- 1Department of Pharmacy, Affiliated Drum Tower Hospital, Nanjing University Medical School, Nanjing, Jiangsu, China
- 2China Pharmaceutical University Nanjing Drum Tower Hospital, Nanjing, Jiangsu, China
- 3Ministry of Education (MOE) Key Laboratory of Model Animal for Disease Study, Model Animal Research Center, Jiangsu Key Laboratory of Molecular Medicine, Medical School, Nanjing University, Nanjing, Jiangsu, China
- 4Department of Pharmacy, Wuhan Fourth Hospital, Wuhan, Hubei, China
- 5Department of Pharmacy, Wuhan No. 1 Hospital, Wuhan, Hubei, China
- 6Department of Neurology, Affiliated Drum Tower Hospital, Nanjing University Medical School, Nanjing, Jiangsu, China
Background: Stress hyperglycemia frequently occurs in patients with acute ischemic stroke (AIS). The influence of stress hyperglycemia on the outcomes of patients with AIS remains ambiguous.
Methods: Data from our institution on patients with AIS between June 2020 and June 2021 were retrospectively analyzed. The severity of the stroke was assessed using the National Institutes of Health Stroke Scale (NIHSS) at admission, and the primary endpoint was functional outcomes. Stress hyperglycemia was measured by the glucose-to-HbA1c ratio. In the multivariable analysis, two models that retained or excluded the NIHSS were adopted to explore the relationship between stress hyperglycemia and outcomes. The receiver operating characteristic curve (ROC) was calculated to determine an optimized cutoff value.
Results: The optimal cutoff value was 1.135. When all patients were included, model 1 did not find an association between the glucose-to-HbA1c ratio and functional outcomes. In model 2, the glucose-to-HbA1c ratio×10 (Glucose-to-HbA1c ratio ×10) was the independent predictor of functional outcomes (OR 1.19, 95% CI 1.07–1.33, p < 0.01). Separately, in patients without diabetes, the glucose-to-HbA1c ratio×10 was the independent predictor of functional outcomes in both model 1 (OR 1.37, 95% CI 1.08–1.73, p = 0.01) and model 2 (OR 1.48, 95% CI 1.22–1.79, p < 0.01), but not in patients with diabetes. In addition, the glucose-to-HbA1c ratio×10 was the independent predictor of stroke severity (OR 1.16, 95% CI 1.05–1.28, p < 0.01).
Conclusion: The glucose-to-HbA1c ratio was associated with more severe AIS. Specifically, the glucose-to-HbA1c ratio was associated with the functional outcomes in patients without diabetes but not in patients with diabetes.
Introduction
Stress hyperglycemia is regarded as transient hyperglycemia secondary to inflammatory and neurohormonal disturbances in the context of acute illnesses (1–3). To our knowledge, the relationship between stress hyperglycemia and clinical outcomes has been studied in patients with AIS (4) and those with cardiovascular disease (5). In addition, studies have shown that stress hyperglycemia in patients with myocardial infarction is associated with an increased risk of in-hospital mortality, whether or not patients have diabetes (5). Stress hyperglycemia is frequently observed in patients with AIS (6), whether or not they have diabetes. Previous studies focused on patients without diabetes (7) or only considered fasting blood glucose (FBG) (8). Therefore, the relationship between stress hyperglycemia and clinical outcomes after AIS in patients with or without diabetes has not been well characterized.
Recently, an increasing number of studies have focused on the role of background blood glucose in assessing stress hyperglycemia. According to Roberts et al. (1), the stress hyperglycemia ratio (SHR), a novel index, can be used for accessing stress hyperglycemia. It was defined as admission blood glucose divided by the estimated mean blood glucose derived from glycated hemoglobin (HbA1c). Furthermore, considering HbA1c is a well-validated index that reflects the background blood glucose levels over the past 8–12 weeks (9) and that FBG is a more recognized index of the current blood glucose level, several studies have begun to use the FBG/HbA1c ratio to assess relative hyperglycemia, which is calculated by dividing FBG by HbA1c (7, 10, 11). Therefore, this calculation formula is convenient, practical, and reasonable.
Using this easy-to-perform method to identify and quantify stress hyperglycemia, our study explored the relationship between stress hyperglycemia and clinical outcomes in patients with AIS.
Methods
Study participants
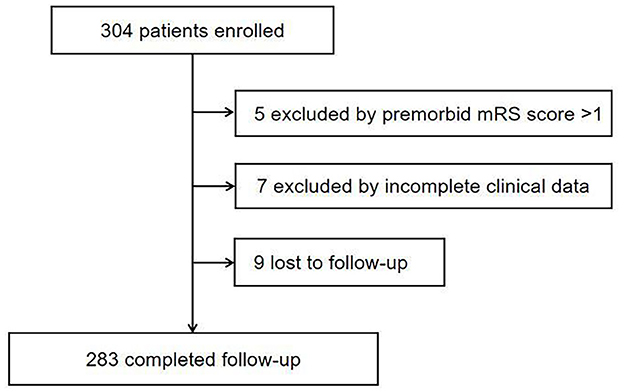
A total of 283 patients with a clinical diagnosis of AIS derived from our institution between June 2020 and June 2021 were finally enrolled. AIS was diagnosed according to the World Health Organization criteria (12) and confirmed by brain computerized tomography (CT) or magnetic resonance imaging (MRI). The severity of the stroke was assessed using the National Institutes of Health Stroke Scale (NIHSS) (13) by trained neurologists at admission. The severity of the stroke was classified as mild stroke (NIHSS score ≤4 at admission) and moderate-to-severe stroke (NIHSS score>4 at admission). Stroke types were classified as large-artery atherosclerosis, cardioembolic, small vessel disease, and others or undetermined.
Patients were eligible for the study if they were >18 years old, were admitted within 7 days after the occurrence of the stroke, underwent routine laboratory investigations after an overnight fast on the first day after admission, underwent MRI or CT, and had the diagnosis of AIS confirmed after admission. Patients were excluded from the study if they had incomplete clinical data or a premorbid mRS score of >1.
Data collection
The clinical data and baseline demographics of patients were consecutively collected through an electronic medical record system. All enrolled patients received routine therapy and nursing care according to their conditions.
Assessment of stress hyperglycemia
Stress hyperglycemia was evaluated by the glucose-to-HbA1c ratio. We used the following formula to calculate the glucose-to-HbA1c ratio: FPG (mmol/L)/HbA1c (%). This index reflected the extent of increase in acute blood glucose level based on the background blood glucose level.
Follow-up and outcomes
All patients completed a 12-month follow-up. During the follow-up period, outcomes were recorded using our hospital's electronic medical record system or through a telephone interview. Functional outcomes were measured using the mRS score at 1 year. A score of 3–6 was defined as a poor functional outcome. Patients' stroke recurrence and all-cause death were also recorded as clinical outcomes.
Statistical analysis
Independent sample t-tests or the Mann-Whitney U-test were used for continuous variables, and the chi-squared test or Fisher's exact test was used for binary variables to perform univariable analyses as appropriate. The receiver operating characteristic curve (ROC) was used to determine an optimized cutoff value for the glucose-to-HbA1c ratio in predicting poor functional outcomes. According to the optimized cutoff, the characteristics of patients with high and low glucose-to-HbA1c ratios were compared.
Univariable analysis variables with significant effects were included in the multivariable regression analysis for further analysis to identify independent predictors of poor functional outcome, stroke recurrence, and stroke severity. To explore whether stress hyperglycemia was related to stroke severity, the two models that retained or excluded the NIHSS score in the multivariable analysis were adopted. The patients were also divided into three groups: those with diabetes, those without diabetes, and all patients. All results were reported using 95% CIs. A p-value of < 0.05 (two-sided) was considered statistically significant. All data were analyzed using the statistical package SPSS (version 23.0; SPSS, Chicago, IL, USA). The study protocol was compliant with the Declaration of Helsinki and was approved by the ethical committee of our institution; individual informed consent was not required. The study was registered in the Chinese Clinical Trial Register (ChiCTR-ROC-17012225).
Results
Participant characteristics
A total of 283 patients with AIS were finally included in our study, with a median age of 65 years, and 196 (69.2%) of them were men. The baseline demographic and disease characteristics of participants are shown in Table 1. The flowchart of the study is displayed in Figure 1.
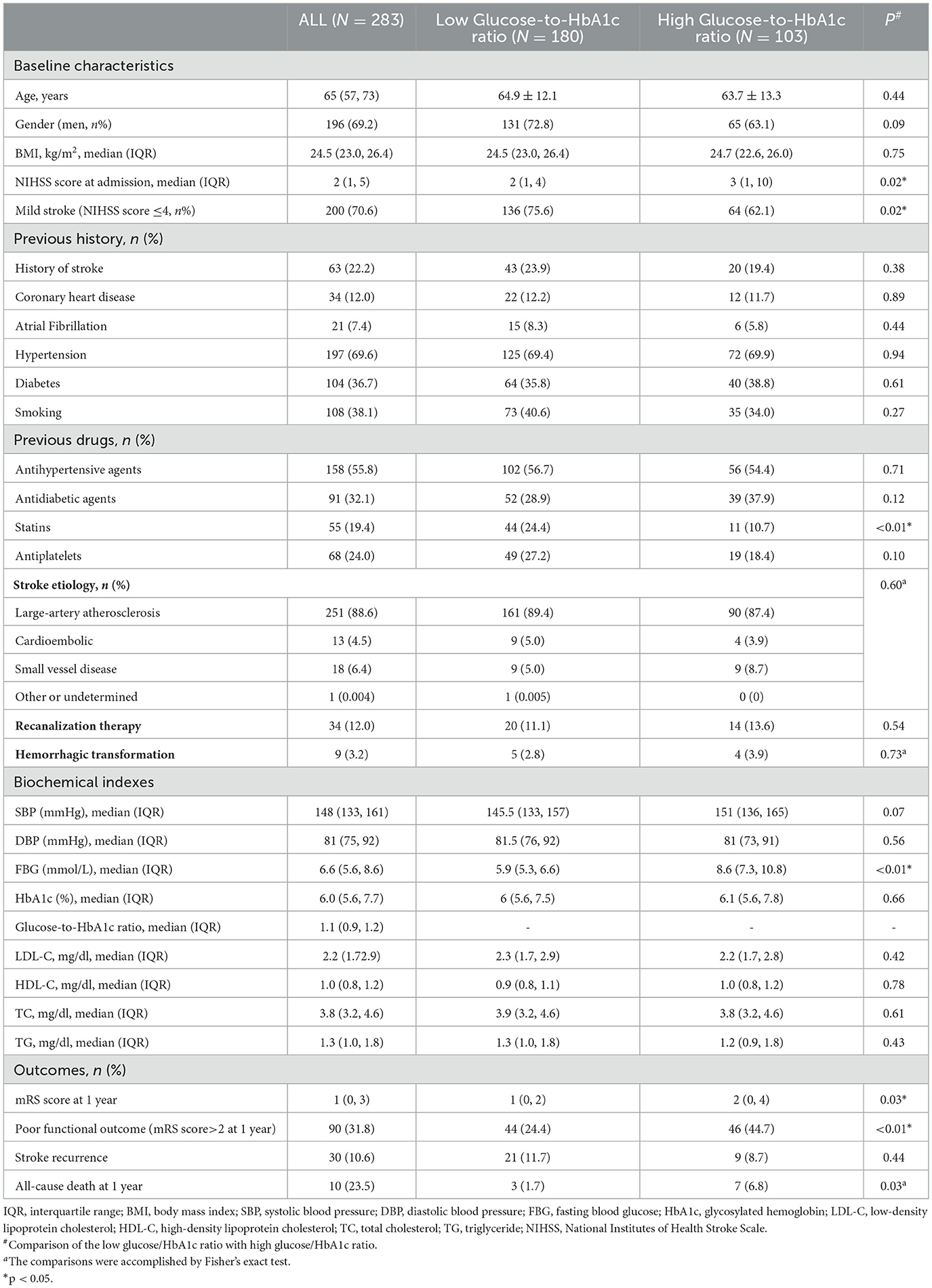
Table 1. Comparison of low-stress hyperglycemia ratio (glucose-to-HbA1c ratio <1.135) vs. high-stress hyperglycemia ratio (glucose-to-HbA1c ≥ 1.135) in patients with acute ischemic stroke.
Characteristics of the patients according to the glucose-to-HbA1c ratio
The ROC curve analysis was employed to determine the predicted value of the glucose-to-HbA1c ratio. The optimal cutoff derived from the glucose-to-HbA1c ratio was 1.135, which helped to predict poor functional outcomes in patients with AIS (area under the curve 0.601, 95% CI 0.53–0.67, p < 0.01), with 50% sensitivity and 70.2% specificity (Figure 2).
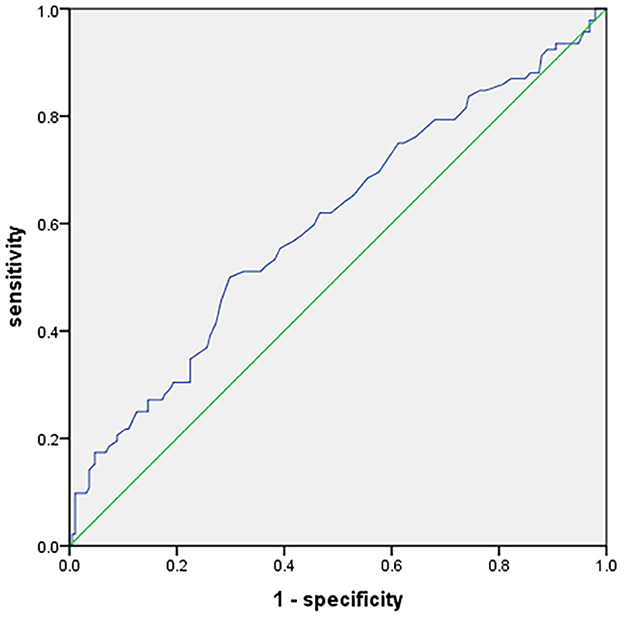
Figure 2. Receiver operating characteristic curve showing optimized cutoff for the glucose-to-HbA1c ratio in predicting poor functional outcomes (mRS score 3–6).
Patients with a higher glucose-to-HbA1c ratio tended to have higher NIHSS scores at admission [3 (1–10) vs. 2 (1–4), p = 0.02]. Furthermore, patients with a higher glucose-to-HbA1c ratio were related to an increased risk of poor functional outcomes and mortality at the end of 12 months of follow-up [44.7 vs. 24.4%, p < 0.01, 6.8 vs. 1.7%, p = 0.03, respectively). However, there was no significant difference in stroke recurrence between the high and low glucose-to-HbA1c ratio groups [8.7 vs. 11.7%, p = 0.44] (Table 1).
We did not observe any significant difference regarding stroke etiology between the two groups (p = 0.55). In terms of previous drugs, patients in the low glucose-to-HbA1c ratio group had a history of a higher statin usage rate [24.4 vs. 10.7%, p < 0.01] (Table 1).
The associations between glucose-to-HbA1c ratio and poor functional outcomes in patients with or without diabetes
A total of 90 patients had poor functional outcomes at 12 months, including 34 in the diabetes group and 56 in the patients without diabetes (Supplementary Table 1). When all patients were included, the multivariable logistic regression analysis (model 1) found that independent predictors of poor functional outcomes were age (OR 1.05, 95% CI 1.01–1.08, p = 0.02), NIHSS score at admission (OR 1.30, 95% CI 1.19–1.42, p < 0.01), atrial fibrillation (OR 4.10, 95% CI 1.18–14.28, p = 0.03), SBP (OR 1.03, 95% CI 1.01–1.05, p < 0.01), and DBP (OR 0.96, 95% CI 0.93–0.99, p = 0.02). When the NIHSS score at admission was removed from the multivariable model (model 2), independent predictors of poor functional outcomes were atrial fibrillation (OR 4.49, 95% CI 1.43–14.14, p = 0.01), SBP (OR 1.03, 95% CI 1.01–1.05, p < 0.01), DBP (OR 0.95, 95% CI 0.92–0.98, p < 0.01), and glucose-to-HbA1c ratio×10 (OR 1.19, 95% CI 1.07–1.33, p < 0.01) (Table 2).
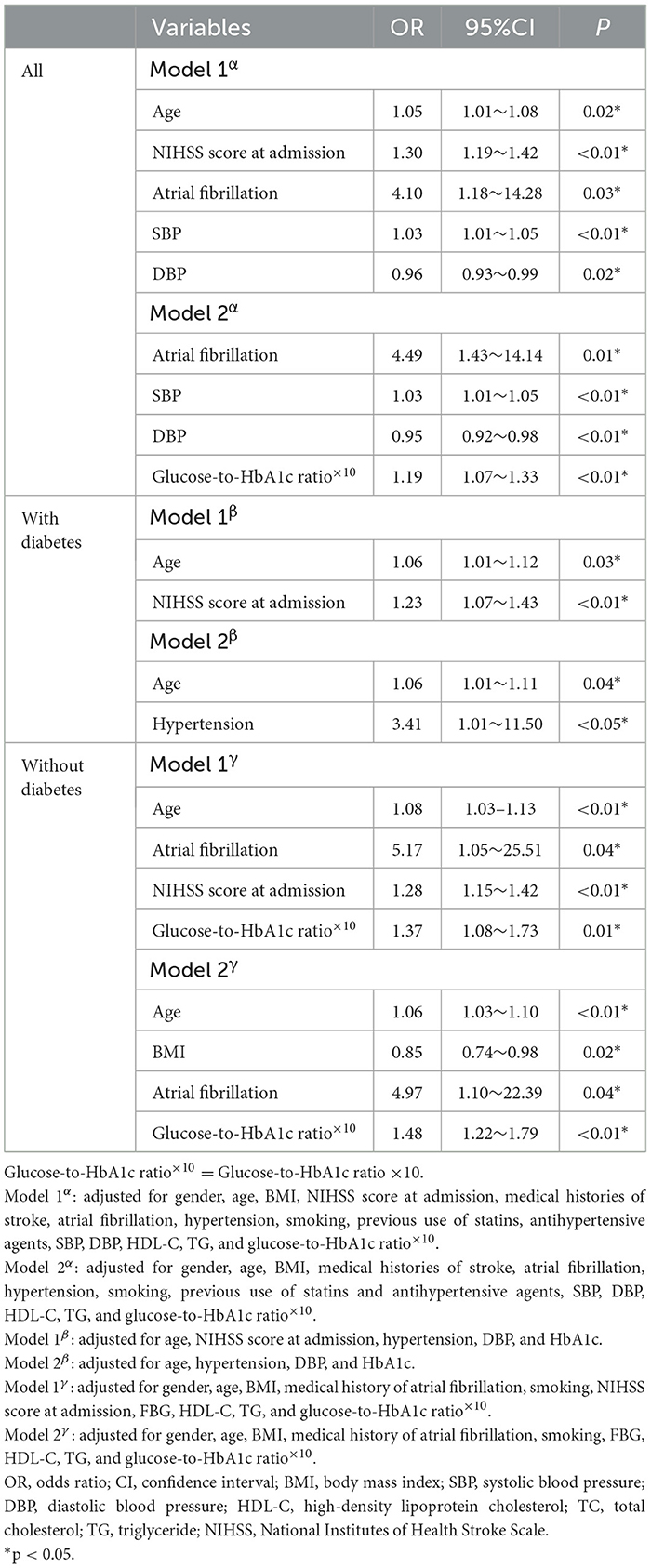
Table 2. Independent predictors of poor functional outcomes after 1 year in binary logistic regression analysis.
In patients with diabetes, we did not observe a relationship between the glucose-to-HbA1c ratio and poor functional outcomes. However, in patients without diabetes, the glucose-to-HbA1c ratio×10 was the independent predictor in both model 1 (OR 1.37, 95% CI 1.08–1.73, p = 0.01) and model 2 (OR 1.48, 95% CI 1.22–1.79, p < 0.01) (Table 2).
The associations between glucose-to-HbA1c ratio and stroke recurrence
In total, 30 patients underwent stroke recurrence during the 12 months of follow-up. There was no difference in the glucose-to-HbA1c ratio between the stroke recurrence and nonrecurrence groups (Supplementary Table 2). In multivariable logistic regression analysis, the glucose-to-HbA1c ratio had no relationship with stroke recurrence (Supplementary Tables 2, 3).
The associations between glucose-to-HbA1c ratio and stroke severity at admission
The patients were divided into two groups according to the NIHSS score at admission as follows: mild stroke was defined as an NIHSS score ≤4 and moderate-to-severe stroke was defined as an NIHSS score >4. A total of 83 patients had a moderate-to-severe stroke at admission (Supplementary Table 4). The glucose-to-HbA1c ratio×10 was related to an increased risk of more severe stroke (OR 1.16, 95% CI 1.05–1.28, p < 0.01). In addition, cardioembolic was also associated with a more severe stroke (OR 4.02, 95% CI 1.23–13.21, p = 0.02) (Table 3).
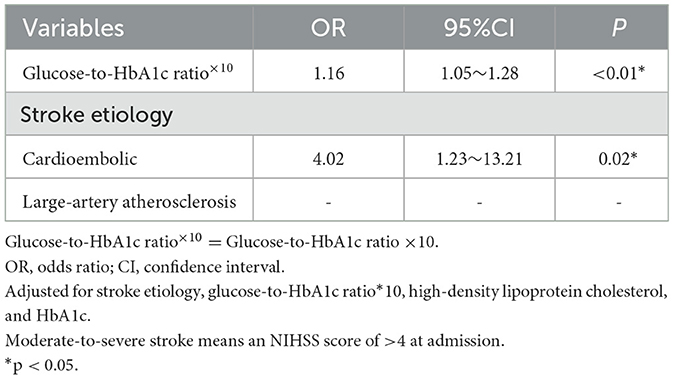
Table 3. Independent predictors of a moderate-to-severe stroke at admission in binary logistic regression analysis.
Discussion
In this study, we explored the relationship between the glucose-to-HbA1c ratio and the clinical outcomes in patients with AIS. The major findings of the present study were as follows: Stress hyperglycemia, via the glucose-to-HbA1c ratio, was related to poor functional outcomes in patients without diabetes but not in patients with diabetes. In addition, regardless of whether patients had diabetes or not, the glucose-to-HbA1c ratio was significantly associated with poor functional outcomes only in model 2, that is, a multivariable analysis excluding NIHSS score at admission. Through analyzing the severity of stroke at admission, we found that the glucose-to-HbA1c ratio was an independent predictor for moderate-to-severe stroke at admission. The association between stress hyperglycemia and poor outcomes tended to be attributed to higher stroke severity in patients with stress hyperglycemia.
Although a range of evidence suggests that stress hyperglycemia is a marker of poor outcomes in patients with AIS (14–21), this relationship is controversial when stroke severity is considered in further analysis (8). However, a recent meta-analysis found that stress hyperglycemia could reflect the extent of ischemic damage and lead to poor clinical outcomes in patients with stroke (22). This finding was consistent with part of our results that stress hyperglycemia is more common in patients with severe stroke, similar to that observed in a previous study (23). However, the underlying mechanism of the relationship between stress hyperglycemia and poor outcomes in patients with AIS is still unclear. In particular, it is necessary to differentiate between patients with and without diabetes.
Our study showed that stress hyperglycemia was a predictor of poor outcomes in patients without diabetes but not in patients with diabetes. Merlino et al. (24) reported that premorbid diabetic status tended to influence outcomes in patients with AIS who were treated with intravenous thrombolysis. Mortality and hemorrhagic complications were significantly increased in patients with more severe stress hyperglycemia only when they were not affected by diabetes. Similar results were also found in several other previous studies (4, 21, 25–27). The underlying mechanism of this phenomenon may be explained by cellular adaptation to hyperglycemia due to physiological readjustments to higher glucose concentrations in patients with diabetes (28, 29). In addition, diabetes may improve antioxidant defenses, which can protect cells from oxidative stress caused by acute hyperglycemia and thus attenuate inflammation caused by oxidative stress (30, 31).
Treatment and risk factor management in patients with AIS greatly influence overall prognosis. Finding indicators that can help us choose different treatments for different patients is therefore crucial, especially in glucose control and intensive glucose-lowering therapy. However, while random blood glucose and FBG are more available and intuitive, both have limitations in ignoring background blood glucose levels and physiological stress responses to AIS. In contrast, using the glucose-to-HbA1c ratio overcomes these shortcomings.
In our study, patients with a higher glucose-to-HbA1c ratio had higher NIHSS scores at admission, which might indicate that stress hyperglycemia was associated with stroke severity, and the glucose-to-HbA1c ratio was an independent predictor of stroke severity. Several previous studies have found that the glucose-to-HbA1c ratio influences clinical outcomes in both patients with and without diabetes (8, 20, 32–34). In these studies, a higher glucose-to-HbA1c ratio was related to poorer outcomes. These results might be explained by poorer outcomes that more severe patients always experience. However, in our study, when the patients were divided into groups with and without diabetes for separate analysis, it was found that the glucose-to-HbA1c ratio did not show a relationship with poor functional outcomes in patients with diabetes, while it was significantly associated with the poor functional outcomes in patients without diabetes.
Regardless, we found that the optimal glucose-to-HbA1c ratio was highly correlated with 1-year functional outcomes and all-cause mortality with the cutoff value of 1.135 but had no relationship with stroke recurrence. Patients with a higher glucose-to-HbA1c ratio had poorer functional outcomes. These results suggested that stress hyperglycemia might influence patients' clinical outcomes. Although the POINT trial found that hyperglycemia was associated with an increased risk of subsequent ischemic stroke (14), there are two possible explanations for the difference. First, the enrolled patients were those who presented with a high-risk TIA or acute minor ischemic stroke, while our study did not limit the stroke characteristics. Second, they defined hyperglycemia as random serum glucose on presentation ≥180 mg/dl, which differs from our study, which used the glucose-to-HbA1c ratio to define hyperglycemia. To our knowledge, diabetes is an independent risk factor for stroke recurrence (35). However, research on the impact of stress hyperglycemia on stroke recurrence is insufficient, and further studies are needed.
Our study had several limitations. First, this was a single-center, retrospective study with a limited sample size, which might reduce the generalizability of the results. Second, since the time of stroke recurrence was not recorded, survival analysis could not be performed, which was a shortcoming of our study. In addition, early control of hyperglycemia might impact the outcomes, as hyperglycemia can exacerbate brain damage in ischemic stroke, which we had not considered. In addition, the time from onset to laboratory investigations might influence our results, which had been ignored. Finally, the absence of 3-month follow-up data was also a shortcoming of this article. Therefore, the impact of stress hyperglycemia on patients with AIS remains a concern, and a multicenter prospective trial with a large sample size is needed in the future. In addition, patients with or without diabetes will also need to be studied separately.
In conclusion, our study showed that the glucose-to-HbA1c ratio was associated with more severe AIS and might be a marker of the severity of the stroke. More specifically, a high glucose-to-HbA1c ratio was associated with poor functional outcomes in patients without diabetes but not in patients with diabetes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Affiliated Drum Tower Hospital, Nanjing University Medical School. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JZ and YX designed the experiments and revised the manuscript. TS enrolled patients and drafted the manuscript. HL did the analysis work and drafted the manuscript with TS. GY, HW, DL, and HN contributed to patient enrollment and data collection. GY helped revise the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Hengrui Hospital Pharmacy Foundation of the Jiangsu Pharmaceutical Association (No. H202006) and the Jiangsu Research Hospital Association for Precision Medication (JY202122).
Acknowledgments
We thank all the doctors and nurses in the Neurology Department for their assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1142084/full#supplementary-material
References
1. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. (2015) 100:4490–7. doi: 10.1210/jc.2015-2660
2. Dungan KM, Braithwaite SS, Preiser J-C. Stress hyperglycaemia. Lancet. (2009) 373:1798–807. doi: 10.1016/S0140-6736(09)60553-5
3. Chen G, Ren J, Huang H, Shen J, Yang C, Hu J, et al. Admission random blood glucose, fasting blood glucose, stress hyperglycemia ratio, and functional outcomes in patients with acute ischemic stroke treated with intravenous thrombolysis. Front Aging Neurosci. (2022) 14:782282. doi: 10.3389/fnagi.2022.782282
4. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. (2001) 32:2426–32. doi: 10.1161/hs1001.096194
5. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. (2000) 355:773–8. doi: 10.1016/S0140-6736(99)08415-9
6. Johnston KC, Bruno A, Pauls Q, Hall CE, Barrett KM, Barsan W, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the shine randomized clinical trial. JAMA. (2019) 322:326–35. doi: 10.1001/jama.2019.9346
7. Zhu B, Pan Y, Jing J, Meng X, Zhao X, Liu L, et al. Stress hyperglycemia and outcome of non-diabetic patients after acute ischemic stroke. Front Neurol. (2019) 10:1003. doi: 10.3389/fneur.2019.01003
8. Tziomalos K, Dimitriou P, Bouziana SD, Spanou M, Kostaki S, Angelopoulou SM, et al. Stress hyperglycemia and acute ischemic stroke in-hospital outcome. Metabolism. (2017) 67:99–105. doi: 10.1016/j.metabol.2016.11.011
9. Emerging Risk Factors Collaboration, Di Angelantonio E, Gao P, Khan H, Butterworth AS, Wormser D, et al. Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA. (2014) 311:1225–33. doi: 10.1001/jama.2014.1873
10. Li J, Quan K, Wang Y, Zhao X, Li Z, Pan Y, et al. Effect of stress hyperglycemia on neurological deficit and mortality in the acute ischemic stroke people with and without diabetes. Front Neurol. (2020) 11:576895. doi: 10.3389/fneur.2020.576895
11. Yuan C, Chen S, Ruan Y, Liu Y, Cheng H, Zeng Y, et al. The stress hyperglycemia ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. Clin Interv Aging. (2021) 16:431–42. doi: 10.2147/CIA.S280808
12. Stroke−1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the who task force on stroke and other cerebrovascular disorders. Stroke. (1989) 20:1407–31. doi: 10.1161/01.STR.20.10.1407
13. Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the nih stroke scale using video training. Stroke. (1994) 25:2220–6. doi: 10.1161/01.STR.25.11.2220
14. Mac Grory B, Piccini JP, Yaghi S, Poli S, De Havenon A, Rostanski SK, et al. Hyperglycemia, risk of subsequent stroke, and efficacy of dual antiplatelet therapy: a post hoc analysis of the point trial. J Am Heart Assoc. (2022) 11:e023223. doi: 10.1161/JAHA.121.023223
15. Merlino G, Pez S, Gigli GL, Sponza M, Lorenzut S, Surcinelli A, et al. Stress hyperglycemia in patients with acute ischemic stroke due to large vessel occlusion undergoing mechanical thrombectomy. Front Neurol. (2021) 12:725002. doi: 10.3389/fneur.2021.725002
16. Merlino G, Smeralda C, Gigli GL, Lorenzut S, Pez S, Surcinelli A, et al. Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic stroke undergoing intravenous thrombolysis. J Thromb Thrombolysis. (2021) 51:789–97. doi: 10.1007/s11239-020-02252-y
17. Tao J, Hu Z, Lou F, Wu J, Wu Z, Yang S, et al. Higher stress hyperglycemia ratio is associated with a higher risk of stroke-associated pneumonia. Front Nutr. (2022) 9:784114. doi: 10.3389/fnut.2022.784114
18. Mi D, Li Z, Gu H, Jiang Y, Zhao X, Wang Y, et al. Stress hyperglycemia is associated with in-hospital mortality in patients with diabetes and acute ischemic stroke. CNS Neurosci Ther. (2022) 28:372–81. doi: 10.1111/cns.13764
19. Roberts G, Sires J, Chen A, Thynne T, Sullivan C, Quinn S, et al. A comparison of the stress hyperglycemia ratio, glycemic gap, and glucose to assess the impact of stress-induced hyperglycemia on ischemic stroke outcome. J Diabetes. (2021) 13:1034–42. doi: 10.1111/1753-0407.13223
20. Roquer J, Giralt-Steinhauer E, Cerda G, Rodriguez-Campello A, Cuadrado-Godia E, Jimenez-Conde J, et al. Glycated hemoglobin value combined with initial glucose levels for evaluating mortality risk in patients with ischemic stroke. Cerebrovasc Dis. (2015) 40:244–50. doi: 10.1159/000440735
21. Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. (2008) 36:2249–55. doi: 10.1097/CCM.0b013e318181039a
22. Huang YW, Yin XS, Li ZP. Association of the stress hyperglycemia ratio and clinical outcomes in patients with stroke: a systematic review and meta-analysis. Front Neurol. (2022) 13:999536. doi: 10.3389/fneur.2022.999536
23. Murros K, Fogelholm R, Kettunen, S, Vuorela, A-L, Valve J. Blood glucose, glycosylated haemoglobin, and outcome of ischemic brain infarction. J Neurol Sci. (1992) 111:59–64. doi: 10.1016/0022-510X(92)90112-X
24. Merlino G, Pez S, Tereshko Y, Gigli GL, Lorenzut S, Surcinelli A, et al. Stress hyperglycemia does not affect clinical outcome of diabetic patients receiving intravenous thrombolysis for acute ischemic stroke. Front Neurol. (2022) 13:903987. doi: 10.3389/fneur.2022.903987
25. Chen X, Liu Z, Miao J, Zheng W, Yang Q, Ye X, et al. High stress hyperglycemia ratio predicts poor outcome after mechanical thrombectomy for ischemic stroke. J Stroke Cerebrovasc Dis. (2019) 28:1668–73. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.022
26. Stollberger C, Exner I, Finsterer J, Slany J, Steger C. Stroke in diabetic and non-diabetic patients: course and prognostic value of admission serum glucose. Ann Med. (2005) 37:357–64. doi: 10.1080/07853890510037356
27. Hu GC, Hsieh SF, Chen YM, Hsu HH, Hu YN, Chien KL. Relationship of initial glucose level and all-cause death in patients with ischaemic stroke: the roles of diabetes mellitus and glycated hemoglobin level. Eur J Neurol. (2012) 19:884–91. doi: 10.1111/j.1468-1331.2011.03647.x
28. Bosarge PL, Shoultz TH, Griffin RL, Kerby JD. Stress-induced hyperglycemia is associated with higher mortality in severe traumatic brain injury. J Trauma Acute Care Surg. (2015) 79:289–94. doi: 10.1097/TA.0000000000000716
29. Krinsley JS, Meyfroidt G, van den Berghe G, Egi M, Bellomo R. The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care. (2012) 15:151–60. doi: 10.1097/MCO.0b013e32834f0009
30. Sechi LA, Ceriello A, Griffin CA, Catena C, Amstad P, Schambelan M, et al. Renal antioxidant enzyme mrna levels are increased in rats with experimental diabetes mellitus. Diabetologia. (1997) 40:23–9. doi: 10.1007/s001250050638
31. Ceriello A. dRP, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture: evidence linking hyperglycemia and oxidative stress. Diabetes. (1996) 45:471–7. doi: 10.2337/diab.45.4.471
32. Stead LG, Gilmore RM, Bellolio MF, Mishra S, Bhagra A, Vaidyanathan L, et al. Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care. (2009) 10:181–6. doi: 10.1007/s12028-008-9080-0
33. Wong AA, Schluter PJ, Henderson RD, O'Sullivan JD, Read SJ. Natural history of blood glucose within the first 48 hours after ischemic stroke. Neurology. (2008) 70:1036–41. doi: 10.1212/01.wnl.0000306635.08410.68
34. Szczudlik A, Slowik A, Turaj W, Wyrwicz-Petkow U, Pera J, Dziedzic T, et al. Transient hyperglycemia in ischemic stroke patients. J Neurol Sci. (2001) 189:105–11. doi: 10.1016/S0022-510X(01)00566-4
Keywords: stress hyperglycemia, acute ischemic stroke, clinical outcomes, fasting blood glucose, glycated hemoglobin
Citation: Shao T, Liu H, Yang G, Wang H, Li D, Ni H, Xu Y and Zhang J (2023) Fasting blood glucose-to-glycated hemoglobin ratio for evaluating clinical outcomes in patients with ischemic stroke. Front. Neurol. 14:1142084. doi: 10.3389/fneur.2023.1142084
Received: 11 January 2023; Accepted: 27 February 2023;
Published: 20 March 2023.
Edited by:
Cheong-Meng Chong, University of Macau, ChinaReviewed by:
Giovanni Merlino, Udine University Hospital, ItalyKlára Fekete, University of Debrecen, Hungary
Copyright © 2023 Shao, Liu, Yang, Wang, Li, Ni, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinping Zhang, empwMTY1MDBAMTYzLmNvbQ==; Yun Xu, eHV5dW4yMDA0MjAwMUBhbGl5dW4uY29t
†These authors have contributed equally to this work and share first authorship
 Tengfei Shao1†
Tengfei Shao1† Hui Liu
Hui Liu Guochao Yang
Guochao Yang Huanyu Ni
Huanyu Ni Yun Xu
Yun Xu