
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 13 April 2023
Sec. Epilepsy
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1134827
This article is part of the Research TopicAdvances in diagnosing and treating new-onset refractory status epilepticus (NORSE)View all 14 articles
Background and purpose: Ketogenic diet (KD) is an emerging treatment option for super-refractory status epilepticus (SRSE). We evaluated the effectiveness of KD in patients presenting SRSE including NORSE (and its subcategory FIRES).
Methods: A retrospective review of the medical records was performed at the Necker Enfants Malades Hospital. All children with SRSE in whom KD was started during the last 10 years were included. A systematic search was carried out for all study designs, including at least one patient of any age with SRSE in whom KD was started. The primary outcome was the responder rate and Kaplan–Meier survival curves were generated for the time-to-KD response. As secondary outcomes, Cox proportional hazard models were created to assess the impact of NORSE-related factors on KD efficacy.
Results: Sixteen children received KD for treatment of SRSE, and three had NORSE presentation (one infectious etiology, two FIRES). In medical literature, 1,613 records were initially identified, and 75 were selected for review. We selected 276 patients receiving KD during SRSE. The most common etiology of SRSE was acute symptomatic (21.3%), among these patients, 67.7% presented with NORSE of immune and infectious etiologies. Other etiologies were remote symptomatic (6.8%), progressive symptomatic (6.1%), and SE in defined electroclinical syndromes (14.8%), including two patients with genetic etiology and NORSE presentation. The etiology was unknown in 50.7% of the patients presenting with cryptogenic NORSE, of which 102 presented with FIRES. Overall, most patients with NORSE benefit from KD (p < 0.004), but they needed a longer time to achieve RSE resolution after starting KD compared with other non-NORSE SRSE (p = 0.001). The response to KD in the NORSE group with identified etiology compared to the cryptogenic NORSE was significantly higher (p = 0.01), and the time to achieve SE resolution after starting KD was shorter (p = 0.04).
Conclusions: The search for underlying etiology should help to a better-targeted therapy. KD can have good efficacy in NORSE; however, the time to achieve SE resolution seems to be longer in cryptogenic cases. These findings highlight the therapeutic role of KD in NORSE, even though this favorable response needs to be better confirmed in prospective controlled studies.
Status epilepticus (SE) is a potentially life-threatening condition resulting either from the failure of the natural homeostatic suppressing mechanisms responsible for seizure termination or from the initiation of mechanisms leading to abnormally prolonged seizure activity (1). About 31%−43% of the patients with SE are not controlled with first- and second-line treatments and enter in refractory SE (RSE), requiring intravenous anesthetic drugs (2). About 15% of the patients will progress further to super-refractory SE (SRSE), defined as SE that persists for more than 24 h after the initiation of anesthesia or recurs on the reduction or withdrawal of anesthetic drugs (3).
New-Onset Refractory Status Epilepticus (NORSE) is the clinical presentation describing a patient without active epilepsy or other preexisting relevant neurological disorder occurring without age limitation. It is characterized by de novo onset of RSE without a clear acute or active structural, toxic, or metabolic cause (4). The diagnosis of FIRES, an identified syndrome within NORSE, requires a prior febrile infection starting between 2 weeks and 24 h before RSE onset (with or without fever at SE onset) (4, 5). NORSE is a rare disorder (4). In Germany, the annual reported incidence and prevalence of FIRES in pediatric age are estimated to be 1:1,000,000 and 1:100,000, respectively (6). Patients presenting with NORSE or FIRES usually have a very poor prognosis, with mortality rates of 12%−27% and severe neurological sequelae, including cognitive impairment, functional disability, and drug resistant epilepsy in most survivors (7–9).
NORSE etiologies include viral or autoimmune causes. Cases with no identified cause after extensive evaluation are considered as “cryptogenic NORSE” or “NORSE of unknown etiology” (5).
So far, there is currently no high evidence to guide NORSE and FIRES treatment since most therapeutic approaches come from expert opinions and few case reports.
The ketogenic diet (KD) is an established, effective non-pharmacological treatment for drug-resistant epilepsy (10), and in the last decade, an increasing number of studies reported on the efficacy and tolerability of KD in intensive care units (ICU) as an emerging treatment option for SRSE (7, 11–13).
We reported our experience at a pediatric single tertiary center on the use of KD in patients with SRSE, specifically assessing the response in those with NORSE presentation. Our results were combined with the evidence provided by a systematic review of the literature. Finally, we aimed to evaluate the effectiveness of KD in patients presenting with SRSE and NORSE, using time to treatment response as the outcome measure, and to assess the impact of NORSE related characteristics on KD efficacy.
A retrospective review of the medical records was performed at the Necker Enfants Malades Hospital from April 2010 to October 2020. All children with SRSE in whom KD was started as adjunctive therapy were included. For each participant, we recorded and analyzed the following variables: age at SRSE onset, gender, previous history of epilepsy, SRSE etiology, number of treatments prior to KD, time lapse from SRSE onset to KD initiation, fasting at KD initiation, KD ratio, time to achieve ketosis from KD initiation, KD efficacy to stop SRSE, time to SRSE resolution after KD initiation, length of KD, side effects, number of antiseizure medications (ASMs) at hospital discharge, time of follow-up, and outcomes. We identified patients with NORSE presentation, specifying those with FIRES or with NORSE with unknown etiology.
A systematic review was performed in the electronic databases MEDLINE (PubMed), EMBASE, and Cochrane Library, with the following search terms: “ketogenic” AND (“refractory status epilepticus” OR “super refractory status epilepticus” OR “intensive care unit” OR “new onset refractory status epilepticus” OR “NORSE” OR “febrile infection related epilepsy syndrome” OR “FIRES”).
The relevant studies have been selected with no date restriction, including children and adult patients. The reference lists of the included articles were also searched manually to find any additional eligible papers. The search was up to date as for the 2nd October 2022.
The results of this systematic review were reported according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (14).
All study designs with individual details, including at least one patient of any age with SRSE in whom KD was started, have been included. Duplicate records were excluded. Reviews, meta-analyses, editorials, commentaries, and expert opinions were excluded. Titles and abstracts were screened for study eligibility, and full-text articles were reviewed by SM and PDL. Any disagreement was resolved by discussion with a third review author (RN).
For each selected study, the following data were extracted on individual bases when available: age at SRSE onset, gender, previous history of epilepsy, etiology of SRSE, number of treatments (ASMs and anesthetic agents) prior to KD start, other treatments (i.e., steroids) prior to start KD, the time lag from SRSE onset to KD initiation, fasting at KD initiation, KD ratio, time to achieve ketosis, KD efficacy to stop SRSE, time to SRSE resolution after KD initiation, length of KD, side effects, number of treatments at hospital discharge, time of follow-up, and outcomes. Patients with NORSE presentation were selected, specifying those with FIRES or with NORSE with unknown etiology.
Demographic and SE characteristics were summarized by standard descriptive measures.
The primary outcome was the responder rate, defined as clinical and electroencephalographic (EEG) resolution. Kaplan–Meier survival curves were generated for the time-to-KD response. As secondary outcomes, Cox proportional hazard models were created to assess the impact of the following factors on KD efficacy: age at SRSE onset, gender, previous history of epilepsy, etiology, the clinical presentation with NORSE/FIRES, number of treatments prior to KD, the time lag from SRSE onset to KD initiation, fasting at KD initiation, KD ratio, time to achieve ketosis from KD initiation, and side effects. A p-value ≤ 0.05 was considered statistically significant. Data were analyzed using STATA/IC version 15 (StataCorp LLC, College Station, TX, USA).
Overall, 16 children (six female) receive KD for treatment of SRSE at the Necker Enfants Malades Hospital. The median age at SRSE onset was 2 years old (IQR: 1–3, range: 1 month−10 years). Before admission for SRSE, 9/16 (56.2%) had a history of epilepsy. SRSE was due to defined epileptic syndromes in six patients (37.5%), and 6/16 (37.5%) had a progressive symptomatic cause. One patient had acute symptomatic etiology of SRSE due to cerebral anoxia. The remaining presented with NORSE due to infectious encephalitis (n = 1) and FIRES (n = 2) of unknown etiology. Before KD initiation, they received a median number of ASMs and anesthetics of 4 (IQR: 3–5; range: 2–7), and other treatments, including steroids (n = 2), IVIg (n = 1), and vitamin therapy (n = 2). The median delay from SRSE onset to KD initiation was 2.5 days (IQR: 2–7; range: 1–20).
KD was effective in achieving SRSE cessation in 5/16 (31.25%), after a median time from starting KD of 4.5 days (IQR: 1.5–16; range: 1–30). Side effects due to KD treatment were detected in 7/16 (43.75%), 3/16 died during the acute phase of SRSE, while at hospital discharge, 12/16 (75%) patients had ongoing seizures and received a median number of ASMs of 1 (IQR: 1–3; range: 1–5).
Table 1 summarizes patients' characteristics and details on KD administration, while Table 2 summarizes the response to KD and outcomes.
One thousand six hundred thirteen records were initially identified. Two hundred and twelve were retrieved for detailed assessment, of which 75 were included in the review (Figure 1). The selected studies were retrospective observational studies (n = 21) (7, 11, 13, 15–32), single cases (n = 43) (33–75), and small case series (n = 10) (76–85); only one study is a prospective, open-label, single-arm observational study (86). There were no randomized or non-randomized clinical trials. All included studies were considered to have a high risk of bias related to the retrospective study design, patient selection and data collection, ascertainment bias, missing data, and reporting of the results.
Table 3 summarizes patients' characteristics and details on KD administration and Table 4 summarizes the response to KD and outcomes.
Overall, the included studies described 276 patients, both of pediatric and adult age, receiving KD during SRSE. One hundred twenty-three/245 (50.2%) were female (information detailed in 71 studies). The majority of reported patients were children (208/276; 75.3%). The median age at SE onset was 9.1 years old [interquartile range (IQR)]: 5.2–20 years; range: 1.2 months−73 years; information available in 73 studies].
The most common etiology of SRSE was acute symptomatic (59/276; 21.3%), among these patients, 67.7% (40/59) presented with NORSE of immune (25/40; 62.5%) and infectious (15/40; 37.5%) etiologies. Other etiologies were remote symptomatic (19/276; 6.8%), progressive symptomatic (17/276; 6.1%), and SE in defined electroclinical syndromes (41/276; 14.8%), including two patients with genetic etiology and NORSE presentation. The etiology was unknown in 50.7% of the patients (140/276) presenting with NORSE, of which 102 presented with FIRES.
Overall, the median time duration of SRSE before KD initiation was 9 days (IQR: 5.2–20; range: 1–73; information available in 56 studies), the median number of treatments (ASMs and anesthetics) prior to KD was 6 (IQR: 5–8; range: 2–14; information detailed in 69 studies), and 143/276 (51.8%) patients also received other treatments prior KD mostly including immunotherapy (133/143; 93%).
KD was considered effective in 197/276 (71.4%) patients after a median time from KD initiation of 6.5 days (IQR 4–9, range 1–28). Overall, the total length of KD was 60 days (IQR 21–180; range: 3–900) in responders and non-responders patients (information available in 47 studies).
In patients with NORSE presentation (182/276, 65.9%), the median time of duration of SRSE before KD initiation was 15 days [interquartile range (IQR): 9–28; range: 2–420], and the median number of other treatments prior to KD was 7 (IQR: 5–8; range: 2–16). KD was considered effective in 117/182 (64.3%) after a median time from KD initiation of 8 days (IQR 6–21, range 1–30).
Overall, adverse effects due to KD were reported in 124/276 (44.9%) patients.
Twenty-seven out of 276 (9.7%) patients died during the acute phase of SRSE, while 7/276 (2.5%) died after achieving SE cessation. At the latest follow-up with a median length after SE cessation of 10 months (IQR 3.3–18, range 9 days−156 months), 50/242 (20.6%) patients achieved seizure freedom, 46/242 (19%) suffering from ongoing seizures, while 44/242 (18.2%) had ongoing seizures associated with cognitive impairment, and 33/242 (13.6%) had cognitive impairment alone (information available in 63 studies). Overall, 88/90 (97.7%) with ongoing seizures received a median number of ASMs of 3 (IQR: 3–4, range: 1–10; information available in 36 studies).
For this analysis, we considered the literature cases in addition to our center cases. The data of 255/292 (82.9%) patients were available for Kaplan–Meier survival curves.
The probability to achieved SRSE cessation after KD initiation is 50.53% at 7 days [95% confidence interval (CI): 44.15–56.57], 33.16% at 14 days (95% CI: 27.21–39.22), and further decreases to 26.34% at 21 days (95% CI: 20.77–32.22), and 25.24% at 28 days (95% CI: 19.73–31.10).
The KD responder rates are different in children compared to adults (HR: 1.47, 95% CI: 1.04–2.06; p < 0.02). The median time to achieve SRSE cessation after starting KD is 8 days in children (IQR: 6–16; range: 1–30) and 5.5 days in adults (IQR 3–10; range 1–30).
A previous history of epilepsy implies a greater likelihood of KD efficacy in achieving SRSE cessation (HR: 1.54, 95% CI: 1.11–2.12; p = 0.009). The detection of known etiology implies a favorable response to KD (HR: 1.70, 95% CI: 1.26–2.30; p < 0.0001); in this regard, patients with an acute symptomatic cause of SRSE have a greater likelihood of KD efficacy (HR: 1.58, 95% CI: 1.13–2.23; p = 0.008).
Otherwise, even though most patients with NORSE benefit from KD (117/185, 63.2% achieving SRSE cessation, p < 0.004), they needed, however, a longer time to achieve SE resolution after starting KD compared with other non-NORSE SRSE (HR: 0.60, 95% CI: 0.44–0.81; p = 0.001; Figure 2). At the Kaplan–Meier survival analysis, the probability of achieving NORSE cessation after KD initiation was 56.13% at 7 days (95% CI: 48.02–63.48), 40.51% at 14 days (95% CI: 32.58–48.28), 32.75% at 21 days (95% CI: 25.20–40.50), and 31.12% at 28 days (95%CI: 23.66–38.85). The response to KD in the NORSE group with identified etiology compared to the cryptogenic NORSE was significantly higher (p = 0.01), and the time to achieve SE resolution after starting KD was shorter (HR: 1.56, 95% CI: 1.01–2.38; p = 0.04; Figure 3).

Figure 2. Kaplan–Meier survival estimates for probability to achieved RSE cessation after KD initiation in patients with NORSE/FIRES presentation vs. non-NORSE (other SRSE).
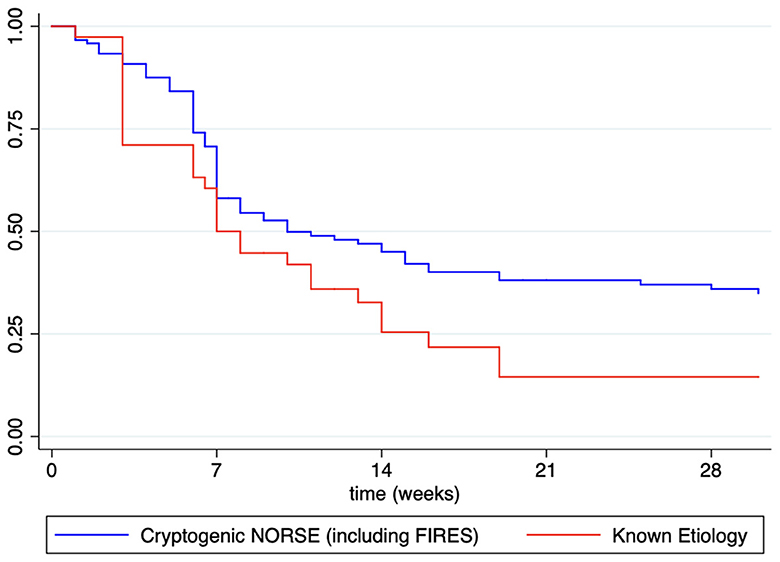
Figure 3. Kaplan–Meier survival estimates for probability to achieved NORSE cessation after KD initiation in patients with known etiology vs. cryptogenic NORSE (including FIRES).
Overall, the number of treatments before KD initiation has a negative impact on the responder rate (HR: 0.93, 95% CI: 0.88–0.98, p = 0.01), while the time from SRSE onset to KD initiation does not significantly impact KD efficacy (HR: 0.99, 95% CI: 0.99–1.00, p = 0.23).
Side effects of KD negatively impact the probability to achieve SRSE cessation after KD initiation (HR: 0.60, 95% CI: 0.44–0.81, p = 0.001). Other KD related factors such as fasting before diet initiation, KD ratio, time to reach ketosis, and total length of KD, do not impact the likelihood of KD efficacy in achieving SRSE cessation.
SRSE is a major neurological emergency, and the therapeutic interventions aim to reduce its duration, mortality, as well as short- and long-term comorbidities. NORSE (with its subcategory FIRES) is one of the most common causes of SRSE. Therapeutic alternatives are scarce, and the use of anesthetic agents as symptomatic treatment could worsen the outcome due to systemic complications that often co-occur. So far, there is currently no high-level evidence to guide NORSE management since most of the therapeutic approaches come from expert opinions and few cases. A few studies and case series on immunotherapy with monoclonal antibodies efficacy have been reported so far (40, 43, 47, 53, 59, 87), but their effectiveness has still to be assessed in large cohort studies.
NORSE outcomes are influenced mainly by non-modifiable variables such as age and underlying etiology, though complications from the NORSE status itself, treatments, and length of stay in ICU also contribute to morbidity and mortality.
KD is an emerging treatment option for RSE and SRSE (88), and most published evidence has shown high efficacy rates (12, 13, 89). The multiple mechanisms of action make KD a good therapeutic option in these conditions. The anti-seizure effect of KD may be due to multiple mechanisms involving neurotransmitters, mitochondria, gut microbiota, DNA methylation, ion channels, inflammation, and G-protein coupled receptors. It mimics ASMs polytherapy (88), and several of these mechanisms can occur rapidly, while others, such as the effects on mitochondria, gut microbiota, and DNA methylation, are likely long-term.
Many case reports and case series have demonstrated the potential efficacy and safety of KD for the acute treatment of SRSE; however, the quality of these studies remains scarce.
More literature reports the use of KD in children compared to adults, but studies on the adult population have shown higher efficacy rates (87.5 vs. 66.8%; p = 0.001) and a shorter time to achieve SE cessation after starting KD. This discrepancy is probably due to more refractory cases being over-represented in the childhood population.
In this systematic review, about half of patients experiencing SRSE, a cause has been identified, and almost a quarter have a previous history of epilepsy. However, half of the cases elude any easily detectable etiology, and previously healthy individuals develop prolonged NORSE without a readily identifiable explanation. Overall, patients with SRSE of known etiology appear to present a better response rate and a shorter time to achieve SRSE cessation after starting KD. SE occurring during the course of epilepsy syndromes, such as genetic and structural epilepsies, may benefit from KD in 75% of the cases. Furthermore, patients with SRSE of remote etiology were also reported as responders to KD in 62.5% (11, 26, 64, 74). Patients with SRSE due to progressive etiologies such as mitochondrial diseases (71) are good candidates for KD to be introduced early. Other etiologies involving immune-mediated pathways, such as Rasmussen encephalitis and autoimmune encephalitis with SRSE were reported to benefit of KD (18, 19, 28, 56, 57, 61, 66, 80, 85). In this regard, the presumed immune etiology in FIRES and NORSE cases, based on the activation of an inflammatory cascade, makes these conditions possible specific targets for KD (90). In this systematic review, NORSE, and its subcategory FIRES, are common causes of SRSE, but these difficult-to-treat conditions imply a longer time to achieve SE resolution after starting KD compared to other SRSE. This might be due to the addition of specific treatment tailored for etiologies and the high level of cases remaining without an etiology (cryptogenic NORSE) or where etiology was much delayed.
The etiology remains unexplained in about two-thirds of the cases of NORSE, representing the so-called “cryptogenic NORSE.” The most identified cause in adult patients is autoimmune encephalitis, while infections are the prevalent etiology in pediatric patients (91).
The analysis of literature data combined with our single center experience highlighted a more favorable response to KD and a shorter SE duration in the NORSE group with identified etiology compared with NORSE of unknown etiology. These findings highlight the alternative therapeutic role of KD in patients affected by NORSE and FIRES, even though this favorable response needs to be better evaluated and confirmed in prospective controlled studies assessing both seizure control and functional outcome. The detection of an underlying cause may also allow an early treatment at the pathogenic level, which may reduce the risk of irreversible sequelae in the long-term. The recent international consensus recommendations for the management of NORSE, including FIRES, provides diagnostic and therapeutic algorithms to aid clinicians in patient care (92, 93). The consensus recommends the initiation of the KD in the first week, or if not already given, KD should be considered in prolonged and severe cases, emphasizing the importance of starting KD very early in the course of NORSE. These management recommendations may allow a faster and more tailored diagnostic process and improve treatment to allow better outcomes.
The main limiting factor for the use of KD in NORSE might be the time lag for efficacy, ketosis is usually reached within 24–72 h, and seizure reduction within the first week in the majority of the patients. This time lag could be challenging to accept in a severe condition such as NORSE.
Another impeding factor for the initiation of KD highlighted by several panelists of the consensus (92) is the limited availability and the lack of experience in its administration, particularly in adult patients. However, the expertise on KD in adult neurology is still increasing, and the number of adult patients with epilepsies, mostly of genetic etiology, treated with KD is on the rise.
The KD is well-tolerated with low rates of side effects in the ICU setting, highlighting that the diet has a safe profile and should be implemented in these settings. The most frequently reported side effects are easily manageable gastrointestinal or biochemical abnormalities, and the few serious adverse events reported in the literature are not necessarily attributable to KD.
The feasibility of implementing the KD in ICUs may be challenging also due to intensive care procedures, the possible occurrence of severe adverse events, and the concurrent administration of glucose-containing medications. A multidisciplinary team, including experienced physicians and dietitians, and standardized protocols should be warranted in these settings to overcome these issues. Most survivors have long-term sequelae in terms of drug-resistant epilepsy and poor functional outcomes, mostly related to the length of stay in the ICU and underlying etiology.
Due to its emergent and rare nature and the heterogeneity of the causes, randomized controlled treatment trials in NORSE are scanty. Literature data on KD in SRSE and NORSE comes mainly from retrospective observational studies, small case series, and anecdotal case reports that mainly report the good efficacy of the diet and rarely detail its failure. These studies have inherent limitations and heterogeneity in etiology, protocols, and assessment criteria. Treating NORSE involves multiple medications and treatments given together, making it difficult to impute SRSE termination to a single therapeutic agent directly. In this regard, it is difficult to assess the primary therapeutic effect of KD or its synergistic action with other treatments. Furthermore, in some patients receiving concurrent medications targeting an underlying etiology, the resolution of SRSE cannot be directly attributed to the KD only. In this regard, the evidence of these reports shares the same weakness with all third-line treatments in RSE and SRSE, where no agent has achieved a high level of evidence-based medicine (3).
Although promising, the current results should be interpreted with caution due to the inherent bias, confounding factors, and small sample size of the included studies.
Evidence-based medicine is dramatically lacking to date, particularly in critical situations such as ICUs. In this regard, prospective, randomized controlled trials are needed to better assess KD as third-line therapy in managing RSE and preventing SRSE, mostly in patients with NORSE presentation. They should evaluate KD effectiveness in these specific settings, identify predictors of treatment response, and determine a ratio-responsive relationship of treatment. Outcomes should be assessed in the short-term, considering SE resolution, and in the long-term, evaluating subsequent seizure burden and neurological functioning.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
RN and SM contributed to the study concept, data acquisition and analysis, and drafting of the manuscript. PD contributed to the data acquisition and drafting of the manuscript. OD and MO contributed to the study concept, data acquisition, and drafting of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus–report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. (2015) 56:1515–23. doi: 10.1111/epi.13121
2. Trinka E, Kälviäinen R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure. (2017) 44:65–73. doi: 10.1016/j.seizure.2016.11.001
3. Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 135(Pt 8):2314–28. Erratum in: Brain. (2013) 136(Pt 7):2326. doi: 10.1093/brain/aws091
4. Hirsch LJ, Gaspard N, van Baalen A, Nabbout R, Demeret S, Loddenkemper T, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. (2018) 59:739–44. doi: 10.1111/epi.14016
5. Gaspard N, Hirsch LJ, Sculier C, Loddenkemper T, van Baalen A, Lancrenon J, et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia. (2018) 59:745–52. doi: 10.1111/epi.14022
6. van Baalen A, Häusler M, Boor R, Rohr A, Sperner J, Kurlemann G, et al. Febrile infection-related epilepsy syndrome (FIRES): a nonencephalitic encephalopathy in childhood. Epilepsia. (2010) 51:1323–8. doi: 10.1111/j.1528-1167.2010.02535.x
7. Kramer U, Chi CS, Lin KL, Specchio N, Sahin M, Olson H, et al. Febrile infection-related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia. (2011) 52:1956–65. doi: 10.1111/j.1528-1167.2011.03250.x
8. Khawaja AM, DeWolfe JL, Miller DW, Szaflarski JP. New-onset refractory status epilepticus (NORSE)–the potential role for immunotherapy. Epilepsy Behav. (2015) 47:17–23. doi: 10.1016/j.yebeh.2015.04.054
9. Gaspard N, Foreman BP, Alvarez V, Cabrera Kang C, Probasco JC, Jongeling AC, et al. New-onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology. (2015) 85:1604–13. doi: 10.1212/WNL.0000000000001940
10. Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. (2018) 3:175–92. doi: 10.1002/epi4.12225
11. Villeneuve N, Pinton F, Bahi-Buisson N, Dulac O, Chiron C, Nabbout R. The ketogenic diet improves recently worsened focal epilepsy. Dev Med Child Neurol. (2009) 51:276–81. doi: 10.1111/j.1469-8749.2008.03216.x
12. Mahmoud SH, Ho-Huang E, Buhler J. Systematic review of ketogenic diet use in adult patients with status epilepticus. Epilepsia Open. (2019) 5:10–21. doi: 10.1002/epi4.12370
13. Schoeler NE, Simpson Z, Zhou R, Pujar S, Eltze C, Cross JH. Dietary management of children with super-refractory status epilepticus: a systematic review and experience in a single UK Tertiary Centre. Front Neurol. (2021) 12:643105. doi: 10.3389/fneur.2021.643105
14. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
15. Chomtho S, Uaariyapanichkul J, Chomtho K. Outcomes of parenteral vs enteral ketogenic diet in pediatric super-refractory status epilepticus. Seizure. (2022) 96:79–85. doi: 10.1016/j.seizure.2022.01.019
16. Breu M, Häfele C, Glatter S, Trimmel-Schwahofer P, Golej J, Male C, et al. Ketogenic diet in the treatment of super-refractory status epilepticus at a pediatric intensive care unit: a single-center experience. Front Neurol. (2021) 12:669296. doi: 10.3389/fneur.2021.669296
17. Wang X, Gao X, Lu G, Lu Z, Zhou S, Wang Y, et al. The ketogenic diet for paediatric patients with super-refractory status epilepticus in febrile infection-related epilepsy syndrome. Acta Epileptologica. (2020) 2:4. doi: 10.1186/s42494-020-00013-1
18. Arayakarnkul P, Chomtho K. Treatment options in pediatric super-refractory status epilepticus. Brain Dev. (2019) 41:359–66. doi: 10.1016/j.braindev.2018.11.011
19. Francis BA, Fillenworth J, Gorelick P, Karanec K, Tanner A. The feasibility, safety and effectiveness of a ketogenic diet for refractory status epilepticus in adults in the intensive care unit. Neurocrit Care. (2019) 30:652–7. doi: 10.1007/s12028-018-0653-2
20. Park EG, Lee J, Lee J. The ketogenic diet for super-refractory status epilepticus patients in intensive care units. Brain Dev. (2019) 41:420–7. doi: 10.1016/j.braindev.2018.12.007
21. Peng P, Peng J, Yin F, Deng X, Chen C, He F, et al. Ketogenic diet as a treatment for super-refractory status epilepticus in febrile infection-related epilepsy syndrome. Front Neurol. (2019) 10:423. doi: 10.3389/fneur.2019.00423
22. Arya R, Peariso K, Gaínza-Lein M, Harvey J, Bergin A, Brenton JN, et al. Efficacy and safety of ketogenic diet for treatment of pediatric convulsive refractory status epilepticus. Epilepsy Res. (2018) 144:1–6. doi: 10.1016/j.eplepsyres.2018.04.012
23. Lee HF, Chi CS. Febrile infection-related epilepsy syndrome (FIRES): therapeutic complications, long-term neurological and neuroimaging follow-up. Seizure. (2018) 56:53–9. doi: 10.1016/j.seizure.2018.02.003
24. Farias-Moeller R, Bartolini L, Pasupuleti A, Brittany Cines RD, Kao A, Carpenter JL. A practical approach to ketogenic diet in the pediatric intensive care unit for super-refractory status epilepticus. Neurocrit Care. (2017) 26:267–72. doi: 10.1007/s12028-016-0312-4
25. Appavu B, Vanatta L, Condie J, Kerrigan JF, Jarrar R. Ketogenic diet treatment for pediatric super-refractory status epilepticus. Seizure. (2016) 41:62–5. doi: 10.1016/j.seizure.2016.07.006
26. Caraballo RH, Flesler S, Armeno M, Fortini S, Agustinho A, Mestre G, et al. Ketogenic diet in pediatric patients with refractory focal status epilepticus. Epilepsy Res. (2014) 108:1912–6. doi: 10.1016/j.eplepsyres.2014.09.033
27. O'Connor SE, Ream MA, Richardson C, Mikati MA, Trescher WH, Byler DL, et al. The ketogenic diet for the treatment of pediatric status epilepticus. Pediatr Neurol. (2014) 50:101–3. doi: 10.1016/j.pediatrneurol.2013.07.020
28. Thakur KT, Probasco JC, Hocker SE, Roehl K, Henry B, Kossoff EH, et al. Ketogenic diet for adults in super-refractory status epilepticus. Neurology. (2014) 82:665–70. doi: 10.1212/WNL.0000000000000151
29. Vaccarezza M, Silva W, Maxit C, Agosta G. Estado de mal epileptico superrefractario: tratamiento con dieta cetogenica en pediatria [Super-refractory status epilepticus: treatment with ketogenic diet in pediatrics]. Rev Neurol. (2012) 55:20–5. doi: 10.33588/rn.5501.2011700
30. Nam SH, Lee BL, Lee CG, Yu HJ, Joo EY, Lee J, et al. The role of ketogenic diet in the treatment of refractory status epilepticus. Epilepsia. (2011) 52:e181–4. doi: 10.1111/j.1528-1167.2011.03289.x
31. Nabbout R, Mazzuca M, Hubert P, Peudennier S, Allaire C, Flurin V. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES). Epilepsia. (2010) 51:2033–7. doi: 10.1111/j.1528-1167.2010.02703.x
32. François LL, Manel V, Rousselle C, David M. Le régime cétogène à visée anti-épileptique: son utilisation chez 29 enfants épileptiques [Ketogenic regime as anti-epileptic treatment: its use in 29 epileptic children]. Arch Pediatr. (2003) 10:300–6. doi: 10.1016/S0929-693X(03)00030-7
33. Aydemir S, Kandula P. High dose cannabidiol (CBD) in the treatment of new-onset refractory status epilepticus (NORSE). Seizure. (2022) 94:126–8. doi: 10.1016/j.seizure.2021.11.020
34. Dutta K, Satishchandra P, Borkotokey M. Medium-chain triglyceride ketogenic diet as a treatment strategy for adult super-refractory status epilepticus. Indian J Crit Care Med. (2022) 26:139–40. doi: 10.5005/jp-journals-10071-24073
35. Giménez-Roca S, Sellées-Galiana F, Canet-Sanz T, Aliaga-Dìaz A. Super-refractory status epilepticus in pregnancy: a clinical challenge. Neurol perspectives. (2022) 2:179–81. doi: 10.1016/j.neurop.2022.02.003
36. Luo T, Wang Y, Lu G, Zhou Y, Wang Y. Vagus nerve stimulation for super-refractory status epilepticus in febrile infection-related epilepsy syndrome: a pediatric case report and literature review. Childs Nerv Syst. (2022) 38:1401–4. doi: 10.1007/s00381-021-05410-6
37. Orlandi N, Giovannini G, Mirandola L, Monti G, Marudi A, Mosca F, et al. An ultra-long new onset refractory status epilepticus: winning the battle but losing the war? Epilepsy Behav Rep. (2022) 18:100537. doi: 10.1016/j.ebr.2022.100537
38. Perulli M, Cicala G, Turrini I, Musto E, Quintiliani M, Gambardella ML, et al. Fighting autoinflammation in FIRES: the role of interleukins and early immunomodulation. Epilepsy Behav Rep. (2022) 18:100531. doi: 10.1016/j.ebr.2022.100531
39. Sivathanu D, Kewalramani D, Kumar Manokaran R. Favorable response to classic ketogenic diet in a child with anti-GAD 65 antibody mediated super refractory status epilepticus. Epilepsy Behav Rep. (2022) 19:100557. doi: 10.1016/j.ebr.2022.100557
40. Varughese RT, Karkare S, Poduri A, Kothare SV. Child neurology: initial presentation of PCDH19-related epilepsy with new-onset refractory status epilepticus and treatment with anakinra. Neurology. (2022) 99:208–11. doi: 10.1212/WNL.0000000000200855
41. Allen CM, Hall CA, Cox NE, Ryan H, De Beer T, O'Donoghue MF. Adjunctive use of the ketogenic diet in a young adult with UBE2A deficiency syndrome and super-refractory status epilepticus. Epilepsy Behav Rep. (2021) 16:100456. doi: 10.1016/j.ebr.2021.100456
42. Baba S, Okanishi T, Ohsugi K, Suzumura R, Niimi K, Shimizu S, et al. Possible role of high-dose barbiturates and early administration of parenteral ketogenic diet for reducing development of chronic epilepsy in febrile infection-related epilepsy syndrome: a case report. Neuropediatrics. (2021) 52:133–7. doi: 10.1055/s-0040-1716903
43. Donnelly JP, Kasatwar N, Hafeez S, Seifi A, Gilbert A, Barthol C, et al. Resolution of cryptogenic new onset refractory status epilepticus with tocilizumab. Epilepsy Behav Rep. (2021) 15:100431. doi: 10.1016/j.ebr.2021.100431
44. Katz JB, Owusu K, Nussbaum I, Beekman R, DeFilippo NA, Gilmore EJ, et al. Pearls and pitfalls of introducing ketogenic diet in adult status epilepticus: a practical guide for the intensivist. J Clin Med. (2021) 10:881. doi: 10.3390/jcm10040881
45. Kaul N, Laing J, Nicolo JP, Nation J, Kwan P, O'Brien TJ. Practical considerations for ketogenic diet in adults with super-refractory status epilepticus. Neurol Clin Pract. (2021) 11:438–44. doi: 10.1212/CPJ.0000000000001009
46. Aurangzeb S, Prisco L, Adcock J, Speirs M, Raby S, Westbrook J, et al. New-onset super refractory status epilepticus: a case-series. Seizure. (2020) 75:174–84. doi: 10.1016/j.seizure.2019.10.005
47. Chee YC, Lim CH, Halim SA, Ong BH. Extinguishing FIRES using tocilizumab. Neurol Clin Neurosci. (2020) 8:192–5. doi: 10.1111/ncn3.12385
48. Chiu M, Datta A. Childhood small vessel primary angiitis of the central nervous system: a treatable cause of super-refractory status epilepticus. J Child Neurol. (2020) 35:31–6. doi: 10.1177/0883073819872579
49. Gupta S, Schwab M, Valdez-Gonzalez K, Segal E. Rare homozygous nonsense variant in AIMP1 causing Early Onset Epileptic Encephalopathy with Burst Suppression (EOEE-BS). Eur J Med Genet. (2020) 63:103970. doi: 10.1016/j.ejmg.2020.103970
50. Koessler M, Haberlandt E, Karall D, Baumann M, Höller A, Scholl-Bürgi S. Ketogenic diet in a patient with refractory status epilepticus due to POLG mutation. JIMD Rep. (2020) 57:3–8. doi: 10.1002/jmd2.12169
51. Noviawaty I, Olaru E, Rondello C, Fitzsimmons B, Raghavan M. Clinical reasoning: ketogenic diet in adult super-refractory status epilepticus. Neurology. (2020) 94:541–6. doi: 10.1212/WNL.0000000000009137
52. Vallecoccia MS, Martinotti A, Siddi C, Dominedò C, Cingolani E. Use of unconventional therapies in super-refractory status epilepticus: a case report and literature review. Clin EEG Neurosci. (2022) 53:70–3. doi: 10.1177/1550059420975612
53. Dilena R, Mauri E, Aronica E, Bernasconi P, Bana C, Cappelletti C, et al. Therapeutic effect of Anakinra in the relapsing chronic phase of febrile infection-related epilepsy syndrome. Epilepsia Open. (2019) 4:344–50. doi: 10.1002/epi4.12317
54. Blunck JR, Newman JW, Fields RK, Croom JE. Therapeutic augmentation of ketogenic diet with a sodium-glucose cotransporter 2 inhibitor in a super-refractory status epilepticus patient. Epilepsy Behav Case Rep. (2018) 10:61–4. doi: 10.1016/j.ebcr.2018.05.002
55. Fox K, Wells ME, Tennison M, Vaughn B. Febrile infection-related epilepsy syndrome (FIRES): a literature review and case study. Neurodiagn J. (2017) 57:224–33. doi: 10.1080/21646821.2017.1355181
56. Uchida Y, Kato D, Toyoda T, Oomura M, Ueki Y, Ohkita K, et al. Combination of ketogenic diet and stiripentol for super-refractory status epilepticus: a case report. J Neurol Sci. (2017) 373:35–7. doi: 10.1016/j.jns.2016.12.020
57. Appavu B, Guido-Estrada N, Lindstrom K, Grebe T, Kerrigan JF, Troester M. Electroclinical phenotypes and outcomes in TBC1D24-related epilepsy. Epileptic Disord. (2016) 18:324–8. doi: 10.1684/epd.2016.0849
58. Chiusolo F, Diamanti A, Bianchi R, Fusco L, Elia M, Capriati T, et al. From intravenous to enteral ketogenic diet in PICU: a potential treatment strategy for refractory status epilepticus. Eur J Paediatr Neurol. (2016) 20:843–7. doi: 10.1016/j.ejpn.2016.08.004
59. Kenney-Jung DL, Vezzani A, Kahoud RJ, LaFrance-Corey RG, Ho ML, Muskardin TW, et al. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol. (2016) 80:939–45. doi: 10.1002/ana.24806
60. Mirás Veiga A, Moreno DC, Menéndez AI, Siscart IM, Fernández MD, Sánchez EG, et al. Effectiveness of electroconvulsive therapy for refractory status epilepticus in febrile infection-related epilepsy syndrome. Neuropediatrics. (2017) 48:45–8. doi: 10.1055/s-0036-1584939
61. Amer S, Shah P, Kommineni V. Refractory status epilepticus from NMDA receptor encephalitis successfully treated with an adjunctive ketogenic diet. Ann Indian Acad Neurol. (2015) 18:256–7. doi: 10.4103/0972-2327.150620
62. Cash, C. (2015). Use of the ketogenic diet in an adult with myoclonic status epilepticus. Abstracts/Clinical Nutrition ESPEN 10: e199 OC51 doi: 10.1016/j.clnesp.2015.03.053
63. Incecik F, Horoz OO, Herguner OM, Yildizdas D, Altunbasak S. Electroconvulsive therapy for refractory status epilepticus in a child: a case report. Ann Indian Acad Neurol. (2015) 18:364–5. doi: 10.4103/0972-2327.157250
64. Lin JJ, Lin KL, Chan OW, Hsia SH, Wang HS, CHEESE Study Group. Intravenous ketogenic diet therapy for treatment of the acute stage of super-refractory status epilepticus in a pediatric patient. Pediatr Neurol. (2015) 52:442–5. doi: 10.1016/j.pediatrneurol.2014.12.008
65. Moriyama K, Watanabe M, Yamada Y, Shiihara T. Protein-losing enteropathy as a rare complication of the ketogenic diet. Pediatr Neurol. (2015) 52:526–8. doi: 10.1016/j.pediatrneurol.2015.01.009
66. Barros P, Brito H, Ferreira PC, Ramalheira J, Lopes J, Rangel R, et al. Resective surgery in the treatment of super-refractory partial status epilepticus secondary to NMDAR antibody encephalitis. Eur J Paediatr Neurol. (2014) 18:449–52. doi: 10.1016/j.ejpn.2014.01.013
67. Fung LW. Re: the ketogenic diet for the treatment of pediatric status epilepticus. Pediatr Neurol. (2014) 51:e7. doi: 10.1016/j.pediatrneurol.2014.03.016
68. Gedik AH, Demirkol D, Tatli B, Bayraktar S, Alkan A, Karabocuoglu M, et al. Therapeutic plasma exchange for malignant refractory status epilepticus: a case report. Pediatr Neurol. (2014) 50:407–10. doi: 10.1016/j.pediatrneurol.2014.01.001
69. Matsuzono K, Kurata T, Deguchi S, Yamashita T, Deguchi K, Abe K. Ketogenic diet therapy is effective in encephalitis with refractory seizures. Neurol Res. (2014) 36:906–10. doi: 10.1179/1743132814Y.0000000371
70. Strzelczyk A, Reif PS, Bauer S, Belke M, Oertel WH, Knake S, et al. Intravenous initiation and maintenance of ketogenic diet: proof of concept in super-refractory status epilepticus. Seizure. (2013) 22:581–3. doi: 10.1016/j.seizure.2013.03.007
71. Martikainen MH, Päivärinta M, Jääskeläinen S, Majamaa K. Successful treatment of POLG-related mitochondrial epilepsy with antiepileptic drugs and low glycaemic index diet. Epileptic Disord. (2012) 14:438–41. doi: 10.1684/epd.2012.0543
72. Cervenka MC, Hartman AL, Venkatesan A, Geocadin RG, Kossoff EH. The ketogenic diet for medically and surgically refractory status epilepticus in the neurocritical care unit. Neurocrit Care. (2011) 15:519–24. doi: 10.1007/s12028-011-9546-3
73. Ismail FY, Kossoff EH. AERRPS, DESC, NORSE, FIRES: multi-labeling or distinct epileptic entities? Epilepsia. (2011) 52:e185–9. doi: 10.1111/j.1528-1167.2011.03293.x
74. Bodenant M, Moreau C, Sejourné C, Auvin S, Delval A, Cuisset JM, et al. Intérêt du régime cétogène dans le traitement d'un état de mal épileptique résistant de l'adulte [Interest of the ketogenic diet in a refractory status epilepticus in adults]. Rev Neurol. (2008) 164:194–9. doi: 10.1016/j.neurol.2007.08.009
75. Baumeister FA, Oberhoffer R, Liebhaber GM, Kunkel J, Eberhardt J, Holthausen H, et al. Fatal propofol infusion syndrome in association with ketogenic diet. Neuropediatrics. (2004) 35:250–2. doi: 10.1055/s-2004-820992
76. Anand S, Vibhute AS, Das A, Pandey S, Paliwal VK. Ketogenic diet for super-refractory status epilepticus: a case series and review of literature. Ann Indian Acad Neurol. (2021) 24:111–5. doi: 10.4103/aian.AIAN_170_20.
77. Camões J, Reis AH, Sousa L, Gomes E. Super-refractory status epilepticus and ketogenic diet in intensive care: a series report. Rev Bras Ter Intensiva. (2022) 33:635–9. doi: 10.5935/0103-507X.20210089
78. Caraballo RH, Valenzuela GR, Armeno M, Fortini S, Mestre G, Cresta A. The ketogenic diet in two paediatric patients with refractory myoclonic status epilepticus. Epileptic Disord. (2015) 17:491–5. doi: 10.1684/epd.2015.0781
79. Cobo NH, Sankar R, Murata KK, Sewak SL, Kezele MA, Matsumoto JH. The ketogenic diet as broad-spectrum treatment for super-refractory pediatric status epilepticus: challenges in implementation in the pediatric and neonatal intensive care units. J Child Neurol. (2015) 30:259–66. doi: 10.1177/0883073813516192
80. Fung EL, Chang SK, Yam KK, Yau PY. Ketogenic diet as a therapeutic option in super-refractory status epilepticus. Pediatr Neonatol. (2015) 56:429–31. doi: 10.1016/j.pedneo.2015.01.010
81. Singh RK, Joshi SM, Potter DM, Leber SM, Carlson MD, Shellhaas RA. Cognitive outcomes in febrile infection-related epilepsy syndrome treated with the ketogenic diet. Pediatrics. (2014) 134:e1431–5. doi: 10.1542/peds.2013-3106
82. Caraballo RH, Reyes G, Avaria MF, Buompadre MC, Gonzalez M, Fortini S, et al. (2013). Febrile infection-related epilepsy syndrome: a study of 12 patients. Seizure. 22:553–9. doi: 10.1016/j.seizure.2013.04.005
83. Sort R, Born AP, Pedersen KN, Fonsmark L, Uldall P. Ketogenic diet in 3 cases of childhood refractory status epilepticus. Eur J Paediatr Neurol. (2013) 17:531–6. doi: 10.1016/j.ejpn.2013.05.001
84. Kumada T, Miyajima T, Kimura N, Saito K, Shimomura H, Oda N, et al. Modified Atkins diet for the treatment of nonconvulsive status epilepticus in children. J Child Neurol. (2010) 25:485–9. doi: 10.1177/0883073809347597
85. Wusthoff CJ, Kranick SM, Morley JF, Christina Bergqvist AG. The ketogenic diet in treatment of two adults with prolonged nonconvulsive status epilepticus. Epilepsia. (2010) 51:1083–5. doi: 10.1111/j.1528-1167.2009.02388.x
86. Cervenka MC, Hocker S, Koenig M, Bar B, Henry-Barron B, Kossoff EH, et al. Phase I/II multicenter ketogenic diet study for adult superrefractory status epilepticus. Neurology. (2017) 88:938–43. doi: 10.1212/WNL.0000000000003690
87. Palacios-Mendoza M, Gómez A, Prieto J, Barrios JC, Orera M, Massot-Tarrús A. Response to anakinra in new-onset refractory status epilepticus: a clinical case. Seizure. (2022) 94:92–4. doi: 10.1016/j.seizure.2021.11.014
88. Kossoff EH, Nabbout R. Use of dietary therapy for status epilepticus. J Child Neurol. (2013) 28:1049–51. doi: 10.1177/0883073813487601
89. Dozières-Puyravel B, Höhn S, Auvin S. Considering safety and patient tolerance in the use of ketogenic diet in the management of refractory and super-refractory status epilepticus: a systematic review. Expert Rev Neurother. (2021) 21:1303–8. doi: 10.1080/14737175.2021.1956905
90. Koh S, Kim TJ, Shin HB, Kim HK, Park B, Moon SY, et al. Expanding indications for a ketogenic diet as an adjuvant therapy in adult refractory status epilepticus: an exploratory study using moderation analysis. Neurotherapeutics. (2022) 19:1526–34. doi: 10.1007/s13311-022-01282-z
91. Lattanzi S, Leitinger M, Rocchi C, Salvemini S, Matricardi S, Brigo F, et al. Unraveling the enigma of new-onset refractory status epilepticus: a systematic review of aetiologies. Eur J Neurol. (2022) 29:626–47. doi: 10.1111/ene.15149
92. Wickstrom R, Taraschenko O, Dilena R, Payne ET, Specchio N, Nabbout R, et al. International consensus recommendations for management of New Onset Refractory Status Epilepticus (NORSE) incl. Febrile Infection-Related Epilepsy Syndrome (FIRES): statements and supporting evidence. Epilepsia. (2022) 63:2840–64. doi: 10.1111/epi.17397
93. Wickstrom R, Taraschenko O, Dilena R, Payne ET, Specchio N, Nabbout R, et al. International consensus recommendations for management of New Onset Refractory Status Epilepticus (NORSE) including Febrile Infection-Related Epilepsy Syndrome (FIRES): summary and clinical tools. Epilepsia. (2022) 63:2827–39. doi: 10.1111/epi.17391
Keywords: NORSE, New-Onset Refractory Status Epilepticus, FIRES, febrile infection-related epilepsy syndrome, SRSE, super refractory status epilepticus, ketogenic diet, KD
Citation: Nabbout R, Matricardi S, De Liso P, Dulac O and Oualha M (2023) Ketogenic diet for super-refractory status epilepticus (SRSE) with NORSE and FIRES: Single tertiary center experience and literature data. Front. Neurol. 14:1134827. doi: 10.3389/fneur.2023.1134827
Received: 30 December 2022; Accepted: 27 March 2023;
Published: 13 April 2023.
Edited by:
Aljoscha Thomschewski, University Hospital Salzburg, AustriaReviewed by:
Xiu-Yu Shi, Chinese People's Liberation Army General Hospital, ChinaCopyright © 2023 Nabbout, Matricardi, De Liso, Dulac and Oualha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rima Nabbout, cmltYW5hYmJvdXRAeWFob28uY29t; cmltYS5uYWJib3V0QGFwaHAuZnI=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.