- Department of Rehabilitation Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Brachial plexus injury (BPI) is one of the most serious peripheral nerve injuries, resulting in severe and persistent impairments of the upper limb and disability in adults and children alike. With the relatively mature early diagnosis and surgical technique of brachial plexus injury, the demand for rehabilitation treatment after brachial plexus injury is gradually increasing. Rehabilitation intervention can be beneficial to some extent during all stages of recovery, including the spontaneous recovery period, the postoperative period, and the sequelae period. However, due to the complex composition of the brachial plexus, location of injury, and the different causes, the treatment varies. A clear rehabilitation process has not been developed yet. Rehabilitation therapy that has been widely studied focusing on exercise therapy, sensory training, neuroelectromagnetic stimulation, neurotrophic factors, acupuncture and massage therapy, etc., while interventions like hydrotherapy, phototherapy, and neural stem cell therapy are less studied. In addition, rehabilitation methods in some special condition and group often neglected, such as postoperative edema, pain, and neonates. The purpose of this article is to explore the potential contributions of various methods to brachial plexus injury rehabilitation and to provide a concise overview of the interventions that have been shown to be beneficial. The key contribution of this article is to form relatively clear rehabilitation processes based on different periods and populations, which provides an important reference for the treatment of brachial plexus injuries.
1. Introduction
Brachial plexus injury (BPI) is one of the most serious peripheral nerve injuries, with severe physical disabilities and long-term financial and psychological consequences. Epidemiological studies conducted in both developing and developed countries have shown that brachial plexus injuries are very common in productive young men. Closed injury is the most common type of injury, caused primarily by strains, fractures, and compression injuries, medical injuries are also quite common (1). When a newborn sustains a brachial plexus injury, it is referred to as a neonatal brachial plexus injury (NBPI), which is caused primarily by a birth incident. The brachial plexus nerve is a complex neural component that arises from the anterior branches of the cervical (C5-8) and thoracic (T1) segments of the spinal cord and creates an interlocking network that innervates the muscles of the upper extremities and transmits cutaneous sensation. It is challenging to pinpoint the damage precisely because of the intricate structure of brachial plexus nerve. Surgery and neurological rehabilitation employ distinct methods and struggle to provide consistent outcomes. Myelography methods and neurophysiological exams have steadily evolved in recent years. Simultaneously, surgical procedures such as nerve repair and grafting have increasingly matured. There is currently no comprehensive rehabilitation program available for individuals with various degrees of brachial plexus injury. To create a more comprehensive treatment plan, we will incorporate the most relevant and successful rehabilitation therapy approaches for various timeframes. To serve as a reference for the clinical management of brachial plexus injury, we will also briefly discuss the fundamental rehabilitation treatments for each period.
2. Brachial plexus injury: Neural localization and clinical symptoms
Brachial plexus injuries (Figure 1) fall under many categories. However, functional placement is more advised since sensory-motor reconstruction and muscle balance restoration are the main goals of therapy after brachial plexus injury. When the affected nerve is C5 and C6, it is categorized as “superior trunk dissection,” which is characterized by impaired shoulder and elbow flexion, abduction, and external rotation while hand function is preserved, and is also known as Duchenne-Erb syndrome. When the involved nerve is C7, it is categorized as “middle trunk dissection.” When the involved nerve is C8 and T1, it is categorized as “inferior trunk dissection,” which is characterized by the impaired hand and wrist function, also known as Dejerine-Klumpke syndrome. Complete brachial plexus palsy (C5-T1) affects the function of the entire arm, resulting in a fully flaccid arm. When this condition is coupled with an eye injury, it is referred to as Horner’s syndrome.
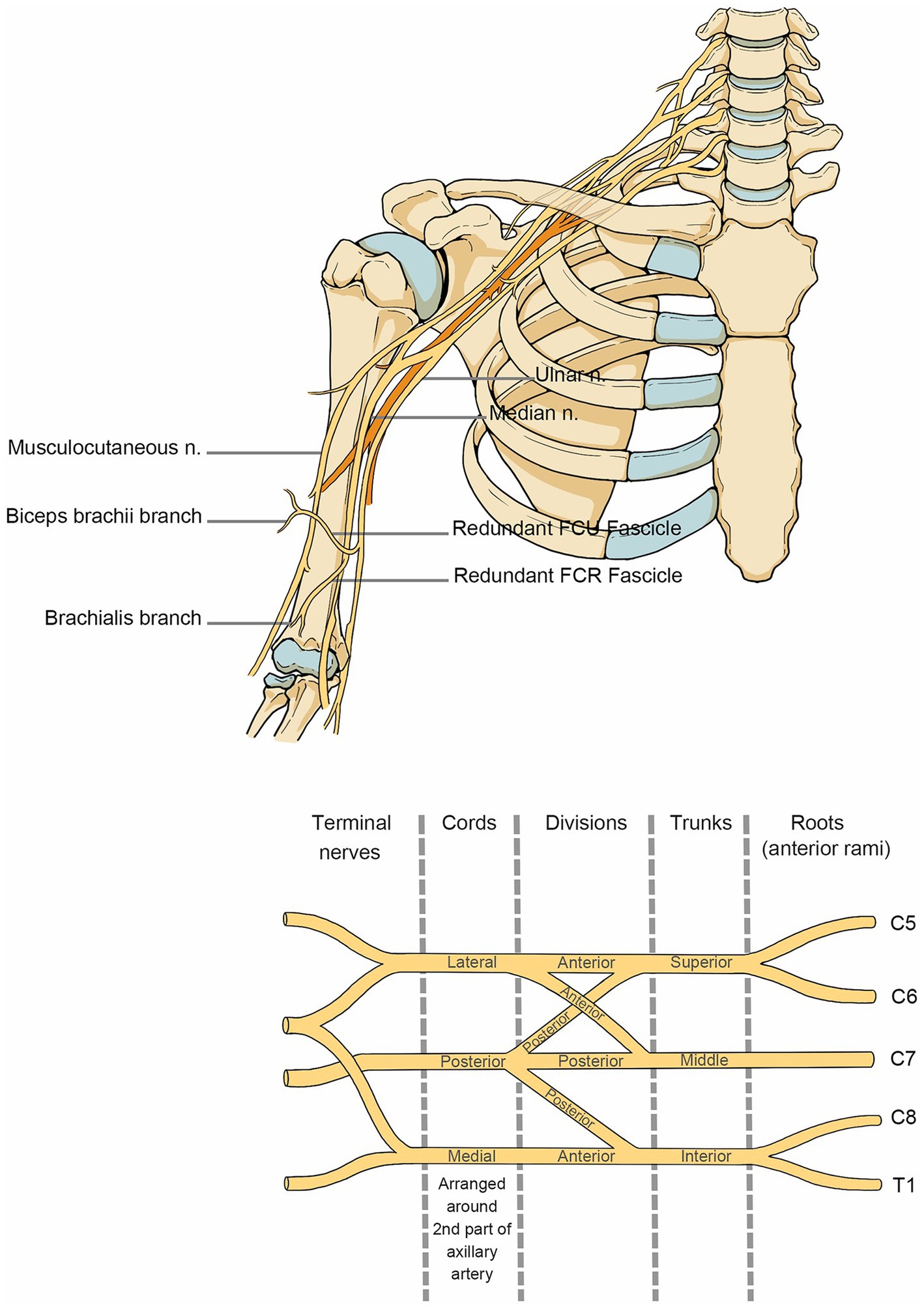
Figure 1. Anatomy of the brachial plexus nerve. The anatomical course of the brachial plexus nerve in the shoulder, neck and upper arm.
3. Adult brachial plexus injury rehabilitation
The management of BPI depends on the type, location, and severity of the injury. It is usually determined by magnetic resonance imaging and/or computed tomography myelography. Surgeons now agree that the best time for surgery is 3–6 months after injury; traction injuries usually heal spontaneously within 3–6 months, and surgical exploration may cause secondary injury, so surgical intervention is recommended if nerve function has not recovered by the end of the spontaneous recovery period (2). Complete tears, ruptures, and lacerations typically do not recover within this time range; hence, early surgical intervention is advised in place of this. Unsurprisingly, rehabilitation, including neurotrophic therapy, is the best course of action before surgery. Following surgery, rehabilitation treatment can aid in the restoration of neurological function. Early intervention for rehabilitation is necessary since brachial plexus injury, with or without surgery, since BPI will cause some degree of muscle loss in the upper extremity and thus can inevitably culminate in fast muscular atrophy. Promoting nerve regeneration and functional recovery while safeguarding and regaining function in joints and intact muscles is the main objective of brachial plexus injury rehabilitation. Based on this premise, subsequent abnormalities such as muscle atrophy, joint stiffness, and limited range of motion are all minimized to the greatest extent feasible.
3.1. Rehabilitation in the spontaneous recovery period
Axonal growth occurs at a pace of approximately 1 mm every day. The basic objectives of the spontaneous recovery period following brachial plexus injury are to ensure adequate muscle nutrition, preserve muscle function, and promote nerve regeneration until the nerve reaches the periphery because it takes a long time for nerves to reinnervate.
3.1.1. Pain management
Pain is present at all stages of brachial plexus injury, including acute neuromuscular injury pain, postoperative pain in the operative area, and chronic neuropathic pain, and it significantly reduces patients’ rehabilitation compliance, mental health, and quality of life. It should be addressed as soon as it is identified. A review by Tom et al. for the functional assessment of brachial plexus injury recommends the use of the Patient Reported Outcome Measure (PROM) preferably for those patients who can report pain autonomously, and the commonly used Visual Analog Scale (VAS) and Numeric Rating Scale (NRS) are also recommended (3). However, there is no efficacy analysis of different pain assessment scales for brachial plexus injury. The various types of pharmaceuticals listed in the overview of pain management after complicated nerve injury (4), such as central analgesics, psychiatric medications, narcotics, and hormones, can be used by both surgical and non-surgical patients. Oral and local injections are just two examples of administration methods. Given that pain is subjective and frequently accompanied by psychological illnesses, psychotherapy, and psychotropic medications may occasionally provide further advantages with lingering effects (5). Yun et al. have demonstrated that continuous interscalene brachial plexus block (CISB) has better effective analgesia, better sleep quality and causes fewer opioid-related complications than single interscalene brachial plexus block (SISB) (6). There have not been many studies comparing the efficacy of various drugs and administration methods. Psychotherapy, along with physiotherapy, exercise therapy, invasive surgery, and others, is one of the non-pharmacological strategies that, when combined with pharmacotherapy, form a comprehensive pain treatment. It is critical to understand that medication is not a substitute for non-drug approaches. Furthermore, while chronic neuropathologic pain is commonly challenging to manage with medication, it can still be used to provide short-term relief in acute nerve injury pain or postoperative pain. Neurostimulation has become increasingly effective for chronic neuropathic pain in recent years, in addition to surgical treatments such as nerve transfer and neuroma excision. Thalamic DBS, for example, has proven to be an effective treatment option for patients suffering from severe and medically incurable pain (7). However, studies on this kind of neural stimulation typically do not include a lot of instances, and there is not a widely accepted standard stimulation paradigm due to the heterogeneity of different stimulation parameters. Future research should be expanded upon, it is advised.
3.1.2. Motor reeducation
Strong external fixation support of the damaged limb is necessary before commencing activities. When shoulder and elbow movements are compromised, shoulder straps and figure-eight bandages can be utilized to restrict the uncontrolled movement of paralyzed joints. When the wrist joint is affected, a splint can be utilized to maintain the wrist in an extended position for 10–20 degrees, assisting in the prevention of joint contracture and easing the patient’s pain (8). The advantages of various types of external fixation have not been studied; thus, the choice may be determined based on the medical profile and tolerance level of the patient.
There is a large amount of literature on motor re-education after brachial plexus injury. Typical therapies include passive range of motion, active-assisted range of motion, active range of motion, and strength, according to a systematic review based on proprioceptive neuromuscular facilitation (PNF) theory (9). This is a progressive procedure that might start with a determination of the strength of the muscles in the upper extremities and the choice of an adequate range-of-motion recovery based on strength grading. The therapeutic concepts indicated in various treatment reports are summarized as follows (10, 11): When the patient has no muscular contraction, a passive range of motion is employed. When the patient maintains sufficient muscular contraction, an active-assisted range of motion is employed. As muscular strength grows, the therapist’s function in joint range of motion is eventually superseded by the patient, and resistance rises.
In comparison to simulative activities or pointless exercise, a systematic study revealed that involvement in purposeful activities for upper extremity motor therapy regularly leads to a greater quantity and quality of muscle building (12). Occupational therapy is frequently utilized after brachial plexus injuries and is based on deliberate and targeted occupational exercises to enhance the patient’s capacity to adapt to daily life and expedite the patient’s return to family and society. A prospective longitudinal study confirmed that postoperative specialist occupational therapy increased motor recovery and upper extremity function in patients following BPI, involving occupational therapy such as folding towels, drinking water, lifting plastic bags, cutting with utensils, etc. (13). The profession of occupational therapy for patients with brachial plexus injuries is not standardized. Nonetheless, it would be beneficial to design more occupational forms that incorporate bending the elbow, bending the shoulder, and extending the wrist, as many doctors expect, which will help the patient regain their capacity to live independently (14). Furthermore, the therapist presses and pulls on the affected limb, or the patient voluntarily touches the object, providing some tactile stimulation that contributes to activity-based sensory recovery. Better outcomes will come from including exposure to different objects at various temperatures during the movement treatment.
3.1.3. Sensory reeducation
Brachial plexus injury can cause sensory deficiencies in the upper extremities, including sensory anomalies and hypoesthesia. Standardized tests can evaluate sensory thresholds and functions in both children and adults including the two-point discrimination test, skin pressure thresholds, vibration perception, and assessments of pain and tactile sensations using needles and cotton (15). Patrick et al. summarized seven rehabilitation techniques for peripheral nerve injuries based on the theory of cortical remodeling. The sensory reeducation component comprises traditional sensory reeducation, activity-based sensory reeducation, mirror visual feedback, cross-modal sensory substitution, and selective deafferentation (16). The above was a description of activity-based sensory re-education. Selective deafferentation is not recommended because it could affect the sensory input of the healthy side.
Traditional sensory re-education involves reestablishing impaired afferent sensory pathways through interactions with the environment to subsequently restore more discriminative senses. The European Consensus on Sensory Retraining in Peripheral Hand Neuropathy provides a systematic strategy to promote reeducation of tactile cognition by interacting with different textures, temperatures, forms, and objects (17). Although sensory abnormalities following brachial plexus injury are not just present in the hand, it is still feasible to employ the traditional methods of sensory re-education mentioned above. Traditional sensory re-education has mostly been proven to be successful as a participating component in comprehensive rehabilitation treatment, but there are unfortunately no clinical investigations on its effectiveness with sufficient sample numbers.
Mirror visual feedback (MVF) is the process of superimposing the motion picture of the healthy hand on the location of the affected hand using a mirror that has been placed vertically on a table. This creates a motor-sensory connection between the hands (18). Such visual input promotes upper extremity mobility and sensory rehabilitation in children with Duchenne-Erb syndrome, according to a randomized controlled trial in children ages 6–12 years (19). It is possible to treat adults with the same technique. However, a randomized controlled trial with insufficient information on sensory or motor recovery found that MVF combined with TDCS over 3 months may lessen neuropathic pain after brachial plexus injury (20). Chen et al. demonstrated the effectiveness of MVF in sensory and motor reeducation, observing improved learning in hand and finger dexterity compared to traditional sensory. Additionally, patients who used MVF demonstrated increased activation in ipsilateral brain areas and the multimodal association cortex on fMRI (21). Unfortunately, Chen only examined 6 cases, which is not representative, and it is advised that future research include more cases and additional follow-up. MVF is a potential therapy for sensory re-education with brachial plexus injury in adults, although its efficacy has not been explicitly validated and requires additional investigation.
Cross-modal sensory replacement translates tactile information across several modalities using sensing technologies (22). Patients must acquire extra sensor systems, which are not commonly employed in the treatment of brachial plexus injuries. It appears to be a possibility to explore persistent sensory abnormalities with brachial plexus injury. We are delighted to see sensing technologies advance and become more widely used.
3.1.4. Neurotrophic treatment
Once brachial plexus injury has been diagnosed, neurotrophic treatment will be used continually and regularly. B vitamins, mecobalamin (23), citicoline, and other pharmaceuticals that are routinely used in clinical practice are the principal neurotrophic medications. Exogenous neurotrophic factors are also a research hotspot in animal studies due to their extensive effectiveness, low risk of side effects, and high level of safety. It has been demonstrated that topical anti-NGF therapy may reduce chronic neuropathic pain, whereas early topical administration of NGF and CNTF-like medicines may assist in preventing the degeneration of injured nerves (24). Regrettably, there have been fewer exogenous neurotrophic factor clinical medication studies overall, and only enkephalin has received marketing approval. A more comprehensive list of neurotrophic factors with potential neuroprotective effects on motor neurons has been published in research and may be a viable pharmaceutical therapy for brachial plexus nerve injury (25). Furthermore, additional clinical investigations are needed to prove the effectiveness of medications such as gangliosides, hormones, Chinese herbs, etc.
3.1.5. Modalities
Numerous studies have demonstrated the effectiveness of early modalities in reducing swelling, promoting edema absorption, relieving pain, and releasing adhesions. This therapy can be applied in a variety of settings, including ultrashort wave, infrared, and low-energy laser irradiation (15).
Electrical nerve stimulation promotes nerve healing and regeneration and is a useful tool. Depending on the stimulation site, neural electrical stimulation can be classified as deep brain stimulation (DBS), transcutaneous neuromuscular electrical stimulation (NMES), spinal cord stimulation, and transcranial direct current stimulation (tDCS) (26). Neuromuscular electrical stimulation, which is frequently utilized for motor and sensory reeducation in the treatment of children and adults with brachial plexus injuries, has been demonstrated to excite both motor nerve fibers and afferent sensory nerve fibers (27). In contrast, DBS, TDCS, and spinal cord stimulation are more commonly applied to alleviate neuropathic pain. The stimulation techniques mentioned above may need to rely on relatively unharmed nerve-down routes to effectively stimulate peripheral nerves, particularly in individuals with diminished fine hand function following brachial plexus injury. Given the aforementioned, there is a huge market and development potential for an implanted peripheral nerve stimulator that could work directly on nerve branches in the axilla while also providing long-term relief from peripheral neuropathic pain following brachial plexus injury (28).
In addition, transcranial magnetic stimulation (TMS) is often used to collect motor-evoked potentials to assess neurological activity or to treat neuropathic pain as a complement to Noninvasive electrical brain stimulation (29). It is worth mentioning that the strong magnetic field generated by TMS can be employed for peripheral neuromuscular regulation in addition to agitating the central nervous system. Consequently, TMS has the potential to replace transcutaneous neuromuscular electrical stimulation with a technique that has a higher safety profile, and it may also represent the future of neuromagnetic stimulation research.
The benefit of neuro-electromagnetic stimulation in the treatment of neuropathic pain, which may be regularly utilized in pain management, should not be overlooked. However, further clinical investigations and systematic assessment research are required to better understand the suggested paradigms due to the diversity of stimulation paradigms resulting in varying effectiveness.
3.1.6. Traditional Chinese medicine treatment
Due to their capacity to stimulate the repair and regeneration of injured nerves, enhance local blood circulation in the treated area, and lessen postoperative pain, acupuncture and tui na are widely used in traditional Chinese medicine to treat brachial plexus nerve injury. Whereas using tui-na or acupuncture alone is preferable to using medication isolated, the combined rehabilitation impact of both with other treatment modalities is noticeably superior to using tui-na or acupuncture alone. Direct comparisons of various tui na massage or acupuncture point therapies are also scarce. Electroacupuncture was initially shown to be more successful than medication therapy alone in treating 54 individuals with peripheral nerve damage in 1995 (30). A further animal investigation revealed that electroacupuncture therapy may protect against brachial plexus injury by lowering the expression of nNOS and slowing the degeneration of injured neurons (31). A series of case reports that were conducted afterward also supported this finding, making the efficacy more solid. Electroacupuncture can be suggested as a conservative treatment for brachial plexus injury in the absence of pertinent contraindications.
3.1.7. Psychosocial support
Emotional and social factors may influence rehabilitation after brachial plexus injury, particularly in a productive young male population. Their high expectations of efficacy lead to an inability to accept the possibility of permanent disability and the long recovery time required for neurological recovery, making them prone to psychological disengagement, denial, and venting behaviors in the later stages of treatment, and highly cooperative in the earlier stages. As a consequence, it is essential to regularly monitor patients’ adherence to treatment during the later phases of recovery, prevent dangerous behaviors, and promptly offer psychosocial assistance such as psychotherapy, psychiatric medication, and family support. For the time being, there is no systematic psychological rehabilitation strategy for brachial plexus injury, and few studies on psychological rehabilitation have been conducted. Studies on psychological aspects in brachial plexus injury patients have demonstrated that these individuals typically employ adaptive coping strategies and self-distraction in the early phases of their recovery to deal with negative psychology (32). Therefore, we investigated pertinent psychological rehabilitation models and came to the conclusion that active positive mindfulness therapy, such as meditation, body scanning, and other forms of active positive mindfulness, can support the above-mentioned positive psychological strategies and lead to a positive psychological state, which reduces stress and eradicates depression. Future research on the effects of various social support measures on the functional rehabilitation and psychological condition of brachial plexus injury patients is advised.
3.2. Rehabilitation in the acute postoperative period
Before starting a structured rehabilitation program after surgery, the muscles and nerves must have had enough time to recover. Controlling pain and edema in the acute postoperative period is the main therapy objective (9). Postoperative patients with complaints of pain might involed pain management because quick pain relief can enhance the patient’s psychological circumstance and speed up recovery. Potential pain relief options include opioids, topical analgesic patches, antiepileptic drugs, antidepressants, and nerve electrical stimulation, according to a systematic review of pain management for complicated nerve injuries (4). There is limited graded pain evaluation after brachial plexus injury, resulting in a wide range of pain management therapy options. Procedures for recommended pain rehabilitation management are not arranged in a hierarchical order. It is critical to highlight that pain control must be utilized throughout the brachial plexus injury treatment procedure.
Additionally, there is general agreement to start early passive mobility of the proximal and distal joints of the trauma and to locally immobilize the nerve anastomosis site in the acute postoperative phase. The purpose is to safeguard the surgical site, promote nerve gliding, and eventually reduce pain and encourage functional recovery. The best period for postoperative fixation is not universally agreed upon; however, it is crucial to hold off until the nerve anastomosis site is tension-free, particularly after repairing a nerve with significant local tension. Based on randomized controlled research of comprehensive postoperative therapy in patients with brachial plexus injuries, the passive motion of the joint within an unrestricted range of motion is advised and needs to be combined flexibly depending on the type of surgery. In conclusion, it is critical to consider the functional recovery of the primary injury site as well as the remaining neuromuscular functional compensation after a surgical transfer, such as after a phrenic nerve transfer to the anterior branch of the superior brachial plexus, which requires both flexions of the elbow joint and deep inspiration (33). Numerous randomized controlled studies have advised thorough rehabilitation following surgery using therapeutic modalities that do not significantly diverge from those performed in spontaneous recovery (34).
3.3. Rehabilitation in the sequela period
Even with complete rehabilitation and satisfactory neurological recovery, associated complications, including pain, muscle atrophy, restricted joint mobility, and subsequent abnormalities, are still possible (15). Considering that complete alleviation of brachial plexus injury is challenging with rehabilitation alone in the sequela period, surgical procedures such as neuroma removal to reduce pain and latissimus dorsi tendon grafting to the rotator cuff to address shoulder mobility limitations are typically employed (35). Postoperative rehabilitation strategies for sequelae are not contradictory and may be adapted to acute postoperative rehabilitation for brachial plexus injuries. The distinction is that patients need to be reevaluated for postoperative immobilization duration and joint range of motion since they are generally related to a loss in muscle performance and joint function. Regrettably, there is no clinical consensus on these specifics (36). Treatment methods may rely on the expertise of experienced physicians and therapists. Power-assisted orthoses can offer a functional replacement in the end phase and have some effectiveness in motor re-education when the surgical benefit is not preferably factored in (37). Rehabilitation of brachial plexus injuries in the sequela period seems to be a neglected area that needs further development.
4. Neonatal brachial plexus injury rehabilitation
Clinical rehabilitation and research on brachial plexus injuries in children have been widely conducted, particularly in children aged 1–12 years. However, the rehabilitation treatment of neonatal brachial plexus injury (NBPI) confronts challenges such as poor patient compliance, difficult efficacy assessment, and high family participation, resulting in slow advancement and becoming an insurmountable barrier for brachial plexus injury. It’s the fact that some demographics indicate a gradually declining frequency of brachial plexus injury following a neonatal birth injury, because of fewer forceps used (38). First, neonatal brachial plexus injuries can cause long-term chronic damage and necessitate early intervention for rehabilitation; second, a high level of home rehabilitation demands specific rehabilitation instructions for parents in preparation. The basic stage in diagnosing NBPP is clinical evaluation and physical examination by an expert doctor. Next, fewer intrusive procedures, frequently magnetic resonance imaging (MRI) and ultrasound scanning (US), are needed. If the diagnosis is still elusive, electrodiagnosis can be utilized (39). The anatomy of a neonate (0–28 days) is less developed than that of an adult; thus, precise localization is more crucial than in adults. Knowing the location and severity of a nerve injury can also help to accurately guide reconstruction procedures and provide advice to families.
For neonates with a clear diagnosis, early referral to an integrated multidisciplinary management model is advised by several systematic reviews of NBPP rehabilitation. The fundamental rehabilitation strategies advised are parallel to those for adults, which include treatments such as passive/active motor exercises, smooth joint motor, sensory stimulation, electrical stimulation, and botulinum toxin injections (40). To serve as a reference, we evaluated the available literature and collected the therapy recommendations that, when applied to newborn brachial plexus injury rehabilitation: firstly, cooperation between the family and the newborn should be considered when selecting a rehabilitation process. As the family rehabilitation model predominates and is more suitable for the care of the infant, simple, straightforward, and repeatable treatments can be selected to allow parents to perform rehabilitation therapy numerous times during the day; Secondly, rehabilitation should intentionally select to encourage active movement to restore function to the injured limb rather than compensating for the healthy side by emphasizing developmental neglect and misuse related to neonatal growth and development (41). In conclusion, there is variability in the efficacy of different treatment methods, which calls for more study to clarify. The agreement on a comprehensive rehabilitation process for NBPP continues to be in the early stages of development.
5. Discussion
The functional recovery of brachial plexus injury is a worldwide challenge. This study compiled the various rehabilitation modalities for clinicians, arranged them into rehabilitation protocols from the time a brachial plexus patient was seen until clinical remission (Figure 2) and enabled the systematic management of BPI patients. A fairly comprehensive evaluation of physical therapy for brachial plexus injuries has been conducted (42). On this basis, we have closely followed the latest advances in current clinical research on brachial plexus injury rehabilitation, and we summarize its intervention strategies and outcomes, details see Table 1.
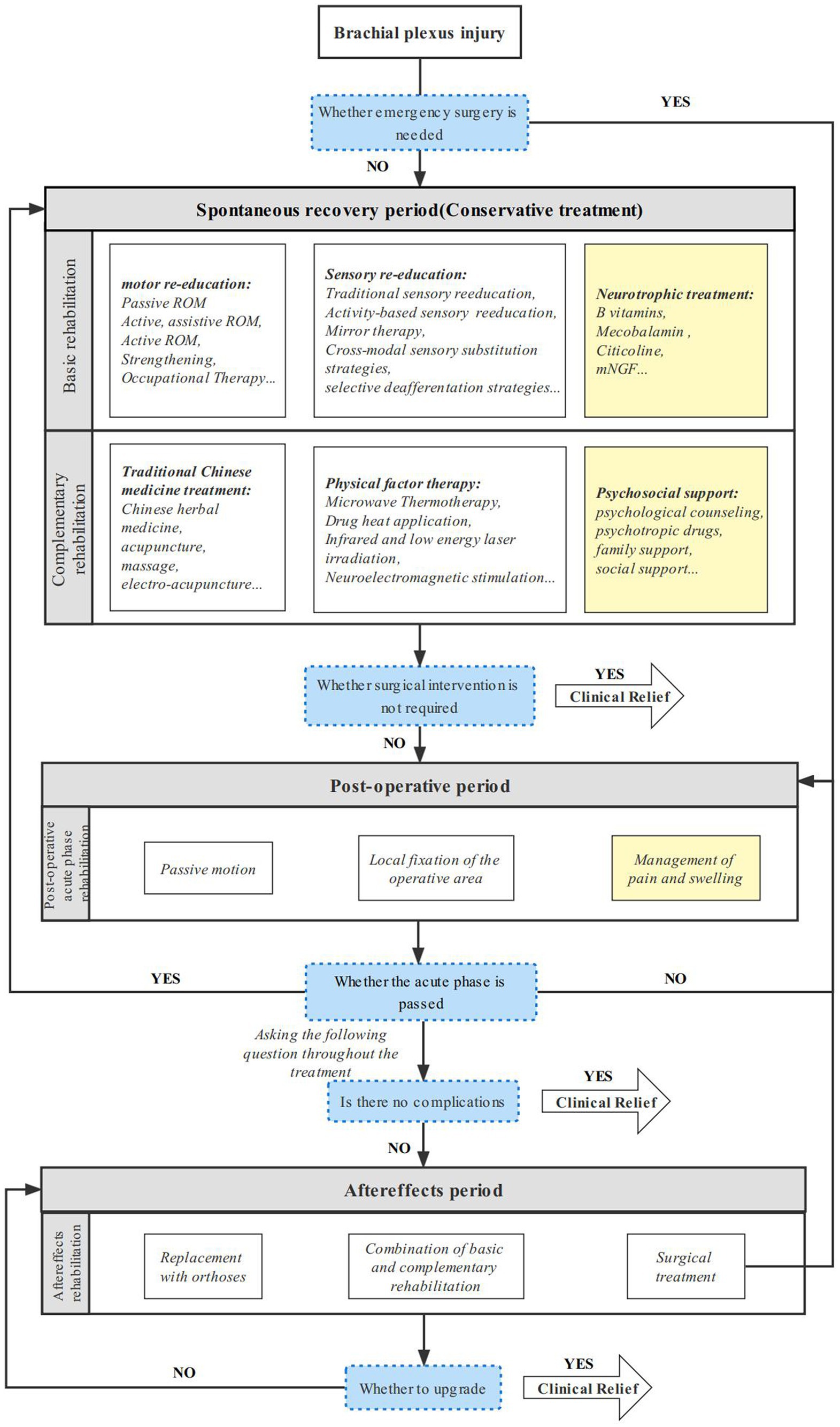
Figure 2. Comprehensive rehabilitation protocol for brachial plexus injury. A sustainable and comprehensive rehabilitation protocol for adult brachial plexus injury in different periods.
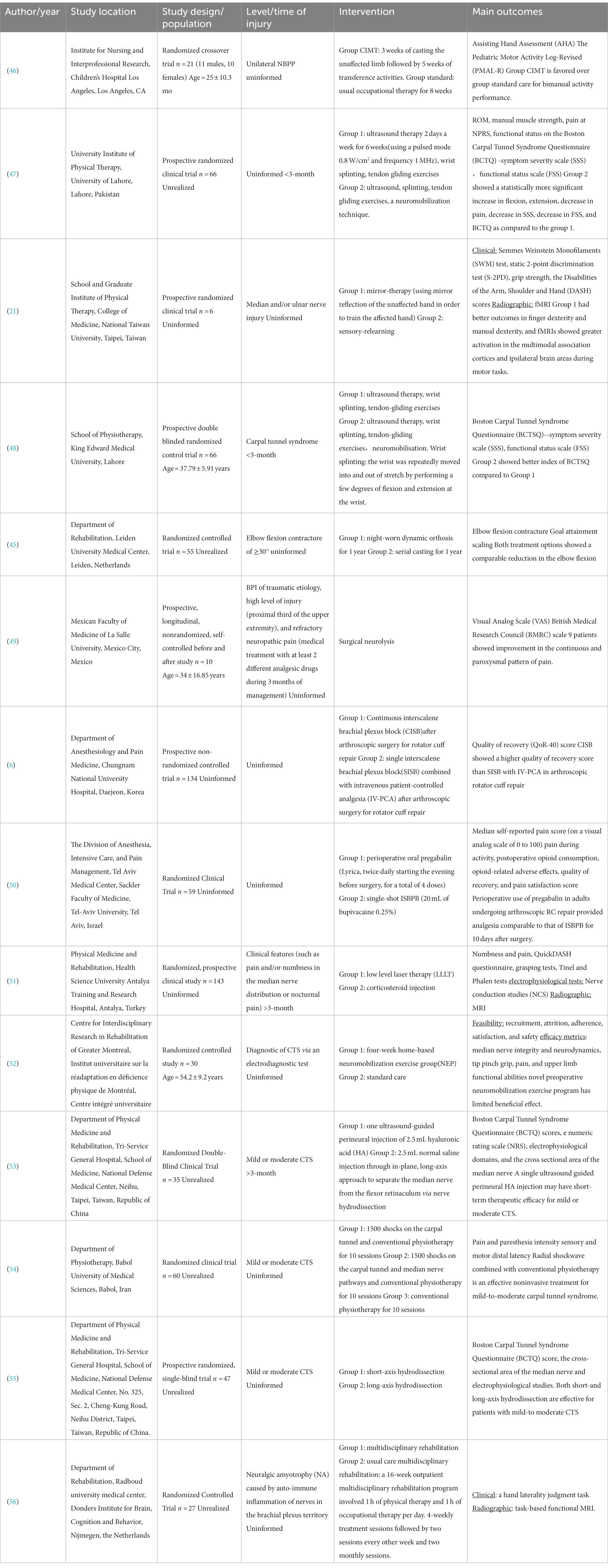
Table 1. Characteristics of the clinical studies on rehabilitation of brachial plexus injuries published in recent years.
Currently, a significant proportion of patients with brachial plexus injury are young adults and newborns, and these patients usually have an expectation of immediate recovery. However, brachial plexus injury typically requires a lengthy recovery period due to the complexity of the injury and the slow recovery tempo. During long-term rehabilitation, the degree of neurological recovery (including motor and sensory recovery, etc.), psychological status, and complications of the affected limb vary widely, requiring the clinician to develop an individualized rehabilitation program, which requires a great deal of experience. Therefore, patients may be in various stages of rehabilitation at the time of consultation, and there is no clinical consensus on how to manage patients in each stage in an integrated manner, making it easy to overlook the various aspects of rehabilitation principles that require attention at various stages of disease development. We will do some sorting later to fill in the gaps in this section.
The efficacy of comprehensive rehabilitation therapy is well established. For patients with brachial plexus who do not need emergency surgery and proceed to the spontaneous recovery stage of rehabilitation. Local external fixation support, motor and sensory re-education, and neurotrophic therapy are common alternatives. When specific treatment requirements are met, physical factor therapy, traditional Chinese medicine treatment, psychosocial support, and other supplementary treatment methods can be applied. For patients undergoing postoperative acute rehabilitation postemergency surgery, passive movement of the afflicted limb and nerve removal site, with suggested mobility within the unrestricted joint range, can be used to reduce pain and edema. For further rehabilitation, conservative therapy options available throughout the spontaneous recovery period may be selected. It is impossible to totally eliminate complications, and when they do arise, the patient moves into the sequelae phase of rehabilitation. These complications include muscular atrophy, joint stiffness, chronic neuropathic pain, etc. To accomplish functional compensation and improve the patient’s quality of life, an end-stage rehabilitation approach might be adopted, mostly employing vigorous replacement therapy. Neurotrophic therapy, pain and swelling control, and psychosocial support can all be consistently employed at different phases because pain and psychiatric illnesses might flare up at various periods. Complete recovery from brachial plexus injury is exceptional, but when clinical remission—that is, increased performance and quality of life—is attained, the patient feels sufficiently better to jump their treatment protocol and return to home rehabilitation. This page also provides an overview of the fundamental principles of rehabilitation for generally slow-progressing neonatal brachial plexus injuries, which can help to hasten the development of treatment approaches to this specific demographic problem.
Artificial intelligence and rehabilitation techniques have been developed in the last 3 years. An electroencephalography (EEG)-based human-machine interface combined with contralateral C7 transfer surgery can treat brachial plexus injury, but further research is required to understand how it promotes cortical remodeling (43). Virtual reality and robotics combined to facilitate upper extremity pain management and sensory recovery after brachial plexus injury (44). The very first mention of dynamic orthoses as having a role in motor re-education came in 2018 (37), but more recent research has revealed that dynamic nocturnal orthoses are equally effective as continuous cast immobilization in treating elbow flexion contractures in kids with brachial plexus nerve birth injuries (45). As children’s upper extremities move less than those of adults, dynamic orthoses may only serve as a fixation device in children with brachial plexus injuries while offering more motor support in adults. Individual rehabilitation is a constant topic, and AI rehabilitation has a higher degree of adjustability, sure AI involvement will become an unstoppable trend.
The literature review deserves some essential conclusions and insights to be presented. The lack of sufficient case studies, inadequate follow-up time, the lack of a recognized control group, and the propensity to employ comprehensive rehabilitation as an intervention to improve treatment results are more or fewer defects of the literature on brachial plexus injury treatment methods. Therefore, techniques like stem cell therapy that remain at the level of animal trials are not included in the clinical approach presented in this work. Our work excludes the mention of efficient treatment methods in case reports involving a small number of patients but keeps useful comments. This takes into account treatment approaches that have all been shown in numerous case reports to be successful. Randomized controlled studies reporting effective rehabilitation treatment modalities and clinical studies with a long-term follow-up process were considered first. The rehabilitation protocols described in this article are experimental and can be supplemented with additional clinical treatment modalities that show benefits. Unfortunately, due to a lack of quantitative assessment methods, this article only develops a comprehensive treatment protocol rather than offering a suitable rehabilitation therapy approach for individuals with varying injury severity and locations. The determination of the optimal treatment approach for a specific patient is still left to the discretion of qualified medical staff and therapists. In summary, it is strongly advised that future research focus on the efficiency of potential comprehensive rehabilitation programs in the management of BPI.
6. Conclusion
The efficacy of comprehensive rehabilitation therapy is well established. Physical therapy, traditional Chinese medicine treatment, psychosocial support, and other supplementary treatment methods can be applied when someone undergoes a brachial plexus injury, in addition, artificial intelligence and rehabilitation techniques are popular in these years which need further research. All the rehabilitation protocols we described are experimental and they show benefits as supplemented with additional clinical treatment modalities. In summary, we strongly advised that future research focus on the efficiency of potential comprehensive rehabilitation programs in the management of BPI, which will facilitate the return of patients, physical activity levels.
Author contributions
HL: visualization and methodology. JC: writing—review and editing, conceptualization. JW: writing—original draft, visualization. TZ: supervision. ZC: authors may have contributed in multiple roles. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kaiser, R, Waldauf, P, Ullas, G, and Krajcova, A. Epidemiology, etiology, and types of severe adult brachial plexus injuries requiring surgical repair: Systematic review and meta-analysis. Neurosurg Rev. (2020) 43:443–52. doi: 10.1007/s10143-018-1009-2
2. Yang, LJ, Chang, KW, and Chung, KC. A systematic review of nerve transfer and nerve repair for the treatment of adult upper brachial plexus injury. Neurosurgery. (2012) 71:417–29; discussion 429. doi: 10.1227/NEU.0b013e318257be98
3. Quick, TJ, and Brown, H. Evaluation of functional outcomes after brachial plexus injury. J Hand Surg Eur. (2020) 45:28–33. doi: 10.1177/1753193419879645
4. Davis, G, and Curtin, CM. Management of Pain in complex nerve injuries. Hand Clin. (2016) 32:257–62. doi: 10.1016/j.hcl.2015.12.011
5. Razak, I, Chung, TY, and Ahmad, TS. A comparative study of two modalities in pain management of patients presenting with chronic brachial neuralgia. J Altern Complement Med. (2019) 25:861–7. doi: 10.1089/acm.2019.0052
6. Yun, S, Jo, Y, Sim, S, Jeong, K, Oh, C, Kim, B, et al. Comparison of continuous and single interscalene block for quality of recovery score following arthroscopic rotator cuff repair. J Orthop Surg. (2021) 29:23094990211000142. doi: 10.1177/23094990211000142
7. Abdallat, M, Saryyeva, A, Blahak, C, Wolf, ME, Weigel, R, Loher, TJ, et al. Centromedian-parafascicular and somatosensory thalamic deep brain stimulation for treatment of chronic neuropathic pain: A contemporary series of 40 patients. Biomedicine. (2021) 9:731. doi: 10.3390/biomedicines9070731
8. Marsh, JL, Mahoney, CR, and Steinbronn, D. External fixation of open humerus fractures. Iowa Orthop J. (1999) 19:35–42.
9. Kinlaw, D. Pre-/postoperative therapy for adult plexus injury. Hand Clin. (2005) 21:103–8. doi: 10.1016/j.hcl.2004.10.003
10. de Oliveira, LAS, Pedron, CA, de Andrade, FG, Horsczaruk, CHR, and Martins, JVP. Motor recovery after bilateral brachial plexus injury using motor irradiation: A case report. Int J Ther Rehabil. (2019) 26:1–12. doi: 10.12968/ijtr.2017.0170
11. Chagas, ACS, Wanderley, D, Barboza, PJM, Martins, JVP, de Moraes, AA, de Souza, FHM, et al. Proprioceptive neuromuscular facilitation compared to conventional physiotherapy for adults with traumatic upper brachial plexus injury: A protocol for a randomized clinical trial. Physiother Res Int. (2021) 26:e1873. doi: 10.1002/pri.1873
12. Collis, JM, Signal, N, Mayland, E, and Wright-St Clair, VA. Influence of purposeful activities on upper extremity motor performance: A systematic review. OTJR. (2020) 40:223–34. doi: 10.1177/1539449220912187
13. Cole, T, Nicks, R, Ferris, S, Paul, E, O’Brien, L, and Pritchard, E. Outcomes after occupational therapy intervention for traumatic brachial plexus injury: A prospective longitudinal cohort study. J Hand Ther. (2020) 33:528–39. doi: 10.1016/j.jht.2019.08.002
14. Moore, AM, and Novak, CB. Advances in nerve transfer surgery. J Hand Ther. (2014) 27:96–104; quiz 5. doi: 10.1016/j.jht.2013.12.007
15. Smania, N, Berto, G, La Marchina, E, Melotti, C, Midiri, A, Roncari, L, et al. Rehabilitation of brachial plexus injuries in adults and children. Eur J Phys Rehabil Med. (2012) 48:483–506.
16. Zink, PJ, and Philip, BA. Cortical plasticity in rehabilitation for upper extremity peripheral nerve injury: A scoping review. Am J Occup Ther. (2020) 74:7401205030p1–7401205030p15. doi: 10.5014/ajot.2020.036665
17. Jerosch-Herold, C. Sensory relearning in peripheral nerve disorders of the hand: A web-based survey and Delphi consensus method. J Hand Ther. (2011) 24:292–9. doi: 10.1016/j.jht.2011.05.002
18. Ramachandran, VS, and RogersRamachandran, D. Synaesthesia in phantom limbs induced with mirrors. Proc R Soc B Biol Sci. (1996) 263:377–86. doi: 10.1098/rspb.1996.0058
19. Yeves-Lite, A, Zuil-Escobar, JC, Martinez-Cepa, C, Romay-Barrero, H, Ferri-Morales, A, and Palomo-Carrion, R. Conventional and virtual reality mirror therapies in upper obstetric brachial palsy: A randomized pilot study. J Clin Med. (2020) 9:3021. doi: 10.3390/jcm9093021
20. Ferreira, CM, de Carvalho, CD, Gomes, R, Bonifacio de Assis, ED, and Andrade, SM. Transcranial direct current stimulation and mirror therapy for neuropathic pain after brachial plexus avulsion: A randomized, double-blind, controlled pilot study. Front Neurol. (2020) 11:568261. doi: 10.3389/fneur.2020.568261
21. Chen, Y-H, Siow, T-Y, Wang, J-Y, Lin, S-Y, and Chao, Y-H. Greater cortical activation and motor recovery following mirror therapy immediately after peripheral nerve repair of the forearm. Neuroscience. (2022) 481:123–33. doi: 10.1016/j.neuroscience.2021.11.048
22. Mendes, RM, Barbosa, RI, Salmon, CEG, Rondinoni, C, Escorsi-Rosset, S, Delsim, JC, et al. Auditory stimuli from a sensor glove model modulate cortical audiotactile integration. Neurosci Lett. (2013) 548:33–7. doi: 10.1016/j.neulet.2013.04.019
23. Waikakul, S, and Hirunyachote, P. Effects of methylcobalamin in neurotisation after brachial plexus injury - a controlled study. Clin Drug Investig. (1999) 17:179–84. doi: 10.2165/00044011-199917030-00002
24. Anand, P, Terenghi, G, Birch, R, Wellmer, A, Cedarbaum, JM, Lindsay, RM, et al. Endogenous Ngf and Cntf levels in human peripheral nerve injury. Neuroreport. (1997) 8:1935–8. doi: 10.1097/00001756-199705260-00028
25. Chu, T-H, and Wu, W. Neurotrophic factor treatment after spinal root avulsion injury. Cent Nerv Syst Agents Med Chem. (2009) 9:40–55. doi: 10.2174/187152409787601914
26. Gordon, T, Chan, KM, Sulaiman, OAR, Udina, E, Amirjani, N, and Brushart, TM. Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery. (2009) 65:A132–44. doi: 10.1227/01.Neu.0000335650.09473.D3
27. Cobo-Vicente, F, San Juan, AF, Larumbe-Zabala, E, Estevez-Gonzalez, AJ, Donadio, MVF, and Perez-Ruiz, M. Neuromuscular electrical stimulation improves muscle strength, biomechanics of movement, and functional mobility in children with chronic neurological disorders: A systematic review and meta-analysis. Phys Ther. (2021) 101:pzab170. doi: 10.1093/ptj/pzab170
28. Stevanato, G, Devigili, G, Eleopra, R, Fontana, P, Lettieri, C, Baracco, C, et al. Chronic post-traumatic neuropathic pain of brachial plexus and upper limb: A new technique of peripheral nerve stimulation. Neurosurg Rev. (2014) 37:473–9; discussion 479-80. doi: 10.1007/s10143-014-0523-0
29. Bonifacio de Assis, ED, Martins, WKN, de Carvalho, CD, Ferreira, CM, Gomes, R, de Almeida Rodrigues, ET, et al. Effects of rTMS and tDCS on neuropathic pain after brachial plexus injury: A randomized placebo-controlled pilot study. Sci Rep. (2022) 12:1440. doi: 10.1038/s41598-022-05254-3
30. Hao, J, Zhao, C, Cao, S, and Yang, S. Electric acupuncture treatment of peripheral nerve injury. J Tradit Chin Med. (1995) 15:114–7.
31. Luo, H, Cheng, X, Tang, Y, Ling, Z, and Zhou, L. Electroacupuncture treatment contributes to the downregulation of neuronal nitric oxide synthase and motoneuron death in injured spinal cords following root avulsion of the brachial plexus. Biomed Rep. (2014) 2:207–12. doi: 10.3892/br.2013.212
32. Sachar, R, Landau, AJ, Ray, WZ, Brogan, DM, and Dy, CJ. Social support and coping strategies in patients with traumatic brachial plexus injury. HSS J. (2020) 16:468–74. doi: 10.1007/s11420-020-09814-z
33. Zhou, JM, Gu, YD, Xu, XJ, Zhang, SY, and Zhao, X. Clinical research of comprehensive rehabilitation in treating brachial plexus injury patients. Chin Med J. (2012) 125:2516–20. doi: 10.3760/cma.j.issn.0366-6999.2012.14.022
34. Kahn, LC, and Moore, AM. Donor activation focused rehabilitation approach: Maximizing outcomes after nerve transfers. Hand Clin. (2016) 32:263–77. doi: 10.1016/j.hcl.2015.12.014
35. Safoury, YA, Eldesoky, MT, Abutaleb, EE, Atteya, MR, and Gabr, AM. Postoperative physical therapy program for latissimus dorsi and teres major tendons transfer to rotator cuff in children with obstetrical brachial plexus injury. Eur J Phys Rehabil Med. (2017) 53:277–85. doi: 10.23736/S1973-9087.16.03910-1
36. Steeg, AMT, Hoeksma, AF, Dijkstra, PF, Nelissen, RGHH, and Jong, BAD. Orthopaedic sequelae in neurologically recovered obstetrical brachial plexus injury. Case study and literature review. Disabil Rehabil. (2009) 25:1–8. doi: 10.1080/09638280210142185
37. Chinchalkar, SJ, Larocerie-Salgado, J, Cepek, J, and Grenier, ML. The use of dynamic assist orthosis for muscle reeducation following brachial plexus injury and reconstruction. J Hand Microsurg. (2018) 10:172–7. doi: 10.1055/s-0038-1642068
38. Gupta, R, and Cabacungan, ET. Neonatal birth trauma: Analysis of yearly trends, risk factors, and outcomes. J Pediatr. (2021) 238:174–180.e3. doi: 10.1016/j.jpeds.2021.06.080
39. van der Looven, R, Le Roy, L, Tanghe, E, van den Broeck, C, de Muynck, M, Vingerhoets, G, et al. Early electrodiagnosis in the management of neonatal brachial plexus palsy: A systematic review. Muscle Nerve. (2020) 61:557–66. doi: 10.1002/mus.26762
40. Frade, F, Gomez-Salgado, J, Jacobsohn, L, and Florindo-Silva, F. Rehabilitation of neonatal brachial plexus palsy: Integrative literature review. J Clin Med. (2019) 8:980. doi: 10.3390/jcm8070980
41. Yang, LJ. Neonatal brachial plexus palsy--management and prognostic factors. Semin Perinatol. (2014) 38:222–34. doi: 10.1053/j.semperi.2014.04.009
42. de Santana Chagas, AC, Wanderley, D, de Oliveira Ferro, JK, Alves de Moraes, A, Morais de Souza, FH, da Silva Tenorio, A, et al. Physical therapeutic treatment for traumatic brachial plexus injury in adults: A scoping review. PM R. (2022) 14:120–50. doi: 10.1002/pmrj.12566
43. Zhang, M, Li, C, Liu, SY, Zhang, FS, and Zhang, PX. An electroencephalography-based human-machine interface combined with contralateral C7 transfer in the treatment of brachial plexus injury. Neural Regen Res. (2022) 17:2600–5. doi: 10.4103/1673-5374.335838
44. Snow, PW, Dimante, D, Sinisi, M, and Loureiro, RCV. Virtual reality combined with robotic facilitated movements for pain management and sensory stimulation of the upper limb following a brachial plexus injury: A case study. Int Conf Rehabil Robot. (2022) 2022:1–6. doi: 10.1109/ICORR55369.2022.9896552
45. Op de Coul, LS, Bleeker, S, de Groot, JH, Nelissen, R, and Steenbeek, D. Elbow flexion contractures in neonatal brachial plexus palsy: A one-year comparison of dynamic orthosis and serial casting. Clin Rehabil. (2023) 37:72–85. doi: 10.1177/02692155221121011
46. Werner, JM, Berggren, J, Loiselle, J, and Lee, GK. Constraint-induced movement therapy for children with neonatal brachial plexus palsy: A randomized crossover trial. Dev Med Child Neurol. (2021) 63:545–51. doi: 10.1111/dmcn.14741
47. Ijaz, MJ, Karimi, H, Ahmad, A, Gillani, SA, Anwar, N, and Chaudhary, MA. Comparative efficacy of routine physical therapy with and without neuromobilization in the treatment of patients with mild to moderate carpal tunnel syndrome. Biomed Res Int. (2022) 2022:2155765. doi: 10.1155/2022/2155765
48. Ijaz, MJ, Karimi, H, Gillani, SA, Ahmad, A, and Chaudhary, MA. Effect of median nerve neuromobilization on functional status in patients with carpal tunnel syndrome: A double blinded randomized control trial. J Pak Med Assoc. (2022) 72:605–9. doi: 10.47391/JPMA.2212
49. Armas-Salazar, A, Tellez-Leon, N, Garcia-Jeronimo, AI, Villegas-Lopez, FA, Navarro-Olvera, JL, and Carrillo-Ruiz, JD. Neuropathic pain relief after surgical neurolysis in patients with traumatic brachial plexus injuries: A preliminary report. Pain Res Manag. (2022) 2022:5660462. doi: 10.1155/2022/5660462
50. Farladansky, E, Hazan, S, Maman, E, Reuveni, AM, Cattan, A, Matot, I, et al. Perioperative oral pregabalin results in postoperative pain scores equivalent to those of interscalene brachial plexus block after arthroscopic rotator cuff repair: A randomized clinical trial. Arthroscopy (2022) 38:31–7. doi: 10.1016/j.arthro.2021.05.022
51. Badil Guloglu, S, Bilgilisoy Filiz, M, Kilic, KK, Koldas Dogan, S, Toslak, IE, and Toraman, NF. Treatment of carpal tunnel syndrome by low-level laser therapy versus corticosteroid injection: A randomized, prospective clinical study. Lasers Med Sci. (2022) 37:2227–37. doi: 10.1007/s10103-021-03489-6
52. Paquette, P, Higgins, J, Danino, MA, Harris, P, Lamontagne, M, and Gagnon, DH. Effects of a preoperative neuromobilization program offered to individuals with carpal tunnel syndrome awaiting carpal tunnel decompression surgery: A pilot randomized controlled study. J Hand Ther. (2021) 34:37–46. doi: 10.1016/j.jht.2019.12.012
53. Su, YC, Shen, YP, Li, TY, Ho, TY, Chen, LC, and Wu, YT. The efficacy of hyaluronic acid for carpal tunnel syndrome: A randomized double-blind clinical trial. Pain Med. (2021) 22:2676–85. doi: 10.1093/pm/pnab109
54. Habibzadeh, A, Mousavi-Khatir, R, Saadat, P, and Javadian, Y. The effect of radial shockwave on the median nerve pathway in patients with mild-to-moderate carpal tunnel syndrome: A randomized clinical trial. J Orthop Surg Res. (2022) 17:46. doi: 10.1186/s13018-022-02941-9
55. Chen, SR, Ho, TY, Shen, YP, Li, TY, Su, YC, Lam, KHS, et al. Comparison of short- and long-axis nerve hydrodissection for carpal tunnel syndrome: A prospective randomized, single-blind trial. Int J Med Sci. (2021) 18:3488–97. doi: 10.7150/ijms.63815
Keywords: brachial plexus injury, rehabilitation, neuropathic pain, neonatal brachial plexus injury, treatment
Citation: Li H, Chen J, Wang J, Zhang T and Chen Z (2023) Review of rehabilitation protocols for brachial plexus injury. Front. Neurol. 14:1084223. doi: 10.3389/fneur.2023.1084223
Edited by:
Shizhang Ling, The First Affiliated Hospital of Wannan Medical College, ChinaReviewed by:
Emmanuel Estrella, University of the Philippines Manila, PhilippinesJie Ma, Shanghai University of Traditional Chinese Medicine, China
Copyright © 2023 Li, Chen, Wang, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zuobing Chen, Y3piMTk3MUB6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Haijun Li
Haijun Li Jinxiu Chen
Jinxiu Chen Juehan Wang
Juehan Wang Tianfang Zhang
Tianfang Zhang Zuobing Chen
Zuobing Chen