
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 23 February 2023
Sec. Neuro-Otology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1106084
 Xia Ling1†
Xia Ling1† Yue-Xia Wu2†
Yue-Xia Wu2† Yu-Fei Feng2
Yu-Fei Feng2 Tong-Tong Zhao2
Tong-Tong Zhao2 Gui-Ping Zhao1
Gui-Ping Zhao1 Ji-Soo Kim3,4
Ji-Soo Kim3,4 Xu Yang2*
Xu Yang2* Zhao-Xia Wang1*
Zhao-Xia Wang1*Objective: To determine the topical diagnosis and etiologies of spontaneous nystagmus (SN) with an upbeat component.
Methods: We retrospectively recruited 43 patients with SN with an upbeat component at a university hospital in China from 2020 to 2022. SN with an upbeat component was divided into pure upbeat nystagmus (UBN), SN with a predominant upbeat component, and SN with a non-predominant upbeat component. We analyzed their clinical and neurotologic findings and the final diagnosis.
Results: Fourteen (32.6%) of them showed pure UBN, while 29 (67.4%) exhibited SN mixed with an upbeat component, mixed upbeat-horizontal in 15, mixed upbeat-horizontal-torsional in 13, and upbeat-torsional in the remaining one. Pure UBN and SN with a predominant upbeat component were more common in central than in peripheral vestibular disorders [16 (80.0%) vs. 0 (0%), Chi-Square test, p < 0.001]. Central vestibular disorders were diagnosed in 20 (46.5%) patients, peripheral in 14 (32.6%), and undetermined in nine (20.9%) patients. The underlying causes mainly included acute unilateral peripheral vestibulopathy (n = 11), posterior circulation infarction (n = 9), benign recurrent vertigo (n = 4), vestibular migraine (VM, n = 3), and VM of childhood (n = 2).
Conclusion: SN with an upbeat component can be seen in both central and peripheral vestibular disorders. Pure UBN was a characteristic sign of central vestibular dysfunction. Central vestibular disorders should be highly suspected when patients show pure UBN or SN with a predominant upbeat component.
Vertigo and dizziness are the most common symptoms of vestibular dysfunction, while one of the most frequent signs is spontaneous nystagmus (SN). Evaluation of SN is an important indicator in differentiating central and peripheral vestibular disorders (1). SN can be classified into pure (horizontal, vertical, or torsional) and mixed nystagmus according to the component of nystagmus. SN with an upbeat component could be divided into pure UBN, SN with a predominant upbeat component, and SN with a non-predominant upbeat component.
Pure UBN and SN with a predominant upbeat component have been reported in patients with brainstem infarction, brainstem hemorrhage, Wernicke's encephalopathy, multiple sclerosis, tumors, episodic ataxia type 2, cerebellar degeneration, Creutzfeldt-Jakob disease, brainstem encephalitis, epilepsy, Behcet's syndrome, Chiari malformation, meningitis, congenital, vestibular migraine (VM), organophosphate toxicity, tobacco, drugs, canalith jam involving the anterior semicircular canal, Meniere's disease (MD), and delayed endolymphatic hydrops (2–11). SN with a non-predominant upbeat component has been reported in patients with both central and peripheral lesions, including lateral medullary infarction and medial medullary infarction (12, 13), and superior vestibular neuritis (VN) (14–16). Few studies have analyzed the topical diagnosis and etiologies of all kinds of SN with an upbeat component and the differences between nystagmus types in peripheral and central vestibular disorders (5–7, 17–20). Hence, we retrospectively analyzed the medical history and examination findings in 43 patients with SN with an upbeat component and explored the topical diagnosis, possible etiologies, and mechanism of SN with an upbeat component.
We retrospectively reviewed the etiology database of dizziness patients established in our hospital for patients with SN with an upbeat component who either visited the emergency department or the outpatient clinic of the Department of Neurology in our hospital from July 2020 to August 2022. Fifty-three consecutive patients were identified in this time period. Ten patients had to be excluded because of strong horizontal nystagmus but no apparent UBN when performing the video-oculography (VOG) test (n = 4), only described UBN on bedside examination but no apparent UBN when performing the VOG test (n = 4), lack of essential laboratory tests (n = 2). Eventually, 43 patients with SN with an upbeat component were included.
The medical records of 43 patients with SN with an upbeat component were reviewed, including disease course, onset form, duration, frequency of attacks, precipitating/relieving factors, symptoms, signs, history, eye movement examination, caloric test, video head impulse test (vHIT, Interacoustics, Middelfart, Denmark), vestibular-evoked myogenic potentials (VEMPs), head magnetic resonance imaging (MRI), three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging (3D-FLAIR MRI) and serum immunology test results and diagnosis. 2D-VOG (Interacoustics, Middelfart, Denmark) was performed for the detection of SN, gaze-evoked nystagmus, saccades, smooth pursuit, optokinetic, head-shaking nystagmus, and positional test. All patients underwent VOG examination during the acute phase or attack phase. The VOG examination was performed on the day of the visit for patients with acute attacks if they could cooperate with the examination. For patients with severe dizziness/vertigo that cannot cooperate with the examination, a VOG test should be performed within seven days of the latest attack.
All experiments followed the tenets of the Declaration of Helsinki and were approved by the Institutional Review Board of Aerospace Center Hospital.
SN with an upbeat component could be divided into pure UBN, SN with a predominant upbeat component, and SN with a non-predominant upbeat component. Since we could not quantify the torsional component of nystagmus due to using a 2D video-oculography, the torsional component was determined by observing the video clips by two experiment neurologists. We mainly focused on comparing the intensity of horizontal and vertical components. When there was only an upbeat component, we defined it as pure UBN. When the intensity of the upbeat component of mixed SN was greater than that of the horizontal component, we defined it as SN with a predominant upbeat component. When the intensity of the upbeat component of mixed SN was equal to or lesser than that of the horizontal component, we defined it as SN with a non-predominant upbeat component.
According to the time characteristics of vestibular symptoms onset, patients can be classified into acute, episodic, or chronic vestibular syndrome (AVS, EVS, or CVS) (21). All diagnoses were made by the senior authors (ZXW and XY) according to widely accepted diagnostic criteria for each vestibular disorder or the international classification of vestibular disorders (ICVD) criteria when available (22–26). The published diagnostic criteria consensus includes acute unilateral vestibulopathy (AUVP)/VN (26), persistent postural-perceptual dizziness (PPPD) (23), VM (25), VM of childhood (24), and MD (22). Besides, probably labyrinthine infarction was diagnosed in older patients with sudden onset of unilateral deafness and vertigo, especially when there is a history of stroke or known vascular risk factors (27). Benign recurrent vertigo (BRV) was diagnosed when patients showed spontaneous rotational vertigo or instability; symptoms that were not triggered by changes in position, lasting longer than 1 min; normal audiogram or symmetric hearing loss; no cochlear symptoms (tinnitus or stuffiness) during the attack phase; no migraine or migraine aura in the acute phase (26, 28). Isolated acute unilateral utricular vestibulopathy was diagnosed in patients with acute onset of postural imbalance, which can be diagnosed by ocular VEMP (26).
All the statistical analyses were performed using SPSS software (version 20.0, IBM SPSS Statistics, N.Y., USA). Continuous variables were expressed as a mean ± SD for parametric values or a median (range) for non-parametric ones. Counting variables were expressed as a percentage. The normality of the data was determined using the Shapiro-Wilk test. The Chi-square test was adopted to compare the proportion of the SN with a predominant upbeat component between peripheral and central disorders. p < 0.05 was considered significant.
Forty-three patients showing SN with an upbeat component were included in the study. There were 26 (60.5%) males and 17 (39.5%) females, with a mean age of 47.0 ± 16.5 years (range 12–78).
Central vestibular disorders were diagnosed in 20 (46.5%) patients, peripheral in 14 (32.6%), and undetermined in 9 (20.9%) patients. Among 14 patients with peripheral vestibular disorders, the underlying causes included AUVP (n = 11, superior AUVP in 10 and complete AUVP in one), probable labyrinthine infarction (n = 1), isolated acute unilateral utricular vestibulopathy (n = 1), and probable MD (n = 1).
Among 20 patients with central vestibular disorders, the underlying causes included posterior circulation infarction (PCI, n = 9), VM (n = 3), VM of childhood (n = 2), Wernicke's encephalopathy (n = 1), brainstem encephalitis (n = 1), postoperative fourth ventricle ependymoma (n = 1), autoimmune encephalitis (n = 1), transient ischemic attack (TIA; n = 1), and combination of VM and PPPD (n = 1). Among nine patients with undetermined central and /or peripheral vestibular disorders, the underlying causes included BRV (n = 4) and undetermined (n = 5).
Fourteen (32.6%) of them showed pure UBN, while 29 (67.4%) exhibited SN mixed with an upbeat component, mixed upbeat-horizontal (UBN-HBN) in 15, mixed upbeat-horizontal-torsional (UBN-HBN-TN) in 13, and upbeat-torsional (UBN-TN) in the remaining one (Table 1). Among patients with pure UBN, central vestibular disorders were noted in eight (57.1%) and undetermined in six (42.9%). Patients with SN with a predominant upbeat component (the intensity of the upbeat component was greater than that of the horizontal component) were only in central vestibular disorders (8, 100%). Among patients with SN with a non-predominant upbeat component, peripheral vestibular disorders were noted in 14 (66.7%) patients, central in four (19.0%), and undetermined in three (14.3%) patients (Table 1, Figure 1).
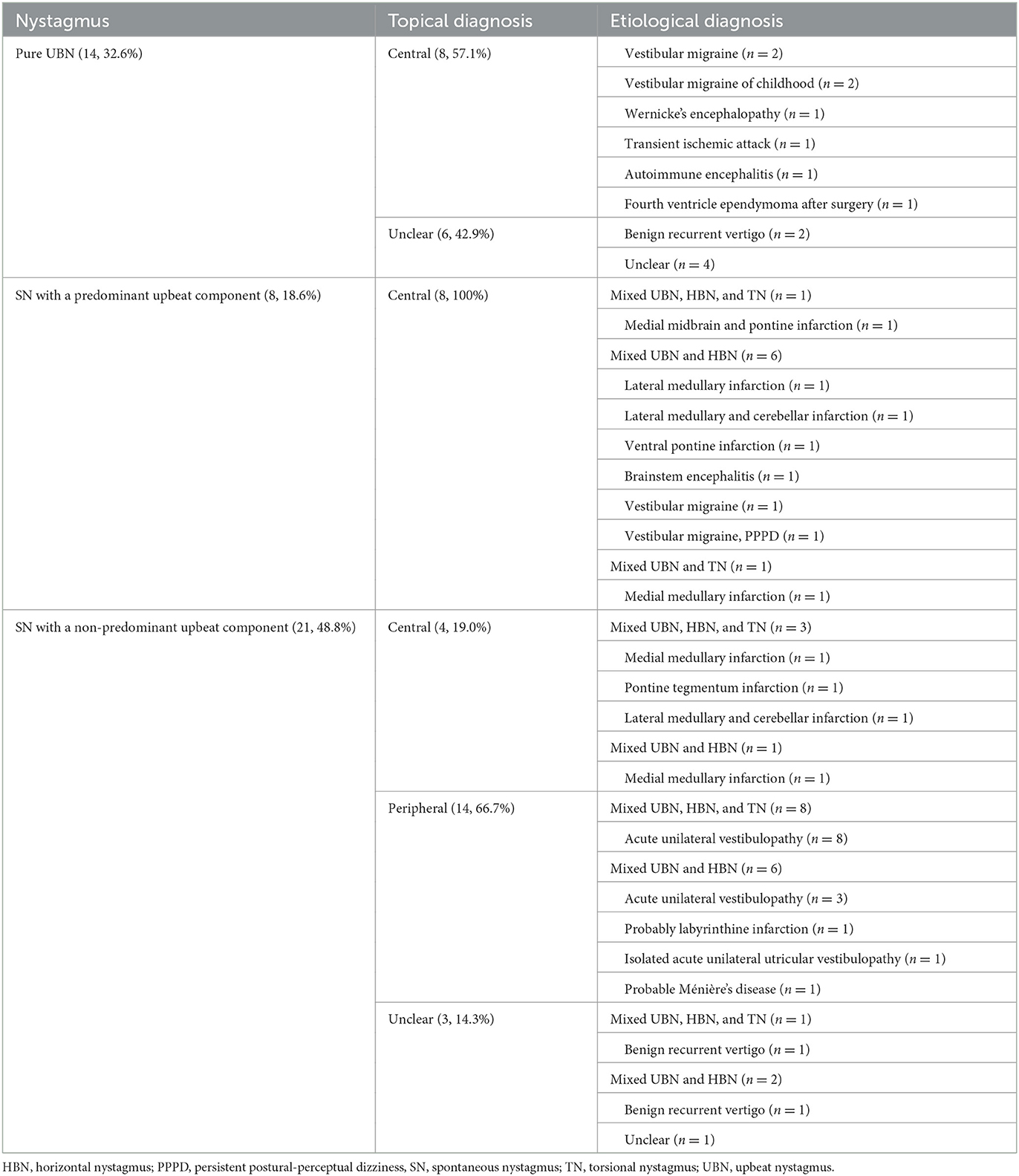
Table 1. Etiology of patients with pure upbeat nystagmus and mixed nystagmus with an upbeat component.
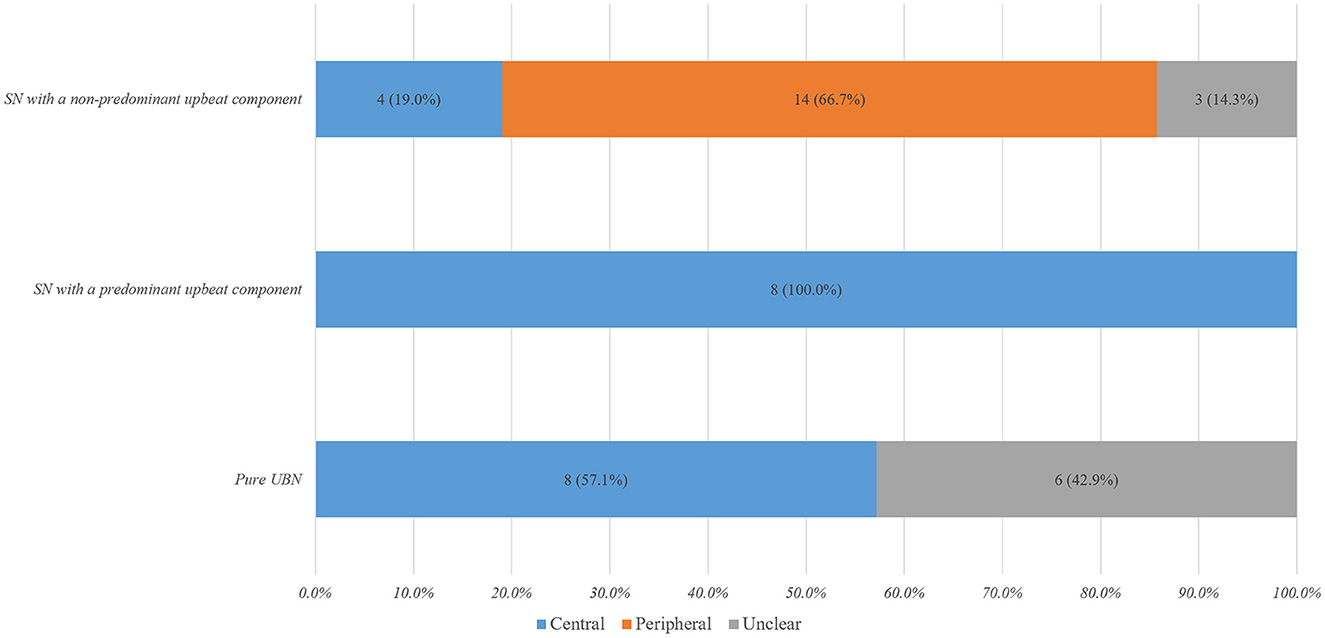
Figure 1. Characteristic distribution of spontaneous nystagmus with an upbeat component in vestibular disorders. SN, spontaneous nystagmus; UBN, upbeat nystagmus.
The horizontal component of SN was greater than the upbeat component in one patient with medial medullary infarction, and one patient with lateral medullary infarction, one patient with pontine tegmentum infarction, and one patient with lateral medullary and cerebellar infarction.
Pure UBN and SN with a predominant upbeat component were more common in central than in peripheral vestibular disorders [16 (80.0%) vs. 0 (0%), Chi-Square test, p < 0.001]. The intensity of upbeat component was significantly weaker than that of the horizontal component in patients with peripheral mixed SN [4.4 ± 2.7°/s (median 4, range 1–11°/s) vs. 10.2 ± 6.4 °/s (median 8.5, range 2–23°/s), Wilcoxon Signed Ranks Test, Z = −2.928, p = 0.003]. There was no significant difference in the intensity of the upbeat component and horizontal component in patients with central mixed SN [6.4 ± 4.1°/s (median: 6, range: 2–16°/s) vs. 6.7 ± 6.5°/s (median: 4.0, range: 1–22), Wilcoxon Signed Ranks Test, Z = −0.045, p = 0.964, Figure 2].
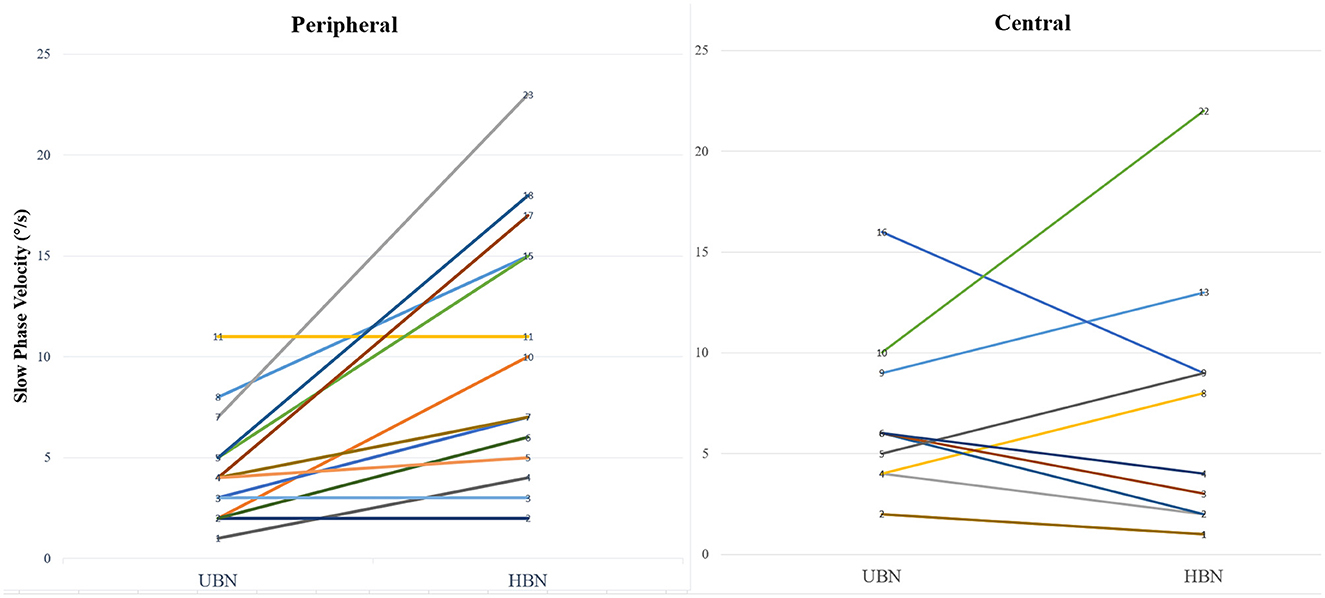
Figure 2. Comparison of the intensity of the horizontal and upbeat components of mixed spontaneous nystagmus in central and peripheral vestibular disorders. UBN, upbeat nystagmus; HBN, horizontal nystagmus.
According to the characteristics of the dizziness/vertigo attacks, 23 (53.5%), 17 (39.5%), and 3 (7.0%) of the patients with SN with an upbeat component had AVS, EVS, and CVS, respectively (Figure 3).
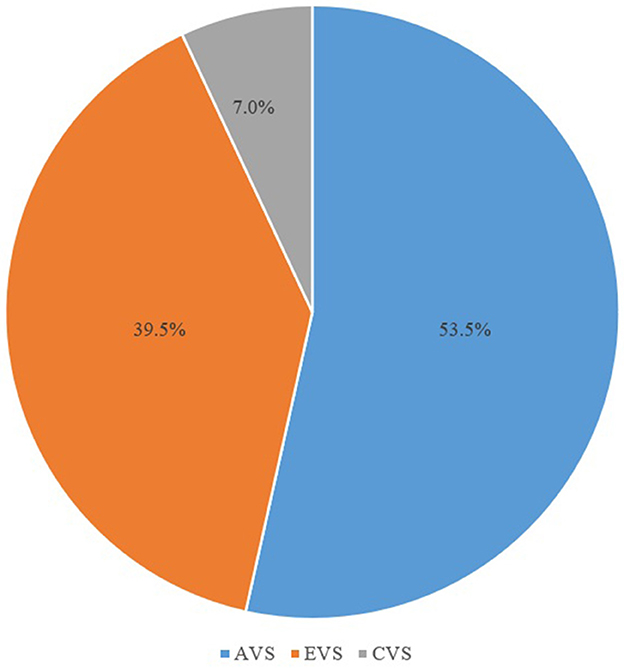
Figure 3. Proportion of vestibular syndrome types in patients showing spontaneous nystagmus with an upbeat component. AVS, acute vestibular syndrome; EVS, episodic vestibular syndrome; CVS, chronic vestibular syndrome.
The AVS group included 11 patients with AUVP, nine patients with PCI (including medullary infarction in 4, pontine infarction in 2, medullary and cerebellar infarction in 2, and midbrain and pontine infarction in 1), one patient with probable labyrinthine infarction, one patient with isolated acute unilateral utricular vestibulopathy, and one patient with brainstem encephalitis.
The EVS group included BRV (n = 4), VM (n = 3), VM of childhood (n = 2), probable MD (n = 1), postoperative fourth ventricle ependymoma (n = 1), autoimmune encephalitis (n = 1), TIA (n = 1), and unclear causes (n = 4). The CVS group included Wernicke's encephalopathy (n = 1), concomitant VM and PPPD (n = 1), and unclear causes (n = 1; Table 2).
Four patients with BRV showed canal paresis on the caloric test, one out of these four patients had a unilateral gain reduction of horizontal and anterior canals, and one patient had a gain reduction of the posterior canal during the vHIT test.
In the present study, we found that central vestibular disorders should be highly suspected when patients showed pure UBN or SN with a predominant upbeat component. For vestibular disorders presenting as SN with an upbeat component, PCI and VM were seen more commonly in patients with central vestibular disorders, whereas AUVP was the most common in peripheral vestibular disorders.
We found that most patients with AVS who showed SN with an upbeat component had AUVP (mainly superior AUVP), and these patients were accompanied by horizontal and anterior semicircular canals hypofunction. The types of SN in these patients were mixed UBN-HBN with or without a torsional component, and the upbeat component of the nystagmus was significantly weaker than the horizontal component. The generation of the upbeat component is mainly associated with hypofunction in the anterior semicircular canal afferents, which would result in relative excitation of the opposite posterior semicircular canal, thus producing UBN with or without a torsional component, whereas the horizontal component was associated with hypofunction in the horizontal semicircular canal afferents.
Previous studies investigated the presence of spontaneous vertical-horizontal-torsional nystagmus in patients with AUVP and found that the vertical component of nystagmus was mostly UBN, and the intensity of the upbeat component was weaker than the horizontal component (14, 16, 29). This may be related to the fact that AUVP/VN primarily involves the superior branches, mainly including the anterior and horizontal semicircular canals, and the utricle (30), because there are anatomical differences between the superior vestibular nerve (SVN) and the inferior vestibular nerve (IVN). The bony channel of the SVN is seven times longer than that of the IVN, and the SVN channel has a larger percentage of bony spicules than the IVN channel, making the SVN more susceptible to entrapment and ischemia (30).
We found that PCI is the second most common cause of spontaneous UBN in patients with AVS. In patients with PCI, the lesions occur mainly in the medial part of the medulla oblongata, pons, or midbrain, and infarction over the ventral part of the pons, lateral part of the medulla or cerebellum can also be seen. The types of nystagmus in these patients were mixed nystagmus, including UBN-HBN-TN, UBN-HBN, and UBN-TN. The upbeat component of the SN was stronger than the horizontal component in 55.6% (5/9) of patients with PCI, which was weaker than the horizontal component in four patients [including lateral medullary infarction (n = 1), medial medullary infarction (n = 1), medial pontine infarction (n = 1), and infarction involving lateral medulla and cerebellum (n = 1)]. A previous study has shown that the presence of SN with an upbeat component in medial medullary infarction is mainly associated with the involvement of the perihypoglossal nucleus (including the nucleus prepositus hypoglossi (31, 32), nucleus intercalatus and nucleus of Roller (19) or damage to the vestibulo-ocular reflex (VOR) pathway of anterior semicircular canal (33). In contrast, the occurrence of SN with an upbeat component in lateral medullary infarction is associated with the involvement of vestibular nuclei, because damage to the vestibular nuclei can lead to the occurrence of various types of SN (2). Similarly, for patients with pontine tegmentum infarction, the presence of upbeat nystagmus is mostly due to the damage of the ascending VOR pathway from the anterior semicircular canal to ocular motor nuclei via the medial longitudinal fasciculus (2). For focal infarct occurs between the base and the tegmentum of the pons at its middle-to-upper level, the presence of UBN may be due to damage to the ventral tegmental tract, which is also one of the ascending VOR pathways (34).
In this study, SN with an upbeat component was often seen in other rare peripheral vestibular disorders, including possible labyrinthine infarction and isolated acute unilateral utricular vestibulopathy. Studies have shown that inner ear ischemia may cause dizziness/vertigo since the inner ear requires high energy metabolism and lacks collateral circulation (35, 36). Because the internal auditory artery is a terminal artery with insufficient collateral circulation, the vestibular labyrinth and its various components appear more vulnerable to ischemia (37). Therefore, internal auditory artery infarction can often lead to severe peripheral vestibular disorders and hearing loss (37). However, it is currently difficult to visually confirm the presence of labyrinthine infarction by imaging (38). One patient in the present study had horizontal and anterior canal hypofunction, ipsilateral hearing loss, risk factors for atherosclerosis, and a normal MRI. We suggest that the patient may have a labyrinthine infarction. In one patient with isolated acute unilateral utricular vestibulopathy included in the present study, the types of SN were UBN-HBN, oVEMP test results on the right side were abnormal, whereas vHIT, caloric test, gaze, saccade, smooth pursuit, and optokinetic reflex test results, and brain MRI findings were normal in the patient. Isolated acute unilateral utricular vestibulopathy has rarely been studied (39). A previous case report of a patient with unilateral utricular dysfunction showed that the patient had SN with horizontal and vertical components during an acute attack phase. vHIT findings showed normal VOR gain in both the horizontal and vertical semicircular canals. It is speculated that the neural input from the otoliths may converge on horizontal semicircular canal neurons in the vestibular nuclei. When the utricular function is asymmetrical, SN, like the nystagmus caused by asymmetrical semicircular canal function, is produced (39). The mechanism underlying the occurrence of SN in isolated acute unilateral utricular vestibulopathy remains to be further investigated.
In recent years, clinicians have found a group of recurrent vertigo syndromes presenting as spontaneous vertigo or instability, which is usually not accompanied by hearing loss, tinnitus, or aural fullness, as well as migraine or migraine aura during the acute phase (28). The etiologies are not the recognized episodic vestibular syndromes, such as VM, MD, benign paroxysmal positional vertigo, and vestibular paroxysmia (40). A previous study has shown that patients with BRV do not have unique clinical features compared to those with VM and MD (41). During the follow-up period, the diagnosis of BRV was changed to VM or MD in a small proportion of patients (42, 43). A previous study documented no statistically significant differences in the frequency of vertigo attacks between patients with BRV and patients with MD and VM after 6 months and 3 years of follow-up (40). MRI revealed endolymphatic hydrops in about 23% of patients with BRV, which was more frequently involved in the horizontal semicircular canal (59%), and patients with BRV often showed abnormal caloric test results (49%) (28). Attyé et al. (44) found that 48.4% of patients with BRV showed vestibular hydrops and/or cochlear hydrops on MRI. Some authors suggest that there is a subtype of vestibular MD presenting with recurrent attacks of vertigo without fluctuating hearing loss and symptoms of aural fullness (45). In the present study, BRV was the most common cause of spontaneous UBN in patients with EVS, two of the four patients with BRV showed mixed SN with a non-predominant upbeat component, and the other two showed pure UBN. Even though these patients showed evidence of lesions involving the peripheral vestibular system on the caloric test or vHIT test, we cannot wholly exclude the central origin, especially in patients with pure UBN.
In this study, three patients with VM showed SN with an upbeat component. Of these three patients, two patients had purely UBN, and one patient had UBN-HBN. Patients with VM may show spontaneous or positional vertigo, and some patients may experience a change from spontaneous vertigo to positional vertigo after a few hours or days (25, 46). Studies have shown that the incidence of ictal SN in VM patients ranged from 9.9 to 71.3% (47–50), which was mostly HBN (47, 48, 50). SN in patients with VM suggests an imbalance in the peripheral or central vestibular system (51). One study described the clinical characteristics of 19 patients with VM and found that one out of these 19 patients developed spontaneous UBN, which resolved after treatment with external trigeminal nerve stimulation (52). Another study showed that the incidence of spontaneous vertical nystagmus in the absence of fixation was 16%. Unfortunately, the authors did not describe the proportion of patients presenting vertical nystagmus with an upbeat component (49). It has been shown that some patients with VM have AUVP, mostly showed unilateral canal paresis of the horizontal semicircular canal during the caloric test and normal vHIT results (50). In the present study, one patient with VM had canal paresis of the horizontal semicircular canal, however, vHIT showed normal VOR gains of the horizontal and vertical semicircular canal.
In the present study, we also found the presence of spontaneous UBN in two children with VM. A population-based study showed that the prevalence of recurrent vertigo that may be associated with migraine in children aged 6–12 years was estimated to be 2.8% (53). Similar to VM in adults, SN, positional nystagmus, or head-shaking nystagmus can also be seen in pediatric patients with VM (54). The rate of spontaneous UBN detected during funduscopy in children with definite VM has been reported to be 6% (54). Marcelli et al. (55) found that the prevalence of positional nystagmus was 44%, while the prevalence of head-shaking nystagmus was 31% in children with migraine and vestibular symptoms.
In this study, one patient with probable MD showed mixed spontaneous UBN-HBN with the horizontal component toward the healthy side, the caloric test showed canal paresis on the left side, but vHIT showed normal gains in all semicircular canals. A previous study showed that 42–77% of patients with unilateral MD had canal paresis on the caloric test (56), and semicircular canal VOR gains were mostly normal during the high-frequency head impulse test (57). Therefore, the presence of mixed spontaneous UBN-HBN in this patient with probable MD may be associated with simultaneous impairment of the horizontal and anterior canal function. In addition, in the present study, one patient was found to have postoperative fourth ventricle ependymoma. Previous studies have reported the presence of spontaneous or positional UBN in patients with occupying lesions within the fourth ventricle (58, 59). The presence of spontaneous UBN may be related to fourth ventricular occupying lesions or surgical damage to the adjacent brainstem or cerebellar structures. Additionally, in the present study, we also found UBN in patients with brainstem encephalitis, autoimmune encephalitis, and TIA. Similar findings have also been reported in previous studies (3, 60, 61).
In the present study, a patient with Wernicke's encephalopathy was observed. Axial FLAIR sequence showed hypersignals in the midbrain periaqueductal gray matter. The patient showed purely UBN, which disappeared after 8 months of follow-up. Wernicke's encephalopathy is caused by thiamine (vitamin B1) deficiency, and patients with Wernicke's encephalopathy may present with spontaneous vertical nystagmus (62). It has been suggested that the presence of spontaneous vertical nystagmus in patients with Wernicke's encephalopathy may be caused not only by imbalances in the semicircular canal-ocular reflex pathways governing the rotational VOR, but also by an imbalance in the otolith-ocular pathways that govern the linear VOR (18). Studies have shown that in the acute phase of Wernicke's encephalopathy, patients presented with spontaneous UBN in primary gaze, which may change to permanent DBN in the chronic phase of Wernicke's encephalopathy (18, 63). Spontaneous UBN may obviously diminish or change to DBN with a change in convergence and horizontal or vertical gaze, head shaking, or mastoid vibration (18, 63). However, in the present study, the conversion of UBN to DBN was not found during the follow-up period. A study analyzed the changes in the characteristics of positional nystagmus in 13 patients with Wernicke's encephalopathy and found a conversion of UBN to permanent DBN in seven patients during the follow-up period. The underlying mechanisms for the occurrence of direction-reversing nystagmus in patients with Wernicke's encephalopathy may be the directional vulnerability of the vertical gaze-holding networks in the dorsomedial medulla to thiamine deficiency and the impairment in the processing of otolithic information (18, 63, 64).
Our study has two main limitations. First, the study was conducted at a single-center tertiary hospital run primarily by neurologists. Therefore, selection bias is unavoidable in the inclusion of cases, and the results cannot be generalized to other primary clinics or clinics mostly run by otolaryngology department doctors. Second, we could not quantify the torsional component of nystagmus due to using 2D video-oculography. When the torsional component of the nystagmus is minimal, the examiner may misjudge the nystagmus as pure UBN due to the limited ability of visual recognition of the torsional component.
Our findings suggested that central vestibular disorders should be highly suspected when pure UBN or SN with a predominant upbeat component was observed. In vestibular disorders presenting as SN with an upbeat component, PCI and VM were the most frequently seen among patients with central vestibular disorders, whereas AUVP was the most common in peripheral vestibular disorders.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by IRB No 2022(023). The patients/participants provided their written informed consent to participate in this study.
XL and Y-XW acquired and analyzed the data and wrote the manuscript. Y-FF and T-TZ collected the data and interpreted the results. G-PZ and J-SK interpreted the results and revised the manuscript. Z-XW and XY conceptualized the study, interpreted the results, and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Hygiene and Health Development Scientific Research Fostering Plan of Haidian District Beijing (No. HP2021-03-50703).
J-SK serves as an associate editor of Frontiers in Neuro-otology and on the editorial boards of the Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, Journal of Neuro-ophthalmology, Journal of Vestibular Research, and Journal of Neurology, Medicine, and Clinical and Translational Neuroscience.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Eggers SD, Bisdorff A, Von Brevern M, Zee DS, Kim J-S, Perez-Fernandez N, et al. Classification of vestibular signs and examination techniques: Nystagmus and nystagmus-like movements. J Vestibul Res. (2019) 29:57–87. doi: 10.3233/VES-190658
2. Kim JS, Yoon B, Choi KD, Oh SY, Park SH, Kim BK. Upbeat nystagmus: Clinicoanatomical correlations in 15 patients. J Clin Neurol. (2006) 2:58–65. doi: 10.3988/jcn.2006.2.1.58
3. Leigh RJ, Zee DS. The Neurology of Eye Movements. 5th ed. New York, NY: Oxford University Press (2015).
4. Kumar A, Patni AH, Charbel F. The Chiari I malformation and the neurotologist. Otol Neurotol. (2002) 23:727–35. doi: 10.1097/00129492-200209000-00021
5. Osborne S, Vivian A. Primary position upbeat nystagmus associated with amitriptyline use. Eye. (2004) 18:106–106. doi: 10.1038/sj.eye.6700529
6. Jay WM, Marcus RW, Jay MS. Primary Position Upbeat Nystagmus With Organophosphate Poisoning. Thorofare, NJ: SLACK Incorporated (1982). p. 318–9.
7. Sibony PA, Evinger C, Manning KA. Tabacco-induced primary-position upbeat nystagmus. Ann Neurol. (1987) 21:53–8. doi: 10.1002/ana.410210110
8. Young AS, Lechner C, Bradshaw AP, MacDougall HG, Black DA, Halmagyi GM, et al. Capturing acute vertigo: A vestibular event monitor. Neurology. (2019) 92:e2743–53. doi: 10.1212/WNL.0000000000007644
9. Castellucci A, Botti C, Martellucci S, Malara P, Delmonte S, Lusetti F, et al. Spontaneous upbeat nystagmus and selective anterior semicircular canal hypofunction on video head impulse test: A new variant of canalith jam? J Audiol Otol. (2022) 26:153–9. doi: 10.7874/jao.2021.00297
10. Martinez-Lopez M, Manrique-Huarte R, Perez-Fernandez N. A puzzle of vestibular physiology in a Meniere's disease acute attack. Case Rep Otolaryngol. (2015) 2015:460757. doi: 10.1155/2015/460757
11. Reynard P, Karkas A, Gavid M, Lelonge Y, Bertholon P. Delayed endolymphatic hydrops. Special emphasis on nystagmus associated with episodes and contribution of chemical labyrinthectomy. Eur Ann Otorhinolaryngol Head Neck Dis. (2018) 135:321–6. doi: 10.1016/j.anorl.2018.08.001
12. Lee SH, Kim JM, Schuknecht B, Tarnutzer AA. Vestibular and ocular motor properties in lateral medullary stroke critically depend on the level of the medullary lesion. Front Neurol. (2020) 11:390. doi: 10.3389/fneur.2020.00390
13. Lee SU, Park SH, Park JJ, Kim HJ, Han MK, Bae HJ, et al. Dorsal medullary infarction: distinct syndrome of isolated central vestibulopathy. Stroke. (2015) 46:3081–7. doi: 10.1161/STROKEAHA.115.010972
14. Fetter M, Dichgans J. Vestibular neuritis spares the inferior division of the vestibular nerve. Brain. (1996) 119:755–63. doi: 10.1093/brain/119.3.755
15. Yagi T, Koizumi Y, Sugizaki K. 3D analysis of spontaneous nystagmus in early stage of vestibular neuritis. Auris Nasus Larynx. (2010) 37:167–72. doi: 10.1016/j.anl.2009.05.008
16. Kim JS. When the room is spinning: Experience of vestibular neuritis by a neurotologist. Front Neurol. (2020) 11:157. doi: 10.3389/fneur.2020.00157
17. Choi H, Kim C-H, Lee K-Y, Lee YJ, Koh S-H. A probable cavernoma in the medulla oblongata presenting only as upbeat nystagmus. J Clin Neurosci. (2011) 18:1567–9. doi: 10.1016/j.jocn.2011.02.043
18. Kattah JC, McClelland C, Zee DS. Vertical nystagmus in Wernicke's encephalopathy: Pathogenesis and role of central processing of information from the otoliths. J Neurol. (2019) 266:139–45. doi: 10.1007/s00415-019-09326-9
19. Meling TR, Nouri A, May A, Guinand N, Vargas MI, Destrieux C. Upbeat vertical nystagmus after brain stem cavernoma resection: A rare case of nucleus intercalatus/nucleus of roller injury. J Neurol. (2020) 267:2865–70. doi: 10.1007/s00415-020-09891-4
20. Hale DE, Green KE. Teaching video neuroimage: Spontaneous upbeat-torsional nystagmus from medial medullary infarction. Neurology. (2021) 97:e2355–6. doi: 10.1212/WNL.0000000000012659
21. Bisdorff A. Vestibular symptoms and history taking. Handb Clin Neurol. (2016) 137:83–90. doi: 10.1016/B978-0-444-63437-5.00006-6
22. Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, et al. Diagnostic criteria for Menière's disease. J Vestibul Res. (2015) 25:1–7. doi: 10.3233/VES-150549
23. Staab JP, Eckhardt-Henn A, Horii A, Jacob R, Strupp M, Brandt T, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): Consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J Vestibul Res. (2017) 27:191–208. doi: 10.3233/VES-170622
24. van de Berg R, Widdershoven J, Bisdorff A, Evers S, Wiener-Vacher S, Cushing SL, et al. Vestibular migraine of childhood and recurrent vertigo of childhood: Diagnostic criteria consensus document of the Committee for the Classification of Vestibular Disorders of the Bárány Society and the International Headache Society. J Vestib Res. (2021) 31:1–9. doi: 10.3233/VES-200003
25. Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J, et al. Vestibular migraine: Diagnostic criteria1. J Vestibul Res. (2022) 32:1–6. doi: 10.3233/VES-201644
26. Strupp M, Bisdorff A, Furman J, Hornibrook J, Jahn K, Maire R, et al. Acute unilateral vestibulopathy/vestibular neuritis: Diagnostic criteria. J Vestibul Res. (2022) 2022:220201. doi: 10.3233/VES-220201
27. Kim JS, Lee H. Vertigo due to posterior circulation stroke. Semin Neurol. (2013) 33:179–84. doi: 10.1055/s-0033-1354600
28. Ducroz C, Dumas G, Quatre R, Attyé A, Fabre C, Schmerber S. Benign recurrent vestibulopathy: MRI and vestibular tests results in a series of 128 cases. Eur Archiv Oto-rhino-laryngol. (2022) 279:169–73. doi: 10.1007/s00405-021-06637-4
29. Jeong SH, Kim HJ, Kim JS. Vestibular neuritis. Semin Neurol. (2013) 33:185–94. doi: 10.1055/s-0033-1354598
30. Gianoli G, Goebel J, Mowry S, Poomipannit P. Anatomic differences in the lateral vestibular nerve channels and their implications in vestibular neuritis. Otol Neurotol. (2005) 26:489–94. doi: 10.1097/01.mao.0000169787.99835.9f
31. Kim SH, Zee DS, du Lac S, Kim HJ, Kim JS. Nucleus prepositus hypoglossi lesions produce a unique ocular motor syndrome. Neurology. (2016) 87:2026–33. doi: 10.1212/WNL.0000000000003316
32. Seo SW, Shin HY, Kim SH, Han SW, Lee KY, Kim SM, et al. Vestibular imbalance associated with a lesion in the nucleus prepositus hypoglossi area. Arch Neurol. (2004) 61:1440–3. doi: 10.1001/archneur.61.9.1440
33. Choi KD, Jung DS, Park KP, Jo JW, Kim JS. Bowtie and upbeat nystagmus evolving into hemi-seesaw nystagmus in medial medullary infarction: Possible anatomic mechanisms. Neurology. (2004) 62:663–5. doi: 10.1212/01.WNL.0000110186.05217.9B
34. Lee SC, Lee SH, Lee KY, Lee YJ, Koh SH. Transient upbeat nystagmus due to unilateral focal pontine infarction. J Clin Neurosci. (2009) 16:563–5. doi: 10.1016/j.jocn.2008.05.027
35. Kim JS, Lopez I, DiPatre PL, Liu F, Ishiyama A, Baloh RW. Internal auditory artery infarction: Clinicopathologic correlation. Neurology. (1999) 52:40–4. doi: 10.1212/WNL.52.1.40
36. Oas JG, Baloh RW. Vertigo and the anterior inferior cerebellar artery syndrome. Neurology. (1992) 42:2274–9. doi: 10.1212/WNL.42.12.2274
37. Mazzoni A. The vascular anatomy of the vestibular labyrinth in man. Acta Otolaryngol Suppl. (1990) 472:1–83. doi: 10.3109/00016489009121137
38. Kim JS, Newman-Toker DE, Kerber KA, Jahn K, Bertholon P, Waterston J, et al. Vascular vertigo and dizziness: Diagnostic criteria. J Vestib Res. (2022) 32:205–22. doi: 10.3233/VES-210169
39. Manzari L, Burgess AM, Curthoys IS. Does unilateral utricular dysfunction cause horizontal spontaneous nystagmus? Eur Archiv Oto-rhino-laryngol. (2012) 269:2441–5. doi: 10.1007/s00405-012-2127-z
40. van Leeuwen RB, Colijn C, van Esch BF, Schermer TR. Benign recurrent vertigo: The course of vertigo attacks compared to patients with Menière's disease and vestibular migraine. Front Neurol. (2022) 13:817812. doi: 10.3389/fneur.2022.817812
41. van Esch BF, van Wensen E, van der Zaag-Loonen HJ, Benthem P, van Leeuwen RB. Clinical characteristics of benign recurrent vestibulopathy: Clearly distinctive from vestibular migraine and Menière's disease? Otol Neurotol. (2017) 38:e357–63. doi: 10.1097/MAO.0000000000001553
42. van Leeuwen RB, Bruintjes TD. Recurrent vestibulopathy: Natural course and prognostic factors. J Laryngol Otol. (2010) 124:19–22. doi: 10.1017/S0022215109991009
43. Pan Q, Zhang Y, Zhang S, Wang W, Jiang H, Fan Y, et al. Clinical features and outcomes of benign recurrent vertigo: A longitudinal study. Acta Neurol Scand. (2020) 141:374–9. doi: 10.1111/ane.13214
44. Attyé A, Dumas G, Troprès I, Roustit M, Karkas A, Banciu E, et al. Recurrent peripheral vestibulopathy: Is MRI useful for the diagnosis of endolymphatic hydrops in clinical practice? Eur Radiol. (2015) 25:3043–9. doi: 10.1007/s00330-015-3712-5
45. Morita Y, Takahashi K, Ohshima S, Yagi C, Kitazawa M, Yamagishi T, et al. Is vestibular Meniere's disease associated with endolymphatic hydrops? Front Surg. (2020) 7:601692. doi: 10.3389/fsurg.2020.601692
46. von Brevern M, Radtke A, Clarke AH, Lempert T. Migrainous vertigo presenting as episodic positional vertigo. Neurology. (2004) 62:469–72. doi: 10.1212/01.WNL.0000106949.55346.CD
47. Polensek SH, Tusa RJ. Nystagmus during attacks of vestibular migraine: An aid in diagnosis. Audiol Neurootol. (2010) 15:241–6. doi: 10.1159/000255440
48. Beh SC, Masrour S, Smith SV, Friedman DI. The spectrum of vestibular migraine: Clinical features, triggers, and examination findings. Headache. (2019) 59:727–40. doi: 10.1111/head.13484
49. ElSherif M, Reda MI, Saadallah H, Mourad M. Eye movements and imaging in vestibular migraine. Acta Otorrinolaringol Esp. (2020) 71:3–8. doi: 10.1016/j.otoeng.2018.10.005
50. Young AS, Nham B, Bradshaw AP, Calic Z, Pogson JM, D'Souza M, et al. Clinical, oculographic, and vestibular test characteristics of vestibular migraine. Cephalalgia. (2021) 41:1039–52. doi: 10.1177/03331024211006042
51. Jeong SH, Oh SY, Kim HJ, Koo JW, Kim JS. Vestibular dysfunction in migraine: Effects of associated vertigo and motion sickness. J Neurol. (2010) 257:905–12. doi: 10.1007/s00415-009-5435-5
52. Beh SC. External trigeminal nerve stimulation: Potential rescue treatment for acute vestibular migraine. J Neurol Sci. (2020) 408:116550. doi: 10.1016/j.jns.2019.116550
53. Abu-Arafeh I, Russell G. Paroxysmal vertigo as a migraine equivalent in children: A population-based study. Cephalalgia. (1995) 15:22–5. doi: 10.1046/j.1468-2982.1995.1501022.x
54. Langhagen T, Lehrer N, Borggraefe I, Heinen F, Jahn K. Vestibular migraine in children and adolescents: Clinical findings and laboratory tests. Front Neurol. (2014) 5:292. doi: 10.3389/fneur.2014.00292
55. Marcelli V, Furia T, Marciano E. Vestibular pathways involvement in children with migraine: A neuro-otological study. Headache. (2010) 50:71–6. doi: 10.1111/j.1526-4610.2009.01454.x
56. Lee SU, Kim HJ, Choi JY, Koo JW, Kim JS. Evolution of caloric responses during and between the attacks of Meniere's disease. J Neurol. (2021) 268:2913–21. doi: 10.1007/s00415-021-10470-4
57. Lee SU, Kim HJ, Koo JW, Kim JS. Comparison of caloric and head-impulse tests during the attacks of Meniere's disease. Laryngoscope. (2017) 127:702–8. doi: 10.1002/lary.26103
58. Daroff RB, Troost BT. Upbeat nystagmus. J Am Med Assoc. (1973) 225:312–312. doi: 10.1001/jama.225.3.312b
59. Power L, Murray K, Drummond KJ, Trost N, Szmulewicz DJ. Fourth ventricle ependymoma mimicking benign paroxysmal positional vertigo. Neurology. (2018) 91:327–8. doi: 10.1212/WNL.0000000000005992
60. Furman JM, Brownstone PK, Baloh RW. Atypical brainstem encephalitis: Magnetic resonance imaging and oculographic features. Neurology. (1985) 35:438–40. doi: 10.1212/WNL.35.3.438
61. Searls DE, Pazdera L, Korbel E, Vysata O, Caplan LR. Symptoms and signs of posterior circulation ischemia in the new England medical center posterior circulation registry. Arch Neurol. (2012) 69:346–51. doi: 10.1001/archneurol.2011.2083
62. Abouaf L, Vighetto A, Magnin E, Nove-Josserand A, Mouton S, Tilikete C. Primary position upbeat nystagmus in Wernicke's encephalopathy. Eur Neurol. (2011) 65:160–3. doi: 10.1159/000324329
63. Kattah JC, Tehrani AS, du Lac S, Newman-Toker DE, Zee DS. Conversion of upbeat to downbeat nystagmus in Wernicke encephalopathy. Neurology. (2018) 91:790–6. doi: 10.1212/WNL.0000000000006385
Keywords: vestibular disorders, topical diagnosis, etiology, upbeat nystagmus, pure upbeat nystagmus, SN with predominant upbeat component
Citation: Ling X, Wu Y-X, Feng Y-F, Zhao T-T, Zhao G-P, Kim J-S, Yang X and Wang Z-X (2023) Spontaneous nystagmus with an upbeat component: Central or peripheral vestibular disorders? Front. Neurol. 14:1106084. doi: 10.3389/fneur.2023.1106084
Received: 23 November 2022; Accepted: 06 February 2023;
Published: 23 February 2023.
Edited by:
Jose Antonio Lopez-Escamez, Universidad de Granada, SpainReviewed by:
Diego Kaski, University College London, United KingdomCopyright © 2023 Ling, Wu, Feng, Zhao, Zhao, Kim, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Yang,  eWFuZ3h1MjAxMUAxNjMuY29t; Zhao-Xia Wang,
eWFuZ3h1MjAxMUAxNjMuY29t; Zhao-Xia Wang,  ZHJ3YW5nenhAMTYzLmNvbQ==
ZHJ3YW5nenhAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.