- Nursing Department, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
Objectives: Malnutrition is an independent risk factor for poor outcomes in patients who suffered an acute ischemic stroke (AIS). The controlling nutritional status (CONUT) score can provide information for nutritional management in AIS patients. However, the risk factors associated with the CONUT score have not been established to date. Therefore, in this study, we aimed to investigate the CONUT score of patients with AIS and explore the potential risk factors associated with it.
Methods: We conducted a retrospective review of the data from consecutive AIS patients who were recruited in the CIRCLE study. Within 2 days of admission, we gathered the CONUT score, the Nutritional Risk Screening 2002, the Modified Rankin Scale, the National Institutes of Health Neurological Deficit Score (NIHSS), and demographic data from medical records. We used chi-squared tests to examine admission, and a logistic regression analysis was performed to explore the risk factors associated with CONUT in patients with AIS.
Results: A total of 231 patients with AIS participated in the study, with a mean age of 62.32 ± 13.0 years and a mean NIHSS of 6.77 ± 3.8. Of these patients, 41(17.7%) had hyperlipidemia. In terms of nutritional assessment, 137(59.3%) patients with AIS had high CONUT scores, 86(37.2%) patients with AIS had low or high BMI, and 117(50.6%) patients with AIS had NRS-2002 scores below 3. The chi-squared tests showed that age, NIHSS, body mass index (BMI), and hyperlipidemia were associated with the CONUT score (P < 0.05). The logistic regression analysis showed that low NIHSS scores (OR = 0.055 95% CI: 0.003–0.893), younger age (OR = 0.159 95% CI: 0.054–0.469), and hyperlipidemia (OR = 0.303 95% CI: 0.141–0.648) were independently associated with lower CONUT scores (P < 0.05), whereas BMI was not found to be independently associated with the CONUT.
Conclusions: More than half of the patients with AIS were at risk of malnutrition, with age and neurological deficits being identified as risk factors for nutritional control. Hyperlipidemia was found to be a protective factor of the CONUT, while NRS-2002 and BMI did not affect the nutritional control in patients with AIS.
Introduction
It is well-known that nutrition is an independent predictor of outcomes in patients with acute ischemic stroke (AIS) (1). AIS patients with malnutrition show a higher frequency of pneumonia, other infections, and bedsores (2). Malnutrition also indicates poor prognosis, such as prolonged hospital stays and disability-related hospital costs (3). In addition, a previous study suggested that malnutrition may negatively affect functional recovery (4). Patients with AIS are vulnerable to the risk of malnutrition because of dysphagia, impaired consciousness, and the inability to maintain an adequate and healthy diet (5). Thus, ensuring proper nutrition is available to patients with AIS is essential to their recovery.
The early evaluation of nutritional status is crucial for developing appropriate nutrition support for patients with AIS. At hospital admission, the prevalence of malnutrition ranged from 7 to 34% in patients with AIS (6). The wide prevalence range may be attributed to the heterogeneous nutritional assessment methods. The European Society for Clinical Nutrition and Metabolism (ESPEN) has the diagnostic criteria for malnutrition but does not assess the risk of malnutrition (7). Although there is no gold standard for screening the risk of malnutrition, there are several nutritional assessment tools, including the Nutritional Risk Screening 2002 (NRS-2002) (8), the Malnutrition Universal Screening Tool (MUST) (9), the Geriatric Nutritional Risk Index (GNRI) (10), the Prognostic Nutritional Index (PNI) (4), the Subjective Global Assessment (SGA) (11), and anthropometric measurements (12). These tools have been used to evaluate nutritional status in patients with various disorders, but they are not always appropriate for patients with emerging diseases such as stroke because of the difficulties in gathering nutritional information (13). Thus, developing a simple and valid tool to assess the risk of malnutrition is significant for nutrition intervention in patients with AIS.
The controlling nutritional status (CONUT) score was initially proposed by Ignacio de Ulíbarri et al. (14) and is used for hospitalized patients. Then, the CONUT score was used for patients with AIS. The CONUT score is a comprehensive and appropriate tool for assessing nutritional status and a more vital prognostic marker for functional recovery in patients with AIS compared to others (15, 16). Furthermore, the CONUT score has predictive validity for all-cause mortality after 3 months in patients who have suffered a stroke (17). In other words, the CONUT score, as a convenient and cost-effective index, can reflect the nutritional status and functional outcomes of patients with AIS.
However, research on the CONUT score focused on its validity and prediction ability in patients with AIS (18). Considering the fact that malnutrition is relatively common in stroke survivors and could result in serious outcomes, identifying the risk factors for the CONUT in patients with AIS is essential. Therefore, this study aimed to identify potential risk factors that might influence the CONUT score in patients with AIS.
Methods
Sample and procedures
In this study, we conducted a retrospective review of the data from consecutive patients who were recruited in the CIRCLE study (19) (ClinicalTrials.gov ID: NCT03702452) between 21 November 2018 and 19 November 2019. The CIRCLE study was designed to verify whether nursing-directed rehabilitation in patients who suffered ischemic strokes can compensate for the lack of professional rehabilitation therapists (20). The inclusion criteria were as follows: (1) patients aged between 18 and 90 years; (2) those having a diagnosis of ischemic stroke by CT or MRI and having met the diagnostic criteria prescribed by the WHO; (3) having an initial ischemic stroke within 7 days with limb dysfunction (muscle strength of the limbs is < 5); (4) those maintaining consciousness (National Institutes of Health Neurological Deficit Score consciousness level 0 or 1); and (5) those who signed an informed consent form. The exclusion criteria were as follows: (1) patients with blood vessels that were recanalized after thrombolysis or thrombectomy; (2) those with cardiopulmonary dysfunction; a history of craniocerebral trauma, fracture trauma, or rheumatoid arthritis; or a physical disability or other diseases that have an impact on the disabled limb; (3) those with cognitive impairment or other mental illness that prevents cooperation with researchers; and (4) those with diseases that affect lymphocyte count. A total of 231 patients were included in this study.
Ethics approval
Informed consent was obtained from the participants before the study, and the human ethics committee approved the protocols of the Second Affiliated Hospital of Zhejiang University. All clinical investigations were conducted according to the principles expressed in the Helsinki Declaration.
Evaluation
All the participants were administered the CONUT score, the Nutritional Risk Screening 2002 (NRS-2002), the Modified Rankin Scale (MRS), and the National Institutes of Health Neurological Deficit Score (NIHSS). Demographic data [age, gender, educational background, body mass index (BMI), the limbs' muscles, hypertension, hyperlipidemia, and diabetes] were gathered from medical records within 2 days from admission.
The controlling nutritional status score
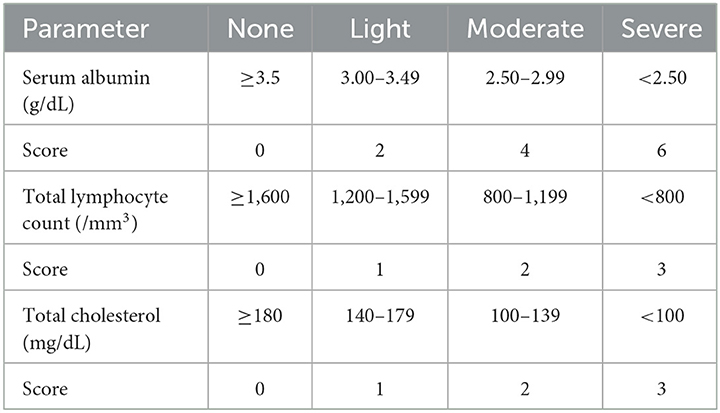
The CONUT scores calculated from the serum albumin concentration, total peripheral lymphocyte count, and total cholesterol concentration are listed in Table 1. The range of the CONUT scores is from 0 to 12; a score of 0 or 1 indicates a normal nutritional status, and higher scores indicate a worse nutritional status (14). According to the original stratification of the CONUT score (normal nutritional status: 0–1; mild malnutrition: 2–4; moderate malnutrition: 5–8; severe malnutrition: 9–12) (21), a CONUT score of 0–4 was used to define the lower risk of malnutrition, and a CONUT score of 5–12 was used to define the higher risk of malnutrition (moderate or severe) in this study. The CONUT score is an effective tool for early detection and continuous control of hospital undernutrition in cases of stroke (18). The samples were collected and analyzed within 2 days of admission.
Nutritional Risk Screening 2002
The NRS-2002 score was calculated from BMI, weight loss over the past 3 months, food intake in recent weeks, and the presence of a fatal disease. Patients with a score of ≥3 were considered at risk of malnutrition (8). The sample was obtained within 2 days of admission.
Modified Rankin Scale
The modified Rankin Scale (MRS) was used to assess the function of patients who suffered a stroke. The MRS ranged from 0 to 6. The range of 0 indicated a normal function status, and higher ranges indicated a worse function status (22). We also recorded the function result 2 days after admission.
National Institutes of Health Neurological Deficit Score
The NIHSS assesses the degree of neurological deficits. It consists of 11 parameters, with a maximum score of 42 points. A score of 0 or 1 indicates a normal neurological status, and higher scores suggest a severe neurological deficit (23). In this study, we recorded the neurological deficit result within 2 days of admission for each patient.
Statistical analysis
Data analysis was conducted using SPSS 24.0. All statistical tests were two-tailed, and an alpha of 0.05 was used to indicate significance. The categorical data were statistical frequencies, including age, gender, education background, muscle of limbs, BMI, NIHSS, MRS, NRS 2002, dysphagia, hypertension, diabetes, and hyperlipidemia, and the chi-squared tests were used to examine the data. Then, a logistic regression analysis with backward stepwise selection (P > 0.10 for exclusion) was performed to examine the association between the CONUT score, age, BMI, hyperlipidemia, and the NIHSS.
Patient and public involvement
Patients and/or the public were not involved in this research's design, conduct, reporting, or dissemination plans.
Results
Clinical characteristics of patients with AIS
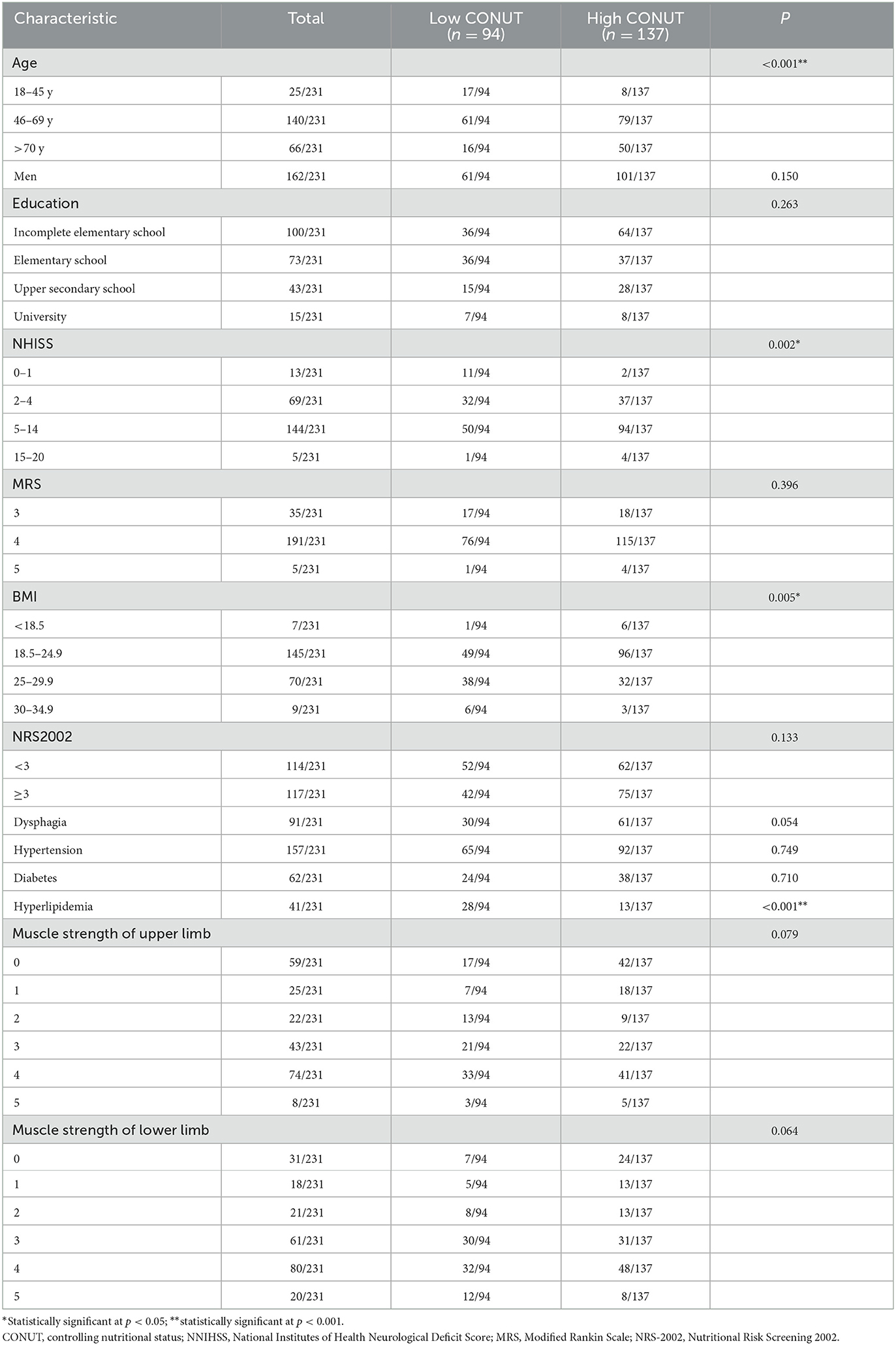
We administered a questionnaire to 231 patients with AIS. The sample comprised 70.1% male participants with a mean age of 62.32 ± 13.0 years. The mean NIHSS score was 6.77 ± 3.8. The mean MRS score was 3.87 ± 0.4. The mean score of the MRC scale was 2.31 ± 1.7 in the upper limbs and 2.87 ± 1.5 in the lower limbs. Comorbidities consisted of hypertension (68.0%), dysphagia (39.4%), hyperlipidemia (17.7%), and diabetes (26.8%). Regarding nutritional assessment, 137 (59.3%) patients with AIS had high CONUT scores, 86 (37.2%) patients with AIS had low or high BMI, and the NRS-2002 scores of 117 (50.6%) patients with AIS were below 3. The demographic characteristics of the 231 patients with AIS are summarized in Table 2.
Risk factors for the CONUT in patients with AIS
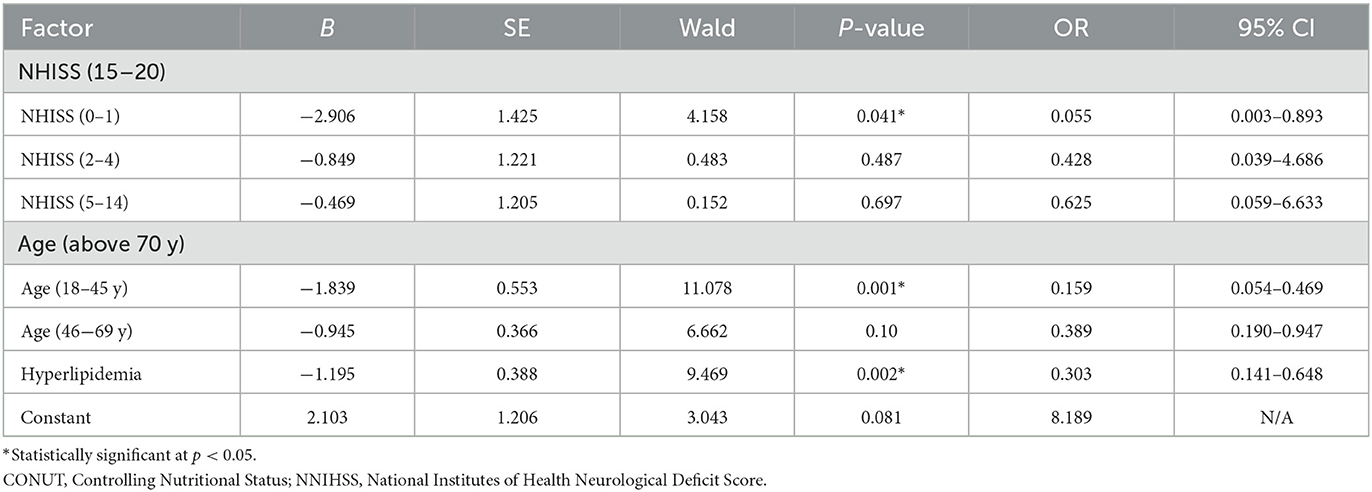
Table 2 shows that age, NIHSS, BMI, and hyperlipidemia were associated with the CONUT score (P < 0.05), according to the results of the univariate analysis. A logistic regression analysis revealed that those patients with AIS who had low NIHSS scores (0–1) were independently associated with lower CONUT scores compared with those with high NIHSS scores (OR = 0.055 95% CI: 0.003–0.893). Younger age (18–44 years) was independently associated with lower CONUT scores than older age (OR = 0.159 95% CI: 0.054–0.469). AIS patients with hyperlipidemia were independently associated with lower CONUT scores (OR = 0.303 95% CI: 0.141–0.648). BMI was not shown to be independently associated with the CONUT scores (Table 3).
Discussion
In this study, the main findings are as follows: (1) More than half of the patients with AIS are at risk for malnutrition or poor nutritional control; (2) older age is independently associated with poor nutritional control; (3) the degree of neurological deficit affects the nutritional control of patients with AIS (severe neurological deficits indicate bad nutritional status); (4) hyperlipidemia is also an independent factor of nutritional status in patients with AIS; and (5) the nutritional assessment of the NRS-2002 and BMI may not affect the nutritional control in patients with AIS.
The CONUT score as a nutritional risk assessment tool is easy to obtain from biochemical parameters, which reflect the risk of malnutrition because of the comprehensive assessment of nutritional status. This study found that more than half of the patients with AIS are under poor nutritional control. This result is similar to the study by Naito et al. (16). In other words, patients who suffered a stroke are likely to experience nutritional problems in the acute stage. It is essential to evaluate the nutritional status of patients with AIS because malnutrition may worsen clinical outcomes (17). Exploring the risk factors for nutritional status can help prevent malnutrition.
In this study, older age was a poor prognosticator for nutritional control. For older adults, natural age-related changes could increase the risk of malnutrition (24). If they suffered acute stroke events, increased protein requirements, alterations to appetite, and declining sensory function could worsen their condition. A previous study showed that advanced age was the main risk factor for malnutrition in patients who suffered a stroke and that it affects recovery (25). In other words, the risk of malnutrition is higher in older patients with AIS, leading to poor outcomes. This study also showed that the risk of malnutrition increases with age.
The NIHSS is a scale that reflects neurological deficits. This study showed that the NIHSS is the independent factor of the CONUT score. In brief, the degree of neurological deficit affects the nutritional control of patients with AIS. AIS patients with critical neurological deficits often have reduced consciousness levels, dysphagia, facial or arm weakness, reduced mobility, cognitive impairments, and poor oral hygiene (26). In the acute stage of a stroke, dysphagia is a significant factor in developing malnutrition, which occurs in 30–50% of patients (27). The disturbance of consciousness may cause patients to not be fed in time after they suffer a stroke, leading to malnutrition. The presence of depression, cognitive impairments, and language deficits could hinder effective communication about food preferences and satiety, leading to malnutrition, particularly with regard to protein intake (28). Moreover, the paralysis of dextromanuality and weakness affects patients' food intake, leading to a premature suspension of feeding (29). In summary, the neurological deficit is an important factor of CONUT in patients with AIS.
In this study, we found an interesting result: AIS patients with hyperlipidemia were associated with lower CONUT scores. In other words, hyperlipidemia may result in a low risk of malnutrition. Cholesterol, an item of the CONUT score, is a part of blood fat, which might be the main reason why hyperlipidemia affects malnutrition. Since hyperlipidemia is a risk factor for cardiovascular and cerebrovascular diseases, maintaining an average blood fat level is crucial for health (30).
In contrast, BMI was not found to be independently associated with the CONUT scores. The most likely explanation is that only seven patients with AIS in the study had a low BMI. Moreover, the risk of malnutrition in the acute stage may not immediately lead to a low BMI soon, meaning that BMI does not reflect current changes in nutrition but is an indicator of malnutrition.
Implications for practice
This study highlights the risk factors associated with malnutrition in patients with AIS and emphasizes the need for early nutritional evaluation and intervention. Close attention should be paid to older patients, especially those with severe neurological deficits. Effective nutritional intervention measures should be developed to decrease the occurrence of malnutrition.
Limitations
This study has some limitations. First, the CONUT score for the chronic stage was not collected; therefore, it was impossible to compare the nutritional control status at different stages. Second, the sample size may be more significant to obtain more stable results. Finally, only one hospital's patients with AIS were included, which may create a representational bias, that is, the representation of all hospitalized individuals was missing, and the study findings might not apply to all patients with AIS.
Conclusion
The risk of malnutrition is high among patients with AIS. The CONUT score can reflect malnutrition. The significant risk factors associated with the CONUT score are older age, severe neurological deficits, and hyperlipidemia. The NRS-2002 and BMI may not be effective screening tools for identifying the risk of malnutrition in the acute stage of patients with stroke.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The Human Ethics Committee approved the protocols of The Second Affiliated Hospital of Zhejiang University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YuC and ML provided critical feedback, edits to drafts of the paper, and managed subsequent revisions. HY contributed to the design of the study and facilitated data acquisition. YaC and HW contributed to the data acquisition and analysis. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No: 81871839).
Acknowledgments
The authors would like to thank the patients with AIS for participating in this research. They are also equally grateful for the assistance of the staff in the department of neurology at The Second Affiliated Hospital, Zhejiang University School of Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yoo SH, Kim JS, Kwon SU, Yun SC, Koh JY, Kang DW. Undernutrition as a predictor of poor clinical outcomes in acute ischemic stroke patients. Arch Neurol. (2008) 65:39–43. doi: 10.1001/archneurol.2007.12
2. FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial. Stroke. (2003) 34:1450–6. doi: 10.1161/01.STR.0000074037.49197.8C
3. Marco E, Sánchez-Rodríguez D, Dávalos-Yerovi VN, Duran X, Pascual EM, et al. Malnutrition according to ESPEN consensus predicts hospitalizations and long-term mortality in rehabilitation patients with stable chronic obstructive pulmonary disease. Clin Nutr. (2019) 38:2180–6. doi: 10.1016/j.clnu.2018.09.014
4. Scrutinio D, Lanzillo B, Guida P, Passantino A, Spaccavento S, Battista P. Association between malnutrition and outcomes in patients with severe ischemic stroke undergoing rehabilitation. Arch Phys Med Rehabil. (2020) 101:852–60. doi: 10.1016/j.apmr.2019.11.012
5. Lieber AC, Hong E, Putrino D, Nistal DA, Pan JS, Kellner CP. Nutrition, energy expenditure, dysphagia, and self-efficacy in stroke rehabilitation: a review of the literature. Brain Sci. (2018) 8:218. doi: 10.3390/brainsci8120218
6. Aliasghari F, Izadi A, Khalili M, Farhoudi M, Ahmadiyan S, Deljavan R. Impact of premorbid malnutrition and dysphagia on ischemic stroke outcome in elderly patients: a community-based study. J Am Coll Nutr. (2019) 38:318–26. doi: 10.1080/07315724.2018.1510348
7. Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition-An ESPEN Consensus Statement. Clin Nutr. (2015) 34:335–40. doi: 10.1016/j.clnu.2015.03.001
8. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M Educational and Clinical Practice Committee European European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr. (2003) 22:415–21. doi: 10.1016/S0261-5614(03)00098-0
9. van der Schueren MA, Guaitoli PR, Jansma EP, de Vet HC. Nutrition screening tools: does one size fit all? a systematic review of screening tools for the hospital setting. Clin Nutr. (2014) 33:39–58. doi: 10.1016/j.clnu.2013.04.008
10. Kang MK, Kim TJ, Kim Y, Nam KW, Jeong HY, Kim SK, et al. Geriatric nutritional risk index predicts poor outcomes in patients with acute ischemic stroke-Automated undernutrition screen tool. PLoS ONE. (2020) 15:e0228738. doi: 10.1371/journal.pone.0228738
11. Martineau J, Bauer JD, Isenring E, Cohen S. Malnutrition determined by the patient-generated subjective global assessment is associated with poor outcomes in acute stroke patients. Clin Nutr. (2005) 24:1073–7. doi: 10.1016/j.clnu.2005.08.010
12. Kimura Y, Yamada M, Kakehi T, Itagaki A, Tanaka N, Muroh Y. Combination of low body mass index and low serum albumin level leads to poor functional recovery in stroke patients. J Stroke Cerebrovasc Dis. (2017) 26:448–53. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.008
13. Akimoto T, Hara M, Morita A, Uehara S, Nakajima H. Relationship between nutritional scales and prognosis in elderly patients after acute ischemic stroke: comparison of controlling nutritional status score and geriatric nutritional risk index. Ann Nutr Metab. (2021) 77:116–23. doi: 10.1159/000515212
14. Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status first validation in a hospital population. Nutr Hosp. (2005) 20:38–45.
15. Kokura Y, Kimoto K, Okada Y, Kawakita S. The Controlling Nutritional Status score as a functional prognostic marker in patients with acute stroke: a multicenter retrospective cohort study. Nutrition. (2020)79–80:110889. doi: 10.1016/j.nut.2020.110889
16. Naito H, Hosomi N, Nezu T, Kuzume D, Aoki S, Morimoto Y, et al. Prognostic role of the controlling nutritional status score in acute ischemic stroke among stroke subtypes. J Neurol Sci. (2020) 416:116984. doi: 10.1016/j.jns.2020.116984
17. López Espuela F, Roncero-Martín R, Zamorano JDP, Rey-Sanchez P, Aliaga-Vera I, et al. Controlling Nutritional Status (CONUT) Score as a predictor of all-cause mortality at three months in stroke patients. Biol Res Nurs. (2019) 21:564–70. doi: 10.1177/1099800419860253
18. Lee EC, Jeong YG, Jung JH, Moon HI. Validity of the controlling nutritional status score as a nutritional assessment tool early after stroke. Int J Rehabil Res. (2022) 45:58–64. doi: 10.1097/MRR.0000000000000503
19. Wang JM, Chen YY, Zhang YP, Li M, Jin JF. Rehabilitation nursing for motor functional recovery of acute ischaemic stroke: study protocol for a randomised controlled trial. BMJ Open. (2020) 10:e037391. doi: 10.1136/bmjopen-2020-037391
20. Wang JM, Zhang YP, Chen YY, Li M, Yang HY, Chen JH, et al. Effectiveness of rehabilitation nursing versus usual therapist-led treatment in patients with acute ischemic stroke: a randomized non-inferiority trial. Clin Interv Aging. (2021) 21:1173–84. doi: 10.2147/CIA.S306255
21. Fukushima K, Ueno Y, Kawagishi N, Kondo Y, Inoue J, Kakazu E, et al. The nutritional index ‘CONUT' is useful for predicting long-term prognosis of patients with end-stage liver diseases. Tohoku J Exp Med. (2011) 224:215–9. doi: 10.1620/tjem.224.215
22. Saver JL, Chaisinanunkul N, Campbell BCV, Grotta JC, Hill MD, Khatri P, et al. Standardized nomenclature for modified rankin scale global disability outcomes: consensus recommendations from stroke therapy academic industry roundtable XI. Stroke. (2021) 52:3054–62. doi: 10.1161/STROKEAHA.121.034480
23. Chalos V, van der Ende NAM, Lingsma HF, Mulder MJHL, Venema E, et al. National institutes of health stroke scale: an alternative primary outcome measure for trials of acute treatment for ischemic stroke. Stroke. (2020) 51:282–90. doi: 10.1161/STROKEAHA.119.026791
24. Bardon LA, Corish CA, Lane M, Bizzaro MG, Loayza Villarroel K, Clarke M, et al. Ageing rate of older adults affects the factors associated with, and the determinants of malnutrition in the community: a systematic review and narrative synthesis. BMC Geriatr. (2021) 21:676. doi: 10.1186/s12877-021-02583-2
25. Sabbouh T, Torbey MT. Malnutrition in stroke patients: risk factors, assessment, and management. Neurocrit Care. (2018) 29:374–84. doi: 10.1007/s12028-017-0436-1
26. Mould J. Nurses must control of the nutritional needs of stroke patients. Br J Nurs. (2009)18:1410–4. doi: 10.12968/bjon.2009.18.22.45572
27. Crary MA, Humphrey JL, Carnaby-Mann G, Sambandam R, Miller L, Silliman S. Dysphagia, nutrition, and hydration in ischemic stroke patients at admission and discharge from acute care. Dysphagia. (2013) 28:69–76. doi: 10.1007/s00455-012-9414-0
28. Corrigan ML, Escuro AA, Celestin J, Kirby DF. Nutrition in the stroke patient. Nutr Clin Pract. (2011) 26:242–52. doi: 10.1177/0884533611405795
29. Westergren A. Nutrition and its relation to mealtime preparation, eating, fatigue and mood among stroke survivors after discharge from hospital - a pilot study. Open Nurs J. (2008) 2:15–20. doi: 10.2174/1874434600802010015
30. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
Keywords: risk factor, acute stage, ischemic stroke, controlling nutritional status, malnutrition
Citation: Chen Y, Yang H, Lan M, Wei H and Chen Y (2023) The controlling nutritional status score and risk factors associated with malnutrition in patients with acute ischemic stroke. Front. Neurol. 14:1067706. doi: 10.3389/fneur.2023.1067706
Received: 12 October 2022; Accepted: 16 February 2023;
Published: 09 March 2023.
Edited by:
Michael V. Mazya, Karolinska University Hospital, SwedenReviewed by:
Susan Alderman, University of Texas Health Science Center at Houston, United StatesMona Laible, Ulm University, Germany
Copyright © 2023 Chen, Yang, Lan, Wei and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meijuan Lan, bGFubWpAemp1LmVkdS5jbg==
 Yuanyuan Chen
Yuanyuan Chen Hongyan Yang
Hongyan Yang Meijuan Lan
Meijuan Lan Hui Wei
Hui Wei