- 1School of Nursing, China Medical University, Shenyang, China
- 2The First Clinical College, China Medical University, Shenyang, China
- 3General Hospital of Pingmei Shenma Group, Pingdingshan, China
Objective: This systematic review and network meta-analysis sought to determine the efficacy of different intensities of transcranial direct current stimulation (tDCS) in patients with dysphagia after stroke to improve swallowing function.
Methods: Randomized-controlled trials (RCTs) of tDCS in post-stroke dysphagia were searched from Pubmed, EMBASE, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Service System (SinoMed), Wanfang database, and Chinese Scientific Journals Database (VIP) from databases' inception to June 22, 2022. Article screening, data extraction, and article quality evaluation were completed by 2 independent researchers. Network meta-analysis was performed using Stata.
Results: A final total of 20 studies involving 838 stroke patients were included. The included control interventions were sham tDCS and conventional therapy (CT). Network meta-analysis showed that 20 min of 1.2, 1.4, 1.5, 1.6, and 2 mA anodal tDCS and 30 min of 2 mA anodal tDCS significantly improved post-stroke dysphagia compared with CT (all P < 0.05). In addition, 20 min of 1, 1.4, 1.6, and 2 mA anodal tDCS also significantly improved post-stroke dysphagia compared with sham tDCS (all P < 0.05). Our results demonstrated that 20 min of stimulation at 1.4 mA was the optimal parameters for anodal tDCS and exhibited superior efficacy to CT [SMD = 1.08, 95% CI (0.46, 1.69)] and sham tDCS [SMD = 1.45, 95% CI (0.54, 2.36)].
Conclusion: Different durations and intensities of anodal tDCS are effective in improving post-stroke dysphagia. However, 20 min of tDCS at 1.4 mA may be the optimal regimen.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/#recordDetails, identifier CRD42022342506.
Introduction
Stroke is a clinical syndrome caused by cerebrovascular disorder and a major disease that seriously threatens human health (1). A recent study showed that there are nearly 113 million stroke-associated disability-adjusted life years worldwide (2). Dysphagia is a common stroke complication (3) found in 20–45% of patients (4, 5). The manifestation of dysphagia differs with varying stroke site, and general cerebral cortex lesions or injuries are common causes of dysphagia (6). Untimely treatment of dysphagia can lead to complications such as aspiration pneumonia, malnutrition, dehydration and suffocation (5, 7), which seriously affects the prevention and treatment of dysphagia and poses a great challenge for medical staff (8, 9). Therefore, how to improve the swallowing functions of patients in the early stage of stroke and their survival remains an actively investigated topic in clinical research.
There are pharmaceutical and non-pharmaceutical treatments for post-stroke dysphagia. Non-pharmaceutical treatments include swallowing exercises, dietary adjustments, feeding exercises, traditional rehabilitation, glacial acid stimulation, balloon dilation, acupuncture, electroacupuncture, neuromuscular electrical stimulation, behavior management, electromyographic biofeedback, and non-invasive brain stimulation (NIBS). In particular, NIBS has been shown to promote better recovery from post-stroke dysphagia than other non-pharmaceutical treatments (10). Transcranial direct current stimulation (tDCS) is a type of NIBS that transcranially delivers a steady flow of low-intensity current (usually within the range of 1–2 mA) through two relatively large electrodes placed on the scalp. Application of a direct current to the projected area on the scalp modulates neuronal resting potential threshold and intervenes with neuron firing. Neuron firing increases when the anode is close to the neuron or dendrite, and decreases when the cathode is close to the neuron or dendrite. Therefore, the therapeutic effect of tDCS is mediated by altering the excitability of the cerebral cortex within the stimulated area through the anode and cathode (11). Due to its non-invasiveness, ease of operation, minimal side effect, ease of equipment management and low cost, tDCS has been widely used in the treatment of various dysfunctions in stroke patients and has demonstrated promising clinical application prospects (12–14). The efficacy of tDCS is highly dependent on the parameters of stimulation, including stimulation intensity, frequency, duration, and electrode placement. Stimulation intensity and duration are the common factors for tDCS and the major factors that influence tDCS safety and efficacy. The optimal stimulation intensity and duration for tDCS are currently unclear. Jefferson et al. (15) conducted neuroelectrophysiological observations on the optimal stimulation parameters of tDCS in the pharyngeal motor cortex and found that 10 min of 1.5 mA and 20 min of 1.0 mA anodal tDCS effectively induced excitation of the pharyngeal motor cortex. Nitsche and Paulus (16) reported that increased stimulation intensity (higher current intensity and longer stimulation time) can lead to better treatment efficacy. However, another study showed that prolonged tDCS stimulation may induce neural adaptation, resulting in decreased neural excitation (17). The guidelines by Bikson et al. (18) recommended a tDCS stimulation intensity of 1–4 mA, but the optimal stimulation intensity and duration were not mentioned. Several published meta-analyses have compared the efficacy of tDCS in improving post-stroke dysphagia (19–21), but there are no evidence-based findings on the optimal current intensity and stimulation time of tDCS in post-stroke dysphagia. Therefore, in order to examine the effect of different stimulation time and current intensities on the efficacy of tDCS in post-stroke dysphagia, we conducted a network meta-analysis of key stimulation parameters (stimulation intensity and duration) of tDCS in existing studies.
In this study, we performed a network meta-analysis of the efficacy of different stimulation time and current intensities of tDCS in post-stroke dysphagia in order to provide reliable support for clinical application.
Data and methods
This study was conducted in accordance with the PRISMA-network meta-analysis extension (Supplementary material 1) (22) and registered on PROSPERO (CRD42022342506).
Search strategy
Randomized-controlled trials (RCTs) of tDCS in post-stroke dysphagia were searched in PubMed, Embase, Cochrane Library, Web of Science, CNKI, Wanfang Data, VIP, and CBM from inception to June 22, 2022 by two researchers (Jianwei Xie and Minghui Wu). Each database was searched using medical subject headings (MeSH) and free search terms. The following search terms were employed: (“stroke” OR “cerebrovascular accident” OR “apoplexy” OR “brain vascular accident” OR “cerebral vascular accident” OR “hemiplegia” OR “CVA” OR “thrombotic stroke”) AND (Deglutition Disorders OR “aphagopraxia” OR “deglutition difficulty” OR “dysphagia” OR “difficulty swallowing”) AND (“transcranial direct current stimulation” OR “Transcranial Electrical Stimulations”). The specific search strategy is described in Supplementary material 2.
Inclusion criteria
(1) Participants: Age > 18 years, definitely diagnosed with dysphagia by CT or MRI and the stroke type was either cerebral hemorrhage or cerebral infarction.
(2) Intervention: Anodal tDCS.
(3) Control treatment: Conventional therapy (CT) or sham tDCS.
(4) Outcomes: The primary outcome measure was the assessment of swallowing function before and after tDCS, and all scales for assessing dysphagia in stroke were included.
(5) Study type: RCTs.
Exclusion criteria
Any other types of articles, such as systematic reviews, letters, case reports, editorials, animal studies, comments, and non-RCTs; Studies that combined other intervention(s); Studies involving patients with severe aphasia or cognitive impairment; Studies with incomparable baseline data or have not reported baseline data; Studies with poor design or improper statistical analysis; Studies with incomplete data or source data or full-text files could not be obtained after contacting the corresponding author; Studies without corresponding results; Studies with unclear diagnostic criteria and intervention time.
Data extraction
Article screening and data extraction were completed by two independent researchers (Chiteng Zhou and Xiaoyu Jiang) according to the study objective and inclusion criteria and cross-verified. In the case of any disagreement, a third researcher (Minghui Wu) was consulted. Data were extracted using a customized data extraction form and included (1) General information of included studies; (2) Baseline characteristics of subjects; (3) Details of intervention; (4) Outcome measures and assessment tools; (5) Methodological quality of the included studies. Missing information were requested from the corresponding author through email or telephone.
Risk of bias of included studies
The risk of bias of the included RCTs was assessed by two researchers (Jianwei Xie and Gilbert Ngaruwenayo) using the Cochrane Handbook for Systematic Review (version 5.1.0) (23). The items of assessment included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, blinding of outcome assessment personnel, selective reporting of study results, and other bias. Each item was evaluated as “low risk of bias,” “unclear risk of bias,” and “high risk of bias.” If all of the above criteria are met, the risk of bias is minimal. If the above criteria are partially met, the risk of bias is moderate. If none of the above criteria are met, the risk of bias is high. A risk of bias graph was generated using Rev Man 5.4.
Statistical analysis
Data were analyzed using Stata 15.0 (StataCorp LLC, College Station, Texas). First, meta-analysis was performed to directly compare different interventions. Since outcome measures are continuous variables and there are differences among questionnaires, standardized mean difference (SMD) and 95% confidence interval (CI) were used for analyses. If the 95% CI does not include 0, a P < 0.05 is considered statistically significant. Heterogeneity among studies was assessed by Q-test and I2. Heterogeneity is not significant when P > 0.05 and I2 < 50%, and a fixed effects model is used for meta-analysis. When P < 0.05 or I2 > 50%, the source of heterogeneity was analyzed. Once clinically significant heterogeneity was excluded, a random effects model was used to combine the data. Sensitivity analysis was conducted by sequentially removing each included study. If pairwise comparison does not lead to significant change in the meta-analysis results, this indicates that the results are relatively stable. Articles that have a relatively large impact on the study results were excluded by sensitivity analysis.
Random effects network meta-analysis was conducted using the Frequentist approach. This approach uses the inverse variance to combine direct and indirect evidence, that is, the inverse variance of each study is used as the weight and the weighted mean is calculated for the effect of each study. The variance of the overall effect is the reciprocal of the sum of weights (24). A network diagram provides an intuitive comparison of different interventions. The size of each dot represents the total sample size of an intervention, and the thickness of the line between two dots represents the number of studies that directly compared the two interventions. The absence of a connecting line indicates no direct comparative study. Consistency in network meta-analysis refers to the similarity between the results of direct and indirect comparisons. When there is a closed loop in the evidence network, consistency is evaluated using the node-splitting method. A P > 0.05 indicates non-significant inconsistency between direct and indirect comparisons (25). Different intensities of the intervention were compared in a pairwise manner, and a P < 0.05 was considered to be statistically significant. The efficacy of each intervention was ranked by surface under the cumulative ranking (SUCRA). SUCRA can range from 0 to 100%, and a greater SUCRA indicates better efficacy. Publication bias of the included articles was evaluated using a funnel plot and Egger's test. Asymmetry in the funnel plot and P < 0.05 in the Egger's test indicate publication bias in the included articles (26).
Results
Search results
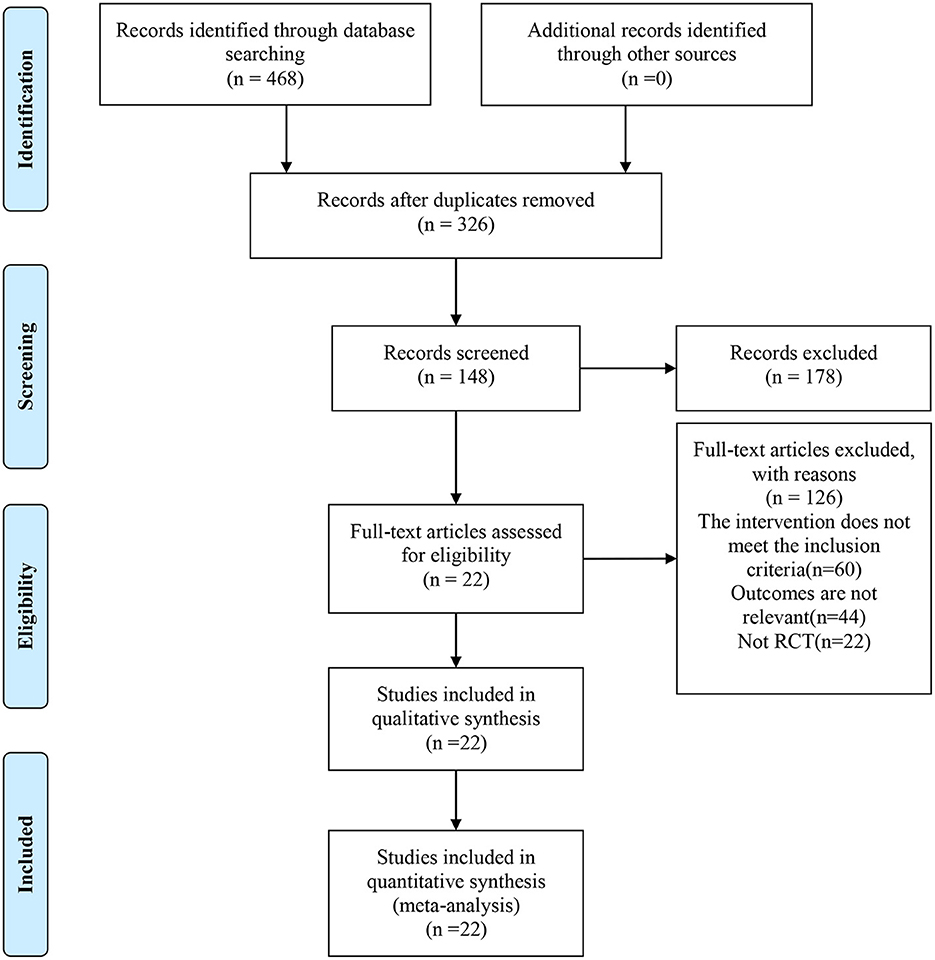
A total of 468 relevant articles were identified through initial search, 326 articles were obtained after removal of duplications by Endnote, and 148 articles remained after reading the study title and excluding studies that did not meet the criteria for study design, subject and outcome measures. A final total of 22 RCTs were included after full-text reading and further screening (27–48). The flow diagram for study inclusion is shown in Figure 1.
General characteristics of included studies
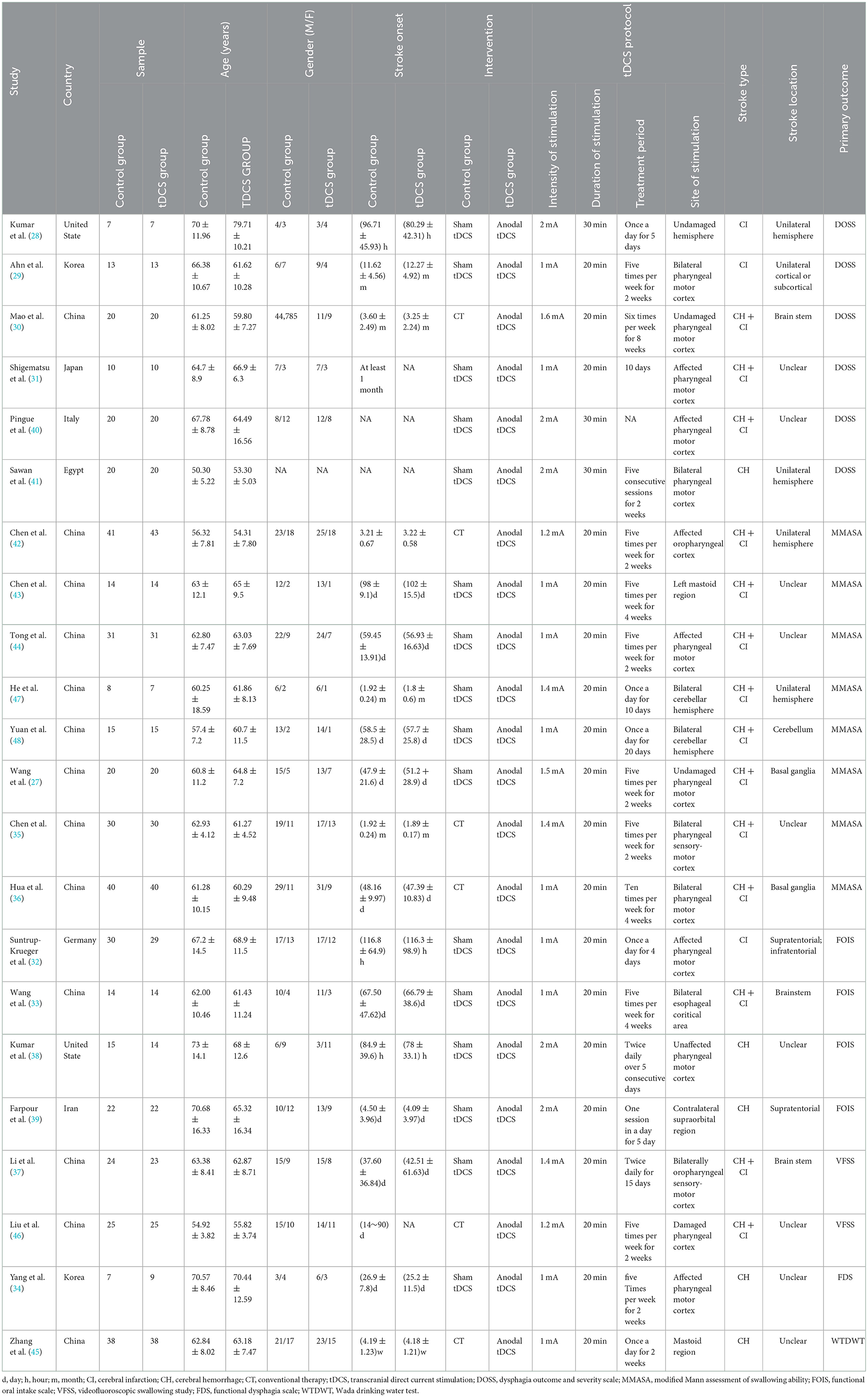
Of the 22 included RCTs, 16 compared the efficacy of anodal tDCS and sham tDCS, and 6 compared the efficacy of anodal tDCS and CT. The included 22 RCTs were published between 2013 and 2022 and involved 928 stroke patients. The current intensity of tDCS used included 1, 1.2, 1.4, 1.5, 1.6, and 2 mA; stimulation durations were 20 and 30 min; and the shortest and longest treatment duration were 5 days and 2 months, respectively. For assessment of dysphagia, 6 RCTs used the Dysphagia Outcome and Severity Scale (DOSS), 8 RCTs used the modified Mann Assessment of Swallowing Ability (MMASA), 4 RCTs used the Functional Oral Intake Scale, 2 RCTs used the Videofluoroscopic Swallowing Study (VFSS), 1 RCT used the Functional Dysphagia Scale (DFS), and 1 RCT used the Wada Drinking Water Test (WTDWT). Detailed characteristics of all included studies are summarized in Table 1.
Methodological quality evaluation
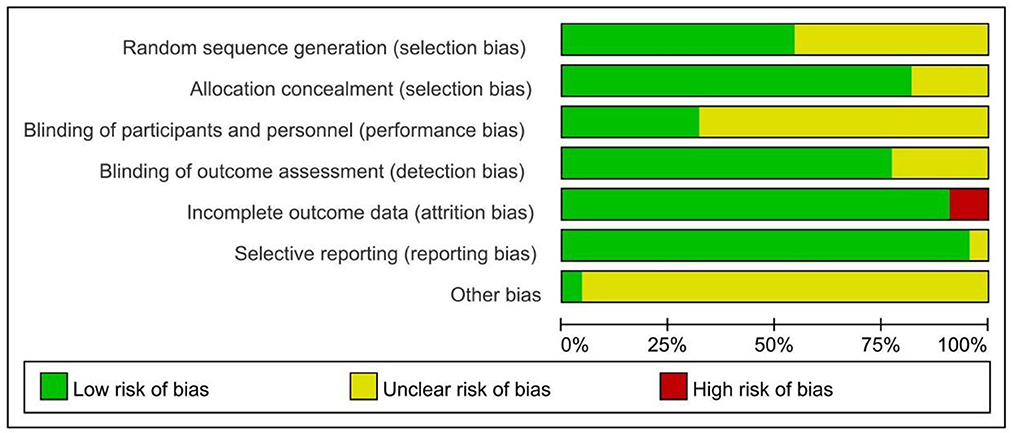
Among the 22 included RCTs, 12 reported the specific method for random sequence generation (primarily using the random number table), 18 reported allocation concealment, 8 reported blinding of participants and personnel, and 16 reported blinding of outcome assessment. One RCT did not show selective reporting and 2 RCTs did not describe subjects who were lost to follow-up. In addition, other bias was unclear for all studies (Figure 2).
Direct meta-analysis
There were nine interventions included, namely CT, sham tDCS, 20 min of anodal tDCS at 6 different current intensities (1, 1.2, 1.4, 1.5, 1.6, and 2 mA), and 30 min of 2 mA anodal tDCS (different intensities and durations of tDCS will hereafter be referred to as 20 min + 1 mA anodal, 20 min + 1 mA anodal, 30 min + 2 mA anodal and so on).
Meta-analysis of direct comparisons showed that 20 min + 1 mA anodal and 20 min + 1.4 mA anodal resulted in significantly better recovery of swallowing functions than sham [SMD = −0.71; 95% CI (−1.05, −0.36) vs. SMD = −0.55; 95% CI (−1.12, −0.01)]. Twenty minutes + 1 mA anodal also led to significantly better recovery of swallowing than CT [SMD = −1.09; 95% CI (−1.51, −0.67)] (Figure 3). Sensitivity analysis of each study revealed that Sawan 2020 and Liu 2020 had the greatest impact on the study results, and hence these two studies were excluded to ensure result stability. A final total of 20 RCTs (27–36, 38–40, 42–48) were included in the network meta-analysis.
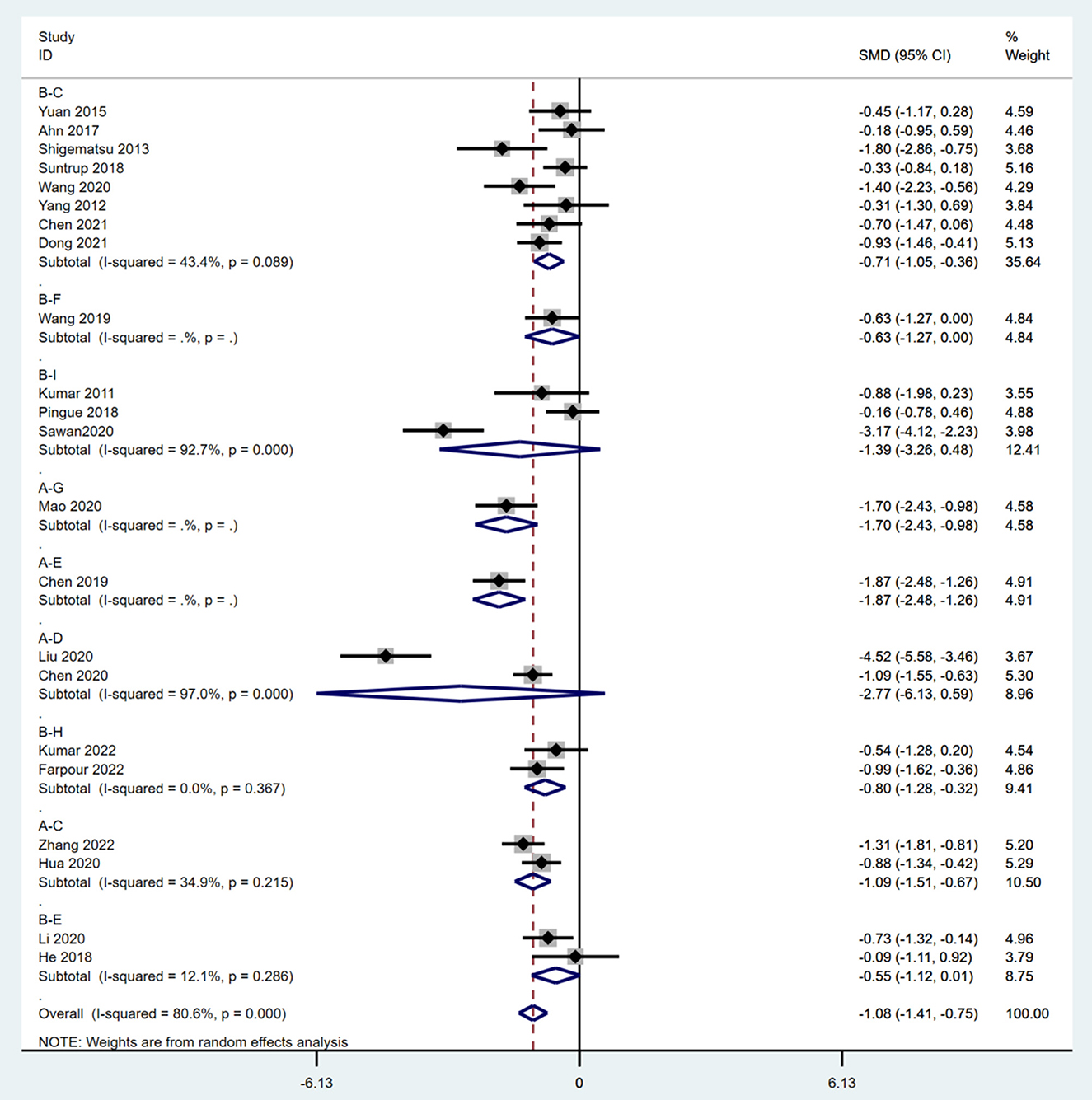
Figure 3. Meta-analysis of pairwise comparisons. A, CT; B, sham; C, 20 min + 1 mA anodal; D, 20 min + 1.2 mA anodal; E, 20 min + 1.4 mAanodal; F, 20 min + 1.5 mA anodal; G, 20 min + 1.6 mA anodal; H, 20 min + 1.6 mA anodal; I, 30 min + 2 mA anodal; SMD, standardized mean difference; CI, confidence interval.
Network meta-analysis
Network results
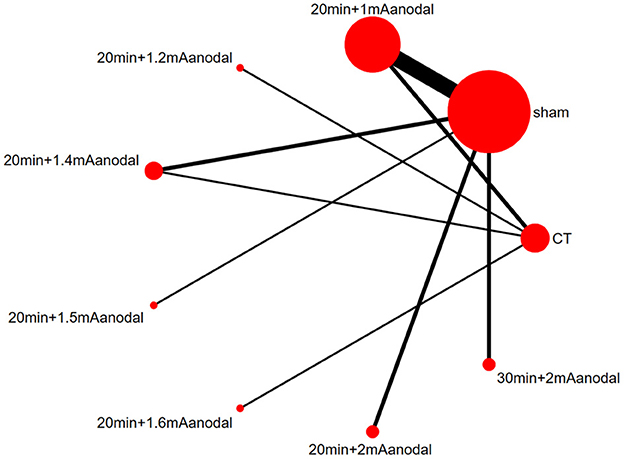
A network diagram was generated based on the results of each study (Figure 4). A line between two dots indicates direct comparison of the two interventions, and the absence of a line indicates no direct comparison. The thickness of the line indicates the number of studies that directly compared the two interventions, and the size of the dot indicates the sample size of the study. The network diagram showed large sample sizes for sham and 20 min+1 mA anodal, and there were numerous studies that compared the two interventions. In addition, there was also a closed loop.
Consistency test
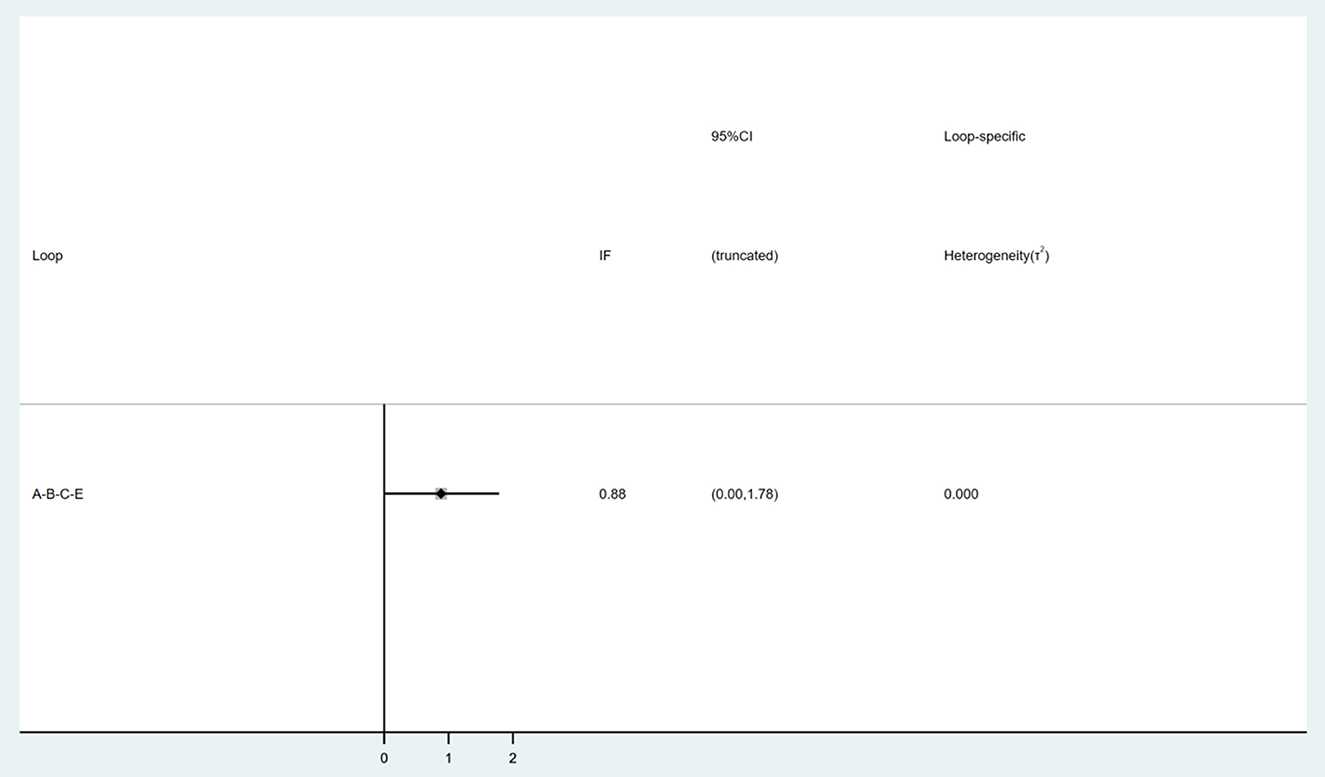
In the inconsistency test, a P = 0.1 and > 0.05 indicates consistency. All P > 0.05 in the partial inconsistency test. Nine interventions formed a triangle loop (Figure 5). Consistency test showed that the IF of the loop was 0.88 and the 95% CI of the IF included the neutral value (0), which indicates that there was no inconsistency within the triangle loop.
Network meta-analysis of different interventions
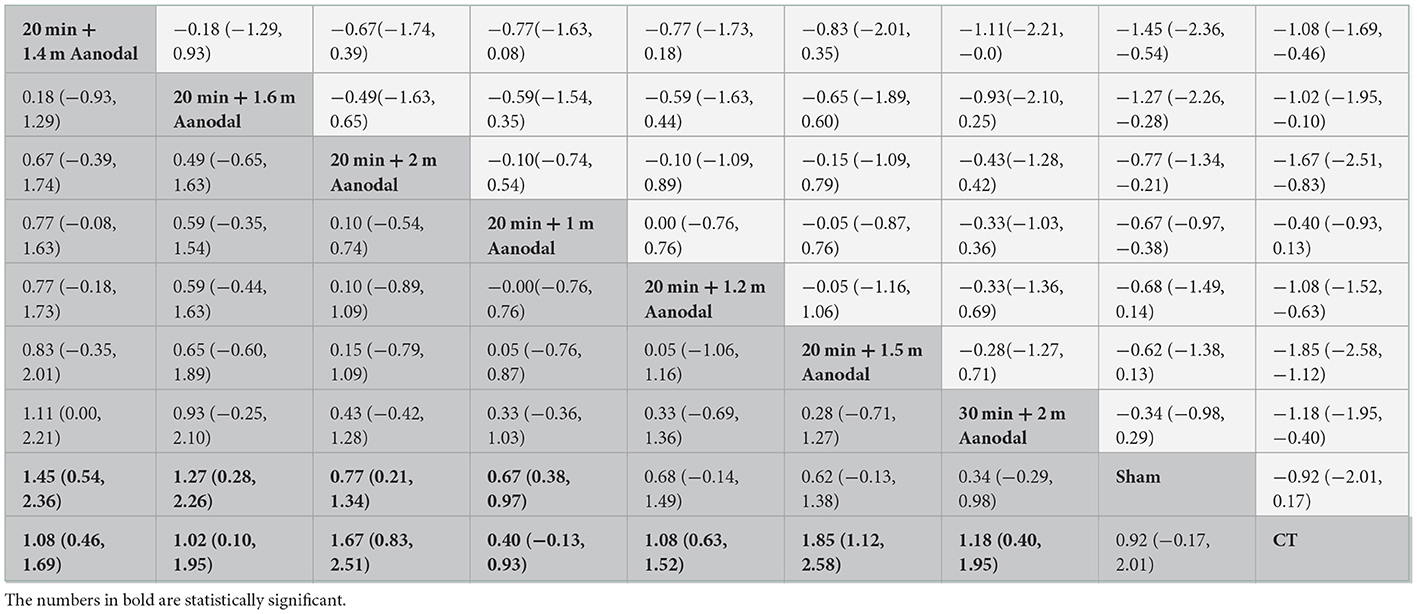
The prediction intervals for the network meta-analysis of different interventions are shown in Table 2. Twenty minutes + 1.4 mA anodal [SMD = 1.08, 95% CI (0.46, 1.69)], 20 min + 16 mA anodal [SMD = 1.02, 95% CI (0.10, 1.96)], 20 min + 2 mA anodal [SMD = 1.67, 95% CI (0.83, 2.51)], 20 min + 1 mA anodal [SMD = 0.67, 95% CI (0.38, 0.97)], 20 min + 1.2 mA anodal [SMD = 1.08, 95% CI (0.63, 1.52)], 20 min + 1.5 mA anodal [SMD = 1.85, 95% CI (1.12, 2.58)], and 30 min + 2 mA anodal [SMD = 1.18, 95% CI (0.40, 1.95)] demonstrated superior efficacy in swallowing functions than CT. Furthermore, 20 min + 1.4 mA anodal [SMD = 1.45, 95% CI (0.54, 2.36)], 20 min + 1.6 mA anodal [SMD = 1.27, 95% CI (0.28, 2.26)], 20 min + 2 mA anodal [SMD = 0.77, 95% CI (0.21, 1.34)], and 20 min + 1 mA anodal [SMD = 0.67, 95% CI (0.38, 0.97)] resulted in better swallowing outcome than sham tDCS. However, there was no significant difference in efficacy among other interventions (P > 0.05).
Ranking of intervention efficacy
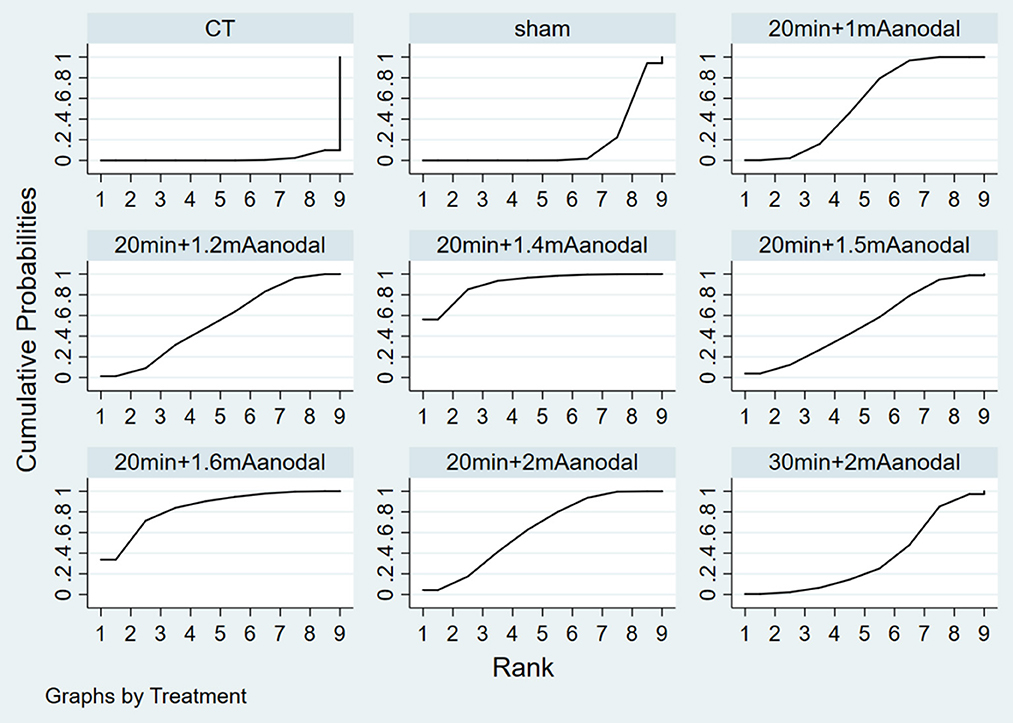
Figure 6 depicts the probability ranking of all treatment protocols. The most likely ranking of the best treatment to the least effective treatment is: 20 min + 1.4 mA anodal > 20 min + 1.6 mA anodal > 20 min + 2 mA anodal > 20 min + 1 mA anodal > 20 min + 1.2 mA anodal > 20 min + 1.5 mA anodal > 30 min + 2 mA anodal > sham > CT.
Publication bias
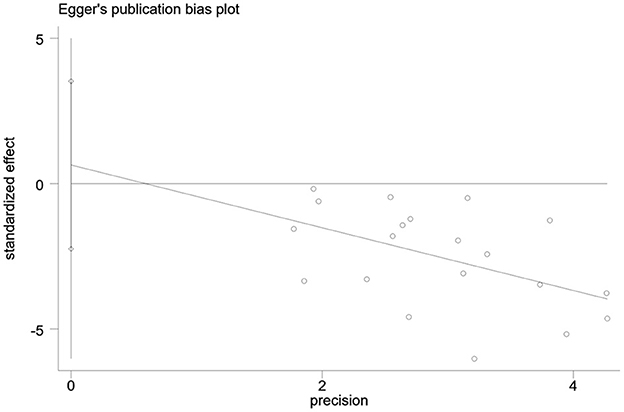
Publication bias in the outcome measures was analyzed. All studies were symmetrically distributed across the x = 0 line, which indicates that there was no small sample size effect or publication bias in the studies. Egger's test showed P = 0.646, supporting the absence of publication bias (Figures 7, 8).
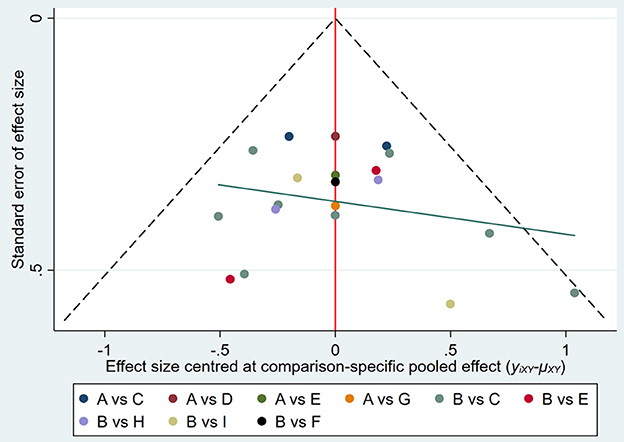
Figure 7. Funnel plot of publication bias of included studies. A, CT; B, sham; C, 20 min + 1 mA anodal; D, 20 min + 1.2 mA anodal; E, 20 min + 1.4 mA anodal; F, 20 min + 1.5 mA anodal; G, 20 min + 1.6 mA anodal; H, 20 min + 2 mA anodal; I, 30 min + 2 mA anodal.
Discussion
Several regions of the brain are damaged after stroke, especially the motor and sensory cortices of the brainstem, thalamus, basal ganglia, and cerebellum, which have been implicated in the control of spontaneous and involuntary swallowing (49, 50). Previous studies showed that dysphagia is an independent risk factor for prolonged hospitalization and occurrence of complications during hospitalization (51, 52). Therefore, early diagnosis and treatment are critical for post-stroke dysphagia. There are numerous swallowing rehabilitation methods that promote patient recovery. Most traditional direct meta-analyses have compared anodal tDCS with CT or sham tDCS (53, 54), but there is currently no high-quality evidence showing the efficacy of different stimulation intensities and durations of anodal tCDS in the treatment of post-stroke dysphagia. In this study, we performed a network meta-analysis to indirectly compare the efficacy of different intervention protocols using common controls, and combined the direct and indirect evidence on different stimulation durations and intensities of anodal tCDS to obtain a probability ranking and hence the optimal regimen. The findings of study can provide insight to the optimal stimulation duration and intensity of anodal tDCS for promoting recovery of swallowing functions in stroke patients.
Our study included 20 RCTs involving 838 participants who were randomly assigned to receive different stimulation duration and intensities of anodal tDCS. Our results showed that 20 min of 1.4 mA anodal tDCS may be most beneficial for the recovery of swallowing functions in stroke patients. This finding demonstrates that the efficacy of anodal tDCS in post-stroke dysphagia is not improved by increasing stimulation duration and intensity, which is consistent with the lack of positive correlation between treatment efficacy and stimulation intensity in another meta-analysis (54). In addition, we should acknowledge that any current stimulation protocol has its own risk and may lead to problems under certain circumstances. There are still many issues that need to be addressed by extensive research and clinical practice. In general, the stimulation duration of tDCS ranges from 3 to 40 min (55) and a current intensity of 1–2 mA is considered to be safe in humans (56). Some of the studies in our analysis did not report any adverse reactions of tDCS in post-stroke dysphagia, this may be attributed to the small sample size. However, a large-cohort study has reported pain, fatigue and pruritus in healthy subjects and patients with other diseases who received tDCS (57). Similar adverse effects were also reported in another safety study of tDCS in stroke (58). Nonetheless, the safety of tDCS has been validated in numerous studies and the currently used stimulation parameters should be safe and reliable. All of the current intensities employed in the included studies were within 1–2 mA. Many studies compared different current intensities, but most of them investigated a stimulation duration of 20 min, except for 1 study that examined a stimulation duration of 30 min. It is possible that when the stimulation intensity is within the safe range, a change in the stimulation duration may impact the efficacy of tDCS in post-stroke dysphagia. However, further studies of stimulation duration and network meta-analyses of different stimulation durations and intensities will be warranted to identify the best anodal tDCS protocol.
Before our study, a previous meta-analysis of 15 RCTs found that tDCS could promote the recovery of swallowing function in post-stroke patients, and high-intensity stimulation might have a better effect (19). Conversely, another meta-analysis of 7 RCTs reported that low-intensity stimulation was more effective in improving dysphagia in patients with stroke (54). The two meta-analyses only explored the effects of stimulation intensity on the improvement of dysphagia but did not comprehensively consider the duration of stimulation. Meanwhile, both of them had a limited number of included studies. In contrast, our study had a larger sample size of updated RCT data, comprehensively analyzed the effects of intensity and duration of stimulation on dysphagia in patients with stroke, and elucidated the results using a consistency model. Furthermore, we performed a surface under the cumulative ranking (SUCRA) analysis for all eligible interventions, which showed that 20 min 1.4 mA transcranial direct current positive electrical stimulation therapy may be the most effective in improving dysphagia in patients with stroke.
There are several limitations in this study. First, the included RCTs performed swallowing exercises for both the treatment and control groups. The slight differences among the various exercises made them difficult to standardize among the studies. In addition, we did not examine the duration of swallowing exercise in the treatment, and control groups of each study. Since swallowing exercise is the current standard treatment, removing such treatment from stroke patients for the purpose of the study objective is unethical. These factors have impeded us from further assessing the impact of swallowing exercises on different stimulation intensities and durations of anodal tDCS. Second, the type of stroke varied among patients in the included RCTs. We cannot exclude the possible effect of different stroke type on the efficacy of different stimulation intensities and durations of anodal tDCS. Third, the instruments used in each study may differ. Only a few studies have reported the name and model of the instruments used, and variations among the instruments may lead to differences in treatment method and hence affect the study results. Fourth, only a limited number of studies provided long-term follow-up data for comparing the lasting effects of different stimulation intensities and durations of anodal tDCS. Fifth, we did not include other important outcomes, such as overall treatment efficacy, nutrition-related measures, and infection-related measures. These important outcomes were barely reported and thus we did not extract the relevant data. Finally, the methodological quality of RCT is generally low. In addition, since economic data was not reported, the cost of different tDCS instruments could not be evaluated. For future studies, we should (1) further validate the findings of this study by strictly following the RCT design, implementation and descriptions and ensuring the quality of the original study; (2) conduct RCT that directly compare tDCS protocols with different stimulation intensities and durations to make up for the drawbacks of indirect comparison; (3) use objective measures such as nutrition- and infection-related measures to make the evaluation of tDCS more objective; and 4) add economic assessment to obtain the most cost-effective current intensity for tDCS.
Conclusion
In this network meta-analysis, we showed that different stimulation intensities of the intervention had different advantages in promoting the recovery of swallowing functions in stroke patients, but the difference in efficacy was not significant. Probability ranking revealed that 20 min of 1.4 mA tDCS may be the most beneficial protocol. However, due to the heterogeneity among studies, caution is required when interpreting these results. Clinicians should develop individualized treatment plans based on the patients' conditions and sensitivity to tDCS in order to bring out the optimal therapeutic effect of tDCS. Given that most included studies focused on a stimulation duration of 20 min, future studies can explore the impact of stimulation duration on the recovery of swallowing functions in stroke patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JX and XL contributed to conception and design of the study and organized the database. CZ and GN performed the statistical analysis. JX and XL wrote the first draft of the manuscript. CZ, GN, MW, and XJ wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1098831/full#supplementary-material
Supplementary material 1. PRISMA_2020_checklist.
Supplementary material 2. The search strategy in all databases.
References
1. Gittler M, Davis AM. Guidelines for adult stroke rehabilitation and recovery. JAMA. (2018) 319:820–1. doi: 10.1001/jama.2017.22036
2. Pandian JD, Gall SL, Kate MP, Silva GS, Akinyemi RO, Ovbiagele BI, et al. Prevention of stroke: a global perspective. Lancet. (2018) 392:1269–78. doi: 10.1016/S0140-6736(18)31269-8
3. Pisegna JM, Kaneoka A, Pearson WG Jr, Kumar S, Langmore SE. Effects of non-invasive brain stimulation on post-stroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. Clin Neurophysiol. (2016) 127:956–68. doi: 10.1016/j.clinph.2015.04.069
4. Rofes L, Muriana D, Palomeras E, Vilardell N, Palomera E, Alvarez-Berdugo D, et al. Prevalence, risk factors and complications of oropharyngeal dysphagia in stroke patients: a cohort study. Neurogastroenterol motil. (2018) 23:e13338. doi: 10.1111/nmo.13338
5. Arnold M, Liesirova K, Broeg-Morvay A, Meisterernst J, Schlager M, Mono ML, et al. Dysphagia in acute stroke: incidence, burden and impact on clinical outcome. PloS one. (2016) 11:e0148424. doi: 10.1371/journal.pone.0148424
7. Smithard DG, O'Neill PA, Parks C, Morris J. Complications and outcome after acute stroke. Does dysphagia matter? Stroke. (1996) 27:1200–4. doi: 10.1161/01.STR.27.7.1200
8. Lin WS, Chou CL, Chang MH, Chung YM, Lin FG, Tsai PY. Vagus nerve magnetic modulation facilitates dysphagia recovery in patients with stroke involving the brainstem - a proof of concept study. Brain Stimul. (2018) 11:264–70. doi: 10.1016/j.brs.2017.10.021
9. Bonilha HS, Simpson AN, Ellis C, Mauldin P, Martin-Harris B, Simpson K. The one-year attributable cost of post-stroke dysphagia. Dysphagia. (2014) 29:545–52. doi: 10.1007/s00455-014-9543-8
10. Li L, Huang H, Jia Y, Yu Y, Liu Z, Shi X, et al. Systematic review and network meta-analysis of noninvasive brain stimulation on dysphagia after stroke. Neural Plast. (2021) 2021:3831472. doi: 10.1155/2021/3831472
11. Bikson M, Hanlon CA, Woods AJ, Gillick BT, Charvet L, Lamm C, et al. Guidelines for TMS/tES clinical services and research through the COVID-19 pandemic. Brain Stimul. (2020) 13:1124–49. doi: 10.1016/j.brs.2020.05.010
12. Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. (2017) 128:56–92. doi: 10.1016/j.clinph.2016.10.087
13. Bandeira ID, Lins-Silva DH, Barouh JL, Faria-Guimarães D, Dorea-Bandeira I, Souza LS, et al. Neuroplasticity and non-invasive brain stimulation in the developing brain. Prog Brain Res. (2021) 264:57–89. doi: 10.1016/bs.pbr.2021.04.003
14. Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev. (2020) 11:CD009645. doi: 10.1002/14651858.CD009645.pub4
15. Jefferson S, Mistry S, Singh S, Rothwell J, Hamdy S. Characterizing the application of transcranial direct current stimulation in human pharyngeal motor cortex. Am J Physiol Gastrointest Liver Physiol. (2009) 297:G1035–40. doi: 10.1152/ajpgi.00294.2009
16. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. (2000) 527 (Pt. 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
17. de Aguiar V, Paolazzi CL, Miceli G. tDCS in post-stroke aphasia: the role of stimulation parameters, behavioral treatment and patient characteristics. Cortex. (2015) 63:296–316. doi: 10.1016/j.cortex.2014.08.015
18. Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. (2016) 9:641–61. doi: 10.1016/j.brs.2016.06.004
19. He K, Wu L, Huang Y, Chen Q, Qiu B, Liang K, et al. Efficacy and safety of transcranial direct current stimulation on post-stroke dysphagia: a systematic review and meta-analysis. J Clin Med. (2022) 11:2297. doi: 10.3390/jcm11092297
20. Zhao N, Sun W, Xiao Z, Fan C, Zeng B, Xu K, et al. Effects of transcranial direct current stimulation on poststroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. (2022) 103:1436–47. doi: 10.1016/j.apmr.2022.03.004
21. Chiang CF, Lin MT, Hsiao MY, Yeh YC, Liang YC, Wang TG. Comparative efficacy of noninvasive neurostimulation therapies for acute and subacute poststroke dysphagia: a systematic review and network meta-analysis. Arch Phys Med Rehabil. (2019) 100:739–50.e4. doi: 10.1016/j.apmr.2018.09.117
22. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
23. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
24. Hu J, Yang ZR, Zhan SY. Methodology and application of indirect comparison and comparison of multiple interventions. Chin J Evid Based Pediatr. (2011) 6:4. doi: 10.3969/j.issn.1673-5501.2011.06.011
25. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. (2013) 33:641–56. doi: 10.1177/0272989X12455847
26. Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. (1993) 2:121–45. doi: 10.1177/096228029300200202
27. Wang S, Shen XY, Mo D, Xu JQ, Tian JY, Sun L. Clinical efficacy of transcranial direct current stimulation combined with swallowing training on swallowing dysfunction after stroke. Nerve Injury Funct Reconstruct. (2019) 14:3. doi: 10.16780/j.cnki.sjssgncj.2019.04.015
28. Kumar S, Wagner CW, Frayne C, Zhu L, Selim M, Feng W, et al. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke. (2011) 42:1035–40. doi: 10.1161/STROKEAHA.110.602128
29. Ahn YH, Sohn HJ, Park JS, Ahn TG, Shin YB, Park M, et al. Effect of bihemispheric anodal transcranial direct current stimulation for dysphagia in chronic stroke patients: a randomized clinical trial. J Rehabil Med. (2017) 49:30–5. doi: 10.2340/16501977-2170
30. Mao HW, Li Y, Xiao ZG, Ni JW, Gan L. Efficacy of transcranial direct current stimulation in the treatment of swallowing disorders in brainstem stroke patients. Chin J Phys Med Rehabil. (2020) 42:3. doi: 10.3760/cma.j.issn.0254-1424.2020.01.007
31. Shigematsu T, Fujishima I, Ohno K. Transcranial direct current stimulation improves swallowing function in stroke patients. Neurorehabil Neural Repair. (2013) 27:363–9. doi: 10.1177/1545968312474116
32. Suntrup-Krueger S, Ringmaier C, Muhle P, Wollbrink A, Kemmling A, Hanning U, et al. Randomized trial of transcranial direct current stimulation for poststroke dysphagia. Ann Neurol. (2018) 83:328–40. doi: 10.1002/ana.25151
33. Wang ZY, Chen JM, Lin ZK, Ni GX. Transcranial direct current stimulation improves the swallowing function in patients with cricopharyngeal muscle dysfunction following a brainstem stroke. Neurol Sci. (2020) 41:569–74. doi: 10.1007/s10072-019-04120-x
34. Yang EJ, Baek SR, Shin J, Lim JY, Jang HJ, Kim YK, et al. Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restorat Neurol Neurosci. (2012) 30:303–11. doi: 10.3233/RNN-2012-110213
35. Yu C. Efficacy of transcranial direct current stimulation in the treatment of post-stroke swallowing dysfunction. Great Phys. (2019) 4:2. Available online at: https://www.zhangqiaokeyan.com/academic-journal-cn_detail_thesis/0201281974399.html
36. Hua HL, Zhang BX, Liu J, Tang NZ, Wang XL, Chen NH. Clinical observation of direct feeding instruction combined with transcranial direct current stimulation in the treatment of post-stroke dysphagia and its effect on the degree of misaspiration. Chin Contemp Med. (2020) 27:4. Available online at: https://www.zhangqiaokeyan.com/academic-journal-cn_china-modern-medicine_thesis/0201282184791.html
37. Liu J, Wang XL, Hua HL. Analysis of clinical treatment effects of post-stroke dysphagia. Chin Health Stand Manage. (2020) 11:3.
38. Kumar S, Marchina S, Langmore S, Massaro J, Palmisano J, Wang N, et al. Fostering eating after stroke (FEASt) trial for improving post-stroke dysphagia with non-invasive brain stimulation. Sci Rep. (2022) 12:9607. doi: 10.1038/s41598-022-14390-9
39. Farpour S, Asadi-Shekaari M, Borhani Haghighi A, Farpour HR. Improving swallowing function and ability in post stroke dysphagia: a randomized clinical trial. Dysphagia. (2022) 1–10. doi: 10.1007/s00455-022-10470-0
40. Pingue V, Priori A, Malovini A, Pistarini C. Dual transcranial direct current stimulation for poststroke dysphagia: a randomized controlled trial. Neurorehabil Neural Repair. (2018) 32:635–44. doi: 10.1177/1545968318782743
41. Sawan SAE, Reda AM, Kamel AH, Ali MAM. Transcranial direct current stimulation (tDCS): its effect on improving dysphagia in stroke patients. Egypt J Neurol Psychiatry Neurosurg. (2020) 56:111. doi: 10.1186/s41983-020-00246-4
42. Chen SL, Hu RL, Deng SY, Yu XJ. Analysis of the effect of transcranial direct current stimulation (tDCS) in the treatment of dysphagia in stroke patients. Mass Rehabil Med. (2019) 10:3.
43. Chen JJ, Wu DC, Shao XZ, Pu XY, Mao YD, Chen B. Effect of transcranial direct current stimulation to modulate vagal excitability on post-stroke dysphagia. Theory Pract Rehabil China. (2021). doi: 10.3969/j.issn.1006-9771.2021.09.012
44. Tong JX, Han F. Effects of transcranial direct current stimulation on post-stroke dysphagia. Gansu Med. (2021). Available online at: https://www.zhangqiaokeyan.com/academic-journal-cn_gansu-medical-journal_thesis/0201291769799.html
45. Zhang Y. Transcranial direct current stimulation in the treatment of swallowing disorders in patients with acute cerebral infarction. Chin Med Guide. (2022). Available online at: https://www.zhangqiaokeyan.com/academic-journal-cn_guide-china-medicine_thesis/0201298530279.html
46. Li XX, Ouyang YY, Yu T, Huang WX, Chen Y, Yang HF. Efficacy of transcranial direct current stimulation in the treatment of swallowing disorders after brainstem stroke. China Rehabil. (2020) 35:4. Available online at: https://www.nstl.gov.cn/paper_detail.html?id=382f3c5ef5e91777e99c6ed703e8d910
47. He H, Fan H, Ao LJ, Wang TT. Study on the efficacy of transcranial direct current stimulation in the treatment of post-stroke dysphagia. China Rehabil. (2018) 33:45–7. doi: 10.3969/j.issn.1001-1242.2016.12.024
48. Yuan Y, Wang J, Wu DY, Song WQ, Zhang DH, Cheng YN, et al. Efficacy of transcranial direct current stimulation in improving post-stroke ataxic dysphagia. Chin J Rehabil Med. (2015) 30:765–70. doi: 10.3969/j.issn.1001-1242.2015.08.003
49. Daniels SK, Foundas AL, Iglesia GC, Sullivan MA. Lesion site in unilateral stroke patients with dysphagia. J Stroke Cerebrovasc Dis. (1996) 6:30–4. doi: 10.1016/S1052-3057(96)80023-1
50. Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Ann Neurol. (1999) 46:281–6. doi: 10.1002/1531-8249(199909)46:3<281::AID-ANA2>3.0.CO;2-L
51. Keser T, Kofler M, Katzmayr M, Schiefecker AJ, Rass V, Ianosi BA, et al. Risk factors for dysphagia and the impact on outcome after spontaneous subarachnoid hemorrhage. Neurocritical care. (2020) 33:132–9. doi: 10.1007/s12028-019-00874-6
52. Dunn K, Rumbach A. Incidence and risk factors for dysphagia following non-traumatic subarachnoid hemorrhage: a retrospective cohort study. Dysphagia. (2019) 34:229–39. doi: 10.1007/s00455-018-9934-3
53. Lin Q, Lin SF, Ke XH, Jia XF, Huang DB. A systematic review and meta-analysis on the effectiveness of transcranial direct current stimulation on swallowing function of poststroke patients. Am J Phys Med Rehabil. (2022) 101:446–53. doi: 10.1097/PHM.0000000000001845
54. Marchina S, Pisegna JM, Massaro JM, Langmore SE, McVey C, Wang J, et al. Transcranial direct current stimulation for post-stroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. J Neurol. (2021) 268:293–304. doi: 10.1007/s00415-020-10142-9
55. Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain stimulation. (2008) 1:206–23. doi: 10.1016/j.brs.2008.06.004
56. Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol. (2017) 128:1774–809. doi: 10.1016/j.clinph.2017.06.001
57. Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. (2007) 72:208–14. doi: 10.1016/j.brainresbull.2007.01.004
Keywords: dosage consideration, transcranial direct current stimulation, post-stroke dysphagia, systematic review, network meta-analysis
Citation: Xie J, Zhou C, Ngaruwenayo G, Wu M, Jiang X and Li X (2023) Dosage consideration for transcranial direct current stimulation in post-stroke dysphagia: A systematic review and network meta-analysis. Front. Neurol. 14:1098831. doi: 10.3389/fneur.2023.1098831
Received: 01 December 2022; Accepted: 04 January 2023;
Published: 24 January 2023.
Edited by:
Federico Posteraro, Azienda USL Toscana Nord Ovest, ItalyReviewed by:
Laura Sagliocco, Versilia Hospital, ItalyXiaorong Tang, Guangzhou University of Chinese Medicine, China
Copyright © 2023 Xie, Zhou, Ngaruwenayo, Wu, Jiang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohan Li,  eGhsaUBjbXUuZWR1LmNu
eGhsaUBjbXUuZWR1LmNu
 Jianwei Xie
Jianwei Xie Chiteng Zhou
Chiteng Zhou Gilbert Ngaruwenayo
Gilbert Ngaruwenayo Minghui Wu3
Minghui Wu3 Xiaohan Li
Xiaohan Li