
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 08 March 2023
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1094364
This article is part of the Research Topic Immune Cells in Glioma Microenvironment and Prognosis View all 5 articles
 Sunhuan Zhang1
Sunhuan Zhang1 Qunqin Ni2*
Qunqin Ni2*Background: The systemic immune-inflammation index (SII) has been recognized as the indicator that reflects the status of immune responses. The SII is related to the prognostic outcome of many malignancies, whereas its role in gliomas is controversial. For patients with glioma, we, therefore, conducted a meta-analysis to determine if the SII has a prognostic value.
Methods: Studies relevant to this topic were searched from 16 October 2022 in several databases. In patients with glioma, the relation of the SII level with the patient prognosis was analyzed based on hazard ratios (HRs) as well as corresponding 95% confidence intervals (CIs). Moreover, subgroup analysis was conducted to examine a possible heterogeneity source.
Results: There were eight articles involving 1,426 cases enrolled in the present meta-analysis. The increased SII level predicted the dismal overall survival (OS) (HR = 1.81, 95% CI = 1.55–2.12, p < 0.001) of glioma cases. Furthermore, an increased SII level also predicted the prognosis of progression-free survival (PFS) (HR = 1.87, 95% CI = 1.44–2.43, p < 0.001) in gliomas. An increased SII was significantly associated with a Ki-67 index of ≥30% (OR = 1.72, 95% CI = 1.10–2.69, p = 0.017). However, a high SII was not correlated with gender (OR = 1.05, 95% CI = 0.78–1.41, p = 0.734), KPS score (OR = 0.64, 95% CI = 0.17–2.37, p = 0.505), or symptom duration (OR 1.22, 95% CI 0.37–4.06, p = 0.745).
Conclusion: There was a significant relation between an increased SII level with poor OS and the PFS of glioma cases. Moreover, patients with glioma with a high SII value have a positive relationship with a Ki-67 of ≥30%.
Primary brain tumors in adults are gliomas, which are the most common and deadliest type of tumors (1). Glioma accounts for 75% of all malignant adult primary brain tumors, and the 5-year survival rate of glioma is <10% (2). Glioblastoma (GBM) is a frequently seen brain cancer, accounting for 57% of all gliomas and 48% of all malignant CNS tumors (3). Among the standard medical treatments for GBM, resections are followed by external beam radiation and temozolomide (TMZ), followed by maintenance chemotherapy (4). In spite of these treatments, the prognosis of adult GBM is extremely dismal, its median survival time after diagnosis is <15 months, and its 5-year survival rate is as low as 5% (5, 6). The dismal prognosis of glioma is partially due to the lack of effective prognostic biomarkers (7). Some circulating biomarkers have been investigated to predict the prognosis of glioma; however, the limited specificity and sensitivity may compromise the clinical application (7). Therefore, to improve the survival of patients with glioma, it is urgently needed to identify cost-effective and reliable prognostic markers.
The systemic immune-inflammation index (SII) represents the plain prediction biomarker for inflammation (8). It was proposed in 2014 that the SII could be used for predicting the prognosis of hepatocellular carcinoma (HCC) cases undergoing surgery (9). The SII formula is as follows: SII = platelet count × neutrophil count/lymphocyte count. It is easy to obtain the SII value from a routine laboratory test in clinical settings, and the SII is cost-effective. In the past, SII has been demonstrated to have a significant prognostic effect in a wide variety of cancers in many studies, including gastric cancer (GC) (10), cervical cancer (CC) (11), renal cell carcinoma (RCC) (12), diffuse large B-cell lymphoma (DLBCL) (13), and melanoma (14). SII has also been investigated in numerous studies to be the prognosis prediction biomarker for glioma cases; however, results have been inconsistent (15–22). For example, some researchers reported elevated SII as the important biomarker to predict the prognosis of glioma cases (17, 19, 20), whereas some other scholars found no evident relation of SII with glioma prognosis (18). These conflicting results confuse the application of SII in the clinical management of patients with glioma. Therefore, for determining whether SII could be used to predict the glioma prognosis, we collected data from the most recent studies and carried out a meta-analysis.
The present meta-analysis was performed following the instruction of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
We searched Embase, PubMed, Cochrane Library, and Web of Science databases to identify related articles using the following keywords: (systemic immune-inflammation index OR SII) AND (glioma OR glioblastoma OR medulloblastoma OR oligodendroglioma OR astrocytoma OR brain tumor). In addition, English publications were considered to be qualified. The search duration was from inception to 16 October 2022. Reference lists from related articles were also manually checked for identifying possibly eligible articles.
Studies conforming to the following criteria were enrolled: (i) the diagnosis of glioma was pathologically or histologically confirmed; (ii) the studies reported the relationship between the SII and survival in patients with glioma, regardless of whether the relationship is statistically significant; (iii) hazard ratios (HRs), as well as associated 95% confidence intervals (CIs), were available or calculable through given information; (iv) a SII threshold was used to classify cases as a high or low SII group; and (v) language of publication, i.e., English. The criteria for exclusion were as follows: (i) comments, reviews, conference abstracts, case reports, and letters; (ii) duplicate studies; (iii) non-human studies; and (iv) lack of sufficient data for analysis in studies.
Qualified articles were included by two reviewers (SZ and QN), and information was collected independently. The disagreements were resolved through discussion and consensus. Data below were recorded, including first author, country, publication year, sample number, age, gender, study duration, pathology, study design, treatment method, follow-up, SII threshold, cutoff determination method, survival, type of survival analysis, HRs, and associated 95% CIs. Overall survival (OS) was the primary outcome, whereas progression-free survival (PFS) was the secondary outcome. Study quality was evaluated by the Newcastle–Ottawa Scale (NOS) (23). It covers three domains, namely, patient screening (0–4 points), research group comparability (0–2 points), and outcome evaluation (0–3 points), yielding a total score of 0–9 points. A NOS score of ≥6 was the criterion to select high-quality articles.
Combined HRs together with associated 95% CIs of patients with glioma were computed for determining whether SII could be used to predict the prognosis of OS and PFS. Inter-study heterogeneity was assessed with Higgin's I2 statistics and Cochran's Q test. The random effects model was utilized to pool data in the case of an obvious heterogeneity (p < 0.10 or I2 > 50%); otherwise, the fixed-effects model was adopted. The potential heterogeneity source was analyzed by subgroup analysis according to a variety of factors. Moreover, this study adopted odds ratios (ORs) together with associated 95% CIs for analyzing the relation of the SII with patient clinicopathological features. By excluding any single study, sensitivity analyses were conducted to identify the source of heterogeneity and demonstrate the stability of the pooled results. Possible publication bias was analyzed using Begg's funnel plot and Egger's test. Stata software version 12.0 (Stata Corporation, College Station, TX, USA) was utilized for statistical analysis. A p < 0.05 was considered to be statistically significant.
The analysis was performed based on previously published literature and did not include personal data, so patient consent and ethical approval were waived for this study.
Initially, 173 records were identified, but only 101 studies remained after duplicate records were removed (Figure 1). Based on the title and abstract of the studies, 88 studies were excluded as they were irrelevant studies on patients without glioma or animal studies, and the full texts of 13 studies were evaluated further. In addition, five studies were discarded based on the reasons such as the nonavailability of survival information (n = 3), no analysis of the SII (n = 1), and the enrollment of overlapped patients (n = 1). As a result of this meta-analysis, we enrolled eight articles including 1,426 cases (15–22) (Figure 1).
Table 1 displays baseline study features. To be specific, articles enrolled in this meta-analysis were published between 2019 and 2022 and were all in English (15–22). A total of four studies were performed in China (15, 16, 19, 22), two studies were carried out in Turkey (17, 18), and one each in Italy (21) and Poland (20), respectively. A sample size of 77 to 358 was obtained, with 168 representing the median. All studies were of retrospective design. A total of six studies included patients with GBM (16–18, 20–22), one study recruited patients with glioma grade III–IV (15), and one study enrolled patients with medulloblastoma (MB) (19). The SII was characterized by a median value of 914.5, with the cut-off value ranging from 324.38 to 2,300. For the following subgroup analysis, we selected the cut-off value of 1,000 for the SII because this value is close to the median value and divides the included data into two equal groups (4:4). A receiver operating characteristic (ROC) curve analysis was conducted to determine the threshold of seven articles (16–22), and there was one article (15) using X-tile software. All eight studies analyzed the prognostic role of the SII for OS (15–22), and three studies reported the association between SII and PFS (17, 18, 22). The HRs and 95% CIs were provided by four studies using multivariate analysis (15, 17, 18, 22), and four studies applied univariate analysis (16, 19–21). There was a range of NOS scores from 6 to 8, suggesting that our enrolled articles were of high quality.
In each of the eight studies involving 1,426 patients, the SII was assessed for its prognostic significance of overall survival in patients with glioma (15–22). Because of insignificant heterogeneity (I2 = 17.2%, Ph = 0.295), this study adopted the fixed-effects model. The pooled results included HR = 1.81, 95% CI = 1.55–2.12, and a p < 0.001, indicating that the high SII level significantly predicted poor OS of glioma cases (Figure 2; Table 2). Furthermore, subgroup analyses were carried out based on the region, threshold level, treatment, and type of survival analysis. According to the subgroup analysis shown in Table 2, a high SII value has an effect on OS in all different subgroups of glioma cases (p < 0.05).
Association between SII with PFS in glioma was presented in three studies with 519 patients (17, 18, 22). Due to the presence of insignificant heterogeneity (I2 = 43.8%, Ph = 0.169), this study utilized the fixed-effects model (Table 2). As suggested by the combined data, an increased SII level predicted dismal PFS (HR = 1.87, 95% CI = 1.44–2.43, p < 0.001) in patients undergoing glioma (Figure 3, Table 2). Due to the limited sample size, the subgroup analysis was carried out by a cut-off value. Based on the subgroup analysis, an increased SII level significantly predicted PFS when the cut-off value of GNRI <1,000 (HR = 2.01, 95% CI = 1.53–2.65, p < 0.001) (Table 2).
The relationship between SII and clinicopathological factors of patients with glioma was reported in five studies including 871 patients (15–17, 19, 22). These clinicopathological characteristics were as follows: gender (male vs. female), Karnofsky performance score (KPS) (<80 vs. ≥80), symptom duration (≥3 vs. < 3 months), and Ki-67 (≥30 vs. < 30%). Based on the combined results, an increased SII level was remarkably related to Ki-67 ≥30% (OR = 1.72, 95%CI = 1.10–2.69, p = 0.017) (Table 3). Nonetheless, SII was not related to sex (OR = 1.05, 95%CI = 0.78–1.41, p = 0.734), KPS (OR = 0.64, 95%CI = 0.17–2.37, p = 0.505), or symptom duration (OR = 1.22, 95%CI = 0.37–4.06, p = 0.745) (Table 3).
In order to identify the source of heterogeneity and to document the stability of our results, we performed a sensitivity analysis by omitting one study at a time. As shown in Figure 4, following the elimination of the included studies, the pooled HRs did not significantly change, indicating that the results of our study were stable.
Egger's test and Begg's test were adopted to analyze publication bias in the present meta-analysis. For OS, the p-value of Begg's test is 1, and the p-value is 0.776 for Egger's test. For PFS, the results included a p-value of 0.296 for Begg's test and a p-value of 0.448 for Egger's test. As shown in Figure 5, the funnel plots are asymmetric, and obvious publication bias was detected for OS or PFS.
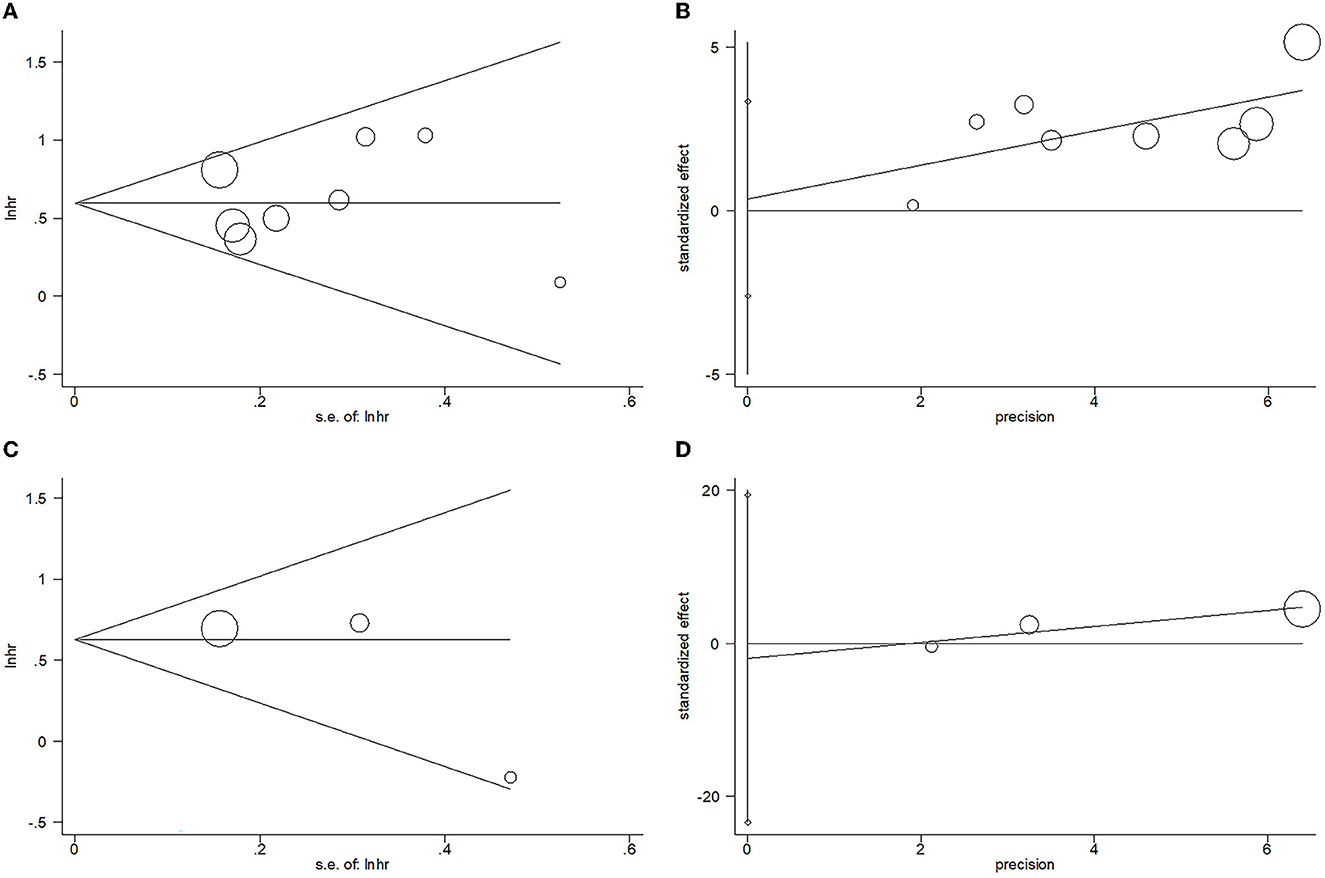
Figure 5. Results of the analysis of publication bias. (A) OS, Begg's test, p = 1; (B) OS, Egger's test, p = 0.776; (C) PFS, Begg's test, p = 0.296; and (D) PFS, Egger's test, p = 0.448.
In previous studies, the SII was explored as the factor to predict the patient prognosis; however, the results were conflicting (15–22). In order to accurately clarify this issue, the data from eight studies comprising 1,426 patients were pooled in this meta-analysis. According to our results, both OS and PFS of patients with glioma were significantly affected by an elevated SII value. Subgroup analyses verified that the SII could be used in predicting the prognosis of glioma. Furthermore, we also identified a positive relationship between SII and Ki-67 ≥30% in patients with glioma. Overall, this meta-analysis indicated the significant relation between SII and OS/PFS of glioma cases. Patients with glioma with high SII may suffer from aggressive cancer of high proliferation. As far as we know, the present study is the first to explore the prognostic and clinicopathological value of SII in glioma.
Increasing evidence suggests that inflammation and immune responses may promote tumor progression by creating conditions that facilitate metastasis within the tumor microenvironment (24, 25). Hematological parameters based on immune cells are easily available for survival prognostications in patients with cancer (26, 27). The SII is calculated by platelet count × neutrophil count/lymphocyte count. Based on the earlier equation, an increased platelet count, an increased neutrophil count, and/or a decreased lymphocyte count could result in an elevated SII. Although the precise mechanisms of SII's prognostic role in glioma are unclear, they can be explained by the following. First, platelets can protect tumor cells from cytolysis and can promote metastasis (28). In addition, platelets can promote tumor progression through platelet factor and vascular endothelial growth factor (VEGF), together with platelet-derived growth factor (PDGF) (29). Platelets can also secrete inflammatory cytokines such as interleukin (IL)-6 and TNF-α to facilitate tumor cell metastases (30). Second, neutrophils have significant tumor-promoting activity. Neutrophils can induce myeloid-derived suppressor cells (MDSCs), and MDSCs are myeloid cells that produce reactive oxygen species and arginase to suppress T lymphocyte activation. Therefore, neutrophils can educate an immunosuppressive activity in the tumor microenvironment. Third, lymphocytes, especially tumor-infiltrating T cells, have an important effect on T- cell-dependent anticancer response. Tumor-infiltrating lymphocytes (TILs) can secrete IL-4, IL-5, and the tumor necrosis factor to regulate the angiogenesis, proliferation, apoptosis, and metastasis of tumor cells. In addition, lymphocyte infiltration in tumors is related to the superior prognosis of patients with cancer (31). Moreover, in a recent quantitative digital analysis, the researchers reported that high expressions of CD8+ and CD163+ cells were significantly connected with inferior survival in patients with GBM (32). According to their observations, a direct correlation was found between CD163 values and CD8 or PDL1/PD1 infiltration in patients with GBM (32). Therefore, the high SII value can be adopted for predicting the dismal prognosis of glioma cases. As reported in this meta-analysis, a high SII value was significantly associated with poor OS and PFS in patients with glioma. Therefore, in clinical settings, patients with glioma with a pretreatment SII value of >1000 may have a high risk of poor survival and disease progression. Notably, the results of included studies differed, which may be caused by selection bias because the eligible studies enrolled patients by different selection criteria (15–18).
The SII is widely suggested to have an essential function in different cancer types by meta-analyses in recent years (33–36). As suggested by Li et al., an increased SII level before treatment predicted low survival in HCC cases after transcatheter arterial chemoembolization in the meta-analysis enrolling 3,557 cases (35). Huang et al. showed with cases of non-small cell lung cancer (NSCLC) who showed an increased SII level had a poor OS and a poor PFS based on their meta-analysis of 17 enrolled studies (36); moreover, they had a higher pathological stage (II–III) relative to patients with normal SII levels (36). According to a recent meta-analysis involving 2,642 patients, breast cancer (BC) cases who had increased SII levels were associated with poor OS, recurrence-free survival (RFS)/disease-free survival (DFS), and dismal distant metastasis-free survival (DMFS) than those with a low SII (37). Head and neck cancer cases who had an increased SII level before treatment were associated with poor OS, DFS, and patient-reported survival in a meta-analysis of 12 studies (33). As a result of a meta-analysis of 1,402 patients with cholangiocarcinoma undergoing invasive surgery, Liu et al. found that an increased SII level independently predicted dismal OS (34). Our findings in the current meta-analysis conformed to SII's role in predicting other cancer prognoses (33–36).
As a meta-analysis, certain limitations should be noted in the present study. First, the enrolled articles were retrospective, which could cause inherent heterogeneity. Second, the total number of studies included was relatively small. There were eight studies involved in this meta-analysis, and only 1,426 patients were recruited. Therefore, selection bias may exist. Third, study cut-off values for the SII were not uniform, which may have affected the combined HRs and 95% CIs. For subgroup analysis, we selected a cut-off value of SII = 1,000 because this value is close to the median value and divides the included data into two equal groups (4:4). The subgroup analysis indicated that high SII was significantly associated with worse OS irrespective of SII cut-off values (Table 2). In addition, an elevated SII value was correlated with poor PFS when the cut-off value is <1,000 (Table 2). Therefore, further prospective studies with a large sample size should be conducted for validating our meta-analysis results.
The overall conclusion of the present meta-analysis was that an increased SII level was related to worse OS and PFS after glioma. Moreover, patients with glioma with high SII have a positive relationship with a Ki-67 of ≥30%.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the article and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
HR, hazard ratio; CI, confidence interval; OS, overall survival; PFS, progression-free survival; SII, systemic immune-inflammation index; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle–Ottawa Scale; OR, odds ratio.
1. Qi Y, Liu B, Sun Q, Xiong X, Chen Q. Immune checkpoint targeted therapy in glioma: status and hopes. Front Immunol. (2020) 11:578877. doi: 10.3389/fimmu.2020.578877
2. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. (2018) 392:432–46. doi: 10.1016/S0140-6736(18)30990-5
3. Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: state of the art and future directions. CA Cancer J Clin. (2020) 70:299–312. doi: 10.3322/caac.21613
4. Bush NA, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. (2017) 40:1–14. doi: 10.1007/s10143-016-0709-8
5. Alexander BM, Cloughesy TF. Adult glioblastoma. Am J Clin Oncol. (2017) 35:2402–9. doi: 10.1200/JCO.2017.73.0119
6. Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. (2010) 60:166–93. doi: 10.3322/caac.20069
7. Müller Bark J, Kulasinghe A, Chua B, Day BW, Punyadeera C. Circulating biomarkers in patients with glioblastoma. Br J Cancer. (2020) 122:295–305. doi: 10.1038/s41416-019-0603-6
8. Engin M, Aydin U, Caran Karaoglu EH, Deveci G, Ata Y. A simple predictive marker of inflammation: systemic immune-inflammation index. J Artif Organs. (2022). doi: 10.1007/s10047-022-01361-0
9. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
10. He K, Si L, Pan X, Sun L, Wang Y, Lu J, et al. Preoperative systemic immune-inflammation index (SII) as a superior predictor of long-term survival outcome in patients with stage I-II gastric cancer after radical surgery. Front Oncol. (2022) 12:829689. doi: 10.3389/fonc.2022.829689
11. Liu P, Jiang Y, Zheng X, Pan B, Xiang H, Zheng M. Pretreatment systemic immune-inflammation index can predict response to neoadjuvant chemotherapy in cervical cancer at stages IB2-IIB. Pathol Oncol Res. (2022) 28:1610294. doi: 10.3389/pore.2022.1610294
12. Stühler V, Herrmann L, Rausch S, Stenzl A, Bedke J. Role of the systemic immune-inflammation index in patients with metastatic renal cell carcinoma treated with first-line ipilimumab plus nivolumab. Cancers (Basel). (2022) 14:12. doi: 10.3390/cancers14122972
13. Wu J, Zhu H, Zhang Q, Sun Y, He X, Liao J, et al. Nomogram based on the systemic immune-inflammation index for predicting the prognosis of diffuse large B-cell lymphoma. Asia Pac J Clin Oncol. (2022). doi: 10.1111/ajco.13806
14. Susok L, Said S, Reinert D, Mansour R, Scheel CH, Becker JC, et al. The pan-immune-inflammation value and systemic immune-inflammation index in advanced melanoma patients under immunotherapy. J Cancer Res Clin Oncol. (2022) 148:3103–8. doi: 10.1007/s00432-021-03878-y
15. Liang R, Li J, Tang X, Liu Y. The prognostic role of preoperative systemic immune-inflammation index and albumin/globulin ratio in patients with newly diagnosed high-grade glioma. Clin Neurol Neurosurg. (2019) 184:105397. doi: 10.1016/j.clineuro.2019.105397
16. Lv Y, Zhang S, Liu Z, Tian Y, Liang N, Zhang J. Prognostic value of preoperative neutrophil to lymphocyte ratio is superior to systemic immune inflammation index for survival in patients with Glioblastoma. Clin Neurol Neurosurg. (2019) 181:24–7. doi: 10.1016/j.clineuro.2019.03.017
17. Topkan E, Besen AA, Ozdemir Y, Kucuk A, Mertsoylu H, Pehlivan B, et al. Prognostic value of pretreatment systemic immune-inflammation index in glioblastoma multiforme patients undergoing postneurosurgical radiotherapy plus concurrent and adjuvant temozolomide. Mediators Inflamm. (2020) 2020:4392189. doi: 10.1155/2020/4392189
18. Yilmaz H, Nigdelioglu B, Oktay E, Meydan N. Clinical significance of postoperatif controlling nutritional status (CONUT) score in glioblastoma multiforme. J Clin Neurosci. (2021) 86:260–6. doi: 10.1016/j.jocn.2021.01.036
19. Zhu S, Cheng Z, Hu Y, Chen Z, Zhang J, Ke C, et al. Prognostic value of the systemic immune-inflammation index and prognostic nutritional index in patients with medulloblastoma undergoing surgical resection. Front Nutr. (2021) 8:754958. doi: 10.3389/fnut.2021.754958
20. Jarmuzek P, Kot M, Defort P, Stawicki J, Komorzycka J, Nowak K, et al. Prognostic values of combined ratios of white blood cells in glioblastoma: a retrospective study. J Clin Med. (2022) 11:3397. doi: 10.3390/jcm11123397
21. Pasqualetti F, Giampietro C, Montemurro N, Giannini N, Gadducci G, Orlandi P, et al. Old and new systemic immune-inflammation indexes are associated with overall survival of glioblastoma patients treated with radio-chemotherapy. Genes (Basel). (2022) 13:1054. doi: 10.3390/genes13061054
22. Shi X, Li H, Xu Y, Nyalali AMK Li F. The prognostic value of the preoperative inflammatory index on the survival of glioblastoma patients. Neurol Sci. (2022) 43:5523–31. doi: 10.1007/s10072-022-06158-w
23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
24. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. (2011) 331:1565–70. doi: 10.1126/science.1203486
25. Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. (2015) 15:73–86. doi: 10.1038/nri3789
26. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:dju124. doi: 10.1093/jnci/dju124
27. Bruni D, Angell HK, Galon J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. (2020) 20:662–80. doi: 10.1038/s41568-020-0285-7
28. Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. (1999) 59:1295–300.
29. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. (2011) 11:123–34. doi: 10.1038/nrc3004
30. Tesfamariam B. Involvement of platelets in tumor cell metastasis. Pharmacol Ther. (2016) 157:112–9. doi: 10.1016/j.pharmthera.2015.11.005
31. Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. (2014) 110:2560–8. doi: 10.1038/bjc.2014.162
32. Idoate Gastearena MA, López-Janeiro Á, Lecumberri Aznarez A, Arana-Iñiguez I, Guillén-Grima F. A quantitative digital analysis of tissue immune components reveals an immunosuppressive and anergic immune response with relevant prognostic significance in glioblastoma. Biomedicines. (2022) 10:1753. doi: 10.3390/biomedicines10071753
33. Wang YT, Kuo LT, Weng HH, Hsu CM, Tsai MS, Chang GH, et al. Systemic immune-inflammation index as a predictor for head and neck cancer prognosis: a meta-analysis. Front Oncol. (2022) 12:899518. doi: 10.3389/fonc.2022.899518
34. Liu XC, Jiang YP, Sun XG, Zhao JJ, Zhang LY, Jing X. Prognostic significance of the systemic immune-inflammation index in patients with cholangiocarcinoma: a meta-analysis. Front Oncol. (2022) 12:938549. doi: 10.3389/fonc.2022.938549
35. Li D, Zhao X, Pi X, Wang K, Song D. Systemic immune-inflammation index and the survival of hepatocellular carcinoma patients after transarterial chemoembolization: a meta-analysis. Clin Exp Med. (2022). doi: 10.1007/s10238-022-00889-y
36. Huang W, Luo J, Wen J, Jiang M. The relationship between systemic immune inflammatory index and prognosis of patients with non-small cell lung cancer: a meta-analysis and systematic review. Front Surg. (2022) 9:898304. doi: 10.3389/fsurg.2022.898304
Keywords: systemic immune-inflammation index, glioma, prognosis, meta-analysis, immune responses
Citation: Zhang S and Ni Q (2023) Prognostic role of the pretreatment systemic immune-inflammation index in patients with glioma: A meta-analysis. Front. Neurol. 14:1094364. doi: 10.3389/fneur.2023.1094364
Received: 10 November 2022; Accepted: 09 February 2023;
Published: 08 March 2023.
Edited by:
Song Han, Sanbo Brain Hospital, Capital Medical University, ChinaCopyright © 2023 Zhang and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qunqin Ni, bnFxODEyMkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.