
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Neurol., 06 February 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1110347
This article is part of the Research TopicOutcomes of Stroke: Prediction and ImprovementView all 38 articles
Objective: To explore the values of neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), neutrophil to albumin ratio (NAR), prognostic nutritional index (PNI), systemic immune inflammatory index (SII) and red cell distribution width to albumin ratio (RA) for evaluating the risk of 30-day mortality of ischemic stroke or hemorrhagic stroke patients.
Methods: In this cohort study, the data of 1,601 patients diagnosed with stroke were extracted from the Medical Information Mart for Intensive Care III (MIMIC-III) database. Among them, 908 were hemorrhagic stroke patients and 693 were ischemic stroke patients. Demographic and clinical variables of patients were collected. Univariate and multivariable Cox regression were performed to evaluate the predictive values of NLR, PLR, SII, NAR, RA, and PNI for 30-day mortality in hemorrhagic stroke or ischemic stroke patients. The receiver operator characteristic (ROC) curves were plotted to assess the predictive values of NLR, NAR, and RA for 30-day mortality of hemorrhagic stroke patients.
Results: At the end of follow-up, 226 hemorrhagic stroke patients and 216 ischemic stroke patients died. The elevated NLR level was associated with increased risk of 30-day mortality in hemorrhagic stroke [hazard ratio (HR) = 1.17, 95% confidence interval (CI): 1.06–1.29]. The increased NAR level was associated with elevated risk of 30-day mortality in hemorrhagic stroke (HR = 1.16, 95% CI: 1.02–1.30). The high RA level was linked with increased risk of 30-day mortality (HR = 1.44, 95% CI: 1.23–1.69). No significant correlation was observed in these inflammation biomarkers with the risk of 30-day mortality in ischemic stroke patients. The area under the curves (AUCs) of NLR, RA, and NAR for evaluating the risk of 30-day mortality of hemorrhagic stroke patients were 0.552 (95% CI: 0.503–0.601), 0.644 (95% CI: 0.590–0.699) and 0.541 (95% CI: 0.490–0.592).
Conclusion: NLR, NAR, and RA were potential prognostic biomarkers for predicting 30-day mortality of hemorrhagic stroke patients, which might provide clinicians an easy and cheap way to quickly identify patients with high risk of mortality.
Stroke is a serious disease affecting a quarter of people during their lifetime with high risk of death and disability (1). Stroke has two main subtypes (ischemic stroke and hemorrhagic stroke), and they have distinct clinical and epidemiological characteristics (2). Ischemic stroke and hemorrhagic stroke are accounted for ~85 and 15% of all stroke cases, respectively (3). Ischemic stroke is caused by the reduction or interruption of blood flow to the brain while hemorrhagic stroke is due to the bleeding in or around the brain (4). Ischemic stroke is the major cause of disability and second cause of deaths globally with a mortality rate of 15% at 90 days (5, 6). As for hemorrhagic strokes, the mortality rate is 25–30% in high-income countries and 30%-48% in low- to middle-income countries (7). Given the prognosis of stroke patients, more reliable biomarkers were essential to help improve the outcomes of these patients.
Numerous studies have demonstrated that neuroinflammatory response plays an essential role in the pathophysiology of ischemic stroke (8, 9). Inflammation associated biomarkers such as monocyte and plateletcrit were reported to be associated with the development of cerebrovascular events including acute ischemic stroke (10, 11). Recently, neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), neutrophil-albumin ratio (NAR), prognostic nutritional index (PNI), systemic immune inflammatory index (SII) and red cell distribution width (RDW) to albumin ratio (RA) have been reported as potential novel biomarkers of baseline inflammatory process and they were identified to be associated with the mortality of stroke (12–17). These studies mainly explored the associations between these inflammation biomarkers and all stroke patients or ischemic stroke patients. They did not compare the differences of these inflammation biomarkers with the mortality of different subtypes of stroke patients. Whether there were differences in the prognostic values of these inflammation biomarkers between ischemic stroke and hemorrhagic stroke was unclear. Which inflammation biomarker was more clearly related to the prognosis of ischemic stroke or hemorrhagic stroke still needs investigation.
In the present study, we hypothesized that ischemic stroke and hemorrhagic stroke might have different prognostic inflammation biomarkers. We planned to explore the prognostic values of NLR, PLR, NAR, PNI, SII, and RA for 30-day mortality of ischemic stroke or hemorrhagic stroke patients based on the data from the Medical Information Mart for Intensive Care III (MIMIC-III) to verify our hypothesis.
In the current cohort study, the data of 3,534 patients diagnosed with stroke were extracted from MIMIC-III database. MIMIC-III is a large, free database involving in de-identified health-related data of over 40,000 patients who stayed in intensive care unit (ICU) of the Beth Israel Deaconess Medical Center (Boston, USA) between 2001 and 2012 (18). The data analyzed using the first measurement data within 24 h after admitting to ICU. Patients who aged < 18 years, and those who stayed in ICU < 24 h were excluded. Those who had no data on SII, NAR, systolic blood pressure (SBP), international normalized ratio (INR), Glasgow coma scale (GCS), or Elixhauser comorbidity index (ECI), and patients with abnormal follow-up time were also excluded. Finally, 1,601 patients were included. Among them, 908 were hemorrhagic stroke patients and 693 were ischemic stroke patients. The project was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). Requirement for individual patient consent was waived because the project did not impact clinical care and all protected health information was deidentified. As the samples were not from The Eighth Medical Center of PLA General Hospital, and this study was exempt from our Institutional Review Board approval.
Main variables analyzed in our study included NLR, PLR, NAP, PNI, SII and RA. NLR (neutrophil to lymphocyte ratio) = neutrophil count/lymphocytes count. PLR (platelet to lymphocyte ratio) = platelet count/lymphocytes count. NAR (neutrophil to albumin ratio) = neutrophil count/albumin. PNI (prognostic nutritional index) = 10 × albumin (g/dL) + 5 × lymphocytes count (109/L). SII (systemic immune inflammatory index) = PLT × neutrophil/lymphocyte. RA (RDW to albumin ratio) = RDW/albumin (g/dL).
Potential covariables analyzed in this study included demographic variables including age (years), gender (female or male), marital status (married, unmarried or unknown), and race [White, or others (Asian, Black, Hispanic or Latino, Unknown)], and clinical variables including respiratory rate (beat/min), SBP (mmHg), diastolic blood pressure (DBP, mmHg), blood oxygen saturation (SpO2), red blood count (RBC, m/μL), INR, hemoglobin (g/dL), hematocrit (%), creatinine (mg/dL), blood urea nitrogen (BUN, mg/dL), fasting blood-glucose (mg/dL), sodium, potassium, chloride, bicarbonate (mEq/L), ECI score, GCS Score, acute kidney failure (AKI, yes or no), infection diseases and treatments.
Infectious diseases was identided from MIMIC-III database based on the ICD-9 code with the first three digits of 001–009, 010–018, 020–027, 030–042, 045–049, 050–059, 060–066, 070–079, 080–088, 090–099, 100–104, 110–118, 120–129, 130–136, and 137–139. Treatments of ischemic stroke included intravenous tissue plasminogen activator (IV-tPA) (ICD-9 procedure code 9910 and 3604), endovascular treatment (ICD-9 procedure code 3974), and the ICD for stent in the procedure (0045, 0046, 0047, and 0048). The main treatments for hemorrhagic stroke were surgery including craniotomy (ICD-9 procedure code: 0120–0129), and minimally invasive surgery (ICD-9 procedure code 0221, 0222, 0139, 0101, 0102, and 0109).
The 30-day mortality of patients was regarded as outcome in our study. The median follow-up was 30 (21.38, 30.00) days. The follow-up was ended when patients died within 30 days. The outcome was obtained through in-hospital observations or through the Social Security Number of patients. At the end of follow-up, 226 hemorrhagic stroke patients and 216 ischemic stroke patients died.
Normally distributed measurement data were described as mean and standard deviation (Mean ± SD), while non-normally distributed measurement data were shown as median and quartile spacing [M (Q1, Q3)]. Mann-whitney U rank-sum test was applied for comparison between groups. Enumeration data were expressed as n (%), and χ2 test was used for comparisons between groups. Univariate cox models were established for 30-day mortality and hazards ratio (HR) and 95% confidence interval (CI) were standardized with P < 0.05 as potential covariables. Univariate and multivariable cox regression were performed to evaluate the prognostic values of NLR, PLR, SII, NAR, RA, and PNI for 30-day mortality of hemorrhagic stroke or ischemic stroke patients. To evaluate the associations between NLR, PLR, SII, NAR, RA, or PNI and 30-day mortality in hemorrhagic stroke patients, confounding factors including age, marital status, respiratory rate, hemoglobin, hematocrit, BUN, fasting blood-glucose, chloride, ECI and AKI were adjusted in the multivariable cox regression model. To assess the associations between NLR, PLR, SII, NAR, RA, or PNI and 30-day mortality in ischemic stroke patients, age, marital status, race, creatinine, BUN, bicarbonate, potassium, ECI, GCS, and AKI were adjusted in the multivariable cox regression model. The receiver operator characteristic (ROC) curves were plotted to evaluate the diagnostic values of NLR, NAR, and RA for 30-day mortality of hemorrhagic stroke. R Studio Version 4.0.3 (2020-10-10) and SAS 9.4 (SAS Institute Inc., Cary, USA) were applied for data analysis.
In total, 3,534 stroke patients were found in MIMIC-III database, among them, 241 people who aged < 18 years and 397 patients who stayed in ICU < 24 h were excluded. Five hundred and thirty-six patients had no data on SII and 728 patients had no data on NAR, and they were excluded. Five patients with abnormal follow-up data (the day admitted to ICU was after the death day) were excluded. Patients without data on SBP (n = 4), INR (n = 6), GCS (n = 6) and ECI (n = 10) were not included. Finally, 1,601 stroke patients were involved in with 908 hemorrhagic stroke patients and 693 ischemic stroke patients. The screen process was shown in Figure 1.
As observed in Table 1, the median NLR (8.39 vs. 7.06) and RA (4.58 vs. 3.97) in the death group were higher than the survival group in hemorrhagic stroke patients. The median PNI in the death group was lower than the survival group in hemorrhagic stroke patients (39.90 vs. 43.17). The median NLR (8.17 vs. 6.82), SII (1.90 vs. 1.58), NAR (2.93 vs. 2.51) and RA (4.52 vs. 4.26) in the death group were higher than the survival group in patients with ischemic stroke. The median PNI in the death group was lower than the survival group in patients with ischemic stroke (38.64 vs. 41.11).
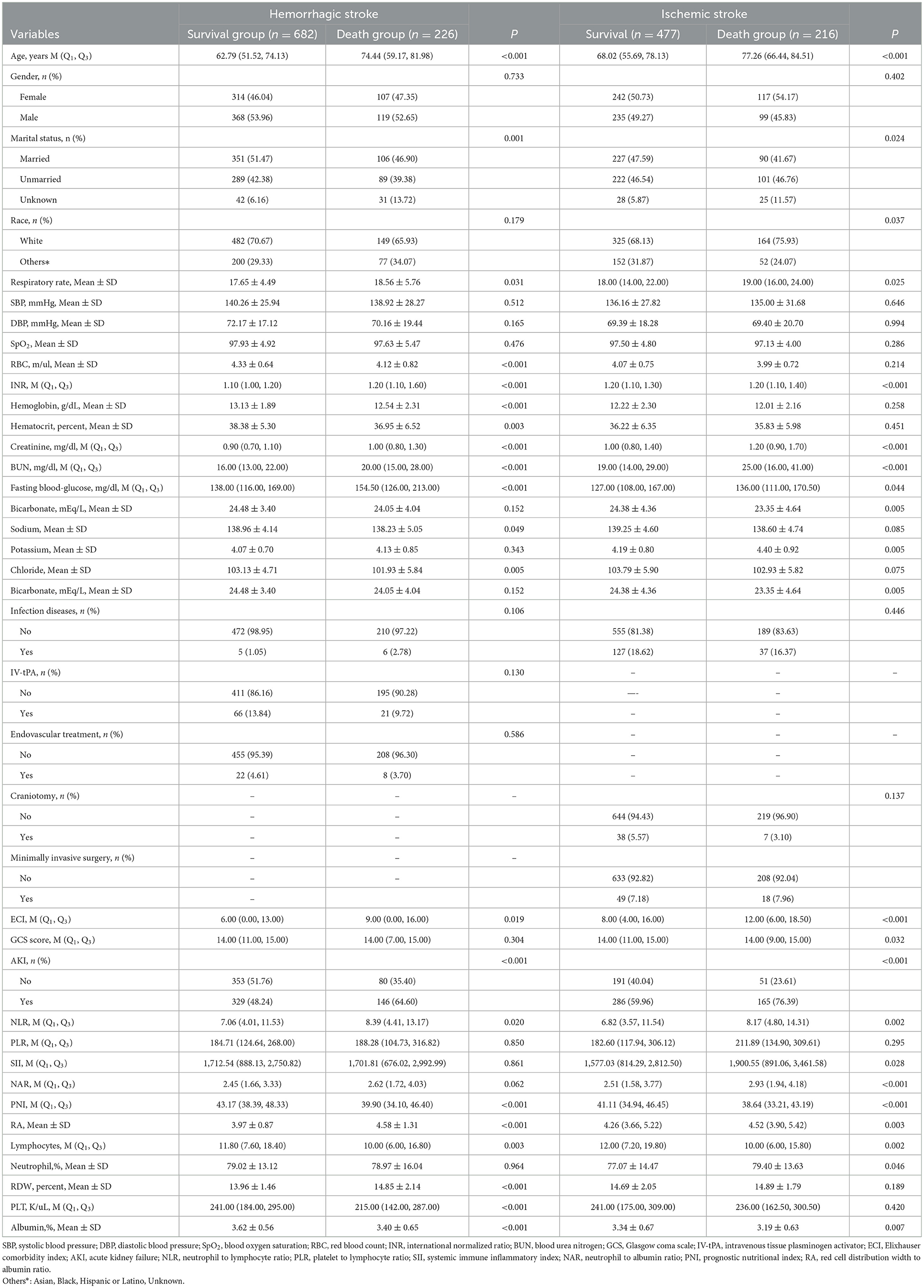
Table 1. Baseline characteristics between patients survived and died within 30 days with ischemic stroke or hemorrhagic stroke.
Potential covariables with statistical difference in Table 1 was involved in univariate cox analysis. The results depicted that age (HR = 1.01, 95% CI: 1.00–1.01), marital status, respiratory rate (HR = 1.03, 95% CI: 1.00–1.06), RBC (HR = 0.60, 95% CI: 0.38–0.95), hemoglobin (HR = 0.74, 95% CI: 0.58–0.95), hematocrit (HR = 0.96, 95% CI: 0.94–0.98), BUN (HR = 1.01, 95% CI: 1.01–1.01), fasting blood–glucose (HR = 1.00, 95% CI: 1.00–1.01), chloride (HR = 0.96, 95% CI: 0.94–0.99), ECI (HR = 1.02, 95% CI: 1.00–1.03) and AKI (HR = 1.74, 95% CI: 1.32–2.29) were covariables that might be associated with 30–day mortality in hemorrhagic stroke patients (Table 2). Age (HR = 1.00, 95% CI: 1.00–1.01), marital status, race (HR = 1.39, 95% CI: 1.02–1.91), creatinine (HR = 1.08, 95% CI: 1.00–1.16), BUN (HR = 1.01, 95% CI: 1.00–1.02), bicarbonate (HR = 0.96, 95% CI: 0.93–0.99), potassium (HR = 1.26, 95% CI: 1.09–1.45), ECI (HR = 1.03, 95% CI: 1.01–1.04), GCS (HR = 0.93, 95% CI: 0.90–0.97) and AKI (HR = 1.95, 95% CI: 1.42–2.67) were covariables that might be associated with 30-day mortality in ischemic stroke patients (Table 3).
As exhibited in Figure 2, univariate analysis revealed that NLR (HR = 1.27, 95% CI: 1.16–1.39), SII (HR = 1.16, 95% CI: 1.07–1.25), NAR (HR = 0.45, 95% CI: 0.32–0.64), RA (HR = 1.23, 95% CI: 1.10–1.39) or PNI (HR = 1.66, 95% CI: 1.46–1.89) might have associations with 30-day mortality in hemorrhagic stroke patients. After adjusting for confounders including age, marital status, respiratory rate, hemoglobin, hematocrit, BUN, fasting blood-glucose, chloride, ECI and AKI, the elevated NLR level was associated with increased risk of 30-day mortality in hemorrhagic stroke (HR = 1.17, 95% CI: 1.06–1.29). The high level of NAR was associated with elevated risk of 30-day mortality in hemorrhagic stroke (HR = 1.16, 95% CI: 1.02–1.30). The increased level of RA was linked with elevated risk of 30-day mortality (HR = 1.44, 95% CI: 1.23–1.69). The higher level of NLR (HR = 1.13, 95% CI: 1.03–1.25), and NAR (HR = 1.18, 95% CI: 1.07–1.32) might correlate with increased risk of 30-day mortality in ischemic stroke patients. No significant correlation was observed in these inflammation biomarkers with the risk of 30-day mortality in ischemic stroke patients after adjusting for age, marital status, race, creatinine, BUN, bicarbonate, potassium, ECI, GCS and AKI (all P > 0.05).
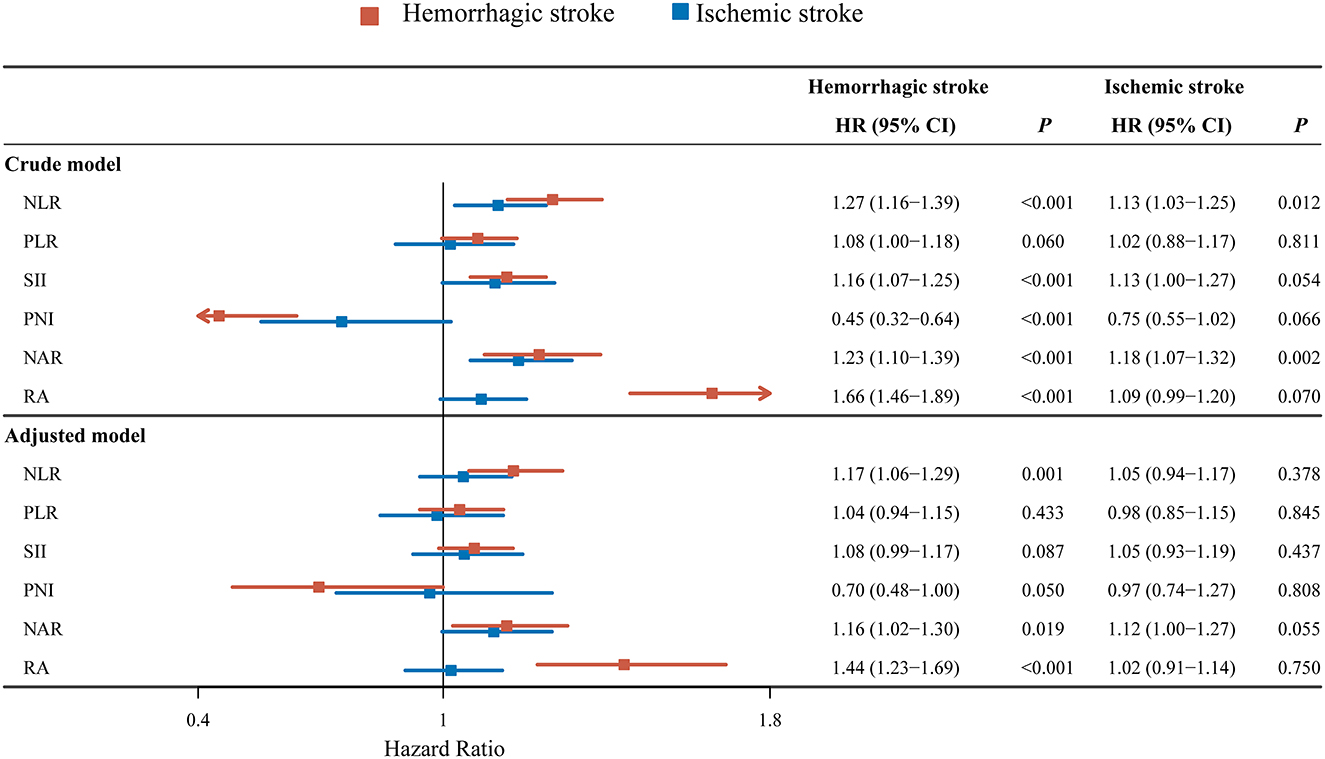
Figure 2. Forest plot showing the associations between NLR, PLR, SII, NAR, RA or PNI and 30-day mortality in hemorrhagic stroke or ischemic stroke patients.
The C-indexes of NLR, NAR, and RA for evaluating the 30-day mortality in hemorrhagic stroke patients were 0.54 (95% CI: 0.50–0.58), 0.53 (95% CI: 0.49–0.57), and 0.61 (95% CI: 0.57–0.65), respectively (Table 4). The AUCs were shown in Figure 3. The AUC values of NLR, NAR and RA for evaluating the risk of 30-day mortality for hemorrhagic stroke patients were 0.552 (95% CI: 0.503–0.601), 0.541 (95% CI: 0.490–0.592) and 0.644 (95% CI: 0.590–0.699). Delong test revealed that the AUCs of NLR and NAR were statistically lower than the AUC of RA(P < 0.001).
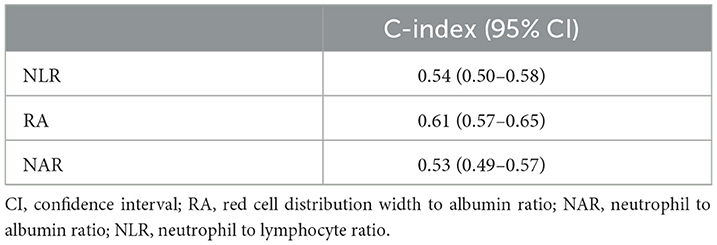
Table 4. The C-index of NLR, NAR, and RA for evaluating the risk of 30-day mortality of hemorrhagic stroke patients.
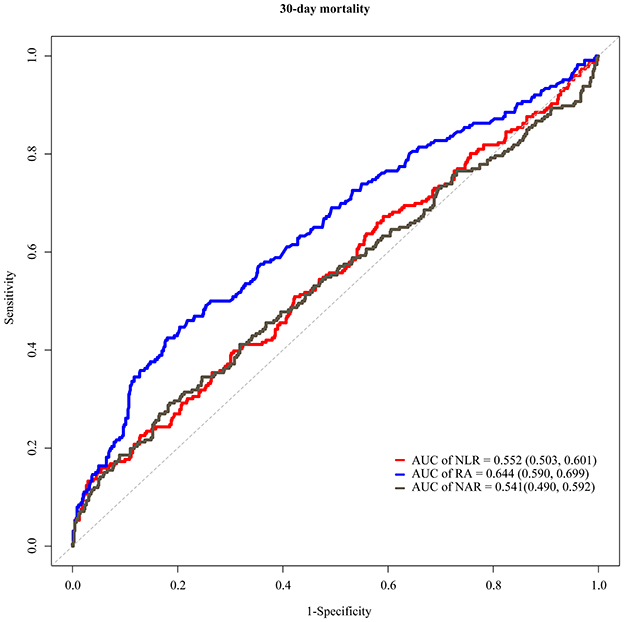
Figure 3. ROC curves presenting the values of NLR, NAR and RA for evaluating the risk of 30-day mortality in hemorrhagic stroke.
In this study, the prognostic values of NLR, PLR, NAR, PNI, SII, and RA for 30-day mortality of ischemic stroke or hemorrhagic stroke patients were investigated based on the data form MIMIC-III database. The results unveiled that high levels of NLR, NAR and RA were linked with increased risk of 30-day mortality in hemorrhagic stroke patients. The AUC values of NLR, NAR, and RA for diagnosing the risk of 30-day mortality in hemorrhagic stroke were 0.552, 0.541, and 0.644. respectively. The findings suggested the values of monitoring the levels of inflammation biomarkers for timely identifying hemorrhagic stroke patients with high risk of mortality within 30 days and provide appropriate interventions to improve their outcomes.
Inflammation is one of the most important pathophysiological mechanisms of stroke and the inflammatory response is activated after stroke, which serves a vital part in secondary brain injury in patients (19). Recently, increasing studies have reported the essential role of immunity in predicting the prognosis and treating patients with acute stroke (20). Immunity is a complex process, and the activation and immunosuppression of different inflammatory cells are induced during the process (21). Neutrophils and lymphocytes are two important inflammatory cells, which were reported to have different roles of in the prognosis after stroke (22, 23). NLR is the ratio of neutrophil to lymphocyte, which can be calculated both from the absolute number of neutrophils and lymphocytes, and from their relative number (24). Previously, a high NLR level was found to associate with poor functional outcomes and increased mortality in patients with spontaneous intracerebral hemorrhage (ICH) (25, 26). These studies provided support to the findings of our study, which depicted that increased NLR was linked with higher risk of 30-day mortality of hemorrhagic stroke patients. NAR is the ratio of neutrophil to albumin, which has become a novel index reflecting systemic inflammation and predicting outcomes of patients in diseases (27). Albumin is an abundant protein in human blood plasma which has osmoregulation, anti-oxidation and anti-inflammation functions (28). A low albumin level was associated with increased mortality risk in hospitalized patients (29). Li et al. found that low serum albumin levels were associated with increased risk of total stroke, ischemic stroke, and ICH (30). In our study, a high level of NAR was correlated to increased risk of 30-day mortality of hemorrhagic stroke patients. RA is another inflammation biomarker derived from the ratio of RDW to albumin, which was reported to be associated with mortality of stroke (17). RDW is a hematologic parameter showing the divergence of red blood cell volume (31). In previous studies, RDW was identified to closely associate with prognosis of cardiovascular events (32, 33). Some other studies revealed that RA might be correlated with hemorrhagic transformation in acute ischemic stroke patients (34). Herein, elevated RA level was associated with higher risk of 30-day mortality of hemorrhagic stroke patients.
The mechanisms underlying the association between NLR, NAR, and RA with 30-day mortality in hemorrhagic stroke patients might be the follows. In hemorrhagic stroke patients, the increased number of neutrophils and decreased number of lymphocytes could induce a cytokine-chemokine storm and, ultimately, lead to more complications (35). Increased neutrophils can release chemical mediators related to increased tissue damage and poor neurological prognosis in stroke patients (36). Lymphocytes were reported to play a brain protective role and the decrease of lymphocytes may lead to deterioration of nerve function (37). Albumin was found to exert an anticoagulant role and inhibitory effect on platelet function by binding antithrombin (38–40), which might aggravate the development of hemorrhagic stroke. In our study, we found that NLR, NAR and RA had potential prognostic values for 30-day mortality in hemorrhagic stroke patients. Previously, ICH score was reported to be a reliable clinical grading scale that allows risk stratification for patients with ICH (41). ICH score includes a basic neurological examination (GCS), a baseline patient characteristic (age), and initial neuroimaging (ICH volume, IVH, infratentorial/supratentorial origin), and compared with ICH score, NLR, NAR and RA are easily available and inexpensive markers that can be routinely detected in clinic. Application of these prognostic biomarkers may help clinicians enhance risk stratification, design individual treatments, and determine follow-up schedules for hemorrhagic stroke patients, which might further improve the outcomes of those patients.
There was evidence indicating that NLR, PLR, or NAR might associate with 30-day mortality of ischemic stroke patients in previous studies (42–44). The mechanisms underlying the findings might be related to the different roles of neutrophils and lymphocytes in the pathophysiologic development of atherosclerosis (45). Neutrophils are found to accumulate in cerebral vessels shortly after stroke and may result in infarctions extension and inhibit microvascular perfusion (46). PNI reflects nutritional status of patients, and previous studies revealed that malnutrition was associated with increased mortality in older Chinese adults with ischemic stroke (47). In our study, no significant association between NLR, PLR, NAR, PNI, SII or RA with 30-day mortality was found in ischemic stroke patients, this might because some other variables related to 30-day mortality of ischemic stroke patients were not included. The association between NAR and 30-day mortality of ischemic stroke patients showed a P-value of 0.055, this suggested that there might be association between NAR and 30-day mortality of ischemic stroke patients.
The strength in our study was that we focused on the prognostic values of LR, PLR, NAR, PNI, SII, and RA for 30-day mortality of different subtypes of stroke including ischemic stroke or hemorrhagic stroke. The finding might help identify potential reliable biomarkers in predicting those with high risk of 30-day mortality of different subtypes of stroke. There were several limitations in the current study. Firstly, this was a retrospective study from single-center, recall bias might exist. Secondly, due to the limitation of the database, some variables including the site or size of the hemorrhage or ischaemic stroke were unavailable, which might affect the results of our study. Thirdly, we analyzed the baseline data of inflammation biomarkers in ICU, and in the future, dynamic changes of the inflammation biomarkers during ICU stay will be analyzed to verify the results of our study. We will also conduct a study based on the samples from our hospital, and more important variables will be included.
This study evaluated the predicitive values of NLR, PLR, NAR, PNI, SII, and RA for 30-day mortality of ischemic stroke or hemorrhagic stroke patients. We found that NLR, NAR and RA were potential prognostic biomarkers for predicting 30-day mortality in hemorrhagic stroke patients, which might help clinicians enhance risk stratification, design individual treatments, and determine follow-up schedules for hemorrhagic stroke patients.
Publicly available datasets were analyzed in this study. This data can be found in MIMIC-III database.
Requirement for individual patient consent was waived because the project did not impact clinical care and all protected health information was deidentified. As the samples were not from The Eighth Medical Center of PLA General Hospital, and this study was exempt from our Institutional Review Board approval.
JZ and JF collected and analyzed the clinical data, reviewed the literature, and drafted the article. CL and FQ designed the study, supervised the initial drafting, and critically revised the article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Guzik A, Bushnell C. Stroke Epidemiology and Risk Factor Management. Continuum. (2017) 23:15–39. doi: 10.1212/CON.0000000000000416
3. Abdu H, Tadese F, Seyoum G. Comparison of ischemic and hemorrhagic stroke in the medical ward of dessie referral hospital, northeast ethiopia: a retrospective study. Neurol Res Int. (2021) 2021:9996958. doi: 10.1155/2021/9996958
4. Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
5. Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. (2013) 369:448–57. doi: 10.1056/NEJMra1201534
6. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
7. Chen S, Zeng L, Hu Z. Progressing haemorrhagic stroke: categories, causes, mechanisms and managements. J Neurol. (2014) 261:2061–78. doi: 10.1007/s00415-014-7291-1
8. Parikh NS, Merkler AE, Iadecola C. Inflammation, autoimmunity, infection, and stroke: epidemiology and lessons from therapeutic intervention. Stroke. (2020) 51:711–8. doi: 10.1161/STROKEAHA.119.024157
9. Dong X, Gao J, Zhang CY, Hayworth C, Frank M, Wang Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. (2019) 13:1272–83. doi: 10.1021/acsnano.8b06572
10. Omar T, Karakayali M, Yesin M, Alaydin HC, Karabag Y, Gümüşdag A. Monocyte to high-density lipoprotein cholesterol ratio is associated with the presence of carotid artery disease in acute ischemic stroke. Biomark Med. (2021) 15:489–95. doi: 10.2217/bmm-2020-0705
11. Aslan S, Demir AR, Demir Y, Taşbulak Ö, Altunova M, Karakayali M, et al. Usefulness of plateletcrit in the prediction of major adverse cardiac and cerebrovascular events in patients with carotid artery stenosis. Vascular. (2019) 27:479–86. doi: 10.1177/1708538119847898
12. Li W, Hou M, Ding Z, Liu X, Shao Y, Li X. Prognostic value of neutrophil-to-lymphocyte ratio in stroke: a systematic review and meta-analysis. Front neurol. (2021) 12:686983. doi: 10.3389/fneur.2021.686983
13. Yan YK, Huang H, Li DP Ai ZY, Li X, Sun Z. Prognostic value of the platelet-to-lymphocyte ratio for outcomes of stroke: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. (2021) 25:6529–38. doi: 10.26355/eurrev_202111_27095
14. Chen Z, Xie D, Li Y, Dai Z, Xiang S, Chen Z, et al. Neutrophil albumin ratio is associated with all-cause mortality in stroke patients: a retrospective database study. Int J Gen Med. (2022) 15:1–9. doi: 10.2147/IJGM.S323114
15. Liu Y, Yang X, Kadasah S, Peng C. Clinical value of the prognostic nutrition index in the assessment of prognosis in critically Ill patients with stroke: a retrospective analysis. Comput Math Methods Med. (2022) 2022:4889920. doi: 10.1155/2022/4889920
16. Ji Y, Xu X, Wu K, Sun Y, Wang H, Guo Y, et al. Prognosis of ischemic stroke patients undergoing endovascular thrombectomy is influenced by systemic inflammatory index through malignant brain edema. Clin Interv Aging. (2022) 17:1001–12. doi: 10.2147/CIA.S365553
17. Zhao N, Hu W, Wu Z, Wu X, Li W, Wang Y, et al. The red blood cell distribution width-albumin ratio: a promising predictor of mortality in stroke patients. Int J Gen Med. (2021) 14:3737–47. doi: 10.2147/IJGM.S322441
18. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Scientific data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
19. Elkind MSV, Boehme AK, Smith CJ, Meisel A, Buckwalter MS. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. (2020) 51:3156–68. doi: 10.1161/STROKEAHA.120.030429
20. Hermann DM, Kleinschnitz C, Gunzer M. Implications of polymorphonuclear neutrophils for ischemic stroke and intracerebral hemorrhage: predictive value, pathophysiological consequences and utility as therapeutic target. J Neuroimmunol. (2018) 321:138–43. doi: 10.1016/j.jneuroim.2018.04.015
21. Davidson S, Coles M, Thomas T, Kollias G, Ludewig B, Turley S, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. (2021) 21:704–17. doi: 10.1038/s41577-021-00540-z
22. Wanrooy BJ, Wen SW, Wong CH. Dynamic roles of neutrophils in post-stroke neuroinflammation. Immunol Cell Biol. (2021) 99:924–35. doi: 10.1111/imcb.12463
23. Xie W, Li P. Visualizing regulatory lymphocytic responses to predict neurological outcome after stroke. CNS Neurosci Ther. (2021) 27:867–8. doi: 10.1111/cns.13698
24. Drăgoescu AN, Pădureanu V, Stănculescu AD, Chiu?u LC, Tomescu P, Geormăneanu C, et al. Neutrophil to lymphocyte ratio (NLR)-a useful tool for the prognosis of sepsis in the ICU. Biomedicines. (2021) 10:75. doi: 10.3390/biomedicines10010075
25. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. (2016) 47:1654–7. doi: 10.1161/STROKEAHA.116.013627
26. Giede-Jeppe A, Bobinger T, Gerner ST, Sembill JA, Sprügel MI, Beuscher VD, et al. Neutrophil-to-lymphocyte ratio is an independent predictor for in-hospital mortality in spontaneous intracerebral hemorrhage. Cerebrovascular Dis. (2017) 44:26–34. doi: 10.1159/000468996
27. Han Z, He X, Peng S. Neutrophil count to albumin ratio as a prognostic indicator for HBV-associated decompensated cirrhosis. J Clin Lab Anal. (2021) 35:e23730. doi: 10.1002/jcla.23730
28. Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. (2008) 582:1783–7. doi: 10.1016/j.febslet.2008.04.057
29. Akirov A, Masri-Iraqi H, Atamna A, Shimon I. Corrigendum to 'low albumin levels are associated with mortality risk in hospitalized patients. Am J Med. (2020) 133:646. doi: 10.1016/j.amjmed.2020.02.001
30. Li J, Imano H, Yamagishi K, Cui R, Muraki I, Umesawa M, et al. Serum albumin and risks of stroke and its subtypes-the circulatory risk in communities study (CIRCS). Circ J. (2021) 85:385–92. doi: 10.1253/circj.CJ-20-0384
31. Zhao H, Zhao Y, Wu Z, Cheng Y, Zhao N. Red cell distribution width is associated with all-cause mortality in patients with acute stroke: a retrospective analysis of a large clinical database. J Int Med Res. (2021) 49:300060520980587. doi: 10.1177/0300060520980587
32. Hou H, Sun T, Li C, Li Y, Guo Z, Wang W, et al. An overall and dose-response meta-analysis of red blood cell distribution width and CVD outcomes. Sci Rep. (2017) 7:43420. doi: 10.1038/srep43420
33. Nakashima K, Ohgami E, Kato K, Yoshitomi S, Maruyama T, Harada M. Prognostic significance of red cell distribution width in hospitalized older patients with heart failure or infection. Geriatr Gerontol Int. (2019) 19:988–92. doi: 10.1111/ggi.13755
34. Fan H, Liu X, Li S, Liu P, Song Y, Wang H, et al. High red blood cell distribution width levels could increase the risk of hemorrhagic transformation after intravenous thrombolysis in acute ischemic stroke patients. Aging. (2021) 13:20762–73. doi: 10.18632/aging.203465
35. Petrone AB, Eisenman RD, Steele KN, Mosmiller LT, Urhie O, Zdilla MJ. Temporal dynamics of peripheral neutrophil and lymphocytes following acute ischemic stroke. Neurol Sci. (2019) 40:1877–85. doi: 10.1007/s10072-019-03919-y
36. Cai W, Liu S, Hu M, Huang F, Zhu Q, Qiu W, et al. Functional dynamics of neutrophils after ischemic stroke. Transl Stroke Res. (2020) 11:108–21. doi: 10.1007/s12975-019-00694-y
37. Gill D, Veltkamp R. Dynamics of T cell responses after stroke. Curr Opin Pharmacol. (2016) 26:26–32. doi: 10.1016/j.coph.2015.09.009
38. Paar M, Rossmann C, Nusshold C, Wagner T, Schlagenhauf A, Leschnik B, et al. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PLoS ONE. (2017) 12:e0182997. doi: 10.1371/journal.pone.0182997
39. Purdon AD, Rao AK. Interaction of albumin, arachidonic acid and prostanoids in platelets. Prostaglandins Leukot Essent Fatty Acids. (1989) 35:213–8. doi: 10.1016/0952-3278(89)90004-5
40. Li N, Zhou H, Tang Q. Red blood cell distribution width: a novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Markers. (2017) 2017:7089493. doi: 10.1155/2017/7089493
41. Hemphill JC. 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC: The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. (2001) 32:891–7. doi: 10.1161/01.STR.32.4.891
42. Huang LY, Sun FR, Yin JJ, Ma YH Li HQ, Zhong XL Yu JT, Song JH, et al. Associations of the neutrophil to lymphocyte ratio with intracranial artery stenosis and ischemic stroke. BMC Neurol. (2021) 21:56. doi: 10.1186/s12883-021-02073-3
43. Yang Y, Xie D, Zhang Y. Increased platelet-to-lymphocyte ratio is an independent predictor of hemorrhagic transformation and in-hospital mortality among acute ischemic stroke with large-artery atherosclerosis patients. Int J Gen Med. (2021) 14:7545–55. doi: 10.2147/IJGM.S329398
44. Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:650–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010
45. Bakogiannis C, Sachse M, Stamatelopoulos K, Stellos K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine. (2019) 122:154157. doi: 10.1016/j.cyto.2017.09.013
46. Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol. (2009) 22:294–301. doi: 10.1097/WCO.0b013e32832b4db3
Keywords: inflammation, biomarkers, 30-day mortality, ischemic stroke, hemorrhagic stroke
Citation: Zhao J, Feng J, Ma Q, Li C and Qiu F (2023) Prognostic value of inflammation biomarkers for 30-day mortality in critically ill patients with stroke. Front. Neurol. 14:1110347. doi: 10.3389/fneur.2023.1110347
Received: 28 November 2022; Accepted: 18 January 2023;
Published: 06 February 2023.
Edited by:
Bin Qiu, Yale University, United StatesReviewed by:
Manuel Cappellari, Integrated University Hospital Verona, ItalyCopyright © 2023 Zhao, Feng, Ma, Li and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Qiu,  cWl1ZmVuZ25ldEBob3RtYWlsLmNvbQ==; Chunlin Li,
cWl1ZmVuZ25ldEBob3RtYWlsLmNvbQ==; Chunlin Li,  TGVlY2wzMTZAMTYzLmNvbQ==
TGVlY2wzMTZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.