
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 08 March 2023
Sec. Experimental Therapeutics
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1117188
 Yun-Yun Hu1,2†
Yun-Yun Hu1,2† Gang Yang3†
Gang Yang3† Xue-Song Liang1,2,4
Xue-Song Liang1,2,4 Xuan-Si Ding1,2
Xuan-Si Ding1,2 De-En Xu5
De-En Xu5 Zhe Li1,6
Zhe Li1,6 Quan-Hong Ma1,2*
Quan-Hong Ma1,2* Rui Chen1*
Rui Chen1* Yan-Yun Sun1,2*
Yan-Yun Sun1,2*Transcranial ultrasound stimulation is a neurostimulation technique that has gradually attracted the attention of researchers, especially as a potential therapy for neurological disorders, because of its high spatial resolution, its good penetration depth, and its non-invasiveness. Ultrasound can be categorized as high-intensity and low-intensity based on the intensity of its acoustic wave. High-intensity ultrasound can be used for thermal ablation by taking advantage of its high-energy characteristics. Low-intensity ultrasound, which produces low energy, can be used as a means to regulate the nervous system. The present review describes the current status of research on low-intensity transcranial ultrasound stimulation (LITUS) in the treatment of neurological disorders, such as epilepsy, essential tremor, depression, Parkinson's disease (PD), and Alzheimer's disease (AD). This review summarizes preclinical and clinical studies using LITUS to treat the aforementioned neurological disorders and discusses their underlying mechanisms.
Treatment modalities for central nervous system (CNS) diseases include drug therapy, surgical therapy, and physical therapy. It is a major challenge to deliver drugs to a brain lesion via the bloodstream due to the particular anatomical structure of the blood–brain barrier (BBB). Physical treatment modalities offer a novel therapeutic opportunity for neurological disorders, especially for those for which effective drugs are not available. Physical treatment modalities currently available for neurological disorders include transcranial direct current stimulation (tDCS), transcranial magnetic stimulation (TMS), photobiomodulation (PBM), deep brain stimulation (DBS), and low-intensity transcranial ultrasound stimulation (LITUS), among others. Although these treatments show great promise for treating neurological disorders, their use is limited in practical clinical applications due to their invasiveness, their low spatial resolution, and the lack of clear mechanisms. TUS, on the other hand, is non-invasive, boasts high spatial resolution, and has high permeability. This review mainly summarizes the potential application of LITUS in neurological disorders, its underlying mechanisms, and the potential development and challenges in its therapeutic application in the future.
Before discussing the application of LITUS in the treatment of neurological disorders, we will provide a brief introduction to the other neuromodulation technologies, which are listed in introduction section. tDCS applies low-amplitude direct current through electrodes placed on the scalp, altering cortical excitability and spontaneous neural activity (1–5). tDCS has several advantages, such as cost-effectiveness, convenience, high tolerability, minimal side effects, and easy operability (6). However, the effect of tDCS is not robust enough for some clinical applications due to the characteristics of the electric field and the magnitude of the current produced by electrodes. Its penetration depth also need to be improved. Large individual differences have been observed in the effects of tDCS treatment, even when patients are subjected to the same parameters (7). TMS uses circular or figure-eight coils to produce a rapidly changing magnetic field. Through electromagnetic induction, its magnetic field generates eddy currents that can cause synchronous neuronal activity in targeted cortical areas at a resolution of several centimeters (8–12). On the basis of frequency, TMS can be divided into two categories: low-frequency repetitive TMS (≤1 Hz) and high-frequency repetitive TMS (≥5 Hz) (13, 14). Low-frequency TMS leads to a transient decrease in local cortical activity, while high-frequency TMS increases the excitability of local cortical neurons (13, 14). Additionally, TMS can be sorted into two stimulation modes: intermittent theta burst (iTBS) and continuous theta burst (cTBS). iTBS can cause local cortical excitation, while cTBS temporarily inhibits brain signals (15–17). Although the depth of the target region can be adjusted through coils, the spatial resolution and penetration depth of TMS are limited by the magnetic field conductivity and permeability (18). Researchers have attempted to develop TMS hardware that can specifically affect the human brain, such as the triple halo coil, which modulates excitability in the subcortical brain regions (as deep as 10 cm), and the quadruple butterfly coil, which reduces the volume of stimulation by approximately 70% (19). DBS delivers a continuous flow of current to specific neuroanatomical targets through electrodes that are surgically inserted into the brain (20). The invasiveness of DBS limits its therapeutic application. PBM, on the other hand, is considered a non-thermal technique because it uses non-ionizing radiation in the visible (400–700 nm) and near-infrared (700–1,100 nm) ranges of the electromagnetic spectrum, such as lasers, light-emitting diodes, and/or broadband light, to cause photophysical and photochemical events (21, 22). However, the length and strength of light delivery to the brain always pose challenges (23). Therefore, novel neuromodulation technology that is non-invasive and has a high spatial resolution is required to treat neurological disorders. In this context, LITUS, due to its non-invasive nature and high spatial resolution with millimeter-grade accuracy, has attracted researchers' attention (24–26). A pioneering study by Fry and colleagues in 1958 discovered the neuromodulatory potential of ultrasound stimulation. They discovered that stimulating the lateral geniculate nucleus of the thalamus with ultrasound reversibly inhibited the visual pathway in cats (27). In 2002, after neuroimaging experiments in patients with psychiatric disorders, Bystritsky proposed that ultrasound could be used for neuromodulation with therapeutic benefits for psychiatric and neurological disorders (28, 29). Since then, an increasing number of studies have demonstrated the neuromodulatory effect of ultrasound.
Ultrasound is a mechanical pressure wave with a frequency >20 kHz that can penetrate soft tissue at a specific wavelength (30). It has strong penetration, good directionality, and high spatial resolution and is non-invasive (18, 31). With these characteristics, ultrasound is used medically as a diagnostic technique (32). As understanding has deepened, it reveals great potential in the treatment of neurological disorders. Unlike diagnostic ultrasound, which requires a frequency range of 1–15 MHz, therapeutic ultrasound generally uses a specific frequency of approximately 1 MHz (33). Ultrasound is applied clinically using high-intensity or low-intensity acoustic waves (34–36). The peak power levels of high-intensity ultrasound can be >1,000 W/cm2, while low-intensity ultrasound is usually 30–500 mW/cm2 (37). High-intensity ultrasound has therapeutic effects, which can be achieved by focusing ultrasound on a specific area or point, causing a rapid temperature increase that destroys the tissue. In contrast, low-intensity ultrasound, which produces less energy, inflicts less damage to the tissue. LITUS mostly uses medium-frequency (650 kHz) or low-frequency (220 kHz) ultrasound (38).
With these characteristics, ultrasound is used medically as a diagnostic technique (32). As understanding has deepened, it reveals great potential in the treatment of neurological disorders
The main components of ultrasonic stimulation systems include a signal generator, a radio-frequency (RF) power amplifier, an ultrasonic transducer, a hydrophone, a transducer fixing device, and an ultrasonic coupling agent (Figure 1). Among these components, the ultrasonic transducer is the core of the whole system, taking advantage of the inverse piezoelectric effect to transform the applied electrical input into mechanical vibration and focus the ultrasound on a target region (18). Curved units that focus the stimulation on oval regions are the most commonly used type of ultrasound transducer (39–46). With this type of ultrasound transducer, the focal volume spans multiple brain subregions in the axial direction of the beam, covering a large area (47) and providing limited target specificity. To overcome this limitation, some researchers use a crossed-beam dual-transducer system (48) to improve the high axial resolution of ultrasound neuromodulation, while others select much higher frequencies, such as 5 MHz, to improve the anatomical specificity (47). The stimulation system enables the adjustment of several key parameters of the ultrasound, including fundamental frequency (FF), pulsed repletion frequency (PRF), stimulation duration (SD), tone-burst duration (TBD), duty cycle (DC), number of tone bursts (NTB), interstimulus interval (ISI), spatial-peak pulse-average intensity (Isppa), and spatial-peak time-average intensity (Ispta) (49, 50). By adjusting these parameters, ultrasound with different frequencies, wavelengths, and acoustic intensities can be generated. Considering its use in the therapy of neurological disorders, it is worth noting that the presence of the skull weakens and distorts the ultrasound signal, affecting the brain tissue (51, 52) and thereby increasing the difficulty of precisely stimulating the brain. Thus, researchers need to set the parameters of ultrasonic stimulation systems with the help of hydrophones to minimize the effects of the skull. Various techniques are available to confirm the neuromodulatory effects of ultrasound stimulation, such as electroencephalography (EEG) (40, 41, 53), electromyography (EMG) (49, 54), functional magnetic resonance imaging (fMRI) (44, 50), and positron emission tomography-computed tomography (PET-CT) (55). In addition, the measurement of extracellular levels of neurotransmitters and metabolic changes can also reflect the effects of ultrasound stimulation (56).
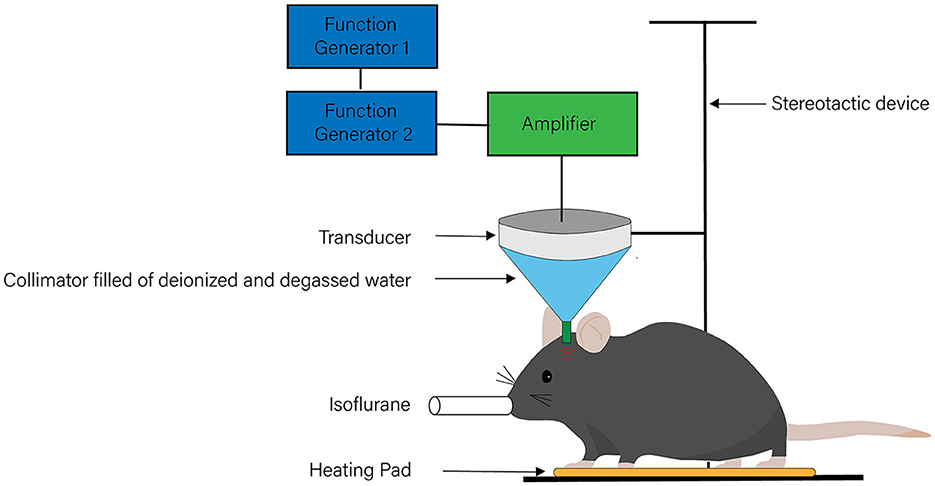
Figure 1. The system of transcranial ultrasound stimulation. The system of transcranial ultrasound stimulation mainly includes a signal generator, a radio-frequency power amplifier, an ultrasonic transducer, a hydrophone, a transducer fixing device, and an ultrasonic coupling agent.
Epilepsy is a highly prevalent neurological disorder characterized by recurrent episodes of neuronal hyperexcitability or inadequate inhibition (57, 58). It can be caused by brain injury or genetic factors involved in neuronal activity. During seizures, the abnormally synchronous activity in the epileptic foci may spread to other brain regions, eventually causing behavioral abnormalities (59–61). Existing treatments for epilepsy include medical therapy, surgical treatment, and neuromodulation. Medically intractable epilepsy can be treated by removing the epileptogenic focus. However, for some epileptogenic foci that are located in eloquent brain areas or are too numerous, diffuse, or bihemispheric, surgery is not suitable. In such cases, non-invasive neuromodulation offers a viable option for seizure control (62). Laser interstitial thermal therapy (LITT) is a new, minimally invasive technology that has been shown to be effective in treating temporal lobe epilepsy (TLE). It uses a laser through an inserted optical fiber to ablate the epileptogenic focus (63). However, this type of treatment also damages the targeted tissue, causing a decline in brain function and memory, although it is less invasive than surgery (64). Therefore, new technology is indeed needed to reduce the damage and decrease the frequency of seizures. LITUS, a non-invasive physical therapy, has been investigated in preclinical and clinical experiments.
As early as 2011, LITUS was applied to the thalamus of a pentetrazol (PTZ)-induced epilepsy model in rats. After inducing acute seizures in model rats, a series of 0.5-ms-long pulses of sonication were delivered to the thalamic region two times for 3 min, each with a repetition rate of 100 Hz and FF = 690 kHz. EEG recordings revealed that the occurrence of epileptic EEG bursts in rat models was significantly reduced after ultrasound treatment (53). This evidence suggests that LITUS holds promise as a therapeutic tool for the non-invasive suppression of epileptic activity. Some researchers injected kainite (KA) into the CA3 region of the hippocampus of mice to induce mesial temporal lobe epilepsy. In these model mice, ultrasound delayed the onset of status epilepticus (SE) and inhibited acute seizure activity (65). Chu PC and colleagues found that, in the KA-induced epilepsy mouse model, LITUS could reduce the occurrence of seizures, and the effects lasted as long as 7 weeks (66).
Additionally, other research indicates that ultrasound stimulation can decrease the power spectrum intensity of low-frequency (<10 Hz) local field potentials (LFPs), weaken the phase-amplitude coupling intensity between slow and fast nerve oscillations, and increase the time interval of seizures. These results indicate the capability of ultrasound to decrease the power spectrum of LFPs, thereby reducing the onset of epilepsy (67, 68). In addition to exploring the effects of LITUS in rodents, other researchers have attempted to investigate the influence of LITUS in non-human primates. Lin Z and colleagues found that ultrasound stimulation lowered the frequency, duration, and interval of seizures in a penicillin-induced non-human primate model of epilepsy (57). Additionally, Zou's study showed that LITUS decreased the number and duration of seizures in a monkey model of acute epilepsy (69). These lines of evidence strengthen the therapeutic potential of ultrasound stimulation in epilepsy. With the development of this technology, some researchers have begun to apply LITUS to patients with epilepsy.
In a recent study, Lee et al. used LITUS in patients with drug-resistant epilepsy. Two of the patients experienced a decrease in seizure frequency, while one patient showed an increase. The results of LIFUS were only observed in the electrode contacts located at the targeted site, as observed in the SEEG recordings taken before, during, and after treatment. In both patients, LIFUS resulted in a significant reduction in spectral power across all frequency bands. Unfortunately, no correlation was established between these short-term effects and changes in seizure frequency (70). Low-frequency stimulation with magnetic resonance-guided focused ultrasound (MRgFUS) was recently reported to be effective in a patient with medically intractable epilepsy. The patient remained seizure-free for up to 12 months (71). In another study, they developed a device platform to deliver pulsed low-intensity focused ultrasound to the brain region under the hippocampus in humans. After multiple sessions, no adverse events occurred (72). The safety and feasibility of ultrasound stimulation need to be evaluated in future studies with a larger number of participants and a longer duration of follow-up. Thus, to date, the therapeutic evidence of LITUS in epilepsy has mainly been limited to preclinical studies (Table 1), where ultrasound stimulation exhibits great potential in epilepsy therapy. Therefore, more preclinical and clinical studies are still needed to determine how to apply LITUS to the clinical treatment of epilepsy.
Several researchers have discovered some potential mechanisms of the therapeutic effect of LITUS on epilepsy. Chen SG et al. showed that LITUS could change the activity of excitatory neurons, activate GABAergic terminals, downregulate S6 phosphorylation, and decrease pAKT expression (73). Lin Z and his colleagues conducted in-depth studies of the potential mechanisms. They found that LITUS can readjust the imbalance of synaptic inputs to inhibit epileptiform discharges and activate interneurons to increase inhibitory synaptic inputs (57). In conclusion, the aforementioned findings suggest that the therapeutic effects of LITUS are associated with the modulation of neuronal activity and the distribution of inhibitory neuronal axons. The effects and potential mechanisms of LITUS in the therapy of epilepsy need more research in preclinical and clinical experiments.
Essential tremor (ET) is one of the most common movement disorders among adults and is characterized by postural and kinetic tremors (74, 75). The most recognized feature of ET is a kinetic tremor of the arms, the hands, or the fingers occurring during voluntary movements (76, 77). During voluntary movements, it occasionally occurs in the head, the vocal cords, or other body parts (78). The clinical therapy of ET mainly relies on drug therapy. The first-line oral agents include propranolol and primidone. However, nearly half of patients fail to respond to these oral drugs (79, 80). Before the 1990s, surgical intervention was the only option for patients with severe ET who were unresponsive to oral medicines. The main surgical intervention then was thalamic lesioning. With the advent of DBS, this treatment modality was gradually replaced by DBS. The implantation sites of DBS electrodes are usually the ventral intermediate nucleus (ViM) (81–83) and the caudal zona incerta (cZI) (84, 85). DBS at these two sites alleviates the symptoms of patients with ET with long-term effects. However, the implantation of electrodes can result in side effects for some patients, such as limb paresthesia (which usually improves with programming adjustments), dysarthria, disequilibrium, and skin infections/breakdown (79). As a less invasive approach, MRgFUS is gradually applied in patients with ET, where the thalamic ViM nucleus remains the main action target. MRgFUS thalamotomy exhibits therapeutic effects in patients with ET (86–90) and has been approved by the Food and Drug Administration (FDA) for unilateral treatment of ET (62, 89). Thermal ablation of the thalamotomy still causes side effects similar to those of MRgFUS, including dizziness (early), nausea/vomiting (early), headache (early), flushing (early), ataxia (late), and paresthesias (late) (79). Thus, researchers have explored the potential of neuromodulatory non-thermal LITUS for tremor suppression. When applied to the inferior olivary (IO) system of the harmaline-induced mouse model of ET with an intensity of 27.2 W/cm2 (Isppa), LITUS significantly reduced the tremor frequency of model mice (91). This study demonstrates the feasibility of the non-thermal effects of LITUS for tremor treatment. However, more studies are required to establish the technical parameters and mechanism of using low-intensity ultrasound for ET therapy.
Depression is one of the most common psychiatric disorders. While antidepressant drugs combined with psychotherapy have shown noticeable therapeutic effects, some patients fail to respond to such therapeutic treatments and may experience serious adverse reactions. In preclinical studies, ultrasound stimulation exhibits excellent therapeutic effects on depression. Stimulation with LIFUS on either the prefrontal cortex or the ventromedial prefrontal cortex (vmPFC) attenuated the depressive behaviors of depressed model rats, accompanied by enhanced brain-derived neurotrophic factor (BDNF) levels, whose downregulation is closely linked with depression. Notably, LIFUS improved BDNF levels in the hippocampus of normal mice, suggesting a common mechanism of BDNF signaling induced by ultrasound stimulation.
Moreover, LIFUS enhanced the proliferation and neurogenesis of adult hippocampal neural stem cells (92). The latter is also an essential mechanism underlying depression and is the effect of antidepressant drugs (93, 94). Sha-Sha Yi et al. recently found that LIFUS can alleviate the behaviors of lipopolysaccharide-induced depressed mice. Moreover, the lipopolysaccharide-mediated upregulation of inflammatory cytokines was significantly reduced by LIFUS (95). As in other neurological disorders, the therapeutic effects of some drugs and other factors on depression are limited by the intrinsic properties of the BBB. Relying on its capability of temporarily opening the BBB, MRgFUS together with microbubbles (MBs) successfully and accurately delivered glial cell line-derived neurotrophic factor (GDNF) to the brain, alleviating the symptoms of chronically stressed mice (96). However, the therapeutic potential of non-thermal ultrasound stimulation has not yet been expanded in clinical research. In clinical research, few studies have tested the potential therapeutic effects of tFUS in depression via thermal ablation of targeted brain areas. Some researchers have recently investigated whether ultrasound may modulate mood. For example, Joseph L. Sanguinetti et al. found that targeting the right ventrolateral prefrontal cortex via tFUS elevated the mood of healthy people after approximately 30 min (97).
Similarly, experiments have shown that LIFUS has the potential to improve mood in healthy subjects (29). Reznik SJ and others performed ultrasound processing on the right frontotemporal cortex of patients with depression and found that it could improve their moods (A double-blind pilot study of transcranial ultrasound (TUS) as a five-day intervention: TUS mitigates worry among depressed patients). These studies provide evidence for the use of LIFUS in the treatment of depression, but more experiments are still needed to verify the improvement effect of LIFUS on depression symptoms.
Parkinson's disease (PD) is the second most common neurodegenerative disease, with clinical symptoms mainly characterized by increased muscle tension, resting tremors, postural instability, and reduced action potentials, accompanied by manifestations of non-motor systems such as autonomic dysfunction and olfactory dysfunction (98). The pathological changes in PD are the gradual loss of nigrostriatal dopaminergic neurons (99, 100). At present, PD therapy mainly relies on levodopa and other drugs to supplement dopamine. Deep brain stimulation (DBS) has exhibited therapeutic effects in reducing the motor symptoms of PD and the side effects associated with long-term dopamine replacement drugs. To date, the stimulation areas have mainly focused on the Vim (101) of the thalamus, the subthalamic nucleus (STN) (102), the globus pallidus interna (GPi) (103), and the cuneiform nucleus (104). However, DBS requires the implantation of electrodes in the corresponding brain regions of patients. It is invasive and poses a risk of infection and cerebral hemorrhage. Jeanmonod et al. reported the feasibility of ultrasound in patients with PD for the first time in 2012. They ablated the fibers that join the thalamus with the globus pallidus by ultrasound delivery. Repeated ultrasound stimulation improved the Unified Parkinson's Disease Rating Scale (UPDRS) score by 57.1% (105). The therapeutic effects of ultrasound stimulation were confirmed by Magara et al. in 2014, who damaged the unilateral pallidothalamic tract in patients with PD using MRgFUS. In this study, 3 months after the surgery, the UPDRS score was significantly improved (106). Afterward, an increasing number of research teams applied ultrasonic ablation in patients with PD, especially with tremor-predominant PD (107–111). However, the treatment modality of high-energy ultrasound ablation also carries the risk of causing serious side effects, such as speech disorders and ataxia (112, 113). As a noninvasive stimulation method, the feasibility of LITUS in PD animal models has been confirmed (Table 2). Hui Zhou et al. first confirmed that the use of ultrasound to stimulate the STN and GP improves locomotor behavior in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse model (114). Subsequently, other researchers also observed the beneficial effect of LITUS in PD animal models (114–119). With the advent of ultrasound combined with microbubble technology, an increasing number of researchers have used this technology to enhance blood-brain-barrier permeability to achieve local drug release in the treatment of PD (120–122).
With regard to the underlying mechanisms for LITUS in the treatment of PD, LITUS was observed to alter the extracellular concentration levels of dopamine and serotonin (5-hydroxytryptamine, or 5-HT), suggesting a capability of ultrasound stimulation in the regulation of the local release and uptake, or degradation, of these neurotransmitters (53). The drug treatment for PD in clinical practice relies mainly on the use of levodopa to increase the level of dopamine. This study provides ideas for ways in which LITUS could improve the symptoms of PD. Exposure to MPTP causes a syndrome that mimics core neurological symptoms and the relatively selective dopaminergic neurodegeneration of PD (123, 124). Pretreatment with LITUS inhibited MPP+-induced neurotoxicity and mitochondrial dysfunction in PC12 cells and N2a cells (124). Consistently stimulating the motor cortex with LITUS enhanced the levels of T-SOD and GSH-PX in the striatum in MPTP-treated mice (114). These results indicate the role of LITUS in attenuating MPTP-induced mitochondrial dysfunction. Recently, Wen-Shin Song and his team discovered that LIFUS could effectively inhibit glial activation and reduce the phosphorylation of nuclear factor-κB p65 in the substantia nigra pars compacta.
Additionally, it helps to maintain normal levels of neurotrophic factors, dopamine transporters, and tight junction proteins in the blood-brain barrier in PD induced by 6-OHDA (125). Moreover, ultrasound stimulation also exhibits the potential to alter cortical excitability. LIFUS reduced parkinsonian-related brain electrical activity in an MPTP-induced mouse model of PD, as shown by the mean power intensity in the beta band in LFPs, as well as the phase-amplitude coupling intensity between the beta and high gamma bands and between the beta and ripple bands (118). Through these multiple mechanisms, LITUS attenuated dopaminergic neurodegeneration and locomotor deficits in various PD animal models. Notably, improving the sensitivity of ultrasound stimulation with the help of mPrestin (N7T, N308S), an engineered auditory-sensing protein, further ameliorated dopaminergic neurodegeneration and the symptoms of PD in MitoPark mice (mice that exhibit several cardinal features of human PD) (126–128). Thus, preclinical studies strengthen the therapeutic effects of LITUS in PD models. However, the longer-term effects of ultrasound stimulation on PD remain to be further investigated.
Alzheimer's disease (AD) is a common neurodegenerative disease clinically manifested through the progressive loss of cognitive and memory function, which is pathologically characterized by the accumulation of β-amyloid plaques (Aβ) and hyperphosphorylated tau. The main therapy for AD is drug therapy. One of the challenges of drug therapy for AD is the low efficacy of drugs entering the brain due to the hindrance of the BBB. For example, the anti-Aβ antibody, which helps to clear Aβ load in the brain, has limited capability to enter the brain. LITUS exhibits the capability to temporally open the BBB to allow such drugs to enter the brain. The BBB can be temporarily opened, and then integrity is restored after 4–6 h by LITUS in combination with microbubbles (129, 130). With such a capability, LITUS enhances the delivery of anti-Aβ antibodies or other drugs to the targeted brain regions, thus reducing plaque load, alleviating the cleavage of Tau protein, and rescuing the function of neurons (131). In addition to helping to deliver drugs, the transient opening of the BBB by FUS has beneficial effects on AD model mice. With the use of microbubbles, focused ultrasound stimulation of the hippocampus, the cortex, or even the whole brain in different transgenic AD mouse models, such as TgCRND8, 3xTg, and 5xFAD, without the need for additional therapeutic agents, was efficient for Aβ clearance (132–140) (Table 3). In the context of the transient opening of the BBB, it is worth noting that the BBB in the brains of AD model mice was compromised.
One explanation for the reduction in Aβ load by LITUS is that it may increase the production of endogenous Aβ antibodies, as Jessica F Jordão et al. found endogenous antibodies bound to Aβ plaques in the cortex of an ultrasound-treated TgCRND8 mouse model of AD (132). Another possibility is that LITUS enhanced the capability of phagocytosis of Aβ by microglia (132, 135, 136, 139). However, in terms of microglial activation, the effects of LITUS seem to be controversial. Eguchiet et al. found that ultrasound stimulation reduced microglial activation in the 5 × FAD transgenic mouse model (135). Leinenga and Gotz et al. observed no change in inflammatory markers in the brains of aged APP23 mice after ultrasound stimulation (139). Thus, the questions of how and whether LITUS-affected glial function contributes to AD pathogenesis remain to be further investigated. In addition, LITUS may enhance neuronal function in AD brains as well. LITUS enhanced axonal neurofilaments in 3 × Tg-AD mice (134) and attenuated the loss of neurons in a 5 × FAD-AD mouse model (137). Burgess et al. observed that ultrasound stimulation increased the number of immature neurons, total dendrite length, and dendrite branching in preexisting or mature neurons in TgCRND8 mice (133). In terms of the molecular mechanisms underlying the beneficial roles of LITUS in AD, some studies have found that this treatment can enhance autophagy, which is compromised in the brains of AD and aging (141, 142).
Through these multiple mechanisms, ultrasound stimulation eventually ameliorates cognitive decline in AD in model animals (133, 135, 137, 139). However, although ultrasound effectively reduces Aβ plaque formation in AD animals, this effect may be attenuated with time after stimulation (132, 143). Therefore, the question of how to prolong the long-term effect of ultrasound on AD therapy remains to be further investigated. The potential of LITUS in AD therapy has also been examined in clinical studies. Ultrasound stimulation combined with microbubbles in the right frontal lobe in patients with AD two times with a 1-month interval successfully opened the BBB but failed to alter the Aβ load (144). The ability of ultrasound stimulation to open the BBB in patients with AD needs further confirmation from other researchers (145–147). Consistent with the observation in preclinical studies that ultrasound stimulation successfully reduces Aβ load, a recent clinical trial showed that Aβ plaques in the hippocampus and entorhinal cortex were reduced 1 week after ultrasound stimulation (interval weeks) in patients with early AD (148). Nicodemus et al. and Beisteiner et al. confirmed that ultrasound stimulation in the cortex of patients with AD for 3 months improved cognitive function (149, 150). Stéphane Epelbaum et al. reported that repeated BBB disruption by ultrasound with microbubbles had a non-significant decline in amyloid accumulation after 4 months (151). The ameliorative effect of LIFUS on pathological parameters in patients with AD in these experiments was based on inducing the opening of BBB. However, Hyeonseok Jeong and colleagues evaluated the safety and efficacy of low-intensity tFUS under the threshold for BBB disruption in patients with AD. They found that, in the absence of an open BBB, the measures of memory, executive function, and global cognitive function were mildly improved (152).
To conclude, LITUS can reduce seizures in models of epileptic disease, improve motor deficits, stimulate dopamine release, reduce EEG activity in PD models, improve depressive phenotypes, and rescue cognitive impairment and neuronal damage in AD models, providing a potential future treatment modality for patients with clear foci who do not wish to undergo invasive treatment.
As a mechanical wave, ultrasound can propagate in solids and liquids and exert biological effects on cells and tissues, mainly including thermal effects, mechanical effects, cavitation effects, and so on Table 4. Focusing ultrasound on the ventrolateral nucleus of the thalamus in rats reversibly inhibits somatosensory evoked potentials (SSEPs) spatially in an intensity-dependent manner. The inhibitory effect is consistent in time with the temperature change in vivo without producing pathological changes at the tissue level. Stereotactic delivery of thermal energy through optical fibers at the same site also produces similar thermal effects and inhibitory effects (153), suggesting that focused ultrasound may cause neuroinhibitory effects through the thermal effect of ultrasound. Although low-intensity ultrasound does not produce thermal ablation of tissue, the accumulation of ultrasonic energy still increases the local temperature without causing damage. However, the existing evidence is still insufficient to determine whether the increased local temperature caused by ultrasonic focusing is involved in its regulatory mechanism. Tyler et al. (37) applied low-intensity and low-frequency ultrasound to hippocampal slices and mouse brains, and they found that low-intensity ultrasound enhances the electrical activity of neurons by activating voltage-gated sodium channels and calcium channels, as well as improving synaptic transmission in the CNS. The neurons of C. elegans expressing TRP4, a stretch-sensitive cationic mechanotransduction channel, are more sensitive to ultrasound stimulation (154). Oh et al. observed that astrocytes are also cellular targets for low-intensity ultrasound stimulation (155). Low-intensity ultrasound-induced neuromodulation is initiated by the opening of TRPA1 channels, a member of the transient receptor potential (TRP) family, in astrocytes. Ca2+ entry via TRPA1 causes the release of gliotransmitters, including glutamate, in astrocytes, which activates NMDA receptors in neighboring neurons to cause action potential firing. In addition, the expression of a mechanosensitive channel (MscL) also makes neurons or cells more susceptible to activation by low-intensity ultrasound (156, 157), suggesting that ultrasound may also modulate the nervous system by activating mechanosensitive ion channels on the cell surface through its mechanical effects. As a unique physical phenomenon of ultrasound, the cavitation effect has been largely studied and utilized in the treatment of diseases. When ultrasound propagates in fluid or soft tissue containing microbubbles, it can control the contraction and expansion of bubbles. Based on this cavitation effect of ultrasound, combined with intravenous injection of microbubbles, the blood–brain barrier in the brain can be temporarily opened to achieve drug delivery in specific brain regions to achieve precise treatment of the lesion site. For example, focused ultrasound can rescue choline function by delivering selective TrkA agonists into the brains of AD mouse models (158). Hameroff and colleagues propose that ultrasound stimulation at specific megahertz frequency bands can resonate with microtubules, causing them to vibrate when the ultrasound beam angle aligns with their long axis (29). This vibration could then modulate electrical signals in the brain by affecting synaptic plasticity through the connection between microtubules and actin filaments in dendritic spines (159). An increasing number of researchers have recently noticed the presence of auditory confounds during ultrasonic stimulation in humans and animals, considering that the auditory signaling pathways may confound the direct regulatory effects of ultrasound. To investigate whether hearing has an effect during ultrasound stimulation, Guo et al. showed that transection of the auditory nerves or removal of cochlear fluid eliminates US-induced cortical and subcortical activity (160). They indicate that ultrasound activates the ascending auditory system through a cochlear pathway, which activates other non-auditory regions through cross-model projections. In contrast to this observation, Wang et al. found that ultrasound was still capable of inducing neural activity and motor responses, even in chemically deafened PD model mice, suggesting that ultrasound induces neuromodulation via multiple action modes that include both direct and indirect effects (118). According to some research, ramping the stimulation onset and offset over several milliseconds can eliminate auditory activation in mice (161). In a clinical experiment, investigators found that a concurrent audio mask applied at the PRF can also reduce auditory perception (162). Other research has shown that ramping and masking TUS stimulation prevent some participants' perception, while the effect of these two methods is not additive (4). Our understanding of the mechanisms underlying ultrasound-induced neuromodulation is currently limited, and more research is needed to advance our knowledge in this area.
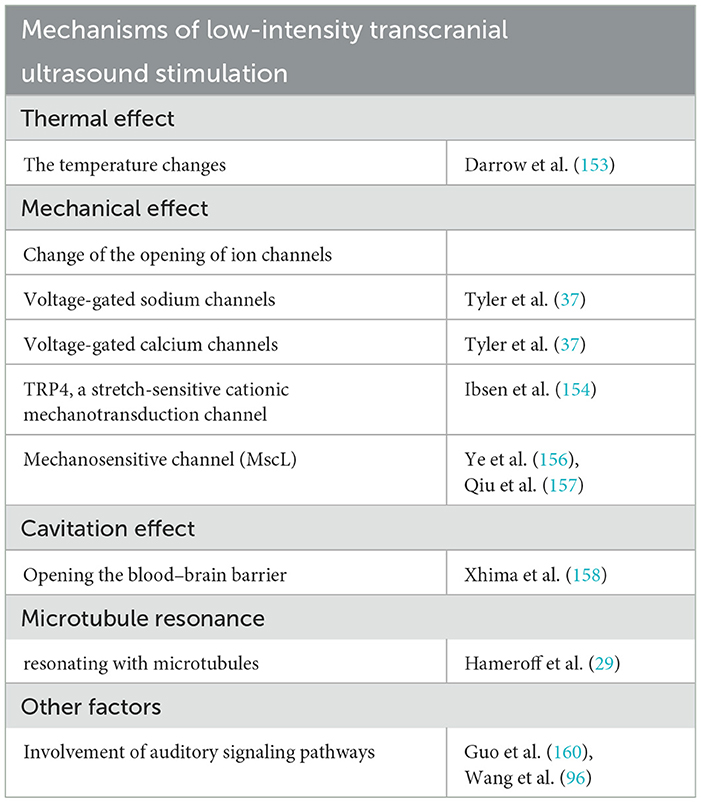
Table 4. Mechanistic study of transcranial ultrasound stimulation in the treatment of central nervous system diseases.
Due to its high spatial resolution and high penetration rate, transcranial ultrasound stimulation is of great significance for the treatment of CNS disorders. It can induce neuromodulation in deep brain regions non-invasively, making it a valuable tool for therapeutic applications. However, this technique is still new, especially for clinical applications. A number of technical problems and challenges urgently need to be addressed by future research (12, 163, 164). First, although preclinical studies show that ultrasound can act on the deep tissue of the brain in the stereotaxic mode, the ultrasonic devices in the existing studies are assembled by individual research teams, which results in a lack of uniform standards for ultrasonic action parameters. From the selection of the devices and the ultrasonic parameters used by each research team, it can be seen that the FF of the focused ultrasound transducer plays a key role in the focal length and focal size of ultrasound (165–167). Second, the voltage wave of ultrasound is converted by the focused ultrasound transducer in a region called the focal spot. The length and width of the focal spot change with the central frequency of the transducer. The larger the frequency, the smaller the focal spot range and the more accurate the active range. However, due to the thermal effect of ultrasound—, the temperature of the action site increases with increasing central frequency, which—may cause thermal damage to the tissue (168, 169). This poses a challenge when selecting different ultrasonic transducers to achieve accurate positioning of the target region. Finally, LITUS requires an ultrasound to act on brain tissue through the skull. Since the acoustic impedance of the skull is greater than that of air, ultrasound will produce different degrees of attenuation when passing through the skull. Therefore, the ultrasonic intensity and energy reaching the target area will be reduced to different levels. Differences in skull thickness among different animals cause varying degrees of ultrasound attenuation upon passing through the skull, which poses challenges for the clinical application of transcranial ultrasound stimulation (170, 171).
In 2003, Norton proposed a new potential technique to stimulate the brain non-invasively; this technique, known as transcranial magnetoacoustic stimulation (TMAS), makes it possible to use LITUS within a static magnetic field (172, 173). TMAS treatment is based on the application of focused ultrasound to a target area within a static magnetic field. In the ultrasonically excited conductive brain, ionic particles induce transient currents generated by Lorentzian forces in a magnetic field. According to Faraday's law, the proportional relationship between the generated electric field and the velocity of ionic particles makes it possible to manipulate the stimulation effect (172, 174). This gives the TMAS an advantage in stimulating specific deep brain regions of small size. Wang H and colleagues first quantified the amplitude and response latency of cortical motor electromyography (EMG) in mice by TMAS compared to LITUS. They found that TMAS could shorten the response time of nerve activity and increase the neuromodulation effect of LITUS on the motor cortex (175). In recent years, more refined and accurate stimulation needs have been proposed with the development of closed-loop brain stimulation techniques, such as DBS, optogenetics, and TMS (176–178). Compared with open-loop brain stimulation, closed-loop brain stimulation can be stimulated as needed according to the received state signal of the brain (179–181), thereby producing the most effective stimulation effect on the brain while reducing the amount of stimulation (182–184). Yang et al. developed a closed-loop transcranial ultrasound stimulation system (CLTUS) for real-time, non-invasive neuromodulation in vivo. The application of CLTUS in a mouse model of temporal lobe epilepsy (TLE) inhibits seizures in real time by detecting epileptic echoes online (185). The ultimate purpose of combining ultrasound with different techniques is to enhance its effectiveness in treating diseases. Further studies are needed in the future to prove the feasibility and effectiveness of these different techniques and finally apply them in clinical practice.
Although transcranial ultrasound stimulation is still a new technique, it has already shown great potential in the treatment of CNS disorders in preclinical studies. Therefore, as an emerging treatment modality, it is believed that the aforementioned problems and challenges will be answered and solved in future studies. In addition, TMAS provides low millimeter-scale spatial resolution even in deep brain regions, with a 10-fold higher focus than TMS due to the use of focused ultrasound.
This article reviews the preclinical and clinical studies of LITUS in the treatment of neurological disorders and summarizes the possible underlying mechanisms. As a non-invasive neuromodulation approach, LITUS exhibits great potential for the therapy of neurological disorders such as epilepsy, ET, PD, and AD, despite their distinct pathological mechanisms. However, the therapeutic application of LITUS for various neurological disorders is far from well-established. Therefore, further exploration is required to enhance the precision and specificity of stimulation by defining the target region and the stimulation parameters in distinct neurological disorders. Moreover, a better understanding of the mechanism underlying the therapeutic effects of LITUS will help accelerate the clinical application of this technology.
Y-YH, Q-HM, and Y-YS contributed to the conception and design of the study. Y-YH, GY, and X-SL organized the database. Y-YH wrote the first draft of the manuscript. GY, X-SD, RC, Q-HM, and Y-YS wrote sections of the manuscript. D-EX, ZL, and RC provided further insights and co-authored the final manuscript alongside Y-YS. All authors contributed to the final revision of the manuscript and read and approved the submitted version.
This study was funded by the National Natural Science Foundation of China (92049120, 81870897, 81770085, 82070095, and 81901296), the Guangdong Key Project in the Development of New Tools for the Diagnosis and Treatment of Autism (2018B030335001), the Natural Science Foundation of Jiangsu Province (BK20181436), the National Major Scientific and Technological Special Project for Significant New Drugs Development (2019ZX09301102), the Discipline Construction Program of the Second Affiliated Hospital of Soochow University (XKTJ-TD202003), STI2030-Major Projects (2021ZD0204001), and Sino German cooperation and exchange project (M-0679).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. (2000) 527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
2. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. (2001) 57:1899–901. doi: 10.1212/WNL.57.10.1899
3. Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. (2012) 5:175–95. doi: 10.1016/j.brs.2011.03.002
4. Johnstone A, Nandi T, Martin E, Bestmann S, Stagg C, Treeby B, et al. range of pulses commonly used for human transcranial ultrasound stimulation are clearly audible. Brain Stimul. (2021) 14:1353–5. doi: 10.1016/j.brs.2021.08.015
5. Wang Y, Wang J, Zhang QF, Xiao KW, Wang L, Yu QP, et al. Neural mechanism underlying task-specific enhancement of motor learning by concurrent transcranial direct current stimulation. Neurosci Bull. (2022). doi: 10.1101/2021.01.31.429080
6. Pacheco-Barrios K, Cardenas-Rojas A, Thibaut A, Costa B, Ferreira I, Caumo W, et al. Methods and strategies of tDCS for the treatment of pain: current status and future directions. Expert Rev Med Devices. (2020) 17:879–98. doi: 10.1080/17434440.2020.1816168
7. Filmer HL, Ballard T, Ehrhardt SE, Bollmann S, Shaw TB, Mattingley JB, et al. Dissociable effects of tDCS polarity on latent decision processes are associated with individual differences in neurochemical concentrations and cortical morphology. Neuropsychologia. (2020) 141:107433. doi: 10.1016/j.neuropsychologia.2020.107433
8. Johnson MD, Lim HH, Netoff TI, Connolly AT, Johnson N, Roy A, et al. Neuromodulation for brain disorders: challenges and opportunities. IEEE Trans Biomed Eng. (2013) 60:610–24. doi: 10.1109/TBME.2013.2244890
9. Wagner T, Valero-Cabre A, Pascual-Leone A. Non-invasive human brain stimulation. Annu Rev Biomed Eng. (2007) 9:527–65. doi: 10.1146/annurev.bioeng.9.061206.133100
10. Gomez LJ, Goetz SM, Peterchev AV. Design of transcranial magnetic stimulation coils with optimal trade-off between depth, focality, and energy. J Neural Eng. (2018) 15:046033. doi: 10.1088/1741-2552/aac967
11. Guo Q, Li C, Wang J. Updated review on the clinical use of repetitive transcranial magnetic stimulation in psychiatric disorders. Neurosci Bull. (2017) 33:747–56. doi: 10.1007/s12264-017-0185-3
12. Zhong G, Yang Z, Jiang T. Precise modulation strategies for transcranial magnetic stimulation: advances and future directions. Neurosci Bull. (2021) 37:1718–34. doi: 10.1007/s12264-021-00781-x
13. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. (2006) 117:2584–96. doi: 10.1016/j.clinph.2006.06.712
14. Cao X, Deng C, Su X, Guo Y. Response and remission rates following high-frequency vs. low-frequency repetitive transcranial magnetic stimulation (rTMS) over right DLPFC for treating major depressive disorder (MDD): a meta-analysis of randomized, double-blind trials. Front Psychiatry. (2018) 9:413. doi: 10.3389/fpsyt.2018.00413
15. Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. (2005) 45:201–6. doi: 10.1016/j.neuron.2004.12.033
16. Huang YZ, Sommer M, Thickbroom G, Hamada M, Pascual-Leonne A, Paulus W, et al. Consensus: new methodologies for brain stimulation. Brain Stimul. (2009) 2:2–13. doi: 10.1016/j.brs.2008.09.007
17. Ozdemir RA, Boucher P, Fried PJ, Momi D, Jannati A, Pascual-Leone A, et al. Reproducibility of cortical response modulation induced by intermittent and continuous theta-burst stimulation of the human motor cortex. Brain Stimul. (2021) 14:949–64. doi: 10.1016/j.brs.2021.05.013
18. Yu K, Niu X, He B. Neuromodulation management of chronic neuropathic pain in the central nervous system. Adv Funct Mater. (2020) 30:1908999. doi: 10.1002/adfm.201908999
19. Rastogi P. Novel Coil Designs for Different Neurological Disorders in transcranial Magnetic Stimulation. Rocky Hill: Iowa State University (2019).
20. Bergfeld IO, Mantione M, Hoogendoorn ML, Ruhe HG, Notten P, van Laarhoven J, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. (2016) 73:456–64. doi: 10.1001/jamapsychiatry.2016.0152
21. Heiskanen V, Hamblin MR. Photobiomodulation: lasers vs. light emitting diodes? Photochem Photobiol Sci. (2018) 17:1003–17. doi: 10.1039/c8pp00176f
22. Buch J, Hammond B. Photobiomodulation of the visual system and human health. Int J Mol Sci. (2020) 21:8020. doi: 10.3390/ijms21218020
23. Mosilhy EA, Alshial EE, Eltaras MM, Rahman MMA, Helmy HI, Elazoul AH, et al. Noninvasive transcranial brain modulation for neurological disorders treatment: a narrative review. Life Sci. (2022) 307:120869. doi: 10.1016/j.lfs.2022.120869
24. Tufail Y, Yoshihiro A, Pati S, Li MM, Tyler WJ. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat Protoc. (2011) 6:1453–70. doi: 10.1038/nprot.2011.371
25. Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. (2010) 66:681–94. doi: 10.1016/j.neuron.2010.05.008
26. Hanson TL, Fuller AM, Lebedev MA, Turner DA, Nicolelis MA. Subcortical neuronal ensembles: an analysis of motor task association, tremor, oscillations, and synchrony in human patients. J Neurosci. (2012) 32:8620–32. doi: 10.1523/JNEUROSCI.0750-12.2012
27. Fry FJ, Ades HW, Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science. (1958) 127:83–4. doi: 10.1126/science.127.3289.83
28. Bystritsky A, Kerwin L, Feusner J. A pilot study of cranial electrotherapy stimulation for generalized anxiety disorder. J Clin Psychiatry. (2008) 69:412–7. doi: 10.4088/JCP.v69n0311
29. Hameroff S, Trakas M, Duffield C, Annabi E, Gerace MB, Boyle P, et al. Transcranial ultrasound (TUS) effects on mental states: a pilot study. Brain Stimul. (2013) 6:409–15. doi: 10.1016/j.brs.2012.05.002
30. Maresca D, Sawyer DP, Renaud G, Lee-Gosselin A, Shapiro MG. Nonlinear X-wave ultrasound imaging of acoustic biomolecules. Phys Rev X. (2018) 8:041002. doi: 10.1103/PhysRevX.8.041002
31. Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. (2014) 17:322–9. doi: 10.1038/nn.3620
32. McKiernan S, Chiarelli P, Warren-Forward H. Diagnostic ultrasound use in physiotherapy, emergency medicine, and anaesthesiology. Radiography. (2010) 16:154–9. doi: 10.1016/j.radi.2009.12.004
33. O'Brien WD. Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. (2007) 93:212–55. doi: 10.1016/j.pbiomolbio.2006.07.010
34. Ter Haar G. Therapeutic ultrasound. Euro J Ultrasound. (1999) 9:3–9. doi: 10.1016/S0929-8266(99)00013-0
35. Warden SJ, Fuchs RK, Kessler CK, Avin KG, Cardinal RE, Stewart RL. Ultrasound produced by a conventional therapeutic ultrasound unit accelerates fracture repair. Phys Ther. (2006) 86:1118–27. doi: 10.1093/ptj/86.8.1118
36. Jiang X, Savchenko O, Li Y, Qi S, Yang T, Zhang W, et al. A review of low-intensity pulsed ultrasound for therapeutic applications. IEEE Trans Biomed Eng. (2019) 66:2704–18. doi: 10.1109/TBME.2018.2889669
37. Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE. (2008) 3:e3511. doi: 10.1371/journal.pone.0003511
38. Krishna V, Sammartino F, Rezai A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology: advances in diagnosis and treatment. JAMA Neurol. (2018) 75:246–54. doi: 10.1001/jamaneurol.2017.3129
39. Deffieux T, Younan Y, Wattiez N, Tanter M, Pouget P, Aubry JF. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr Biol. (2013) 23:2430–3. doi: 10.1016/j.cub.2013.10.029
40. Lee W, Lee SD, Park MY, Foley L, Purcell-Estabrook E, Kim H, et al. Image-guided focused ultrasound-mediated regional brain stimulation in sheep. Ultrasound Med Biol. (2016) 42:459–70. doi: 10.1016/j.ultrasmedbio.2015.10.001
41. Kim H, Park MY, Lee SD, Lee W, Chiu A, Yoo SS. Suppression of EEG visual-evoked potentials in rats through neuromodulatory focused ultrasound. Neuroreport. (2015) 26:211–5. doi: 10.1097/WNR.0000000000000330
42. Pang N, Huang X, Zhou H, Xia X, Liu X, Wang Y, et al. Transcranial ultrasound stimulation of hypothalamus in aging mice. IEEE Trans Ultrason Ferroelectr Freq Control. (2021) 68:29–37. doi: 10.1109/TUFFC.2020.2968479
43. Yuan Y, Wang Z, Liu M, Shoham S. Cortical hemodynamic responses induced by low-intensity transcranial ultrasound stimulation of mouse cortex. Neuroimage. (2020) 211:116597. doi: 10.1016/j.neuroimage.2020.116597
44. Yoo SS, Bystritsky A, Lee JH, Zhang Y, Fischer K, Min BK, et al. Focused ultrasound modulates region-specific brain activity. Neuroimage. (2011) 56:1267–75. doi: 10.1016/j.neuroimage.2011.02.058
45. Wattiez N, Constans C, Deffieux T, Daye PM, Tanter M, Aubry JF, et al. Transcranial ultrasonic stimulation modulates single-neuron discharge in macaques performing an antisaccade task. Brain Stimul. (2017) 10:1024–31. doi: 10.1016/j.brs.2017.07.007
46. Mueller J, Legon W, Opitz A, Sato TF, Tyler WJ. Transcranial focused ultrasound modulates intrinsic and evoked EEG dynamics. Brain Stimul. (2014) 7:900–8. doi: 10.1016/j.brs.2014.08.008
47. Li GF, Zhao HX, Zhou H, Yan F, Wang JY, Xu CX, et al. Improved anatomical specificity of noninvasive neuro-stimulation by high frequency (5 MHz) ultrasound. Sci Rep. (2016) 6:24738. doi: 10.1038/srep24738
48. Kim S, Jo Y, Kook G, Pasquinelli C, Kim H, Kim K, et al. Transcranial focused ultrasound stimulation with high spatial resolution. Brain Stimul. (2021) 14:290–300. doi: 10.1016/j.brs.2021.01.002
49. King RL, Brown JR, Newsome WT, Pauly KB. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med Biol. (2013) 39:312–31. doi: 10.1016/j.ultrasmedbio.2012.09.009
50. Kim H, Chiu A, Lee SD, Fischer K, Yoo SS. Focused ultrasound-mediated noninvasive brain stimulation: examination of sonication parameters. Brain Stimul. (2014) 7:748–56. doi: 10.1016/j.brs.2014.06.011
51. Gerstenmayer M, Fellah B, Magnin R, Selingue E, Larrat B. Acoustic transmission factor through the rat skull as a function of body mass, frequency and position. Ultrasound Med Biol. (2018) 44:2336–44. doi: 10.1016/j.ultrasmedbio.2018.06.005
52. Wang X, Luo Y, Chen Y, Chen C, Yin L, Yu T, et al. A skull-removed chronic cranial window for ultrasound and photoacoustic imaging of the rodent brain. Front Neurosci. (2021) 15:673740. doi: 10.3389/fnins.2021.673740
53. Min BK, Yang PS, Bohlke M, Park SR, Vago D, Maher TJ, et al. Focused ultrasound modulates the level of cortical neurotransmitters: Potential as a new functional brain mapping technique. Int J Imag Syst Technol. (2011) 21:232–40. doi: 10.1002/ima.20284
54. Ye PP, Brown JR, Pauly KB. Frequency dependence of ultrasound neurostimulation in the mouse brain. Ultrasound Med Biol. (2016) 42:1512–30. doi: 10.1016/j.ultrasmedbio.2016.02.012
55. Kim H, Park MA, Wang S, Chiu A, Fischer K, Yoo SS, et al. Imaging evidence of FUS-mediated (18)F-FDG uptake changes in rat brain. Med Phys. (2013) 40:033501. doi: 10.1118/1.4789916
56. Yang PS, Kim H, Lee W, Bohlke M, Park S, Maher TJ, et al. Transcranial focused ultrasound to the thalamus is associated with reduced extracellular GABA levels in rats. Neuropsychobiology. (2012) 65:153–60. doi: 10.1159/000336001
57. Lin Z, Meng L, Zou J, Zhou W, Huang X, Xue S, et al. Noninvasive ultrasonic neuromodulation of neuronal excitability for treatment of epilepsy. Theranostics. (2020) 10:5514–26. doi: 10.7150/thno.40520
58. Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, et al. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. (2012) 75:1022–34. doi: 10.1016/j.neuron.2012.08.002
59. Brennan KC, Jurewicz EC, Ford B, Pullman SL, Louis ED. Is essential tremor predominantly a kinetic or a postural tremor? A clinical and electrophysiological study. Mov Disord. (2002) 17:313–6. doi: 10.1002/mds.10003
60. Handforth A. Linking essential tremor to the cerebellum-animal model evidence. Cerebellum. (2016) 15:285–98. doi: 10.1007/s12311-015-0750-0
61. Plaksin M, Kimmel E, Shoham S. Cell-type-selective effects of intramembrane cavitation as a unifying theoretical framework for ultrasonic neuromodulation. eNeuro. (2016) 3:3. doi: 10.1523/ENEURO.0136-15.2016
62. Ranjan M, Boutet A, Bhatia S, Wilfong A, Hader W, Lee MR, et al. Neuromodulation beyond neurostimulation for epilepsy: scope for focused ultrasound. Expert Rev Neurother. (2019) 19:937–43. doi: 10.1080/14737175.2019.1635013
63. Chee K, Razmara A, Geller AS, Harris WB, Restrepo D, Thompson JA, et al. The role of the piriform cortex in temporal lobe epilepsy: a current literature review. Front Neurol. (2022) 13:1042887. doi: 10.3389/fneur.2022.1042887
64. Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. (2016) 57:325–34. doi: 10.1111/epi.13284
65. Hakimova H, Kim S, Chu K, Lee SK, Jeong B, Jeon D. Ultrasound stimulation inhibits recurrent seizures and improves behavioral outcome in an experimental model of mesial temporal lobe epilepsy. Epilepsy Behav. (2015) 49:26–32. doi: 10.1016/j.yebeh.2015.04.008
66. Chu PC Yu HY, Lee CC, Fisher R, Liu HL. Pulsed-focused ultrasound provides long-term suppression of epileptiform bursts in the kainic acid-induced epilepsy rat model. Neurotherapeutics. (2022) 19:1368–80. doi: 10.1007/s13311-022-01250-7
67. Li X, Yang H, Yan J, Wang X, Yuan Y, Li X. Seizure control by low-intensity ultrasound in mice with temporal lobe epilepsy. Epilepsy Res. (2019) 154:1–7. doi: 10.1016/j.eplepsyres.2019.04.002
68. Zhang M, Li B, Lv X, Liu S, Liu Y, Tang R, et al. Low-intensity focused ultrasound-mediated attenuation of acute seizure activity based on EEG brain functional connectivity. Brain Sci. (2021) 11:711. doi: 10.3390/brainsci11060711
69. Zou J, Meng L, Lin Z, Qiao Y, Tie C, Wang Y, et al. Ultrasound neuromodulation inhibits seizures in acute epileptic monkeys. iScience. (2020) 23:101066. doi: 10.1016/j.isci.2020.101066
70. Lee CC, Chou CC, Hsiao FJ, Chen YH, Lin CF, Chen CJ, et al. Pilot study of focused ultrasound for drug-resistant epilepsy. Epilepsia. (2022) 63:162–75. doi: 10.1111/epi.17105
71. Abe K, Yamaguchi T, Hori H, Sumi M, Horisawa S, Taira T, et al. Magnetic resonance-guided focused ultrasound for mesial temporal lobe epilepsy: a case report. BMC Neurol. (2020) 20:160. doi: 10.1186/s12883-020-01744-x
72. Brinker ST, Preiswerk F, White PJ, Mariano TY, McDannold NJ, Bubrick EJ. Focused ultrasound platform for investigating therapeutic neuromodulation across the human hippocampus. Ultrasound Med Biol. (2020) 46:1270–4. doi: 10.1016/j.ultrasmedbio.2020.01.007
73. Chen SG, Tsai CH, Lin CJ, Lee CC Yu HY, Hsieh TH, et al. Transcranial focused ultrasound pulsation suppresses pentylenetetrazol induced epilepsy in vivo. Brain Stimul. (2020) 13:35–46. doi: 10.1016/j.brs.2019.09.011
74. Zesiewicz TA, Chari A, Jahan I, Miller AM, Sullivan KL. Overview of essential tremor. Neuropsychiatr Dis Treat. (2010) 6:401–8. doi: 10.2147/NDT.S4795
75. Louis ED. Essential tremor then and now: how views of the most common tremor diathesis have changed over time. Parkinsonism Relat Disord. (2018) 46:S70–S4. doi: 10.1016/j.parkreldis.2017.07.010
76. Puschmann A, Wszolek ZK. Diagnosis and treatment of common forms of tremor. Semin Neurol. (2011) 31:65–77. doi: 10.1055/s-0031-1271312
77. Thenganatt MA, Louis ED. Distinguishing essential tremor from Parkinson's disease: bedside tests and laboratory evaluations. Expert Rev Neurother. (2012) 12:687–96. doi: 10.1586/ern.12.49
78. Benito-Leon J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol. (2006) 2:666–78. doi: 10.1038/ncpneuro0347
79. Shanker V. Essential tremor: diagnosis and management. BMJ. (2019) 366:l4485. doi: 10.1136/bmj.l4485
80. Louis ED, Rios E. Henchcliffe C. How are we doing with the treatment of essential tremor (ET)?: Persistence of patients with ET on medication: data from 528 patients in three settings. Eur J Neurol. (2010) 17:882–4. doi: 10.1111/j.1468-1331.2009.02926.x
81. Lee JY, Kondziolka D. Thalamic deep brain stimulation for management of essential tremor. J Neurosurg. (2005) 103:400–3. doi: 10.3171/jns.2005.103.3.0400
82. Graff-Radford J, Foote KD, Mikos AE, Bowers D, Fernandez HH, Rosado CA, et al. Mood and motor effects of thalamic deep brain stimulation surgery for essential tremor. Eur J Neurol. (2010) 17:1040–6. doi: 10.1111/j.1468-1331.2010.02958.x
83. Cury RG, Fraix V, Castrioto A, Perez Fernandez MA, Krack P, Chabardes S, et al. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology. (2017) 89:1416–23. doi: 10.1212/WNL.0000000000004295
84. Sandvik U, Koskinen LO, Lundquist A, Blomstedt P. Thalamic and subthalamic deep brain stimulation for essential tremor: where is the optimal target? Neurosurgery. (2012) 70:840–5. doi: 10.1227/NEU.0b013e318236a809
85. Plaha P, Javed S, Agombar D. G OF, Khan S, Whone A, et al. Bilateral caudal zona incerta nucleus stimulation for essential tremor: outcome and quality of life. J Neurol Neurosurg Psychiatry. (2011) 82:899–904. doi: 10.1136/jnnp.2010.222992
86. Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. (2013) 369:640–8. doi: 10.1056/NEJMoa1300962
87. Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. (2013) 12:462–8. doi: 10.1016/S1474-4422(13)70048-6
88. Chang WS, Jung HH, Kweon EJ, Zadicario E, Rachmilevitch I, Chang JW. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: practices and clinicoradiological outcomes. J Neurol Neurosurg Psychiatry. (2015) 86:257–64. doi: 10.1136/jnnp-2014-307642
89. Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. (2016) 375:730–9. doi: 10.1056/NEJMoa1600159
90. Meng Y, Solomon B, Boutet A, Llinas M, Scantlebury N, Huang Y, et al. Magnetic resonance-guided focused ultrasound thalamotomy for treatment of essential tremor: A 2-year outcome study. Mov Disord. (2018) 33:1647–50. doi: 10.1002/mds.99
91. Sharabi S, Daniels D, Last D, Guez D, Zivli Z, Castel D, et al. Non-thermal focused ultrasound induced reversible reduction of essential tremor in a rat model. Brain Stimul. (2019) 12:1–8. doi: 10.1016/j.brs.2018.08.014
92. Scarcelli T, Jordao JF, O'Reilly MA, Ellens N, Hynynen K, Aubert I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. (2014) 7:304–7. doi: 10.1016/j.brs.2013.12.012
93. Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. (2011) 36:2589–602. doi: 10.1038/npp.2011.220
94. Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. (2012) 338:72–5. doi: 10.1126/science.1222941
95. Yi SS, Zou JJ, Meng L, Chen HM, Hong ZQ, Liu XF, et al. Ultrasound stimulation of prefrontal cortex improves lipopolysaccharide-induced depressive-like behaviors in mice. Front Psychiatry. (2022) 13:864481. doi: 10.3389/fpsyt.2022.864481
96. Wang F, Li N, Wei X, Jia X, Liu H, Wang Y, et al. MRI-guided focused ultrasound-induced blood brain barrier disruption to deliver glial cell line derived neurotropic factor proteins into brain to treat rat depression. J Biomed Nanotechnol. (2020) 16:626–39. doi: 10.1166/jbn.2020.2914
97. Sanguinetti JL, Hameroff S, Smith EE, Sato T, Daft CMW, Tyler WJ, et al. Transcranial focused ultrasound to the right prefrontal cortex improves mood and alters functional connectivity in humans. Front Hum Neurosci. (2020) 14:52. doi: 10.3389/fnhum.2020.00052
98. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. (1988) 51:745–52. doi: 10.1136/jnnp.51.6.745
99. Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson's disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. (1976) 38:277–301. doi: 10.1007/BF01249445
100. Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. (2009) 8:1150–7. doi: 10.1016/S1474-4422(09)70238-8
101. Guiot G, Brion S. [Treatment of abnormal movement by pallidal coagulation]. Rev Neurol (Paris). (1953) 89:578–80.
102. Limousin P, Pollak P, Benazzouz A, Hoffmann D, Broussolle E, Perret JE, et al. Bilateral subthalamic nucleus stimulation for severe Parkinson's disease. Mov Disord. (1995) 10:672–4. doi: 10.1002/mds.870100523
103. Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal vs. subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. (2010) 362:2077–91. doi: 10.1056/NEJMoa0907083
104. Cui W, Xue B, Xie J, Xu H. Targeting the cuneiform nucleus in parkinson's disease: option to improve locomotor activity. Neurosci Bull. (2022) 38:976–8. doi: 10.1007/s12264-022-00870-5
105. Moser D, Zadicario E, Schiff G, Jeanmonod D. MR-guided focused ultrasound technique in functional neurosurgery: targeting accuracy. J Ther Ultrasound. (2013) 1:3. doi: 10.1186/2050-5736-1-3
106. Magara A, Buhler R, Moser D, Kowalski M, Pourtehrani P, Jeanmonod D. First experience with MR-guided focused ultrasound in the treatment of Parkinson's disease. J Ther Ultrasound. (2014) 2:11. doi: 10.1186/2050-5736-2-11
107. Moosa S, Martinez-Fernandez R, Elias WJ, Del Alamo M, Eisenberg HM, Fishman PS. The role of high-intensity focused ultrasound as a symptomatic treatment for Parkinson's disease. Mov Disord. (2019) 34:1243–51. doi: 10.1002/mds.27779
108. Bond AE, Shah BB, Huss DS, Dallapiazza RF, Warren A, Harrison MB, et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant parkinson disease: a randomized clinical trial. JAMA Neurol. (2017) 74:1412–8. doi: 10.1001/jamaneurol.2017.3098
109. Schlesinger I, Eran A, Sinai A, Erikh I, Nassar M, Goldsher D, et al. MRI Guided focused ultrasound thalamotomy for moderate-to-severe tremor in Parkinson's disease. Parkinsons Dis. (2015) 2015:219149. doi: 10.1155/2015/219149
110. Zaaroor M, Sinai A, Goldsher D, Eran A, Nassar M, Schlesinger I. Magnetic resonance-guided focused ultrasound thalamotomy for tremor: a report of 30 Parkinson's disease and essential tremor cases. J Neurosurg. (2018) 128:202–10. doi: 10.3171/2016.10.JNS16758
111. Martinez-Fernandez R, Rodriguez-Rojas R, Del Alamo M, Hernandez-Fernandez F, Pineda-Pardo JA, Dileone M, et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson's disease: a pilot study. Lancet Neurol. (2018) 17:54–63. doi: 10.1016/S1474-4422(17)30403-9
112. De Bie RM, Schuurman PR, Esselink RA, Bosch DA, Speelman JD. Bilateral pallidotomy in Parkinson's disease: a retrospective study. Mov Disord. (2002) 17:533–8. doi: 10.1002/mds.10090
113. Alomar S, King NK, Tam J, Bari AA, Hamani C, Lozano AM. Speech and language adverse effects after thalamotomy and deep brain stimulation in patients with movement disorders: A meta-analysis. Mov Disord. (2017) 32:53–63. doi: 10.1002/mds.26924
114. Zhou H, Niu L, Meng L, Lin Z, Zou J, Xia X, et al. Noninvasive ultrasound deep brain stimulation for the treatment of Parkinson's disease model mouse. Research. (2019) 2019:1748489. doi: 10.34133/2019/1748489
115. Chen X, Wang D, Zhang L, Yao H, Zhu H, Zhao N, et al. Neuroprotective effect of low-intensity pulsed ultrasound on the mouse MPTP/MPP(+) model of dopaminergic neuron injury. Ultrasound Med Biol. (2021) 47:2321–30. doi: 10.1016/j.ultrasmedbio.2021.03.034
116. Dong Y, Liu D, Zhao Y, Yuan Y, Wang W, Wu S, et al. Assessment of neuroprotective effects of low-intensity transcranial ultrasound stimulation in a parkinson's disease rat model by fractional anisotropy and relaxation time T2(*) value. Front Neurosci. (2021) 15:590354. doi: 10.3389/fnins.2021.590354
117. Sung CY, Chiang PK, Tsai CW, Yang FY. Low-intensity pulsed ultrasound enhances neurotrophic factors and alleviates neuroinflammation in a rat model of Parkinson's disease. Cereb Cortex. (2021) 32:176–85. doi: 10.1093/cercor/bhab201
118. Wang Z, Yan J, Wang X, Yuan Y, Li X. Transcranial ultrasound stimulation directly influences the cortical excitability of the motor cortex in Parkinsonian mice. Mov Disord. (2020) 35:693–8. doi: 10.1002/mds.27952
119. Yuan Y, Zhao Z, Wang Z, Wang X, Yan J, Li X. The effect of low-intensity transcranial ultrasound stimulation on behavior in a mouse model of Parkinson's disease induced by MPTP. IEEE Trans Neural Syst Rehabil Eng. (2020) 28:1017–21. doi: 10.1109/TNSRE.2020.2978865
120. Feng Y, An R, Zhang Y, Chen M, Wang L, Duan Y, et al. AHNAK-modified microbubbles for the intracranial delivery of triptolide: In-vitro and in-vivo investigations. Int J Pharm. (2022) 629:122351. doi: 10.1016/j.ijpharm.2022.122351
121. Yan YR, Chen Y, Liu ZX, Cai FY, Niu WT, Song LM, et al. Brain delivery of curcumin through low-intensity ultrasound-induced blood-brain barrier opening via lipid-PLGA nanobubbles. Int J Nanomed. (2021) 16:7433–47. doi: 10.2147/IJN.S327737
122. Meng Y, Pople CB, Huang YX, Jones RM, Ottoy J, Goubran M, et al. Putaminal recombinant glucocerebrosidase delivery with magnetic resonance-guided focused ultrasound in Parkinson's disease: a phase I study. Movement Disord. (2022) 37:2134. doi: 10.1002/mds.29190
123. Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. (2003) 39:889–909. doi: 10.1016/S0896-6273(03)00568-3
124. Zhao L, Feng Y, Shi A, Zhang L, Guo S, Wan M. Neuroprotective effect of low-intensity pulsed ultrasound against MPP(+)-induced neurotoxicity in PC12 cells: involvement of K2P channels and stretch-activated ion channels. Ultrasound Med Biol. (2017) 43:1986–99. doi: 10.1016/j.ultrasmedbio.2017.04.020
125. Song WS, Sung CY, Ke CH, Yang FY. Anti-inflammatory and neuroprotective effects of transcranial ultrasound stimulation on Parkinson's disease. Ultrasound Med Biol. (2022) 48:265–74. doi: 10.1016/j.ultrasmedbio.2021.10.001
126. Fan CH, Wei KC, Chiu NH, Liao EC, Wang HC, Wu RY, et al. Sonogenetic-based neuromodulation for the Amelioration of Parkinson's disease. Nano Lett. (2021) 21:5967–76. doi: 10.1021/acs.nanolett.1c00886
127. Ekstrand MI, Galter D. The MitoPark Mouse-an animal model of Parkinson's disease with impaired respiratory chain function in dopamine neurons. Parkinsonism Relat Disord. (2009) 15:S185–8. doi: 10.1016/S1353-8020(09)70811-9
128. Galter D, Pernold K, Yoshitake T, Lindqvist E, Hoffer B, Kehr J, et al. MitoPark mice mirror the slow progression of key symptoms and L-DOPA response in Parkinson's disease. Genes Brain Behav. (2010) 9:173–81. doi: 10.1111/j.1601-183X.2009.00542.x
129. Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, et al. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. (2006) 105:445–54. doi: 10.3171/jns.2006.105.3.445
130. Mehta RI, Carpenter JS, Mehta RI, Haut MW, Ranjan M, Najib U, et al. Blood-brain barrier opening with mri-guided focused ultrasound elicits meningeal venous permeability in humans with early Alzheimer disease. Radiology. (2021) 298:654–62. doi: 10.1148/radiol.2021200643
131. Jordao JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS ONE. (2010) 5:e10549. doi: 10.1371/journal.pone.0010549
132. Jordao JF, Thevenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K, et al. Amyloid-beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol. (2013) 248:16–29. doi: 10.1016/j.expneurol.2013.05.008
133. Burgess A, Dubey S, Yeung S, Hough O, Eterman N, Aubert I, et al. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology. (2014) 273:736–45. doi: 10.1148/radiol.14140245
134. Shen Y, Hua L, Yeh CK, Shen L, Ying M, Zhang Z, et al. Ultrasound with microbubbles improves memory, ameliorates pathology and modulates hippocampal proteomic changes in a triple transgenic mouse model of Alzheimer's disease. Theranostics. (2020) 10:11794–819. doi: 10.7150/thno.44152
135. Eguchi K, Shindo T, Ito K, Ogata T, Kurosawa R, Kagaya Y, et al. Whole-brain low-intensity pulsed ultrasound therapy markedly improves cognitive dysfunctions in mouse models of dementia - Crucial roles of endothelial nitric oxide synthase. Brain Stimul. (2018) 11:959–73. doi: 10.1016/j.brs.2018.05.012
136. Bobola MS, Chen L, Ezeokeke CK, Olmstead TA, Nguyen C, Sahota A, et al. Transcranial focused ultrasound, pulsed at 40 Hz, activates microglia acutely and reduces Abeta load chronically, as demonstrated in vivo. Brain Stimul. (2020) 13:1014–23. doi: 10.1016/j.brs.2020.03.016
137. Lee Y, Choi Y, Park EJ, Kwon S, Kim H, Lee JY, et al. Improvement of glymphatic-lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer's disease model. Sci Rep. (2020) 10:16144. doi: 10.1038/s41598-020-73151-8
138. Poon CT, Shah K, Lin C, Tse R, Kim KK, Mooney S, et al. Time course of focused ultrasound effects on beta-amyloid plaque pathology in the TgCRND8 mouse model of Alzheimer's disease. Sci Rep. (2018) 8:14061. doi: 10.1038/s41598-018-32250-3
139. Leinenga G, Gotz J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer's disease mouse model. Sci Transl Med. (2015) 7:278. doi: 10.1126/scitranslmed.aaa2512
140. Leinenga G, Gotz J. Safety and Efficacy of Scanning Ultrasound Treatment of Aged APP23 Mice. Front Neurosci. (2018) 12:55. doi: 10.3389/fnins.2018.00055
141. Karakatsani ME, Kugelman T, Ji R, Murillo M, Wang S, Niimi Y, et al. Unilateral Focused Ultrasound-Induced Blood-Brain Barrier Opening Reduces Phosphorylated Tau from The rTg4510 Mouse Model. Theranostics. (2019) 9:5396–411. doi: 10.7150/thno.28717
142. Pandit R, Leinenga G, Gotz J. Repeated ultrasound treatment of tau transgenic mice clears neuronal tau by autophagy and improves behavioral functions. Theranostics. (2019) 9:3754–67. doi: 10.7150/thno.34388
143. O'Reilly MA, Jones RM, Barrett E, Schwab A, Head E, Hynynen K. Investigation of the safety of focused ultrasound-induced blood-brain barrier opening in a natural canine model of aging. Theranostics. (2017) 7:3573–84. doi: 10.7150/thno.20621
144. Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, et al. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun. (2018) 9:2336. doi: 10.1038/s41467-018-04529-6
145. Meng Y, Abrahao A, Heyn CC, Bethune AJ, Huang Y, Pople CB, et al. Glymphatics visualization after focused ultrasound-induced blood-brain barrier opening in humans. Ann Neurol. (2019) 86:975–80. doi: 10.1002/ana.25604
146. Meng Y, MacIntosh BJ, Shirzadi Z, Kiss A, Bethune A, Heyn C, et al. Resting state functional connectivity changes after MR-guided focused ultrasound mediated blood-brain barrier opening in patients with Alzheimer's disease. Neuroimage. (2019) 200:275–80. doi: 10.1016/j.neuroimage.2019.06.060
147. Rezai AR, Ranjan M, D'Haese PF, Haut MW, Carpenter J, Najib U, et al. Noninvasive hippocampal blood-brain barrier opening in Alzheimer's disease with focused ultrasound. Proc Natl Acad Sci USA. (2020) 117:9180–2. doi: 10.1073/pnas.2002571117
148. D'Haese PF, Ranjan M, Song A, Haut MW, Carpenter J, Dieb G, et al. Beta-amyloid plaque reduction in the hippocampus after focused ultrasound-induced blood-brain barrier opening in Alzheimer's disease. Front Hum Neurosci. (2020) 14:593672. doi: 10.3389/fnhum.2020.593672
149. Nicodemus NE, Becerra S, Kuhn TP, Packham HR, Duncan J, Mahdavi K, et al. Focused transcranial ultrasound for treatment of neurodegenerative dementia. Alzheimers Dement. (2019) 5:374–81. doi: 10.1016/j.trci.2019.06.007
150. Beisteiner R, Matt E, Fan C, Baldysiak H, Schonfeld M, Philippi Novak T, et al. Transcranial pulse stimulation with ultrasound in Alzheimer's disease-a new navigated focal brain therapy. Adv Sci. (2020) 7:1902583. doi: 10.1002/advs.201902583
151. Epelbaum S, Burgos N, Canney M, Matthews D, Houot M, Santin MD, et al. Pilot study of repeated blood-brain barrier disruption in patients with mild Alzheimer's disease with an implantable ultrasound device. Alzheimers Res Ther. (2022) 14:40. doi: 10.1186/s13195-022-00981-1
152. Jeong H, Im JJ, Park JS, Na SH, Lee W, Yoo SS, et al. A pilot clinical study of low-intensity transcranial focused ultrasound in Alzheimer's disease. Ultrasonography. (2021) 40:512–9. doi: 10.14366/usg.20138
153. Darrow DP, O'Brien P, Richner TJ, Netoff TI, Ebbini ES. Reversible neuroinhibition by focused ultrasound is mediated by a thermal mechanism. Brain Stimul. (2019) 12:1439–47. doi: 10.1016/j.brs.2019.07.015
154. Ibsen S, Tong A, Schutt C, Esener S, Chalasani SH. Sonogenetics is a noninvasive approach to activating neurons in Caenorhabditis elegans. Nat Commun. (2015) 6:8264. doi: 10.1038/ncomms9264
155. Oh SJ, Lee JM, Kim HB, Lee J, Han S, Bae JY, et al. Ultrasonic neuromodulation via astrocytic TRPA1. Curr Biol. (2019) 29:3386–401. doi: 10.1016/j.cub.2019.08.021
156. Ye J, Tang S, Meng L, Li X, Wen X, Chen S, et al. Ultrasonic control of neural activity through activation of the mechanosensitive channel MscL. Nano Lett. (2018) 18:4148–55. doi: 10.1021/acs.nanolett.8b00935
157. Qiu Z, Kala S, Guo J, Xian Q, Zhu J, Zhu T, et al. Targeted neurostimulation in mouse brains with noninvasive ultrasound. Cell Rep. (2021) 34:108595. doi: 10.1016/j.celrep.2020.108595
158. Xhima K, Markham-Coultes K, Nedev H, Heinen S, Saragovi HU, Hynynen K, et al. Focused ultrasound delivery of a selective TrkA agonist rescues cholinergic function in a mouse model of Alzheimer's disease. Sci Adv. (2020) 6:eaax6646. doi: 10.1126/sciadv.aax6646
159. Lasser M, Tiber J, Lowery LA. The role of the microtubule cytoskeleton in neurodevelopmental disorders. Front Cell Neurosci. (2018) 12:165. doi: 10.3389/fncel.2018.00165
160. Guo H, Hamilton Ii M, Offutt SJ, Gloeckner CD Li T, Kim Y, et al. Ultrasound produces extensive brain activation via a cochlear pathway. Neuron. (2018) 99:866. doi: 10.1016/j.neuron.2018.07.049
161. Mohammadjavadi M, Ye PP, Xia A, Brown J, Popelka G, Pauly KB. Elimination of peripheral auditory pathway activation does not affect motor responses from ultrasound neuromodulation. Brain Stimul. (2019) 12:901–10. doi: 10.1016/j.brs.2019.03.005
162. Braun V, Blackmore J, Cleveland RO, Butler CR. Transcranial ultrasound stimulation in humans is associated with an auditory confound that can be effectively masked. Brain Stimul. (2020) 13:1527–34. doi: 10.1016/j.brs.2020.08.014
163. Akhtar H, Bukhari F, Nazir M, Anwar MN, Shahzad A. Therapeutic efficacy of neurostimulation for depression: techniques, current modalities, and future challenges. Neurosci Bull. (2016) 32:115–26. doi: 10.1007/s12264-015-0009-2
164. Yang LZ, Yang Z, Zhang X. Noninvasive brain stimulation for the treatment of nicotine addiction: potential and challenges. Neurosci Bull. (2016) 32:550–6. doi: 10.1007/s12264-016-0056-3
165. Sassaroli E, Vykhodtseva N. Acoustic neuromodulation from a basic science prospective. J Ther Ultrasound. (2016) 4:17. doi: 10.1186/s40349-016-0061-z
166. Fomenko A, Neudorfer C, Dallapiazza RF, Kalia SK, Lozano AM. Low-intensity ultrasound neuromodulation: an overview of mechanisms and emerging human applications. Brain Stimul. (2018) 11:1209–17. doi: 10.1016/j.brs.2018.08.013
167. Tyler WJ, Lani SW, Hwang GM. Ultrasonic modulation of neural circuit activity. Curr Opin Neurobiol. (2018) 50:222–31. doi: 10.1016/j.conb.2018.04.011
168. Barnett SB. Intracranial temperature elevation from diagnostic ultrasound. Ultrasound Med Biol. (2001) 27:883–8. doi: 10.1016/S0301-5629(01)00367-2
169. Fatar M, Stroick M, Griebe M, Alonso A, Hennerici MG, Daffertshofer M. Brain temperature during 340-kHz pulsed ultrasound insonation: a safety study for sonothrombolysis. Stroke. (2006) 37:1883–7. doi: 10.1161/01.STR.0000226737.47319.aa
170. Pinton G, Aubry JF, Bossy E, Muller M, Pernot M, Tanter M. Attenuation, scattering, and absorption of ultrasound in the skull bone. Med Phys. (2012) 39:299–307. doi: 10.1118/1.3668316
171. Tsai PC, Gougheri HS, Kiani M. Skull impact on the ultrasound beam profile of transcranial focused ultrasound stimulation. Annu Int Conf IEEE Eng Med Biol Soc. (2019) 2019:5188–91. doi: 10.1109/EMBC.2019.8857269
172. Yuan Y, Chen Y, Li X. Theoretical analysis of transcranial magneto-acoustical stimulation with hodgkin-huxley neuron model. Front Comput Neurosci. (2016) 10:35. doi: 10.3389/fncom.2016.00035
173. Norton SJ. Can ultrasound be used to stimulate nerve tissue? Biomed Eng Online. (2003) 2:6. doi: 10.1186/1475-925X-2-6
174. Bystritsky A, Korb AS, Douglas PK, Cohen MS, Melega WP, Mulgaonkar AP, et al. A review of low-intensity focused ultrasound pulsation. Brain Stimul. (2011) 4:125–36. doi: 10.1016/j.brs.2011.03.007
175. Wang H, Zhou X, Cui D, Liu R, Tan R, Wang X, et al. Comparative study of transcranial magnetoacoustic stimulation and transcranial ultrasound stimulation of motor cortex. Front Behav Neurosci. (2019) 13:241. doi: 10.3389/fnbeh.2019.00241
176. Kraus D, Naros G, Bauer R, Khademi F, Leao MT, Ziemann U, et al. Brain state-dependent transcranial magnetic closed-loop stimulation controlled by sensorimotor desynchronization induces robust increase of corticospinal excitability. Brain Stimul. (2016) 9:415–24. doi: 10.1016/j.brs.2016.02.007
177. Milosevic L, Kalia SK, Hodaie M, Lozano AM, Fasano A, Popovic MR, et al. Neuronal inhibition and synaptic plasticity of basal ganglia neurons in Parkinson's disease. Brain. (2018) 141:177–90. doi: 10.1093/brain/awx296
178. Zhang Z, Russell LE, Packer AM, Gauld OM, Hausser M. Closed-loop all-optical interrogation of neural circuits in vivo. Nat Methods. (2018) 15:1037–40. doi: 10.1038/s41592-018-0183-z
179. Muller J, Bakkum DJ, Hierlemann A. Sub-millisecond closed-loop feedback stimulation between arbitrary sets of individual neurons. Front Neural Circuits. (2012) 6:121. doi: 10.3389/fncir.2012.00121
180. Cagnan H, Pedrosa D, Little S, Pogosyan A, Cheeran B, Aziz T, et al. Stimulating at the right time: phase-specific deep brain stimulation. Brain. (2017) 140:132–45. doi: 10.1093/brain/aww286
181. Zhou A, Santacruz SR, Johnson BC, Alexandrov G, Moin A, Burghardt FL, et al. A wireless and artefact-free 128-channel neuromodulation device for closed-loop stimulation and recording in nonhuman primates. Nat Biomed Eng. (2019) 3:15–26. doi: 10.1038/s41551-018-0323-x
182. Berenyi A, Belluscio M, Mao D, Buzsaki G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science. (2012) 337:735–7. doi: 10.1126/science.1223154
183. Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. (2013) 4:1376. doi: 10.1038/ncomms2376
184. Liu A, Voroslakos M, Kronberg G, Henin S, Krause MR, Huang Y, et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun. (2018) 9:5092. doi: 10.1038/s41467-018-07233-7
Keywords: low-intensity transcranial ultrasound stimulation, neuromodulation, central nervous system diseases, Alzheimer's disease, Parkinson's disease, depression, epilepsy
Citation: Hu Y-Y, Yang G, Liang X-S, Ding X-S, Xu D-E, Li Z, Ma Q-H, Chen R and Sun Y-Y (2023) Transcranial low-intensity ultrasound stimulation for treating central nervous system disorders: A promising therapeutic application. Front. Neurol. 14:1117188. doi: 10.3389/fneur.2023.1117188
Received: 06 December 2022; Accepted: 10 February 2023;
Published: 08 March 2023.
Edited by:
Xiaoli Li, Beijing Normal University, ChinaReviewed by:
Hyungmin Kim, Korea Institute of Science and Technology, Republic of KoreaCopyright © 2023 Hu, Yang, Liang, Ding, Xu, Li, Ma, Chen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Chen, Y2hlbnJ1aWdvb2RAMTI2LmNvbQ==; Quan-Hong Ma, bWFxdWFuaG9uZ0BzdWRhLmVkdS5jbg==; Yan-Yun Sun, eXlzdW5Ac3VkYS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.