
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 05 May 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1122744
This article is part of the Research Topic Current issues in diagnosis and therapy of cerebral amyloid angiopathy View all 5 articles
 Yu-jia Jin1†
Yu-jia Jin1† Jia-wen Li1†
Jia-wen Li1† Jian Wu1
Jian Wu1 Yu-hui Huang2
Yu-hui Huang2 Kai-cheng Yang1
Kai-cheng Yang1 Hong-na An3
Hong-na An3 Chang-zheng Yuan2
Chang-zheng Yuan2 Feng Gao1*
Feng Gao1* Lu-sha Tong1*
Lu-sha Tong1*Background: Previous studies have shown that cortical superficial siderosis (cSS) can increase hematoma volume and predict poor outcomes following primary intracerebral hemorrhage (ICH).
Objective: We aimed to determine whether a large hematoma volume was the essential factor contributing to worse outcomes of cSS.
Methods: Patients with spontaneous ICH underwent a CT scan within 48 h after ictus. Evaluation of cSS was performed using magnetic resonance imaging (MRI) within 7 days. The 90-day outcome was assessed using the modified Rankin Scale (mRS). In addition, we investigated the correlation between cSS, hematoma volume, and 90-day outcomes using multivariate regression and mediation analyses.
Results: Among the 673 patients with ICH [mean (SD) age, 61 (13) years; 237 female subjects (35.2%); median (IQR) hematoma volume, 9.0 (3.0–17.6) ml], 131 (19.5%) had cSS. There was an association between cSS and larger hematoma volume (β = 4.449, 95% CI 1.890–7.009, p < 0.001) independent of hematoma location and was also related to worse 90-day mRS (β = 0.333, 95% CI 0.008–0.659, p = 0.045) in multivariable regression. In addition, mediation analyses revealed that hematoma volume was an essential factor mediating the effect of cSS on unfavorable 90-day outcomes (proportion mediated:66.04%, p = 0.01).
Conclusion: Large hematoma volume was the major charge of directing cSS to worse outcomes in patients with mild to moderate ICH, and cSS was related to a larger hematoma in both lobar and non-lobar areas.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT04803292, identifier: NCT04803292.
Cortical superficial siderosis (cSS) has been defined as the previous extravasation of blood on the superficial layers of the cortex or subarachnoid space, which can be detected as a hypointense curvilinear signal on the susceptibility-weighted image (SWI) on magnetic resonance imaging (MRI) (1, 2). Observations from large cohorts revealed that patients with cSS were more likely to have large intracerebral hemorrhage (ICH) volume and hematoma expansion, especially in the lobar area. cSS was also reported to correlate with poor outcomes after ICH (1, 3–8). However, whether cSS leads to worse outcomes solely by itself or through a specific pathway (especially hematoma volume) and whether cSS-related enlarged hematoma is restricted to the lobar area because of the origin of cSS remain unknown. A better understanding of this relationship could provide evidence of the latent mechanisms by which cSS induces hemorrhage. In this context, we extrapolated several factors that might be the essential factors mediating the association between cSS and worse outcomes, and we tested the relationship between cSS and hematoma volume separately in lobar and non-lobar areas.
This study collected data from consecutively enrolled patients with ICH from a single center (Department of Neurology, the Second Affiliated Hospital of Zhejiang University, Hangzhou) between November 2016 and February 2021 (ClinicalTrials.gov Identifier: NCT 04803292). The cohort included patients who had an initial diagnosis of primary ICH in the emergency room (according to their past medical history and emergency examinations) and did not undergo emergency surgery. This study was approved by the Human Ethics Committee of the Second Affiliated Hospital of Zhejiang University and followed the tenets specified in the 1975 Declaration of Helsinki. Written informed consent was obtained from all participants.
Patients ≥ 18 years of age admitted to the hospital within 48 h of primary ICH were included. Suspected secondary ICH caused by trauma, aneurysm or vascular malformation, systemic disease-related coagulopathy, hemorrhagic venous infarct, or hemorrhagic transformation of ischemic stroke was omitted. The diagnoses were determined by experienced neurologists based on medical history and neuroimaging findings. Patients with isolated intraventricular hemorrhage (IVH) and those who underwent neurosurgical procedures were also excluded.
We included patients who underwent a 3.0 T MRI examination with SWI sequences after ICH ictus for this study focusing on cSS. In addition, patients with a modified Rankin scale (mRS) score of ≥3 before ICH (according to medical records or inquiries to relatives) were excluded (Figure 1).
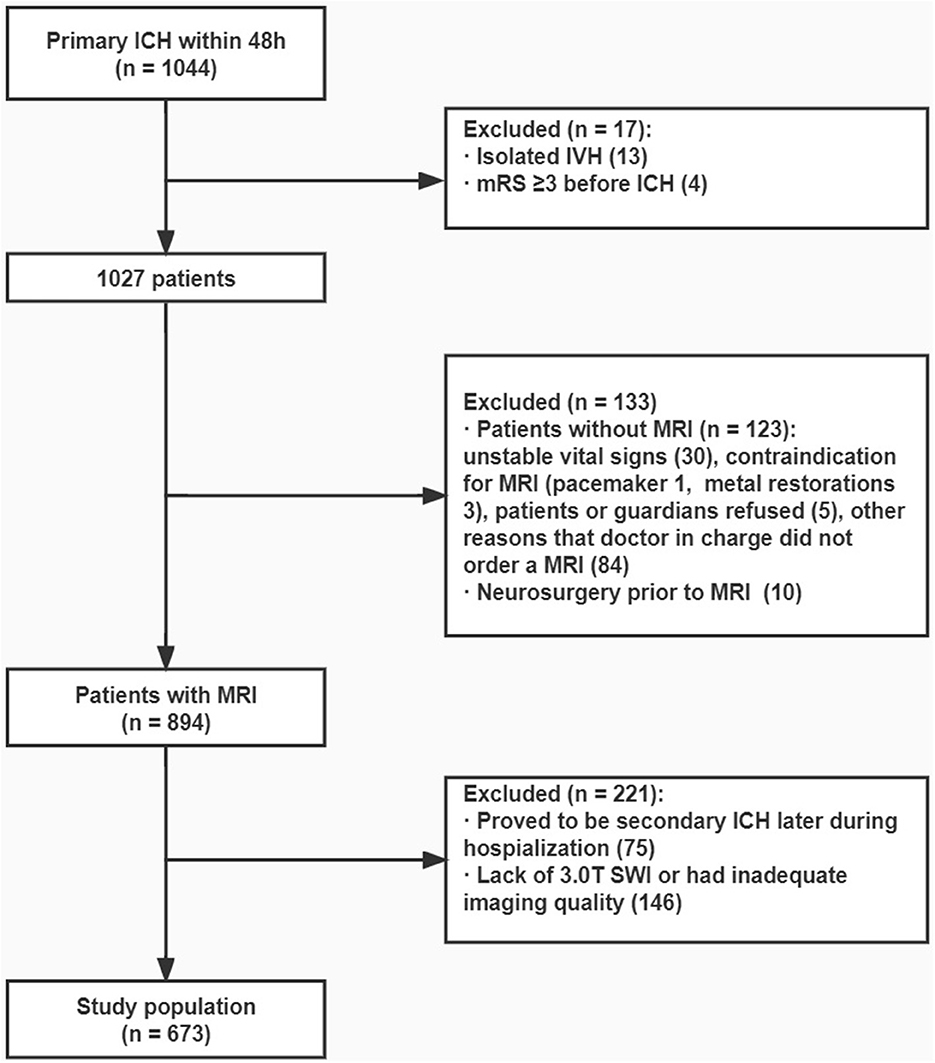
Figure 1. Flowchart. From the original cohort of 1,044 patients with primary ICH that occurred within 48 h, 673 patients were finally included in analysis.
We prospectively collected the following information: (i) demographic characteristics, including age and sex; (ii) presence of vascular risk factors and medical history (smoking, alcohol intake, hypertension, diabetes mellitus, coronary heart disease, ischemic stroke or TIA, ICH) according to the description from the patients or their relatives, or medical records using previously published definitions; (9) (iii) atrial fibrillation based on the electrocardiogram obtained during hospitalization or previous medical records; (iv) medication at the baseline, including antiplatelet drugs, anticoagulant drugs, and statins; and (v) Glasgow Coma Score (GCS) on admission.
Patients underwent a non-contrast CT scan at admission within 48 h after ictus on multidetector row scanners (Optima CT540, General Electric Healthcare, Connecticut, USA; or SOMATOM Sensation 16, Siemens, Germany) with the following parameters: slice thickness, 5 mm; 120 kV, and 100–300 mAs. Images were reviewed in the Digital Imaging and Communications in Medicine (DICOM) format by two board-certified neurologists blinded to the data and not involved in clinical management. Hematoma volume was calculated per protocol on the baseline and stability CT using semi-automatic software (ITK-SNAP software, University of Pennsylvania, Philadelphia, USA; www.itksnap.org). IVH was defined as an intraventricular hyperdense image that was not attributable to the choroid plexus or calcification and was not included in the hematoma volume. Lobar ICH was defined as a hematoma restricted to the frontal, temporal, parietal, or occipital areas. The origin of the hemorrhage appeared at the cortical and subcortical junctions.
All patients underwent an MRI during hospitalization. If data from more than one MRI scan were available, the earliest scan was chosen to retrieve the data (median delay, 5 days; IQR, 4–7 days after ICH). MRI was performed using a 3.0-Telsa scanner (Signa HDxt, GE Healthcare, USA) with a standardized protocol consisting of at least T1, T2, and SWI sequences (axial acquisition plane 3.0 T, TR 5,200 ms, TE 75 ms, b = 0/1,000 s/mm2, 6-mm slice thickness, 0.5-mm gap, FOV 240 × 240 mm). In total, two board-certified neurologists assessed the presence of cSS, defined as a curvilinear signal loss on SWI in compliance with the gyral cortical surface within the subarachnoid space, away from at least two sulci of the macrohemorrhage with no corresponding signal hyperintensity on the baseline CT scan (2, 8). We categorized cSS as focal (restricted to ≤3 sulci) or disseminated (affecting at least four sulci) (2).
We also recorded the presence of cerebral microbleeds (CMB), defined as round or ovoid signal voids of 2 and 10 mm in diameter, with associated blooming on the SWI sequences (10–12). CMB locations were categorized as lobar, deep structures (basal ganglia, thalamus, and internal capsule), the brainstem, or the cerebellum. Probable cerebral amyloid angiopathy (CAA) was evaluated according to the modified Boston criteria (13). We evaluated white matter hyperintensity (WMH) visually on axial FLAIR images using the 4-point Fazekas' rating scale. The total WMH score was the addition of the scores for periventricular white matter and deep white matter hyperintensities (11).
The 90-day mRS was obtained through face-to-face or telephone interviews with the patients using standardized questionnaires based on the Rankin Focused Assessment, as described previously (14). At least one telephone number was collected on admission to ensure maximum follow-up integrity, and most patients had two telephone numbers recorded (telephone number of relatives). Patients were defined as lost to follow-up if five or more calls could not contact them. Furthermore, we provided free visits to the Neurology Clinic of the Second Affiliated Hospital of Zhejiang University, School of Medicine, for those unwilling to share recovery conditions. In addition, as the only regional advanced stroke center responsible for quality control, we maintained good contact with other hospitals at different levels in Zhejiang Province. Additionally, we entrusted local doctors with follow-up if necessary.
Continuous variables are presented as mean (SD) or median (IQR) and were compared using the t-test or the Mann–Whitney U-test. Categorical variables were presented as counts (percent) and were analyzed using the Pearson χ2 test or Fisher's exact test, as appropriate. Clinical data (demographics, vascular risk factors, medical history, medication and GCS on admission, and imaging characteristics) were compared between the two groups of patients (with vs. without cSS). The same comparisons were made between the patients with focal and disseminated cSS. Hematoma volume and 90-day mRS score were calculated as continuous variables (15). According to published literature and pathophysiological consideration, we first selected clinical information including age, sex, hypertension, diabetes mellitus, atrial fibrillation, coronary heart disease, previous ischemic stroke or TIA, previous ICH, alcohol intake, smoking, antiplatelet drugs, anticoagulant drugs, statin, lobar ICH, intraventricular extension, cSS, CMB, and GCS on admission in univariable analysis. Variables with a p-value of <0.1 in univariable analysis were included in multivariable linear regression analyses to determine the risk factors for hematoma volume and 90-day mRS. An additional multivariable regression model was performed to evaluate the relationship between cSS and hematoma volume after discriminating lobar or non-lobar hematoma location.
Regression-based mediation analyses were then performed to distinguish the direct effect of cSS on 90-day mRS, and the indirect effect mediated by hematoma volume (16). Three estimates were calculated: (i) total effect, which indicated the whole association between cSS and 90-day mRS, composed of direct and indirect effects; (ii) direct effect, disclosing the sole association between cSS itself and 90-day mRS, except for the part caused by the mediator; and (iii) indirect effect, the part of causal relationship mediated by the mediator, that is, hematoma volume, intraventricular hemorrhage extension, and recurrent hemorrhage at present (Figure 2; Supplementary Tables 6, 7). All mediation calculations were non-parametric bootstrapped and estimated at a 95% CI when estimating the coefficients and residuals. Additional analyses were performed for patients with and without probable CAA. A sensitivity analysis was conducted among patients who had a CT scan within 6 h after ICH ictus.
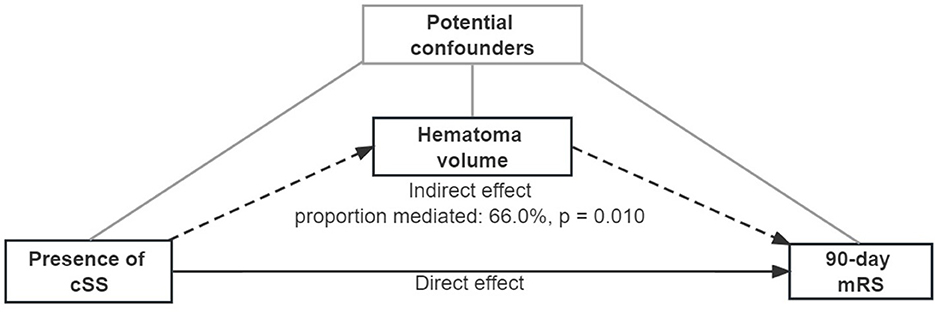
Figure 2. Mediation analysis of associations between cortical superficial siderosis and the 90-day modified Rankin scale, mediated by hematoma volume. A continuous arrow and the indirect effect by dotted arrows represent the direct effect. Adjusted potential confounders (age, sex, previous ICH, intraventricular extension, and GCS score) were selected from the multiple regression model.
Statistical analyses were performed using Empower® (http://www.empowerstats.com; X&Y Solutions, Inc., Boston, MA) and R (https://www.R-project.org/). A P-value of <0.05 was considered to be statistically significant. All the significance was two-tailed.
From the original cohort of 1,044 patients with primary ICH that occurred within 48 h, 673 patients were included in hematoma volume evaluation, and 663 patients were included when evaluating 90-day mRS because 10 patients failed to keep up with the follow-up. The baseline characteristics were compared between the included and excluded patients (Supplementary Table 1).
In the entire cohort, 131 out of 673 patients (19.5%) presented with cSS on acute-phase MRI, and disseminated cSS was observed in 29 patients (22.1%). Probable CAA was identified in 48 patients (7.2%). The demographic and clinical characteristics of patients with and without cSS were compared (Table 1). Patients with cSS were older and had a larger hematoma volume, more lobar CMBs, and a higher prevalence of previous ICH, lobar hematoma, intraventricular extension, and probable CAA. In addition, worse neurological presentation and long-term outcomes (GCS on admission and 90-day mRS) were observed in these patients. No significant differences were found in other characteristics, including sex, past medication, and other vascular risk factors (with the exception of previous ICH).
The same characteristics were compared between patients with focal and disseminated cSS (Supplementary Table 2). We found that patients with disseminated cSS were older and had a larger hematoma volume, whereas vascular risk factors, medication, and neurological impairment (GCS on admission) did not differ significantly.
We performed univariate and multivariate analyses of hematoma volume (Supplementary Table 3; Table 2). Multivariable regression analyses revealed that diabetes mellitus, lobar ICH, intraventricular extension, cSS, and fewer deep CMBs were independently associated with larger hematoma volume. Additionally, in stratified analyses according to hematoma location, cSS was independently associated with a larger hematoma volume in both lobar and non-lobar ICH (Table 2). Hematoma volume remained significantly different compared to patients without cSS after stratification as focal (β = 2.99, 95% CI 0.24–5.73, p = 0.033) and disseminated (β = 10.01, 95% CI 5.34–14.68, p < 0.001, Supplementary Table 4).
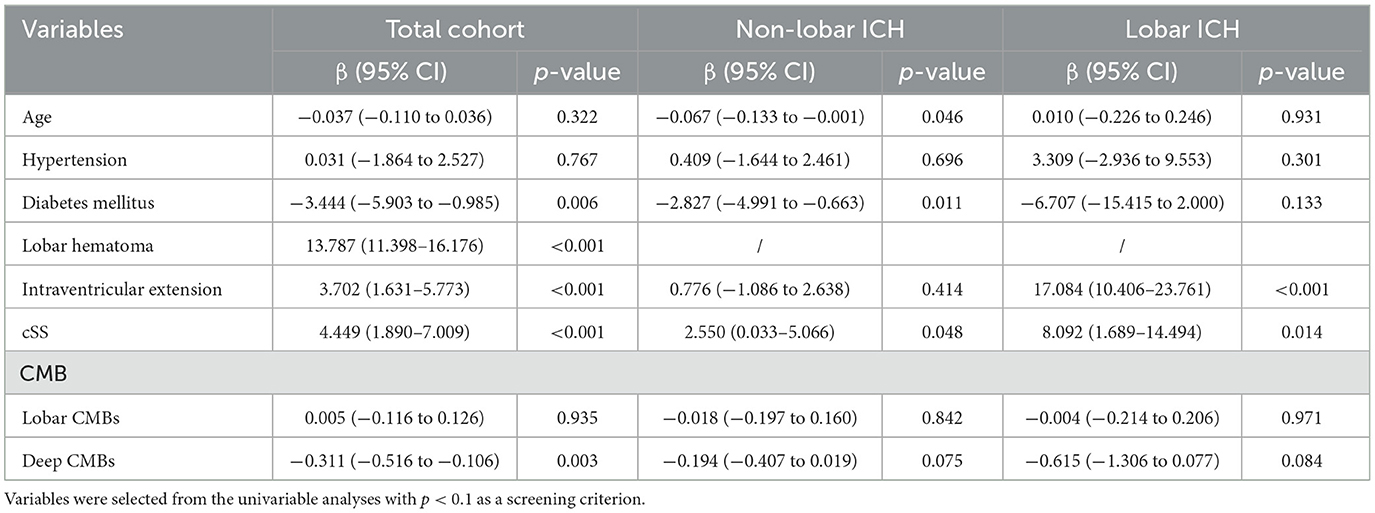
Table 2. Multivariable regression analyses of factors associated with hematoma volume stratified by hematoma location.
Univariate and multivariate analyses were performed for the 90-day mRS (Supplementary Table 5; Table 3). In total, 663 patients were included in the analysis. Multivariable regression analyses revealed that the characteristics independently associated with worse 90-day mRS were older age, female sex, larger hematoma volume, intraventricular extension, and low GCS score on admission (Table 3). However, cSS was not independently associated with a higher 90-day mRS when adjusted for these covariates, including hematoma volume. After removing hematoma volume from the multivariable analyses, cSS was an independent risk factor for poor outcomes (Table 4).
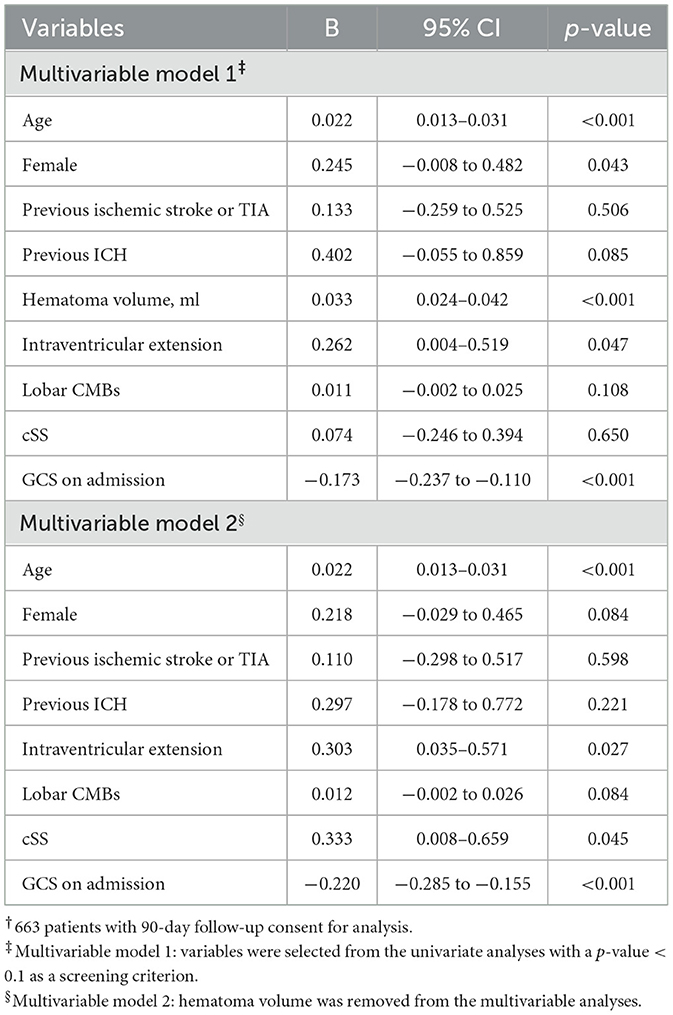
Table 3. Multivariable regression analyses of factors associated with the 90-day modified Rankin scale†.
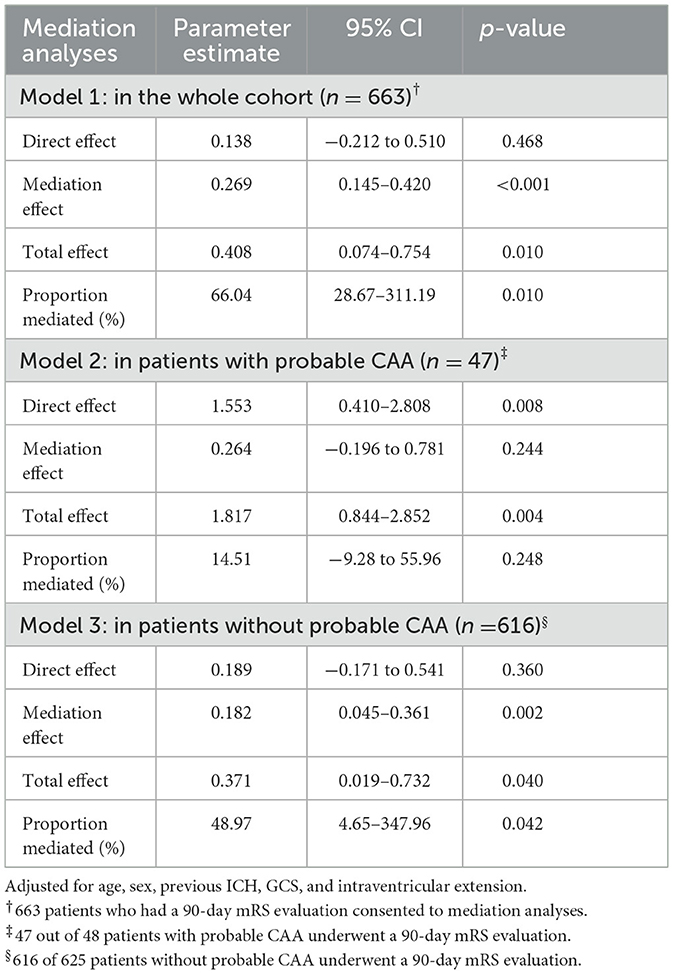
Table 4. Mediation analyses of the association between cortical superficial siderosis and the 90-day modified Rankin scale mediated by hematoma volume.
The distribution of 90-day mRS scores is presented in Supplementary Figure 1, in which data from patients with and without cSS, and with focal or disseminated cSS were compared.
Based on the analysis of cSS with hematoma volume and 90-day mRS, we tested the hypothesis that hematoma volume might be an essential mediator between cSS occurrence and unfavorable 90-day outcomes (Figure 2; Table 4). The association between cSS and unfavorable outcomes can be subdivided into a direct effect of cSS and an indirect effect mediated by large hematoma volume induced by cSS. It revealed that the association between cSS and worse 90-day outcomes was mainly mediated by induction of a larger hematoma volume (proportion mediated: 66.0%, p = 0.010), whereas the direct effect of cSS on 90-day mRS was not significant.
Additional mediation analyses were performed for other probable factors, including probable CAA, previous ICH, and intraventricular extension. In patients without probable CAA, the mediation effect was consistent with that in the whole cohort (proportion mediated: 49%, p = 0.042, Table 4). Recurrent ICH showed an unremarkable mediation effect, whereas intraventricular extension presented a minor effect (proportion mediated: 15.8%, p = 0.044) on cSS and 90-day mRS (Supplementary Tables 6, 7).
The sensitivity analysis including 449 patients who had a CT scan within 6 h after ICH ictus suggested that hematoma volume was still a significant mediator between cSS and 90-day outcome (proportion mediated: 68.1%, p = 0.006); the direct effect of cSS on 90-day mRS was not remarkable (Supplementary Table 8).
For WMH, we performed univariable analysis for hematoma volume and found that WMH was not associated with large hematoma volume (β = −0.342, 95% CI −0.920–0.235, p = 0.246; Supplementary Table 3), and therefore, WMH should not be included in multivariable regression analysis. With regard to 90-day mRS, WMH had a p-value of 0.001 in univariable analysis (β = 0.124, 95% CI 0.053–0.196, p = 0.001; Supplementary Table 5) but was not associated with worse 90-day mRS in multivariable regression analysis (β = −0.044, 95% CI −0.125 to −0.037, p = 0.287; Supplementary Table 9). We also performed mediation analysis after including WMH as an adjusted factor, and the results showed that hematoma volume remained a significant mediator (proportion mediated: 55.09%, p = 0.010; Supplementary Table 10).
In this prospective cohort of 673 patients with spontaneous ICH, cSS was observed in one out of five patients. The presence of cSS led to a large hematoma volume, independent of hematoma location and unfavorable 90-day outcomes. The relationship between cSS and 90-day mRS was mainly mediated by a large hematoma volume, as identified in this study, while the sole effect of cSS on 90-day mRS was unremarkable.
For decades, cSS has been proven to be a strong risk factor for unfavorable neurologic outcomes and is related to other clinical characteristics that correlate with worse outcomes, such as recurrent ICH and large hematoma (1, 3–8). However, it is unclear whether the deleterious outcome is determined by cSS or by other potential factors induced by cSS. We made the hypothesis that part of the effect (indirect effect) of cSS and 90-day mRS was mediated by hematoma volume based on the results of numerous cohort studies that concluded those as follows: (i) cSS is associated with large hematoma volume in patients with ICH; (ii) patients with cSS are more likely to have poor outcomes after ICH, including higher risk of recurrent ICH and poor functional recovery; and (iii) hematoma volume is the most significant factor deciding prognosis of ICH, and large hematoma volume leads to poor outcomes (1, 3–8). Therefore, a probable relationship chain is that cSS leads to large hematoma volume and then caused poor outcomes. We used mediation analysis to test this hypothesis and identified large hematoma as the essential pathway leading to worse neurologic recovery because of cSS and quantified the prominent mediation effect.
In our study, cSS was related to large hematoma in both lobar and non-lobar areas. This contradicts previous findings reported on patients from Western countries, indicating that cSS is mainly associated with large lobar hemorrhage (1). In in vivo and ex vivo MRI examinations and neuropathological studies on CAA-related patients, most affected arteries tended to be located in the intra-sulcal part of the hematoma. Thus, the origin of cSS appeared from the meningeal arteriole, with larger diameters than those in bulging areas on the cortex (17, 18). It was speculated that successive vessel ruptures in the sulci initiated the formation of an intra-sulcal hematoma, accompanied by surrounding hemorrhagic or anemic infarct in the cerebral cortex, which then extended into the brain parenchyma and generated a lobar ICH. The presence of cSS, therefore, emerges as a history of leakage of blood from small vessels located in the sulci, also indicating a higher risk of larger hematoma volume and hematoma expansion into brain parenchyma (17). The affected vessel tended to have a meningeal rather than cortical location, which was assumed to be because of APOE e2genotype of, assumed to aggravate vessel fragility and enhanced blood leakage (19). However, in this study, including Asian patients with primary ICH, we found that cSS was not only related to a larger hematoma in the lobar area but also in non-lobar areas, which may suggest an underlying etiology other than CAA of cSS in Asian patients (Supplementary Figure 2). This result might be explained by recent evidence testing imaging-diagnosed CAA-probable patients with PiB-PET in Taiwan, which found that in Asian populations, imaging markers indicating CAA may display a much lower predictive effect (20).
Although both are recognized as indicators of chronic blood extravasation in the brain, cSS showed clinical characteristics as opposed to CMB. Recent cohort studies have reported an inverse relationship between CMB and ICH volume and hematoma expansion, regardless of CMB location (lobar or deep) (1, 10). The results of CMB in our cohort presented similar results. We found that patients with higher CMB counts had smaller ICH volumes at admission. However, the results of our study were different from those previously reported in patients with cSS (1). This interesting difference between CMB and cSS suggests different mechanisms regulating ICH formation and hematoma enlargement, which warrants further exploration.
In the entire cohort, 48 patients (7.2%) were diagnosed with probable CAA according to the modified Boston criteria. Surprisingly, the mediation analyses in patients with and without probable CAA identified hematoma volume as an important mediator for 90-day mRS only in patients without probable CAA. We speculate that patients with probable CAA had more extensive and profound destruction of vessels and brain structure caused by CAA, which led to a series of pathological statuses and might fundamentally affect long-term recovery; thus, neurological dysfunction may be integrated with other noxious effectors, such as dementia and depression, in this particular case. Earlier studies also reported that dementia and depression were common in CAA patients, which is independent of ICH occurrence and recurrence (21–27). Herein, we inferred that these deleterious aspects of CAA might take over the central role of hematoma volume, leading to unfavorable outcomes.
Nevertheless, this study has some limitations. In this single-center cohort study in Hangzhou, China, the proportion of patients with lobar ICH was lower than that reported in other studies in the European cohort (8). Thus, the proportion of patients with probable CAA was low. The etiologies of these patients are quite different from those found in patients with ICH from Western countries. Second, cSS was assessed on MRI with a median delay of 5 (IQR, 4–7) days after ICH, but not before ICH ictus. The assumption is that cSS was chronic or former minimum leakage of blood before ICH, and we identified gyral high density on CT to exclude the newly formed cortical hemorrhage (2, 28). In addition, mRS was analyzed as a continuous variable in this study to retain the complete information in mediation analyses; this is less common but has been proven feasible in a previous article (15). We excluded patients with surgical procedures, mRS ≥ 3 before ICH, and those without MRI during hospitalization. Patients did not undergo MRI if they had a pacemaker, prosthetic heart valve, severe neurologic impairment, and unstable vital signs. Therefore, the severity of the patients included in this study was relatively mild; this explains why the patients had better outcomes than those in other studies (29–31).
This study highlighted the critical role of hematoma volume as a mediator between cSS and poor outcomes after mild to moderate ICH. In contrast, the noxious effect of a large hematoma because of cSS was independent of hematoma location, either in lobar or non-lobar areas.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Human Investigation Committee (IRB) of Second Affiliated Hospital of Zhejiang University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Y-jJ and L-sT: conceptualization. Y-jJ, Y-hH, and C-zY: methodology. J-wL, JW, K-cY, and H-nA: formal analysis and investigation. Y-jJ: writing—original draft preparation. L-sT and FG: writing—review and editing. L-sT: funding acquisition. FG: resources. J-wL, L-sT, and FG: supervision. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Natural Science Foundation of China (NSFC) 81971155 and 81500991.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1122744/full#supplementary-material
CAA, cerebral amyloid angiopathy; CMB, cerebral microbleed; cSS, cortical superficial siderosis; GCS, Glasgow Coma Score; ICH, intracerebral hemorrhage; IVH, isolated intraventricular hemorrhage; mRS, modified Rankin scale.
1. Boulouis G, van Etten ES, Charidimou A, Auriel E, Morotti A, Pasi M, et al. Association of key magnetic resonance imaging markers of cerebral small vessel disease with hematoma volume and expansion in patients with lobar and deep intracerebral hemorrhage. JAMA Neurol. (2016) 73:1440–7. doi: 10.1001/jamaneurol.2016.2619
2. Charidimou A, Linn J, Vernooij MW, Opherk C, Akoudad S, Baron JC, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. (2015) 138:2126–39. doi: 10.1093/brain/awv162
3. Shoamanesh A, Akoudad S, Himali JJ, Beiser AS, DeCarli C, Seshadri S, et al. Cortical superficial siderosis in the general population: The Framingham Heart and Rotterdam studies. Int J Stroke. (2021) 16:798–808. doi: 10.1177/1747493020984559
4. Charidimou A, Boulouis G, Roongpiboonsopit D, Xiong L, Pasi M, Schwab KM, et al. Cortical superficial siderosis and recurrent intracerebral hemorrhage risk in cerebral amyloid angiopathy: large prospective cohort and preliminary meta-analysis. Int J Stroke. (2019) 14:723–33. doi: 10.1177/1747493019830065
5. Wollenweber FA, Opherk C, Zedde M, Catak C, Malik R, Duering M, et al. Prognostic relevance of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology. (2019) 92:e792–801. doi: 10.1212/WNL.0000000000006956
6. Beitzke M, Enzinger C, Wünsch G, Asslaber M, Gattringer T, Fazekas F. Contribution of convexal subarachnoid hemorrhage to disease progression in cerebral amyloid angiopathy. Stroke. (2015) 46:1533–40. doi: 10.1161/STROKEAHA.115.008778
7. Warrier AR, Bhatia R, Garg A, Padma Srivastava MV, Dash D, Tripathi M, et al. Do imaging markers of cerebral small vessel disease predict hematoma volume and outcome in acute intracerebral hemorrhage? Ann Indian Acad Neurol. (2021) 24:204–10. doi: 10.4103/aian.AIAN_183_20
8. Moulin S, Casolla B, Kuchcinski G, Boulouis G, Rossi C, Hénon H, et al. Cortical superficial siderosis: a prospective observational cohort study. Neurology. (2018) 91:e132–8. doi: 10.1212/WNL.0000000000005778
9. Cordonnier C, Rutgers MP, Dumont F, Pasquini M, Lejeune JP, Garrigue D, et al. Intra-cerebral haemorrhages: are there any differences in baseline characteristics and intra-hospital mortality between hospitaland population-based registries? J Neurol. (2009) 256:198–202. doi: 10.1007/s00415-009-0030-3
10. Magid-Bernstein JR, Li Y, Cho SM, Piran PJ, Roh DJ, Gupta A, et al. Cerebral microbleeds and acute hematoma characteristics in the ATACH-2 and MISTIE III trials. Neurology. (2022) 98:e1013-20-e20. doi: 10.1212/WNL.0000000000013247
11. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
12. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. (2009) 8:165–74. doi: 10.1016/S1474-4422(09)70013-4
13. Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke. (2018) 49:491–7. doi: 10.1161/STROKEAHA.117.016990
14. Saver JL, Filip B, Hamilton S, Yanes A, Craig S, Cho M, et al. Improving the reliability of stroke disability grading in clinical trials and clinical practice: The Rankin Focused Assessment (RFA). Stroke. (2010) 41:992–5. doi: 10.1161/STROKEAHA.109.571364
15. Raseta M, Bazarova A, Wright H, Parrott A, Nayak S. A novel toolkit for the prediction of clinical outcomes following mechanical thrombectomy. Clin Radiol. (2020) 75:795.e15-21. doi: 10.1016/j.crad.2020.06.026
16. Imai K, Keele L, Tingley D, A. general approach to causal mediation analysis. Psychol Methods. (2010) 15:309–34. doi: 10.1037/a0020761
17. Takeda S, Hinokuma K, Yamazaki K, Onda K, Miyakawa T, Ikuta F, et al. The hemorrhage caused by sporadic-type cerebral amyloid angiopathy occurs primarily in the cerebral sulci. Neuropathology. (2012) 32:38–43. doi: 10.1111/j.1440-1789.2011.01219.x
18. Charidimou A, Perosa V, Frosch MP, Scherlek AA, Greenberg SM, van Veluw SJ. Neuropathological correlates of cortical superficial siderosis in cerebral amyloid angiopathy. Brain. (2020) 143:3343–51. doi: 10.1093/brain/awaa266
19. Shoamanesh A, Martinez-Ramirez S, Oliveira-Filho J, Reijmer Y, Falcone GJ, Ayres A, et al. Interrelationship of superficial siderosis and microbleeds in cerebral amyloid angiopathy. Neurology. (2014) 83:1838–43. doi: 10.1212/WNL.0000000000000984
20. Tsai HH, Pasi M, Tsai LK, Huang CC, Chen YF, Lee BC, et al. Centrum semiovale perivascular space and amyloid deposition in spontaneous intracerebral hemorrhage. Stroke. (2021) 52:2356–62. doi: 10.1161/STROKEAHA.120.032139
21. Pasi M, Casolla B, Kyheng M, Boulouis G, Kuchcinski G, Moulin S, et al. Long-term functional decline of spontaneous intracerebral haemorrhage survivors. J Neurol Neurosurg Psychiatry. (2021) 92:249–54. doi: 10.1136/jnnp-2020-324741
22. Avadhani R, Thompson RE, Carhuapoma L, Yenokyan G, McBee N, Lane K, et al. Post-stroke depression in patients with large spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2021) 30:106082. doi: 10.1016/j.jstrokecerebrovasdis.2021.106082
23. Yamada M. Cerebral amyloid angiopathy: emerging concepts. J Stroke. (2015) 17:17–30. doi: 10.5853/jos.2015.17.1.17
24. Hirohata M, Yoshita M, Ishida C, Ikeda SI, Tamaoka A, Kuzuhara S, et al. Clinical features of non-hypertensive lobar intracerebral hemorrhage related to cerebral amyloid angiopathy. Eur J Neurol. (2010) 17:823–9. doi: 10.1111/j.1468-1331.2009.02940.x
25. Cordonnier C, Leys D, Dumont F, Deramecourt V, Bordet R, Pasquier F, et al. What are the causes of pre-existing dementia in patients with intracerebral haemorrhages? Brain. (2010) 133:3281–9. doi: 10.1093/brain/awq246
26. Moulin S, Labreuche J, Bombois S, Rossi C, Boulouis G, Hénon H, et al. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol. (2016) 15:820–9. doi: 10.1016/S1474-4422(16)00130-7
27. Smith EE, Crites S, Wang M, Charlton A, Zwiers A, Sekhon R, et al. Cerebral amyloid angiopathy is associated with emotional dysregulation, impulse dyscontrol, and apathy. J Am Heart Assoc. (2021) 10:e022089. doi: 10.1161/JAHA.121.022089
28. Koeppen AH, Michael SC Li D, Chen Z, Cusack MJ, Gibson WM, et al. The pathology of superficial siderosis of the central nervous system. Acta Neuropathol. (2008) 116:371–82. doi: 10.1007/s00401-008-0421-z
29. Fernando SM, Qureshi D, Talarico R, Tanuseputro P, Dowlatshahi D, Sood MM, et al. Intracerebral hemorrhage incidence, mortality, and association with oral anticoagulation use: a population study. Stroke. (2021) 52:1673–81. doi: 10.1161/STROKEAHA.120.032550
30. Béjot Y, Blanc C, Delpont B, Thouant P, Chazalon C, Daumas A, et al. Increasing early ambulation disability in spontaneous intracerebral hemorrhage survivors. Neurology. (2018) 90:e2017–e24. doi: 10.1212/WNL.0000000000005633
Keywords: cSS, ICH volume and outcome, mediation analysis, prospective studies, cerebral hemorrhage, hematoma
Citation: Jin Y-j, Li J-w, Wu J, Huang Y-h, Yang K-c, An H-n, Yuan C-z, Gao F and Tong L-s (2023) Cortical superficial siderosis, hematoma volume, and outcomes after intracerebral hemorrhage: a mediation analysis. Front. Neurol. 14:1122744. doi: 10.3389/fneur.2023.1122744
Received: 22 December 2022; Accepted: 03 April 2023;
Published: 05 May 2023.
Edited by:
Hsin-Hsi Cynthia Tsai, National Taiwan University, TaiwanReviewed by:
Zhaohui He, First Affiliated Hospital of Chongqing Medical University, ChinaCopyright © 2023 Jin, Li, Wu, Huang, Yang, An, Yuan, Gao and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu-sha Tong, MjMxMDA0MEB6anUuZWR1LmNu; Feng Gao, MjIwMjAxMkB6anUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.