
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol. , 27 March 2023
Sec. Movement Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1147008
 Roongroj Bhidayasiri1,2*
Roongroj Bhidayasiri1,2* Michinori Koebis3
Michinori Koebis3 Takanori Kamei3
Takanori Kamei3 Takayuki Ishida3
Takayuki Ishida3 Ippei Suzuki4
Ippei Suzuki4 Jin Whan Cho5,6
Jin Whan Cho5,6 Shey-Lin Wu7
Shey-Lin Wu7Safinamide is a selective, reversible, monoamine oxidase B inhibitor for the treatment of patients with Parkinson's disease (PD) and motor fluctuations. This was a post hoc analysis of the SETTLE study, in which patients with PD and motor fluctuations were randomly assigned to 24-week treatment with safinamide (50 mg/day for 2 weeks, increased to 100 mg/day if tolerated) or placebo. In the present analysis, responders were defined according to their treatment responses at Week 2 and Week 24 based on changes in ON-time without troublesome dyskinesia from baseline with cutoffs of 1 hour. It was found that 81% (103/127) of the responders at Week 2 maintained the response through Week 24 in the safinamide group. Other outcomes did not necessarily coincide with the ON-time response; however, “Early” responders who showed a treatment response at both Week 2 and Week 24 had substantial improvements from baseline in OFF-time, UPDRS Part II and III scores, and PDQ-39 summary index scores through Week 24. The safinamide group had a higher proportion of early responders than the placebo group (39% vs 20%, p < 0.0001). At baseline, early responders in the safinamide group had significantly higher UPDRS Part II and III scores, shorter ON-time, and longer OFF-time than the other responder populations. In conclusion, the results of the present post hoc analysis suggest that patients with a short ON-time, severe motor symptoms, and highly compromised activities of daily living can benefit from safinamide early in treatment and over the long term.
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in the substantia nigra of the midbrain. Dopamine replacement therapy with levodopa is the gold standard pharmacotherapy for PD; however, with pulsatile dopaminergic stimulation by levodopa and disease progression, motor fluctuation or wearing-off develops (1).
The onset of wearing-off often requires additional adjunct treatment to levodopa, such as dopamine agonists, monoamine oxidase B inhibitors, or catechol-O-amine transferase inhibitors. Although these drugs have been shown in clinical studies to be superior to placebo in reducing wearing-off, not all subjects achieve clinically significant changes (2, 3). In clinical practice, the treatment of PD is individualized, depending on various factors, including patients' demographic characteristics, symptoms manifested and their severity, concomitant drugs, and/or comorbidities (4, 5). Several subgroup analyses have been conducted according to these factors, showing robustness of treatment efficacy and safety [e.g., (6–10)]. However, few have successfully characterized responders to treatment (10, 11) by methods other than the pharmacogenomic approach (12–14).
Safinamide is a monoamine oxidase B inhibitor that is unique for its reversible monoamine oxidase B inhibition and voltage-dependent sodium channel blockade effect (15), and its efficacy in reducing wearing-off has been demonstrated by three Phase III trials and a Phase II/III trial (16–19). In these trials, 57.7% to 64.3% of subjects showed a clinical response to 24-week treatment with safinamide 100 mg/day, as defined either by a 1-h or greater increase in ON-time without troublesome dyskinesia or by Clinical Global Impression—Change. The responder rate was significantly greater than with placebo (25.7 vs. 48.1–61.7%) (18). A meta-analysis of the trials demonstrated that the safinamide groups also showed significant improvements in the Unified Parkinson's Disease Rating Scale (UPDRS) Part II score and the 39-item Parkinson's Disease Questionnaire (PDQ-39) score (20, 21). However, which type of patients can benefit from safinamide treatment is still largely unknown.
The present study was a post hoc analysis of one of the Phase III trials, the SETTLE study. The SETTLE study was a double-blind, parallel-group, 24-week trial of safinamide (50–100 mg/day orally) vs placebo in patients taking stable dosages of levodopa and concomitant PD medications (17). One of the characteristics of this study is that efficacy assessments were conducted both as early as at Week 2 and after long-term treatment for up to 6 months. By using the design of the SETTLE study, responder subgroups were defined and analyzed based on the response to treatment at Week 2 and the end of the 24-week treatment (Figure 1). The demographic characteristics and characteristics associated with treatment response were then determined in the responder subgroups.
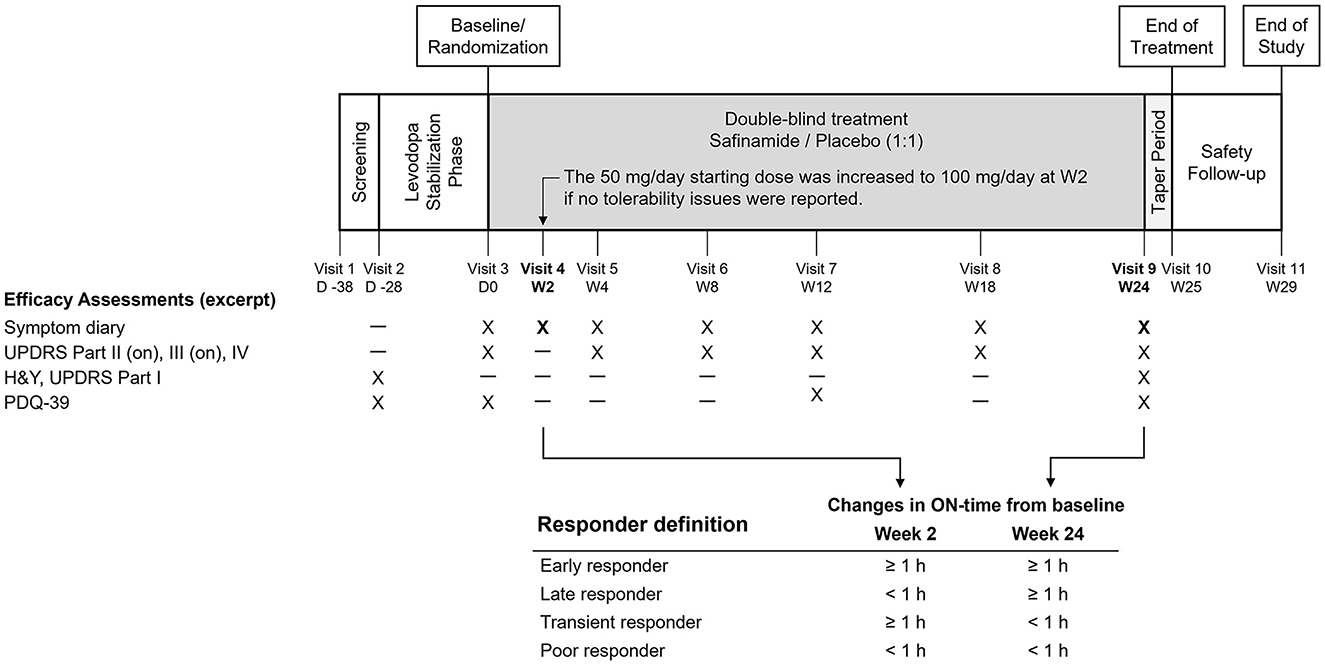
Figure 1. Design of the SETTLE study and the responder definition in the present analysis. H&Y, Hoehn and Yahr staging, PDQ-39, 39-item Parkinson's Disease Questionnaire; UPDRS, Unified Parkinson's Disease Rating Scale.
This was a post hoc analysis of a Phase III study of safinamide (SETTLE study), which was conducted in 21 countries in Europe, Asia, and North America between 2009 and 2012 and was registered at Clinical trials.gov (NCT00627640). The study was conducted in accordance with the International Conference on Harmonization Tripartite Guideline for good clinical practices and the Declaration of Helsinki. The design of the SETTLE study has been reported elsewhere (17). Briefly, the study enrolled PD patients with a disease duration of at least 3 years, Hoehn and Yahr stage 1–4, and an average daily off-time of at least 1.5 h. Before randomization, pharmacotherapy for PD was adjusted to minimize motor fluctuations and was required to be stable for at least 4 weeks. Eligible patients were then randomly assigned in a 1:1 ratio to receive safinamide or placebo. Patients received the study drug at a dose of 50 mg/day for 2 weeks, and if well-tolerated, the dose was increased to 100 mg/day (Figure 1).
The primary endpoint of the SETTLE study was the change from baseline to 24 weeks in ON-time without troublesome dyskinesia (hereafter, ON-time) as recorded in the 24-h diary. Other efficacy assessments included diary entries, UPDRS scores during the ON-phase, and PDQ-39 scores. For efficacy assessment at Week 2, only diary entries were evaluated (Figure 1).
The subjects were divided into four responder subgroups: Early responder, ≥ 1-h increase from baseline in ON-time both at Week 2 and at Week 24; Late responder, ≥ 1-h increase in ON-time not at Week 2, but at Week 24; Transient responder, ≥ 1-h increase in ON-time at Week 2, but not at Week 24; and Poor responder, ≥ 1-h increase in ON-time neither at Week 2 nor at Week 24 (Figure 1). The present study defined responders as subjects who achieved an increase in ON-time of at least 1 h. One reason was that this definition was in accordance with similar studies of levodopa adjuncts, in which subjects with a change in ON- or OFF-time of at least 1 h were defined as responders (22–25). Another is the reported minimal clinically important difference for OFF-time being 1.0 to 1.3 (2, 26), which supports the use of a 1-h cutoff for ON-time in the definition of responders who achieve a clinically significant change.
To analyze the responders according to the treatment responses in ON-time at Week 2 and Week 24 (end of the treatment), only those who had a diary assessment at Week 2 were included in the post hoc analysis population. The proportions of these responder subgroups in the safinamide and placebo groups were compared with the Chi-squared test, and the subgroup with the larger contribution to the intergroup difference was identified by analysis of residuals.
To identify factors that contributed to changes in ON-time at Week 24, multiple regression analysis was performed in each treatment group with the change in ON-time at Week 24 as the response variable and baseline values and the change in ON-time at Week 2 as explanatory variables.
To compare treatment responses by responder subgroup, summary statistics of the value at baseline and change from baseline at Week 24 were calculated by each responder subgroup and treatment group. Analysis of covariance was used to compare treatment groups within each subgroup, with the change from baseline to the Week 24 assessment as the response variable, the treatment group as the fixed effect, and the baseline value and region as the covariates.
To compare the demographic characteristics and baseline values between the early population and the others and between safinamide and placebo within subgroups, Welch's t-test was used for continuous variables, Fisher's exact test was used for categorical variables, and the Wilcoxon rank-sum test was used for the numbers of concomitant non-levodopa antiparkinsonian drugs.
Since this was a post hoc analysis, statistical methods were not pre-specified. Dropout and missing data at the last assessment point were imputed using the last observation carried forward methodology. All tests had a significance level of 5% (two-tailed), and no adjustments were made for multiplicity. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
In the SETTLE study, 274 and 275 patients were randomized to safinamide and placebo, respectively. Of these, 263 and 270 subjects, respectively, had ON-time assessment at Week 2 and were included in this post hoc analysis.
The safinamide and placebo groups were divided into four responder subgroups according to the change from baseline in ON-time at Weeks 2 and 24 of treatment (Figure 1). In the safinamide group, more than one-third of the subjects were classified as Early responders (103 subjects, 39%), another one-third were Poor responders (92, 35%), and the Late and Transient responders were few (44, 17; 24, 9%, respectively) (Figure 2). On the other hand, in the placebo group, the largest population was the Poor responders (120, 44%), followed by the Late responders (58, 22%), the Early responders (55, 20%), and the Transient responders (37, 14%). The distribution of the responders was significantly different between the safinamide and placebo groups (p < 0.0001, Chi-squared test), with significant differences in the proportion of Early responders (p < 0.0001) and Poor responders (p = 0.03).
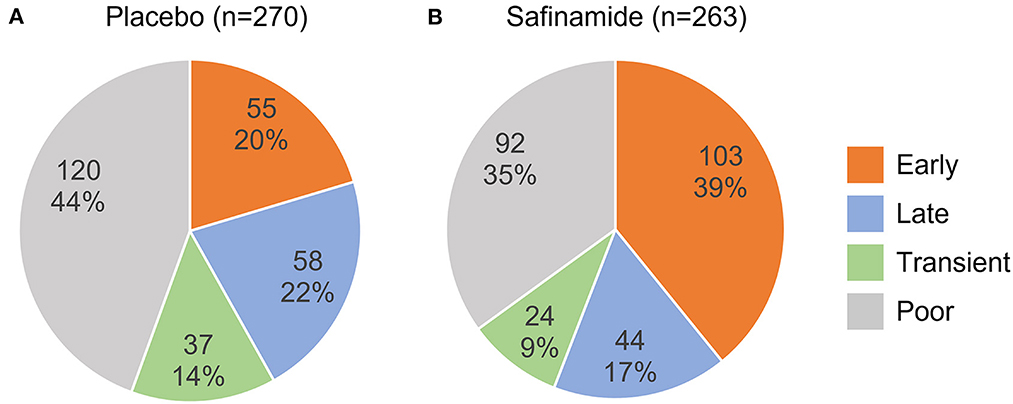
Figure 2. Distribution of responder subgroups in the placebo (A) and safinamide (B) groups. The upper and lower numbers are the number and the percentage, respectively, of each responder subgroup in the treatment group. The distributions are significantly different between the treatment groups (p < 0.0001, Chi-squared test).
The proportion of responders who achieved an increase in ON-time of at least 1 h as of Week 24, i.e., the total of Early and Late responders, was significantly higher (56%) in the safinamide group than in the placebo group (42%; p = 0.001, Chi-squared test).
In the safinamide group, 127 subjects showed a 1-h or greater increase in ON-time at Week 2, and most of them (103 subjects, 81%) maintained the treatment response at Week 24. A similar trend was found in the placebo group: 55 of 92 (60%) subjects who showed a response at Week 2 maintained their response at Week 24, but the rate was significantly greater in the safinamide group than in the placebo group (p = 0.0005, Chi-squared test).
These results were supported by the multivariable regression analyses, which showed that the change in ON-time from baseline to Week 2 was positively associated with the change in ON-time from baseline to Week 24 in both treatment groups (Supplementary Table 1).
Changes in the outcomes from baseline at Week 24 in the individual responder subgroups are shown in Figure 3, Supplementary Figure 1, and Supplementary Tables 2–4. Reflective of the defining of responders according to changes in ON-time, ON-time changes in the responder subgroups did not differ substantially between the safinamide and placebo groups. Changes in scores in UPDRS Parts II and III and the PDQ-39 summary index, however, did not necessarily mirror this trend in ON-time changes: UPDRS Part III scores, for example, decreased substantially from baseline even among the Poor responders.
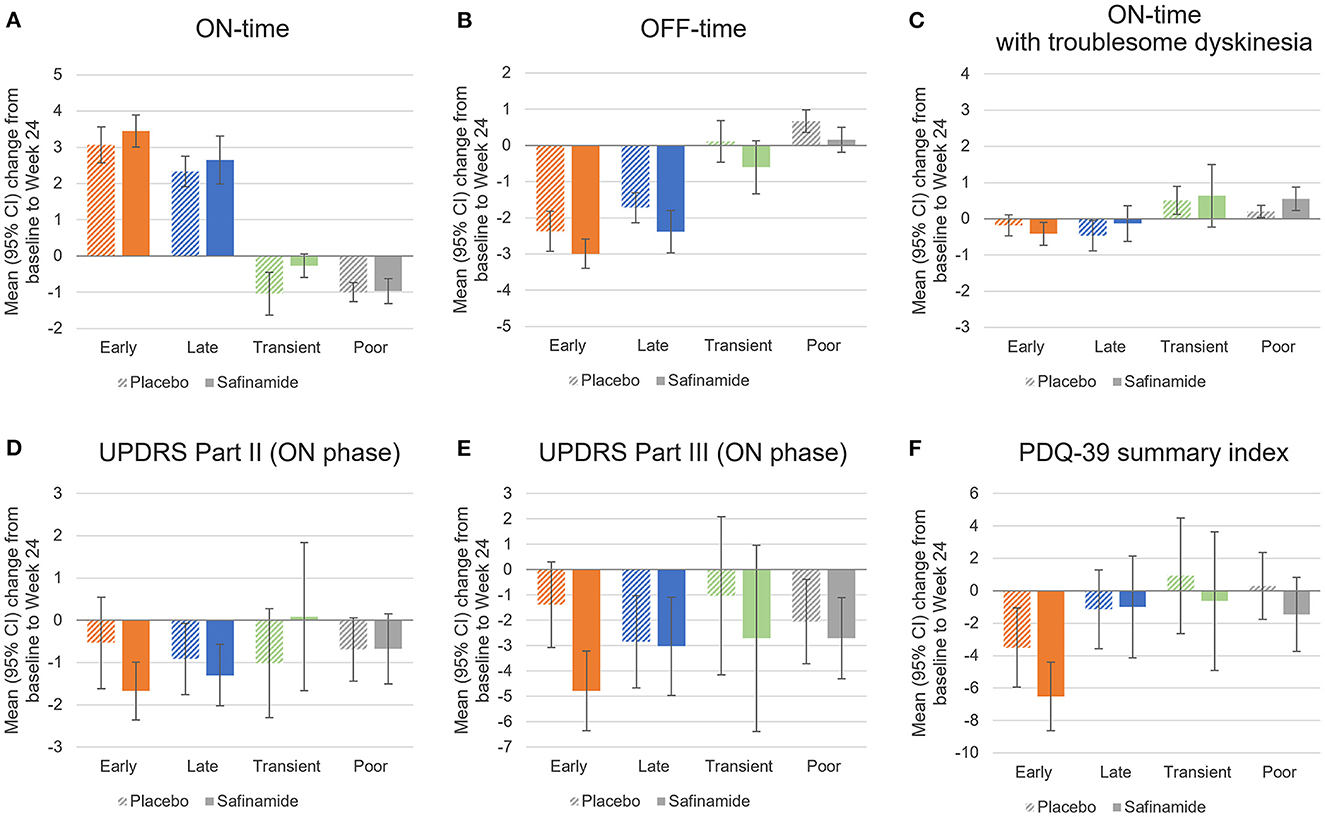
Figure 3. Changes from baseline to Week 24 in diary outcomes (A–C), UPDRS scores (D, E), and the PDQ-39 summary index (F) by responder subgroup in the placebo and safinamide groups. 95%CI, 95% confidence interval; PDQ-39, 39-item Parkinson's Disease Questionnaire; UPDRS, Unified Parkinson's Disease Rating Scale.
At Week 24, the Early responders in the safinamide group achieved a substantial reduction from baseline in OFF-time, UPDRS Part II and III scores, and PDQ-39 summary index scores. The change in the UPDRS Part III score was significant compared with the Early responders in the placebo group. At Week 24, the Late responders achieved a reduction from baseline in OFF-time and UPDRS Part II and III scores. Their reduction in OFF-time was significant relative to the placebo group. The few Transient responders had broad confidence intervals in changes in UPDRS and PDQ-39 scores. The Poor responders in the safinamide group had a significant reduction in OFF-time and a significant increase in ON-time with troublesome dyskinesia compared with the placebo group.
The demographic characteristics, baseline values, and concomitant use of antiparkinsonian drugs in the different responder subgroups are summarized in Table 1 and Supplementary Table 5. In the safinamide group, the Early responders had, at baseline, significantly higher UPDRS Part II and III scores, shorter ON-time, and longer OFF-time than the other responder populations (Table 1). In the placebo group as well, the Early responders had significantly shorter ON-time and longer OFF-time than the other responder populations, but UPDRS Part II and III scores did not differ significantly and tended to be lower in the Early responders (Supplementary Table 5). No significant differences were seen in the other demographic characteristics, baseline values, or concomitant drugs. A comparison of the safinamide and placebo groups in each responder subgroup showed significantly higher UPDRS Part II and III and total scores in the safinamide group than in the placebo group among Early responders (35.78 vs. 29.57, p = 0.04) and significantly lower UPDRS Part II and III and total scores among the Poor responders (30.36 vs. 34.88, p = 0.04).
The present study demonstrated that the safinamide group in the SETTLE study, relative to the placebo group, had a significantly higher proportion of Early responders, i.e., subjects who achieved a clinically significant increase in ON-time early in treatment and maintained the response through Week 24. The change in Part III scores was significantly greater in the Early responders in the safinamide group than in the placebo group. Although the Early responders in both treatment groups had substantial improvements from baseline in OFF-time, UPDRS Part II and III scores, and PDQ-39 summary index scores through Week 24, the safinamide group had greater changes in Part II and III and PDQ-39 summary index scores than the placebo group. The differences were not significant; however, they exceeded the minimal clinically important differences (Part II, −0.7; Part III, −2.4; PDQ-39 summary index, −1.6) (2, 3). This finding suggests that Early responders are one group of patients that benefits greatly from safinamide treatment.
Characteristically, Early responders achieve a large early improvement in ON-time at Week 2 that persists through Week 24. In the safinamide group, about 80% of the subjects with an improvement of at least 1 h in ON-time at Week 2 were Early responders. Change from baseline in ON-time at Week 2 was significantly associated with the change at Week 24. Thus, the initial response may help to predict the long-term therapeutic response to safinamide.
The baseline shorter ON-time was another characteristic of Early responders. Reportedly, the more severe the symptoms at baseline were, the larger the magnitude of therapeutic response would be expected (10, 11, 27). For example, Poewe et al. showed that baseline OFF-time was significantly correlated with levodopa/carbidopa intestinal gel-induced OFF-time improvement at month 24 (27). This relationship was also found in the placebo-treated subjects (28). Patients with shorter ON-time would have a higher expectation for the study medication to elongate ON-time, so they might show greater response in ON-time.
Early responders in the safinamide group also had higher UPDRS Part II and III scores at baseline, and importantly, this was not found in the placebo group. These baseline characteristics indicated that Early responders in the safinamide group were those whose baseline treatment was suboptimal for symptomatic improvement during the ON phase. Since Early responders showed a tendency toward a higher UPDRS Part IV score and longer ON-time with troublesome dyskinesia at baseline, dyskinesia during the ON-phase might limit dopaminergic treatments in this population. Nonetheless, safinamide treatment brought a large increase in ON-time without troublesome dyskinesia, with a slight decrease in ON-time with troublesome dyskinesia from baseline in Early responders. This is in accordance with the previous reports, which showed that long-term safinamide treatment did not exacerbate dyskinesia in a subgroup of patients with dyskinesia at baseline (29, 30). Safinamide has both dopaminergic and non-dopaminergic actions, and this “dual action” may help overcome the limit of dopaminergic treatments in Early responders. For example, safinamide was reported to inhibit glutamate release in the basal ganglia in rats via its sodium channel inhibitory effect (15). Excessive glutamate signaling has been suggested in dyskinesia pathophysiology (31, 32), and abnormal cortical facilitation, which indicates hyperactive glutamatergic neurotransmission, was reported in patients with levodopa-induced dyskinesia (33). Guerra et al. have shown that long-term safinamide treatment ameliorated cortical facilitation, and the change in cortical facilitation and the change in dyskinesia severity were positively correlated (34). Although it is not clear that the non-dopaminergic action of safinamide was involved in the decrease in cortical facilitation, this suggests that inhibition of glutamatergic neurotransmission may lead to dyskinesia alleviation.
The proportions of patients on placebo and safinamide in the different responder subgroups differed significantly. The proportion of Early responders in the safinamide group (39%) was almost double that in the placebo group (20%), which suggests that safinamide is effective in reducing wearing-off. Likewise, the proportion of subjects whose ON-time was increased at least 1 h at Week 24 (i.e., Early + Late responders) was significantly higher in the safinamide group (56%) than in the placebo group (42%). This high responder rate at Week 24 was reproduced in a Japanese Phase II/III study of safinamide (18). In addition, it is comparable to the responder rates found in the clinical studies of other levodopa adjuncts for advanced PD (22–25). For example, in the LARGO study, the proportions of subjects with a decrease from baseline of at least 1 h in OFF-time at Week 18 were 51% in the rasagiline 1 mg group, 45% in the entacapone 200 mg group, and 32% in the placebo group (22). In a Phase III clinical study of opicapone, the proportions of subjects with an increase in ON-time of at least 1 h at Week 14–15 was 57% in the opicapone 25 mg group, 65% in the opicapone 50 mg group, 58% in the entacapone 200 mg group, and 46% in the placebo group (25).
The proportion of Transient responders in the safinamide group (24 of 263 subjects, or 9%) was not substantially different from that in the placebo group (37 of 270 subjects, or 14%). Since beginning treatment often entails a psychological placebo effect, the transient responses seen may be indicative of such an effect. Alternatively, Transient responders may have rapid disease progression that may mask long-term efficacy. It is also possible to assume that the Transient responders may have responded only to 50 mg because the subjects in the SETTLE study started safinamide treatment at a dose of 50 mg/day, and about 90% of them were subsequently escalated to 100 mg/day at Week 2 (17). A lack of response to high doses was found in a clinical study in patients with early PD (Study 015) (35): Although treatment with 100 mg of safinamide in Study 015 significantly improved UPDRS Part III scores over placebo, no significant improvement was achieved at the higher dose of 200 mg. The present analysis may have shown the presence of a population of transient responders among PD patients with wearing-off who respond sufficiently to 50 mg of safinamide and gain no benefits from higher doses. If this proves true, proper dose adjustment should help maintain a therapeutic effect. The reason for the lack of efficacy at the high dose in Study 015, however, is unclear (35). A high dropout rate in the higher dose group was also proposed as a cause of the lack of efficacy. It is, therefore, unknown whether a lack of efficacy at higher doses could be seen in clinical practice.
The outcomes at Week 24 were more varied among the subjects without an improvement of at least 1 h in ON-time at Week 2. One-third of them (Late responders) achieved an increase of more than 1 h in ON-time at Week 24, and the rest did not (Poor responders). Furthermore, even in the Poor responders, some outcomes other than ON-time were improved; for example, the safinamide group had a significantly greater decrease in OFF-time from baseline through Week 24 than the placebo group, and the difference between the safinamide and placebo groups in the change in PDQ-39 summary index scores exceeded the minimal clinically important difference (3). Given the nature of PD with its diverse symptoms, the continuation of treatment should be considered with their problematic symptoms factored in.
The present study has several limitations. First, it was a post hoc analysis with no predefined conditions. Given that the responder subgroups were defined by post-baseline characteristics, the present study did not adequately control the demographic characteristics and baseline values of the responders among the groups. Second, since subjects in the SETTLE study who tolerated 50 mg/day were escalated to 100 mg/day (17), it is unclear whether the initial treatment response is predictive of the long-term response when the dose is maintained at 50 mg/day. Moreover, as stated previously, the dose may not have been optimized in some patients. Third, the present study defined responders as subjects who achieved an increase in ON-time of at least 1 h. Some studies, however, have defined responders as those subjects who achieved a change from baseline in ON- or OFF-time of at least 30% or at least 3 h (11, 27, 36–38). The use of different cutoff values may yield different results.
In conclusion, the present post hoc analysis showed that, when a patient responded significantly to safinamide treatment early on, the response was more likely to last for a longer period of time. Furthermore, patients with a short ON-time, severe motor symptoms, and highly compromised activities of daily living may benefit from safinamide early in the treatment and over the long term. These results suggest that the evaluation of activities of daily living, as well as wearing-off and motor functions, is crucial for the most effective treatment with safinamide. Recently, genetic biomarkers for other antiparkinsonian drugs that predict patients' therapeutic responses have been discovered (12–14). To provide precision treatment with safinamide, further research may be warranted to discover biomarkers that predict response to safinamide treatment.
The data analyzed in this study was obtained from Eisai Co., Ltd, the following licenses/restrictions apply: access to these datasets must be approved by Eisai Co., Ltd. Requests to access these datasets should be directed to RB, cmJoQGNodWxhcGQub3Jn.
The studies involving human participants were reviewed and approved by an appropriately constructed Independent Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Statistical analysis: IS. Preparation of the first draft: MK. Conception and design of the study, interpretation of data, review and critique of the statistical analysis, and the entire draft: All authors. All authors have approved the final manuscript.
This work was sponsored by Eisai Co., Ltd. The original clinical trial was sponsored by Newron Pharmaceuticals and Merck Serono.
The authors would like to thank the patients who participated in this trial and their families, as well as the staff at all investigational sites.
RB, SW, and JC received support for the present manuscript including the provision of study materials and medical writing support from Eisai Co. Ltd., as well as consulting feed from Eisai Co. Ltd. RB has royalties or licenses with Springer Science+ Business Media (Humana Press) and John Wiley & Sons, Inc., received consulting fees from H Lundbeck A/S, B. L. Hua Co. Ltd, Abbott Laboratories, Ipsen SA, Teva Pharmaceutical Industries Ltd, Takeda Pharmaceutical Company Limited, and Otsuka Pharmaceutical Co. Ltd, received payment or honoraria from the International Parkinson's and Movement Disorder Society and the Korean Movement Disorder Society, and has patents for a Nocturnal Monitoring Device, a Tremor Analysis Software and Device, and a Laser-guided Walking Stick, as well as leadership or fiduciary roles in the International Parkinson's and Movement Disorder Society, Chulalongkorn University, and the World Health Organization. MK, TI, TK, and IS are current employees of Eisai Co., Ltd.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1147008/full#supplementary-material
Supplementary Figure 1. Change from baseline in ON-time by responder subgroup.
Supplementary Table 1. Multivariable regression analysis to find factor(s) associated with change from baseline to Week 24 in ON-time.
Supplementary Table 2. The baseline values and changes from baseline to Week 24 in diary outcomes by responder subgroups in the placebo and safinamide groups.
Supplementary Table 3. The baseline values and changes from baseline to Week 24 in UPDRS Part II and III scores by responder subgroups in the placebo and safinamide groups.
Supplementary Table 4. The baseline values and changes from baseline to Week 24 in PDQ-39 summary index and subdomain scores by responder subgroups in the placebo and safinamide groups.
Supplementary Table 5. Demography and baseline characteristics by responder subgroups in the placebo group.
PD, Parkinson's disease; PDQ-39, 39-item Parkinson's Disease Questionnaire; UPDRS, Unified Parkinson's Disease Rating Scale.
1. Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Lanciego JL, Artieda J, Gonzalo N, et al. Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci. (2000) 23:S8–19. doi: 10.1016/S1471-1931(00)00028-8
2. Hauser RA, Auinger P. Parkinson Study Group. Determination of minimal clinically important change in early and advanced Parkinson's disease. Mov Disord. (2001) 26:813–8. doi: 10.1002/mds.23638
3. Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the PDQ-39 Parkinson's disease questionnaire. Age Ageing. (2001) 30:299–302. doi: 10.1093/ageing/30.4.299
4. Orayj K, Lane E. Patterns and Determinants of Prescribing for Parkinson's Disease: A Systematic Literature Review. Parkinsons Dis. (2019) 2019:9237181. doi: 10.1155/2019/9237181
5. Jankovic J. Parkinson's disease therapy [colon] tailoring choices for early and late disease, young and old patients. Clin Neuropharmacol. (2000) 23:252–61. doi: 10.1097/00002826-200009000-00003
6. Elmer LW. Rasagiline adjunct therapy in patients with Parkinson's disease: post hoc analyses of the PRESTO and LARGO trials. Parkinsonism Relat Disord. (2013) 19:930–6. doi: 10.1016/j.parkreldis.2013.06.001
7. Cattaneo C, Sardina M, Bonizzoni E. Safinamide as add-on therapy to levodopa in mid- to late-stage parkinson's disease fluctuating patients: post hoc analyses of studies 016 SETTLE. J Parkinsons Dis. (2016) 6:165–73. doi: 10.3233/JPD-150700
8. Giladi N, Nicholas AP, Asgharnejad M, Dohin E, Woltering F, Bauer L, et al. Efficacy of rotigotine at different stages of parkinson's disease symptom severity and disability: a post hoc analysis according to baseline hoehn and yahr stage. J Parkinsons Dis. (2016) 6:741–9. doi: 10.3233/JPD-160847
9. Rocha JF, Ebersbach G, Lees A, Tolosa E, Ferreira JJ, Poewe W, et al. The added benefit of opicapone when used early in Parkinson's disease patients with levodopa-induced motor fluctuations: a post-hoc analysis of BIPARK-I -II. Front Neurol. (2021) 12:754016. doi: 10.3389/fneur.2021.754016
10. Hattori N, Kitabayashi H, Kanda T, Nomura T, Toyama K, Mori A, et al. Pooled analysis from phase 2b and 3 studies in japan of Istradefylline in Parkinson's disease. Mov Disord. (2020) 35:1481–7. doi: 10.1002/mds.28095
11. Standaert DG, Boyd JT, Odin P, Robieson WZ, Zamudio J, Chatamra K. Systematic evaluation of levodopa-carbidopa intestinal gel patient-responder characteristics. NPJ Parkinsons Dis. (2018) 4:4. doi: 10.1038/s41531-017-0040-2
12. Masellis M, Collinson S, Freeman N, Tampakeras M, Levy J, Tchelet A, et al. Dopamine D2 receptor gene variants and response to rasagiline in early Parkinson's disease: a pharmacogenetic study. Brain. (2006) 139:2050–62. doi: 10.1093/brain/aww109
13. MacDonald HJ, Stinear CM, Ren A, Coxon JP, Kao J, Macdonald L, et al. Dopamine gene profiling to predict impulse control and effects of dopamine agonist ropinirole. J Cogn Neurosci. (2016) 28:909–19. doi: 10.1162/jocn_a_00946
14. Naito T, Satake W, Cha PC, Kobayashi K, Murata M, Toda T. Comparative whole transcriptome analysis of Parkinson's disease focusing on the efficacy of zonisamide. J Neurol Neurosurg Psychiatry. (2022) 93:509–12. doi: 10.1136/jnnp-2021-328742
15. Morari M, Brugnoli A, Pisanò CA, Novello S, Caccia C, Melloni E, et al. Safinamide differentially modulates in vivo glutamate and GABA release in the rat hippocampus and basal ganglia. J Pharmacol Exp Ther. (2018) 364:198–206. doi: 10.1124/jpet.117.245100
16. Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt M, Chirilineau D, et al. Randomized trial of safinamide add-on to levodopa in Parkinson's disease with motor fluctuations. Mov Disord. (2014) 29:229–37. doi: 10.1002/mds.25751
17. Schapira AH, Fox SH, Hauser RA, Jankovic J, Jost WH, Kenney C, et al. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol. (2017) 74:216–24. doi: 10.1001/jamaneurol.2016.4467
18. Hattori N, Tsuboi Y, Yamamoto A, Sasagawa Y, Nomoto M, ME2125-3 Study Group. Efficacy and safety of safinamide as an add-on therapy to L-DOPA for patients with Parkinson's disease: a randomized, double-blind, placebo-controlled, phase II/III study. Parkinsonism Relat Disord. (2020) 75:17–23. doi: 10.1016/j.parkreldis.2020.04.012
19. Wei Q, Tan Y, Xu P, Tao E, Lu Z, Pan X, et al. The XINDI Study: a randomized phase iii clinical trial evaluating the efficacy and safety of safinamide as add-on therapy to levodopa in Chinese patients with Parkinson's disease with motor fluctuations. CNS Drugs. (2022) 36:1217–27. doi: 10.1007/s40263-022-00958-6
20. Yan R, Cai H, Cui Y, Su D, Cai G, Lin F, et al. Comparative efficacy and safety of monoamine oxidase type B inhibitors plus channel blockers and monoamine oxidase type B inhibitors as adjuvant therapy to levodopa in the treatment of Parkinson's disease: a network meta-analysis of randomized controlled trials. Eur J Neurol. (2022) 30:1118–34. doi: 10.1111/ene.15651
21. Giossi R, Carrara F, Mazzari M, Lo Re F, Senatore M, Schicchi A, et al. Overall efficacy and safety of safinamide in parkinson's disease: a systematic review and a meta-analysis. Clin Drug Investig. (2021) 41:321–39. doi: 10.1007/s40261-021-01011-y
22. Rascol O, Brooks DJ, Melamed E, Oertel W, Poewe W, Stocchi F, et al. Rasagiline as an adjunct to levodopa in patients with Parkinson's disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet. (2005) 365:947–54. doi: 10.1016/S0140-6736(05)71083-7
23. Lees AJ, Ferreira J, Rascol O, Poewe W, Rocha JF, McCrory M, et al. Opicapone as adjunct to levodopa therapy in patients with parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol. (2017) 74:197–206. doi: 10.1001/jamaneurol.2016.4703
24. Takeda A, Takahashi R, Tsuboi Y, Nomoto M, Maeda T, Nishimura A, et al. Randomized, controlled study of opicapone in japanese Parkinson's patients with motor fluctuations. Mov Disord. (2021) 36:415–23. doi: 10.1002/mds.28322
25. Ferreira JJ, Lees A, Rocha JF, Poewe W, Rascol O, Soares-da-Silva P, et al. Opicapone as an adjunct to levodopa in patients with Parkinson's disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol. (2016) 15:154–65. doi: 10.1016/S1474-4422(15)00336-1
26. Hauser RA, Gordon MF, Mizuno Y, Poewe W, Barone P, Schapira AH, et al. Minimal clinically important difference in Parkinson's disease as assessed in pivotal trials of pramipexole extended release. Parkinsons Dis. (2014) 2014:467131. doi: 10.1155/2014/467131
27. Poewe W, Bergmann L, Robieson WZ, Antonini A. Predictors of response for “Off” time improvement with levodopa-carbidopa intestinal gel treatment: an analysis of the GLORIA registry. Front Neurol. (2020) 11:419. doi: 10.3389/fneur.2020.00419
28. Shin CW, Hahn S, Park BJ, Kim JM, Park EO, Jeon B. Predictors of the placebo response in clinical trials on Parkinson's disease: a meta-analysis. Parkinsonism Relat Disord. (2016) 29:83–9. doi: 10.1016/j.parkreldis.2016.05.019
29. Cattaneo C, Ferla RL, Bonizzoni E, Sardina M. Long-term effects of safinamide on dyskinesia in mid- to late-stage parkinson's disease: a post-hoc analysis. J Parkinsons Dis. (2015) 5:475–81. doi: 10.3233/JPD-150569
30. Hattori N, Kamei T, Ishida T, Suzuki I, Nomoto M, Tsuboi Y. Long-term effects of safinamide adjunct therapy on levodopa-induced dyskinesia in Parkinson's disease: post-hoc analysis of a Japanese phase III study. J Neural Transm (Vienna). (2022) 129:1277–87. doi: 10.1007/s00702-022-02532-2
31. Espay AJ, Morgante F, Merola A, Fasano A, Marsili L, Fox SH, et al. Levodopa-induced dyskinesia in Parkinson disease: current and evolving concepts. Ann Neurol. (2018) 84:797–811. doi: 10.1002/ana.25364
32. Cenci MA, Skovgård K, Odin P. Non-dopaminergic approaches to the treatment of motor complications in Parkinson's disease. Neuropharmacology. (2022) 210:109027. doi: 10.1016/j.neuropharm.2022.109027
33. Guerra A, Suppa A, D'Onofrio V, Di Stasio F, Asci F, Fabbrini G, et al. Abnormal cortical facilitation and L-dopa-induced dyskinesia in Parkinson's disease. Brain Stimul. (2019) 12:1517–25. doi: 10.1016/j.brs.2019.06.012
34. Guerra A, Asci F, Zampogna A, D'Onofrio V, Suppa A, Fabbrini G, et al. Long-term changes in short-interval intracortical facilitation modulate motor cortex plasticity and L-dopa-induced dyskinesia in Parkinson's disease. Brain Stimul. (2022) 15:99–108. doi: 10.1016/j.brs.2021.11.016
35. Stocchi F, Borgohain R, Onofrj M, Schapira AH, Bhatt M, Lucini V, et al. A randomized, double-blind, placebo-controlled trial of safinamide as add-on therapy in early Parkinson's disease patients. Mov Disord. (2012) 27:106–12. doi: 10.1002/mds.23954
36. LeWitt PA, Lyons KE, Pahwa R. SP 650 Study Group. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER study. Neurology. (2007) 68:1262–7. doi: 10.1212/01.wnl.0000259516.61938.bb
37. Zhang ZX, Liu CF, Tao EX, Shao M, Liu YM, Wang J, et al. Rotigotine transdermal patch in Chinese patients with advanced Parkinson's disease: a randomized, double-blind, placebo-controlled pivotal study. Parkinsonism Relat Disord. (2017) 44:6–12. doi: 10.1016/j.parkreldis.2017.08.015
Keywords: safinamide, Parkinson's disease, levodopa, monoamine oxidase B inhibitor, motor fluctuation, post hoc analysis, treatment response
Citation: Bhidayasiri R, Koebis M, Kamei T, Ishida T, Suzuki I, Cho JW and Wu S-L (2023) Sustained response in early responders to safinamide in patients with Parkinson's disease and motor fluctuations: A post hoc analysis of the SETTLE study. Front. Neurol. 14:1147008. doi: 10.3389/fneur.2023.1147008
Received: 18 January 2023; Accepted: 06 March 2023;
Published: 27 March 2023.
Edited by:
Antonella Conte, Sapienza University of Rome, ItalyReviewed by:
Arjun Tarakad, Baylor College of Medicine, United StatesCopyright © 2023 Bhidayasiri, Koebis, Kamei, Ishida, Suzuki, Cho and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roongroj Bhidayasiri, cmJoQGNodWxhcGQub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.