- 1Department of Neurology, University of Colorado School of Medicine, Aurora, CO, United States
- 2Department of Neurology, Behavioral Neurology Section, University of Colorado School of Medicine, Aurora, CO, United States
- 3Department of Neurology, Movement Disorders Section, University of Colorado School of Medicine, Aurora, CO, United States
- 4Department of Psychiatry, University of Colorado School of Medicine, Aurora, CO, United States
- 5Marcus Institute for Brain Health, Aurora, CO, United States
- 6Department of Radiology, University of Colorado School of Medicine, Aurora, CO, United States
- 7Department of Ophthalmology, University of Colorado School of Medicine, Aurora, CO, United States
Background: The Colorado Posterior Cortical Questionnaire (CPC-Q) is a self-report, 15-item screening questionnaire for posterior cortical symptoms, including visuospatial and visuoperceptual difficulties. Changes in white matter connectivity may precede obvious gray matter atrophy in neurodegenerative conditions, especially posterior cortical atrophy. Integration of CPC-Q scores and measures of white matter integrity could contribute to earlier detection of posterior cortical syndromes.
Methods: We investigated the relationships between posterior cortical symptoms as captured by the CPC-Q and diffusion tensor imaging fractional anisotropy (DTI FA) of white matter regions of interest localized to posterior brain regions (posterior thalamic radiations, splenium of corpus callosum, tapetum). Comparisons were also made by diagnostic group [healthy older adult (n = 31), amnestic Alzheimer's disease (AD, n = 18), and posterior cortical atrophy (PCA, n = 9)] and by SENAS battery visuospatial composite score quartile. Exploratory comparisons of all available individual white matter region DTI FA to CPC-Q, as well as comparisons of DTI FA between diagnostic groups and visuospatial quartiles, were also made.
Results: CPC-Q score was correlated with the average DTI FA for the averaged posterior white matter regions of interest (r = −0.31, p = 0.02). Posterior thalamic radiation DTI FA was most strongly associated with CPC-Q (r = −0.34, p = 0.01) and visuospatial composite (r = 0.58, p < 0.01) scores and differed between the PCA and AD groups and the lower and higher visuospatial quartiles. The DTI FA of body and splenium of the corpus callosum also demonstrated this pattern but not the DTI FA of the tapetum.
Conclusion: The integrity of posterior white matter tracts is associated with scores on the CPC-Q, adding to the validation evidence for this new questionnaire. White matter regions that may be related to posterior cortical symptoms detected by the CPC-Q, and distinct from those affected in amnestic syndromes, include the posterior thalamic radiations and body and splenium of the corpus callosum. These findings are in line with previous neuroimaging studies of PCA and support continued research on white matter in posterior cortical dysfunction.
Introduction
Posterior cortical symptoms, including visuospatial and visuoperceptual impairments, are under-recognized by both clinicians and patients (1). Patients with these symptoms may first present to ophthalmology or optometry clinics for perceived eye problems; alternatively, they may seek neurological evaluation for perceived memory problems. Solicitation of qualitative description of these problems from patients uncovers non-ocular, non-amnestic symptoms. In addition to visually-based deficits, posterior cortical dysfunction can also cause difficulty with calculations, left/right orientation, and praxis (2). Posterior cortical symptoms are often more disabling and anxiety-provoking than other types of cognitive symptoms, with more prominent and earlier safety issues (e.g., driving impairment, falls) (3, 4). Systematic screening for posterior cortical symptoms could improve accuracy and timeliness of diagnoses, leading to improved symptom management, counseling, and outcomes. To contribute to this goal, our group recently developed a brief self-report posterior cortical symptom screening tool, the Colorado Posterior Cortical Questionnaire (CPC-Q) (5). The CPC-Q demonstrates strong psychometric properties, correlates strongly with visuospatial neuropsychological measures, and can distinguish participants with expert consensus posterior cortical atrophy (PCA) diagnosis from participants who are asymptomatic or have amnestic Alzheimer's disease (AD).
To extend this prior work and further validate the CPC-Q, we assessed the neuroanatomical correlates of the measure with a specific focus on white matter integrity. Visuospatial and visuoperceptual symptoms are not restricted to dysfunction of posterior cortical regions. More widespread structural and functional networks also play a major role, including fronto-parietal and temporal-parietal regions. Connectivity disruption may be more sensitive for higher order visual dysfunction than regional atrophy patterns, especially at the earliest stages of neurodegenerative conditions (6). Changes in white matter tracts have been demonstrated in several diffusion tensor imaging (DTI) studies of PCA, with these changes exceeding what would be expected based on gray matter atrophy of posterior regions alone (7–10). One study found significantly more white matter degeneration in PCA than in logopenic primary progressive aphasia, another “atypical” form of AD, suggesting that white matter may be particularly vulnerable in PCA (10). Specific white matter tracts implicated in PCA by these imaging studies include the posterior thalamic radiations, splenium of the corpus callosum, inferior longitudinal fasciculus, and the cingulum.
For our goal of early detection of posterior cortical syndromes, including but not limited to PCA, we included a broader group of participants with a range of quality and severity of cognitive symptoms. We aimed to explore the relationship between the CPC-Q and structural white matter alterations associated with posterior cortical dysfunction. The clinical utility of this screening questionnaire would improve if it were also associated with subtle and early white matter changes, even prior to obvious cortical atrophy. Informed by previous work focused on PCA, our primary hypothesis was that total CPC-Q scores would negatively correlate with the fractional anisotropy (FA) of white matter tracts localized to posterior brain regions (i.e., greater posterior cortical symptom burden, reflected by higher CPC-Q scores, are associated with lower integrity posterior white matter tracts), namely the posterior thalamic radiations, the splenium of the corpus callosum, and the tapetum. However, because posterior cortical symptoms involve broader structural connections with the temporal and frontal cortices, we also examined additional white matter tracts in exploratory analyses to determine if more widespread white matter alterations were associated with self-reported posterior cortical symptoms on the CPC-Q.
Materials and methods
Participants and clinical assessment
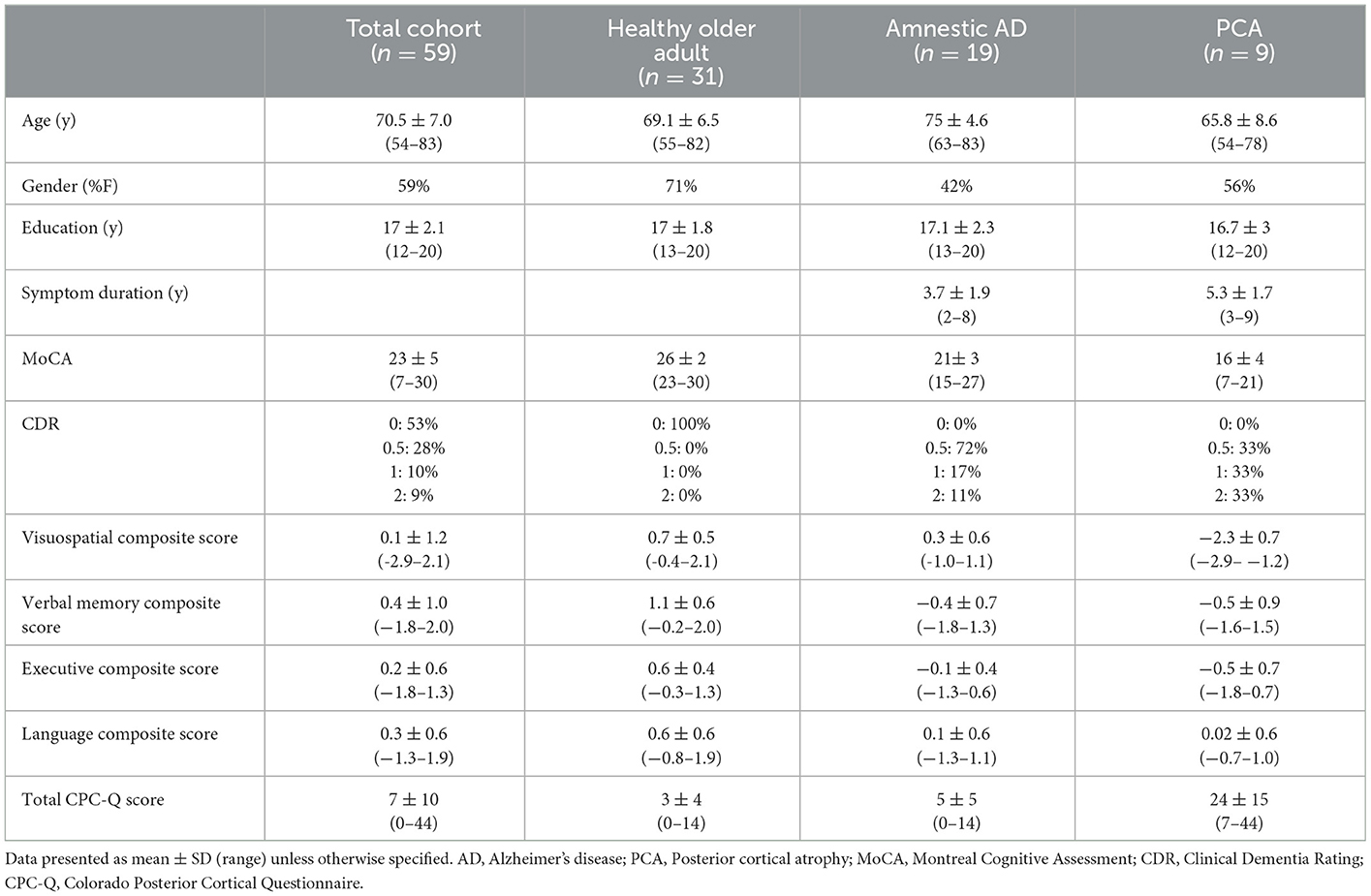
This cross-sectional study took place within an ongoing longitudinal, observational study of cognitive aging at the University of Colorado Alzheimer's and Cognition Center, the Longitudinal Biomarker and Clinical Phenotyping (Bio-AD) study. Inclusion and exclusion criteria for the Bio-AD study, as well as methods for cognitive and syndromic classification by consensus conference have been described previously (5). Fifty-nine Bio-AD participants completed the CPC-Q, along with clinical interview, neurological examination, cognitive testing [Montreal Cognitive Assessment (MoCA) (11) and Spanish and English Neuropsychological Assessment Scales (SENAS) (12)]. Informant-based interviews were performed for determination of presence and severity of functional impairment [Clinical Dementia Rating (CDR) (13)]. Cognitive domain (visuospatial, executive, memory, and language) composite scores were calculated from the SENAS battery item response theory (IRT) composite scores. Administration procedures, measure development, and psychometric characteristics of the SENAS battery are described in detail elsewhere (14). All participants provided written informed consent and the study protocol was approved by the Colorado Multiple Institutional Review Board (Protocol #15-1774).
MRI acquisition and neuroimaging analysis
Whole brain MRI scans were obtained on a 3.0 Tesla Siemens (Iselin, NJ) Skyra scanner equipped with a 20- channel head coil. Diffusion imaging data were acquired utilizing multi-shell 2D echo planar imaging sequence (56 slices; TR/TE = 8,400/105 milliseconds, acquisition matrix = 112 × 112; 2 × 2 × 2 mm3 isotropic spatial resolution; 64 x b = 2,500 s/mm2 A>P direction; 32 x b = 700 s/mm2 P>A direction; 18 x b = 0 s/mm2). Diffusion data were first pre-processed to correct for susceptibility induced distortions using FMIRB Software Library (FSL) tools topup function, as well as eddy current distortions and motion artifacts using FSL's eddy function (15, 16). The corrected diffusion data were imported into DSI studio (https://dsi-studio.labsolver.org) to create the diffusion maps. Region of Interest (ROI) analyses were performed by first registering individual subject's FA maps to the MNI152_T1_1mm template using an affine transformation in DSI studio. Mean FA values were then extracted from 48 white matter regions (including left and right hemisphere regions separately) obtained from the Johns Hopkins University ICBM-DTI-81 atlas (17).
Posterior white matter tracts of interest included the posterior thalamic radiations, splenium of the corpus callosum, and tapetum, with the justification that these tracts are localized only to posterior brain regions without more diffuse projections and would therefore be expected to be strongly associated with clinical symptoms captured by the CPC-Q (Figure 1). FA measurement for these three tracts were averaged with an equal weighting to create a localized posterior tracts FA variable, allowing for greater variability.
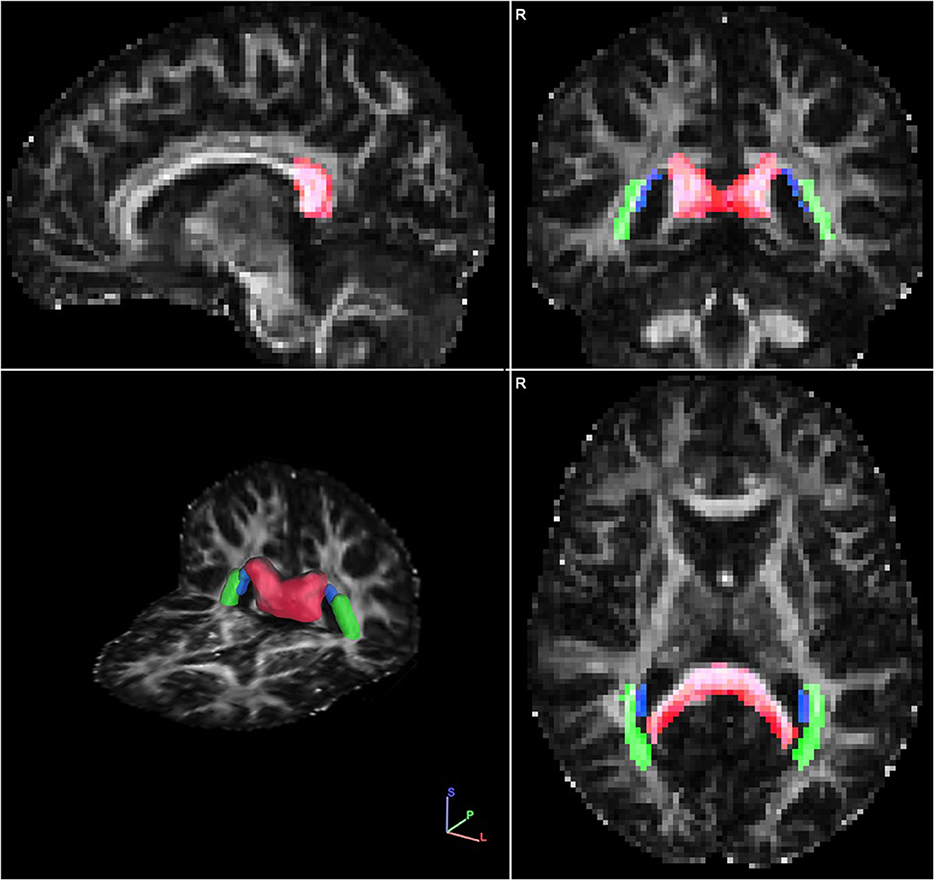
Figure 1. Single representative diffusion fractional anisotropy (FA) map with the localized posterior tract ROIs overlayed in sagittal (Upper left quadrant), coronal (Upper right quadrant), axial (Bottom right quadrant) and 3-dimensional (Bottom left quadrant) views. The ROIs include: splenium of the corpus callosum (red), tapetum (blue), and posterior thalamic radiations (green). Images are displayed in radiological view.
Statistical analyses
Demographic and clinical characteristics of participants were evaluated using descriptive statistics. To evaluate the white matter integrity of the full cohort by objectively measured visuospatial function, independent of diagnostic group (healthy older adult, amnestic AD, or PCA), the cohort was divided into quartiles based on visuospatial composite score from the SENAS battery. Mean DTI FA for all available white matter regions/tracts was calculated and compared for the resulting four groups, with Quartile 1 representing the lowest visuospatial composite scores and Quartile 4 representing the highest. Mean DTI FA for all available white matter regions/tracts was also compared by diagnostic group. Normality of CPC-Q and DTI FA data was tested using Shapiro-Wilk W tests; based on these results, one-way ANOVA or Kruskal-Wallis one-way ANOVA analyses were then performed, as appropriate, to evaluate for groupwise differences in DTI FA for each white matter region/tract. Groupwise differences were then tested by either t-tests or Mann Whitney U tests, as appropriate.
The association between the CPC-Q and FA of the localized posterior tracts was tested with pairwise correlation. A linear regression model, with CPC-Q total score as the dependent variable, was also used to evaluate the relationship with the localized posterior tract FA variable, with relevant covariates of participant age and education. As exploratory analysis into the relationship between the CPC-Q and integrity of white matter, pairwise correlations (with Bonferroni correction for multiple comparisons) were also calculated for all available regions from our dataset, including those tracts that should not be associated with posterior cortical function (e.g., corticospinal tracts). For comparison, pairwise correlations were also calculated for all ROIs and visuospatial composite scores, evaluating the hypothesis that the strength of the relationship between CPC-Q score and posterior white matter tracts should mirror that of the relationship between objectively measured visuospatial function and posterior white matter tracts. Statistical analyses were performed using the Stata statistical software package (StataCorp 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
Participant characteristics
Demographic and clinical characteristics of the participants are presented in Table 1. There was a broad range of age (54–83 years), education (12–20 years), and global cognitive abilities [Montreal Cognitive Assessment (MoCA) (11) score range 7–30] among participants. Of the 59 participants, 31 (53%) were healthy older adults, 19 (32%) had amnestic AD, and 9 (15%) had PCA. Scores on the CPC-Q ranged from 0 to 44, with a mean score of 7 and median score of 4, with a positive skew of data distribution. The PCA group was significantly younger (mean diff 9.2 years, p < 0.01) with significantly longer duration of symptoms (mean diff 1.6 years, p = 0.04) compared to the amnestic AD group. Visuospatial composite scores ranged from −2.9 to 0.03 for Quartile 1 (n = 17, mean CPC-Q score 14.2 ± 15.4); 0.03–0.45 for Quartile 2 (n = 12, mean CPC-Q score 3.1 ± 3.4); 0.45–0.86 for Quartile 3 (n = 12, mean CPC-Q score 3.1 ± 3.4); and 0.86–2.1 for Quartile 4 (n = 18, mean CPC-Q score 3.7 ± 4.0).
White matter integrity by diagnostic group and visuospatial quartile
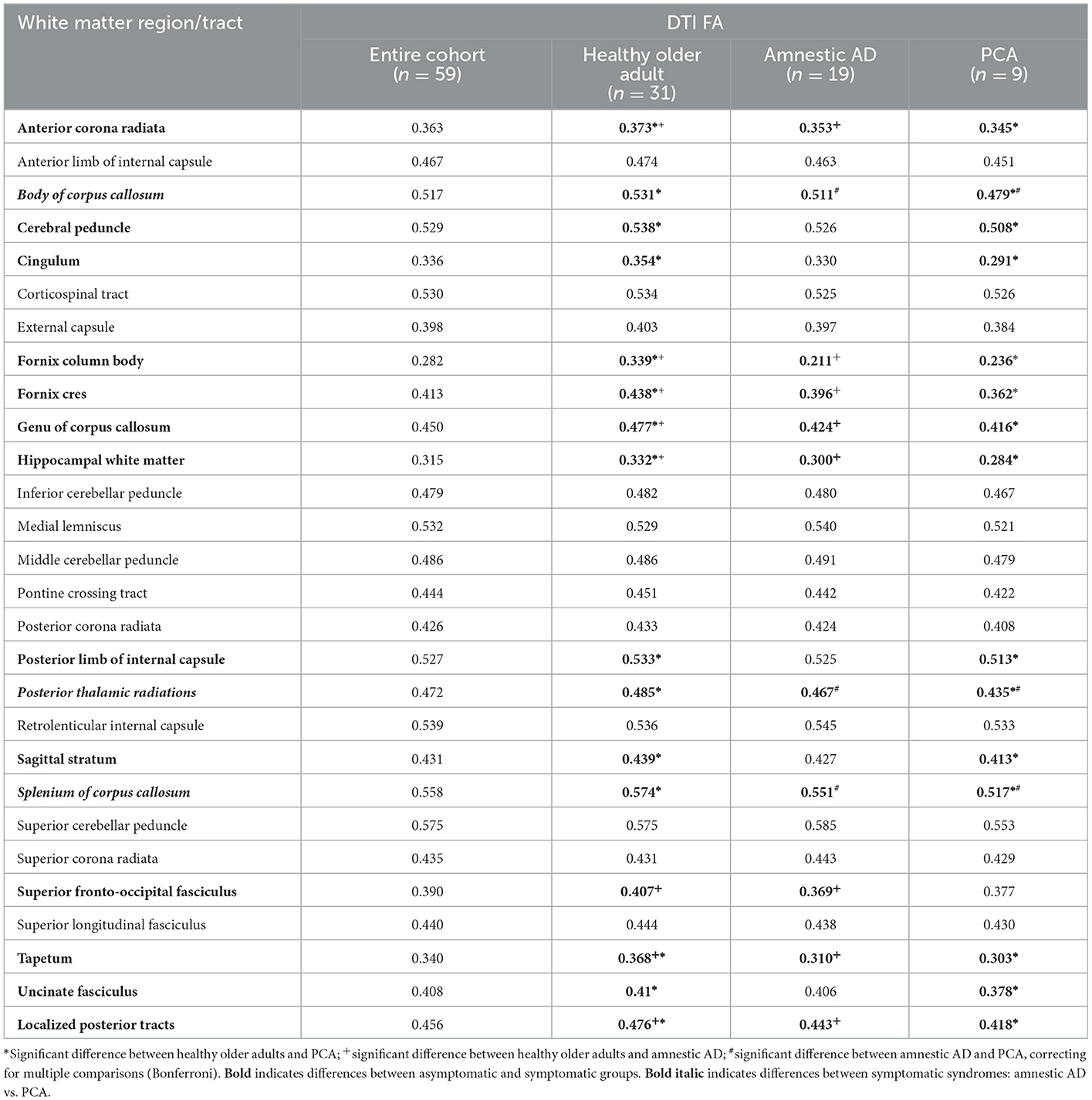
Mean DTI FA measurements of all available white matter region/tracts are presented in Table 2, for the entire cohort and by diagnostic groups. Several white matter tracts demonstrated significant differences in FA between the healthy older adult and either amnestic AD or PCA groups, with lower FA in the symptomatic groups. Only three tracts were significantly different between the two symptomatic groups, with lower FA in the PCA group for the body of the corpus callosum, posterior thalamic radiations, and splenium of the corpus callosum when compared to the amnestic AD group.
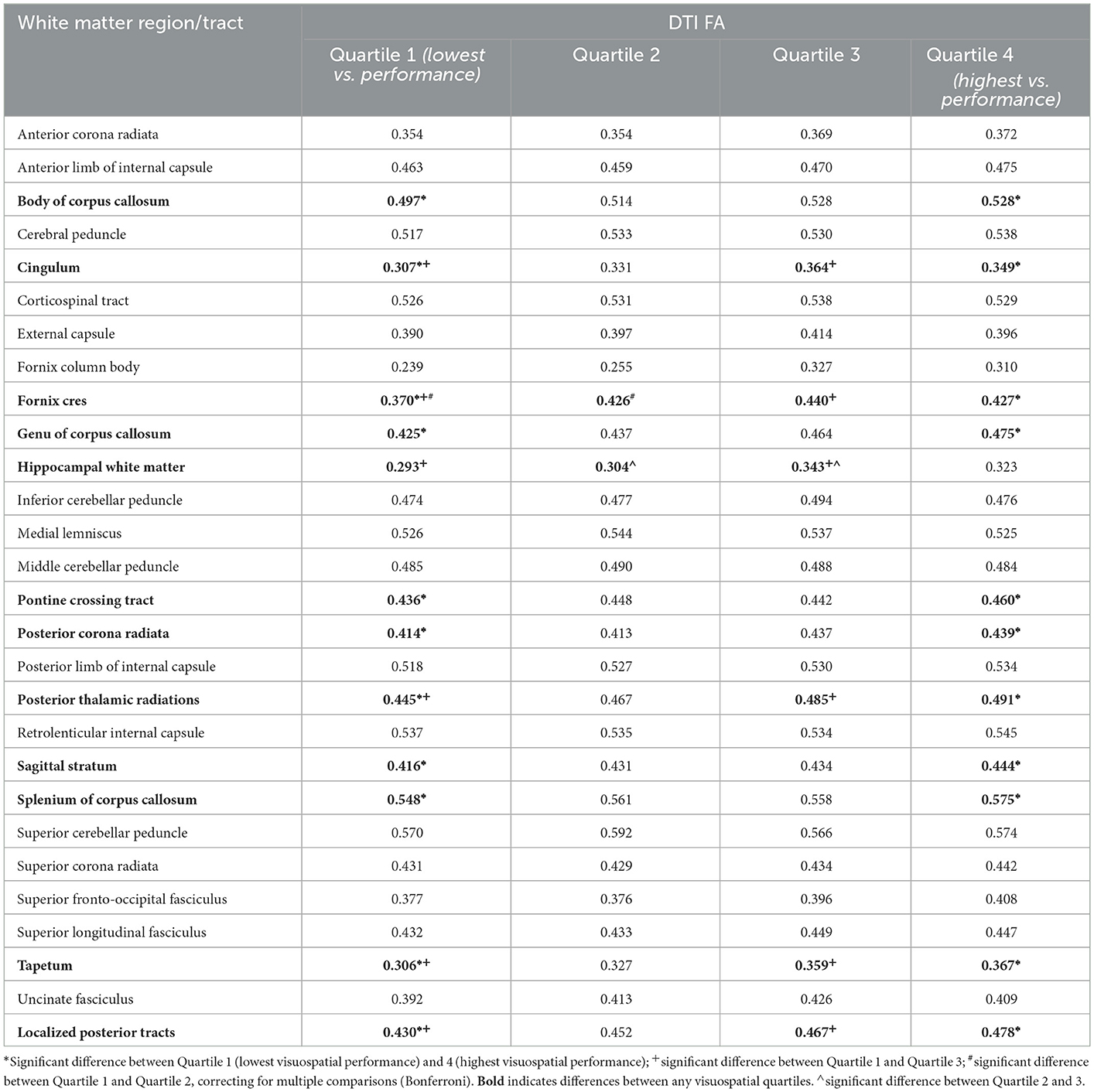
Table 3 displays mean white matter region/tract FA by visuospatial composite quartiles and statistical differences between groups. Ten tracts demonstrated significant groupwise differences between the lowest (Quartile 1) and highest (Quartile 4) visuospatial composite score groups. The FA of five tracts were significantly different between Quartile 1 and Quartile 3, but only two tracts were significantly different between Quartile 1 and 2 (fornix cres and hippocampal white matter).
White matter integrity and CPC-Q scores
In univariate analyses, the FA of our main a priori white matter region of interest, the mean localized posterior tracts, significantly correlated with total CPC-Q score (r = −0.31, p = 0.018, Figure 2). When controlling for demographic covariates of age and education, the localized posterior tracts remained significantly associated with total CPC-Q score [F (3, 55) = 9.56, p < 0.001].
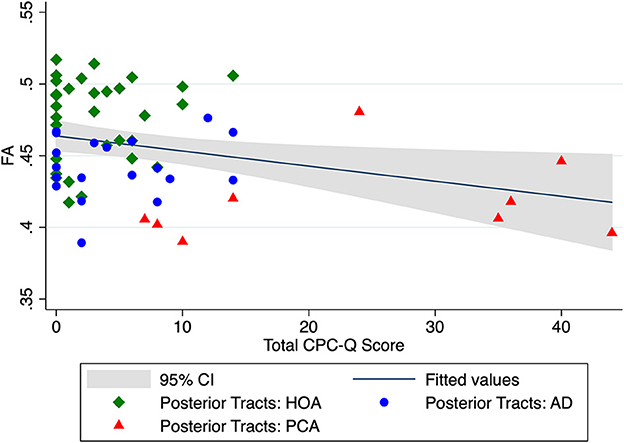
Figure 2. Unadjusted relationship between mean DTI FA of localized posterior tracts and total CPC-Q score.
Correlations for DTI FA of all white matter region/tracts to both total CPC-Q and visuospatial composite scores are presented in Table 4. Relationships between individual tract/region FA and total CPC-Q score did not survive correction for multiple comparisons; uncorrected values are presented as exploratory results. Two white matter regions chosen a priori for the localized posterior tract variable were associated with both CPC-Q and visuospatial composite scores: the posterior thalamic radiations and splenium of the corpus callosum. In addition, the body of the corpus callosum and fornix cres were also associated with both scores. The medial lemniscus was associated with the CPC-Q, but not the visuospatial composite. The posterior thalamic radiations were most strongly associated with CPC-Q score (r = −0.34, p = 0.009); the corticospinal tracts were the least associated (r = −0.08, p = 0.55).
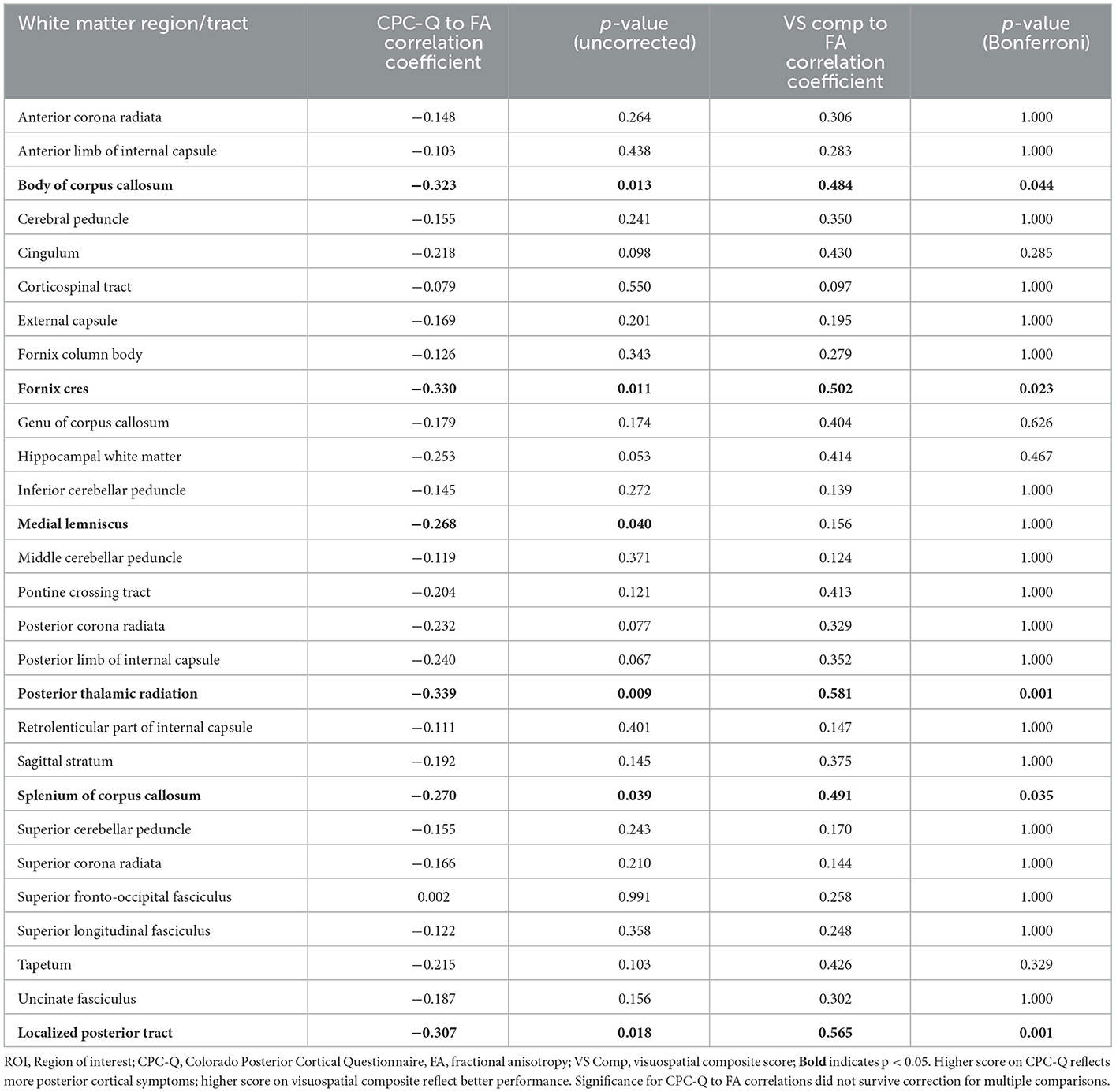
Table 4. Exploratory correlational analyses between mean DTI FA for all white matter region/tracts and CPC-Q and visuospatial composite scores.
Discussion
Early and accurate detection of posterior cortical symptoms, with distinction from ocular or ophthalmologic symptoms, is a major need in the clinical care and research of cognitive syndromes. The CPC-Q is significantly correlated with visuospatial neuropsychological measures and discriminates PCA from amnestic AD and asymptomatic groups (5), offering a quick self-report scale that could easily be deployed within clinical settings. To support future dissemination, the presented preliminary neuroanatomical analyses further validate the CPC-Q and deepen its clinical and pathophysiological implications. A focus on white matter is supported by previous neuroimaging studies in PCA (7–10). The integrity of our a priori white matter region of interest, the averaged localized posterior tract, was significantly associated with CPC-Q scores. Furthermore, the FA of this region of interest significantly differed between the symptomatic and asymptomatic groups, as well as between the lower and higher visuospatial performance quartiles. The localized posterior tract FA was not significantly different between the PCA and amnestic AD groups, however. In our exploratory comparisons, the FA of the posterior thalamic radiations and the body and splenium of the corpus callosum were significantly different between the PCA and amnestic AD groups.
The FA of the fornix cres and medial lemniscus were also associated with CPC-Q score in uncorrected comparisons for future hypothesis generation; the medial lemniscus finding could reflect vestibular abnormalities that have been reported by people with PCA, including upside down vision or tilt of the vertical (18). The FA of the posterior thalamic radiations, body and splenium of the corpus callosum, and the fornix cres were also significantly correlated with visuospatial composite scores, though the FA of the medial lemniscus was not. These observations may indicate that the CPC-Q is detecting unique visuospatial/perceptual symptoms that traditional neuropsychological testing cannot. The correlation between the FA of any individual white matter region/tract and CPC-Q did not survive correction for multiple comparisons, but future studies will consider these five regions as areas of interest in additional validation of the CPC-Q.
Separate from the analyses focused on relationships between white matter integrity and the CPC-Q, we more broadly explored white matter integrity by diagnostic group. Our PCA group demonstrated lower FA than healthy older adults in 14 of the 27 available white matter region/tracts; the amnestic AD group had lower FA in 10 of these 27 regions compared to the controls. These results support previous findings of more diffuse and severe white matter degradation in PCA compared to other syndromic presentations of AD (10). However, our PCA group had significantly longer symptom duration, despite younger age, and more severe impairment by cognitive test scores. Therefore, the degree of white matter abnormality, measured by comparisons of individual FA values or number of affected region/tracts, may reflect more advanced disease stage rather than cognitive syndrome.
The findings of reduced FA in a variety of posterior cerebral white matter tracts are of interest from two perspectives. First, with respect to PCA, our data add to existing evidence that white matter is significantly affected in this disease (7–10). Longitudinal studies to identify the sequence of neuropathological events in PCA are clearly needed, however, to establish the role of white matter in pathogenesis. Second, our data showing reduced FA in many posterior white matter tracts among the lowest performing visuospatial quartile support the use of the CPC-Q to identify individuals with prominent visuospatial and visuoperceptual impairments, regardless of their diagnosis. These observations suggest that the CPC-Q may prove useful as a clinical tool for the detection of symptoms reflecting disrupted posterior tracts in white matter disorders of diverse etiologies. Such an instrument would be welcomed in clinical settings where the diagnosis of PCA, and other disorders of posterior cortical regions, presents substantial challenges, including in optometry and ophthalmology clinics.
Limitations of this study include a small sample size, including only nine participants with PCA. Though likely more common than currently thought due to limited screening tools and misdiagnoses, PCA is a relatively rare condition overall. Current efforts toward multi-center collaborations, with harmonization of PCA clinical measures and data sharing, will help to confirm and expand our initial pilot findings (19, 20). The small sample size likely affected the power of the CPC-Q to DTI FA comparisons, especially given the non-normality of CPC-Q data; as can be appreciated in Figure 2, most CPC-Q scores cluster in the <20 range. While the significance of the CPC-Q to FA correlations did not survive correction for multiple comparisons in this pilot study, the uncorrected correlation coefficients are hypothesis generating for planned larger studies moving forward. Despite these limitations, our initial results suggest that integration of a self-reported questionnaire with measures of white matter integrity from neuroimaging may provide additional avenues for early detection of posterior cortical dysfunction and underlying neurodegenerative etiologies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Colorado Multiple Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SH, BB, and VP contributed to conception and design of the study. SH, BB, and DL-P organized the database. SH performed the statistical analysis and wrote the first draft of the manuscript. SH, BB, CF, and VP wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by grants from the National Institute on Aging (NIA; PI; R01 AG058772, BB, PI), NIH/NCATS Colorado CTSA UL1 TR002535 (R. Sokol, PI), and support from the State of Colorado and many generous philanthropists.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The contents of this article are the authors' sole responsibility and do not necessarily represent official NIH views.
References
1. Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. (2017) 13:870–84. doi: 10.1016/j.jalz.2017.01.014
2. Possin KL. Visual spatial cognition in neurodegenerative disease. Neurocase. (2010) 16:466–87. doi: 10.1080/13554791003730600
3. Isella V, Villa G, Mapelli C, Ferri F, Appollonio IM, Ferrarese C. The neuropsychiatric profile of posterior cortical atrophy. J Geriatr Psychiatry Neurol. (2015) 28:136–44. doi: 10.1177/0891988714554713
4. Holden SK, Bettcher BM, Pelak VS. Update on posterior cortical atrophy. Curr Opin Neurol. (2020) 33:68–73. doi: 10.1097/WCO.0000000000000767
5. Holden SK, Pelak VS, Sooy T, Heffernan KS, McConnell BV, Pressman PS, et al. Development of the Colorado posterior cortical questionnaire within an Alzheimer's disease study cohort. J Clin Exp Neuropsychol. (2022) 44:226–36. doi: 10.1080/13803395.2022.2105820
6. Stricker NH, Salat DH, Foley JM, Zink TA, Kellison IL, McFarland CP, et al. Decreased white matter integrity in neuropsychologically defined mild cognitive impairment is independent of cortical thinning. J Int Neuropsychol Soc. (2013) 19:925–37. doi: 10.1017/S1355617713000660
7. Overman MJ, Zamboni G, Butler C, Ahmed S. Splenial white matter integrity is associated with memory impairments in posterior cortical atrophy. Brain Commun. (2021) 3:fcab060. doi: 10.1093/braincomms/fcab060
8. Agosta F, Mandic-Stojmenovic G, Canu E, Stojkovic T, Imperiale F, Caso F, et al. Functional and structural brain networks in posterior cortical atrophy: a two-centre multiparametric MRI study. Neuroimage Clin. (2018) 19:901–10. doi: 10.1016/j.nicl.2018.06.013
9. Glick-Shames H, Keadan T, Backner Y, Bick A, Levin N. Global brain involvement in posterior cortical atrophy: multimodal MR imaging investigation. Brain Topogr. (2020) 33:600–12. doi: 10.1007/s10548-020-00788-z
10. Sintini I, Schwarz CG, Martin PR, Graff-Radford J, Machulda MM, Senjem ML, et al. Regional multimodal relationships between tau, hypometabolism, atrophy, and fractional anisotropy in atypical Alzheimer's disease. Hum Brain Mapp. (2019) 40:1618–31. doi: 10.1002/hbm.24473
11. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
12. Mungas D, Reed BR, Haan MN, González H. Spanish and English neuropsychological assessment scales: relationship to demographics, language, cognition, and independent function. Neuropsychology. (2005) 19:466–75. doi: 10.1037/0894-4105.19.4.466
13. Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. (1993) 43:2412–4. doi: 10.1212/WNL.43.11.2412-a
14. Mungas D, Reed BR, Crane PK, Haan MN, González H. Spanish and English neuropsychological assessment scales (SENAS): further development and psychometric characteristics. Psychol Assess. (2004) 16:347–59. doi: 10.1037/1040-3590.16.4.347
15. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. (2004) 23 Suppl 1:S208–19. doi: 10.1016/j.neuroimage.2004.07.051
16. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. (2016) 125:1063–78. doi: 10.1016/j.neuroimage.2015.10.019
17. Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. (2008) 40:570–82. doi: 10.1016/j.neuroimage.2007.12.035
18. Zwergal A, Büttner-Ennever J, Brandt T, Strupp M. An ipsilateral vestibulothalamic tract adjacent to the medial lemniscus in humans. Brain. (2008) 131:2928–35. doi: 10.1093/brain/awn201
19. Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol. (2012) 11:170–8. doi: 10.1016/S1474-4422(11)70289-7
Keywords: white matter, posterior cortex, outcome measure assessment, posterior cortical atrophy (PCA), self-report measures, diffusion tensor imaging
Citation: Holden SK, Bettcher BM, Filley CM, Lopez-Paniagua D and Pelak VS (2023) Posterior white matter integrity and self-reported posterior cortical symptoms using the Colorado Posterior Cortical Questionnaire. Front. Neurol. 14:1072938. doi: 10.3389/fneur.2023.1072938
Received: 18 October 2022; Accepted: 16 January 2023;
Published: 01 February 2023.
Edited by:
Tomomichi Iizuka, Fukujuji Hospital, JapanReviewed by:
Robert I. Reid, Mayo Clinic, United StatesYe Wu, Nanjing University of Science and Technology, China
Copyright © 2023 Holden, Bettcher, Filley, Lopez-Paniagua and Pelak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samantha K. Holden,  c2FtYW50aGEuaG9sZGVuQGN1YW5zY2h1dHouZWR1
c2FtYW50aGEuaG9sZGVuQGN1YW5zY2h1dHouZWR1
 Samantha K. Holden
Samantha K. Holden Brianne M. Bettcher
Brianne M. Bettcher Christopher M. Filley
Christopher M. Filley Dan Lopez-Paniagua
Dan Lopez-Paniagua Victoria S. Pelak
Victoria S. Pelak