
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 09 March 2023
Sec. Neurological Biomarkers
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1096358
This article is part of the Research TopicBiomarkers in ischemic strokeView all 11 articles
Objectives: Patients with minor ischemic stroke (MIS) frequently suffer from early neurological deterioration (END) and become disabled. Our study aimed to explore the association between serum neurofilament light chain (sNfL) levels and END in patients with MIS.
Methods: We conducted a prospective observational study in patients with MIS [defined as a National Institutes of Health Stroke Scale (NIHSS) score 0–3] admitted within 24 h from the onset of symptoms. sNfL levels were measured at admission. The primary outcome was END, defined as an increase in the NIHSS score by ≥2 points within 5 days after admission. Univariate and multivariate analyses were performed to explore the risk factors associated with END. Stratified analyses and interaction tests were conducted to identify variables that might modify the association between sNfL levels and END.
Results: A total of 152 patients with MIS were enrolled, of which 24 (15.8%) developed END. The median sNfL level was 63.1 [interquartile range (IQR), 51.2–83.4] pg/ml on admission, which was significantly higher than that of 40 age- and sex-matched healthy controls (median 47.6, IQR 40.8–56.1 pg/ml; p < 0.001). Patients with MIS with END had a higher level of sNfL (with ND: median 74.1, IQR 59.5–89.8 pg/ml; without END: median 61.2, IQR 50.5–82.2 pg/ml; p = 0.026). After adjusting for age, baseline NIHSS score, and potential confounding factors in multivariate analyses, an elevated sNfL level (per 10 pg/mL) was associated with an increased risk of END [odds ratio (OR) 1.35, 95% confidence interval (CI) 1.04–1.77; p = 0.027). Stratified analyses and interaction tests demonstrated that the association between sNfL and END did not change by age group, sex, baseline NIHSS score, Fazekas' rating scale, hypertension, diabetes mellitus, intravenous thrombolysis, and dual antiplatelet therapy in patients with MIS (all p for interaction > 0.05). END was associated with an increased risk of unfavorable outcomes (modified Rankin scale score ranging from 3 to 6) at 3 months.
Conclusion: Early neurological deterioration is common in minor ischemic stroke and is associated with poor prognosis. The elevated sNfL level was associated with an increased risk of early neurological deterioration in patients with minor ischemic stroke. sNfL might be a promising biomarker candidate that can help to identify patients with minor ischemic stroke at high risk of neurological deterioration, for reaching individual therapeutic decisions in clinical practice.
Minor ischemic stroke (MIS) is fairly common and accounts for about 30% of all strokes (1). Although most patients with MIS have favorable outcomes, a small but significant proportion of individuals suffer neurological deterioration in the early stages of acute ischemic stroke (AIS) and become disabled (2, 3). It also has been demonstrated that early neurological deterioration (END) after ischemic stroke is an independent predictor of poor prognosis (4–6). Several hypotheses have been proposed regarding the mechanisms of END, including the propagation of thrombus in situ, inflammation, excitotoxicity, oxidative stress, and cortical spreading depolarization (7). However, until recently, the underlying pathophysiology of END in patients with MIS is still unclear (8). Once END occurs, there are no effective therapies to arrest it. Thus, the early identification and rational prevention of END are essential for this ominous event.
Neuronal damage and loss are the pathological basis of disability caused by cerebral infarction. As a part of the neuronal cytoskeleton that is exclusively expressed in neurons, neurofilaments are suitable candidate biomarkers for neuronal injury (9). When ischemic damage occurs, neurofilament light chain (NfL) protein is released into the extracellular fluid, the cerebrospinal fluid, and to a lower concentration in the peripheral blood (10). With the application of single-molecule array (SiMoA) assays that enabled a sensitive detection of NfL in blood samples, neurofilaments are gaining increasing attention in various neurologic diseases such as traumatic brain injury, multiple sclerosis, dementias, and different neurodegenerative diseases (9). In patients with ischemic stroke, serum NfL (sNfL) levels have been correlated with initial stroke severity assessed by the NIHSS score on admission (11–14). Meanwhile, the baseline NIHSS score has been shown to be a good predictor of the course of END (15–17). Some studies have suggested that baseline sNfL is a valuable biomarker of the functional outcome at 3 months after cerebral infarction (12, 14), but others have reached a different conclusion (11, 13). sNfL has also been shown to be associated with active small vessel disease (18). Until recently, whether sNfL levels are associated with END in patients with MIS has not been elucidated.
Therefore, the current study aimed to explore the potential association of sNfL levels with END in Chinese patients with MIS.
Patients with AIS admitted to Deyang People's Hospital were prospectively and consecutively registered from 1 March 2020 to 31 June 2021. Patients with MIS who were admitted within 24 h from the symptom onset and with magnetic resonance diffusion-weighted imaging (DWI) diagnoses of cerebral infarction were eligible for this observational study. MIS was defined as having a National Institutes of Health Stroke Scale (NIHSS) score of ≤3 points at admission (19). All patients received an extensive stroke etiologic workup (computed tomographic angiography or magnetic resonance angiography, color duplex ultrasound, Holter monitoring, echocardiography, and blood sampling) and were routinely followed up after 3 months by telephone interview or by mail. We excluded cases with incomplete hospital records or missing imaging that would prevent complete data collection. We also excluded subjects with a preexisting score of more than 2 on the modified Rankin scale (mRS, a scale of 0 to 6, with 0 indicating no symptoms and 6 indicating death) and lived dependently (20). Meanwhile, cases with comorbid disorders that could lead to neuronal damage, such as traumatic brain injury, multiple sclerosis, dementia, and other neurological diseases were excluded. The study was approved by the Ethics Committee of Deyang People's Hospital (Reference No. 2019-01-142-K01) and was carried out under the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all patients before they were enrolled. The study described here is registered at http://www.chictr.org/ (unique identifier: ChiCTR2000029902). The date of trial registration was 16 February 2020. All methods in the present study were performed according to relevant guidelines and regulations.
Baseline data on age, sex, onset to admission time, baseline NIHSS score, systolic and diastolic blood pressure on admission, baseline serum glucose, vascular risk factors, and potential stroke etiology were recorded, which has been described in our previous study (21). Results of routine laboratory tests such as triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), uric acid, fibrinogen, d-dimer, and C-reactive protein (CRP) were also collected. The white matter lesions (WMLs) were visually evaluated by experienced neuroradiologists using the modified Fazekas scale (22, 23). The Fazekas scale is a 4-point rating scale: 0 (no WML), 1 (mild WML), 2 (moderate WML), and 3 (severe WML). White matter changes were divided into two groups: 0–1 (absent or mild) or 2–3 (moderate or severe). In-hospital treatments analyzed in our study included intravenous thrombolysis and antiplatelet therapy. Intravenous thrombolysis was performed according to the Chinese guidelines, which had similar inclusion and exclusion criteria compared with the American guidelines (24, 25). The final treatment decision was made in consultation with the neurologist and the patient's family. Antiplatelet therapies were administered at the physicians' discretion. Patients enrolled in the present study received either (1) aspirin or clopidogrel only or (2) clopidogrel plus aspirin (dual antiplatelet therapy) at admission.
Whole blood samples (4 ml) were drawn from all patients with MIS at admission, and serum samples were isolated following centrifugation for 20 min at 2,000 g at room temperature. Then, serum samples were stored at −80°C until analysis. At the same time, 40 age- and sex-matched healthy controls were selected, and their serum samples were obtained after their enrollment in the study. Serum NfL (sNfL) concentrations were measured using a SiMoAplatform (Quanterix, Lexington, MA, United States) as described (26). All serum samples were analyzed in duplicates for inter-test validation, and the two results were averaged to determine the mean concentration. The mean intra-assay variability (the coefficient of the variation of concentrations) was <10%, and the inter-assay coefficient of variation was <15%.
The primary outcome of the present study was END, which was defined as an increase in NIHSS score by 2 or more points within 5 days after admission, after excluding the hemorrhagic transformation of the brain infarct or a new infarct in another vascular territory (27). Trained neurologists assessed the neurological severity of the patients daily between admission and discharge from the stroke unit. The secondary outcome measures in our study were 3-month death and an unfavorable outcome [defined as having an mRS score of 3–6 (20)].
Continuous variables are presented as mean with standard deviation (SD) or median with interquartile range (IQR), and categorical variables are presented as frequencies with percentages. The normality of data was tested using a Shapiro–Wilk test. Baseline characteristics, laboratory values, and in-hospital treatment were compared between patients with MIS with or without END. The χ2 tests or Fisher's exact tests were used for differences in categorical data, while Student's t-tests or the Mann–Whitney U-test were used for differences in continuous data. Multivariate logistic regression analysis was performed using the forced entry method, including variables with a p-value of <0.1 in univariate analyses, to identify the association between sNfL levels and END in patients with MIS. Then, stratified analyses and interaction tests were conducted to identify variables that might modify the association between sNfL levels and END. All statistical analyses were performed using SPSS v21.0 (IBM, Chicago, IL, USA), the statistical software packages R (http://www.R-project.org, The R Foundation, version 3.4.3), and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA), which have been described in our previous study (28). A two-sided p-value of < 0.05 denoted statistical significance.
During the study period, 798 patients with AIS were registered. Of those patients, 152 (19.0%) patients with MIS admitted within 24 h were enrolled in the present study (median baseline NIHSS score: 2, IQR: 1–3). A flow diagram of included and excluded patients is provided in Figure 1. Their age varied from 32 to 95 years (mean age 67.7 and standardized difference 11.1), and 104 (68.4%) were men. The median onset to admission time was 8.5 h (IQR: 3.0–20.8 h). On admission, the median sNfL levels of enrolled patients with MIS were 63.1 pg/ml (IQR: 51.2–83.4 pg/ml). We did not observe a significant correlation between the baseline NIHSS score and sNfL values at hospital admission (Spearman correlation analysis, rho = 0.150, p = 0.066) (Supplemental Figure 1). A total of 30 (19.7%) cases were treated with intravenous thrombolysis, and 109 (71.7%) were treated with dual antiplatelet therapy at admission (Table 1). A total of 24 (15.8%) patients experienced END within 5 days after admission. The median time from the stroke onset to the development of END was 48 h (IQR: 30–49.75 h). In 22 out of the 24 patients (91.7%), END was observed on days 2 and 3 after the stroke onset (Figure 2). All enrolled cases completed 3-month follow-up. In total, three (2.0%) patients died, and 10 (6.6%) patients had unfavorable outcomes at 3 months (Table 1).
Baseline characteristics and clinical outcomes were compared between patients with MIS with and without END (Table 2). The median sNfL levels of enrolled patients with MIS were significantly higher than the levels of 40 age- and sex-matched healthy controls (patients with MIS: median 63.1; IQR: 51.2–83.4 pg/ml; healthy controls median: 47.6; IQR: 40.8–56.1 pg/ml; p < 0.001), while patients with MIS with END had a higher level of sNfL compared with those patients without END (with END median: 74.1; IQR: 59.5–89.8; without END median: 61.2, IQR: 50.5–82.2; p = 0.026) (Figure 3). Meanwhile, the END group had a higher systolic blood pressure level on admission (168.1 ± 28.5 vs. 156.0 ±2 4.5 mmHg, p = 0.033) and less frequently received dual antiplatelet therapy (54.2 vs. 75.0%, p = 0.038). There was no difference in the age, sex, onset to admission time, baseline NIHSS score, diastolic blood pressure, vascular risk factors, stroke etiology, moderate–severe WML (defined as Fazekas' rating scale score 2–3), and other laboratory values between the two groups (all p > 0.05). Although there was no difference in the 3-month death between the two groups, the incidence rate of 3-month unfavorable outcome was significantly higher in patients with MIS with END (with END: 25.0%; without END: 3.1%; p < 0.001). After adjusting for age, sex, and baseline NIHSS score, END was still associated with an increased risk of the unfavorable outcome at 3 months [odds ratio (OR) 12.4, 95 % confidence interval (CI) 2.7 to 56.3, p = 0.001].
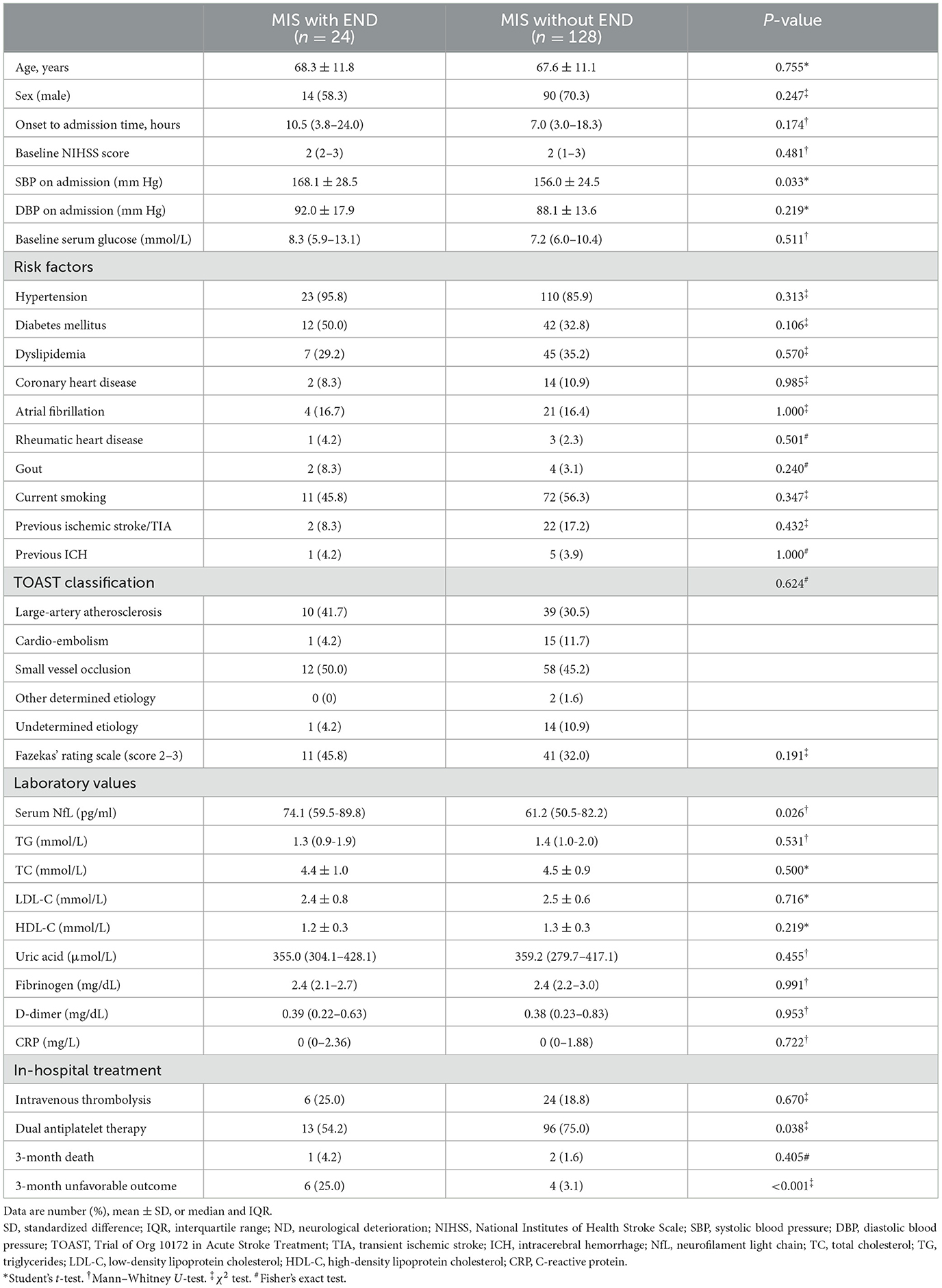
Table 2. Baseline characteristics and clinical outcomes between patients with MIS with and without END.

Figure 3. Serum neurofilament light chain (sNfL) levels between groups. (A) sNfL levels between patients with minor ischemic stroke (MIS) and healthy controls are shown as violin plots (patients with MIS median: 63.1, IQR: 51.2–83.4 pg/ml vs. healthy controls median: 47.6, IQR: 40.8–56.1 pg/ml; p < 0.001). (B) sNfL levels between patients with MIS with END and without END are shown as violin plots (with END median: 74.1, IQR: 59.5–89.8 pg/ml vs. without END median: 61.2, IQR: 50.5–82.2 pg/ml; p = 0.026).
Variables that potentially affect END in patients with MIS (p < 0.1) were included in multivariate logistic regression analyses; the results are shown in Table 3. After adjusting for the baseline NIHSS score and potential confounding factors (Model 1), an elevated sNfL level (per 10 pg/mL) was associated with an increased risk of END (OR 1.36, 95% CI 1.04–0.77; p = 0.026) in multivariate analyses. When age was included in the multivariate logistic regression (Model 2), an elevated sNfL level (per 10 pg/mL) remained an independent risk factor for END (OR 1.35, 95% CI 1.04–1.77; p = 0.027). Moreover, systolic blood pressure on admission (OR 1.25, 95% CI 1.03–1.51) and dual antiplatelet therapy (OR 0.22, 95% CI 0.08–0.63) were independently associated with END in patients with MIS in the two multivariate logistic regression models (both with p < 0.05). An increased baseline NIHSS score also tended to be associated with a higher risk of END (OR 1.66, 95% CI 0.93–2.99; p = 0.090).
Stratified analyses and interaction tests were further employed to explore the association between sNfL levels and END in patients with MIS. Stratified logistic regression analyses demonstrated that the association between sNfL and END did not change by age group, sex, baseline NIHSS score, Fazekas' rating scale, hypertension, diabetes mellitus, intravenous thrombolysis, and dual antiplatelet therapy in patients with MIS (all p > 0.05) (Table 4).
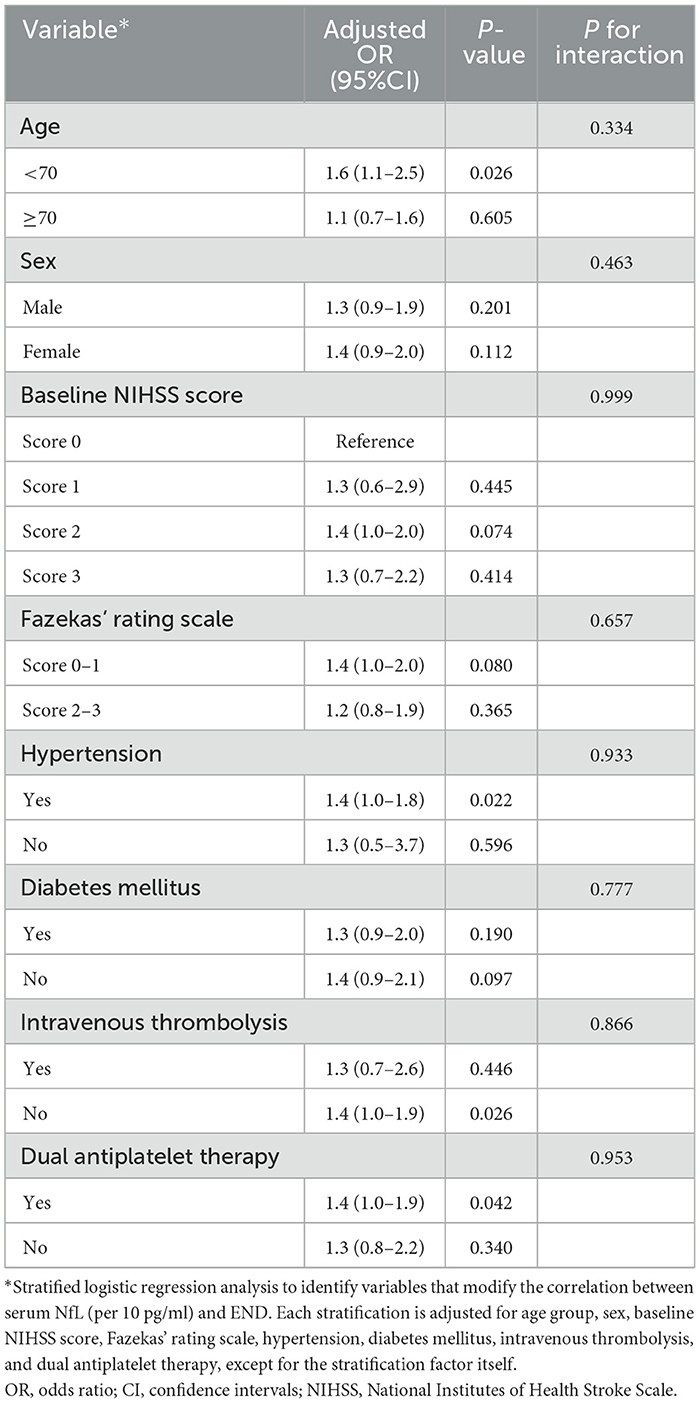
Table 4. Stratified logistic regression analyses to identify variables that might modify the association between sNfL and END in patients with MIS.
Epidemiological studies showed that there are approximately 3 million new-onset strokes every year in China and approximately 1 million are MIS (29, 30). Since the baseline NIHSS score can strongly predict outcomes after stroke, the outcomes for patients with MIS are generally favorable (2). Yet, prospective data suggest that 4.5–26.4% of patients with MIS are also affected by early neurological worsening and become disabled (3, 27, 31, 32). The incidence rate of END in patients with MIS is 15.8% in our cohort. Differences in the incidence rate of END in patients with MIS may reflect heterogeneity in demographics (age, sex, and ethnicity) of the enrolled patients, the definition of MIS, and the way END was defined and measured, highlighting the need for a standardized definition of MIS and END. The median time from the stroke onset to the development of END in our cohort was 48 h (IQR: 30–49.75 h), similar to previous studies (3, 27, 32). Although the association between END and the outcome of patients with MIS remains to be established, our study suggested that MIS patients with END had a significantly higher rate of the 3-month unfavorable outcome than those without (25.0 vs. 3.1%). END was also associated with an increased risk of a 3-month unfavorable outcome in multivariate analysis after adjusting for age, sex, and baseline NIHSS score, as found in many other studies conducted in patients with ischemic stroke (4–6, 33, 34) and is consistent with our results. Until recently, the mechanisms underlying END in patients with MIS are still unclear, and no consensus has been reached on the risk factors of END (8, 15). Understanding the mechanisms underlying END in patients with MIS could provide valuable insights for rational prevention of END in patients with MIS. Moreover, the early targeting of patients at higher risk of END is of great importance for improving the outcome of MIS.
In a previous study, NfL was shown to be higher in patients with ischemic stroke than in healthy controls, whereas NfL in patients with transient ischemic stroke (TIA) was comparable to those in healthy controls (35). In patients with ischemic stroke, NfL levels have been correlated with initial stroke severity (11–14). In the present study, we found that the sNfL levels of patients with MIS were significantly higher than that of age- and sex-matched healthy controls, while patients with MIS with END had a higher level of sNfL compared with those patients without END. Although it has been demonstrated that the baseline NIHSS score could strongly predict the course of END (15–17), multivariate analyses adjusting for confounders, including age and baseline NIHSS score, also suggested that an elevated sNfL level was independently associated with END in patients with MIS. Stratified analyses and interaction tests demonstrated that the association between sNfL and END did not change by age group, sex, baseline NIHSS score, Fazekas' rating scale, hypertension, diabetes mellitus, intravenous thrombolysis, and dual antiplatelet therapy in patients with MIS. Some studies have suggested an association between baseline sNfL levels and final infarct size on MRI (13, 26, 36), but others have reached a different conclusion (11, 12). Therefore, our results could not be explained by the effect of final infarct volume on the sNfL levels. Neuronal damage and loss are the pathological substrates of disability caused by an AIS. As a part of the neuronal cytoskeleton that is exclusively expressed in neurons, neurofilaments are suitable candidate biomarkers of ischemic neuronal injury (9, 37). It has been demonstrated that sNfL levels increased during the first few days after the stroke onset and remained increased over 3–6 months (26, 36). Experimental studies suggest that synaptic NfL plays an essential role in controlling synaptic function, neurotransmission, and stabilizing NMDA receptors in the neuronal cell membrane (38–40). According to the currently available evidence, elevated sNfL levels after AIS seem to reflect the extent of neuronal injury, persistent blood–brain barrier breakdown, and ongoing post-ischemic immunological or inflammatory processes. Meanwhile, elevated sNfL levels may act as a biomarker of neural plasticity and a positive predictor of functional improvement (9, 41). All these findings suggest a potential molecular mechanism that links the sNfL with the risk of neurologic worsening and functional disability. The present study is the first to report a positive correlation between sNfL levels and END in Chinese patients with MIS. Therefore, sNfL might be a promising biomarker candidate that can help identify MIS patients at high risk of END, for reaching individual therapeutic decisions in clinical trials. Further studies with large sample sizes are needed to determine the optimal cutoff value of sNfL as an indicator for END and validate sNfL as a biomarker for END in patients with MIS.
It is also worth noting that dual antiplatelet therapy was associated with decreased risk of END in patients with MIS in our cohort (OR 0.22, 95% CI 0.08–0.63), which are in line with previous studies conducted in patients with AIS (42–44). In addition, higher baseline systolic blood pressure (OR 1.25, 95% CI 1.03–1.51) was associated with increased END risk in patients with MIS. These results support that platelet aggregation might be an important mechanism of END (45). Our results also support the view that an impaired cerebral hemodynamic response due to hypertension might be another contributor to END (45). Therefore, dual antiplatelet therapy in the acute phase of MIS and premorbid personalized antihypertensive treatment may potentially reduce END and subsequently improve the outcome of patients with MIS. However, the present study was not specifically targeted at this treatment effect, thus, our results should be interpreted cautiously. Further studies targeting patients with MIS at high risk of END are warranted to determine the usefulness of different acute therapy strategies.
The results of the present study should be interpreted with caution, given its limitations. First, it was a single hospital-based study conducted in China, with limited generalizability. Second, the sample size of our study was relatively small, and only 24 cases suffered END. We could not determine the optimal cutoff value of sNfL as an indicator for END in patients with MIS. Third, sNfL levels may change dynamically after acute ischemic stroke. In the present study, sNfL levels were tested one time on admission. We did not have longitudinal data on sNfL levels. Further studies are needed to evaluate the association between dynamic changes in sNfL levels and END in patients with MIS. In addition, the number and location of the infarcts were not assessed in MRI imaging, as well as the infarct volume, which might have an association with END in patients with ischemic stroke. Meanwhile, the renal function might affect the level of sNfL. However, due to a lack of data, we did not include the eGFR levels in the multivariate analyses. In addition, although non-neurological complications, such as infections, might cause clinical deterioration and an increase in the NIHSS score by causing a confusional state and decreasing the level of consciousness, we did not include medical complications in the analyses because of a lack of data. Moreover, we performed the follow-up by telephone interview or a mailed questionnaire instead of a clinic visit which may result in reporting bias. Finally, our study was an observational study. No causal link could be drawn. Thus, well-designed studies with large sample sizes are needed to validate our findings in the future.
We conducted a prospective observational study in Chinese patients with acute MIS admitted within 24 h from the symptom onset. We identified that END is common in patients with MIS and associated with 3-month unfavorable outcomes. An elevated sNfL level was independently associated with END in patients with MIS. sNfL might be a promising biomarker candidate that can help identify patients with MIS at high risk of END, for reaching individual therapeutic decisions in clinical practice.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Deyang People's Hospital (Reference No. 2019-01-142-K01). The patients/participants provided their written informed consent to participate in this study.
JL and XY: conceived the study, analyzed and interpreted the data, as well as drafted the manuscript. HC and CW: contributed to study supervision. PZ, YZ, YD, SL, and JF: participated in data collection. JL and PZ: participated in statistical analysis, data interpretation, and revised the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final manuscript.
This research was funded by the Science and Technology Research Foundation of Deyang City (2021SZZ065, 2020SZZ069, and 2022SZ055) in China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1096358/full#supplementary-material
1. Ayis SA, Coker B, Rudd AG, Dennis MS, Wolfe CDA. Predicting independent survival after stroke: a European study for the development and validation of standardised stroke scales and prediction models of outcome. J Neurol Neurosurg Psychiatry. (2013) 84:288–96. doi: 10.1136/jnnp-2012-303657
2. Jr HPA, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the trial of Org 10172 in acute stroke treatment (TOAST). Neurology. (1999) 53:126–31. doi: 10.1212/WNL.53.1.126
3. Kim J-T, Heo S-H, Yoon W, Choi K-H, Park M-S, Saver JL, et al. Clinical outcomes of patients with acute minor stroke receiving rescue IA therapy following early neurological deterioration. J Neurointerv Surg. (2016) 8:461–5. doi: 10.1136/neurintsurg-2015-011690
4. Dávalos A, Cendra E, Teruel J, Martinez M, Genís D. Deteriorating ischemic stroke: risk factors and prognosis. Neurology. (1990) 40:1865–9. doi: 10.1212/WNL.40.12.1865
5. Toni D, Fiorelli M, Gentile M, Bastianello S, Sacchetti ML, Argentino C, et al. Progressing neurological deficit secondary to acute ischemic stroke. A study on predictability, pathogenesis, and prognosis. Arch Neurol. (1995) 52:670–5. doi: 10.1001/archneur.1995.00540310040014
6. Liu P, Liu S, Feng N, Wang Y, Gao Y, Wu J. Association between neurological deterioration and outcomes in patients with stroke. Ann Transl Med. (2020) 8:4. doi: 10.21037/atm.2019.12.36
7. Saia V, Pantoni L. Progressive stroke in pontine infarction. Acta Neurol Scand. (2009) 120:213–5. doi: 10.1111/j.1600-0404.2009.01161.x
8. Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. (2015) 86:87–94. doi: 10.1136/jnnp-2014-308327
9. Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. (2018) 14:577–89. doi: 10.1038/s41582-018-0058-z
10. Skillbäck T, Farahmand B, Bartlett JW, Rosén C, Mattsson N, Nägga K, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. (2014) 83:1945–53. doi: 10.1212/WNL.0000000000001015
11. De Marchis GM, Katan M, Barro C, Fladt J, Traenka C, Seiffge DJ, et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol. (2018) 25:562–8. doi: 10.1111/ene.13554
12. Uphaus T, Bittner S, Gröschel S, Steffen F, Muthuraman M, Wasser K, et al. NfL (neurofilament light chain) levels as a predictive marker for long-term outcome after ischemic stroke. Stroke. (2019) 50:3077–84. doi: 10.1161/STROKEAHA.119.026410
13. Onatsu J, Vanninen R, Jäkälä P, Mustonen P, Pulkki K, Korhonen M, et al. Serum neurofilament light chain concentration correlates with infarct volume but not prognosis in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2019) 28:2242–9. doi: 10.1016/j.jstrokecerebrovasdis.2019.05.008
14. Pedersen A, Stanne TM, Nilsson S, Klasson S, Rosengren L, Holmegaard L, et al. Circulating neurofilament light in ischemic stroke: temporal profile and outcome prediction. J Neurol. (2019) 266:2796–806. doi: 10.1007/s00415-019-09477-9
15. Thanvi B, Treadwell S, Robinson T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgrad Med J. (2008) 84:412–7. doi: 10.1136/pgmj.2007.066118
16. Seners P, Baron JC. Revisiting ‘progressive stroke’: incidence, predictors, pathophysiology, and management of unexplained early neurological deterioration following acute ischemic stroke. J Neurol. (2018) 265:216–25. doi: 10.1007/s00415-017-8490-3
17. Nam KW, Kang MK, Jeong HY, Kim TJ, Lee EJ, Bae J, et al. Triglyceride-glucose index is associated with early neurological deterioration in single subcortical infarction: early prognosis in single subcortical infarctions. Int J Stroke. (2021) 16:944–52. doi: 10.1177/1747493020984069
18. Duering M, Konieczny MJ, Tiedt S, Baykara E, Tuladhar AM, van Leijsen E, et al. Serum neurofilament light chain levels are related to small vessel disease burden. J Stroke. (2018) 20:228–38. doi: 10.5853/jos.2017.02565
19. Fischer U, Baumgartner A, Arnold M, Nedeltchev K, Gralla J, Marco De Marchis G. What is a minor stroke? Stroke. (2010) 41:661–6. doi: 10.1161/STROKEAHA.109.572883
20. De Haan R, Limburg M, Bossuyt P, Van der Meulen J, Aaronson N. The clinical meaning of Rankin ‘handicap’ grades after stroke. Stroke. (1995) 26:2027–30. doi: 10.1161/01.STR.26.11.2027
21. Li J, Wang D, Tao W, Dong W, Zhang J, Yang J, et al. Early consciousness disorder in acute ischemic stroke: incidence, risk factors and outcome. BMC Neurol. (2016) 16:140. doi: 10.1186/s12883-016-0666-4
22. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging AJR. Am J Roentgenol. (1987) 149:351–6. doi: 10.2214/ajr.149.2.351
23. Pantoni L, Basile AM, Pracucci G, Asplund K, Bogousslavsky J, Chabriat H, et al. Impact of age-related cerebral white matter changes on the transition to disability—the LADIS study: rationale, design and methodology. Neuroepidemiology. (2005) 24:51–62. doi: 10.1159/000081050
24. Qiu S, Xu Y. Guidelines for acute ischemic stroke treatment. Neurosci Bull. (2020) 36:1229–32. doi: 10.1007/s12264-020-00534-2
25. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. American heart association stroke council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2018) 49:e46–e110. doi: 10.1161/STR.0000000000000158
26. Tiedt S, Duering M, Barro C, Kaya AG, Boeck J, Bode FJ, et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischemic stroke. Neurology. (2018) 91:e1338–47. doi: 10.1212/WNL.0000000000006282
27. Yi X, Han Z, Zhou Q, Lin J, Liu P. 20-Hydroxyeicosatetraenoic acid as a predictor of neurological deterioration in acute minor ischemic stroke. Stroke. (2016) 47:3045–7. doi: 10.1161/STROKEAHA.116.015146
28. Li J, Gao L, Zhang P, Liu Y, Zhou J, Yi X, et al. Vulnerable plaque is more prevalent in male individuals at high risk of stroke: a propensity score-matched study. Front Physiol. (2021) 12:642192. doi: 10.3389/fphys.2021.642192
29. Zhao D, Liu J, Wang W, Zeng Z, Cheng J, Liu J, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke. (2008) 39:1668–74. doi: 10.1161/STROKEAHA.107.502807
30. Wang YL, Wu D, Liao X, et al. Burden of stroke in China. Int J Stroke. (2007) 2:211–3. doi: 10.1111/j.1747-4949.2007.00142.x
31. Khatri P, Conaway MR, Johnston KC. acute stroke accurate prediction study (ASAP) Investigators. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke. (2012) 43:560–2. doi: 10.1161/STROKEAHA.110.593897
32. Ferrari J, Knoflach M, Kiechl S, Willeit J, Schnabl S, Seyfang L, et al. Austrian stroke unit registry collaborators. Early clinical worsening in patients with TIA or minor stroke: the Austrian Stroke Unit Registry. Neurology. (2010) 74:136–41. doi: 10.1212/WNL.0b013e3181c9188b
33. Vynckier J, Maamari B, Grunder L, Goeldlin MB, Meinel TR, Kaesmacher J, et al. Early neurologic deterioration in lacunar stroke: clinical and imaging predictors and association with long-term outcome. Neurology. (2021) 97:e1437–46. doi: 10.1212/WNL.0000000000012661
34. Kim YD, Song D, Kim EH, Lee KJ, Lee HS, Nam CM, et al. Long-term mortality according to the characteristics of early neurological deterioration in ischemic stroke patients. Yonsei Med J. (2014) 55:669–75. doi: 10.3349/ymj.2014.55.3.669
35. Nielsen HH, Soares CB, Høgedal SS, Madsen JS, Hansen RB, Christensen AA, et al. Acute neurofilament light chain plasma levels correlate with stroke severity and clinical outcome in ischemic stroke patients. Front Neurol. (2020) 11:448. doi: 10.3389/fneur.2020.00448
36. Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology. (2017) 89:2108–14. doi: 10.1212/WNL.0000000000004645
37. Barro C, Chitnis T, Weiner HL. Blood neurofilament light: a critical review of its application to neurologic disease. Ann Clin Transl Neurol. (2020) 7:2508–23. doi: 10.1002/acn3.51234
38. Ehlers MD, Fung ET, O'Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci. (1998) 18:720–30. doi: 10.1523/JNEUROSCI.18-02-00720.1998
39. Ratnam J, Teichberg VI. Neurofilament-light increases the cell surface expression of the N-methyl-D-aspartate receptor and prevents its ubiquitination. J Neurochem. (2005) 92:878–85. doi: 10.1111/j.1471-4159.2004.02936.x
40. Gafson AR, Barthélemy NR, Bomont P, Carare RO, Durham HD, Julien JP, et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain. (2020) 143:1975–98. doi: 10.1093/brain/awaa098
41. Pekny M, Wilhelmsson U, Stokowska A, Tatlisumak T, Jood K, Pekna M. Neurofilament light chain (NfL) in blood-a biomarker predicting unfavourable outcome in the acute phase and improvement in the late phase after stroke. Cells. (2021) 10:1537. doi: 10.3390/cells10061537
42. Yi X, Zhou Q, Wang C, Lin J, Chai Z. Aspirin plus clopidogrel may reduce the risk of early neurologic deterioration in ischemic stroke patients carrying CYP2C19*2 reduced-function alleles. J Neurol. (2018) 265:2396–403. doi: 10.1007/s00415-018-8998-1
43. Wang C, Yi X, Zhang B, Liao D, Lin J, Chi L. Clopidogrel plus aspirin prevents early neurologic deterioration and improves 6-month outcome in patients with acute large artery atherosclerosis stroke. Clin Appl Thromb Hemost. (2015) 21:453–61. doi: 10.1177/1076029614551823
44. Berberich A, Schneider C, Herweh C, Hielscher T, Reiff T, Bendszus M, et al. Risk factors associated with progressive lacunar strokes and benefit from dual antiplatelet therapy. Eur J Neurol. (2020) 27:817–24. doi: 10.1111/ene.14159
Keywords: minor ischemic stroke, neurological deterioration, neurofilament light chain, odds ratio, biomarker
Citation: Li J, Zhang P, Zhu Y, Duan Y, Liu S, Fan J, Chen H, Wang C and Yi X (2023) Serum neurofilament light chain levels are associated with early neurological deterioration in minor ischemic stroke. Front. Neurol. 14:1096358. doi: 10.3389/fneur.2023.1096358
Received: 12 November 2022; Accepted: 16 February 2023;
Published: 09 March 2023.
Edited by:
Pedro Ramos-Cabrer, CIC biomaGUNE, SpainReviewed by:
Stefano Forlivesi, IRCCS Institute of Neurological Sciences of Bologna (ISNB), ItalyCopyright © 2023 Li, Zhang, Zhu, Duan, Liu, Fan, Chen, Wang and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Li, bGlqaWU4NjAxMTRAMTYzLmNvbQ==; Xingyang Yi, WWlYaW5nWWFuZzY0QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.