- Department of Neurosurgery, Renji Hospital, Shanghai Jiaotong University School of Medicine, Pudong, Shanghai, China
Background: The rarity and complex angioarchitecture of foramen magnum dural arteriovenous fistulas (DAVFs) make its treatment difficult and controversial. We aimed to describe their clinical features, angio-architectural phenotypes, and treatments, through a case series study.
Methods: We first retrospectively studied cases of foramen magnum DAVFs treated in our Cerebrovascular Center, and then reviewed the published cases on Pubmed. The clinical characteristics, angioarchitecture, and treatments were analyzed.
Results: A total of 55 patients were confirmed with foramen magnum DAVFs, which included 50 men and 5 women, with a mean age of 52.8 years. Most patients presented with subarachnoid hemorrhage (SAH) (21/55) or myelopathy (30/55), depending on the venous drainage pattern. In this group, 21 DAVFs were supplied by only the vertebral artery (VA), three by only the occipital artery (OA), three by only the ascending pharyngeal artery (APA), and the remaining 28 DAVFs were supplied by two or three of these feeding arteries. Most cases (30/55) were treated with only endovascular embolization, 18 cases (18/55) with only surgical disconnection, five cases (5/55) with combined therapy, and two cases rejected treatment. The angiographic outcome of complete obliteration was achieved in most patients (50/55). In addition, two cases of foramen magnum DAVFs were treated by us in a Hybrid Angio-Surgical Suite (HASS) with good outcomes.
Conclusions: Foramen magnum DAVFs are rare and their angio-architectural features are complicated. The treatment option (microsurgical disconnection or endovascular embolization) should be weighed carefully, and combined therapy in HASS could be a more feasible and less invasive treatment option.
Introduction
Foramen magnum dural arteriovenous fistula (DAVF) is a subset of craniocervical DAVF with the fistula point around the foramen magnum. Generally, the foramen magnum region lies in the bilateral occipital area that runs laterally up to the jugular foramen and includes both the inner and outer sides of the occipital bone (1). Most foramen magnum DAVFs are presented with myelopathy or subarachnoid hemorrhage, depending on the pattern of venous drainage (1). Foramen magnum DAVFs are rare, representing only 1.5–2.3% of all intracranial DAVFs (1, 2), and their rarity and complicated angioarchitectures make the treatment difficult and controversial.
Generally, microsurgical disconnection of the shunt was the predominant treatment option for the foramen magnum DAVFs before 2010 (3–5). However, in the past decade, with remarkable advances in the endovascular techniques providing highly flexible hydrophilic-coated catheters and new non-adhesive liquid embolic agents, such as Onyx and N-butyl-cyanoacrylate (NBCA) glue, endovascular embolization of the fistula has become an alternative option for treating the foramen magnum DAVFs (6–8). It has been reported that ~80% of the foramen magnum DAVFs in the literature were treated endovascularly (9). Nevertheless, the incomplete obliteration and reoccurrence of the fistula after endovascular embolization could not be underestimated, and some cases even required staged or salvage combined surgeries (1, 10). Recently, a combined surgical-endovascular technique in a hybrid operating room (Hybrid Angio-Surgical Suite, HASS) has emerged as a solution to the complexity of cerebrovascular surgery, and this technique has been an effective treatment option for DAVFs complicated by inaccessible arterial and transvenous approaches (11, 12). Coincidentally, we applied this concept of a HASS to treat two complicated cases of foramen magnum DAVFs with good outcomes.
In this retrospective observational case series, we first studied the cases of foramen magnum DAVFs treated in our Cerebrovascular Center, and then reviewed the published cases of foramen magnum DAVFs (4, 5, 7–10, 13–29), aiming to obtain a comprehensive understanding of the clinical symptoms, angioarchitectures, pathophysiological mechanisms and treatments for foramen magnum DAVFs.
Methods
Patients
In this retrospective case series, we reviewed clinical charts, radiological images, and operative notes of 13 consecutive patients who were diagnosed with foramen magnum DAVFs from January 2002 until April 2021. All patients were managed at the Neurosurgery Department, Renji Hospital, Shanghai Jiao Tong University School of Medicine. All available imaging studies were reviewed and analyzed. All the patients had digital subtraction angiography (DSA) of the bilateral internal and external carotid arteries and the vertebral arteries that carefully analyzed the arterial feeders, the location of the fistula, and the venous drainage patterns. Patients who presented with myelopathy had a complete spine MRI and spine DSA. All the patients gave consent to be enrolled and have their data published.
In addition, we found 78 published reports when we searched for “dural arteriovenous fistula foramen magnum” on PubMed, 26 of which included sufficient clinical descriptions and adequate angiographic architecture of the foramen magnum DAVFs.
Data collection
The variables collected and analyzed include demographic profiles such as age and gender, clinical presentation and the duration of symptoms, and angio-architectural features of the fistulas including the location, arterial supply, venous drainage pattern, and presence of venous aneurysms. Treatment modalities, outcomes, and follow-up information were also documented and analyzed.
Data analysis
All the data were analyzed and interpreted by the senior investigator with extensive knowledge of vascular neuroanatomy and angio-architectural interpretation. No statistical analysis software was warranted.
Results
There were 55 patients (including 13 of our current cases) who were angiographically confirmed with foramen magnum DAVFs, which included 50 men (90.9%) and 5 women (9.1%). The mean age was 52.8 years, ranging from 20 to 83 years (Supplementary Table 1).
Clinical presentation
Most patients presented with subarachnoid hemorrhage (SAH) or myelopathy (debility of bilateral limbs and/or urinary retention) after symptoms appeared, with 30 cases (54.5%) of myelopathy, 21 cases (38.2%) of SAH, and 4 cases (7.3%) of other etiologies (three intracranial hematomas, and one trigeminal neuralgia).
Angioarchitecture
Generally, foramen magnum DAVFs have three important arterial supplies: the neuromeningeal trunk of the ascending pharyngeal artery (APA), the meningeal branches of the vertebral artery (VA), and the mastoid branches of the occipital artery (OA). As listed in Table 1, of the 55 cases in this case series study, 21 cases were supplied by only the VA; 3 cases were supplied by only the OA; 3 cases were supplied only by the APA, and the remaining 28 cases were supplied by two or three of these feeding arteries. Thus, more than half of these cases were not supplied by only one feeding artery, but by two or even three feeding arteries. In addition, as listed in Table 1, foramen magnum DAVFs usually first drain into the bridging medullary veins, but some DAVFs then drain into the intracranial cerebral veins or the spinal veins.
Treatments
Almost all the patients adopted treatments (two patients rejected treatment). Most cases (30/55) were treated with only endovascular embolization, while 18 cases (18/55) were treated with only surgical disconnection, and 5 cases (5/55) with combined therapy (endovascular embolization and microsurgical disconnection) because of incomplete embolization or DAVF reoccurrence.
Follow-up and outcome
With follow-up duration ranging from 6 months to 2 years, all the treated patients recovered well. All the patients were followed with cerebral DSA. The angiographic outcome of complete obliteration was achieved in all the patients who underwent surgery or combined therapy, and in 30 of 35 patients who received endovascular embolization.
Illustrative cases
Case 12
A 62-year-old male patient presented with a 2-month history of progressive difficulty in walking and bilateral extremity weakness and numbness. He reported no pain but described balance issues and urinary and bowel incontinence episodes. Physical examinations revealed the muscle strength of his distal limbs was grade 4. His Hoffman's and Babinski's signs were both positive. The superficial sensation of his lower body was not intact, with a sensory level at T10.
Cervical spine magnetic resonance imaging (MRI) showed an expanded spinal brain stem and a longitudinal extensive cervical cord enlargement with obvious edema. Vascular flow voids were also demonstrated in front of the cervical cord (Figure 1A). The presence of brainstem and cervical cord edema, as well as the vascular flow voids in front of the cervical cord, were strong indicators of a DAVF. The clinicians strongly advised pursuing further angiographic investigations. A complete spine DSA was negative, while the cerebral DSA demonstrated a DAVF located at the foramen magnum supplied by the meningeal branch of the left VA (Figures 1B–D). The fistula was draining through the bridge-medullary veins into the dilated tortuous anterior spinal vein (SPV) (Figures 1B–D; Supplementary material Video 1). After consultation with the multidisciplinary team of the Cerebrovascular Center, a surgical disconnection of the fistula in HASS was considered to be the treatment option. During the operation, the right femoral artery catheterization was first performed. With the patient in the park bench position on his right side, the foramen magnum was osteoclastically enlarged, followed by a partial hemilaminectomy of C1(Figure 1E; Supplementary material Video 2). After opening the dura, a large tortuous vein was visible at the level of the foramen magnum. It originated near the dural penetration of the left vertebral artery and formed a “C” pattern crawling between the vertebral artery and the medulla (Figure 1F). After an aneurysm min-clip was temporarily applied to the origin of the fistula (Figure 1F), intra-operative DSA was performed and revealed the complete disappearance of the DAVF (Figure 1G; Supplementary material Video 3). Thus, electrical coagulation and disconnection of the fistula at its origin from the dura were performed. After the operation, a repeated cerebral DSA confirmed the complete obliteration of the fistula (Figure 1H; Supplementary material Video 4). The postoperative course was uneventful, and his limb's motor and sensory function gradually recovered. Cerebral angiography 6 months later confirmed the disappearance of the DAVF.
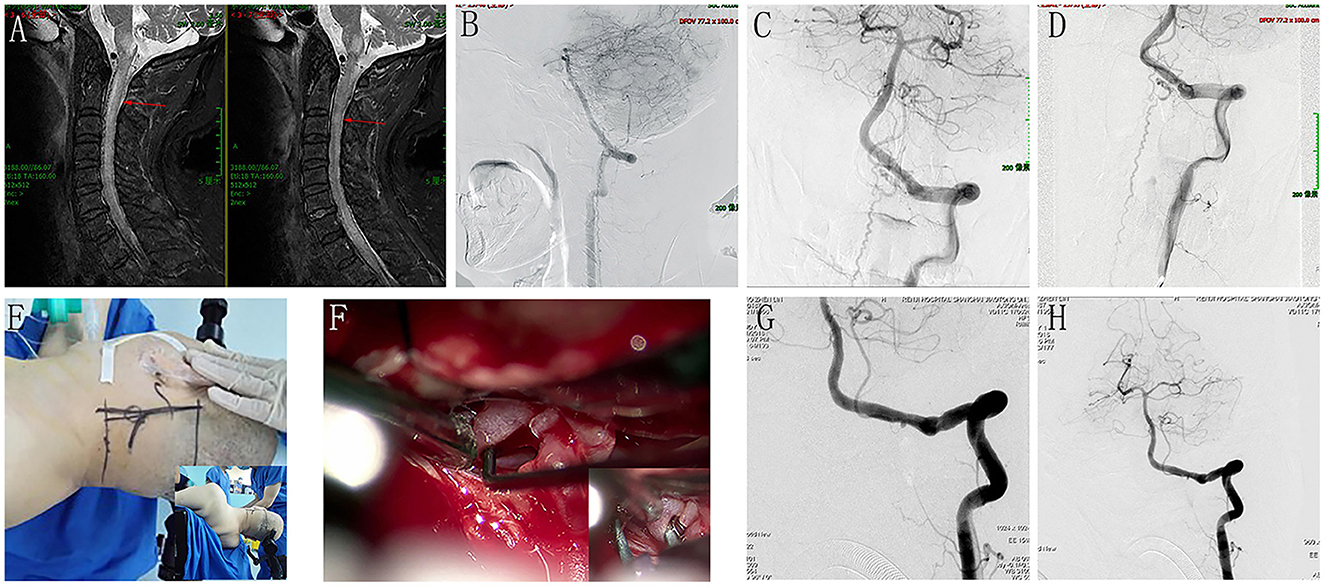
Figure 1. Perioperative neuroimages of Case 12. A cervical spine MRI showed an expanded spinal brain stem and a longitudinal extensive cervical cord enlargement with obvious edema, and vascular flow voids was also demonstrated in front of the cervical cord (A). The cerebral DSA demonstrated a DAVF located at the foramen magnum supplied by the meningeal branch of left VA, and the fistula was draining through the bridge-medullary veins into the dilated tortuous anterior spinal vein (B), lateral view; (C), anteroposterior view; (D), oblique view with magnification. (E) showed the incision line and the operation position (park bench position). F demonstrated a large tortuous vein was visible at the level of the foramen magnum, and it originated near the dural penetration of the left vertebral artery and formed a “C” pattern crawling between the vertebral artery and the medulla. (F) showed an aneurysm min-clip was temporarily applied to the origin of the fistula to occlude the shunt. Intraoperative DSA revealed the complete disappearance of the DAVF (G). After electrical coagulation and disconnection of the fistula at its origin from the dura, repeat cerebral DSA confirmed the complete obliteration of the fistula (H).
Case 13
A 60-year-old male presented with a sudden onset of severe headache and loss of consciousness for 2 h. Emergent cranial CT revealed diffuse SAH and 4th ventricular hematoma (Figure 2A) (Fisher grade IV). The patient was in a deep coma with tracheal intubation (GCS 7, Hunt Hesse IV). Emergent complete cerebral DSA revealed a DAVF located at the foramen magnum supplied by the meningeal branch of the right VA, draining into the enlarged tortuous medullary vein (Figure 2B). As the patient's neurological state was very poor and the only feeding artery was not small, trans-arterial embolization via the meningeal branch of the VA in HASS was considered as the treatment option. In addition, microsurgical disconnection of the fistula was regarded as the alternative option, if the endovascular approach failed.
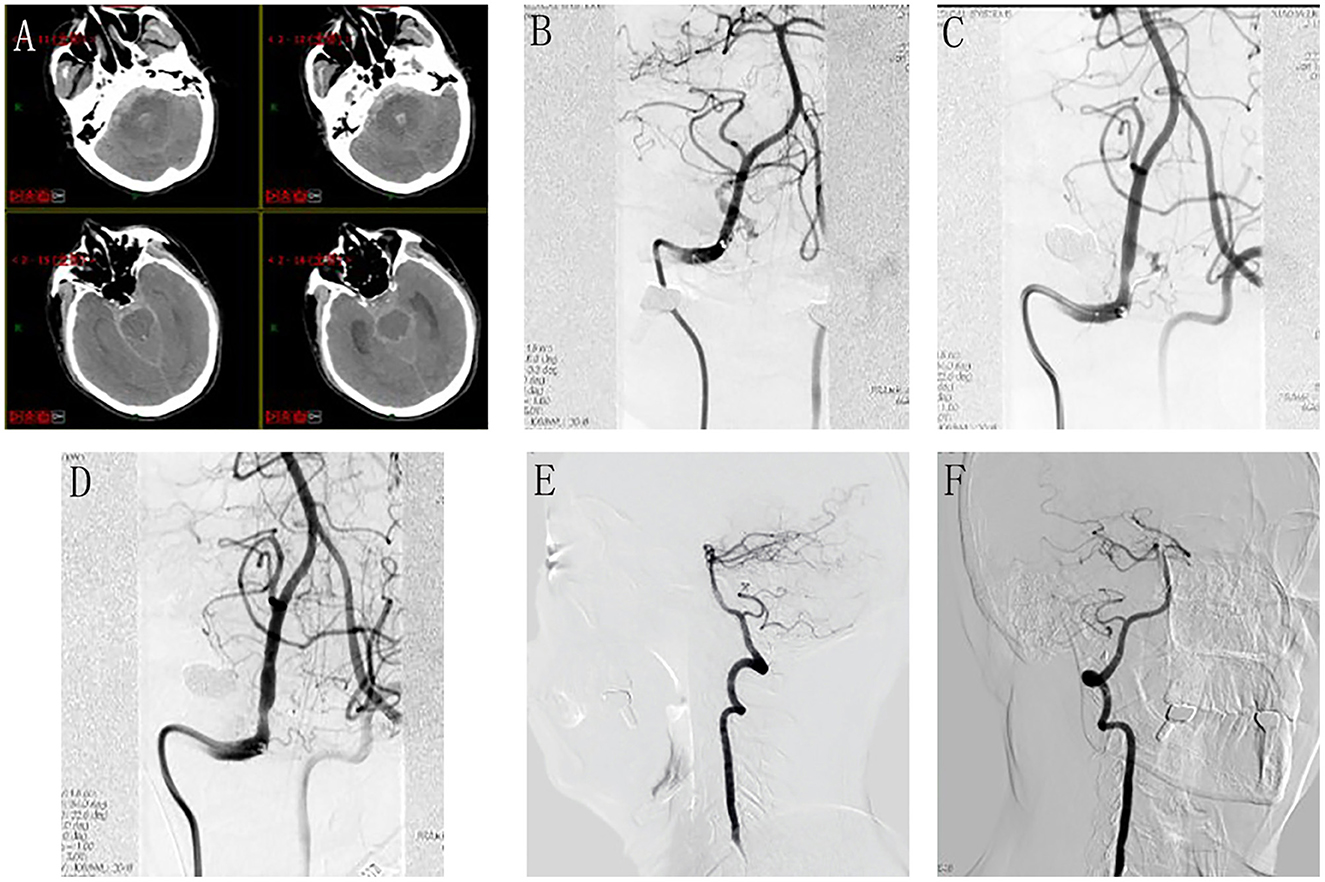
Figure 2. Perioperative neuroimages of Case 13. Emergent cranial CT revealed diffuse SAH and 4th ventricular hematoma (A). Emergent cerebral DSA revealed a DAVF located at the foramen magnum supplied by the meningeal branch of the right VA, draining into the enlarged tortuous medullary vein (B). During the procedure, the microcatheter tip was advanced to reach the optimal position (C); the injection of Onyx was continued until the DAVF was completely obliterated (D). Follow-up angiography at 6 months did not reveal any fistula residual or recurrence (E), lateral view; (F), anteroposterior view.
Under general anesthesia, catheterization was performed via the transfemoral approach. The Marathon microcatheter (MTI-EV3, Irvine, CA, USA) was dimethylsulfoxide (DMSO)-compatible, and smoothly entered the feeding artery. The microcatheter tip was placed as close as possible to the DAVF nidus to ensure that the liquid embolic agent could penetrate and occlude the lesion. Once the microcatheter tip was advanced to reach the optimal position (Figure 2C), the injection of Onyx-18 (MTI-EV3) was carried out. Whenever any venous migration appeared, the injection was stopped to allow for solidification and subsequently the injection was continued until the DAVF was completely obliterated (Figure 2D). After the embolization, continuous lumbar drainage of bloody cerebrospinal fluid was applied to the patient for 5 days. The patient woke up from a coma 1 week later and recovered well without any neurological deficit 1 month later. Follow-up angiography at 6 months did not reveal any fistula residual or recurrence (Figures 2E, F).
Discussion
From these 55 cases, foramen magnum DAVFs appeared to be much more common in men (90.9%), with a median age of 52.8 years. Most patients presented with myelopathy or SAH after symptoms appeared, with 30 cases (54.5%) of myelopathy, 21 cases (38.2%) of SAH, and 4 cases (7.3%) of other etiologies (three intracranial hematomas, and one trigeminal neuralgia). The pathophysiological mechanism underlying SAH or myelopathy is presumed to involve venous hypertension, although it is not entirely understood (1, 3, 10). The DAVFs around the foramen magnum could drain into the bridging medullary veins and then to the intracranial cerebral veins or the spinal veins (1). Drainage into the cortical or perimedullary spinal veins increases the pial venous pressure, resulting in aggressive neurologic outcomes including SAH, cerebral hemorrhage, or congestive venous myelopathy (3, 10). Almost all the patients in this study experienced symptoms associated with SAH (38.2%) or myelopathy (54.5%), depending on the pattern of venous drainage.
Anatomically, foramen magnum DAVFs have three important arterial supplies: the neuromeningeal trunk of the APA, the meningeal branches of the VA, and the mastoid branches of the OA (1, 3, 5). As listed in Table 1, among the 55 cases, 21 cases were supplied by only the VA; 3 cases were supplied by only the OA; 3 cases were supplied only by the APA, and the remaining 28 cases were supplied by two or three of these feeding arteries. Therefore, to reduce the incidence of missed or misdiagnosed foramen magnum DAVFs, accurate recognition of six arteries (including the bilateral external carotid arteries, the internal carotid arteries, and the vertebral arteries) and their branches is necessary during the angiography (3).
The dangerous clinical presentations of foramen magnum DAVFs require rapid and aggressive treatment. However, their rarity and complicated angioarchitectures make the treatment difficult and controversial. Generally, microsurgical disconnection of the fistula has been the predominant treatment option for foramen magnum DAVFs before 2010 (3–5). In the past decade, with remarkable advances in endovascular techniques providing highly flexible hydrophilic-coated catheters and new non-adhesive liquid embolic agents, such as Onyx and N-butyl-cyanoacrylate (NBCA) glue, endovascular embolization of the fistula has become an alternative option for treating foramen magnum DAVFs (6–8).
There are abundant anastomoses with surrounding blood vessels in the meningeal branches of VA at the craniocervical junction (1, 3, 5, 30), and embolized materials like Onyx or N-butyl-cyanoacrylate glue (NBCA) can easily migrate or reflux to the basilar and vertebral arteries (1). Therefore, the foramen magnum DAVFs supplied exclusively by the VA are preferably treated with direct microsurgery. As in our current cases, microsurgical disconnection (6/9) was dominantly chosen to treat the foramen magnum DAVFs with VA to be the only feeding artery. On the other hand, in most of the cases supplied by the external carotid artery, the microcatheter was guided sufficiently proximal to the fistula site and the fistula was completely obliterated by using Onyx or NBCA from the external carotid artery. Thus, for DAVFs supplied by the external carotid artery and its branches, endovascular embolization could be preferred (7, 25). During the embolization, the catheter tip should be advanced beyond the hypoglossal or jugular foramina to prevent occlusion of the vasa nervosum.
As shown in Table 1, most cases (30/55) were treated with only endovascular embolization, 18 cases (18/55) with only surgical disconnection, and 5 cases (5/55) with combined therapy (endovascular embolization and microsurgical disconnection) because of incomplete embolization or DAVF reoccurrence. The angiographic outcome of complete obliteration was achieved in all the patients who underwent surgery or combined therapy, and in 30 of 35 patients who received endovascular embolization. Nevertheless, the incomplete obliteration and reoccurrence of the fistula after endovascular embolization could not be underestimated, and some cases even required staged or salvage combined surgeries (1, 10). According to Hiramatsu et al., complication rates of the overall embolization procedure is 15.8% in craniocervical junction DAVF (20). Specifically, cranial nerve palsy may occur after Onyx injection, possibly due to the neurotoxicity of Onyx or dimethyl sulfoxide, or occlusion of the vasa nervorum due to dangerous extracranial-intracranial anastomoses (31–33). Drawing from our experience, for the patients with extracranial-intracranial anastomoses, a non-detachable balloon catheter was inflated in the VA across the C1 branch origin to prevent reflux of the embolized materials into the VA during the embolization.
Notwithstanding alternative treatment options in recent years, microsurgical disconnection of the shunt appears to be a more effective and reliable method of treatment. The surgery can be performed via the far lateral suboccipital craniotomy with a C1 laminectomy or hemilaminectomy (3). Microsurgery has also been useful in cases with symptoms caused by the mass effect of the dilated venous pouch and the shunt fistula (1). Chen et al. reported that trigeminal neuralgia due to a foramen magnum DAVF compression was completely relieved after surgical disconnection (9). In addition, although seldom reported, surgery-related complications (intracranial hematoma, intracranial infection, etc.,) cannot be completely avoided.
No treatment is perfect. To achieve complete obliteration of the fistula through minimally invasive procedures, combined therapy might be a better option, especially for the complex foramen magnum DAVFs. However, it often needs staging or additional surgery. Early studies with intraoperative portable DSA (early “hybrid surgery”) showed that intraoperative angiogram altered surgical management, frequently by avoiding additional surgery. Recently, the concept of a Hybrid Angio-Surgical Suite (HASS) has emerged as a solution to the complexity of cerebrovascular surgery and the need for immediate intraoperative feedback (11, 12). It combines the capacity of both the operating room and interventional suite—a standard operating room equipped with a biplane or single-plane angiogram machine. The HASS is becoming a standard facility for many hospitals around the world, and many neurosurgeons and neuro-interventionists use it for the treatment of cerebrovascular diseases (11).
Coincidentally, we treated two cases of foramen magnum DAVFs in HASS with good outcomes. The use of HASS for foramen magnum DAVFs could have the following advantages: 1) the HASS can afford immediate intraoperative feedback (for example, the accurate anatomic location of the fistula, the complete obliteration of the fistula after surgical disconnection); 2) the HASS could immediately provide surgical obliteration when the endovascular approach fails; 3) the HASS with open surgery could supply alternative access for endovascular embolization when the regular trans-arterial and trans-venous approaches fail. In short, HASS provides a less invasive and more feasible alternative for the treatment of difficult foramen magnum DAVFs.
Conclusion
Foramen magnum DAVFs are rare, with only 55 cases being reported till now. Their angioarchitecture is complicated, with three possible feeder arteries (APA, VA, and OA), draining into the bridging medullary veins and then to the intracranial cerebral veins or the spinal veins, leading to SAH or myelopathy. Their rarity and angio-architectural complexity make their treatment difficult and controversial. Generally, microsurgical disconnection of the fistula appears to be a more effective and reliable method of treatment, while endovascular embolization is a more recent and popular treatment option for selected cases. However, we suggest that all foramen magnum DAVFs should be managed in HASS if available because HASS could provide a less invasive and more feasible alternative treatment strategy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiaotong University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LG, ZX, and WG designed the study, analyzed data, and wrote the article. HZ and XZ collected the data. JD and JW assisted in analyzing the data and revised the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1121075/full#supplementary-material
References
1. Kakizaki S, Ishibashi T, Kato N, Kan I, Nishimura K, Murayama Y. Complete obliteration of a foramen magnum dural arteriovenous fistula by microsurgery after failed endovascular treatment using onyx: case report and literature review. World Neurosurg. (2020) 144:43–9. doi: 10.1016/j.wneu.2020.08.077
2. Baltsavias G, Valavanis A. Endovascular treatment of 170 consecutive cranial dural arteriovenous fistulae: results and complications. Neurosurg Rev. (2014) 37:63–71. doi: 10.1007/s10143-013-0498-2
3. Guo LM, Zhou HY, Xu JW, Wang GS, Tian X, Wang Y, et al. Dural arteriovenous fistula at the foramen magnum presenting with subarachnoid hemorrhage: case reports and literature review. Eur J Neurol. (2010) 17:684–91. doi: 10.1111/j.1468-1331.2009.02895.x
4. Reinges MH, Thron A, Mull M, Huffmann BC, Gilsbach JM. Dural arteriovenous fistulae at the foramen magnum. J Neurol. (2001) 248:197–203. doi: 10.1007/s004150170226
5. Takami T, Ohata K, Nishio A, Nishikawa M, Goto T, Tsuyuguchi N, et al. Microsurgical interruption of dural arteriovenous fistula at the foramen magnum. J Clin Neurosci. (2005) 12:580–3. doi: 10.1016/j.jocn.2005.02.003
6. Guo L, Qiu Y, A. dural arterioveous fistula at the foramen magnum treated with transarterial Onyx embolization. Neurol India. (2012) 60:661–3. doi: 10.4103/0028-3886.105215
7. Liang G, Gao X, Li Z, Wang X, Zhang H, Wu Z. Endovascular treatment for dural arteriovenous fistula at the foramen magnum: report of five consecutive patients and experience with balloon-augmented transarterial Onyx injection. J Neuroradiol. (2013) 40:134–9. doi: 10.1016/j.neurad.2012.09.001
8. Spiotta AM, Hughes G, Masaryk TJ, Hui FK. Balloon-augmented Onyx embolization of a dural arteriovenous fistula arising from the neuromeningeal trunk of the ascending pharyngeal artery: technical report. J Neurointerv Surg. (2011) 3:300–3. doi: 10.1136/jnis.2010.003095
9. Chen H, Chen R, Yang H, Li H, Wang J, Yu J. Resolution of trigeminal neuralgia after surgical disconnection of a foramen magnum Dural arteriovenous fistula. World Neurosurg. (2020) 135:209–13. doi: 10.1016/j.wneu.2019.07.063
10. Iampreechakul P, Liengudom A, Lertbutsayanukul P, Wattanasen Y, Siriwimonmas S. Medullary hemorrhage caused by foramen magnum dural arteriovenous fistula successfully obliterated using combination of endovascular and surgical treatments: a case report and literature review. Asian J Neurosurg. (2019) 14:1256–67. doi: 10.4103/ajns.AJNS_259_19
11. Ren Z, Wang S, Xu K, Mokin M, Zhao Y, Cao Y, et al. The working road map in a neurosurgical Hybrid Angio-Surgical suite—development and practice of a neurosurgical Hybrid Angio-Surgical suite. Chin Neurosurg J. (2018) 4:7. doi: 10.1186/s41016-017-0108-1
12. Gruter BE, Strange F, Burn F, Remonda L, Diepers M, Fandino J, et al. Hybrid operating room settings for treatment of complex dural arteriovenous fistulas. World Neurosurg. (2018) 120:e932–e9. doi: 10.1016/j.wneu.2018.08.193
13. Rivierez M, Gazengel J, Chiras J, Dorwling-Carter D, Debussche D, Dubbs M, et al. Vertebro-dural arteriovenous fistulae of the foramen magnum with perimedullary venous drainage. Neurochirurgie. (1991) 37:179–84.
14. Slaba S, Smayra T, Hage P, Okais N, Atallah N. An unusual cause of acute myelopathy: a dural arteriovenous fistula at the craniocervical junction. J Med Liban. (2000) 48:168–72.
15. Kim MS, Han DH, Han MH, Oh CW. Posterior fossa hemorrhage caused by dural arteriovenous fistula: case reports. Surg Neurol. (2003) 59:512-6. doi: 10.1016/S0090-3019(03)00077-6
16. Chng SM, Sitoh YY, Hui F. Intracranial dural arteriovenous fistula presenting with tetraparesis due to cervicomedullary junction compression. A case report. Interv Neuroradiol. (2004) 10:347–51. doi: 10.1177/159101990401000410
17. Gilard V, Curey S, Tollard E, Proust F. Coincidental vascular anomalies at the foramen magnum: dural arteriovenous fistula and high flow aneurysm on perimedullary fistula. Neurochirurgie. (2013) 59:210–3. doi: 10.1016/j.neuchi.2013.06.003
18. Mendes GA, Caire F, Saleme S, Ponomarjova S, Mounayer C. Retrograde leptomeningeal venous approach for dural arteriovenous fistulas at foramen magnum. Interv Neuroradiol. (2015) 21:244–8. doi: 10.1177/1591019915582942
19. Pop R, Manisor M, Aloraini Z, Chibarro S, Proust F, Quenardelle V, et al. Foramen magnum dural arteriovenous fistula presenting with epilepsy. Interv Neuroradiol. (2015) 21:724–7. doi: 10.1177/1591019915609783
20. Hiramatsu R, Kuroiwa T, Nozaki K, Kuroiwa T. Foramen magnum dural arteriovenous fistula treated by a microsurgical technique combined with a feeder occlusion using transarterial coil embolization. Turk Neurosurg. (2015) 25:971–5. doi: 10.5137/1019-5149.JTN.11860-14.3
21. Llacer JL, Suay G, Piquer J, Vazquez V. Dural arteriovenous fistula at the foramen magnum: Report of a case and clinical-anatomical review. Neurocirugia (Astur). (2016) 27:199–203. doi: 10.1016/j.neucir.2016.01.006
22. Raheja A, Taussky P, Couldwell WT. Successful microsurgical management of high-risk foramen magnum dural arteriovenous fistula with cortical venous reflex using far-lateral approach: 3-dimensional operative video. Oper Neurosurg. (2017) 13:644. doi: 10.1093/ons/opx055
23. Do AS, Kapurch J, Kumar R, Port J, Miller JW, Van Gompel JJ. The long and winding road: thoracic myelopathy associated with occipitocervical dural arteriovenous fistula. World Neurosurg. (2017) 108:998e7–e16. doi: 10.1016/j.wneu.2017.09.073
24. Kim H, Lee YS, Kang HJ, Lee MS, Suh SJ, Lee JH, et al. A rare case of subarachnoid hemorrhage caused by ruptured venous varix due to dural arteriovenous fistula at the foramen magnum fed solely by the ascending pharyngeal artery. J Cerebrovasc Endovasc Neurosurg. (2018) 20:120–6. doi: 10.7461/jcen.2018.20.2.120
25. Motebejane MS, Choi IS. Foramen magnum dural arteriovenous fistulas: clinical presentations and treatment outcomes, a case-series of 12 patients. Oper Neurosurg. (2018) 15:262–9. doi: 10.1093/ons/opx229
26. Sattur MG, Abi-Aad KR, Richards AE, Chong BW, Welz ME, Tian F, et al. Microsurgical treatment of foramen magnum cognard type V dural arteriovenous fistula: 2-dimensional operative video. Oper Neurosurg. (2019) 17:E203. doi: 10.1093/ons/opz030
27. Artemiadis A, Magoufis G, Papadopoulos TS, Zekiou K, Nikolaou G. Foramen magnum arteriovenous fistula presenting as transverse myelitis. Acta Neurol Belg. (2020) 120:779–81. doi: 10.1007/s13760-020-01334-1
28. Gadot R, Gopakumar S, Wagner K, Xu DS, Raper DMS, Burkhardt JK, et al. Foramen magnum dural arteriovenous fistula presenting with thoracic myelopathy: technical case report with 2-dimensional operative video. Oper Neurosurg. (2021) 21:E55–E9. doi: 10.1093/ons/opab077
29. Caton MT, Narsinh KH, Baker A, Dowd CF, Higashida RT, Cooke DL, et al. Dural arteriovenous fistulas of the foramen magnum region: clinical features and angioarchitectural phenotypes. AJNR Am J Neuroradiol. (2021) 42:1486–91. doi: 10.3174/ajnr.A7152
30. Rhoton AL. The foramen magnum. Neurosurgery. (2000) 47:S155–93. doi: 10.1097/00006123-200009001-00017
31. Lee JM, Whang K, Cho SM, Kim JY, Oh JW, Koo YM, et al. Cranial nerve palsy after onyx embolization as a treatment for cerebral vascular malformation. J Cerebrovasc Endovasc Neurosurg. (2017) 19:189–95. doi: 10.7461/jcen.2017.19.3.189
32. Pei W, Huai-Zhang S, Shan-Cai X, Cheng G, Di Z. Isolated hypoglossal nerve palsy due to endovascular treatment of a dural arteriovenous fistula with Onyx-18. Interv Neuroradiol. (2010) 16:286–9. doi: 10.1177/159101991001600310
Keywords: foramen magnum DAVF foramen magnum, dural arteriovenous fistula, subarachnoid hemorrhage, myelopathy, Hybrid Angio-Surgical Suite
Citation: Xiao Z, Gao W, Zhou H, Zhang X, Dai J, Wan J and Guo L (2023) Clinical features, angio-architectural phenotypes, and treatment strategy of foramen magnum dural arteriovenous fistulas: a retrospective case series study. Front. Neurol. 14:1121075. doi: 10.3389/fneur.2023.1121075
Received: 11 December 2022; Accepted: 24 March 2023;
Published: 18 April 2023.
Edited by:
Osama O Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Feres Chaddad-Neto, Federal University of São Paulo, BrazilGuilin Li, Capital Medical University, China
Copyright © 2023 Xiao, Gao, Zhou, Zhang, Dai, Wan and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liemei Guo, Z3VvbG0wMDFAMTI2LmNvbQ==
 Zhipeng Xiao
Zhipeng Xiao Weizhen Gao
Weizhen Gao Liemei Guo
Liemei Guo