- 1Department of Otorhinolaryngology Head and Neck Surgery, Fujian Institute of Otorhinolaryngology, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 2National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 3Department of Otorhinolaryngology Head and Neck Surgery, First Hospital of Putian City, Putian, Fujian, China
- 4Department of Otolaryngology, Fujian Provincial Geriatric Hospital, Fuzhou, China
- 5Department of Clinical Research and Translation Center, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 6Clinical Laboratory, Fujian Provincial Hospital South Branch, Fuzhou, China
- 7Central Laboratory, Key Laboratory of Radiation Biology of Fujian Higher Education Institutions, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China
Background: Sudden sensorineural hearing loss (SSNHL) is a global problem threatening human health. Early and rapid diagnosis contributes to effective treatment. However, there is a lack of effective SSNHL prediction models.
Methods: A retrospective study of SSNHL patients from Fujian Geriatric Hospital (the development cohort with 77 participants) was conducted and data from First Hospital of Putian City (the validation cohort with 57 participants) from January 2018 to December 2021 were validated. Basic characteristics and the results of the conventional coagulation test (CCT) and the blood routine test (BRT) were then evaluated. Binary logistic regression was used to develop a prediction model to identify variables significantly associated with SSNHL, which were then included in the nomogram. The discrimination and calibration ability of the nomogram was evaluated by receiver operating characteristic (ROC), calibration plot, and decision curve analysis both in the development and validation cohorts. Delong’s test was used to calculate the difference in ROC curves between the two cohorts.
Results: Thrombin time (TT), red blood cell (RBC), and granulocyte–lymphocyte ratio (GLR) were found to be associated with the diagnosis of SSNHL. A prediction nomogram was constructed using these three predictors. The AUC in the development and validation cohorts was 0.871 (95% CI: 0.789–0.953) and 0.759 (95% CI: 0.635–0.883), respectively. Delong’s test showed no significant difference in the ROC curves between the two groups (D = 1.482, p = 0.141).
Conclusion: In this study, a multifactor prediction model for SSNHL was established and validated. The factors included in the model could be easily and quickly accessed, which could help physicians make early diagnosis and clinical treatment decisions.
Introduction
Sudden sensorineural hearing loss (SSNHL) is defined as a rapid hearing loss of at least 30 dB at three consecutive frequencies within 72 h (1). As an otological emergency, SSNHL has an annual incidence of 5–30/100,000 cases, with an increasing trend worldwide (1). Only 4.47–15% of cases of SSNHL can be identified with a final diagnosis in the acute stage (2), such as labyrinthine hemorrhage (3) or vascular events (4). The prognosis of SSNHL is poor due to unclear pathophysiology, delayed diagnosis, and treatment. If the disease can be predicted in advance, it will help to give timely treatment and improve the therapeutic effect of SSNHL. Therefore, such prediction models are very important to SSNHL.
Ischemia and hypoxia caused by the alteration of blood flow are important causes of SSNHL (5). Several routine hematological parameters have been identified as prognostic factors, including platelet-to-lymphocyte ratio (PLR), neutrophil–lymphocyte ratio (NLR), and fibrinogen–albumin ratio (FAR) (6–9). However, the relationship between thrombin time (TT), red blood cell (RBC), granulocyte lymphocyte ratio (GLR), and SSNHL is not fully understood. It is worth noting that most of these studies have only predicted the prognosis of SSNHL, and these prognosis models were developed using data from single-center data with different clinical outcome settings leading to different conclusions. Even if several studies previously investigated diagnostic markers (10–12), few studies focused on prediction models for SSNHL.
Therefore, the purpose of this study was to develop a prediction model in the development cohort and verify its reliability in the validation cohort, thus establishing a promising prediction model for SSNHL.
Methods
Study participants
SSNHL refers to a sudden occurrence of sensorineural hearing loss of unknown etiology within 72 h with at least three consecutive frequency losses of 30 dB (1). In the development cohort, Fujian Provincial Geriatric Hospital recruited 39 patients diagnosed with SSNHL and 38 adults who were free of any ear disease, cancer, or any other blood disease in the physical examination as healthy controls from January 2018 to December 2021. All participants ranged in age from 18 to 79 years and underwent conventional coagulation test (CCT) and blood routine test (BRT). We excluded pregnant women and patients who are taking anticoagulant drugs, as well as patients with blood diseases, Meniere’s disease, herpes zoster oticus, or any other disease with a known cause of hearing loss. Because low-frequency hearing loss is one of the symptoms of Meniere’s disease, patients with low-frequency hearing loss were also excluded. At the same time, 57 adults (29 SSNHL patients and 28 healthy controls) from First Hospital of Putian City were incorporated into the validation cohort. The exclusion criteria were the same as the development cohort as depicted in Figure 1.
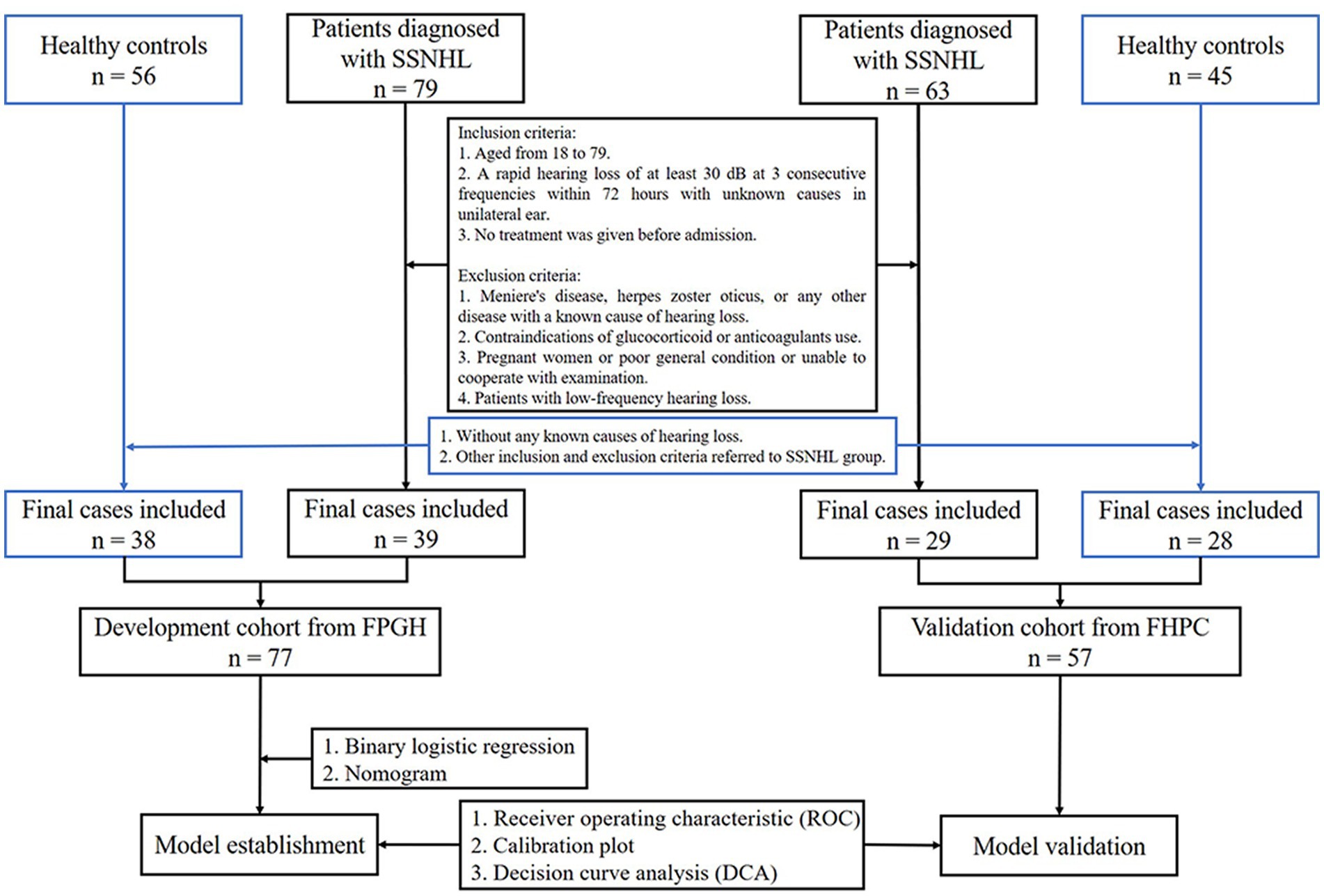
Figure 1. Flow chart (FPGH, Fujian Provincial Geriatric Hospital; FHPC, First Hospital of Putian City).
This study was reviewed and approved by the institutional review board and ethics committee of Fujian Geriatric Hospital (Ethics Committee No.2020-03-01). All the methods were carried out in accordance with the relevant guidelines and regulations. Informed consent was obtained from all participants.
Data collection
Detailed medical history, which includes details of baseline characteristics (including age, gender, hypertension, diabetes, hypertriglyceridemia, hypercholesterolemia, and autoimmune disease), clinical characteristics (including affected side), accompanying symptoms (such as tinnitus, vertigo, headache, and dizziness), was obtained from all participants.
Clinical and other measurements
Hearing loss was determined by pure-tone audiometry (Conera, Denmark) before and after treatment. Pure-tone average (PTA) was calculated as the average of thresholds (dB HL) at seven frequencies of 0.125, 0.25, 0.5, 1, 2, 4, and 8 kHz. The coagulation function parameters were measured by an automatic coagulation analyzer (Sysmex CS5100 or CS2000i, Japan), and the blood routine parameters were measured by a fully automatic hematology analyzer (Sysmex XN 1000 or CN3000, Japan).
Statistical analysis
Post hoc assessments of sample size were performed. In the development cohort, 39 samples in the positive group and 38 samples in the negative group were tested using a two-sided z-test between the area under the receiver operating characteristic (ROC) curve (AUC) under the null hypothesis of 0.500 and an AUC at the alternative hypothesis of 0.871, achieving>99% power at a significance level of 0.050 and a difference of 0.371 was detected. In the validation cohort, 29 samples in the positive group and 28 samples in the negative group achieved 95% power and the detected difference was 0.259.
Continuous variables were expressed as mean ± standard deviation (SD), while categorical variables were presented as frequencies and percentages (%). To compare the difference between groups, the Chi-square test or the Fisher exact test was used for categorical variables and the t-test was used for continuous variables.
The prediction model for the risk of SSNHL was established based on the existing information. The steps are as follows:
First, the baseline characteristics of the control group and SSNHL group in the development cohort were balanced, and the 12 items of CCT and BRT parameters were initially selected for candidate predictors. Second, the potential predictors with p < 0.05 in the univariate analyses were selected to be included in a multivariate logistic model. Third, a backward step-down selection process was performed by a threshold of p < 0.05 to establish a parsimonious model, and a nomogram was formulated in the training cohort. Fourth, the discriminative ability, predictive accuracy, and clinical application value of the model in the training cohort were assessed using a ROC curve, calibration plot, and decision curve analysis (DCA). Finally, the external validity of model performance was assessed in the validation cohort, and Delong’s test was conducted to compare the ROC curves of the development cohort and the validation cohort.
The statistical software used in this study includes SPSS software version 17.0 (IBM) and R software (version 4.2.1). The p-values less than 0.05 were considered statistically significant in each statistical analysis.
Results
Baseline characteristics, blood CCT, and BRT parameters in the development and validation cohort
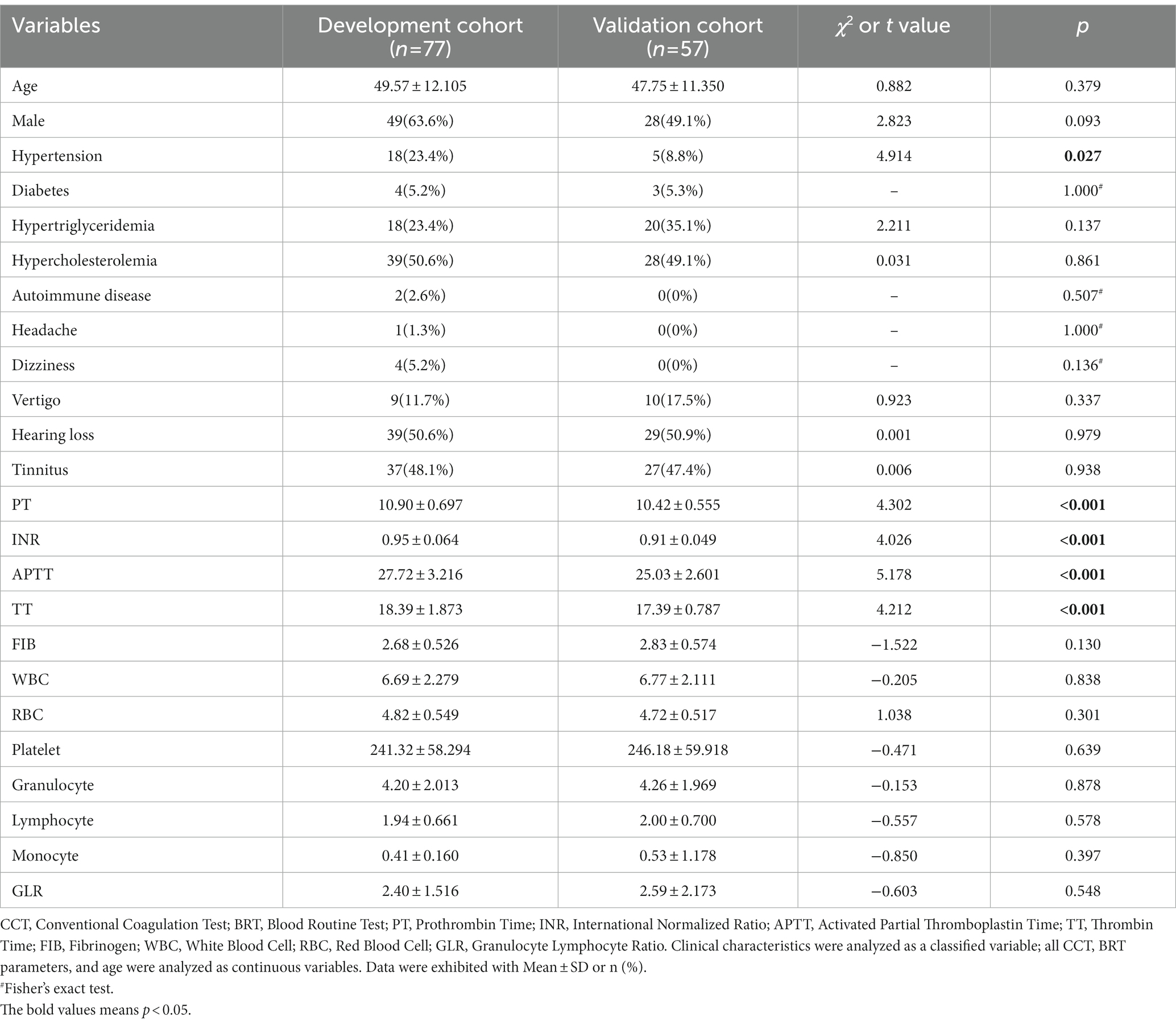
In total, 134 participants were enrolled, 77 in the development cohort and 57 in the validation cohort. The baseline characteristics, CCT, and BRT parameters are detailed in Table 1. Simply, the participants comprised the development cohort (28 women and 49 men) with a median age of 49.57 years and the validation cohort (27 women and 28 men) with a median age of 47.75 years. Both cohorts were comparable not only in terms of age, gender, hypertension, diabetes, hypertriglyceridemia, hypercholesterolemia, and autoimmune disease but also hearing loss, tinnitus, vertigo, dizziness, and headache. The probability of all the basic characteristics in the development cohort was similar to the validation cohort except for hypertension (p = 0.027).
Five coagulation function parameters and seven blood routine test parameters were within the normal range in both cohorts. The development cohort exhibited a significantly prolonged PT, INR, TT, and APTT compared with the validation cohort (all p < 0.001). Fibrinogen (FIB) in the development cohort was lower but had no statistical difference (p = 0.130) than in the validation cohort. The seven blood routine test parameters showed no statistical difference in both cohorts (all p > 0.05).
Factors selection for SSNHL prediction model construction in the development cohort
In the development cohort, all baseline characteristics were similar (p > 0.05) except for the SSNHL-specific characteristics, including hearing loss (p < 0.001), tinnitus (p < 0.001), vertigo (p = 0.002), in control and SSNHL group (Table 2). The parameters in CCT and BRT, including PT, INR, TT, RBC, granulocyte, lymphocyte, monocyte, and granulocyte to Lymphocyte ratio (GLR), were significantly different (all p < 0.05) between the control and the SSNHL group (Table 2).
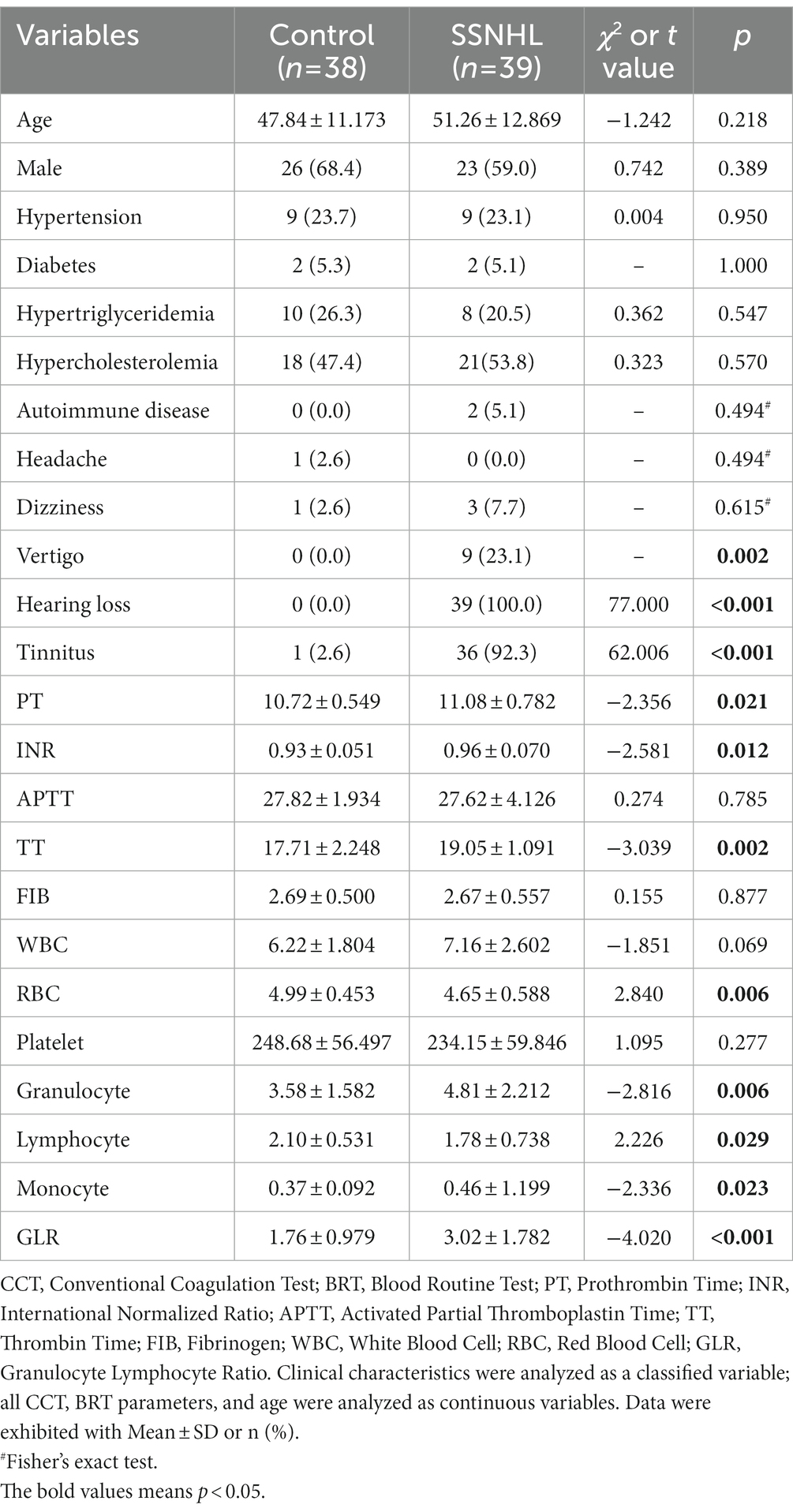
Table 2. Baseline characteristics, CCT and BRT parameters of control and SSNHL group in the development cohort.
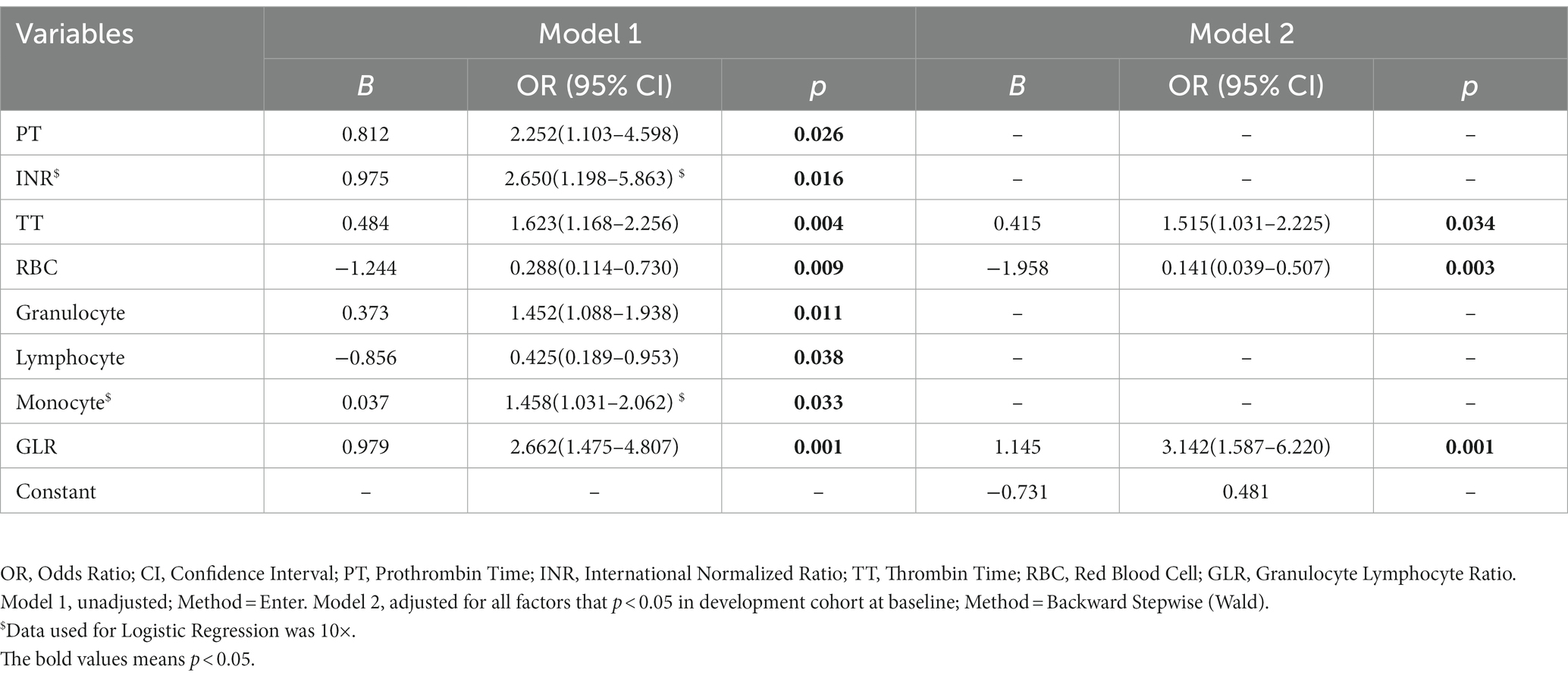
The factors (p < 0.05) were incorporated for the predicted model using logistic regression analysis among the abovementioned CCT and BRT factors. TT (OR = 1.515, 95% CI: 1.031–2.225, p = 0.034), RBC (OR = 0.141, 95% CI: 0.039–0.507, p = 0.003), and GLR (OR = 3.142, 95% CI: 1.587–6.220, p = 0.001) in model 2 can be used for SSNHL prediction model construction (Table 3).
Construction of the prediction risk model
The three candidate factors (TT, RBC, and GLR) were chosen to construct the prediction risk model. According to the logistic regression derived β coefficients, an individual’s risk of SSNHL might be calculated as follows:
To evaluate the prediction model more readable and convenient, a nomogram was constructed, as shown in Figure 2.
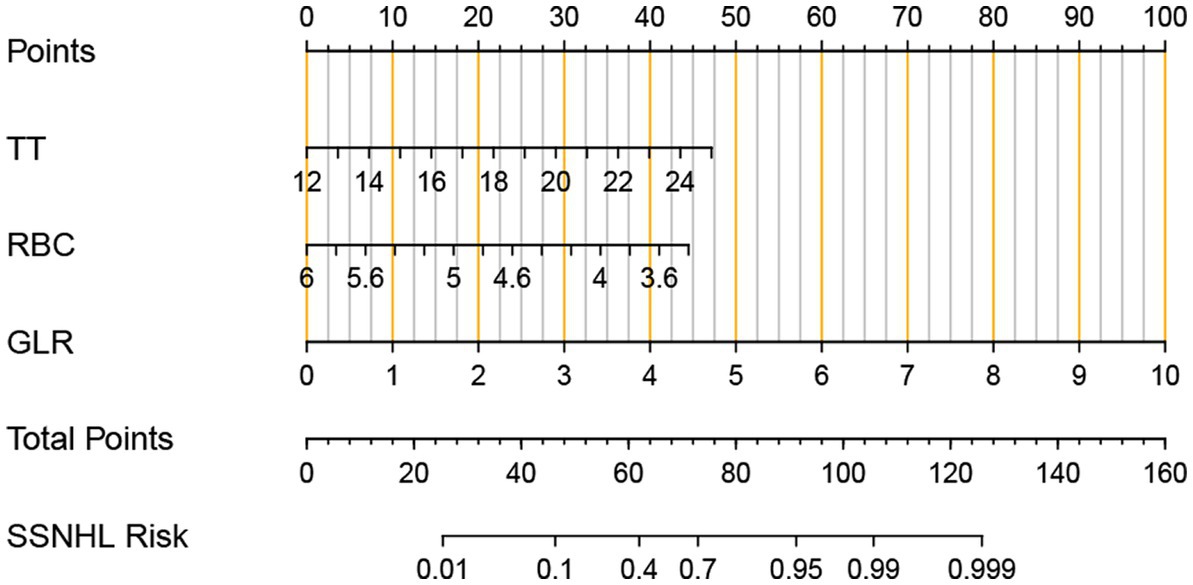
Figure 2. Nomogram for predicting the risk of an individual adult sudden sensorineural hearing loss (SSNHL). The values of thrombin time (TT), red blood cell (RBC), granulocyte lymphocyte Ratio (GLR), and points are acquired from each variable axis. The total points on the axis are the sum values of these three factors, which can predict the SSNHL risk.
Validation of the risk model
Based on the development cohort, the ROC analysis was significantly different between the control group and the SSNHL group (p < 0.001) (Figure 3A). The AUC value of the prediction model was 0.871 (95% CI: 0.789–0.953). The calibration plot is shown in Figure 3B, and DCA is shown in Figure 3C.
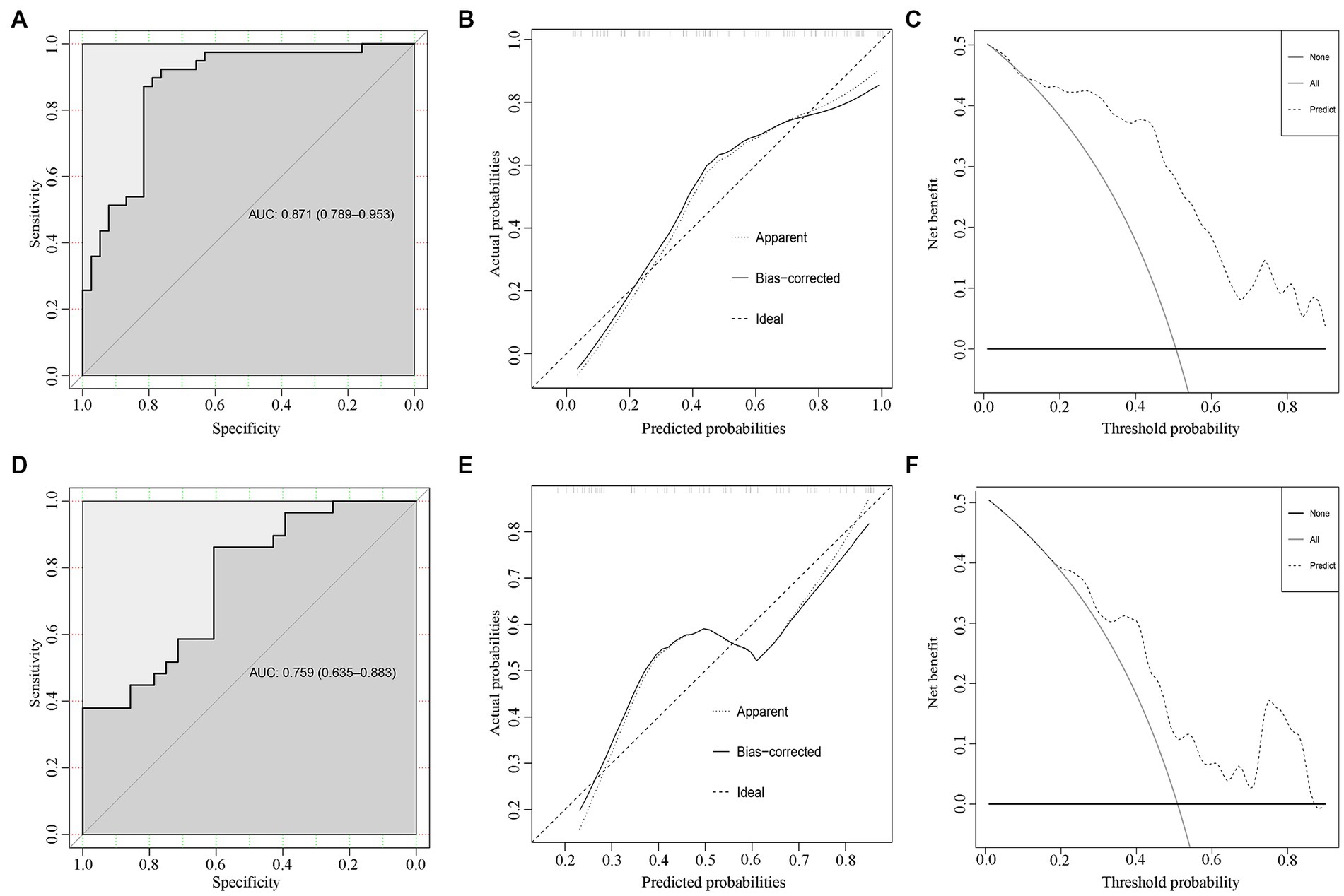
Figure 3. Internal and external validations for the SSNHL prediction model. The ROC, calibration plots, and decision curve analysis for the predicting model in the development cohort (A–C) and the validation cohort (D–F).
The SSNHL prediction model was evaluated in the validation cohort. The ROC analysis was significantly different between the two groups (p < 0.001) (Figure 3D). The AUC value of the prediction model was 0.759 (95% CI: 0.635–0.883).
Delong’s test for two ROC curves did not find a significant difference between the AUC in the development cohort and the validation cohort (D = 1.482, p = 0.141). The calibration plot is shown in Figure 3E, and DCA is shown in Figure 3F.
Discussion
The diagnosis of SSNHL is mainly based on the audiogram; however, a subjective audiogram alone cannot accurately diagnose SSNHL, and may also misdiagnose prognosis. In this study, a prediction model of SSNHL was established via binary logistic regression and verified externally. TT, RBC, and GLR were identified as the diagnostic factors for SSNHL and used to build a reliable nomogram. Nomogram has been shown to predict SSNHL in both the development and the validation cohorts and is strongly recommended for clinical use.
In this study, TT was considered to be a risk factor for SSNHL. Prolonged TT was seen in diseases with reduced plasma fibrinogen, such as disseminated intravascular coagulation (12) and dysfibrinogenemia (13). A previous study has shown that dysfunction of inner ear microcirculation plays an important role in SSNHL (14); however, due to the lack of effective evidence-based evidence, the use of anticoagulants has not reached an international consensus (11). Labyrinthine hemorrhage has also been reported as a potential factor for SSNHL (3); meanwhile, oral anticoagulants may interfere with microcirculation in the inner ear by influencing the viscosity of the plasma (15). Our data showed that TT was prolonged before treatment in the SSNHL group. Therefore, TT should be paid more attention to avoid bleeding events or aggravating the condition in older patients with SSNHL or patients who are taking anticoagulants.
RBC has been identified as a risk factor for myometrial invasion in endometrioid endometrial carcinoma patients with metabolic syndrome (16) and as a significant predictor of the risk of adverse cardiovascular events (17). Studies have indicated that the cochlea is more susceptible to hypoxia at high frequencies in rats (18), and hypoxia can impair hearing function in patients with chronic obstructive pulmonary disease (19). In this study, low RBC count is a risk factor for SSNHL and an indicator of blood viscosity. A low RBC count means reduced blood viscosity and the use of anticoagulants should be carefully considered in patients with SSNHL. Furthermore, the labyrinthian artery is the only small-diameter artery in the cochlea, and the decreased RBC count will lead to the reduction of oxygenation (20), which may aggravate cochlear ischemia and hypoxia, then causes SSNHL or exacerbate the condition.
GLR can be regarded as a reliable indicator to predict infectious complications after gastrectomy (21) or systemic spread of streptococcus pyogenes with acute skin infection (22), which is associated with inflammatory reactions. It suggested that increased GLR can be used as a predicted marker of SSNHL. The mechanism of SSNHL is associated with inflammation, which is well demonstrated by steroid therapy (23). Increased granulocyte and/or decreased lymphocyte (especially viral infection) will lead to increased GLR in the acute inflammation phase of patients with SSNHL. Therefore, GLR as a predictor of SSNHL is reliable and necessary.
In this study, after excluding not statistically significant variables, a prediction model of SSNHL was established by combining TT, RBC, and GLR, and visualized by nomogram. In the development and validation cohorts, the AUC values of the prediction model were 0.871 (95% CI: 0.789–0.953) and 0.759 (95% CI: 0.635–0.883), respectively, showing a certain predictive ability. Meanwhile, the calibration plot and DCA show good performance in the development and validation cohorts. However, it is difficult for all instruments in both centers to be the same. It has been found that the performance of the instruments is stable, and the measurement reference values are the same in both centers. In addition, the highly consistent results between the two centers further indicate the stability of our model. It is also limited by the retrospective design and small sample size, and a prospective cohort study with a larger sample size is needed to further refine this study. Nevertheless, we believe that the prediction model and nomogram will play an important role in guiding the clinical diagnosis and management of SSNHL.
Conclusion
In this study, a prediction model and nomogram of SSNHL were established with good discrimination and calibration and were helpful for clinicians to diagnose SSNHL timely and to take reasonable treatment decisions.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding authors
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Fujian Geriatric Hospital (Ethics Committee No. 2020-03-01). Written informed consent to participate in this study was provided by the patient/participants or patient/participants legal guardian/next of kin. Written informed consent was obtained from the individual(s) and/or minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
CZ and JC were involved in study conception and design, designed the experiment, analyzed the data, drafted the manuscript, discussed results, and revised and finalized the manuscript. YY performed the SSNHL diagnosis, collected the data, evaluated the treatment outcome, and discussed the results. SH analyzed the data and confirmed the statistical results. WH collected the data from physical examination persons. ZC and DH performed the SSNHL diagnosis, collected the data, and evaluated the treatment outcome. CL was involved in study conception and design, discussed the results, and revised and finalized the manuscript. All authors approved the final copy of this manuscript.
Funding
This study was supported by the Natural Science Foundation of Fujian Province (grant nos: 2022J01215 and 2020J01097) and the Research Project of the Treatment of Auditory System Dysfunction in Aging Population (grant no: 2020YFC2005204).
Acknowledgments
The authors would like to thank all the participants of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stachler, RJ, Chandrasekhar, SS, Archer, SM, Rosenfeld, RM, Schwartz, SR, Barrs, DM, et al. Clinical practice guideline. Otolaryngol Head Neck Surg. (2012) 146:S1–S35. doi: 10.1177/0194599812436449
2. Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Society of Otorhinolaryngology Head and Neck Surgery CMA. Guideline of diagnosis and treatment of sudden deafness. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2015) 50:443–7.
3. Chen, XH, Zeng, CJ, Fang, ZM, Zhang, R, Cheng, JM, and Lin, C. The natural history of labyrinthine hemorrhage in patients with sudden sensorineural hearing loss. Ear Nose Throat J. (2019) 98:E13–20. doi: 10.1177/0145561319834862
4. Kim, JY, Hong, JY, and Kim, DK. Association of sudden sensorineural hearing loss with risk of cardiocerebrovascular disease: a study using data from the Korea National Health Insurance Service. JAMA Otolaryngol Head Neck Surg. (2018) 144:129–35. doi: 10.1001/jamaoto.2017.2569
5. Zhu, WY, Jin, X, Ma, YC, and Liu, ZB. MIF protects against oxygen-glucose deprivation-induced ototoxicity in HEI-OC1 cochlear cells by enhancement of Akt-Nrf2-HO-1 pathway. BiochemBiophys Res Commun. (2018) 503:665–70. doi: 10.1016/j.bbrc.2018.06.058
6. Diao, T, Ke, Y, Zhang, J, Jing, Y, and Ma, X. Correlation between the prognosis of sudden total deafness and the peripheral blood inflammation markers. Front Neurol. (2022) 13:927235. doi: 10.3389/fneur.2022.927235
7. Kang, DW, Kim, S, and Sunwoo, W. Correlation of neutrophil-to-lymphocyte ratio and the dilation of the basilar artery with the potential role of vascular compromise in the pathophysiology of idiopathic sudden sensorineural hearing loss. J Clin Med. (2022) 11:5943. doi: 10.3390/jcm11195943
8. Cayir, S, Kayabasi, S, and Hizli, O. Predictor parameters for poor prognosis in patients with sudden sensorineural hearing loss: fibrinogen to albumin ratio vs c-reactive protein to albumin ratio. Braz J Otorhinolaryngol. (2021) 87:457–61. doi: 10.1016/j.bjorl.2020.06.010
9. Ni, W, Song, SP, and Jiang, YD. Association between routine hematological parameters and sudden sensorineural hearing loss: a meta-analysis. J Otol. (2021) 16:47–54. doi: 10.1016/j.joto.2020.07.006
10. Zheng, Z, Liu, C, Shen, Y, Xia, L, Xiao, L, Sun, Y, et al. Serum albumin levels as a potential marker for the predictive and prognostic factor in sudden sensorineural hearing loss: aprospective cohort study. Front Neurol. (2021) 12:747561. doi: 10.3389/fneur.2021.747561
11. Guo, Y, and Liu, J. The roles played by blood inflammatory parameters in sudden sensorineural hearing loss. Ear Nose Throat J. (2021) 18:145561320960355. doi: 10.1177/0145561320960355
12. Haupt, L, Vieira, M, Brits, H, de Beer, J, Erasmus, E, Esterhuyse, W, et al. An audit of disseminated intravascular coagulation screen requests at an academic hospital laboratory in Central South Africa. Int J Lab Hematol. (2021) 43:1174–80. doi: 10.1111/ijlh.13461
13. Marchi, R, Neerman-Arbez, M, Gay, V, Mourey, G, Fiore, M, Mouton, C, et al. Comparison of different activators of coagulation by turbidity analysis of hereditary dysfibrinogenemia and controls. Blood Coagul Fibrinolysis. (2021) 32:108–14. doi: 10.1097/MBC.0000000000001000
14. Yamada, S, Kita, J, Shinmura, D, Nakamura, Y, Sahara, S, Misawa, K, et al. Update on findings about sudden sensorineural hearing loss and insight into its pathogenesis. J Clin Med. (2022) 11:6387. doi: 10.3390/jcm11216387
15. Mierzwa, K, Schneider, G, and Müller, A. Sudden sensorineural hearing loss during oral anticoagulant therapy. J Laryngol Otol. (2004) 118:872–6. doi: 10.1258/0022215042703778
16. Tong, Y, Xie, X, Mao, X, Lei, H, Chen, Y, and Sun, P. Low red blood cell count as an early indicator for myometrial invasion in women with endometrioid endometrial carcinoma with metabolic syndrome. Cancer Manag Res. (2020) 12:10849–59. doi: 10.2147/CMAR.S271078
17. Paquette, M, Bernard, S, and Baass, A. Hemoglobin concentration, hematocrit and red blood cell count predict major adverse cardiovascular events in patients with familial hypercholesterolemia. Atherosclerosis. (2021) 335:41–6. doi: 10.1016/j.atherosclerosis.2021.09.015
18. Sonbay Yilmaz, ND, Saka, C, AlicuraTokgöz, S, Çalişkan, M, Vuralkan, E, and Beşalti, Ö. Effects of acute hypoxia on auditory pathway of Westar albino rats. Turk J Med Sci. (2020) 50:1580–4. doi: 10.1016/j.atherosclerosis.2021.09.015
19. Sonbay Yilmaz, ND, Saka, C, Oktay Arslan, B, Aygener Yeşilyurt, N, Saka, D, Ardıç, S, et al. The effect of hypoxia on hearing function. Turk J Med Sci. (2019) 49:1450–4. doi: 10.3906/sag-1902-210
20. Yoshida, T, Prudent, M, and D'alessandro, A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. (2019) 17:27–52. doi: 10.2450/2019.0217-18
21. Lu, J, Liu, H, Cao, LL, Zheng, CH, Li, P, Xie, JW, et al. The granulocyte-to-lymphocyte ratio as a marker of surgical stress and a predictor of postoperative infectious complications after gastric cancer surgery: an analysis of patients enrolled in a prospective randomized trial. Ann Surg Oncol. (2017) 24:2688–97. doi: 10.1245/s10434-017-5846-y
22. Loof, TG, Sohail, A, Bahgat, MM, Tallam, A, Arshad, H, Akmatov, MK, et al. Early lymphocyte loss and increased granulocyte/lymphocyte ratio predict systemic spread of streptococcus pyogenes in a mouse model of acute skin infection. Front Cell Infect Microbiol. (2018) 8:101. doi: 10.3389/fcimb.2018.00101
Keywords: sudden sensorineural hearing loss, prediction, nomogram, thrombin time, red blood cell, granulocyte lymphocyte ratio
Citation: Zeng C, Yang Y, Huang S, He W, Cai Z, Huang D, Lin C and Chen J (2023) Development and validation for multifactor prediction model of sudden sensorineural hearing loss. Front. Neurol. 14:1134564. doi: 10.3389/fneur.2023.1134564
Edited by:
Lisheng Yu, Peking University People's Hospital, ChinaReviewed by:
Eduardo Martin-Sanz, Hospital de Getafe, SpainBenyu Nan, Wenzhou Medical University, China
Copyright © 2023 Zeng, Yang, Huang, He, Cai, Huang, Lin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang Lin, bGluYzMwMUBzaW5hLmNvbQ==; Junying Chen, anVueWluZ2NoZW4wMUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Chaojun Zeng
Chaojun Zeng Yunhua Yang4†
Yunhua Yang4† Chang Lin
Chang Lin Junying Chen
Junying Chen