to measure upper limb apraxia (TULIAS): a reliability study. Mov Disord. (2007) 119:e12–S214. doi: 10.1016/j.clinph.2007.09.103
38. Vanbellingen, T, Ottiger, B, Maaijwee, N, Pflugshaupt, T, Bohlhalter, S, Müri, RM, et al. Spatial neglect predicts upper limb use in the activities of daily living. Cerebrovasc Dis. (2017) 44:122–7. doi: 10.1159/000477500
39. Mallinson, T, Kozlowski, AJ, Johnston, MV, Weaver, J, Terhorst, L, Grampurohit, N, et al. A. Rasch reporting guideline for rehabilitation research (RULER): the RULER statement. Arch Phys Med Rehabil. (2022) 103:1477–86. doi: 10.1016/j.apmr.2022.03.013
40. Van de Winckel, A, Kozlowski, AJ, Johnston, MV, Weaver, J, Grampurohit, N, Terhorst, L, et al. Reporting guideline for RULER: Rasch reporting guideline for rehabilitation research – Explanation & Elaboration manuscript. Arch Phys Med Rehabil. (2022) 103:1487–98. doi: 10.1016/j.apmr.2022.03.019
41. Linacre, JM. (2014) Dimensionality: contrasts & variances. A user’s guide to Winsteps Ministep Rasch-model computer programs (version 3 81 0) Available at: http://www.winsteps.com/winman/principalcomponents.htm
42. Reliability and separation of measures. Available at: https://www.winsteps.com/winman/reliability.htm (Accessed 30 January 2021).
43. Christensen, KB, Makransky, G, and Horton, M. Critical Values for Yen’s Q3: Identification of Local Dependence in the Rasch Model Using Residual Correlations. Appl Psychol Meas. (2017) 41:178–94. doi: 10.1177/0146621616677520
44. Power, M, Fell, G, and Wright, M. Principles for high-quality, high-value testing. Evid Based Med. (2013) 18:5–10. doi: 10.1136/eb-2012-100645
45. Walker, MF, and Lincoln, NB. Factors influencing dressing performance after stroke. J Neurol Neurosurg Psychiatry. (1991) 54:699–701. doi: 10.1136/jnnp.54.8.699
46. Edmans, JA, and Lincoln, NB. The relation between perceptual deficits after stroke and Independence in activities of daily living. Br J Occup Ther. (1990) 53:139–42. doi: 10.1177/030802269005300404
47. Walker, MF, Sunderland, A, Fletcher-Smith, J, Drummond, A, Logan, P, Edmans, JA, et al. The DRESS trial: a feasibility randomized controlled trial of a neuropsychological approach to dressing therapy for stroke inpatients. Clin Rehabil. (2012) 26:675–85. doi: 10.1177/0269215511431089
48. Van de Winckel, A, Feys, H, van der Knaap, S, Messerli, R, Baronti, F, Lehmann, R, et al. Can quality of movement be measured? Rasch analysis and inter-rater reliability of the motor evaluation scale for upper extremity in stroke patients (MESUPES). Clin Rehabil. (2006) 20:871–84. doi: 10.1177/0269215506072181
49. Nijland, RHM, van Wegen, EEH, Harmeling-van der Wel, BC, Kwakkel, G, and Investigators, EPOS. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke. (2010) 41:745–50. doi: 10.1161/STROKEAHA.109.572065
50. Stinear, CM, Barber, PA, Petoe, M, Anwar, S, and Byblow, WD. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain. (2012) 135:2527–35. doi: 10.1093/brain/aws146
51. van der Vliet, R, Selles, RW, Andrinopoulou, E-R, Nijland, R, Ribbers, GM, Frens, MA, et al. Predicting upper limb motor impairment recovery after stroke: a mixture model. Ann Neurol. (2020) 87:383–93. doi: 10.1002/ana.25679
52. Selles, RW, Andrinopoulou, E-R, Nijland, RH, van der Vliet, R, Slaman, J, van Wegen, EE, et al. Computerised patient-specific prediction of the recovery profile of upper limb capacity within stroke services: the next step. J Neurol Neurosurg Psychiatry. (2021) 92:574–81. doi: 10.1136/jnnp-2020-324637
53. Stinear, CM, Byblow, WD, Ackerley, SJ, Smith, M-C, Borges, VM, and Barber, PA. PREP2: a biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol. (2017) 4:811–20. doi: 10.1002/acn3.488
54. Krakauer, JW, and Marshall, RS. The proportional recovery rule for stroke revisited. Ann Neurol. (2015) 78:845–7. doi: 10.1002/ana.24537
Keywords: outcome, Rasch Measurement Theory, Rasch analysis, upper limb, disability, health, stroke, activities of daily living
Citation: Van de Winckel A, Ottiger B, Veerbeek JM, Nyffeler T and Vanbellingen T (2023) Rasch validation of a new scale to measure dependency in arm use in daily life: the Upper Limb Lucerne ICF-based Multidisciplinary Observation Scale. Front. Neurol. 14:1154322. doi: 10.3389/fneur.2023.1154322
Edited by:
Ana Catarina Fonseca, University of Lisbon, PortugalReviewed by:
Mustafa Kutlu, Sakarya University of Applied Sciences, TürkiyeAlan Tennant, University of Leeds, United Kingdom
Copyright © 2023 Van de Winckel, Ottiger, Veerbeek, Nyffeler and Vanbellingen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ann Van de Winckel, YXZhbmRld2lAdW1uLmVkdQ==
 Ann Van de Winckel
Ann Van de Winckel Beatrice Ottiger
Beatrice Ottiger Janne Marieke Veerbeek
Janne Marieke Veerbeek Thomas Nyffeler
Thomas Nyffeler Tim Vanbellingen
Tim Vanbellingen