
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 13 April 2023
Sec. Neurological Biomarkers
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1148450
Introduction: We aimed to investigate whether lipid profiles and homocysteine levels in patients with anti-N-methyl-D-aspartate receptor encephalitis are related to clinical presentation and prognosis, which may contribute to further research on the pathogenesis and treatment of this disease.
Methods: This study included a total of 43 patients with anti-N-methyl-D-aspartate receptor encephalitis and 43 sex–age-matched healthy controls. Baseline demography, clinical data, patient outcomes, and ancillary examination results were recorded. Patients were followed up every 2–3 months during the first year. The modified Rankin Scale score was used to evaluate the therapeutic effect and clinical outcome.
Results: Among the 43 patients included in this study, 55.81% were male, the mean age of onset was 27 years old, and the median modified Rankin Scale score on admission was 3.0. Apolipoprotein A-1 was significantly lower in patients with anti-N-methyl-D-aspartate receptor encephalitis compared with healthy controls (p = 0.004). Compared with healthy controls, homocysteine (p = 0.002), apolipoprotein B (p = 0.004), Lpa (p = 0.045), and apolipoprotein B/apolipoprotein A-1 (p = 0.001) were significantly increased in patients with anti-N-methyl-D-aspartate receptor encephalitis. According to the modified Rankin Scale scores, 6 months after discharge, 72.09% of patients had a good prognosis and 27.91% had a poor prognosis. In the good prognosis group, age (p = 0.031), lipoprotein a (p = 0.023), apolipoprotein A-1 (p = 0.027) at baseline, and the modified Rankin Scale score on admission (p = 0.019) were significantly higher than those in the poor prognosis group.
Conclusion: This study suggests the possibility that serum lipid profile and homocysteine play an important role in the pathogenesis of anti-N-methyl-D-aspartate receptor encephalitis, providing support for lipid-lowering treatment of anti-N-methyl-D-aspartate receptor encephalitis patients.
Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis was first proposed by Dalmau et al. in 2007 and is currently the most common autoimmune encephalitis (1). Clinical manifestations of anti-NMDAR encephalitis include cognitive impairment, behavioral disorder, autonomic dysfunction, epileptic seizures, and changes of consciousness (2–4). The pathogenesis of anti-NMDAR encephalitis remains to be clarified, and potential biomarkers indicating disease progression need to be explored.
It has been reported that high-density lipoprotein (HDL-C) has immune regulation, anti-oxidant, and anti-inflammatory effects (5–7). Researchers indicate that higher serum high-density lipoprotein cholesterol (HDL-C) is associated with lower levels of blood–brain barrier (BBB) injury in multiple sclerosis (MS) (8, 9). In neuromyelitis optic spectrum disorder (NMOSD), low HDL-C and high triglyceride (TG) levels were positively associated with poor recovery and relapse (10, 11).
Apolipoprotein A1 (ApoA-1) is an important component of HDL-C, which has anti-inflammatory effects (12). Studies have found that ApoA-1 is associated with a variety of neurological diseases. ApoA-1 levels were inversely associated with disease severity in MS (13, 14), and lower HDL-C and apoA-I levels were also associated with disease severity in SLE patients (15, 16).
Apolipoprotein B (ApoB) is believed to be atherogenic and has been associated with neurological diseases, such as the future risk of Parkinson's disease (PD) (17). Given the association between lipid profiles and these autoimmune diseases, we hypothesized that lipid profiles might be relevant to the prognosis of patients with NMDAR encephalitis.
Homocysteine (Hcy) is a non-essential sulfur-containing amino acid produced during methionine metabolism and is considered to be a pro-inflammatory and immunomodulatory molecule (18). Hyperhomocysteinemia has been found in a variety of neurological and autoimmune diseases, such as Alzheimer's and Parkinson's diseases (19–21), multiple sclerosis (MS) (22–26), systemic lupus erythematosus (SLE) (27), and rheumatoid arthritis (RA) (28–30). The excitotoxicity and pro-inflammatory effects of Hcy may be induced by the overactivation of NMDA receptors (which exist in neurons, neutrophils, and macrophages) to destroy the blood–brain barrier (BBB) (31), induce excitotoxicity of neurons (32–36), and activate the pro-inflammatory cascade (37).
We aimed to investigate whether lipid profiles and homocysteine levels in patients with anti-NMDAR encephalitis are related to clinical presentation and prognosis, which may contribute to further research on the pathogenesis and treatment of this disease.
This retrospective study was conducted at Qilu Hospital of Shandong University in China from January 2016 to December 2020 and included 43 patients diagnosed with anti-NMDAR encephalitis according to published diagnostic criteria (38). The inclusion criteria were as follows: (1) acute or subacute seizures with one or more clinical features, including seizures, memory deficits, mental and behavioral disorders, and speech disorders related to the limbic system, (2) positive serum and/or cerebrospinal fluid (CSF) for neurosurface antibodies, and (3) reasonable exclusion of other diseases. The exclusion criteria were as follows: (1) patients who had received corticosteroids or lipid-lowering drugs prior to the study; (2) patients having clinical symptoms that affect lipid profiles, such as diabetes, hypertension, hypothyroidism, severe infections, abnormal liver function, abnormal kidney function, or Cushing's syndrome; and (3) patients with incomplete clinical data. In addition, 43 sex- and age-matched healthy individuals were included as a control group.
The modified Rankin Scale (mRS) score was used to evaluate the therapeutic effect and clinical outcome (39). The mRS scores were defined as follows: 0 = asymptomatic; 1 = no significant disability in performing all daily activities despite some symptoms; 2 = having a minor disability in handling personal matters without assistance, but being unable to carry out all previous activities; 3 = moderately disabled, requiring some assistance, but able to walk independently; 4 = moderately severe disability, i.e., inability to take care of physical needs without assistance and inability to walk unassisted; 5 = severe disability requiring constant care and nursing, as well as bedridden and incontinence; and 6 = death.
Patients were followed up every 2–3 months during the first year after discharge; from the second year onward, follow-up was conducted every 4–6 months. Patients were followed up for at least 1 year. Follow-up data were carefully retrieved from hospital records or through interviews with patients and their families (directly or via phone and WeChat). After 6 months of discharge, patients with an mRS score of ≤ 2 were defined as the good prognosis group, and patients with an mRS score of >2 were defined as the poor prognosis group.
The study was approved by the Ethics Committee of Shandong University Qilu Hospital (Approval number: KYLL202008-044) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was given to all study participants or their legal guardians.
Baseline demography (sex, age, and past medical history), clinical data, patient outcomes, and ancillary examination results were recorded. Autoantibodies to NMDAR were evaluated in 43 patients by indirect immunofluorescence. The initial dilution titers of serum and cerebrospinal fluid were 1:10 and 1:1, respectively. Blood samples were taken from all patients (fasting) on the morning of the second day after the initial admission. Serum lipid profiling and homocysteine levels were measured in our hospital using a chemiluminescent analyzer (Cobas E601, Shanghai, China). The lipid profile included total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), HDL-C, apolipoprotein A-I (apoA-I), apolipoprotein B, and apolipoprotein A-I/apoB.
SPSS IBM 25.0 software was used for statistical analysis. The Shapiro–Wilk test was used to determine the normality of the values, and the normally distributed continuous variables were expressed as mean and standard deviation. Non-normal data were expressed as median and interquartile spacing (IQR). Class variables were expressed as ratios. Data were analyzed by the chi-square test or Fisher's exact test. Student t-test was used for normally distributed continuous variables, and the Mann–Whitney U-test was used for non-normal continuous variables. Bilateral values with a p-value of < 0.05 were considered to be statistically significant.
From January 2016 to December 2020, clinical data were collected from 138 patients, of whom 95 were excluded because they did not meet the inclusion criteria, had incomplete clinical data, or were lost to follow-up. A total of 43 patients with anti-NMDAR encephalitis and 43 sex–age-matched healthy controls (HCs) were enrolled. Figure 1 depicts the flowchart of patient recruitment. Among the 43 patients included in this study, 55.81% (24/43) were men, the mean age of onset was 27 years (IQR, 18–41), and the median mRS score on admission was 3.0 (3.0, 4.0). The main symptoms of the patients on admission were seizure (23/43), abnormal mental behavior (17/43), memory deficit (7/43), movement disorders (6/43), and consciousness disorders (3/43). In total, two patients were diagnosed with ovarian teratoma after admission and underwent tumor resection during hospitalization. According to the mRS scores, 6 months after discharge, 72.09% (31/43) of patients had a good prognosis and 27.91% (12/43) had a poor prognosis (Table 1). No deaths were recorded during the hospitalization. Overall, one male patient died of severe pneumonia within 6 months of discharge.
As shown in Table 1, ApoA-1 was significantly lower in patients with anti-NMDAR encephalitis compared with healthy controls (p = 0.004). Compared with the HCs, Hcy (p = 0.002), ApoB (p = 0.004), Lpa (p = 0.045), and ApoB/ApoA-1(p = 0.001) were significantly increased in patients with anti-NMDAR encephalitis. In addition, TG, TC, HDL-C, and LDL-C showed no statistical difference (p = 0.873, 0.527, 0.11, 0.56, respectively).
According to the mRS scores after 6 months of follow-up, the patients were divided into two groups with mRS scores of ≤ 2 (good prognosis) and mRS scores of >2 (poor prognosis), as shown in Table 2, using the clinical data collected at the baseline for analysis. In the good prognosis group, age (27.34 ± 2.26 vs. 43.58 ± 6.41, p = 0.031), lipoprotein a [19.3 (6.5, 41.9) vs. 57.75 ± 11.00, p = 0.023], mRS score on admission [3.0 (3.0, 4.0) vs. 4.0 (3.0, 5.0), p = 0.019], and ApoA-1 were significantly higher than that in the poor prognosis group [1.15 ± 0.03 vs. 0.99 (0.88, 1.04), p = 0.027]. The main symptoms of the good prognosis group on admission were seizure (16/31), abnormal mental behavior (13/31), memory loss (6/31), movement disorders (3/31), and consciousness disorders (3/31), and there was no statistical difference between the good prognosis group and the poor prognosis group. Both patients with ovarian teratoma had a good prognosis.
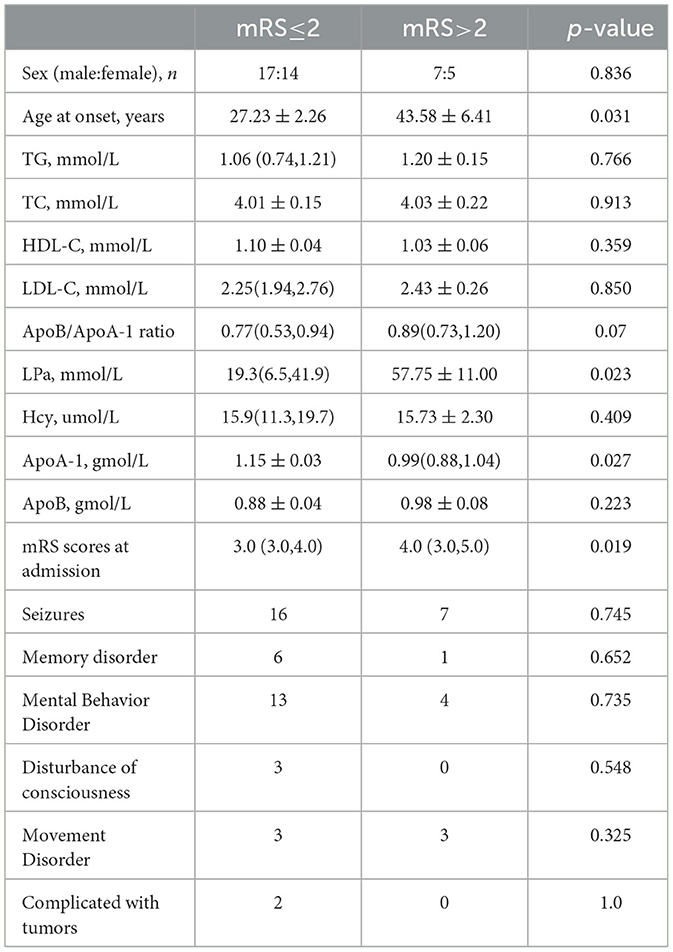
Table 2. Subgroup comparison of anti-NMDAR encephalitis patients based on mRS Scores at follow-up after 6 months.
In this regression study, the lipid profile and homocysteine of patients with anti-NMDAR encephalitis were significantly abnormal compared with the HCs. At the baseline, there were statistical differences in ApoA-1, ApoB, ApoB/ApoA-1, LPa, and Hcy between the anti-NMDAR encephalitis patients and the HCs. At the follow-up stage, compared with the poor prognosis group, there were significant differences in age, LPa, mRS score, and ApoA-1 in the good prognosis group.
HDL-C has anti-inflammatory and immune function regulation (5–7). In our study, HDL-C levels did not show statistical significance between the groups. However, in the same type of study on anti-NmdAR encephalitis, it was found that the level of HDL-C in patients was significantly reduced and was associated with poor prognosis and recurrence of patients (40, 41). This may be because our study was small and needed to be larger to show more accurate results.
ApoA-1, as the main component of HDL-C, is involved in a variety of functions, including anti-oxidative stress (42) and inflammatory response (43). ApoA-1 can inhibit endothelial cell apoptosis, pro-oxidation, and pro-inflammatory processes and induce vascular dilation, inhibit platelet activation, and promote innate immunity (44). Although HDL-C did not show statistical significance in this study, ApoA-1 showed low levels in both patients with anti-NMDAR encephalitis and in the group with a poor prognosis. Consistent with our conclusion, low ApoA-1 levels have been found in all the existing studies on anti-NMDAR encephalitis.
In contrast to the anti-inflammatory effects of ApoA-1, ApoB promotes inflammatory progression. Thus, an elevated ApoB/ApoA-I ratio may indicate a pro-inflammatory effect over an anti-inflammatory effect of lipoprotein, indicating the progression of inflammation and severity of disease (45). Our study found that serum ApoB levels and the ApoB/ApoA-I ratio were increased in patients with anti-NmdAR compared with the HCs. In addition, ApoB and ApoB/ApoA-1 in the good and poor prognosis groups showed a higher trend than that in the good prognosis group, although there was no statistical significance. A regression study showed that the level of ApoB/ApoA-1 in patients was significantly higher than that in healthy people but was not related to the outcome of the disease (46). However, another study suggested that ApoB/ApoA-1 was associated with adverse outcomes in patients (41). Given our small sample size, it is possible that a larger study could give a clearer trend.
There is evidence that lipoprotein a (LPa) plays a role in promoting the progression of inflammation in most cases (47). High levels of LPa have been found in both rheumatoid arthritis and systemic lupus erythematosus (48–51). In this study, the level of LPa in anti-NmdAR patients was significantly higher than that of the HCs, and the level of LPa in the poor prognosis group was significantly higher than that in the good prognosis group. This may be related to its pro-inflammatory effect.
Hyperhomocysteinemia has been found in a variety of neurological and autoimmune diseases (19–30). A large number of studies have shown that excessive Hcy can cause neurotoxicity through its dual effect on NMDAR (42), and Hcy can also induce neuronal excitatory toxicity through overstimulation of NMDAR (32–36). In addition, hyperhomocysteinemia may also promote an inflammatory cascade (37). In this study, Hcy levels were significantly increased in patients with anti-NMDAR encephalitis compared with the levels in the HCs, and there was no statistical difference in Hcy levels between the good prognosis group and the poor prognosis group. Studies have shown that the Hcy level of patients with anti-NMDAR encephalitis is significantly higher than that of healthy groups, and that of male patients is higher than that of female patients (52).
There are several limitations to this study that need to be addressed. First, this was a single-center, small sample size, and retrospective study, so more centers and larger sample sizes are needed to further explore the relationship between lipid profiles and homocysteine and anti-NMDAR encephalitis. Second, the potential impact of treatment remains a confounding variable. Finally, due to the limited sample size, anti-NMDAR encephalitis patients were only divided into good prognosis and poor prognosis groups for subgroup analysis. A more detailed subgroup analysis may be more helpful in studying the role of lipid profiles and homocysteine in the disease.
In conclusion, our results showed that ApoB, ApoB/ApoA-1, Hcy, and LPa levels significantly increased and ApoA-1 levels decreased in patients with anti-NMDAR encephalitis at the early stage of the disease. Follow-up results of 6 months after discharge showed that mRS scores and the LPa level in the poor prognosis group were significantly higher than those in the good prognosis group at the age of onset and the early stage of onset, and ApoA-1 levels were significantly lower at the early stage of onset than those in the good prognosis group. In addition, there are several threshold variables in this study, and larger studies may be needed to further clarify the actual situation of such variables. With our research, it is still difficult to determine the role of lipids and homocysteine in the pathogenesis of anti-NMDAR encephalitis, but in view of their differences between patients with anti-NMDAR encephalitis and healthy people, as well as between different prognoses, we believe that lipids and homocysteine can be used to detect or predict patients' condition. Larger samples are needed to further elucidate the relationship between serum lipid profiles and anti-NMDAR encephalitis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Shandong University Qilu Hospital (Approval number: KYLL202008-044) and was conducted in accordance with the Declaration of Helsinki. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Z-hW and SQ conceptualized and designed the study, collected and organized the data, drafted the initial manuscript, reviewed, and revised the manuscript. LW, KW, RZ, YJ, and Z-hW assisted in collecting data and provided important suggestions for the design of research scheme and the writing of manuscript. XL conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This study was supported by the National Natural Science Foundation (No. 81873786). The funder did not participate in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
NMDAR: N-methyl-D-aspartate receptor; BBB: blood–brain barrier; Hcy: homocysteine; mRS: modified Rankin Scale; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; ApoA-1: apolipoprotein A-1; ApoB: apolipoprotein B; HCs: healthy controls; LPa: lipoprotein a; MS: multiple sclerosis; SLE: systemic lupus erythematosus; RA: rheumatoid arthritis.
1. Dalmau J, Tüzün E, Wu H, Masjuan J, Rossi JE, Volocshin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. (2007) 61:25–36. doi: 10.1002/ana.21050
2. Gleichman AG, Spruce LA, Dalmau J, Seeholzer SH, Lynch DR. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci. (2012) 32:11082–94. doi: 10.1523/JNEUROSCI.0064-12.2012
3. Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. (2013) 12:157–65. doi: 10.1016/S1474-4422(12)70310-1
4. Dong X, Zheng D, Nao J. Clinical characteristics and factors associated with short-term prognosis in adult patients with autoimmune encephalitis of non-neoplastic etiology. Neurol Sci. (2019) 40:1567–75. doi: 10.1007/s10072-019-03883-7
5. Soran H, Hama S, Yadav R, Durrington PN, HDL. functionality. Curr Opin Lipidol. (2012) 23:353–66. doi: 10.1097/MOL.0b013e328355ca25
6. Saemann MD, Poglitsch M, Kopecky C, Haidinger M, Horl WH, Weichhart T. The versatility of HDL: a crucial anti-inflammatory regulator. Eur J Clin Invest. (2010) 40:1131–43. doi: 10.1111/j.1365-2362.2010.02361.x
7. Zhu X, Parks JS. New roles of HDL in inflammation and hematopoiesis. Annu Rev Nutr. (2012) 32:161–82. doi: 10.1146/annurev-nutr-071811-150709
8. Fellows K, Uher T, Browne RW, Weinstock-Guttman B, Horakova D, Posova H, et al. Protective associations of HDL with blood-brain barrier injury in multiple sclerosis patients. J Lipid Res. (2015) 56:2010–8. doi: 10.1194/jlr.M060970
9. Weinstock-Guttman B, Zivadinov R, Horakova D, Havrdova E, Qu J, Shyh G, et al. Lipid profiles are associated with lesion formation over 24 months in interferon-beta treated patients following the first demyelinating event. J Neurol Neurosurg Psychiatry. (2013) 84:1186–91. doi: 10.1136/jnnp-2012-304740
10. Wu K, Wen L, Duan R, Li Y, Yao Y, Jing L, et al. Triglyceride level is an independent risk factor in first-attacked Neuromyelitis Optica spectrum disorders patients. Front Neurol. (2019) 10:1230. doi: 10.3389/fneur.2019.01230
11. Cho EB, Cho H-J, Choi M, Seok JM, Shin HY, Kim BJ, et al. Low high- density lipoprotein cholesterol and high triglycerides lipid profile in neuromyelitis optica spectrum disorder: associations with disease activity and disability. Mult Scler Relat Disord. (2020) 40:101981. doi: 10.1016/j.msard.2020.101981
12. Mangaraj M. Nanda Rachita, Panda S, Apolipoprotein A-I: a molecule of diverse function. Indian J Clin Biochem. (2016) 31:253–9. doi: 10.1007/s12291-015-0513-1
13. Tettey P, Simpson S, Taylor B, Blizzard L, Ponsonby A-L, Dwyer T, et al. K. An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult Scler. (2014) 20:1737–44. doi: 10.1177/1352458514533162
14. Meyers L, Groover CJ, Douglas J, Lee S, Brand D, Levin MC, et al. A role for Apolipoprotein A-I in the pathogenesis of multiple sclerosis. J Neuroimmunol. (2014) 277:176–85. doi: 10.1016/j.jneuroim.2014.10.010
15. Atta AM, Silva JPCG, Santiago MB, Oliveira IS, Oliveira RC, Sousa Atta MLB. Clinical and laboratory aspects of dyslipidemia in Brazilian women with systemic lupus erythematosus. Clin Rheumatol. (2018) 37:1539–46. doi: 10.1007/s10067-018-4051-0
16. Ardoin SP, Schanberg LE, Sandborg C, Yow E, Barnhart HX, Mieszkalski KI, et al. Laboratory markers of cardiovascular risk in pediatric SLE: the APPLE baseline cohort. Lupus. (2010) 19:1315–25. doi: 10.1177/0961203310373937
17. Fang F, Zhan Y, Hammar N, Shen X, Wirdefeldt K, Walldius G, et al. Lipids, apolipoproteins, and the risk of parkinson disease. Circ Res. (2019) 125:643–52. doi: 10.1161/CIRCRESAHA.119.314929
18. Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, et al. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun Rev. (2007) 6:503–9. doi: 10.1016/j.autrev.2007.03.008
19. Kuhn W, Hummel T, Woitalla D, Muller T. Plasma homocysteine and MTHFR C677T genotype in levodopa-treated patients with PD. Neurology. (2001) 56:281–2. doi: 10.1212/WNL.56.2.281
20. Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in animal models of Alzheimer's disease. J Neurosci. (2002) 22:1752–62. doi: 10.1523/JNEUROSCI.22-05-01752.2002
21. Morris MS. Homocysteine and Alzheimer's disease. Lancet Neurol. (2003) 2:425–8. doi: 10.1016/S1474-4422(03)00438-1
22. Vrethem M, Mattsson E, Hebelka H, Leerbeck K, Osterberg A, Landtblom AM, et al. Increased plasma homocysteine levels without signs of vitamin B12 deficiency in patients with multiple sclerosis assessed by blood and cerebrospinal fluid homocysteine and methylmalonic acid. Mult Scler. (2003) 9:239–45. doi: 10.1191/1352458503ms918oa
23. Ramsaransing GSM, Fokkema MR, Teelken A, Arutjunyan AV, Koch M, Keyser JD. Plasma homocysteine levels in multiple sclerosis. J Neurol Neurosurg Psychiatry. (2006) 77:189–92. doi: 10.1136/jnnp.2005.072199
24. Sahin S, Aksungar FB, Topkaya AE, Yildiz Z, Boru UT, Ayalp S, et al. Increased plasma homocysteine levels in multiple sclerosis. Mult Scler. (2007) 13:945–6. doi: 10.1177/1352458506075503
25. Stefano Zoccolella, Carla Tortorella, Pietro Iaffaldano, et al. Elevated plasma homocysteine levels in patients with multiple sclerosis are associated with male gender. J Neurol. (2012) 259:2105–10. doi: 10.1007/s00415-012-6464-z
26. Guzel I, Mungan S, Oztekin ZN, Ak F. Is there an association between the expanded disability status scale and inflammatory markers in multiple sclerosis? J Chin Med Assoc. (2016) 79:54–7. doi: 10.1016/j.jcma.2015.08.010
27. Chung CP, Avalos I, Oeser A, Gabretsadik T, Shintani A, Raggi P, et al. High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: association with disease characteristics and cardiovascular risk factors. Ann Rheum Dis. (2007) 66:208–14. doi: 10.1136/ard.2006.054973
28. Lazzerini PE, Capecchi PL, Bisogno S, Galeazzi M, Marcolongo R, Pasini FL. Reduction in plasma homocysteine levels in patients with rheumatoid arthritis given pulsed glucocorticoid treatment. Ann Rheum Dis. (2003) 62:694–5. doi: 10.1136/ard.62.7.694
29. Chiang E-P I, Bagley PJ, Selhub J, Nadeau M, Roubenoff R. Abnormal vitamin B status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. (2003) 114:283–7. doi: 10.1016/S0002-9343(02)01528-0
30. Lopez-Olivo MA, Gonzalez-Lopez L, Garcia-Gonzalez A, Villa-Manzano AI, Cota-Sanchez AR, Salazar-Paramo M, et al. Factors associated with hyperhomocysteinemia in Mexican patients with rheumatoid arthritis. Scand J Rheumatol. (2006) 35:112–6. doi: 10.1080/03009740510026922
31. Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, et al. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. (2006) 107:591–3. doi: 10.1182/blood-2005-06-2506
32. Lipton SA, Kim WK, Choi YB, Kumar S, D'Emilia DM, Rayudu PV, et al. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. (1997) 94:5923–8. doi: 10.1073/pnas.94.11.5923
33. Ho PI, Ortiz D, Rogers E, Shea TB. Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res. (2002) 70:694–702. doi: 10.1002/jnr.10416
34. Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. (2003) 26:137–46. doi: 10.1016/S0166-2236(03)00032-8
35. Tjiattas L, Ortiz DO, Dhivant S, Mitton K, Rogers E, Shea TB. Folate deficiency and homocysteine induce toxicity in cultured dorsal root ganglion neurons via cytosolic calcium accumulation. Aging Cell. (2004) 3:71–6. doi: 10.1111/j.1474-9728.2004.00086.x
36. Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. (2006) 580:2994–3005. doi: 10.1016/j.febslet.2006.04.088
37. Boldyrev A, Bryshkova E, Mashkina A, Vladychenskaya E. Why is homocysteine toxic for the nervous and immune systems? Curr Aging Sci. (2013) 6:29–36. doi: 10.2174/18746098112059990007
38. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
39. Wang W, Li J, Hu F, Wang R, Hong Z, He L, et al. Anti-NMDA receptor encephalitis: clinical characteristics, predictors of outcome and the knowledge gap in southwest China. Eur J Neurol. (2016) 23:621–9. doi: 10.1111/ene.12911
40. Shu Y, Qin B, Xu Y, Sun X, Chen Z, et al. Lipid metabolism in patients with anti-N-Methyl- D -aspartate receptor encephalitis. Neuroimmunomodulation. (2017) 24:256–63. doi: 10.1159/000485623
41. Liu F, Huang T, Wang B, Wang C, Guo S. Low high-density lipoprotein cholesterol and apolipoprotein A-I levels are associated with poor outcome and relapse in autoimmune encephalitis. Neurosci Lett. (2022) 775:136546. doi: 10.1016/j.neulet.2022.136546
42. Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. (2008) 13:216–26.
43. Orsó E, Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. (2017) 12:31–7. doi: 10.1007/s11789-017-0084-1
44. Georgila K, Vyrla D, Drakos E. Apolipoprotein A-I (ApoA-I), immunity, inflammation and cancer. Cancers. (2019) 11:1097. doi: 10.3390/cancers11081097
45. Wu J, Wang Y, Li H, Tan W, Chen X, Ye S. Serum apolipoprotein B-to-apolipoprotein A1 ratio is independently associated with disease severity in patients with acute pancreatitis. Sci Rep. (2019) 9:7764. doi: 10.1038/s41598-019-44244-w
46. Liu Y, Ma X, Ma L, Su Z, Li D, Chen X. Elevated ApoB/ApoA-I ratio is associated with acute anti-N-Methyl-D-aspartate receptor encephalitis, but not disease outcomes. Front Neurol. (2022) 13:896656. doi: 10.3389/fneur.2022.896656
47. Huang M, Gong Y, Grondolsky J, Hoover-Plow J. Lp(a)/apo(a) modulate MMP-9 activation and neutrophil cytokines in vivo in inflammation to regulate leukocyte recruitment. Am J Pathol. (2014) 184:1503–17. doi: 10.1016/j.ajpath.2014.01.010
48. Garcia-Gomez C, Martin-Martinez MA, Castaneda S, Sanchez-Alonso F, Uriarte-Ecenarro M, Gonzalez-Juanatey C, et al. Lipoprotein(a) concentrations in rheumatoid arthritis on biologic therapy: results from the CARdiovascular in rheuMAtology study project. J Clin Lipidol. (2017) 11:749–756, e3. doi: 10.1016/j.jacl.2017.02.018
49. Govindan KPS, Basha S, Ramesh V, Kumar CN, Swathi S. A comparative study on serum lipoprotein (a) and lipid profile between rheumatoid arthritis patients and normal subjects. J Pharm Bioallied Sci. (2015) 7:S22–25. doi: 10.4103/0975-7406.155767
50. Dursunoglu D, Evrengul H, Polat B, Tanriverdi H, Cobankara V, Kaftan A, et al. Lp(a) lipoprotein and lipids in patients with rheumatoid arthritis: serum levels and relationship to inflammation. Rheuma Int. (2005) 25:241–5. doi: 10.1007/s00296-004-0438-0
51. Borba EF, Santos RD, Bonfa E, Vinagre CG, Pileggi FJ, Cossermelli W, et al. Lipoprotein(a) levels in systemic lupus erythematosus. J Rheumatol. (1994) 21:220–3.
Keywords: anti-N-methyl-D-aspartate receptor encephalitis, lipid profile, homocysteine, ApoB/ApoA-1, prognosis
Citation: Wang Z-h, Qiao S, Wang L, Wang K, Zhang R, Jin Y, Wu H-k and Liu X (2023) Plasma lipid profiles and homocysteine levels in anti-N-methyl-D-aspartate receptor encephalitis. Front. Neurol. 14:1148450. doi: 10.3389/fneur.2023.1148450
Received: 20 January 2023; Accepted: 15 March 2023;
Published: 13 April 2023.
Edited by:
Veronica Rivas, Manuel Velasco Suárez National Institute of Neurology and Neurosurgery, MexicoReviewed by:
Massimiliano Castellazzi, University of Ferrara, ItalyCopyright © 2023 Wang, Qiao, Wang, Wang, Zhang, Jin, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuewu Liu, c25seHcxOTY2QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.