
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 06 April 2023
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1126444
This article is part of the Research Topic Neuromuscular Disorders and Peripheral Neuropathies – Case Report Collection 2022 View all 32 articles
 Yao Xie1
Yao Xie1 Lesang Li2
Lesang Li2 Le Xie1
Le Xie1 Junlin Jiang1
Junlin Jiang1 Ting Yao1
Ting Yao1 Guo Mao3
Guo Mao3 Shiliang Wang1
Shiliang Wang1 Anchao Lin1
Anchao Lin1 Jinwen Ge1
Jinwen Ge1 Dahua Wu1*
Dahua Wu1*Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an immune-mediated neuropathy. First-line treatments for CIDP include corticosteroids, intravenous immunoglobulin, and plasma exchange. However, the application is always limited by high costs, effectiveness, and adverse events. This study investigated a new potentially effective and safe therapeutic treatment to alleviate CIDP symptoms and improve the quality of life. In the present case, a 47-year-old rural woman presented with weakness and numbness of progressive extremities. She was diagnosed with CIDP based on abnormal cerebrospinal fluid and electromyography. The patient was treated with intravenous dexamethasone for 1 week and with Huangqi-Guizhi-Wuwu and Bu-Yang-Huan-Wu decoctions for 90 days. Surprisingly, after the treatment, the weakness and numbness were eliminated, and the quality of life improved. The varying INCAT, MRC, and BI scores also reflected the treatment effects. After 8 months of discharge, the symptoms did not relapse during the follow-up. We also searched “traditional Chinese medicine (TCM)” and “CIDP” in PubMed, EMBASE, the Web of Science, the Cochrane Library, the Chinese National Knowledge Infrastructure Databases, Wanfang Data, and the Chongqing Chinese Science and Technology Periodical Database. Finally, only ten studies were included in the literature review. Three studies were randomized controlled trials, and seven were case reports or case series. There were 419 CIDP patients, but all study sites were in China. Nine TCM formulas involving 44 herbs were reported, with Huang Qi (Astragalus membranaceus) being the most important herb. In conclusion, the case and literature demonstrated that TCM treatment might be a more effective, low-cost, and safe option for treating CIDP. Although these preliminary findings are promising, a larger sample size and higher-quality randomized clinical trials are urgently required to confirm our findings.
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a rare immune-mediated neuropathy in which an abnormal immune response causes peripheral nerve demyelination and axonal damage (1). It usually results in progressive weakness of the extremities with sensory dysfunction. Symptoms should appear for at least 2 months, and the course can be progressive or relapsing (2). The annual cost of treating CIDP illness can reach USD$ 116,330 per patient, and patients may also experience physical and psychosocial burdens such as impaired physical function, pain, depression, and adverse events (3).
The guidelines recommended intravenous immunoglobulin (IVIg), corticosteroids, and plasma exchange as first-line treatments for CIDP (4, 5). However, the potential side effects of corticosteroids, such as gastric ulceration, diabetes, arterial hypertension, osteoporosis, avascular necrosis of long bones, and cataracts, may outweigh the benefits. Moreover, long-term corticosteroid treatment may cause significant side effects (6). IVIg may result in rapid improvement in disability, but the benefit requires long-term repeated infusions, which carry a significant economic burden, particularly in developing countries (7, 8). Plasma exchange necessitates good vascular access and specialized equipment.
Although first-line treatments can improve symptoms in some patients, they also require ongoing maintenance therapy and the patient's willingness to tolerate the adverse effects or financial burden. The International CIDP Outcome Study (ICOS) revealed that only one-third of CIDP patients were in remission 1 year after the start of first-line treatment, and residual symptoms and deficits were common regardless of treatment (9). There is also a lack of formal efficacy evidence for alternative immunomodulatory agents (4, 10). Because of the side effects, high cost, and limited effectiveness of these treatments, there is an urgent need for new, effective, and safe CIDP treatments.
Traditional Chinese medicine (TCM) has a long history of boosting weakness and sensory dysfunction. TCM treatments have been widely used as a supplement to CIDP treatment in China. Many recent clinical trials have found that TCM treatments can improve clinical symptoms and the quality of life in patients with peripheral neuropathy (PN). Furthermore, TCM therapy can also enhance the sensory nerve conduction velocity (SNCV) and motor nerve conduction velocity (MNCV) in PN (11–13). The therapeutic mechanisms of TCM were elucidated by regulating inflammatory responses and repairing nerve injury (14).
However, no English literature reports the clinical efficacy of Chinese herbs for CIDP. In the present study, we attempted to use short-term corticosteroids combined with Chinese herbs on a patient with CIDP. Surprisingly, we identified that this therapeutic regimen completely relieved limb weakness and numbness and improved the quality of life. A literature review for TCM in CIDP was also conducted.
This case report was conducted in accordance with the Case Report (CARE) Guidelines (15).
On 25 November 2021, a 47-year-old rural woman complained of progressive weakness and numbness in her extremities. Initially, her symptoms were mild, causing her to experience difficulty grabbing objects or walking. After 3 months, the symptoms gradually worsened; she could not hold anything and only walked with assistance. On 23 February 2022, she was diagnosed with PN at another hospital. However, no IVIg or corticosteroids were administered to her, and her symptoms did not improve. Then, on 10 March 2022, she was transferred to our hospital (Hunan Hospital of Integrated Traditional Chinese and Western Medicine, Changsha, China). Upon arrival, the woman exhibited signs of both fatigue and limb paralysis, and she specifically emphasized that the numbness she was experiencing felt like she was wearing gloves and socks. She had no other particular medical history (Figure 1).

Figure 1. Timeline of the case report. HGW and BYHW decoctions, Huangqi-Guizhi-Wuwu and Bu-Yang-Huan-Wu decoctions; CSF, cerebrospinal fluid; EMG, electromyography.
The proximal end of the upper and lower limbs had grade 3 muscle strength, while the distal end of all limbs had grade 2 muscle strength. Muscular atrophy was observed in the bilateral first dorsal interosseous. Tendon reflexes decreased or disappeared, and limb muscular tension also decreased. Pain and temperature sensibility on the distal limbs decreased. Pyramidal tract signs were not elicited. There was no problem with eye montraosvement, pupil size, or swallowing function.
The cerebrospinal fluid (CSF) result revealed that the white blood cell count was one, and the micro-amount of protein was 652.6 mg/L (normal reference value: 150–450 mg/L). Other anti-ganglioside antibodies in CSF and serum were all negative, except for anti-sulfatide (aSF) IgG in CSF. The erythrocyte sedimentation rate (ESR) was 36 mm/h (normal reference value: 0–20 mm/h). Antinuclear antibodies, rheumatoid factors, HBsAg, HCV-Ab, rapid plasma regain, and HIV-Ab, were all negative. The routine blood, stool, and urine examinations, renal and liver functions, myocardial enzymes, and thyroid functions were all normal.
Multiple nerves in the upper and lower limbs were observed with decreased MNCV, SNCV, and amplitude during an electromyography (EMG) examination. Demyelination and axonal damage were identified in the bilateral median, ulnar, tibial, and peroneal nerves. Furthermore, no waveforms were elicited from the left peroneal motor nerve, the left median sensory nerve, or the bilateral superficial peroneal sensory nerves (Supplementary Table S1). The brain and spinal MRIs revealed no noticeable abnormalities.
Over 8 weeks, the patient developed progressive, symmetric upper- and lower-limb weakness and numbness and reduced tendon reflexes in all limbs. In addition, abnormal CSF and EMG were observed. The patient was diagnosed with CIDP using the EFNS/PNS consensus guideline (4).
Subsequently, intravenous dexamethasone (20 mg daily) was used for 1 week. Simultaneously, the patient was given TCM. The TCM formulas used in this case report were Huangqi-Guizhi-Wuwu and Bu-Yang-Huan-Wu decoctions (HGW and BYHW), which is composed of 12 herbs: Huang Qi (Astragalus membranaceus), 60 g; guizhi (Cassia Twig), 10 g; chishao (Radix Paeoniae Rubra), 10 g; danggui (Angelica sinensis), 10 g; chuangxiong (Ligusticum wallichii), 10 g; dilong (Lumbricus), 10 g; weilingxian (Radix Clematidis), 10 g; fuling (Poria Cocos), 10 g; baizhu (Rhizoma Atractylodis macrocephalae), 10 g; dangshen (Codonopsis pilosula), 10 g; Roucongrong (Cistanche), 15 g; and gancao (liquorice), 6 g. The quality of these herbs was consistent with the 2020 Chinese Pharmacopeia (16). The decoction was prepared with a standardized procedure, with each formula unit yielding 400 ml of decoction. The HGW and BYHW decoctions were prescribed for 90 days (200 ml orally two times daily).
Upon admission, the Inflammatory Neuropathy Cause and Treatment (INCAT) Disability Score was 6. The Medical Research Council (MRC) score was 32. The Barthel Index (BI) was 50. After 20 days of treatment, the MRC and BI scores increased to 38 and 60, respectively. However, the INCAT score remained unchanged. Moreover, 3 months later, the patient reported no limb weakness or numbness, and his limb muscle strength could reach grade 5. INCAT, MRC, and BI scores changed to 0, 60, and 100, respectively (Figure 2). After eight months of discharge, the symptoms did not recur during the follow-up.
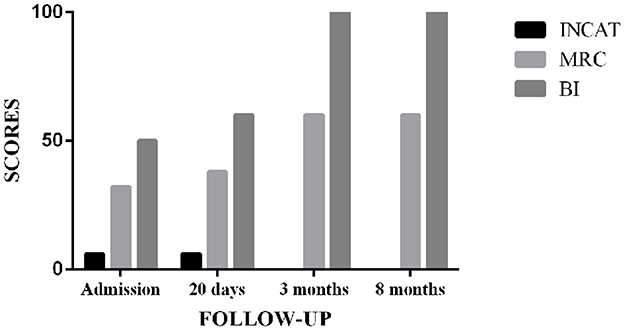
Figure 2. Outcome assessment for the CIDP patient in the follow-up. INCAT, Inflammatory Neuropathy Cause and Treatment; MRC, Medical Research Council; BI, Barthel Index.
Most importantly, no side effects were reported. It was unfortunate that she refused to accept an EMG to assess nerve recovery. The patient was delighted with the treatment outcome and was free of adverse events, and the cost was acceptable for a rural family in a developed country. She stated that, after coming to our hospital, her symptoms improved and her quality of life significantly improved. All follow-ups were conducted face to face.
From their inception to 11 November 2022, we screened the following databases for literature reviews: PubMed, EMBASE, Web of Science, the Cochrane Library, the Chinese National Knowledge Infrastructure Databases (CNKI), Wanfang Data, and the Chongqing Chinese Science and Technology Periodical Database (VIP). The search strategy included “traditional Chinese medicine” and “chronic inflammatory demyelinating polyradiculoneuropathy” (Supplementary Table S2).
Supplementary Figure S1 depicts the selection of studies. Finally, only 10 studies were included in the literature review (Table 1) (17–26). Three studies were randomized controlled trials, while seven were case reports or case series (Figure 3). The earliest study was conducted in 1998. A total of 419 CIDP patients were involved, but all study sites were in China. The age of CIPD cases ranged from 6 to 70 years, with the most enrolled patients being men. The duration of CIDP disease was 2.5 months to 3 years. Most studies did not report comorbidities.
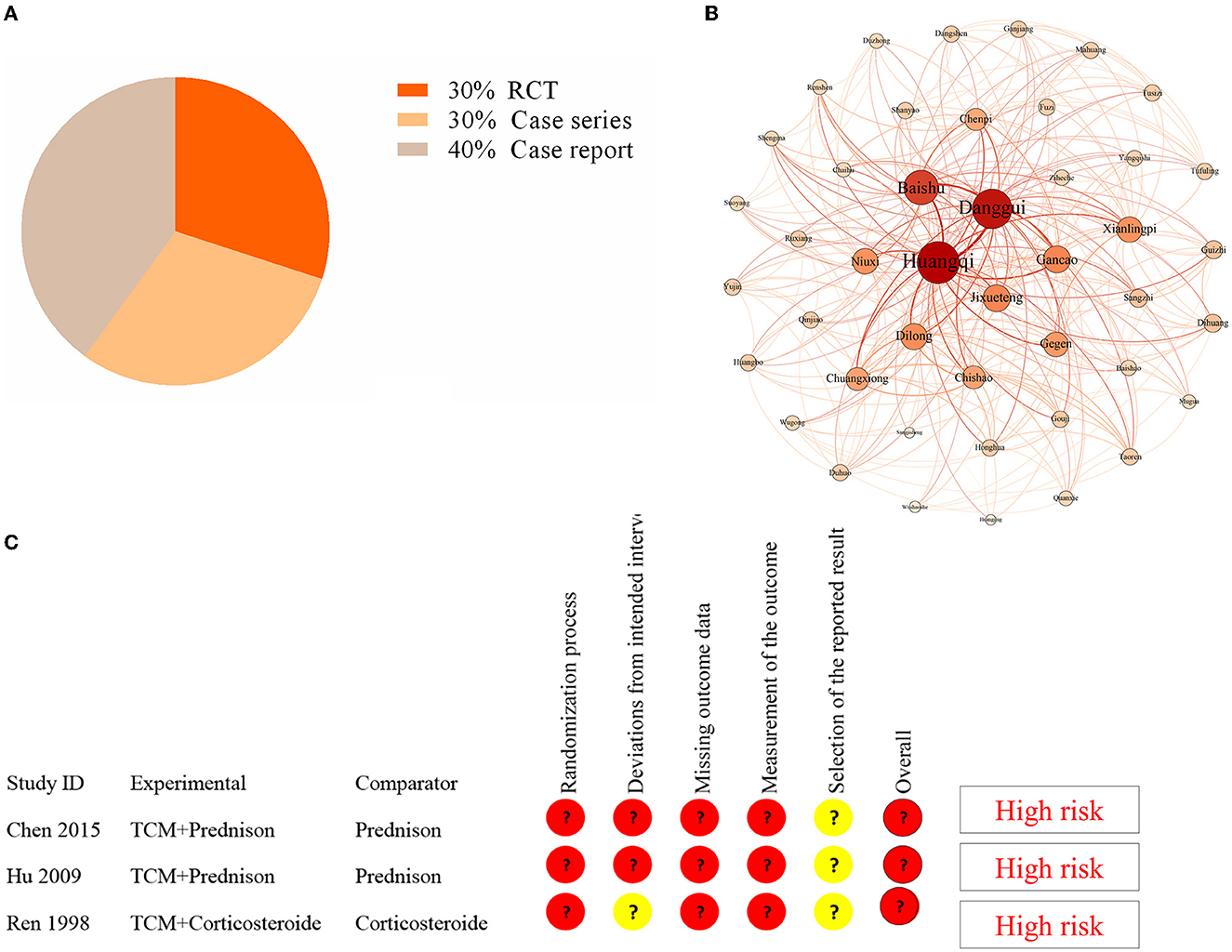
Figure 3. Analysis in the literature review. (A) The distribution of study design in the included studies. (B) Complex network analysis of selected TCM formulas from the included studies. (C) The results of the quality assessment of three RCT studies by using the risk bias assessment tool (RoB2).
TCM decoctions were used in all included studies, and the treatment duration ranged from 4 weeks to 6 months. In 60% of the studies, TCM was combined with corticosteroids or IVIg. The average duration of follow-up ranged from 6 months to 16 months. TCM therapies improved muscular strength, sensory disorders (SNCV and MNCV), daily life ability, and relapse rate. However, many studies did not report any adverse event.
There have been reports of nine TCM formulas involving 44 herbs (Supplementary Table S3). The most common herbs were Huang Qi (Astragalus membranaceus) (100%), Banggui (Angelica sinensis) (77.8%), Baizhu (Rhizoma Atractylodis macrocephalae) (44.4%), and Dilong (Lumbricus) (44.4%). We used Gephi 0.9.2 and SPSS Modeler 18.0 to perform a complex network analysis to find the core herb. We identified that Huang Qi was the core herb. The results revealed that the number of triangles, clustering coefficient, and eigenvector centrality were 291, 0.32, and 1, respectively (Figure 3). Furthermore, the dose of Huang Qi in various formulas ranged from 30 to 60 g.
Three RCT studies had sample sizes ranging from 40 to 115. The risk bias assessment tool (RoB2) (27) was used to assess the quality of three RCT studies. The randomization process, deviations from the intended intervention, missing outcome data, outcome measurement, and selection of the reported result were all evaluated. However, the bias in these studies indicated a high risk (Figure 3). Therefore, the efficacy of TCM for CIDP requires further validation in a high-quality RCT.
The typical CIDP is a chronic, progressive, monophasic, or recurrent demyelinating polyradiculoneuropathy with progressive weakness, sensory dysfunction, and absent or reduced tendon reflexes (28). CIDP variants include distal, multifocal, focal, motor, and sensory CIDP, all of which share the characteristics of demyelination and the immune therapy response (4). The diagnosis of CIDP is mainly based on clinical, electrodiagnostic, and laboratory features. However, the heterogeneity of CIDP creates challenges and delays in diagnosis. The ICOS study indicated that the time between disease onset and treatment was 11 months, and delayed-treated patients had a more severe disability (9).
The CIDP patient discussed in the above care report presented with symmetrical and progressive weakness, numbness, loss of tendon reflexes, abnormal protein levels in the CSF, and demyelination on the EMG. The progressive phase lasted more than 3 months. Furthermore, this patient responded to corticosteroid treatment, one of the characteristics of CIDP (29). Therefore, there was sufficient evidence to confirm a CIDP diagnosis for the patient. However, treatment was delayed due to economic hardship and initial mild symptoms.
Interestingly, the patient's CSF ASF-IgG was positive. One of the glycolipid antibodies against the peripheral nerve membrane surface is aSF. Sulfatides constitute a significant class of myelin-specific lipids, and their depletion can result in region-specific effects on non-compact myelin (30). Only 1–3% of CIDP patients had positive aSF (31, 32), raising questions about its diagnostic value. However, aSF-positive patients can exhibit typical clinical symptoms and respond well to immunomodulatory therapy (33), and this case supports the conclusion. The pathological mechanism and clinical significance of aSF should be investigated further.
Almost 20% of the CIDP patients did not respond to first-line therapy (5). Furthermore, 85.7% and 76.9% of CIDP patients experienced worsening after discontinuing IVIg and intravenous methylprednisolone, respectively (34). In addition, the long-term treatment course, high costs, inconvenience, and adverse events limit the application. There is a pressing need to explore alternative therapies that are both effective and safe in order to avoid those problems. Some studies have demonstrated that pulsed high-dose corticosteroid treatment might have fewer side effects and a faster response than daily oral corticosteroid treatment (35, 36). However, the evidence in the guideline is only low to moderate (4).
Meanwhile, some researchers have focused on subcutaneous immunoglobulin, which is well tolerated, effective, and affordable. However, the duration of maintenance treatment is at least 24 weeks, and local reactions at the infusion site are common (37). Better therapies must be explored further.
Traditional Chinese medicine and intravenous dexamethasone were used to treat this case. The intravenous dexamethasone course lasted only 1 week, and TCM was used for 3 months. With no adverse events and an extremely positive response, the combined treatment may be a more effective and safe option. The short-term corticosteroid treatment was well tolerated. Moreover, Chinese herbs were inexpensive, had few or no side effects, and were readily available. In the literature review, 50% of the studies (17, 20–22, and 25) used TCM in combination with corticosteroids, resulting in improvements in weakness, sensory disorder, daily life ability, and relapse rate. Therefore, it can potentially solve the CIDP dilemma, particularly in developing and rural countries. It is generally known that CIDP is an autoimmune disorder that affects peripheral nerve components. Macrophage and T-cell infiltration into peripheral nerves or nerve roots can result in demyelination and axonal damage (2). Corticosteroids are believed to treat CIDP by suppressing various immune components of its presumed autoimmune inflammatory process. Higher concentrations of corticosteroids also interact with DNA recognition sites to activate the transcription of anti-inflammatory genes (6).
Traditional Chinese medicine treatment of this reported CIDP case included Huangqi-Guizhi-Wuwu (HGW) and Bu-Yang-Huan-Wu (BYHW) decoctions, which are the most famous formulas in ancient TCM books. The network pharmacology analysis revealed that HGW could regulate cytokines and the inflammatory response, elements of the cytokine network, the T-cell receptor, NF-kappa B, HIF-1, and PI3K-Akt signaling pathways (14). BYHW can also regulate inflammatory cytokine production and facilitate axonal regeneration (38). Moreover, BYHW may assist in the timely interaction of regenerating axons and distal Schwann cells (39). Furthermore, some studies have indicated that quercetin, formononetin, isorhamnetin, and kaempferol were the main active compounds of HGW, while hydroxysafflor yellow A, astragaloside IV, ferulic acid, ligustrazine, and Z-ligustilide were mainly present in BYHW (40). In the literature review, the herb Huang Qi was used in all studies, with doses ranging from 30 to 60 g in different formulas, and it was used as the core herb in the complex network analysis. This female CIDP patient was also treated with 60 g Huang Qi. More than 100 compounds have been identified in Huang Qi, including flavonoids, saponins, polysaccharides, and amino acids (41). Huangqi can promote the development of immune organs, enhance mucosal immune function, increase the quantity and phagocytic capacity of innate immunity, promote the maturation and differentiation of acquired immunity cells, and improve antibody expression in acquired immunity (42). It appears promising to conduct extensive research on these formulas and Huang Qi for CIDP.
The Inflammatory Neuropathy Cause and Treatment Disability Score, MRC, and BI were used in this case to assess the treatment effect at multiple time points. The INCAT disability score is a valuable tool for evaluating upper and lower limb dysfunctions in inflammatory polyneuropathy studies, with the advantages of feasibility, high face validity, and high reliability (43). The MRC scale was used to assess muscle strength, with a score ranging from 0 for paralysis to 5 for normal and the sum of six pairs of muscles to represent a patient's overall strength. For inflammatory neuropathies, the MRC grading system was frequently used as an outcome measure (44). The BI is a reliable indicator of independence in daily activities. The scale described ten tasks based on the amount of time or assistance the patient requires, with lower scores indicating greater nursing dependency (45, 46). Surprisingly, after 3 months of TCM treatment, the INCAT, MRC, and BI scores were close to normal, and the treatment effect was sustainable. There was no relapse in the 8 months of follow-up.
Adverse effects were common with short-, medium-, and long-term immunotherapy (47). Adverse effects of corticosteroids include diabetes mellitus, hypertension, gastric ulceration, weight gain, osteopenia, and the long-term risk of severe side effects. Transient hypertension, headache, venous thrombosis, and acute renal dysfunction are the common side effects of IVIg. Adverse events associated with citrate use, difficulty with venous access, and hemodynamic changes have been reported in plasma exchange patients. In some cohorts, 60–70% of the CIDP patients reported problems with mobility, self-care, usual activities, pain, or discomfort in IVIg and methylprednisolone (9). It is inspiring to be here and see how TCM can be used to ensure efficacy and safety in the literature review and case report. Reducing adverse events is particularly important for CIDP patients.
To the best of our knowledge, to date, no English literature has reported the clinical efficacy of Chinese herbs for CIDP. This is the first article to summarize TCM trial or case report findings for CIDP. The article also presented potentially effective herbs or formulas containing specific ingredients. However, evidence of efficacy in case reports is lacking. Longer follow-up efficacy and an EMG exam should be investigated further. All the studies in the review were published in Chinese literature, had a single study site, and had low study quality. Large-sample, multi-center, high-quality studies are required to obtain a high level of evidence for TCM. Therefore, TCM has broad potential applications in CIDP because TCM is well tolerated, effective, inexpensive, and readily available.
In conclusion, the case demonstrated that TCM treatment combined with short-term corticosteroid therapy eliminated the weakness and numbness of CIDP and improved her quality of life. Huangqi (Astragalus membranaceus) may be important for regulating the inflammatory response. Although these preliminary findings are promising, larger sample sizes and higher-quality RCTs are urgently required to confirm our findings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
The idea for this research was proposed by YX. This protocol's drafting was undertaken by YX and LL. DW and JG revised the manuscript for intellectual content. Data collection and illness discussion were performed by YX, LX, JJ, GM, TY, SW, and AL. All authors approved the final version of the manuscript.
This research was supported by the General Project of the Health Commission of Hunan Province (No. 202218014505), the General Project of the Hunan Administration of Traditional Chinese Medicine (No. D2022060), the Hunan Province Clinical Medical Technology Innovation Guidance Project (No. 2021SK51001), and the Natural Science Foundation of Hunan Province (No. 2022JJ40241).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1126444/full#supplementary-material
1. Mathey EK, Park SB, Hughes RA, Pollard JD, Armati PJ, Barnett MH, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry. (2015) 86:973–85. doi: 10.1136/jnnp-2014-309697
2. Querol L, Lleixà C. Novel immunological and therapeutic insights in Guillain-Barré syndrome and CIDP. Neurotherapeutics. (2021) 18:2222–35. doi: 10.1007/s13311-021-01117-3
3. Querol L, Crabtree M, Herepath M, Priedane E, Viejo VI, Agush S, et al. Systematic literature review of burden of illness in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol. (2021) 268:3706–16. doi: 10.1007/s00415-020-09998-8
4. Van den Bergh P, van Doorn PA, Hadden R, Avau B, Vankrunkelsven P, Allen JA, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint Task Force-Second revision. Eur J Neurol. (2021) 28:3556–83. doi: 10.1111/ene.14959
5. Chinese Chinese Society of Neurology Peripheral Peripheral Neuropathy Collaboration Group of Chinese Society of Neurology Chinese Chinese Society of Electromyography and Clinical Neuroelectrophysiology Chinese Chinese Society of Neuromuscular Disease. Chinese guidelines for diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy 2019. Chin J Neurol. (2019) 52:883–8. doi: 10.3760/cma.j.issn.1006-7876.2019.11.003
6. Hughes RA, Mehndiratta MM, Rajabally YA. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. (2017) 11:D2062. doi: 10.1002/14651858.CD002062.pub4
7. Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. (2013) 2013:D1797. doi: 10.1002/14651858.CD001797.pub3
8. Kuwabara S, Mori M, Misawa S, Suzuki M, Nishiyama K, Mutoh T, et al. Intravenous immunoglobulin for maintenance treatment of chronic inflammatory demyelinating polyneuropathy: a multicentre, open-label, 52-week phase III trial. J Neurol Neurosurg Psychiatry. (2017) 88:832–8. doi: 10.1136/jnnp-2017-316427
9. Bus S, Broers MC, Lucke IM, Bunschoten C, van Lieverloo G, Adrichem ME, et al. Clinical outcome of CIDP one year after start of treatment: a prospective cohort study. J Neurol. (2022) 269:945–55. doi: 10.1007/s00415-021-10677-5
10. Hughes R, Dalakas MC, Merkies I, Latov N, Léger JM, Nobile-Orazio E, et al. Oral fingolimod for chronic inflammatory demyelinating polyradiculoneuropathy (FORCIDP Trial): a double-blind, multicentre, randomised controlled trial. Lancet Neurol. (2018) 17:689–98. doi: 10.1016/S1474-4422(18)30202-3
11. Li Z, Jin H, Yan Q, Sun L, Wasan HS, Shen M, et al. The method of activating blood and dredging collaterals for reducing Chemotherapy-Induced peripheral neuropathy: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2019) 2019:1029626. doi: 10.1155/2019/1029626
12. Pang B, Zhao TY, Zhao LH, Wan F, Ye R, Zhou Q, et al. Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy: a meta-analysis of 16 randomized controlled trials. Neural Regen Res. (2016) 11:1347–58. doi: 10.4103/1673-5374.189202
13. Li QY, Cai FH, Lu Y, Liu H, Wang X, Li FL, et al. External treatment with Chinese herbal medicine for chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Front Pharmacol. (2022) 13:764473. doi: 10.3389/fphar.2022.764473
14. Gu JL, Wei GL, Ma YZ, Zhang JZ Ji Y, Li LC, et al. Exploring the possible mechanism and drug targets of Huang-Qi-Gui-Zhi-Wu-Wu decoction for the treatment of Chemotherapy-Induced peripheral neuropathy on network pharmacology. Evid Based Complement Alternat Med. (2020) 2020:2363262. doi: 10.1155/2020/2363262
15. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
16. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China. Beijing: China Medical Science and Technology Press (2020).
17. Sha D, Lingjuan H, Qiliang X, Suicheng X. TCM in the treatment of childhood chronic inflammatory demyelinating polyneuropathy: a case report. World Chin Med. (2018) 13:138–41. doi: 10.3969/j.issn.1673-7202.2018.01.035
18. Jinliang C, Xiaoli Y, Junyong HU. Therapy of integrated medicine in the treatment of chronic inflammatory demyelinating polyneuropathy for 58 cases. Chin Med Modern Distance Educ China. (2015) 13:71–3. doi: 10.3969/j.issn.1672-2779.2015.17.036
19. Kuang S, Liu C. Discussion on treating chronic Guillain-Barre syndrome with integrated traditional and western medicine. Shanxi J Trad Chin Med. (2015) 31:1–3. doi: 10.3969/j.issn.1000-7156.2015.06.001
20. Wei Y, Zhou Z, Liu G, Tang C. Eighteen cases with chronic inflammatory demyelinating polyradiculoneuropathy were treated with traditional Chinese medicine. Hebei J Trad Chin Med. (2014): 537-8, 539.
21. Yu S, Yang S, Zheng M. Summary of 7 cases with chronic Guillain-Barre syndrome treated with traditional Chinese medicine therapy. Hunan J Tradit Chin Med. (2014) 30:29–30. doi: 10.16808/j.cnki.issn1003-7705.2014.02.015
22. Liu M, Zheng S, Liu J, A. case of chronic Guillain-Barre syndrome was treated with acupuncture and traditional Chinese medicine. Jiangxi J Tradit Chin Med. (2012) 43:57–8. doi: 10.3969/j.issn.0411-9584.2012.04.030
23. Hu J, Chen J, Zhang Z. Clinical observation on treatment of 30 patients with chronic inflammatory demyelinating polyneuropathy by Guilong Tongluo capsule combined with prednisone. Chin J Integr Med. (2009) 29:649–51. doi: 10.3321/j.issn:1003-5370.2009.07.021
24. Li W, Fan X. Analysis of 15 cases of chronic Guillain-Barre syndrome treated with integrated traditional Chinese and western medicine. Shanxi Med J. (2007) 637.
25. Lin X, Wu X, Zhong J. Effect of Buyang-Huanwu decoction combined with gamma globulin therapy on 11 cases of chronic Guillain-Barre syndrome. J New Chin Med. (2005) 37:50–1. doi: 10.3969/j.issn.0256-7415.2005.03.023
26. Ren M, Yang R, Wang X, Hu J. Clinical study of integrated traditional Chinese and western medicine in the treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Chin J Med. (1998) 55–7.
27. Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
28. Bunschoten C, Jacobs BC, Van den Bergh P, Cornblath DR, van Doorn PA. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. (2019) 18:784–94. doi: 10.1016/S1474-4422(19)30144-9
29. Shije J, Brannagan TR. Chronic inflammatory demyelinating polyradiculoneuropathy. Semin Neurol. (2019) 39:596–607. doi: 10.1055/s-0039-1693008
30. Palavicini JP, Wang C, Chen L, Ahmar S, Higuera JD, Dupree JL, et al. Novel molecular insights into the critical role of sulfatide in myelin maintenance/function. J Neurochem. (2016) 139:40–54. doi: 10.1111/jnc.13738
31. Nobile-Orazio E, Giannotta C, Briani C. Anti-ganglioside complex IgM antibodies in multifocal motor neuropathy and chronic immune-mediated neuropathies. J Neuroimmunol. (2010) 219:119–22. doi: 10.1016/j.jneuroim.2009.11.012
32. Giannotta C, Di Pietro D, Gallia F, Nobile-Orazio E. Anti-sulfatide IgM antibodies in peripheral neuropathy: to test or not to test? Eur J Neurol. (2015) 22:879–82. doi: 10.1111/ene.12658
33. Klehmet J, Märschenz S, Ruprecht K, Wunderlich B, Büttner T, Hiemann R, et al. Analysis of anti-ganglioside antibodies by a line immunoassay in patients with chronic-inflammatory demyelinating polyneuropathies (CIDP). Clin Chem Lab Med. (2018) 56:919–26. doi: 10.1515/cclm-2017-0792
34. Nobile-Orazio E, Cocito D, Jann S, Uncini A, Messina P, Antonini G, et al. Frequency and time to relapse after discontinuing 6-month therapy with IVIg or pulsed methylprednisolone in CIDP. J Neurol Neurosurg Psychiatry. (2015) 86:729–34. doi: 10.1136/jnnp-2013-307515
35. van Schaik IN, Eftimov F, van Doorn PA, Brusse E, van den Berg LH, van der Pol WL, et al. Pulsed high-dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double-blind, randomised, controlled trial. Lancet Neurol. (2010) 9:245–53. doi: 10.1016/S1474-4422(10)70021-1
36. Eftimov F, Vermeulen M, van Doorn PA, Brusse E, van Schaik IN. Long-term remission of CIDP after pulsed dexamethasone or short-term prednisolone treatment. Neurology. (2012) 78:1079–84. doi: 10.1212/WNL.0b013e31824e8f84
37. van Schaik IN, Bril V, van Geloven N, Hartung HP, Lewis RA, Sobue G, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. (2018) 17:35–46. doi: 10.1016/S1474-4422(17)30378-2
38. Kim KJ, Namgung U. Facilitating effects of Buyang Huanwu decoction on axonal regeneration after peripheral nerve transection. J Ethnopharmacol. (2018) 213:56–64. doi: 10.1016/j.jep.2017.10.036
39. Kim KJ, Hwang J, Park JY, Namgung U. Augmented Buyang Huanwu Decoction facilitates axonal regeneration after peripheral nerve transection through the regulation of inflammatory cytokine production. J Ethnopharmacol. (2020) 260:113063. doi: 10.1016/j.jep.2020.113063
40. Wang K, Lei L, Cao J, Qiao Y, Liang R, Duan J, et al. Network pharmacology-based prediction of the active compounds and mechanism of Buyang Huanwu Decoction for ischemic stroke. Exp Ther Med. (2021) 22:1050. doi: 10.3892/etm.2021.10484
41. Fu J, Wang Z, Huang L, Zheng S, Wang D, Chen S, et al. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother Res. (2014) 28:1275–83. doi: 10.1002/ptr.5188
42. Chen Z, Liu L, Gao C, Chen W, Vong CT, Yao P, et al. Astragali Radix (Huangqi): a promising edible immunomodulatory herbal medicine. J Ethnopharmacol. (2020) 258:112895. doi: 10.1016/j.jep.2020.112895
43. Breiner A, Barnett C, Bril V. INCAT disability score: a critical analysis of its measurement properties. Muscle Nerve. (2014) 50:164–9. doi: 10.1002/mus.24207
44. Vanhoutte EK, Faber CG, Merkies IS. 196Th ENMC international workshop: outcome measures in inflammatory peripheral neuropathies 8–10 February 2013, Naarden, the Netherlands. Neuromuscul Disord. (2013) 23:924–33. doi: 10.1016/j.nmd.2013.06.006
45. Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. (1988) 10:61–3. doi: 10.3109/09638288809164103
46. Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: development, properties, and application. Stroke. (2011) 42:1146–51. doi: 10.1161/STROKEAHA.110.598540
Keywords: chronic inflammatory demyelinating polyradiculoneuropathy, traditional Chinese medicine, case report, review, Astragalus membranaceus
Citation: Xie Y, Li L, Xie L, Jiang J, Yao T, Mao G, Wang S, Lin A, Ge J and Wu D (2023) Beneficial effects and safety of traditional Chinese medicine for chronic inflammatory demyelinating polyradiculoneuropathy: A case report and literature review. Front. Neurol. 14:1126444. doi: 10.3389/fneur.2023.1126444
Received: 17 December 2022; Accepted: 09 March 2023;
Published: 06 April 2023.
Edited by:
Giovanni Meola, University of Milan, ItalyReviewed by:
Xinxing Lai, Dongzhimen Hospital, Beijing University of Chinese Medicine, ChinaCopyright © 2023 Xie, Li, Xie, Jiang, Yao, Mao, Wang, Lin, Ge and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dahua Wu, d2RoODM4NjkyQHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.