
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol., 05 May 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1152992
This article is part of the Research TopicMultiple Sclerosis and Neuroimmunology – Case Report Collection, Volume IIView all 35 articles
Introduction: Rituximab (RTX) showed good efficacy and safety for patients with myasthenia gravis. However, the percentage of peripheral CD20+ B cell may be absent for years after low dose of RTX treatment. Persistent hypogammaglobulinemia and opportunistic infection may occur in patients under treatment of RTX with thymoma relapse.
Case representation: We report a case of refractory myasthenia gravis. After two doses of 100 mg rituximab, the patient developed transient neutropenia. The peripheral blood CD20+ B cell percentage was 0 more than 3 years. Eighteen months later, the patient's symptoms relapsed with thymoma recurred. She had persistent hypogammaglobulinemia and multiple opportunistic infections.
Conclusion: In MG patient under B cell depletion therapy had thymoma relapse, Good's syndrome may induce prolonged B cell depletion, hypogammaglobulinemia and opportunistic infections.
Many studies have indicated that rituximab (RTX) is effective for myasthenia gravis (MG) (1). Although most studies have suggested that rituximab is safe, serious adverse events have been reported, including severe skin reactions, blood system diseases, hepatitis B reactivation, progressive multifocal leukoencephalopathy and other severe opportunistic infections (2). Conditions may become more complicated in patients with MG and thymoma.
Herein, we report a patient who received rituximab and radiotherapy for MG with thymoma. She suffered from transient severe agranulocytosis, persistent hypogammaglobulinemia, opportunistic infection and haemolytic anemia.
The patient is a 34-year-old female who was diagnosed with generalized myasthenia gravis 12 years prior. She underwent thymectomy and radiotherapy. Previously, she had three episodes of myasthenia gravis crises induced by infection. In addition to prednisone, she received azathioprine and cyclosporine, which failed to control her symptoms. Her dose of tacrolimus was halved due to the high blood concentration.
Physical examination showed bilateral mild ptosis, and the distal muscle strength of the lower extremities was Grade IV. She was positive for anti-acetylcholine receptor (anti-AChR) antibody. The myasthenia gravis Foundation of America (MGFA) classification was IIb, and the Activities of Daily Living (ADL) and Quantitative Myasthenia Gravis Score for disease severity (QMG) score was 11 and 15, respectively. The baseline total T lymphocytes, total B lymphocytes, CD20+ B cells and CD22+ B cells were 79% (50–82%), 9.6% (5–21%), 9.5 and 9.6%, respectively. The patient was treated with rituximab (100 mg) intravenously in February 2019. After discharge, she continued to take prednisone (20 mg) daily. After 3 months, the percentage of CD20+ B cells in the peripheral blood was 0.1%. She was given another intravenous infusion of rituximab (100 mg) in May 2019, and the number of CD20+ B cells decreased to 0 on the second day. Her symptoms were quite stable, and she was prescribed 60 mg of pyridostigmine bromide and 20 mg of prednisone orally for maintenance therapy.
One month later, the patient presented with intermittent low fever, night sweats and herpes around the mouth, accompanied by ptosis of the right eyelid, dysarthria and chewing difficulty. The peripheral blood leukocyte count was significantly decreased to 1.65 × 109, and the neutrophil count was 0. Peripheral platelet count and red blood cell count were normal. The total peripheral blood B lymphocytes and CD20+ B cells were still 0. The immunoglobulin G (IgG) level was 8.51 (7.23–16.85) g/L. The immunoglobulin A (IgA) level was 0.78 (0.69–3.82) g/L. The immunoglobulin M (IgM) level was 0.83 (0.62–2.77) g/L. The patient's symptoms improved after empiric anti-infection treatment with cefatriaxone, acyclovir and colony stimulating factor. The peripheral counts of leukocytes and neutrophils returned to normal 3 months later. The dose of prednisone was tapered to 17.5 mg.
In August 2020, the patient's symptoms were aggravated. Her chest computed tomography (CT) showed that the thymoma recurred. She received intravenous infusion 20g of immunoglobulin (IVIG) for 5 days and 25 sessions of thymic intensity modulated radiotherapy, with total dose of 5000 gy in May 2021. Her immunotherapy regimen was adjusted to 20 mg and 12.5 mg of prednisone every other day, and her symptoms stabilized.
In August 2021, the patient had fever again. Her chest CT showed that both lungs had new multiple patchy ground glass foci. The percentage of total T lymphocytes was 96.5%, the percentage of CD4+ and CD8+ T cells was 23.7 and 72.5% respectively, and the percentage of B cells was still 0% in peripheral blood. The level of IgG was 29.3 g/L, the level of IgA was 0.49 g/L and the level of IgM was 0.58 g/L. Bronchoscopic alveolar lavage indicates pneumocystis pneumonia. IVIG (20 g) was administered intravenously for 5 days. Sulfamethoxazole tablets were given as treatment. Her prednisone dosage was reduced to 20 mg and 5 mg orally every other day. Two months later, the level of IgG was 7.86 g/L, the level of IgM returned to 0.65 g/L, and the level of IgA was still as low as 0.47 g/L.
In October 2021, the patient experienced decreased vision in her left eye, and she was diagnosed with cytomegalovirus infectious retinitis. Additionally, the IgG, IgM and IgA levels were all below normal limits (6.86 g/L, 0.43 g/l and 0.51 g/L). The patient received ganciclovir intravitreal injection, tobramycin and prednisone acetate eye drops, and the left eye vision of the patient was maintained at 0.15. Steroids were discontinued, and 20 g IVIG was administered every month. After 5 administrations, the interval was extended to 2 months, and the patient's immunoglobulin level was monitored monthly. The IgG level returned to normal, but IgM and IgA levels fluctuated between 0.45–0.5 g/L (0.63–2.77 g/L) and 0.27–0.4 g/L (0.69–3.82 g/L), respectively, for a long time.
In May 2022, the percentage of peripheral CD20+ B cells was 11.65% (5.0–21%). The patient's myasthenia symptoms were relatively stable, leaving only extraocular muscle paralysis and mild limb weakness. In August 2022, however, the patient felt fatigue and dizziness. Routine blood tests revealed anemia. HGB levels decreased to 50 mg/dl at minimum. Coomb's test was positive, and the patient was diagnosed with erythroblastic anemia. IVIG was given with 40 mg prednisone. HGB levels returned to 94 mg/dl. At present, the patient's symptoms are quite stable except for low immunoglobin levels. Her IgG level was 6.87 g/L, IgA and IgM was 0.39 g/L and 0.46 g/L, respectively. Her corticosteroids were tapered slowly, and IVIG was administered every 2–3 months. We summarized the patients clinical events and laboratory results in Figure 1.
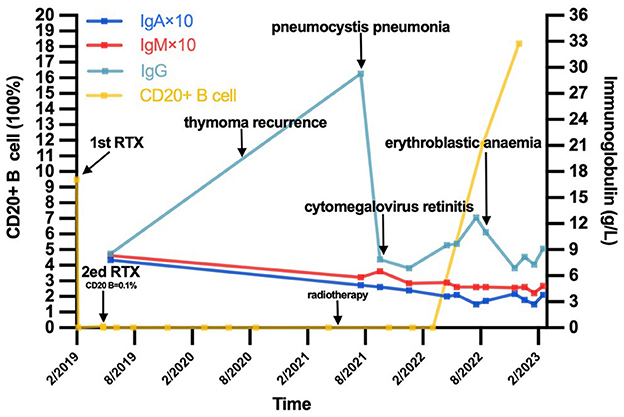
Figure 1. The timeline of this patient's course and the trend of CD20+ B cell as well as immunoglobulin level. IgA, immunoglobulin A; IgM, immunoglobulin M; IgG, immunoglobulin M.
In the current case, transient neutropenia was induced by RTX and persistent hypogammaglobulinemia preceded by thymoma relapse, resulting in multiple opportunistic infections.
The incidence of neutropenia has been reported to be approximately 6.5% after RTX treatment in patients with rheumatoid arthritis. Most patients have a good prognosis without severe infections (3). Due to the low dosage of RTX and the patient was asymptomatic, the level of immunoglobin was not evaluated before and after treatment of RTX. Three months after thymoma relapse and radiotherapy, hypogammaglobulinemia was found, while peripheral B cell was still 0. These leads to multiple opportunistic infections. Both RTX and thymoma can induce hypogammaglobulinemia.
The incidence of hypogammaglobulinemia following RTX treatment ranges from 13 to 56% in patients with non-Hodgkin lymphoma and autoimmune diseases, which is more common and severe than neutropenia (4, 5). Most studies believe that persistent IgG deficiency is linked to an increased risk of serious infections after RTX and that decreased IgM and IgA are less clinically significant (5–8). At present, the etiology of hypogammaglobulinemia after RTX has not been fully clarified, and many studies believe that it is the result of the interaction of multiple factors (9). RTX mainly acts on the surface antigen of CD20, which is mainly expressed in peripheral blood circulation B cells but not bone marrow stem cells and CD20-negative plasma cells (10). Therefore, RTX will not completely inhibit humoral immunity. However, in patients with haematologic diseases, combined chemotherapy may lead to persistent hypogammaglobulinemia related to the stagnation of B-cell reconstruction (11). It is unclear whether the differences in dosage and interval of RTX will have a variable impact on the degree and duration of B-cell depletion (12, 13).
Theoretically, with the regeneration of peripheral blood CD20+B cells, the immunoglobulin level should return to the normal level. After two doses of rituximab 100 mg, the level of B lymphocytes in this patient remained at 0 for 3 years. As the level of IgG returned to normal, the levels of IgA and IgM in peripheral blood continued to be lower than the normal limit, even after CD20+ B cells returned to the normal level. There might be other factors influencing immunological function.
Immunological disturbances such as Good's Syndrome may occur in patients with thymoma, including a reduction in circulating B lymphocytes, hypogammaglobulinemia and decreased T lymphocytes (14), presenting with a variable phenotype of immunodeficiency. The current patient had persistent hypogammaglobulinemia, B-cell deficiency and thymoma, Good's syndrome should be considered. Decreased T lymphocytes, inversion of the CD4+/CD8+ ratio also occurred in this patient (11). Comparing with the treatment of RTX, Good's syndrome is more accountable for the subsequent immunodeficiency.
At present, there are no proven diagnoses or treatments. Immunoglobulin replacement reduces the risk of infection with primary and secondary immunodeficiency associated with hypogammaglobulinemia. Some studies believe that the clinical manifestations of severe infection are more reliable as indicators of alternative treatment than laboratory indicators (15). The B-cell phenotype of patients receiving immunoglobulin replacement therapy after rituximab is mainly naive B cells, and the switching memory B cells are reduced (16).
In MG patient under B cell depletion therapy had thymoma relapse, Good's syndrome may induce prolonged B cell depletion, persistent hypogammaglobulinemia and opportunistic infections.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JR and RL analyzed data and wrote the main manuscript text. JW and JL followed up patients and collected data. JG and YY examined immunoglobulin and lymphocyte level in serum. HH helped interpret relevant indicators. FG provided ideas and guidance for this article. All the authors read and approve the final revision of the manuscript.
We are grateful to all the members for participation in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Howard JF Jr., Barohn RJ, Cutter GR, Freimer M, Juel VC, Mozaffar T, et al. A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. (2013) 48:76–84. doi: 10.1002/mus.23839
2. Schmidt E, Bröcker EB, Goebeler M. Rituximab in treatment-resistant autoimmune blistering skin disorders. Clin Rev Allergy Immunol. (2008) 34:56–64. doi: 10.1007/s12016-007-8021-6
3. Monaco WE, Jones JD, Rigby WF. Rituximab associated late-onset neutropenia-a rheumatology case series and review of the literature. Clin Rheumatol. (2016) 35:2457–62. doi: 10.1007/s10067-016-3313-y
4. Roberts DM, Jones RB, Smith RM, Alberici F, Kumaratne DS, Burns S, et al. Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun. (2015) 57:60–5. doi: 10.1016/j.jaut.2014.11.009
5. Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. (2013) 13:106–11. doi: 10.1016/j.clml.2012.11.011
6. Kado R, Sanders G, McCune WJ. Suppression of normal immune responses after treatment with rituximab. Curr Opin Rheumatol. (2016) 28:251–8. doi: 10.1097/BOR.0000000000000272
7. Venhoff N, Effelsberg NM, Salzer U, Warnatz K, Peter HH, Lebrecht D, et al. Impact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. PLoS ONE. (2012) 7:e37626. doi: 10.1371/journal.pone.0037626
8. Hansen DA, Robbins BA, Bylund DJ, Piro LD, Saven A, Ellison DJ. Identification of monoclonal immunoglobulins and quantitative immunoglobulin abnormalities in hairy cell leukemia and chronic lymphocytic leukemia. Am J Clin Pathol. (1994) 102:580–5. doi: 10.1093/ajcp/102.5.580
9. Adeli MM, Eichner BH, Thornburg C, Williams L. Persistent antibody depletion after rituximab in three children with autoimmune cytopenias. Pediatr Hematol Oncol. (2009) 26:566–72. doi: 10.3109/08880010903271697
10. Eisenberg RA, Jawad AF, Boyer J, Maurer K, McDonald K, Prak ET, et al. Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol. (2013) 33:388–96. doi: 10.1007/s10875-012-9813-x
11. Irie E, Shirota Y, Suzuki C, Tajima Y, Ishizawa K, Kameoka J, et al. Severe hypogammaglobulinemia persisting for 6 years after treatment with rituximab combined chemotherapy due to arrest of B lymphocyte differentiation together with alteration of T lymphocyte homeostasis. Int J Hematol. (2010) 91:501–8. doi: 10.1007/s12185-010-0528-6
12. Kewalramani T, Zelenetz AD, Nimer SD, Portlock C, Straus D, Noy A, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. (2004) 103:3684–8. doi: 10.1182/blood-2003-11-3911
13. Bredemeier M, De Oliveira FK, Rocha CM. Low- versus high-dose rituximab for rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. (2014) 66:228–35. doi: 10.1002/acr.22116
14. Guevara-Hoyer K, Fuentes-Antrás J, Calatayud Gastardi J, Sánchez-Ramón S. Immunodeficiency and thymoma in good syndrome: two sides of the same coin. Immunol Lett. (2021) 231:11–7. doi: 10.1016/j.imlet.2020.12.010
15. Sacco KA, Abraham RS. Consequences of B-cell-depleting therapy: hypogammaglobulinemia and impaired B-cell reconstitution. Immunotherapy. (2018) 10:713–28. doi: 10.2217/imt-2017-0178
Keywords: myasthenia gravis (MG), rituximab (RTX), hypogammaglobulinemia, infections, Good's syndrome (GS)
Citation: Ren J, Wang J, Liu R, Guo J, Yao Y, Luo J, Hao H and Gao F (2023) Case report: Persistent hypogammaglobulinemia in thymoma-associated myasthenia gravis: the impact of rituximab or Good's syndrome? Front. Neurol. 14:1152992. doi: 10.3389/fneur.2023.1152992
Received: 01 February 2023; Accepted: 12 April 2023;
Published: 05 May 2023.
Edited by:
Hans-Peter Hartung, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Elena Grebenciucova, Northwestern University, United StatesCopyright © 2023 Ren, Wang, Liu, Guo, Yao, Luo, Hao and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Liu, ZHl5eHlzQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.