- 1Department of Neurology, Minhang Hospital, Fudan University, Shanghai, China
- 2Department of Neurosurgery, Minhang Hospital, Fudan University, Shanghai, China
Introduction: A crucial aspect of stroke progression is the inflammatory response. As novel inflammatory and prognostic markers, the systemic immune inflammation index (SII) and the systemic inflammation response index (SIRI) have recently been studied. The objective of our study was to evaluate the prognostic value of SII and SIRI in mild acute ischemic stroke (AIS) patients following intravenous thrombolysis (IVT).
Methods: Our study screened the clinical data of patients with mild AIS admitted to the Minhang Hospital of Fudan University for retrospective analysis. The SIRI and SII were examined by the emergency laboratory before IVT. Functional outcome was evaluated 3 months after the onset of stroke using the modified Rankin Scale (mRS). mRS ≥ 2 was defined as an unfavorable outcome. The relationship between SIRI and SII and the 3-month prognosis was determined using both univariate and multivariate analysis. Receiver operating characteristic curve was performed to evaluate the predictive value of SIRI for AIS prognosis.
Results: A total of 240 patients were included in this study. Both SIRI and SII were higher in the unfavorable outcome group than in the favorable outcome group [1.28 (0.70–1.88) vs. 0.79 (0.51–1.08), P < 0.001 and 531.93 (377.55–797.12) vs. 397.23 (263.32–577.65), P < 0.001]. Multivariate logistic regression analyses showed that SIRI was significantly associated with 3-month unfavorable outcome of mild AIS patients [odds ratio (OR) = 2.938, 95% confidence interval (CI) = 1.805–4.782, P < 0.001], conversely, SII had no prognostic value. When SIRI combined with the established clinical factors, the area under the curve (AUC) showed a significant improvement (0.773 vs. 0.683, P for comparison = 0.0017).
Conclusions: Higher SIRI could be valuable in predicting poor clinical outcomes for patients with mild AIS following IVT.
Introduction
Worldwide, acute ischemic stroke (AIS) is a frequent condition characterized by high morbidity, disability, and mortality. Previous studies reported that more than half of AIS patients present with mild stroke (1, 2). Currently, there is no formal definition for mild AIS, but the majority of studies describe it as a National Institutes of Health stroke scale (NHISS) score ≤5 at admission (3, 4). In the clinical guidelines published in December 2019, intravenous thrombolysis (IVT) was recommended for mild AIS patients with disabling symptoms (5). However, ~30% of mild AIS patients have an unfavorable outcome after IVT (6, 7). Therefore, identification of risk factors linked to poor outcomes in patients with mild AIS after IVT is crucial. Age, diabetes, the baseline NHISS, and large vessel occlusion are some of the factors that have been mentioned in prior investigations (3, 8, 9). Moreover, it has been proposed that serum biomarkers may play an important role in the prognosis of mild AIS in recent years. Serum biomarkers, however, have received little research attention.
The blood-brain barrier disruption, oxidative stress, and the direct induction of neurocyte death that occurs as a result of inflammation response (IR) have all been identified as being important pathogenic processes of AIS (10, 11). Systemic immune inflammation index (SII) and systemic inflammation response index (SIRI), which are composed of platelets and three subtypes of white blood cells, have recently been reported as new inflammatory biomarkers that can reflect IR (12). Several studies have investigated the association between serum inflammatory indicators and functional outcomes in AIS patients (13–15). Additionally, it has been noted that the neutrophil-to-lymphocyte ratio (NLR) is a helpful inflammatory biomarker for predicting a bad short-term outcome in individuals with mild AIS following IVT, but their predictive effectiveness is not clarified (9).
Recently, SII and SIRI have been proven to predict the prognosis of some diseases such as ischemic stroke, coronary artery disease, subarachnoid hemorrhage and several cancers (16–19). However, the prognostic importance of SII and SIRI in mild AIS patients who have undergone thrombolysis has not yet been reported. Therefore, we aimed to systematically investigate the association of SII and SIRI with functional outcomes in mild AIS patients who received thrombolysis.
Materials and methods
Patients recruitment
This retrospective observational study was conducted from January 2017 to May 2022. All AIS patients receiving IVT therapy alone were consecutively included in the study and collected from the Minhang Hospital of Fudan University. An admission NHISS score ≤ 5 was considered to be mild AIS. The following were the inclusion requirements: (1) aged 18 years or older; (2) NHISS score ≤ 5 at admission; (3) stroke symptoms appearing within 4.5 h and receiving IVT treatment. Patients were disqualified if they fulfilled the following requirements: (1) score on the modified Rankin Scale (mRS) ≥ 2 before the stroke; (2) patients with malignant tumor, autoimmune disease and hepatic or renal diseases; (3) patients with acute infection, including pneumonia or other active concomitant infections. This study was reviewed and approved by the Ethical Review Board of Minhang Hospital of Fudan University.
Data collection
Onset to treatment time (ONT), NHISS score and stroke subtype were evaluated by experienced clinicians. The Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria were used to classify stroke subtypes. Stroke nurses gathered demographic and baseline information on patients with age, gender, smoking, diabetes, hypertension, and atrial fibrillation. Before IVT, initial counts for platelets, monocytes, neutrophils, and white blood cells were also obtained. We calculated the SIRI and SII as follows: SIRI = NEUT × Mo/TLC, SII = PLT × NEUT/TLC, where PLT is the platelet count, NEUT is the neutrophil count, TLC is the total lymphocyte count and Mo is the monocyte count.
Laboratory tests
Before thrombolysis, 2 mL of venous blood was collected with EDTA-K2 anticoagulant vacuum tube. Within 30 min after reversing evenly, Complete blood counts including white blood cells, neutrophils, lymphocytes, platelets, and other parameters were carried out using an automatic Mindray cal 8000 blood cell analyzer.
Clinical outcome
Functional outcome was evaluated by mRS score at 3 months after the onset of stroke. There was a neurologist in charge of the follow-up using the phone and face-to-face follow-up. Favorable outcome was considered to be an mRS score ≤ 1, while an unfavorable outcome was considered an mRS score ≥ 2. Safety outcomes included hemorrhagic transformation (HT) and symptomatic intracerebral hemorrhage (sICH). HT was confirmed by computed tomography (CT) scan within 7 days after IVT therapy. Any intracranial hemorrhage that raises the overall NIHSS score by 4 points is considered to be a sICH.
Imaging analysis
To determine the location of the infarction, all patients underwent brain magnetic resonance imaging (MRI). Ipsilateral severe vessel stenosis (ISVS) and ipsilateral severe vessel occlusion (ISVO) were evaluated by computed tomographic angiography (CTA). For patients with incomplete CTA, brain magnetic resonance angiography (MRA) examination was performed after admission. An intracranial or extracranial artery on the same side of the infarction with a diameter loss higher than 70% was categorized as ISVS. ISVO was defined as the absence of a blood flow signal from the ipsilateral infarction. Blinded to the clinical data, two seasoned MRI professional neuroradiologists separately assessed each subject's imaging manifestations, including the degree of artery stenosis and HT.
Statistical analysis
Statistical analyses were performed using SPSS (version 26.0, IBM Corp, Armonk, NY, USA). Receiver operating characteristic (ROC) analysis was conducted to identify the optimal cutoff values of SII and SIRI for predictive clinical outcomes in mild AIS patients after IVT. All subjects were divided into two groups according to the mRS score at 3 months (favorable 0–1 vs. unfavorable 2–6). Normal-distribution continuous data were reported as mean ± SD and compared using the t-test, whereas non-normal-distribution continuous variables were expressed as median (interquartile range) and compared using the Mann–Whitney U test. Categorical variables were presented numerically (percentages, %) and compared between the groups using the relevant Fisher exact or Pearson χ2 tests. A subsequent multivariate logistic regression analysis included the variables for which P < 0.1 in the univariate analysis. P < 0.05 (two-sided) was used to determine statistical significance.
Results
Clinical characteristics of patients
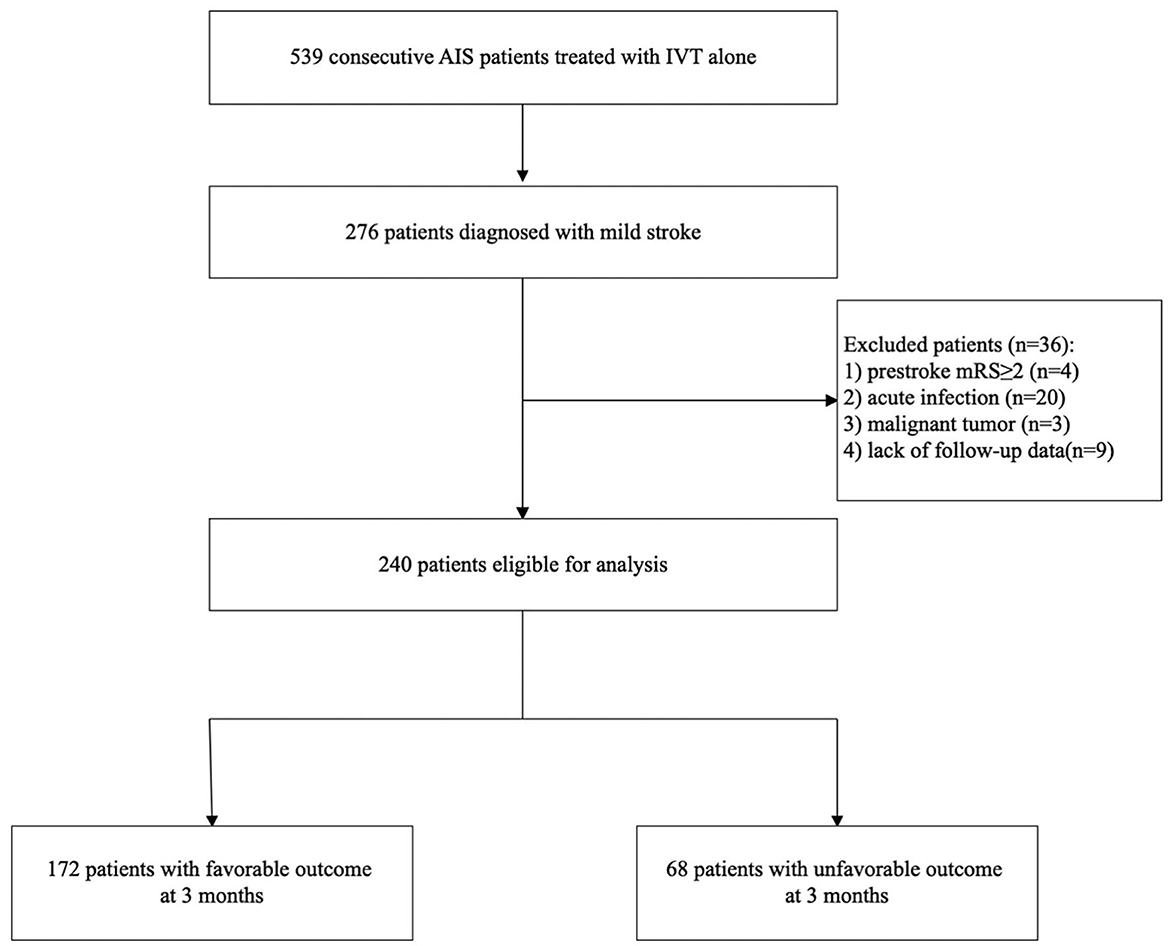
During the study period, we collected 539 consecutive AIS patients treated with IVT alone. Of these patients, 276 patients were diagnosed with mild stroke. Finally, 240 patients were involved in our analysis after excluding those with pre-stroke mRS ≥ 2 (n = 4), acute infection (n = 20) including 18 patients with pneumonia and 2 patients with cholecystitis, malignant tumor (n = 3), lack of follow-up data (n = 9). Figure 1 shows a diagram depicting study recruitment.
There were 159 men and 81 women, aged 32–92 (median 66) years. Vascular risk factors included diabetes mellitus (n = 74, 30.8%), atrial fibrillation (n = 41, 17.1%), hypertension (n = 140, 58.3%), and smoking (n = 73, 30.4%). All patients were categorized into two subgroups according to the mRS score at 3 months. Compared with the patients in the favorable outcome group, the patients in the unfavorable group were older (66.00 vs. 65.00, P = 0.039). In terms of laboratory findings, the white blood cell level (7.42 vs. 6.51, P < 0.001), neutrophil count level (4.97 vs. 3.96, P < 0.001), monocyte count level (0.42 vs. 0.38, P < 0.001) were significantly increased in the unfavorable outcome group. The unfavorable outcome group had significantly higher SIRI (1.28 vs. 0.79, P < 0.001) and SII (531.93 vs. 397.23, P < 0.001) than the favorable outcome group and exhibited more serious neurological deficits on admission (NIHSS score 4.0 vs. 3.0, P = 0.002). The proportion of ISVS (25.0 vs. 13.4%, P = 0.029), ISVO (11.8 vs. 4.7%, P = 0.047), and ISVS/ISVO (36.8 vs. 18.0%, P = 0.002) in unfavorable outcome group were higher than in the favorable outcome group. Demographic features and risk factors are summarized in Table 1.

Table 1. Baseline characteristics of mild AIS stroke patients receiving IVT therapy based on favorable vs. unfavorable outcome at 3 months.
Multivariate analysis of factors related to unfavorable outcome
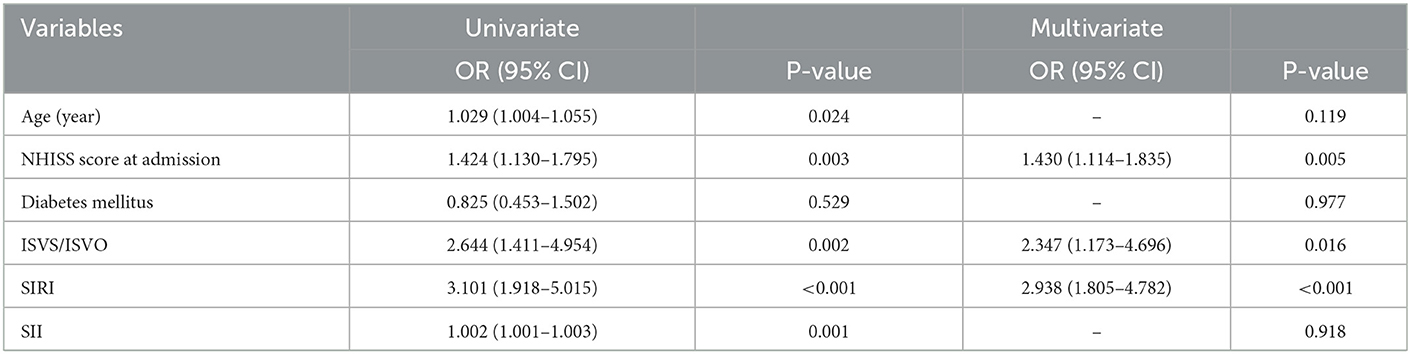
In mild AIS patients who were only receiving IVT, univariate and multivariate logistic regression analyses were run to assess the prognostic significance of SIRI and SII. We included age, NHISS score at admission, diabetes mellitus, ISVS/ISVO, SIRI and SII into the multivariate analysis.
In multivariate analysis, the NHISS score at admission [OR = 1.430, 95% confidence interval (CI) = 1.114–1.835, P = 0.005], ISVS/ISVO (OR = 2.347, 95% CI = 1.173–4.696, P = 0.016), and SIRI (OR = 2.938, 95% CI = 1.805–4.782, P < 0.001) were the predictors of an unfavorable outcome (Table 2).
Association of SIRI with clinical outcome
According to the ROC analysis (Figure 2), the optimal cut-off threshold of SIRI was 1.00 × 109/L [area under the curve (AUC) = 0.714, 95% CI = 0.652–0.770, P < 0.001]. The ROC curve showed that the AUC of NHISS score combined with ISVS/ISVO to predict the adverse outcome after thrombolysis in mild stroke was 0.683 (95% CI = 0.620–0.742, P < 0.001). When SIRI combined the above indicators, the AUC increased to 0.773 (95% CI = 0.715–0.825, P < 0.001). There was a significant statistical difference between the two ROC curves (P = 0.0017).
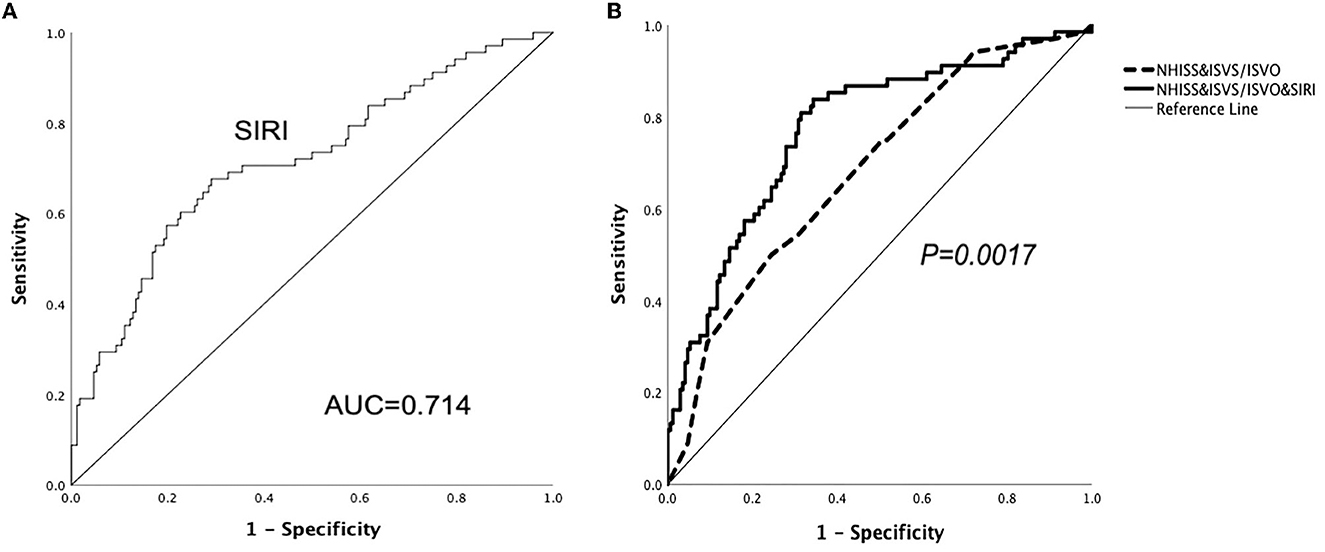
Figure 2. ROC curves demonstrate the predictive ability of SIRI and show SIRI improves the predictive ability of ISVS/ISVO&NHISS for unfavorable outcomes in mild AIS.
Discussion
For the first time, the current study examined the relationship between SIRI and SII and the 3-month functional outcome of mild AIS after IVT therapy alone. Our analysis demonstrated that SIRI and SII in patients with 3-month unfavorable outcomes of mild AIS treated with IVT are higher than those in patients with favorable outcomes. SIRI was an independent predictor for unfavorable outcome. Most importantly, when SIRI was combined, it can enhance the prognostic usefulness of traditional risk variables in people who had mild stroke after IVT.
It has been proved that the IR after AIS is related to the secondary brain damage after infarction (20, 21). The inflammatory mediators, cytokines, adhesion molecules, and chemokines released by immune inflammatory cells exacerbate tissue damage. According to previous studies, IR can be activated immediately after stroke event has occurred and is associated with poor prognosis (22–24). The mentioned mechanisms explain why the biomarkers now being researched are based on numerous inflammatory variables linked to stroke.
SIRI is a novel inflammation index, including peripheral neutrophils, lymphocytes and monocytes. Peripheral circulating neutrophils are regarded as the first inflammatory cells to penetrate the ischemic parenchyma after the onset of AIS, which exacerbate brain injury by releasing particles containing antibacterial enzymes and chemicals (25, 26). Higher neutrophil levels in early AIS were associated with larger infarction size. This may be because the increase in neutrophil concentration will promote the enhanced expression of matrix metalloproteinase-9 (MMP-9), a protein related to blood brain barrier damage (27, 28). In addition, monocytes are another important type of inflammatory cell after AIS, which can infiltrate infarcted areas and aggravate brain injury (29–31). Nevertheless, in contrast to neutrophils and monocytes, certain lymphocytes largely serve a protective role in the IR following AIS, controlling and reducing local IR (32). As a result, high SIRI (an increase in neutrophils, monocytes, and a decrease in lymphocytes) may accurately reflect adaptive immune response and IR, which are crucial processes for the incidence of stroke and may be effective biomarkers for prognosis.
Several earlier research has shown that SIRI is an effective marker for assessing the prognosis of varieties of inflammation-related diseases, which was consistent with our research results. According to Qi et al. (33), SIRI can be used to evaluate survival in pancreatic cancer patients receiving chemotherapy. Recently, Yun et al. (34) analyzed 680 aneurysmal subarachnoid hemorrhage (aSAH) patients and concluded that SIRI is an independent risk factor for unfavorable outcomes in aSAH patients. In addition, a retrospective study found that SIRI may serve as a predictive index for patients undergoing mechanical thrombectomy (MT) due to large artery occlusion (12). However, no study has found an association between SIRI and mild AIS treated with IVT. To the best of our knowledge, this is the first study to show a potential correlation between SIRI and the outcome of mild AIS after IVT.
Inflammatory markers based on platelets as well as leukocyte-based markers have been investigated in AIS patients. During AIS, platelets aggregate rapidly after blood vessel damage and play an important role in thrombosis. Furthermore, platelets also participate in immune inflammatory reaction. By altering the surface expression of P-selectin, platelets can directly interact with circulating leukocytes to create platelet-leukocyte aggregates and activate innate immunity in ischemic organs (35). Recently, the prognosis of AIS patients is thought to be reflected by SII, which is based on a combination of platelets, neutrophils, and lymphocytes (13). Even though the SII level in the group with a poor prognosis was higher than that in the group with a good prognosis in our study of thrombolytic patients with mild stroke, we did not discover that the SII was directly associated with the unfavorable outcome at 3 months. As it might be a viable clinical tool for determining mild AIS and its potential consequences, a more thorough and well-designed study on large populations of patients is required.
It has been demonstrated that the NHISS score at admission is a reliable predictor of a poor outcome in moderate AIS following IVT (3, 6). Our findings are consistent with those of earlier research. Additionally, we found that ISVS/ISVO was a predictor of a worse clinical outcome at 3 months, which is similar to a finding from a prior study (9). It reflects the bad condition of intracranial vessels and extracranial vessels.
There are some strengths in our study. First off, to the best of our knowledge, it is the first article that has specifically addressed the correlation between the short-term unfavorable outcome in patients with mild AIS following IVT and novel inflammation index, including SIRI and SII. Second, our study has a benefit over many earlier studies in that we investigated the predictive potency for the prognosis of mild AIS. However, our study also has some limitations that need to be considered. First of all, this analysis was performed retrospectively and without blinding, which has a chance of selection bias. Second, the absence of dynamic SIRI and SII data prevented us from making predictions that might be more accurate. Third, given the limited sample size, the results should be confirmed in future, larger populations.
Conclusion
In conclusion, the current investigation shows for the first time that mild AIS patients with poor outcomes after IVT had increased SIRI and SII. Also, SIRI was an independent predictor for unfavorable outcomes at 3 months. Our work provides an economically convenient way to refine risk stratification for adverse outcomes in mild AIS. Further large-scale prospective studies are needed for dynamically monitoring SIRI and SII to understand the value of the index better.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Minhang Hospital of Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MC and JZ designed the study. YaL and YW collected the data. MC, YuL, and DaW performed the statistical analysis and wrote the paper. DeW and JZ revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China grants/awards 82173646 and 81973157.
Acknowledgments
Special thanks to the participants involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reeves M, Khoury J, Alwell K, Moomaw C, Flaherty M, Woo D, et al. Distribution of national institutes of health stroke scale in the Cincinnati/Northern Kentucky Stroke study. Stroke. (2013) 44:3211–3. doi: 10.1161/STROKEAHA.113.002881
2. Wang Y, Li Z, Wang Y, Zhao X, Liu L, Yang X, et al. Chinese stroke center alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vascul Neurol. (2018) 3:256–62. doi: 10.1136/svn-2018-000154
3. Tang H, Yan S, Wu C, Zhang Y. Characteristics and outcomes of intravenous thrombolysis in mild ischemic stroke patients. Front Neurol. (2021) 12:744909. doi: 10.3389/fneur.2021.744909
4. Duan H, Cheng Z, Yun H, Cai L, Tong Y, Han Z, et al. Serum bilirubin associated with stroke severity and prognosis: preliminary findings on liver function after acute ischemic stroke. Neurol Res. (2022) 2022:1–8. doi: 10.1080/01616412.2022.2119724
5. Powers W, Rabinstein A, Ackerson T, Adeoye O, Bambakidis N, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
6. Romano J, Smith E, Liang L, Gardener H, Camp S, Shuey L, et al. Outcomes in mild acute ischemic stroke treated with intravenous thrombolysis: a retrospective analysis of the get with the guidelines-stroke registry. JAMA Neurol. (2015) 72:423–31. doi: 10.1001/jamaneurol.2014.4354
7. Yu A, Hill M, Coutts S. Should minor stroke patients be thrombolyzed? A focused review and future directions. Int J Stroke Off J Int Stroke Soc. (2015) 10:292–7. doi: 10.1111/ijs.12426
8. Kenmuir C, Hammer M, Jovin T, Reddy V, Wechsler L, Jadhav A. Predictors of outcome in patients presenting with acute ischemic stroke and mild stroke scale scores. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. (2015) 24:1685–9. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.042
9. Liu Y, Wu Z, Qu J, Qiu D, Luo G, Yin H, et al. High neutrophil-to-lymphocyte ratio is a predictor of poor short-term outcome in patients with mild acute ischemic stroke receiving intravenous thrombolysis. Brain Behav. (2020) 10:e01857. doi: 10.1002/brb3.1857
10. Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. (2011) 10:471–80. doi: 10.1016/S1474-4422(11)70066-7
11. Jenny N, Callas P, Judd S, McClure L, Kissela B, Zakai N, et al. Inflammatory cytokines and ischemic stroke risk: the regards cohort. Neurology. (2019) 92:e2375–e84. doi: 10.1212/WNL.0000000000007416
12. Yi HJ, Sung JH, Lee DH. Systemic inflammation response index and systemic immune-inflammation index are associated with clinical outcomes in patients treated with mechanical thrombectomy for large artery occlusion. World Neurosurg. (2021) 153:e282–e9. doi: 10.1016/j.wneu.2021.06.113
13. Huang L. Increased systemic immune-inflammation index predicts disease severity and functional outcome in acute ischemic stroke patients. Neurologist. (2022). doi: 10.1097/NRL.0000000000000464
14. Zhou Y, Zhang Y, Cui M, Zhang Y, Shang X. Prognostic value of the systemic inflammation response index in patients with acute ischemic stroke. Brain Behav. (2022) 12:e2619. doi: 10.1002/brb3.2619
15. Zhang Y, Xing Z, Zhou K, Jiang S. The predictive role of systemic inflammation response index (siri) in the prognosis of stroke patients. Clin Intervent Aging. (2021) 16:1997–2007. doi: 10.2147/CIA.S339221
16. Li J, Yuan Y, Liao X, Yu Z, Li H, Zheng J. Prognostic significance of admission systemic inflammation response index in patients with spontaneous intracerebral hemorrhage: a propensity score matching analysis. Front Neurol. (2021) 12:718032. doi: 10.3389/fneur.2021.718032
17. Dziedzic E, Gasior J, Tuzimek A, Paleczny J, Junka A, Dabrowski M, et al. Investigation of the associations of novel inflammatory biomarkers-systemic inflammatory index (Sii) and systemic inflammatory response index (Siri)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. (2022) 23:9553. doi: 10.3390/ijms23179553
18. Pacheco-Barcia V, Mondéjar Solís R, France T, Asselah J, Donnay O, Zogopoulos G, et al. A systemic inflammation response index (Siri) correlates with survival and predicts oncological outcome for mfolfirinox therapy in metastatic pancreatic cancer. Pancreatol Off J Int Assoc Pancreatol. (2020) 20:254–64. doi: 10.1016/j.pan.2019.12.010
19. Wang J, Zhang X, Tian J, Li H, Tang H, Yang C. Predictive values of systemic inflammatory responses index in early neurological deterioration in patients with acute ischemic stroke. J Integ Neurosci. (2022) 21:94. doi: 10.31083/j.jin2103094
20. Wu F, Liu Z, Zhou L, Ye D, Zhu Y, Huang K, et al. Systemic immune responses after ischemic stroke: from the center to the periphery. Front Immunol. (2022) 13:911661. doi: 10.3389/fimmu.2022.911661
21. Sadeghi F, Sarkady F, Zsóri K, Szegedi I, Orbán-Kálmándi R, Székely E, et al. High neutrophil-lymphocyte ratio and low lymphocyte-monocyte ratio combination after thrombolysis is a potential predictor of poor functional outcome of acute ischemic stroke. J Personal Med. (2022) 12:1221. doi: 10.3390/jpm12081221
22. Feng Y, Bai X, Li W, Cao W, Xu X, Yu F, et al. Postoperative neutrophil-lymphocyte ratio predicts unfavorable outcome of acute ischemic stroke patients who achieve complete reperfusion after thrombectomy. Front Immunol. (2022) 13:963111. doi: 10.3389/fimmu.2022.963111
23. Wu F, Wang Q, Qiao Y, Yu Q, Wang F. A new marker of short-term mortality and poor outcome in patients with acute ischemic stroke: mean platelet volume-to-lymphocyte ratio. Medicine. (2022) 101:e30911. doi: 10.1097/MD.0000000000030911
24. Stuckey S, Ong L, Collins-Praino L, Turner R. Neuroinflammation as a key driver of secondary neurodegeneration following stroke? Int J Mol Sci. (2021) 22:101. doi: 10.3390/ijms222313101
25. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. (2013) 13:159–75. doi: 10.1038/nri3399
26. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. (2015) 35:888–901. doi: 10.1038/jcbfm.2015.45
27. Buck BH, Liebeskind DS, Saver JL, Bang OY, Yun SW, Starkman S, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. (2008) 39:355–60. doi: 10.1161/STROKEAHA.107.490128
28. Garau A, Bertini R, Colotta F, Casilli F, Bigini P, Cagnotto A, et al. Neuroprotection with the Cxcl8 inhibitor repertaxin in transient brain ischemia. Cytokine. (2005) 30:125–31. doi: 10.1016/j.cyto.2004.12.014
29. Kaito M, Araya S, Gondo Y, Fujita M, Minato N, Nakanishi M, et al. Relevance of distinct monocyte subsets to clinical course of ischemic stroke patients. PLoS ONE. (2013) 8:e69409. doi: 10.1371/journal.pone.0069409
30. Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. (2010) 87:779–89. doi: 10.1189/jlb.1109766
31. Ray MJ, Walters DL, Bett JN, Cameron J, Wood P, Aroney CN. Platelet-monocyte aggregates predict troponin rise after percutaneous coronary intervention and are inhibited by abciximab. Int J Cardiol. (2005) 101:249–55. doi: 10.1016/j.ijcard.2004.03.033
32. Liesz A, Zhou W, Na SY, Hämmerling GJ, Garbi N, Karcher S, et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci. (2013) 33:17350–62. doi: 10.1523/JNEUROSCI.4901-12.2013
33. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (Siri) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
34. Yun S, Yi H, Lee D, Sung J. Systemic inflammation response index and systemic immune-inflammation index for predicting the prognosis of patients with aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. (2021) 30:105861. doi: 10.1016/j.jstrokecerebrovasdis.2021.105861
Keywords: systemic immune inflammation index, systemic inflammation response index, mild acute ischemic stroke, intravenous thrombolysis, 3-month outcome
Citation: Chu M, Luo Y, Wang D, Liu Y, Wang D, Wang Y and Zhao J (2023) Systemic inflammation response index predicts 3-month outcome in patients with mild acute ischemic stroke receiving intravenous thrombolysis. Front. Neurol. 14:1095668. doi: 10.3389/fneur.2023.1095668
Received: 20 November 2022; Accepted: 20 January 2023;
Published: 08 February 2023.
Edited by:
Lian Duan, Chinese PLA General Hospital, ChinaReviewed by:
En Xu, The Second Affiliated Hospital of Guangzhou Medical University, ChinaMasahiro Mishina, Tokyo Rosai Hospital, Japan
Copyright © 2023 Chu, Luo, Wang, Liu, Wang, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhao,  emhhb19qaW5nQGZ1ZGFuLmVkdS5jbg==
emhhb19qaW5nQGZ1ZGFuLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Min Chu
Min Chu Yunhe Luo
Yunhe Luo Daosheng Wang2†
Daosheng Wang2† Yang Liu
Yang Liu Yong Wang
Yong Wang Jing Zhao
Jing Zhao