
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 27 March 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1129062
This article is part of the Research Topic Understanding PFO-associated stroke View all 9 articles
 Fanfan Shi1
Fanfan Shi1 Leihao Sha2
Leihao Sha2 Hua Li2
Hua Li2 Yusha Tang2
Yusha Tang2 Litao Huang1
Litao Huang1 Huizhen Liu1
Huizhen Liu1 Xu Li1
Xu Li1 Lin Li1
Lin Li1 Wenjie Yang1
Wenjie Yang1 Deying Kang1,3
Deying Kang1,3 Lei Chen2*
Lei Chen2*Patent foramen ovale (PFO) is a common congenital cardiac abnormality when the opening of the interatrial septum is not closed in adulthood. This abnormality affects 25% of the general population. With the development of precision medicine, an increasing number of clinical studies have reported that PFO is closely related to various neurological diseases such as stroke, migraine, obstructive sleep apnea, and decompression syndrome. It has also been suggested that PFO closure could be effective for preventing and treating these neurological diseases. Therefore, increasing attention has been given to the prevention, diagnosis, and treatment of PFO-related neurological diseases. By reviewing existing literature, this article focuses on the pathogenesis, epidemiology, and clinical characteristics of PFO-related neurological diseases, as well as the prevention and treatment of different neurological diseases to discuss, and aims to provide current progress for this field and decision-making evidence for clinical practice.
The foramen ovale is an opening in the interatrial septum in the fetal heart. After the fetus is born, with the establishment of pulmonary circulation, the left atrial pressure increases, pushing the primary septum to fuse with the secondary septum and then prompting the foramen ovale to close. Foramen ovale closes before the age of 2 years in most people, and approximately 25% fail to close in adulthood, resulting in the formation of congenital heart abnormalities—patent foramen ovale (PFO) (1).
An increasing number of studies have reported that PFO is closely related to many neurological diseases, such as stroke, migraine, obstructive sleep apnea (OSA), and decompression sickness (DCS) (2). For stroke, large-scale clinical studies have suggested that PFO could be a novel risk factor for cryptogenic stroke, especially for younger adults. Three randomized controlled trials (RCTs) from the New England Journal of Medicine also indicated that PFO closure was effective in preventing stroke recurrence (3–5). Based on these, the concept of “PFO-related stroke” was separately proposed in the U.S. SCAI (Society for Cardiovascular Angiography and Interventions) guidelines in 2022, strongly recommending that PFO closure should be used for patients aged 18–60 years with previous PFO-related stroke to prevent stroke recurrence (6). However, some studies have shown that PFO closure has poor therapeutic effects in patients with long-tunnel PFO (7), and the best beneficiaries are not yet clear. For migraine, several observational clinical studies have shown an association with PFO, especially for migraine with aura. However, the efficacy of PFO closure was mixed in three RCTs (8–10), and post-hoc analysis found PFO closure was only effective in migraine with aura. The relationship between PFO and migraine remains questionable. For other neurological diseases (such as OSA and DCS), even though some previous literature has also explored the association with PFO, limited evidence supported it. Since neurological diseases are a major burden to human health, and PFO could be a potential novel risk factor and treatment target for neurological diseases, it is very necessary to focus on and carry out related research.
Based on the earlier mentioned text, this article reviews the research progress of PFO diagnosis, as well as epidemiology, clinical characteristics, pathogenesis, prevention, and treatment of PFO-related neurological diseases to provide strong supporting evidence to carry out future research in this field.
Patent foramen ovale was first discovered by autopsy in 1877 (11), and the prevalence reported ranged from 15–35% (12–14). In the 1980s, with the advances of echocardiography and aerated saline contrast media, screening for PFO became common in clinical practice. Common diagnostic techniques include transesophageal echocardiography contrast-enhanced acoustics (cTEE), transthoracic echocardiography contrast-enhanced acoustics (cTTE), and transcranial Doppler contrast-enhanced ultrasound (cTCD), each of which has advantages and disadvantages (refer to details in Table 1). cTEE is the best tool for obtaining an anatomical confirmation of PFO and provides some essential details such as measure (width and length) and presence of an atrial septal aneurysm, and these characteristics have important guidance for treatment decision-making. However, cTEE is also limited by high cost and poor patient tolerance. Therefore, it is not suitable for primary screening. cTTE and cTCD are often presented as potential screening tools. But according to the results of a meta-analysis of diagnostic tests, compared with cTEE, the pooled sensitivity and specificity for cTTE were just 45.1% and 99.6%, and the respective measures for cTCD were 96.1% and 92.4% (18). In view of the lower sensitivity of cTTE, and cTCD can only detect right to left shunt (RLS), but cannot confirm the presence of PFO, the current clinical guidelines of several countries suggest the combination of multiple diagnostic screening tools for screening and diagnosis of PFO (15, 19). Generally, when using cTTE or cTCD, it is recommended to detect both resting and implementing Valsalva. If either item has many microbubbles, there is no need to repeat the examination. If it is negative and PFO is still suspected, it is recommended to (I) repeat Valsalva or cough at an appropriate time (three–four times) for contrast media injection detection of normal saline, (II) use a mixture of blood saline and air, and (III) use TEE detection (image acquisition should begin before the appearance of saline contrast medium in the right atrium and continue for at least 10 cardiac cycles after the appearance of contrast medium) (20).
In terms of any diagnostic techniques, it is essential to strictly follow the operation process. Clinical practice and research have found that the accuracy of PFO diagnosis is easily affected by the operational details, and even the gold-standard cTEE may have a false negative rate of 7.9% due to inadequate pressures generated during Valsalva maneuvers (21). In addition, although some countries have issued corresponding guidelines or consensus (22, 23), there are still disputes over some details. For example, the choice of Valsalva implementation time during cTCD, although most recommended after contrast agent (CA) injection, some studies recommended before or during CA injection and different implementation times, will lead to completely different examination results (24, 25). Another example, most previous studies diagnosed PFO by echocardiography only using left heart contrast and cardiac cycles after right atrium (RA) opacification, but the definition for quantity of left heart contrast and cardiac cycles varies among studies (20). All these problems need more detailed and standardized clinical practice guidelines to guide them.
It is worth mentioning that with the innovation of diagnostic technology, intracardiac echocardiography (ICE) has been gradually explored in the diagnosis and PFO closure. Jeonggeun Moon et al. compared the procedural efficacy and safety of TEE-guide and ICE-guide PFO device closure for the first time and found that the fluoroscopy time, radiation dose, and total procedural time in the catheter laboratory were significantly lower in the ICE group than those in the TEE group while achieving similar procedural outcomes and hospital stay duration (26). Compared with TEE and TTE, ICE has a higher image resolution and can accurately assess the size, location, and edge of PFO from different angles, which makes it easy to capture anatomical information such as atrial myxoma, Chiari network with thrombi, and additional septal defects (27). The development of this technology will bring new prospects for PFO diagnosis and closure. However, the possible disagreement in the anatomical evaluation of PFO between preprocedural TEE and intraprocedural ICE needs to be further studied and considered.
Thus far, stroke and migraine are the most discussed diseases in the research of PFO-related neurological diseases. According to the results from community-based and multi-center epidemiological studies conducted in different countries, the prevalence of PFO or RLS for stroke ranges from 23.5 to 61.1% (28–32), for migraine with aura from 19 to 77.9% (33–36), and for migraine without aura from a relatively low 11–34.1% (33, 34). The prevalence of patients in most studies was generally greater than the general population. Furthermore, the results of two meta-analyses showed that compared with healthy people, the prevalence of PFO was higher in patients with stroke and migraine (OR = 3.1 and 2.54, respectively) (37, 38), which indicates that there is a close relationship. Studies on the correlation between PFO and OSA, DCS, and dementia (refer to Supplementary Table 1) are relatively few. Some retrospective studies showed the prevalence of PFO in OSA was higher than that in healthy controls (39, 40), and the incidence of PFO in DCS patients is 62.5% (41). In addition, more direct evidence found that venous to arterial circulation shunt (v-aCS) of PFO was more common in both patients with AD and vascular dementia (VaD) than in healthy controls, suggesting that PFO is associated with cognitive dysfunction, especially AD (42, 43). These are worthy of further exploration.
Patent foramen ovale-related strokes were mainly cryptogenic stroke (CS) or embolic stroke of unknown origin (ESUS). According to the proportion of CS in ischemic stroke (44) and the global incidence of ischemic stroke in 2019 (45), the incidence of PFO-related stroke is estimated to be 19 to 28 cases per 100 000 and is more common in people under 55 years of age (46). For elderly patients, Mazzucc. et al. found the prevalence of RLS with cryptogenic transient ischemic attacks and non-disabling strokes reached 28.21% (31). It also needs attention. At present, there is no sex or racial difference in the prevalence of PFO-related stroke, but the incidence of stroke in young women is increasing, which may be caused by risk factors such as blood coagulation and hormonal changes during pregnancy (47). For pregnant women with PFO, the risk of recurrent stroke is higher (48), which deserves more attention too. Migraine is the second leading cause of non-fatal burden globally (49), and few studies reported the unique epidemiological characteristics of PFO-related migraine. But unlike most previous studies, two cross-sectional studies conducted in China found that the incidence of migraine without aura in PFO was significantly higher than in healthy people, suggesting that PFO may be also associated with migraine without aura (50, 51). The correlation deserves further exploration in other countries and populations.
The results of some clinical studies indicated that clinical characteristics and symptoms of PFO-related neurological diseases might be related to the volume of RLS shunt. PFO-related stroke lesions mostly involve vertebrobasilar circulation (52). The greater the amount of RLS shunts, the higher the proportion of small lesions, the greater the likelihood of posterior circulation involvement, and the greater the frequency of multiple cortical lesions (53). But for migraines, the findings were inconsistent. Some studies have shown that the frequency, intensity, and duration of headaches in migraine patients with moderate and severe RLS shunting were significantly higher than those in patients with mild RLS and patients with non-RLS (54), but some found the greater RLS severity, the younger was onset age (35, 55). These findings will encourage further research to explore the characteristics of the high-risk population for RLS screening and assessment. In addition, the results of multiple clinical studies (refer to Supplementary Table 1) show that patients with high-risk PFO anatomical features, such as long tunnels, small angles, excessive atrial septal movement, and prominent eustachian tubes or Chiari networks, are also at relatively high risk of neurological disease, and these should be focused on.
Although it was initially proposed that PFO is associated with these neurological diseases based on observational studies and clinical trials, mechanistic research remains at the hypothesis level. Figure 1 shows the research progress and hypothesized hypotheses on the pathogenesis of PFO-related neurological diseases. Currently, it is believed that the pathogenesis of PFO-related neurological diseases mainly includes ischemic hypoxic changes caused by microembolism, the 5-HT abnormal metabolism hypothesis, the platelet-based mechanism hypothesis, and the genetic susceptibility hypothesis.
The pathophysiology of PFO is characterized by abnormal right-to-left shunting of cardiac chambers, and abnormal hemodynamics in the left atrium and left atrial appendage easily lead to the formation of local microembolism (56). In addition, elevated platelet functions could be another reason for microembolism, which needs to be further investigated. It is currently believed that central system ischemia and hypoxia caused by PFO-related microembolism are the main mechanisms of nervous system diseases. Animal models have demonstrated that microembolism can cause pathological changes related to stroke and migraine when introduced into experimental animals (57, 58). Microembolization with larger sizes and higher doses can cause brain tissue injury, necrosis, neuroinflammation, and blood–brain barrier damage in mice (58). Microembolism of a smaller size and lower dose, especially air embolism, can cause cortical diffusion suppression (CSD) in mice, which is the hallmark pathological change of migraine with aura (57). It is worth mentioning that moderate microembolism can cause delayed stroke-like changes after inducing cortical diffusion inhibition, which may be related to the increased susceptibility to stroke caused by disseminated depolarization after migraine.
The hypothesis of abnormal metabolism of 5-HT is mainly related to the occurrence of migraine, which is characterized by increased production and decreased inactivation of 5-HT. Peripheral 5-HT production is mainly performed by platelets, and clinical studies have reported increased platelet activity in patients with PFO (59) and increased production of 5-HT (60). The inactivation process of peripheral 5-HT is mainly completed in the lung, while the atrial shunt of PFO is increased, leading to the decreased pulmonary circulation and decreased inactivation (60). However, the hypothesis of abnormal metabolism of 5-HT lacks sufficient evidence in animal experiments, and whether it is related to the occurrence of other neurological diseases is not clear.
The platelet-based mechanism hypothesis was reported in a recent article. Trabattoni et al. found that PFO had a prothrombotic potential sustained by an altered oxidative stress status in patients with migraine with aura, which could be associated with abnormal 5-HT levels (61). Furthermore, this association could be reverted after PFO closure by P2Y12-blockade. This hypothesis highlighted the role of oxidative stress in the pathology of PFO-related migraine. Future studies could provide a more detailed pathophysiologic pathway with basic experiments.
The genetic susceptibility hypothesis is still not mature. PFO closure is mediated by NOTCH signaling, particularly NOTCH3, a highly variable gene involved in CADASIL and migraine with aura (62). These genes could be related to the comorbidity of PFO and neurological diseases. However, the specific mechanism has not been confirmed by relevant tests.
In addition, whether there is a causal relationship between obstructive sleep apnea and PFO has not been determined. In terms of pathophysiology, they share a common mechanism, namely pulmonary circulation ischemia and hypoxia, which leads to a further increase in right-to-left shunting (63). Therefore, obstructive sleep apnea may aggravate PFO-related neurological disorders, which may be a new pathogenic mechanism of PFO, but no clinical studies have investigated this relationship.
With further research, proteomic-based studies have revealed more mechanistic hypotheses. The proteomic characteristics of serum albumin-binding proteins have recently been reported to change before and after PFO closure in patients with PFO-related stroke (64), which will be conducive to the exploration of targeted biomarkers. In the future, it may be more necessary to carry out multimodal in-depth studies combining peripheral blood and brain radiomics.
Secondary prevention is central to the management of patients with stroke. At present, specific preventive measures for PFO-related stroke are dominated by antiplatelet therapy (such as aspirin and clopidogrel), anticoagulation (mainly warfarin), and PFO closure. Several RCTs (The main information is shown in Table 2) provide clinical evidence for PFO-related stroke prevention.
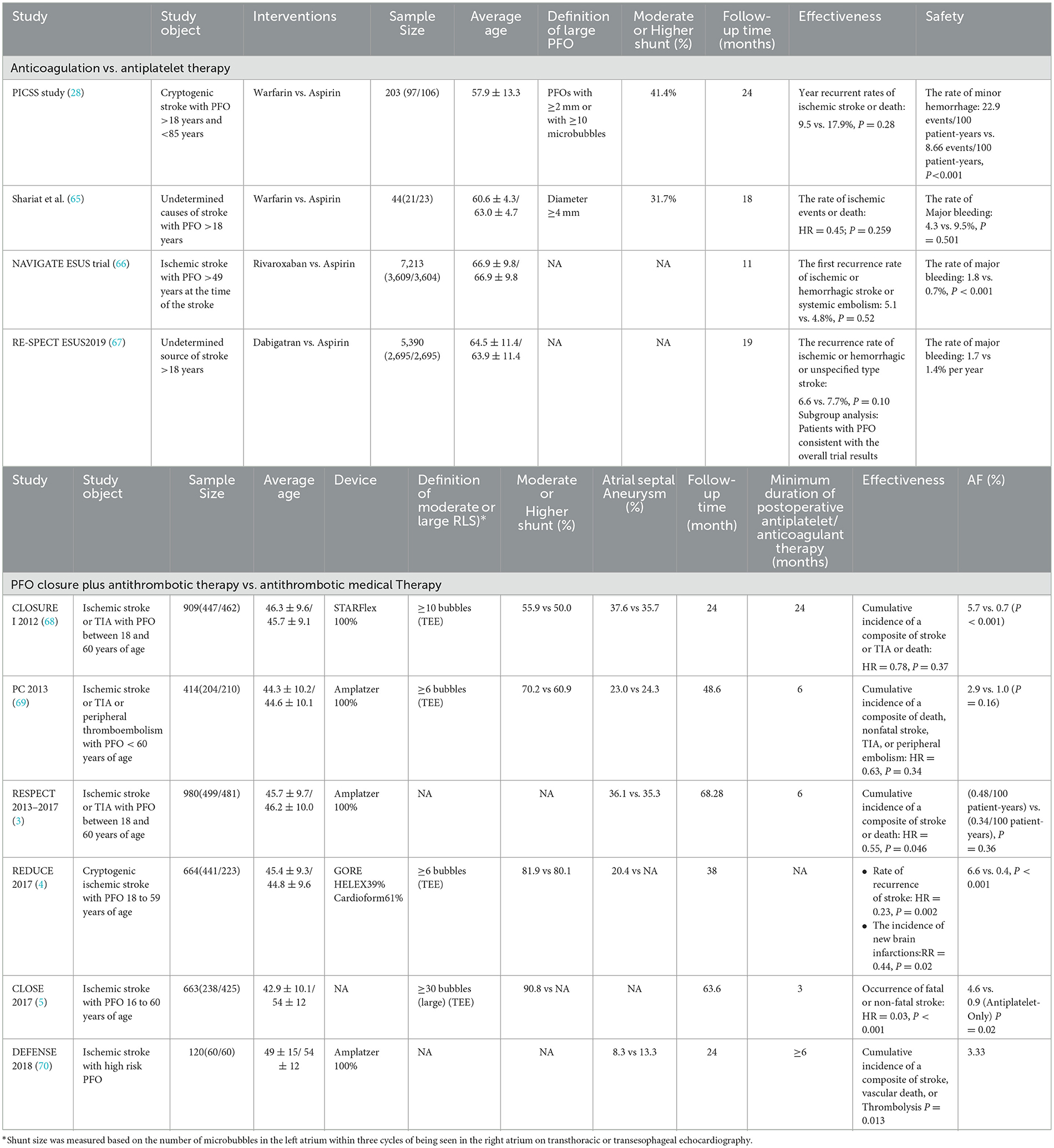
Table 2. Summary of important information from randomized controlled clinical trials for the treatment of PFO-related stroke.
According to the guidelines, antiplatelet or anticoagulant therapy may be recommended in patients with low or uncertain associations between PFO and stroke and patients with contraindications for PFO closure (e.g., atrial fibrillation) (6, 15), but which drug is the best is still a matter of debate. No benefit of anticoagulant therapy over antiplatelet therapy has been found in the four RCTs conducted so far (Table 2). Even the latest ATTICUS trial found no significant difference in the efficacy of apixaban compared to aspirin for ESUS (reported from the last ESOC congress in 2022). Contrary to the results of multiple RCTs, two meta-analyses showed that the recurrence rate of stroke in the anticoagulant group was lower than that in the antiplatelet group (71, 72). It is possible that anticoagulant therapy can effectively prevent deep vein thrombosis, and the mechanism of action is more consistent with the hypothesis of the microemboli pathogenesis of PFO-related stroke. However, because of the risk of bleeding, there is insufficient evidence to support anticoagulants. It is still necessary to further combine the characteristics of the disease and the mechanism of action to seek the best drug therapy.
The efficacy of PFO closure in preventing stroke recurrence has basically reached consensus, and PFO closure has been strongly recommended for patients aged 18–60 years. However, there are still some disputes and problems to be solved urgently. (I) Definition of the best benefit group: In view of the inconsistent definition of the high-risk population for PFO in the existing RCT studies (73), at present, the determination of the optimal benefit population is still controversial. The SCAI guidelines still use a risk of paradoxical embolism (RoPE) score >7 as the recommended criterion (6). However, the French consensus holds that RoPE scoring has certain limitations (74). In the future, various diagnostic techniques should be used to explore more focused benefit groups by combining morphological characteristics of PFO, related anatomical characteristics of the adjacent atrial septum, and disease history. In addition, it is unclear whether patients older than 60 will benefit. (II) Duration of postoperative medication: Dual antiplatelet therapy is usually recommended within 1–6 months after PFO closure to prevent device thrombotic complications, but there is no accepted guideline for the duration of medication. A meta-regression analysis found that the duration of dual antiplatelet therapy after PFO closure was significantly associated with the incidence of TIA (15), while the effect on the recurrence of postoperative stroke was unknown. It is necessary to confirm whether the occasional postoperative bleeding was related to postoperative medication. (III) Device selection and postoperative atrial fibrillation (AF): New-onset atrial fibrillation is a common postoperative complication of PFO closure, mostly occurring in the first 45 days after surgery. Two recent studies reported a significantly higher incidence of postoperative AF (37% and 20.9%, respectively) than routinely reported (<6%) (75, 76), suggesting that the incidence of postoperative AF may be underestimated. Enough attention should be paid to exploring the risk factors of AF. Guedeney et al. found it was associated with older age, male sex, and device size. In addition, new-onset atrial fibrillation may be caused by device stimulation and endothelialization (77, 78). Therefore, the choice of device is also particularly important. At present, circumstantial evidence suggests that the use of Amplatzer (AMP) and GORE HELEX/CARDIOFORM Septal Occluder (GORE) is more effective and has a lower risk of postoperative atrial fibrillation than StarFlex (79). However, there is a lack of original research evidence for direct comparison between devices, and future real-world studies to explore the impact of devices may be considered. In addition, studies have shown that patients with lower RoPE scores have a higher risk of atrial fibrillation (80), which indicates that the cause of postoperative atrial fibrillation is not only related to devices but also that occult atrial fibrillation may be the mechanism of occurrence. At present, to reduce the risk of postoperative atrial fibrillation, the American Academy of Neurology guidelines strongly recommend electrocardiogram testing and atrial fibrillation evaluation for all patients considering PFO closure (6, 81).
As with PFO-related stroke, PFO-related migraine is treated primarily with medication and PFO closure. Medication therapy includes thienopyridine antiplatelet agents (e.g., clopidogrel and prasugrel) (82, 83) and nonthienopyridine P2Y12 inhibitors (e.g., ticagrelor). Although studies have reported that clopidogrel resistance is widespread (40% of migraine patients do not respond to treatment), Trabattoni et al. found the mechanisms of action of P2Y12 antagonists in the treatment of migraine with aura. Specifically, P2Y12 antagonists effectively inhibited the oxidative stress-induced platelet-associated tissue factor (TF) and reactive oxygen species (ROS) expression and on microvesicle information (61). This will bring new hope for the drug treatment of PFO-related migraine with aura.
For PFO closure in migraine, the results of three RCTs performed negatively in primary outcomes (shown in Table 3), but the PRIMA trial and PREMIUM trial obtained positive results in the migraine with aura subgroup analysis (9, 10). Based on this, PFO closure has been recommended for migraine with aura in the European position paper, but there are still issues that need further clarity, including (I) an appropriate crowd. Patients with refractory migraine combined with moderate-to-large RLS shunts in PFO were included in the three RCTs. The clinical symptoms of these patients were more severe, and it was difficult to improve the symptoms, which could be seen from the MIST study. To exclude two patients with long migraine days in the PFO closure group, the results of the study changed from negative to positive. In addition, the treatment effect for migraine without aura is not clear. (II) Selection of outcome indicators. The primary outcomes selected by the three RCTs were heterogeneous. Due to the lack of more clear or objective outcome indicators, migraine assessment is mainly based on a scale (such as the HIT-6 questionnaire), and the scores are largely affected by the variability of pain tolerance in different individuals (84). Thus, the results may be due to chance. Therefore, further consideration is needed to select more sensitive and convincing end points.
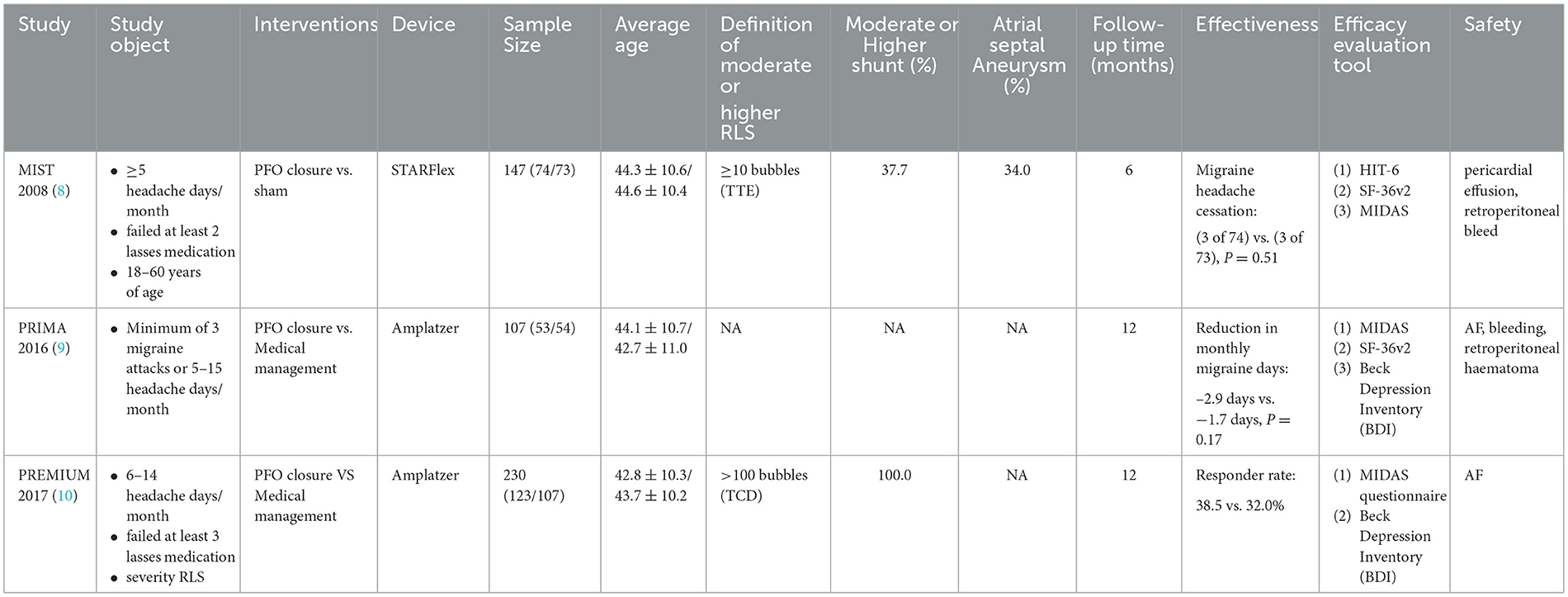
Table 3. Summary of important information from randomized controlled clinical trials for the treatment of PFO-related migraine.
Experience with PFO closure for other neurological diseases has been reported in observational studies (refer to Supplementary Table 1). The results of several clinical studies have demonstrated that PFO closure can improve sleep-disordered breathing and nocturnal oxygenation in patients with OSA. However, due to the small sample size, non-randomized controlled design, and low level of evidence, the effectiveness of PFO closure in improving clinical symptoms of OSA remains controversial (85, 86). In the field of decompression sickness research, a meta-analysis of four observational studies showed that PFO closure by divers can reduce the incidence of decompression sickness (87). However, the current international consensus primarily recommends behavioral and technical (B&T) changes to prevent DCS (88). If B&T changes are not possible or not effective, PFO closure can be proposed with shared decision-making underscoring the lack of evidence.
The prevalence of PFO in the general population is approximately 25%. Since the great shunt of RLS may be a high-risk factor for the occurrence and development of various PFO-related neurological diseases, it is necessary to conduct long-term follow-ups for such people to understand the law of the occurrence and development of diseases (such as exploring the peak age of various related neurological diseases). However, most of the current epidemiological studies are from patients with preexisting diseases, and few studies based on community populations may have a certain selection bias. There is still a lack of cluster random sampling-based representative samples to carry out prospective cohort studies. In addition, whether PFO has familial heritability is still unclear and needs to be further explored by pedigree studies. At the same time, studies have shown that the anatomical characteristics of PFO are not only a risk factor for the occurrence of diseases but also a key factor affecting the effect of PFO closure. Therefore, PFO-related studies are more dependent on accurate PFO diagnosis technology. It is urgent to develop more refined and standardized technical operation standards and norms to guide, and whether in vitro non-invasive testing techniques can be developed to improve patient compliance is still the focus of future research. For the correlation between PFO and various neurological diseases, since the mechanism hypothesis has not been confirmed and the therapeutic effect of PFO closure is still controversial, it is necessary to further explore the potential association based on basic and clinical research to scientifically guide the diagnosis and treatment of related diseases.
FS, LS, HLi, and LC contributed to the conception and design of this study. YT, LH, HLiu, XL, LL, and WY searched and collected literature and materials. FS and LS wrote the first draft of the manuscript. LH, HLiu, and XL wrote sections of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Grant No. 82271500).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1129062/full#supplementary-material
1. Kerut EK, Norfleet WT, Plotnick GD, Giles TD. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol. (2001) 38:613–23. doi: 10.1016/S0735-1097(01)01427-9
2. Alakbarzade V, Keteepe-Arachi T, Karsan N, Ray R, Pereira AC. Patent foramen ovale. Pract Neurol. (2020) 20:225–33. doi: 10.1136/practneurol-2019-002450
3. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. (2017) 377:1022–32. doi: 10.1056/NEJMoa1610057
4. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. (2017) 377:1033–42. doi: 10.1056/NEJMoa1707404
5. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. New England J Med. (2017) 377:1011–21. doi: 10.1056/NEJMoa1705915
6. Kavinsky CJ, Szerlip M, Goldsweig AM, Amin Z, Boudoulas KD, Carroll JD, et al. Scai guidelines for the management of patent foramen ovale. J Soc Cardiovasc Angiogr Intervent. (2022) 1:100039. doi: 10.1016/j.jscai.2022.100039
7. Vitarelli A, Mangieri E, Capotosto L, Tanzilli G, D'Angeli I, Toni D, et al. Echocardiographic findings in simple and complex patent foramen ovale before and after transcatheter closure. Eur Heart J Cardiovasc Imaging. (2014) 15:1377–85. doi: 10.1093/ehjci/jeu143
8. Dowson A, Mullen MJ, Peatfield R, Muir K, Khan AA, Wells C, et al. Migraine intervention with starflex technology (mist) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with starflex septal repair implant to resolve refractory migraine headache. Circulation. (2008) 117:1397–404. doi: 10.1161/CIRCULATIONAHA.107.727271
9. Mattle HP, Evers S, Hildick-Smith D, Becker WJ, Baumgartner H, Chataway J, et al. Percutaneous closure of patent foramen ovale in migraine with aura, a randomized controlled trial. Eur Heart J. (2016) 37:2029–36. doi: 10.1093/eurheartj/ehw027
10. Tobis JM, Charles A, Silberstein SD, Sorensen S, Maini B, Horwitz PA, et al. Percutaneous closure of patent foramen ovale in patients with migraine: the premium trial. J Am Coll Cardiol. (2017) 70:2766–74. doi: 10.1016/j.jacc.2017.09.1105
11. Homma S, Messé SR, Rundek T, Sun YP, Franke J, Davidson K, et al. Patent foramen ovale. Nat Rev Dis Primers. (2016) 2:15086. doi: 10.1038/nrdp.2015.86
12. Penther P. Patent Foramen Ovale: an anatomical study. Apropos of 500 consecutive autopsies. Archives des maladies du coeur et des vaisseaux. (1994)87:15–21.
13. Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am College Cardiol. (2006) 47:440–5. doi: 10.1016/j.jacc.2005.10.044
14. Di Tullio MR. Patent foramen ovale: echocardiographic detection and clinical relevance in stroke. J Am Soc Echocardiogr. (2010) 23:144–55. doi: 10.1016/j.echo.2009.12.008
15. Pristipino C, Sievert H, D'Ascenzo F, Louis Mas J, Meier B, Scacciatella P, et al. European position paper on the management of patients with patent foramen ovale. General approach left circulation thromboembolism. Eur Heart J. (2019) 40:3182–95.
16. Takaya Y, Watanabe N, Ikeda M, Akagi T, Nakayama R, Nakagawa K, et al. Importance of abdominal compression valsalva maneuver and microbubble grading in contrast transthoracic echocardiography for detecting patent foramen ovale. J Am Soc Echocardiogr. (2020) 33:201–6. doi: 10.1016/j.echo.2019.09.018
17. Mojadidi MK, Bogush N, Caceres JD, Msaouel P, Tobis JM. Diagnostic accuracy of transesophageal echocardiogram for the detection of patent foramen ovale: a meta-analysis. Echocardiography. (2014) 31:752–8. doi: 10.1111/echo.12462
18. Katsanos AH, Psaltopoulou T, Sergentanis TN, Frogoudaki A, Vrettou AR, Ikonomidis I, et al. Transcranial doppler vs. transthoracic echocardiography for the detection of patent foramen ovale in patients with cryptogenic cerebral ischemia: a systematic review and diagnostic test accuracy meta-analysis. Ann Neurol. (2016) 79:625–35. doi: 10.1002/ana.24609
19. Messé SR, Gronseth GS, Kent DM, Kizer JR, Homma S, Rosterman L, et al. Practice advisory update summary: patent foramen ovale and secondary stroke prevention: report of the guideline subcommittee of the american academy of neurology. Neurology. (2020) 94:876–85. doi: 10.1212/WNL.0000000000009443
20. Lee M, Oh JH. Echocardiographic diagnosis of right-to-left shunt using transoesophageal and transthoracic echocardiography. Open Heart. (2020) 7:e001150. doi: 10.1136/openhrt-2019-001150
21. Van H, Poommipanit P, Shalaby M, Gevorgyan R, Tseng CH, Tobis J. Sensitivity of transcranial doppler vs. intracardiac echocardiography in the detection of right-to-left shunt. JACC Cardiovascular Imaging. (2010) 3:343–8. doi: 10.1016/j.jcmg.2009.12.012
22. Zetola VF, Lange MC, Scavasine VC, Bazan R, Braga GP, Leite A, et al. Latin American consensus statement for the use of contrast-enhanced transcranial ultrasound as a diagnostic test for detection of right-to-left shunt. Cerebrovascular Dis. (2019) 48:99–108. doi: 10.1159/000503851
23. Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, Hijazi ZM, et al. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the american society of echocardiography and society for cardiac angiography and interventions. J Am Soc Echocardiography. (2015) 28:910–58. doi: 10.1016/j.echo.2015.05.015
24. Lange MC, Zétola VF, Piovesan EJ, Werneck LC. Valsalva maneuver procedures in the diagnosis of right-to-left shunt by contrast-enhanced transcranial doppler using agitated saline solution with blood as a contrast agent. Arq Neuropsiquiatr. (2010) 68:410–3. doi: 10.1590/S0004-282X2010000300016
25. Liu C, Lu T, Zhai NN, Bu N, Wang HQ, Chen MY, et al. Different valsalva manoeuvre procedures for the diagnosis of right-to-left shunt by contrast-transcranial doppler. Ultrasound Med Biol. (2017) 43:1716–21. doi: 10.1016/j.ultrasmedbio.2017.04.006
26. Moon J, Park Y, Park SJ, Oh PC, Jang AY, Chung WJ, et al. Comparison of intracardiac echocardiography and transesophageal echocardiography for image guidance in percutaneous patent foramen ovale closure. Medicina. (2020) 56:401. doi: 10.3390/medicina56080401
27. Han KN, Ma XT, Yang SW, Zhou YJ. Intracardiac echocardiography in the diagnosis and closure of patent foramen ovale. J Geriatric Cardiol. (2021) 18:697–701. doi: 10.11909/j.issn.1671-5411.2021.09.009
28. Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in cryptogenic stroke study. Circulation. (2002) 105:2625–31. doi: 10.1161/01.CIR.0000017498.88393.44
29. Weimar C, Holle DN, Benemann J, Schmid E, Schminke U, Haberl RL, et al. Current management and risk of recurrent stroke in cerebrovascular patients with right-to-left cardiac shunt. Cerebrovasc Dis. (2009) 28:349–56. doi: 10.1159/000229553
30. Serena J, Marti-Fàbregas J, Santamarina E, Rodríguez JJ, Perez-Ayuso MJ, Masjuan J, et al. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (Codicia) Study. Stroke. (2008) 39:3131–6. doi: 10.1161/STROKEAHA.108.521427
31. Mazzucco S, Li L, Binney L, Rothwell PM. Prevalence of patent foramen ovale in cryptogenic transient ischaemic attack and non-disabling stroke at older ages: a population-based study, systematic review, and meta-analysis. Lancet Neurol. (2018) 17:609–17. doi: 10.1016/S1474-4422(18)30167-4
32. Consoli D, Paciaroni M, Galati F, Aguggia M, Melis M, Malferrari G, et al. Prevalence of patent foramen ovale in ischaemic stroke in italy: results of sisifo study. Cerebrovascular Dis. (2015) 39:162–9. doi: 10.1159/000375152
33. Küper M, Rabe K, Holle D, Savidou I, Dommes P, Frings M, et al. Prevalence of cardiac right left shunts in migraine: a population-based case-control study. Neurol Sci. (2013) 34:205–8. doi: 10.1007/s10072-012-0986-0
34. Rundek T, Elkind MS, Di Tullio MR, Carrera E, Jin Z, Sacco RL, et al. Patent foramen ovale and migraine: a cross-sectional study from the northern manhattan study (Nomas). Circulation. (2008) 118:1419–24. doi: 10.1161/CIRCULATIONAHA.108.771303
35. Anzola GP, Meneghetti G, Zanferrari C, Adami A, Dinia L, Del Sette M. Is migraine associated with right-to-left shunt a separate disease? Results of the sam study. Cephalalgia Int J Headache. (2008) 28:360–6. doi: 10.1111/j.1468-2982.2008.01539.x
36. Jiang XH, Wang SB, Tian Q, Zhong C, Zhang GL Li YJ, et al. Right-to-left shunt and subclinical ischemic brain lesions in Chinese migraineurs: a multicentre MRI study. BMC Neurol. (2018) 18:18. doi: 10.1186/s12883-018-1022-7
37. Koutroulou I, Tsivgoulis G, Tsalikakis D, Karacostas D, Grigoriadis N, Karapanayiotides T. Epidemiology of patent foramen ovale in general population and in stroke patients: a narrative review. Front Neurol. (2020) 11:281. doi: 10.3389/fneur.2020.00281
38. Schwedt TJ, Demaerschalk BM, Dodick DW. Patent foramen ovale and migraine: a quantitative systematic review. Cephalalgia Int J Headache. (2008) 28:531–40. doi: 10.1111/j.1468-2982.2008.01554.x
39. Beelke M, Angeli S, Del Sette M, Gandolfo C, Cabano ME, Canovaro P, et al. Prevalence of patent foramen ovale in subjects with obstructive sleep apnea: a transcranial Doppler ultrasound study. Sleep Med. (2003) 4:219–23. doi: 10.1016/S1389-9457(02)00256-3
40. Guchlerner M, Kardos P, Liss-Koch E, Franke J, Wunderlich N, Bertog S, et al. Pfo and right-to-left shunting in patients with obstructive sleep apnea. J Clin Sleep Med. (2012) 8:375–80. doi: 10.5664/jcsm.2026
41. Billinger M, Zbinden R, Mordasini R, Windecker S, Schwerzmann M, Meier B, et al. Patent foramen ovale closure in recreational divers: effect on decompression illness and ischaemic brain lesions during long-term follow-up. Heart. (2011) 97:1932–7. doi: 10.1136/heartjnl-2011-300436
42. Purandare N, Welsh S, Hutchinson S, Riding G, Burns A, McCollum C. Cerebral emboli and paradoxical embolisation in dementia: a pilot study. Int J Geriatr Psychiatry. (2005) 20:12–6. doi: 10.1002/gps.1202
43. Purandare N, Oude Voshaar RC, Burns A, Velupandian UM, McCollum C. Paradoxical embolization: a potential cause of cerebral damage in Alzheimer's disease? Neurol Res. (2006) 28:679–84. doi: 10.1179/016164106X130425
44. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. (2014) 13:429–38. doi: 10.1016/S1474-4422(13)70310-7
45. Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
46. Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke. (2009) 40:2349–55. doi: 10.1161/STROKEAHA.109.547828
47. Hovsepian DA, Sriram N, Kamel H, Fink ME, Navi BB. Acute cerebrovascular disease occurring after hospital discharge for labor and delivery. Stroke. (2014) 45:1947–50. doi: 10.1161/STROKEAHA.114.005129
48. Chen L, Deng W, Palacios I, Inglessis-Azuaje I, McMullin D, Zhou D, et al. Patent foramen ovale (Pfo), stroke and pregnancy. J Investig Med. (2016) 64:992–1000. doi: 10.1136/jim-2016-000103
49. Safiri S, Pourfathi H, Eagan A, Mansournia MA, Khodayari MT, Sullman MJM, et al. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain. (2022) 163:e293–309. doi: 10.1097/j.pain.0000000000002275
50. Tang Y, Peng A, Peng B, He S, Zhao X, Zhu Y, et al. Association between patent foramen ovale and migraine without aura: a community-based cross-sectional study in China. BMJ Open. (2022) 12:e056937. doi: 10.1136/bmjopen-2021-056937
51. Wang SB, Liu KD, Yang Y, Li YJ, Hu MY, Lin P, et al. Prevalence and extent of right-to-left shunt on contrast-enhanced transcranial Doppler in Chinese patients with migraine in a multicentre case-control study. Cephalalgia Int J Headache. (2018) 38:690–6. doi: 10.1177/0333102417708203
52. Kim BJ, Kim NY, Kang DW, Kim JS, Kwon SU. Provoked right-to-left shunt in patent foramen ovale associates with ischemic stroke in posterior circulation. Stroke. (2014) 45:3707–10. doi: 10.1161/STROKEAHA.114.007453
53. He D, Li Q, Xu G, Hu Z, Li X, Guo Y, et al. Clinical and imaging characteristics of Pfo-related stroke with different amounts of right-to-left shunt. Brain Behav. (2018) 8:e01122. doi: 10.1002/brb3.1122
54. He Q, Zhang Y, Wang F, Li C, Guo R, Li X, et al. Impact of right-to-left shunt and transcatheter closure on the clinical features of migraine. Int J Neurosci. (2020) 130:270–5. doi: 10.1080/00207454.2019.1672681
55. Altamura C, Paolucci M, Costa CM, Brunelli N, Cascio Rizzo A, Cecchi G, et al. Right-to-left shunt and the clinical features of migraine with aura: earlier but not more. Cerebrovasc Dis. (2019) 47:268–74. doi: 10.1159/000501544
56. Rigatelli G, Zuin M, Fong A. Computational flow dynamic analysis of right and left atria in patent foramen ovale: potential links with atrial fibrillation. J Atr Fibrillation. (2018) 10:1852. doi: 10.4022/jafib.1852
57. Nozari A, Dilekoz E, Sukhotinsky I, Stein T, Eikermann-Haerter K, Liu C, et al. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann Neurol. (2010) 67:221–9. doi: 10.1002/ana.21871
58. Rapp JH, Hollenbeck K, Pan XM. An experimental model of lacunar infarction: embolization of microthrombi. J Vasc Surg. (2008) 48:196–200. doi: 10.1016/j.jvs.2008.01.038
59. Demir B, Caglar IM, Ungan I, Ugurlucan M, Tureli HO, Karakaya O. Mean platelet volume is elevated in patients with patent foramen ovale. Arch Med Sci. (2013) 9:1055–61. doi: 10.5114/aoms.2013.38687
60. Borgdorff P, Tangelder GJ. Migraine: possible role of shear-induced platelet aggregation with serotonin release. Headache. (2012) 52:1298–318. doi: 10.1111/j.1526-4610.2012.02162.x
61. Trabattoni D, Brambilla M, Canzano P, Becchetti A, Teruzzi G, Porro B, et al. Migraine in patients undergoing Pfo closure: characterization of a platelet-associated pathophysiological mechanism: the learner study. JACC Basic Translat Sci. (2022) 7:525–40. doi: 10.1016/j.jacbts.2022.02.002
62. Elliott GC, Gurtu R, McCollum C, Newman WG, Wang T. Foramen ovale closure is a process of endothelial-to-mesenchymal transition leading to fibrosis. PLoS ONE. (2014) 9:e107175. doi: 10.1371/journal.pone.0107175
63. Lau EM, Yee BJ, Grunstein RR, Celermajer DS. Patent foramen ovale and obstructive sleep apnea: a new association? Sleep Med Rev. (2010) 14:391–5. doi: 10.1016/j.smrv.2010.02.002
64. Lopez MF, Krastins B, Sarracino DA, Byram G, Vogelsang MS, Prakash A, et al. Proteomic signatures of serum albumin-bound proteins from stroke patients with and without endovascular closure of Pfo are significantly different and suggest a novel mechanism for cholesterol efflux. Clin Proteomics. (2015) 12:2. doi: 10.1186/1559-0275-12-2
65. Shariat A, Yaghoubi E, Farazdaghi M, Aghasadeghi K, Borhani Haghighi A. Comparison of medical treatments in cryptogenic stroke patients with patent foramen ovale: a randomized clinical trial. J Res Med Sci. (2013) 18:94–8.
66. Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. (2018) 378:2191–201. doi: 10.1056/NEJMoa1802686
67. Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. (2019) 380:1906–17. doi: 10.1056/NEJMoa1813959
68. Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. (2012) 366:991–9. doi: 10.1056/NEJMoa1009639
69. Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. (2013) 368:1083–91. doi: 10.1056/NEJMoa1211716
70. Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, et al. Cryptogenic stroke and high-risk patent foramen ovale: the defense-Pfo trial. J Am Coll Cardiol. (2018) 71:2335–42. doi: 10.1016/j.jacc.2018.02.046
71. Angelini F, Fortuni F, Tsivgoulis G, Agnelli G, Bocchino PP, Franchin L, et al. Comparison of antithrombotic strategies in patients with cryptogenic stroke and patent foramen ovale: an updated meta-analysis. Cardiovasc Drugs Therapy. (2021) 35:987–93. doi: 10.1007/s10557-020-07068-9
72. Mir H, Siemieniuk RAC, Ge L, Foroutan F, Fralick M, Syed T, et al. Patent foramen ovale closure, antiplatelet therapy or anticoagulation in patients with patent foramen ovale and cryptogenic stroke: a systematic review and network meta-analysis incorporating complementary external evidence. BMJ Open. (2018) 8:e023761. doi: 10.1136/bmjopen-2018-023761
73. Huber C, Wachter R, Pelz J, Michalski D. Current challenges and future directions in handling stroke patients with patent foramen ovale-a brief review. Front Neurol. (2022) 13:855656. doi: 10.3389/fneur.2022.855656
74. Mas JL, Derex L, Guérin P, Guillon B, Habib G, Juliard JM, et al. Transcatheter closure of patent foramen ovale to prevent stroke recurrence in patients with otherwise unexplained ischaemic stroke: expert consensus of the French neurovascular society and the French society of cardiology. Arch Cardiovasc Dis. (2019) 112:532–42. doi: 10.1016/j.acvd.2019.06.002
75. Krishnamurthy Y, Ben-Ami J, Robbins BT, Sommer RJ. Incidence and time course of atrial fibrillation following patent foramen ovale closure. Catheterization Cardiovas Intervent. (2022) 100:219–24. doi: 10.1002/ccd.30247
76. Guedeney P, Laredo M, Zeitouni M, Hauguel-Moreau M, Wallet T, Elegamandji B, et al. Supraventricular arrhythmia following patent foramen ovale percutaneous closure. JACC Cardiovasc Intervent. (2022) 15:2315–22. doi: 10.1016/j.jcin.2022.07.044
77. Vukadinović D, Scheller B, Ukena C, Ewen S, Mahfoud F, Böhm M. Device-related risk of atrial fibrillation after closure of patent foramen ovale: a systematic review and meta-analysis. Clin Res Cardiol. (2022) 111:583–7. doi: 10.1007/s00392-021-01964-2
78. Kent DM, Saver JL, Kasner SE, Nelson J, Carroll JD, Chatellier G, et al. Heterogeneity of treatment effects in an analysis of pooled individual patient data from randomized trials of device closure of patent foramen ovale after stroke. JAMA. (2021) 326:2277–86. doi: 10.1001/jama.2021.20956
79. Tsivgoulis G, Katsanos AH, Mavridis D, Frogoudaki A, Vrettou AR, Ikonomidis I, et al. Percutaneous patent foramen ovale closure for secondary stroke prevention: network meta-analysis. Neurology. (2018) 91:e8–e18. doi: 10.1212/WNL.0000000000005739
80. Strambo D, Sirimarco G, Nannoni S, Perlepe K, Ntaios G, Vemmos K, et al. Embolic stroke of undetermined source and patent foramen ovale: risk of paradoxical embolism score validation and atrial fibrillation prediction. Stroke. (2021) 52:1643–52. doi: 10.1161/STROKEAHA.120.032453
81. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
82. Sommer RJ, Nazif T, Privitera L, Robbins BT. Retrospective review of thienopyridine therapy in migraineurs with patent foramen ovale. Neurology. (2018) 91:1002–9. doi: 10.1212/WNL.0000000000006572
83. Guo Y, Shi Y, Zhu D, Liu R, Qi Y, Luo G. Clopidogrel can be an effective complementary prophylactic for drug-refractory migraine with patent foramen ovale. J Invest Med. (2020) 68:1250–5. doi: 10.1136/jim-2020-001342
84. He YD, Yan XL, Qin C, Zhang P, Guo ZN, Yang Y. Transcatheter patent foramen ovale closure is effective in alleviating migraine in a 5-year follow-up. Front Neurol. (2019) 10:1224. doi: 10.3389/fneur.2019.01224
85. Hoole SP, Hernández-Sánchez J, Davies WR, McNab DC, Calvert PA, Rana BS, et al. Effects of patent foramen ovale closure on obstructive sleep apnea syndrome: pcosa study. Can J Cardiol. (2017) 33:1708–15. doi: 10.1016/j.cjca.2017.09.005
86. Shaikh ZF, Jaye J, Ward N, Malhotra A, de Villa M, Polkey MI, et al. Patent foramen ovale in severe obstructive sleep apnea: clinical features and effects of closure. Chest. (2013) 143:56–63. doi: 10.1378/chest.12-0334
87. Abdelfattah OM, Sayed A, Elgendy IY, Munir M, Saleh Y, Kapadia SR, et al. Patent foramen ovale closure and decompression sickness among divers. Cardiovasc Revasculariz Med Including Mol Intervent. (2021). 40:160–2. doi: 10.1016/j.carrev.2021.11.017
Keywords: patent foramen ovale, stroke, migraine, obstructive sleep apnea, dementia, decompression sickness
Citation: Shi F, Sha L, Li H, Tang Y, Huang L, Liu H, Li X, Li L, Yang W, Kang D and Chen L (2023) Recent progress in patent foramen ovale and related neurological diseases: A narrative review. Front. Neurol. 14:1129062. doi: 10.3389/fneur.2023.1129062
Received: 08 January 2023; Accepted: 20 February 2023;
Published: 27 March 2023.
Edited by:
Gilles Montalescot, Sorbonne Universités, FranceReviewed by:
Thomas Wallet, Hôpitaux Universitaires Pitié Salpêtrière, FranceCopyright © 2023 Shi, Sha, Li, Tang, Huang, Liu, Li, Li, Yang, Kang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Chen, bGVpbGVpXzI1QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.